Abstract
Recent research on chiropteran parasites suggests a high prevalence and diversity, and extensive spatial distribution of filarial species; however, ecological and phylogenetic studies are still in their infancy. We sampled blood from 78 bat specimens, collected 1181 ectoparasites at summer colonies in Armenia and Georgia, and used nested-PCR targeting the cytochrome c oxidase subunit 1 (cox1) gene to detect and genotype filarial parasites. The overall prevalence of filarial DNA was 17.9% in blood samples from Myotis blythii, Myotis emarginatus, Miniopterus schreibersii, and Rhinolophus ferrumequinum, and 8.5% in ectoparasites, including two mite species (Eyndhovenia euryalis and Spinturnix myoti) and two bat flies (Nycteribia kolenatii and Penicillidia dufouri). The prevalence of microfilarial infection was significantly higher in mite samples (13.8%) than in bat fly samples (4.1%). Bats with ectoparasites positive for filarial DNA had a significantly higher total number of ectoparasites. Phylogenetic analysis placed the 18 sequences obtained into different closely related clades of onchocercid nematodes, with four different species recorded: two belonging to the genus Litomosa and two to a newly observed genus of the family Onchocercidae. Additionally, two new species of these parasites, one Litomosa sp. and one Onchocercid sp., were genetically recognised. As predicted, the diversity of filarial parasites reflects the diversity of bat hosts in the Caucasus. Extending the sampling effort to more Caucasian bat species will likely reveal previously unknown filarial species. Non-lethal and non-invasive sampling of blood and ectoparasites for molecular screening proved effective for gaining insights into parasite diversity and phylogenetic relationships of bat-infecting filarial nematodes.
Keywords: Vector-borne parasites, Bat blood parasites, Filarioidea, Nematodes, Onchocercidae, Bat mites, Bat flies, cox1
Graphical abstract
Highlights
-
•
High prevalence and diversity of filarial species in the blood of Caucasian bats.
-
•
Filarial DNA in potential vectors: bat ectoparasites (mites and bat flies).
-
•
Four filarial species: two Litomosa and two from a new genus of the Onchocercidae.
-
•
Non-invasive sampling and molecular screening are effective for studying filarial bat diversity and phylogeny.
1. Introduction
Biological diversity refers to the variety of life forms on Earth, encompassing different levels of biological organization, including genetic diversity, species diversity, and ecosystem diversity (Gaston and Spicer, 2012). Some biogeographical regions with significant biodiversity qualify as biodiversity conservation hotspots (Myers et al., 2000). Two biodiversity hotspots integrate in Armenia and Georgia, the Caucasus and the Irano-Anatolian, where species richness, rarity, and endemism are surprisingly high for a terrestrial temperate zone region due to topographic and ecosystem heterogeneity (Mittermeier et al., 2004). A simple approach to measure biodiversity is through species counts, and in this ecoregion, the chiropteran fauna consists of 35 species of bats (Bukhnikashvili et al., 2009). However, understanding biodiversity involves more than just counting species; various components of evolutionary biodiversity, such as phylogenetic diversity, play a vital role in maintaining the functioning of ecosystems (Faith, 1992).
Parasites are thought to make up a large fraction of the total biodiversity on Earth (Poulin, 2014). Parasite diversity is closely linked to the diversity of their hosts (Poulin, 2014); as such, a greater variety of parasites signifies a well-functioning ecosystem with a complex network of host-parasite interactions (Lafferty, 2012). Importantly, the presence of cryptic and undiscovered species, occurring in both bats (Mayer et al., 2007) and parasites (Poulin, 2014), has complicated the situation regarding the revelation of true diversity; however, this is changing with the application of molecular taxonomy methods.
Traits that make bats unique hosts include their ancient origin and co-evolution with pathogens, the ability to fly and disperse infectious agents over large geographical ranges, the ability to enter daily torpor and seasonal hibernation associated with the suppression of immune responses to infectious agents, their long life spans, high population densities, crowded roosting behaviour, multi-species colonies that promote direct contact and intra- and inter-specific transmission of infectious agents, and fidelity to roosts allowing indirect spread of pathogens (Calisher et al., 2006). In a meta-analysis, Kamiya et al. (2014) concluded that host body size, geographical range, and population density consistently predict parasite diversity across host species.
Threadlike filarioid nematodes parasitise all classes of vertebrates except fish (Notarnicola, 2024). These slender nematodes reside in tissues, vessels, heart ventricles, and body cavities and have an indirect life cycle via hematophagous arthropods. Female filariae are ovoviviparous, producing microfilariae that migrate through tissues and circulate in the lymph and blood. When ingested by a competent ectoparasite vector, microfilariae continue to develop into infective larvae and may be transmitted during blood-feeding to the next host.
Parasites of bats are an understudied topic compared to viruses (Hayman, 2016) and emerging fungal agents that threaten chiropteran biodiversity (Zukal et al., 2016). An exception to this is the filarioid nematodes from the family Onchocercidae, of the genera Litomosa and Litomosoides, with many species parasitic in bats in the Old and New World, respectively (Junker et al., 2009). Around two dozen species of Litomosa are known (Martin et al., 2006), mostly based on morphological characteristics, as taxonomic identification of the species combined with sequences of 12S rDNA and/or cox1 genes is available for Litomosa chiropterorum and Litomosa westi only (Casiraghi et al., 2004; Junker et al., 2009). Filariae diversity has recently been detected in bats from Madagascar (Ramasindrazana et al., 2016) and in parti-coloured bats (Vespertilio murinus) in the Czech Republic and Russia (Pikula et al., 2023; Bednarikova et al., 2025). Chiropteran ectoparasites comprise many arthropod species (Léger, 2020). Bednarikova et al. (2025), for example, found a macronyssid mite Steatonyssus spinosus collected from V. murinus positive for mixed microfilarial infection, suggesting a role of this ectoparasite as a potential vector of filarial infection. However, further epidemiological studies are needed to describe the diversity of filarial species in bats and their ectoparasites, life cycles, host ranges, infection prevalence and intensity, parasite pathogenicity, and competent vectors.
The protected status of bats limits our ability to study their parasite diversity using traditional morphological methods. Since data on the diversity of filariae circulating in the South Caucasus are completely lacking, we subjected blood samples and ectoparasites collected from bats in Armenia and Georgia to molecular screening for onchocercid parasites. We predict that parasite diversity will reflect both bat host species diversity and sampling effort, and generalist filarial parasites will reflect the possibility of interspecific transmission in crowded multi-species bat colonies.
2. Materials and methods
2.1. Sampling bat blood and ectoparasites
In 2023, blood (100 μl per bat) and ectoparasites were collected from 38 bats at two Armenian localities (Gethashen Cave, Gndevang Canyon, 39°41′59.8″N, 45°33′43.8″E; and Arakelots Monastery Complex, Acharkut village, 41°02′01.1″N, 45°04′00.9″E) and from 40 bats at one Georgian locality (Ghliana Cave, Tskaltubo town, 42°22′24.6″N, 42°35′50.7″E). The bats were identified as the lesser mouse-eared bat Myotis blythii (n = 40), Geoffroy’s bat Myotis emarginatus (n = 11), the pale bent-wing bat Miniopterus pallidus (n = 10), Schreiber’s bent-wing bat Miniopterus schreibersii (n = 8), and the greater horseshoe bat Rhinolophus ferrumequinum (n = 9). Sex, age, morphometric parameters (weight, forearm length), and ectoparasite counts were recorded on site.
All 78 blood samples were collected from the distal plagiopatagial vessel (vena cephalica) using a sterile needle and heparinized pipette tip after disinfecting the skin with alcohol, as described in Pikula et al. (2023). The samples were then stored at −20 °C for further analysis.
A total of 1181 ectoparasites were removed from the bats using forceps and identified based on external morphological characteristics under a Leica EZ4 stereomicroscope (Leica, Wetzlar, Germany). Identification was carried out using the published guides of Estrada-Peña et al. (2017) for ixodid ticks, Beĭ;-Bienko (1970) for Nycteribiidae bat flies and Siphonaptera, Hutson (1984) for Nycteribiidae, and Orlova et al. (2015) for Spinturnicidae and Macronyssidae. The ectoparasites were then grouped into 165 pooled samples according to their species and bat origin (see Supplementary Table S1 for more details) and stored in 70% ethanol for further analysis.
2.2. Molecular screening for filarial DNA
DNA was isolated from blood samples using the NucleoSpin® Blood Kit (Macherey-Nagel, Dueren, Germany) and from ectoparasites using the NucleoSpin® Tissue Kit (Macherey-Nagel), according to the manufacturer’s instructions. An Implen NanoPhotometer (Implen, Munich, Germany) was used to evaluate the quantity and quality of the isolated DNA, after which the DNA samples were stored at −20 °C until further use.
All samples were screened for filarial DNA using nested PCRs targeting the partial gene of mitochondrial cytochrome c oxidase subunit 1 (cox1). PCR reactions followed the protocol published in Bednarikova et al. (2025) (for primers and PCR conditions, see Table 1). All PCR reactions were carried out using a GeneExplorer Thermal Cycler (Hangzhou Bioer Technology Co. Ltd., Hangzhou, China), with a negative (PCR grade water) and positive (DNA isolated from Dirofilaria immitis) control included in each run. All PCR products visualized on a 1.5% agarose gel stained with Serva DNA Stain G (Serva, Heidelberg, Germany) under UV light like amplicons of the appropriate size 689 bp were purified using the NucleoSpin® Gel and PCR Clean-up Kit (Macherey-Nagel) and then commercially sequenced using Sanger sequencing (SEQme s.r.o, Dobříš, Czech Republic). The sequences obtained were then compared with available sequences in the GenBank databases using MegaBLAST and edited using Geneious Prime software (Biomatters Ltd., Auckland, New Zealand).
Table 1.
Primers and PCR conditions used for amplification of the cox1 gene in the present study.
| Primer | Sequence (5‘-3‘) | Product length | Tann | Reference |
|---|---|---|---|---|
| CF F3 | TTCTGTTTTDACTATRCATGG | 957 bp | 53 °C | Bednarikova et al. (2025) |
| CF R5 | GCHACAACATAATAAGTATCATG | |||
| COI int F | TGATTGGTGGTTTTGGTAA | 689 bp | 53 °C | Casiraghi et al. (2001) |
| COI int R | ATAAGTACGAGTATCAATATC |
Abbreviations: Tann, annealing temperature.
2.3. Phylogenetic analysis
Two phylogenetic trees of the cox1 gene were constructed to assess the sequences detected. First, a phylogenetic tree covering the entire superfamily Filaroidea was built to confirm and specify the identity and phylogenetic position of sequences from the present study (data not shown). A detailed analysis of Litomosa spp., Litomosoides spp., and closely related genera was then performed based on the initial analysis. For this analysis, all unique cox1 sequences longer than 300 bp available in the GenBank database were used, while representative sequences were used to construct the second phylogeny (Fig. 1 and Supplementary file 2: Fig. S1). All phylogenies were inferred by IQ-TREE v.1.6.12 (Nguyen et al., 2015), with the best-fit evolution model selected based on the Bayesian information criterion, computed and implemented using ModelFinder (Kalyaanamoorthy et al., 2017). Branch supports were assessed by ultrafast bootstrap (UFBoot) approximation (Minh et al., 2013) and the Shimodaira-Hasegawa-like approximate likelihood ratio test (SH-aLRT) (Guindon and Gascuel, 2003). Trees were then visualised and edited in FigTree v.1.4.4 and Inkscape v.1.3, respectively. The final length of the alignment for the phylogenies was 1653 bp, and the alignment contained 138 sequences (18 generated in the present study). The tree was constructed using the evolution model TN + F + I + G4. Two sequences of Acanthocheilonema viteae were used as the outgroup.
Fig. 1.
Schematic representation of a maximum likelihood phylogenetic tree based on the cox1 gene sequences of genera closely related to Litomosa spp. (A) and detailed phylogeny of Litomosa spp., Litomosoides spp., and closely related Onchocercidae spp. (B). The final length of the alignment was 1653 bp and contained 138 sequences, 18 of which were generated in this study. The tree was constructed using the evolution model TN+F+I+G4. Two sequences of Acanthocheilonema viteae were used as the outgroup. Sequences from this study are marked in bold and blue. The scale-bar indicates the number of nucleotide substitutions per site. Bootstrap values (SH-aLRT/UFB) above the 80/95 threshold are displayed. Sequences are labelled by accession number, species, host, and country of origin, if available.
2.4. Statistical analysis
All variables were tested for normality of distribution using the Shapiro-Wilk test. As most parameters were non-normally distributed, even after log-transformation, patterns of filarial infection distribution in all samples, with respect to combinations of species and locality, were assessed using the non-parametric Mann-Whitney and Chi-square tests. The percentage of positive samples in different groups was compared by testing the difference between the two proportions. All statistical analyses were conducted using the TIBCO Statistica® software package v.14.0.0 (TIBCO Software Inc., Palo Alto, California, USA).
A bipartite interaction network was created in which bat and ectoparasite species are represented by nodes and interacting species are linked by lines, with the width of the line proportional to the abundance of ectoparasites found on the bat species. The same procedure was also used to create an ectoparasite-filariae interaction network. The networks were built and analysed using the bipartite R package (Dormann et al., 2008), based on the parameters outlined in Ospina-Pérez et al. (2023). To characterize the properties of the interaction network, we calculated four metrics: the entropy index (H2′) (Blüthgen et al., 2006), quantitative modularity (QuanBiMo) (Fortuna et al., 2010), connectance (C) (Blüthgen et al., 2006), and nestedness (wNODF) (Almeida-Neto and Ulrich, 2011). H2′ quantifies species-level specialization, while QuanBiMo detects the existence of a modular structure within the network. The C index measures the proportion of realized interactions, and wNODF assesses the degree to which less-connected species interact with those of highly connected species (Blüthgen et al., 2006; Fortuna et al., 2010; Almeida-Neto and Ulrich, 2011).
3. Results
A total of 14 bat blood samples and 14 ectoparasite pools tested positive for filarial nematodes on nested PCR targeting cox1. All positive samples were successfully sequenced, producing 18 unique sequences. The sequences obtained were aligned and separated into four groups based on sequence distance and similarity. Each group was aligned separately, and the consensus of the alignment was used for the BLAST analysis (Table 2). All unique nucleotide sequences of the cox1 gene produced in this study were deposited in GenBank under the accession numbers PV023858-PV023875.
Table 2.
BLAST analysis for sequences obtained in this study.
| Sequence group | No. of sequences | Sequence homology (%) | Length of group alignment | BLAST result (% query cover/% identity)a |
|---|---|---|---|---|
| Group 1 | 5 | 97.3–99.6 | 660 bp | PQ042402 (100/99.70) |
| Group 2 | 3 | 98.6–99.5 | 661 bp | KP728059 (97/90.39) |
| Group 3 | 3 | 99.2–99.4 | 663 bp | PQ042407 (100/99.70) |
| Group 4 | 7 | 99.7–99.9 | 663 bp | PQ042415 (100/89.14) |
Consensus of the group alignment used for BLAST analysis.
The cox1 phylogeny of all available sequences for the superfamily Filaroidea clearly placed the sequences obtained in this study into a cluster containing the bat-infecting genera Litomosa and Litomosoides (data not shown). The detailed phylogeny of Litomosa, Litomosoides, and closely related genera placed the sequences obtained in this study into four well-defined clades corresponding to the groups used for the BLAST analysis, representing four filarial nematode species (Fig. 1). Sequences of Group 1 formed a clade with sequences of Litomosa sp. detected in V. murinus in the Czech Republic, while sequences of Group 2 formed a separate well-defined clade in a sister position to L. chiropterorum. The remaining sequences formed two well-supported clades separated from both Litomosa spp. and Litomosoides spp. The first clade containing Group 3 along with sequences of undescribed filarial nematodes reported as Onchocercid sp. in our previous study, obtained from V. murinus in the Czech Republic or its ectoparasites in Russia (Bednarikova et al., 2025). Sequences of Group 4 formed a new clade in a sister position to the clade containing Group 3.
Molecular analysis of 78 blood samples using nested PCR targeting the partial cox1 gene showed a total prevalence of 17.9% (14/78) filarial infection. Positive findings came from all bat species tested except M. pallidus, and only from females (14/70), corresponding to a prevalence of 20.0%. There were no significant differences in the distribution of negative and positive blood and bat ectoparasite samples between the species or localities examined (χ2 = 7.847, P = 0.097 and χ2 = 3.166, P = 0.530, respectively). Based on sequence comparisons and phylogenetic analysis, occurrence of two species of Litomosa sp. was determined in both cases in two samples, and, in a further 10 positive samples, an undescribed genus of the family Onchocercidae containing two species: the Onchocercid sp. already recorded by us (Group 3, n = 4) and a completely new species (Group 4, n = 6), with 1–3 species of filariae per bat species (Table 3).
Table 3.
Prevalence of filarial infection in bat blood samples.
| Species (Group) | Overall |
Armenia |
Georgia |
M. blythii |
M. emarginatus |
M. schreibersii |
R. ferrumequinum |
|---|---|---|---|---|---|---|---|
| % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) | |
| Litomosa sp. (Group 1) | 2.6 (2/78) | 2.5 (1/40) | 2.6 (1/38) | 0 (0/40) | 0 (0/11) | 12.5 (1/8) | 11.1 (1/9) |
| Litomosa sp. (Group 2) | 2.6 (2/78) | 5.0 (2/40) | 0 (0/38) | 0 (0/40) | 0 (0/11) | 0 (0/8) | 22.2 (2/9) |
| Onchocercid sp. (Group 3) | 5.1 (4/78) | 5.0 (2/40) | 5.3 (2/38) | 7.5 (3/40) | 0 (0/11) | 0 (0/8) | 11.1 (1/9) |
| Onchocercid sp. (Group 4) | 7.7 (6/78) | 10.0 (4/40) | 5.3 (2/38) | 10.0 (4/40) | 18.2 (2/11) | 0 (0/8) | 0 (0/9) |
| Total | 17.9 (14/78) | 22.5 (9/40) | 13.2 (5/38) | 17.5 (7/40) | 18.2 (1/11) | 12.5 (1/8) | 44.4 (4/9) |
Abbreviations: %, prevalence in %; n, number of positive samples; N, total number examined.
Seventy-six of the bats monitored were parasitized by arthropods. Nested PCR of the 165 pooled bat ectoparasite samples obtained indicated the presence of filarial DNA in 8.5% of the samples (14/165; Table 4). Bats with filarial DNA-positive ectoparasites had a significantly higher total number of ectoparasites (Mann-Whitney test, Z = −2.317, P = 0.020) than those without. Of the 12 ectoparasite species identified to the species level (2.2 species of ectoparasite per bat on average), only two mite species (Spinturnix myoti and Eyndhovenia euryalis) and two bat fly species (Nycteribia kolenatii and Penicillidia dufouri) tested positive. The interaction networks consisted of a relatively low number of species (Fig. 2, Fig. 3). The quantitative modularity QuanBiMo and specialization calculated for the interaction networks were low in both cases (bat-ectoparasite network: H2′ = 0.44 and Q = 0.20; filaria-ectoparasite network: H2′ = 0.15 and Q = 0.21), indicating a lack of niche differentiation in the networks. This was confirmed by the low connectance (C = 0.39 and C = 0.45, respectively) and nestedness (wNODF = 28.75 and wNODF = 28.13, respectively) observed in both networks. Each of the positive ectoparasite species hosted 1–3 species of filariae, and each filarial species was detected in 1–4 ectoparasite species. The prevalence of microfilarial infection was significantly higher in mite samples (13.8%) than bat fly samples (4.1%; Difference test, z = −2.076, P = 0.038), regardless of location and bat species. Likewise, we also confirmed a significantly higher prevalence of microfilariae DNA in mites (21.9%) than bat flies (5.3%; Difference test, z = −2.065, P = 0.039) in samples of M. blythii from Ghliana Cave (Georgia). Nevertheless, we found no significant impact of filarial infection in this bat species in relation to morphological parameters (Mann- Whitney test, forearm length Z = −0.512, P = 0.608; body weight Z = 0.952, P = 0.341).
Table 4.
Prevalence of filarial infection in pooled bat ectoparasite samples.
| Species (Group) | Overall |
Armenia |
Georgia |
S. myoti |
E. euryalis |
N. kolenatii |
P. dufouri |
|---|---|---|---|---|---|---|---|
| % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) | % (n/N) | |
| Litomosa sp. (Group 1) | 4.2 (7/165) | 1.35 (1/74) | 6.6 (6/91) | 6.6 (4/61) | 20.0 (1/5) | 3.3 (1/30) | 6.3 (1/16) |
| Litomosa sp. (Group 2) | 0.6 (1/165) | 1.35 (1/74) | 0 (0/91) | 0 (0/61) | 20.0 (1/5) | 0 (0/30) | 0 (0/16) |
| Onchocercid sp. (Group 3) | 1.8 (3/165) | 0 (0/74) | 3.3 (3/91) | 3.3 (2/61) | 0 (0/5) | 3.3 (1/30) | 0 (0/16) |
| Onchocercid sp. (Group 4) | 1.8 (3/165) | 2.7 (2/74) | 1.1 (1/91) | 4.9 (3/61) | 0 (0/5) | 0 (0/30) | 0 (0/16) |
| Total | 8.5 (14/165) | 5.4 (4/74) | 11.0 (10/91) | 14.5 (9/61) | 40.0 (2/5) | 6.5 (2/30) | 6.3 (1/16) |
Abbreviations: %, prevalence in %; n, number of positive samples; N, total number examined.
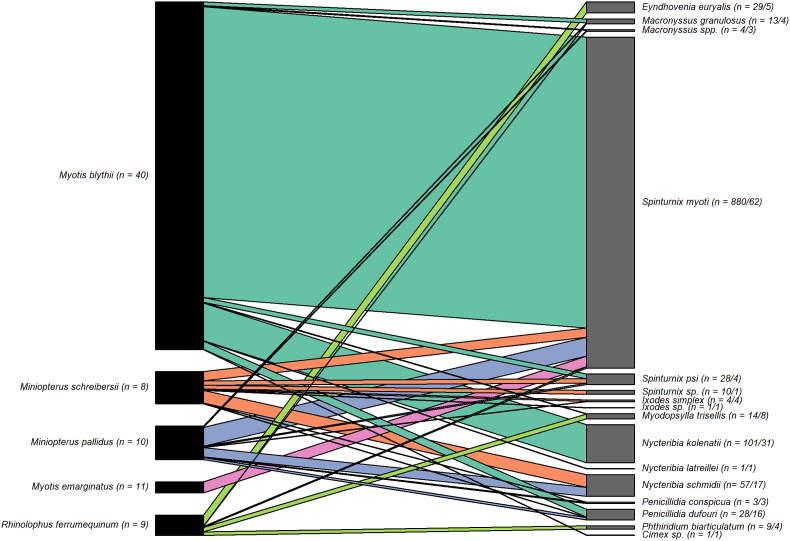
Fig. 2.
Bipartite bat-ectoparasite quantitative network. The size of the black bars on the left is proportional to the number of ectoparasite species found on a given bat species. The value of “n” on the left indicates the number of bats of that species examined. The size of the grey bars on the right is proportional to the abundance (number of individuals) of ectoparasites per species observed. The value of “n” on the right represents the number of ectoparasites/number of pooled samples. The width of the coloured lines/bars indicates the abundance of ectoparasites found on a given bat species.
Fig. 3.
Bipartite ectoparasite-filariae quantitative network. The size of the grey bars on the left is proportional to the number of filarial species found in a given ectoparasite species. The number “n” on the left is the number of positive pooled ectoparasite samples for a given ectoparasite species. The size of the black bars on the right is proportional to the number of ectoparasite samples positive for filariae of that species. The value of “n” on the right indicates the number of pooled ectoparasite samples testing positive for a given filarial species. The width of the coloured bars indicates the abundance of filariae found for a given ectoparasite species.
Of the 14 bats whose blood tested positive for presence of filarial DNA, only 3 tested positive for ectoparasites, of which only 2 were hosting the same filarial species. On the other hand, 14 positive pooled ectoparasite samples matched positive bats in four cases, meaning that 10 positive pooled ectoparasite samples were collected from bats that were negative for filarial DNA.
The filarial DNA related to L. chiropterorum (Group 2) was only present in R. ferrumequinum from the Getashen Cave (Armenia). In one case, it was present in both blood and E. euryalis mites collected from the same individual. Similarly, Onchocercid sp. (Group 4) was detected in both blood and S. myoti ectoparasites from a single bat, one of two cases in which M. blythii carried two ectoparasite species, S. myoti and N. kolenatii, positive for different species of filariae. In this case, while Onchocercid sp. from Group 4 and Onchocercid sp. from Group 3 were identified in S. myoti and N. kolenatii, respectively, collected from one bat, mites and bat flies of the second bat were positive for Onchocercid sp. from Group 3 and Litomosa sp. from Group 1, respectively. Filarial DNA of Onchocercid sp. from Group 4 was only found in three pooled samples of S. myoti collected from M. blythii and M. pallidus bats. This filaria was not detected in any other ectoparasite species, even when these ectoparasites were collected from an infected animal and/or from the same animal as a positive S. myoti pooled sample. Filarial DNA of Onchocercid sp. from Group 3 was detected in pooled samples of S. myoti (n = 2) and N. kolenatii (n = 1), whereas DNA of Litomosa sp. from Group 1 was detected in four different ectoparasite species (E. euryalis, S. myoti, N. kolenatii, and P. dufouri).
4. Discussion
In this study, we used molecular detection methods to assess the diversity of filariae circulating in five South Caucasian bat species and their ectoparasites. We found a significant prevalence of single and mixed infections with four filarial species, two species of Litomosa and two species of a genus of the family Onchocercidae, for which sequence data are now available for the first time. Notably, filarial infection had no apparent impact on bat health, measured as body size (forearm length) or body mass. Our data suggest that each of these filarial parasites is a generalist occurring in several bat species. Since there are 35 bat species known from this geographical region, if we assume one to three filarial parasite species per bat species, the overall diversity can be estimated as ranging between four and 28 filarial species. Interestingly, while M. pallidus proved negative as a host, its ectoparasite S. myoti tested positive. Two mite species and two bat fly species were found positive, suggesting that they may be vectors of microfilariae; however, it would be necessary to confirm their vectorial competence experimentally.
4.1. Filarial diversity in bat hosts
An important finding of our study is that circulating microfilariae can be identified in the peripheral blood of bats; consequently, the information gained regarding their DNA sequences offers the possibility of using blood samples for the detection of onchocercid nematodes in these wild mammals. Molecular detection revealed blood microfilariae in 17.9% of bat blood samples, a similar level to our previous study (Bednarikova et al., 2025), indicating a high rate of parasitism in bats. Detection of microfilariae in peripheral blood is easier than finding adult worms in the animal body, which is often a limiting factor in the study of free-living animals due to the need for euthanasia of examined hosts (McKeand, 1999; Bain et al., 2008). This is also complicated by similarities in the morphology of microfilariae (Jiménez et al., 2021). On the other hand, molecular methods have simplified the detection of filariae in both definitive hosts and vectors and provides the opportunity to discover new filariae species or to supplement existing morphological descriptions with genetic data.
While non-lethal approaches may be less precise, the screening of large numbers of individuals, as in our study, helps offset this. We believe that invasive, non-lethal alternatives, such as blood sampling combined with molecular techniques, provide sufficient data on filarial species diversity. The method can also serve as a predictor of filarial occurrence, and also has the advantage of relatively quick testing time compared to collecting and dissecting the cadavers of wild animals (Bednarikova et al., 2025). However, it is important to note that the prevalence reported in the present study may not be accurate, as microscopic detection of microfilariae or necropsy were not performed. The sole presence or lack of detectable DNA in blood may not represent actual infection of the animal. Indeed, Bednarikova et al. (2025) confirmed noticeable variability in their samples, with only one of four bats testing positive for Litomosa sp. in blood-associated organs, despite the presence of adult worms in the body. On the other hand, 30.4% of heart and spleen samples proved positive for bats in which adult filariae were not found.
Very few bat helminth taxa show significant, non-random host species preference; indeed, the genus Litomosa (Onchocercidae) is often cited as an example of narrow host specificity at the host-parasite species level. At the same time, it should be recalled that host preference correlates with the bat host’s preference for a particular habitat type and/or roost (Hosek and Horacek, 1987). Nevertheless, we found no significant difference in the distribution of positive and negative samples between species and locations, suggesting that filarial infections may be evenly distributed over the South Caucasus, and that environmental factors may not have a significant impact on the parasite prevalence. Our results also suggest that filariae are not specific to one host species. One exception may be Litomosa sp. (Group 2), which occurred in the blood of one bat species only, i.e. R. ferrumequinum. Whether it truly is a parasite specific to this distinct Yinpterochiroptera group of bats requires further investigation. Other species of onchocercid nematode (Groups 1, 3, and 4) were consistently found in two bat species in different combinations, regardless of genera, i.e. Myotis or Miniopterus. Furthermore, Litomosa sp. (Group 1) was previously recorded in V. murinus (Pikula et al., 2023; Bednarikova et al., 2025), a bat with a different taxonomic classification (family Vespertilionidae) and life strategy than M. schreibersii (family Miniopteridae). It should be noted that the monitored bats came from species-mixed colonies (see Supplementary Table S1).
Hosek and Horacek (1987) noted that the genus Litomosa was found almost exclusively in bats that form large breeding colonies; this is also supported by our positive detections in M. schreibersii and R. ferrumequinum. On the other hand, Litomosa was absent from tree-dwelling species such as Nyctalus spp., Myotis bechsteini, and Myotis nattereri, which refutes our previous studies focused on V. murinus, a bat with a lifestyle partly tied to forests (Pikula et al., 2023; Bednarikova et al., 2025). Hosek and Horacek (1987) also mentioned the frequent occurrence of Litomosa spp. in bat pups, suggesting very early vector-borne or transplacental infection (Haque and Capron, 1982; Eberhard et al., 1993). Indeed, Bednarikova et al. (2025) recently confirmed two adult Litomosa sp. worms in the uterine cavity of a mid-gestation pregnant V. murinus female.
Most species of filariae are known only from their original morphological descriptions that lack context for understanding host specificity and distribution. Some species of Litomosa, such as Litomosa desportesi (Bain, 1966) or Litomosa adami (Petit, 1980), have been found only once, while others, such as Litomosa filaria (Bain, 1966) or Litomosa ottavianii (Esteban et al., 1991, 1999; Horvat et al., 2015), have undergone multiple precise morphological analyses. These studies revealed significant diversity among filarial species. Current possibilities regarding use of molecular data could contribute significantly to a better understanding of these parasites and their relationships with host species (Jiménez et al., 2021).
Our study relied on cox1, one of the most frequently used markers for identifying filariae. Based on our phylogenetic data, four species of filarial nematode were detected in South Caucasian bats and their ectoparasites. Two of these species were previously detected in V. murinus in the Czech Republic and in its ectoparasites in Russia, both forming uniform clades with their respective sequences, confirming the BLAST results (Pikula et al., 2023; Bednarikova et al., 2025). Compared to the previously published phylogeny (Bednarikova et al., 2025), both onchocercid species formed a separate, well-defined cluster with high support, suggesting a novel filarial genus in bats. While the sequences of L. westi formed a distinct clade separated from other Litomosa spp., they remained part of the genus, contrary to our previously published phylogenetic trees (Bednarikova et al., 2025). Interestingly, the newly observed Litomosa sp. (Group 2) formed a distinct clade in sister position to L. chiropterorum, which is a parasite of Miniopterus bats (M. natalensis, previously included as a subspecies of M. schreibersii) in South Africa (Junker et al., 2009). This could suggest that the range of this Litomosa sp. stretches further south than that of the Litomosa sp. found in V. murinus.
Some authors have claimed that, based on morphology, L. chiropterorum is closely related to L. adami (ex Miniopterus m. minor, Gabon), L. goodmani (ex Miniopterus gleni, Madagascar), Litomosa sp. (ex Miniopterus manavi, Madagascar), L. seurati (ex R. ferrumequinum, Algeria), and L. ottavianii (ex M. blythii, Sardinia, Europe) (Martin et al., 2006; Junker et al., 2009). As Rhinolophus ferrumequinum is the type-species host for L. seurati (Algeria, studied by Seurat, 1921, and transcribed by Bain, 1966; Martin et al., 2006) and L. filaria (Turkey, Merdivenci, 1964), the sequences of the newly moleculary detected Litomosa sp. (Group 2) could belong to one of these species. Furthermore, since no morphological data are available for the detected and yet undescribed species of the Onchocercidae (Groups 3 and 4), these may also represent filarial species historically identified as Litomosa spp.
4.2. Filarial diversity in bat ectoparasites
To our knowledge, no other study has reported detecting microfilariae DNA in both the blood and ectoparasites of insectivorous bats. Bat ectoparasites cover a range of evolutionary lineages, including diverse mites, ticks, bat flies, and fleas. Many of these parasites exhibit high host specificity, often parasitizing a limited spectrum of closely related bat species. In addition to strictly host-specific parasites, several species occur on multiple bat species of the same genus (e.g. Myotis) or on members of other bat families (Jaunbauere et al., 2008). Furthermore, some can also infect humans and other mammals (Reeves et al., 2016). By feeding on the blood of bats, these arthropods have the potential to transmit filariae. Notably, mites and bat flies have been found to harbour microfilariae in their intestines or hemocoel, indicating their role as possible vectors for filariae in bats (Yunker and Chitwood, 1972; Reeves et al., 2016).
To date, mites of the family Macronyssidae have generally been put forward as the main vectors of bat microfilariae (Yunker and Chitwood, 1972; Bain et al., 2002; Guerrero et al., 2002, 2006; Bednarikova et al., 2025), although filarial DNA has also been observed in the Spinturnicidae mite of the Mexican fruit bat Artibeus jamaicensis (Reeves et al., 2016; Espinal-Palomino et al., 2024). To the best of our knowledge, however, all records of filariae in bat ectoparasites, apart from our own previous research (Bednarikova et al., 2025), are from tropical regions.
Mites are the most abundant ectoparasites on cave-dwelling bats (Zahn and Rupp, 2004), with species of the Spinturnicidae being the exclusive, and species of the Macronyssidae almost exclusive ectoparasites of Chiroptera (Rudnick, 1960). For example, the mite S. myoti is recorded as most numerous on bats of the genus Myotis, but it has also been found on specimens from other genera (Stanyukovich, 1990; Ivanova-Aleksandrova et al., 2022; Orlova et al., 2015). The exception of high abundance of mites appears to be Myotis emarginatus (Zahn and Rupp, 2004), which were also the least infested bats in our study, though two of 11 ectoparasite pools still hosted mites positive for filariae.
Cosmopolitan bat flies that parasitize chiropterans are divided into two families, the Streblidae and Nycteribiidae, with the latter being more common in the Eastern Hemisphere, comprising 222 species out of a total of 275. The subfamily Nycteribiinae includes genera such as Nycteribia, Penicillidia, and Phthiridium, which are predominantly associated with the bat families Vespertilionidae and Rhinolophidae (Dick and Patterson, 2006). Nycteribiid flies have been linked to the transmission of protozoan parasites of bats (Hoare, 1972; Gardner and Molyneux, 1988), while streblid flies have been associated with the transmission of filarial parasites in frugivorous bats (Reeves et al., 2016).
In our study, only two mite species (S. myoti and E. euryalis) and two bat fly species (N. kolenatii and P. dufouri) tested positive for filarial DNA, supporting the specificity of filariae to particular vectors. This is further supported by the average of 2.2 ectoparasite species per bat, despite 12 species being found in total. On the other hand, the interaction networks between bats, their ectoparasites and filariae exhibited a low degree of specialization and structure, suggesting ecological homogeneity and a weakly developed network organization. This may reflect a generalist mode of transmission of filariae via diverse ectoparasitic vectors. The number of ectoparasite individuals contained in a positive pooled sample ranged from 1 to 48. Samples consisting of only one P. dufouri or two E. euryalis individuals demonstrate the high sensitivity of the detection method used. The prevalence of microfilarial infection was significantly higher in mite samples (13.8%) than in bat fly samples (4.1%), suggesting that mites may play a more significant role as vectors of these parasitic nematodes. Confirmation of the increased prevalence of microfilarial infections in mites collected on M. blythii from the Ghliana Cave in Georgia (21.9% in mite samples vs 5.3% in bat fly samples) further strengthens this assumption.
Microfilarial production and release occur in periodic patterns, with the result that numbers in peripheral blood vary between different filarial species. This variation coincides with the circadian rhythm and biting activity of their primary vectors, such as Culex spp., Anopheles spp., and Aedes spp. (Shriram et al., 2015; Ughasi et al., 2012; Bhuvaneswari et al., 2023). These patterns are not known or may not exist in bat filariae since many ectoparasites are constantly present on bats. Our results showed that 10 out of 14 bats proved negative for filariae despite hosting filaria-positive ectoparasites and, conversely, 11 filaria-positive bats did not harbour filaria-positive ectoparasites. This suggests that the length and frequency of blood-feeding by various ectoparasites is an important factor. It may also suggest a non-continuous release of microfilariae into the bloodstream and/or migration of infected ectoparasites between hosts, which would support the spread of filarial infection among bats in a colony. Also consistent with these explanations are the findings of ectoparasites with different filarial species than those in the blood of the bat on which they were captured.
Our results also showed a close correlation between increasing levels of ectoparasite infection intensity and filaria-positive ectoparasites, suggesting a higher probability of infection in bats with higher ectoparasite infestation. Positive findings were exclusively in females, with a prevalence of 20.0%, which may suggest that the reproductive cycle of female bats plays a role in the transmission and circulation of these parasites. From a transmission perspective, the reproductive activity of filarial worms appears more likely to occur during the summer months when bats in colonies are most heavily infested with ectoparasites. It is the aggregation of bats and the formation of abundant summer colonies that creates conditions facilitating the movement of ectoparasites between hosts and thereby promoting the transmission of filarial infection (Espinal-Palomino et al., 2024; Montes de Oca-Aguilar et al., 2024).
Studies by Lourenço and Palmeirim (2007) and Christe et al. (2000) on hematophagous parasitic mites of bats (S. psi and S. myoti) have demonstrated that these ectoparasites can adapt their life cycles to their hosts, utilizing the reproductive period of bats to spread from adult to juvenile bats. For example, Spinturnix mites were rarely found when hosts were in hibernation; however, their numbers increased as summer colonies formed and during the gestation period, peaking during the nursing season, with overall abundance of mites during the nursing season nearly double that of other periods (Lourenço and Palmeirim, 2007). Other studies have shown that juvenile bats and female bats during gestation and lactation carry significantly more mites than males and non-reproductive females (Christe et al., 2000; Zahn and Rupp, 2004; Lourenço and Palmeirim, 2007), and that males roosting among females and juveniles do not have higher parasite loads than solitary males (Zahn and Rupp, 2004). This strong seasonal variation in mite load also relates to the reproductive cycle of the mites, which appears to be synchronized with that of their host (Lourenço and Palmeirim, 2007). Mites of the families Spinturnicidae and Macronyssidae, for example, reach maximum numbers in June or July, between the birth and departure of the host’s young (Zahn and Rupp, 2004). In the case of Spinturnicidae, minimum values were reached at the end of September and in October (Zahn and Rupp, 2004). The population size of nycteribiid ectoparasites declines to its lowest levels during the winter months (Lehane, 2005). Although nycteribiids continue to feed on blood during this time, their reproductive activity slows down significantly or ceases altogether due to the hibernating state of their bat hosts (Jaunbauere et al., 2008). From this perspective, it may be supposed that these ectoparasites would be effective vectors of microfilariae in summer female colonies, which corresponds with our findings of positive spinturnicids (E. euryalis and S. myoti) and nycteribids (N. kolenattii and P. dufouri) in females (except for one sample collected from a subadult male).
5. Conclusions
Vector-borne parasitic diseases of Palaearctic bats are poorly investigated and deserve further study, allowing us to increase our understanding of species diversity, life cycles, host ranges, infection prevalence and intensity, and parasite pathogenicity, as well as to identify competent vectors. In this study, we confirmed our hypothesis that the diversity of filarial parasites reflects the species diversity of bat hosts in the Caucasus. Our findings also suggest that extending sampling effort to the other Caucasian bat species will probably reveal previously unknown filarial species. Non-lethal sampling of blood and non-invasive sampling of ectoparasites for molecular screening proved to be an effective approach for gaining insights into parasite diversity. However, in the future, it will be necessary to obtain adult worms through necropsy not only to improve morphological descriptions and achieve identification to the species level, but also to integrate morphological and molecular parasite descriptions and advance knowledge on bat-infecting filarial nematodes in the Caucasus. The higher the number of available sequences of filarial species, the better the clarification of phylogenetic relationships of unresolved clades within the family Onchocercidae.
Ethical approval
Each bat was handled in such a way as to minimize stress during sampling, and in all cases, bats were released near their roosts within one hour of capture and sampling. Bat blood and ectoparasite samples were collected under permissions No. #2302/01 and No. 3/29.7/1043 issued by the Ministry of Environmental Protection and Agriculture of Georgia and the Ministry of Nature Protection of the Republic of Armenia, respectively. All team members are authorised to handle wild bats according to Czech Certificates of Competency Nos. CZ01341 and CZ04344 (§17, Act No. 246/1992 Coll.).
CRediT authorship contribution statement
Sarka Bednarikova: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. Ondrej Danek: Validation, Formal analysis, Visualization, Writing – review & editing. Erik Bachorec: Formal analysis, Writing – review & editing. Heliana Dundarova: Investigation, Writing – review & editing. Astghik Ghazaryan: Investigation, Writing – review & editing. Nadya Ivanova-Aleksandrova: Investigation, Writing – review & editing. Sophio Maglakelidze: Investigation, Writing – review & editing. Monika Nemcova: Investigation, Methodology, Writing – review & editing. Vladimir Piacek: Investigation, Writing – review & editing. Katerina Zukalova: Investigation, Writing – review & editing. Jan Zukal: Conceptualization, Formal analysis, Investigation, Writing – review & editing. Jiri Pikula: Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Writing – review & editing.
Funding
This research was supported through Project IGA 224/2024/FVHE. The funder had no role in the study design, data analysis, the decision to publish, or the preparation of the manuscript. The fieldwork in Armenia was financially supported through Project No. 20TTWS-1F031 of the Ministry of Education, Science, Culture and Sport of the Republic of Armenia.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We are grateful to Dr Kevin Roche for the correction and improvement of the English text.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2025.100304.
Contributor Information
Sarka Bednarikova, Email: bednarikovas@vfu.cz.
Jan Zukal, Email: 49992@mail.muni.cz.
Appendix A. Supplementary data
The following is the supplementary data to this article:
Data availability
All data supporting the findings of this study are available within the article and its supplementary files. The newly generated sequences were deposited in GenBank under the accession numbers PV023858-PV023875.
References
- Almeida-Neto M., Ulrich W. A straightforward computational approach for measuring nestedness using quantitative matrices. Environ. Model. Software. 2011;26:173–178. [Google Scholar]
- Bain O. Diversité et étroite spécificité parasitaire des filaires de chauves-souris, confondues sous le nom de Litomosa filaria (van Beneden, 1872) Bull. Mus. Natl. Hist. Nat. 1966;38:928–939. [Google Scholar]
- Bain O., Babayan S., Gomes J., Rojas G., Guerrero R. First account on the larval biology of a Litomosoides filaria, from a bat. Parassitologia. 2002;44:89–92. [PubMed] [Google Scholar]
- Bain O., Casiraghi M., Martin C., Uni S. The Nematoda Filarioidea: Critical analysis linking molecular and traditional approaches. Parasite. 2008;15:342–348. doi: 10.1051/parasite/2008153342. [DOI] [PubMed] [Google Scholar]
- Bednarikova S., Danek O., Dundarova H., Nemcova M., Piacek V., Zukalova K., et al. Filariasis of parti-colored bats: Phylogenetic analysis, infection prevalence, and possible vector mite identification. Front. Vet. Sci. 2025;12 doi: 10.3389/fvets.2025.1546353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beĭ-Bienko G.Ia. Nauka; Leningrad: 1970. Keys to the Insects of the European Part of the USSR: Diptera and Siphonaptera II. [DOI] [Google Scholar]
- Bhuvaneswari A., Shriram A.N., Raju K.H.K., Kumar A. Mosquitoes, lymphatic filariasis, and public health: A systematic review of Anopheles and Aedes surveillance strategies. Pathogens. 2023;12:1406. doi: 10.3390/pathogens12121406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüthgen N., Menzel F., Blüthgen N. Measuring specialization in species interaction networks. BMC Ecol. 2006;6:9. doi: 10.1186/1472-6785-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhnikashvili A., Gazaryan S., Kandaurov A., Natradze I., Rakhmatulina I., Yavruyan E. In: Status and Protection of Globally Threatened Species in the Caucasus. Zazanashvili N., Mallon D., editors. CEPF, WWF. Contour Ltd.; Tbilisi: 2009. Current status of Chiroptera conservation in the Caucasus.https://d29l0tur8ol1gj.cloudfront.net/sites/default/files/status-and-protection-globally-threatened-species-caucasus.pdf [Google Scholar]
- Calisher C.H., Childs J.E., Field H.E., Holmes K.V., Schountz T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casiraghi M., Anderson T.J.C., Bandi C., Bazzocchi C., Genchi C. A phylogenetic analysis of filarial nematodes: Comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122:93–103. doi: 10.1017/S0031182000007149. [DOI] [PubMed] [Google Scholar]
- Casiraghi M., Bain O., Guerrero R., Martin C., Pocacqua V., Gardner S.L., et al. Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: Evidence for symbiont loss during evolution. Int. J. Parasitol. 2004;34:191–203. doi: 10.1016/j.ijpara.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Christe P., Arlettaz R., Vogel P. Variation in intensity of a parasitic mite (Spinturnix myoti) in relation to the reproductive cycle and immunocompetence of its bat host (Myotis myotis) Ecol. Lett. 2000;3:207–212. doi: 10.1046/j.1461-0248.2000.00142.x. [DOI] [Google Scholar]
- Dick C.W., Patterson B.D. In: Micromammals and Macroparasites. Morand S., Krasnov B.R., Poulin R., editors. Springer; Japan, Tokyo: 2006. Bat flies: Obligate ectoparasites of bats; pp. 179–194. [DOI] [Google Scholar]
- Dormann C.F., Gruber B., Fründ J. Introducing the bipartite package: Analysing ecological networks. R News. 2008;8:8–11. https://uni-goettingen.de/de/document/download/96729eb9d30a6f2dc4403df15854305c.pdf/Rnews2008,8_8-11_open.pdf [Google Scholar]
- Eberhard M.L., Hitch W.L., McNeeley D.F., Lammie P.J. Transplacental transmission of Wuchereria bancrofti in Haitian women. J. Parasitol. 1993;79:62. doi: 10.2307/3283278. [DOI] [PubMed] [Google Scholar]
- Espinal-Palomino R., Montes de Oca-Aguilar A.C., Ibarra-López M.P., Vidal-Martínez V.M., Ibarra-Cerdeña C.N. Bat microfilariae in the cityscape: A transmission tale between bats, mites, and bat flies. Int. J. Parasitol. S0020751924002017. 2024 doi: 10.1016/j.ijpara.2024.11.001. [DOI] [PubMed] [Google Scholar]
- Esteban J.G., Botella P., Toledo R., Oltra-Ferrero J.L. Helminth fauna of bats in Spain. IV. Parasites of Rhinolophus ferrumequinum (Schreber, 1774) (Chiroptera: Rhinolophidae) Res. Rev. Parasitol. 1999;59:57–68. [Google Scholar]
- Esteban J.G., Oltra-Ferrero J.L., Botella P., Mas-Coma S. Helmintos de quirópteros en España: Espectro faunístico e interés aplicado de su estudio. In: Los murciélagos de España y Portugal. Colección Técnica. Ministerio de Agricultura, Pesca y Alimentación; 1991. pp. 282–304. [Google Scholar]
- Estrada-Peña A., Mihalca A.D., Petney T.N., editors. Ticks of Europe and North Africa. Springer International Publishing; Cham: 2017. [DOI] [Google Scholar]
- Faith D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992;61:1–10. doi: 10.1016/0006-3207(92)91201-3. [DOI] [Google Scholar]
- Fortuna M.A., Stouffer D.B., Olesen J.M., Jordano P., Mouillot D., Krasnov B.R., et al. Nestedness versus modularity in ecological networks: Two sides of the same coin? J. Anim. Ecol. 2010;79:811–817. doi: 10.1111/j.1365-2656.2010.01688.x. [DOI] [PubMed] [Google Scholar]
- Gardner R.A., Molyneux D.H. Polychromophilus murinus: A malarial parasite of bats: life-history and ultrastructural studies. Parasitology. 1988;96:591–605. doi: 10.1017/S0031182000080215. [DOI] [PubMed] [Google Scholar]
- Gaston K.J., Spicer J.I. 2nd ed. Blackwell Publishers; Malden, Mass, USA: 2012. Biodiversity: An Introduction. [Google Scholar]
- Guerrero R., Bain O., Attout T., Martin C. The infective larva of Litomosoides yutajensis Guerrero et al., 2003 (Nematoda: Onchocercidae), a Wolbachia-free filaria from bat. Parasite. 2006;13:127–130. doi: 10.1051/parasite/2006132127. [DOI] [PubMed] [Google Scholar]
- Guerrero R., Martin C., Gardner S.L., Bain O. New and known species of Litomosoides (Nematoda: Filarioidea): Important adult and larval characters and taxonomic changes. Comp. Parasitol. 2002;69:177–195. doi: 10.1654/1525-2647(2002)069[0177:NAKSOL]2.0.CO. 2. [DOI] [Google Scholar]
- Guindon S., Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Haque A., Capron A. Transplacental transfer of rodent microfilariae induces antigen-specific tolerance in rats. Nature. 1982;299:361–363. doi: 10.1038/299361a0. [DOI] [PubMed] [Google Scholar]
- Hayman D.T.S. Bats as viral reservoirs. Annu. Rev. Virol. 2016;3:77–99. doi: 10.1146/annurev-virology-110615-042203. [DOI] [PubMed] [Google Scholar]
- Hoare C.A. Blackwell Scientific Publications; Oxford: 1972. The Trypanosomes of Mammals. A Zoological Monograph. [Google Scholar]
- Horvat Ž., Čabrilo B., Paunovic M., Karapandža B., Josipovic J., Budinski I., Bjelić-Čabrilo O. The helminth fauna of the greater horseshoe bat (Rhinolophus ferrumequinum) (Chiroptera: Rhinolophidae) on the territory of Serbia. Biol. Serbica. 2015;37:64–67. https://ojs.pmf.uns.ac.rs/index.php/dbe_serbica/article/view/4174 [Google Scholar]
- Hosek J., Horacek I. In: European Bat Research. Hanák V., Horáček I., Gaisler J., editors. Charles University Press; Praha: 1987. Nematodes parasitizing the Palearctic bats: Host-parasite relations; pp. 465–473. [Google Scholar]
- Hutson A.M. Diptera, Hippoboscidae and Nycteribiidae. Royal Entomological Society; London: 1984. Handbooks for the Identification of British Insects. [Google Scholar]
- Ivanova-Aleksandrova N., Dundarova H., Neov B., Emilova R., Georgieva I., Antova R., et al. Ectoparasites of cave-dwelling bat species in Bulgaria. Proc. Zool. Soc. 2022;75:463–468. doi: 10.1007/s12595-022-00451-4. [DOI] [Google Scholar]
- Jaunbauere G., Salmane I., Spuis V. Occurrence of bat ectoparasites in Latvia. Latv. Entomol. 2008;45:38–42. [Google Scholar]
- Jiménez F.A., Notarnicola J., Gardner S.L. Host-switching events in Litomosoides Chandler, 1931 (Filarioidea: Onchocercidae) are not rampant but clade-dependent. J. Parasitol. 2021;107:320–335. doi: 10.1645/20-35. [DOI] [PubMed] [Google Scholar]
- Junker K., Barbuto M., Casiraghi M., Martin C., Uni S., Boomker J., Bain O. Litomosa chiropterorum Ortlepp, 1932 (Nematoda: Filarioidea) from a South African miniopterid: Redescription, Wolbachia screening and phylogenetic relationships with Litomosoides. Parasite. 2009;16:43–50. doi: 10.1051/parasite/2009161043. [DOI] [PubMed] [Google Scholar]
- Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., Von Haeseler A., Jermiin L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T., O'Dwyer K., Nakagawa S., Poulin R. What determines species richness of parasitic organisms? A meta‐analysis across animal, plant and fungal hosts. Biol. Rev. 2014;89:123–134. doi: 10.1111/brv.12046. [DOI] [PubMed] [Google Scholar]
- Lafferty K.D. Biodiversity loss decreases parasite diversity: Theory and patterns. Phil. Trans. Biol. Sci. 2012;367:2814–2827. doi: 10.1098/rstb.2012.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léger C. Bat parasites (Acari, Anoplura, Cestoda, Diptera, Hemiptera, Nematoda, Siphonaptera, Trematoda) in France (1762–2018): A literature review and contribution to a checklist. Parasite. 2020;27:61. doi: 10.1051/parasite/2020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehane M.J. 2nd ed. Cambridge University Press; Cambridge: 2005. The Biology of Blood-Sucking in Insects. [DOI] [Google Scholar]
- Lourenço S.I., Palmeirim J.M. Can mite parasitism affect the condition of bat hosts? Implications for the social structure of colonial bats. J. Zool. 2007;273:161–168. doi: 10.1111/j.1469-7998.2007.00322.x. [DOI] [Google Scholar]
- Martin C., Bain O., Jouvenet N., Raharimanga V., Robert V., Rousset D. First report of Litomosa spp. (Nematoda: Filarioidea) from Malagasy bats; review of the genus and relationships between species. Parasite. 2006;13:3–10. doi: 10.1051/parasite/2006131003. [DOI] [PubMed] [Google Scholar]
- Mayer F., Dietz C., Kiefer A. Molecular species identification boosts bat diversity. Front. Zool. 2007;4:4. doi: 10.1186/1742-9994-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeand J.B. Molecular diagnosis of parasitic nematodes. Parasitology. 1999;117:87–96. doi: 10.1017/S0031182099004096. [DOI] [PubMed] [Google Scholar]
- Merdivenci A. [The occurrence of Litomosa filaria (van Beneden, 1873) in bats, in Turkey] Acta Biol. Turcica. 1964;14:30–34. (In Turkish) [Google Scholar]
- Minh B.Q., Nguyen M.A.T., Von Haeseler A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013;30:1188–1195. doi: 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittermeier R.A., Mittermeier R.A., Cemex S.A., de C.V., editors. Hotspots Revisited (1st English Edition). Cemex; Mexico City, Mexico: 2004. [Google Scholar]
- Montes de Oca-Aguilar A.C., Ibarra-López M.P., Ibarra-Cerdeña C.N. A five-year study on infestation and abundance of bat flies (Hippoboscoidea: Streblidae) under severe dry season conditions in the tropical dry forest of Yucatan, Mexico. Neotrop. Entomol. 2024;53:439–454. doi: 10.1007/s13744-024-01130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers N., Mittermeier R.A., Mittermeier C.G., Da Fonseca G.A.B., Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Nguyen L.-T., Schmidt H.A., Von Haeseler A., Minh B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarnicola J. In: Concepts in Animal Parasitology. Gardner S.L., Gardner S.A., editors. Zea Books; Lincoln, Nebraska, USA.: 2024. Chapter 55: Filarioidea (Superfamily) [DOI] [Google Scholar]
- Orlova M., Stanyukovich M., Orlov O. Publishing House of Tomsk State University; Tomsk, Russia: 2015. Gamasid Mites (Mesostigmata: Gamasina) Parasitizing Bats (Chiroptera: Rhinolophidae, Vespertilionidae, Molossidae) of Palearctic Boreal Zone (Russia and Adjacent Countries)https://core.ac.uk/download/pdf/287487631.pdf [Google Scholar]
- Ospina-Pérez E.M., Rivera-Páez F.A., Ramírez-Chaves H.E. Exploring the relationship between bats (Mammalia, Chiroptera) and ectoparasitic flies (Diptera, Hippoboscoidea) of the Orinoquia Region in South America. ZooKeys. 2023;1179:1–34. doi: 10.3897/zookeys.1179.103479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit G. Sur les Filaires du genre Litomosa (Nematoda, Filarioidea), parasites de Chauves-souris. Bull. Mus. Natl. Hist. Nat. 1980;2:365–374. doi: 10.5962/p.283844. [DOI] [Google Scholar]
- Pikula J., Piacek V., Bandouchova H., Bartlova M., Bednarikova S., Burianova R., et al. Case report: Filarial infection of a parti-coloured bat: Litomosa sp. adult worms in abdominal cavity and microfilariae in bat semen. Front. Vet. Sci. 2023;10 doi: 10.3389/fvets.2023.1284025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin R. Parasite biodiversity revisited: Frontiers and constraints. Int. J. Parasitol. 2014;44:581–589. doi: 10.1016/j.ijpara.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Ramasindrazana B., Dellagi K., Lagadec E., Randrianarivelojosia M., Goodman S.M., Tortosa P. Diversity, host specialization, and geographic structure of filarial nematodes infecting Malagasy bats. PLoS One. 2016;11 doi: 10.1371/journal.pone.0145709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W.K., Beck J., Orlova M.V., Daly J.L., Pippin K., Revan F., Loftis A.D. Ecology of bats, their ectoparasites, and associated pathogens on Saint Kitts Island. J. Med. Entomol. 2016;53:1218–1225. doi: 10.1093/jme/tjw078. [DOI] [PubMed] [Google Scholar]
- Rudnick A. University of California Publications in Entomology. University of California Press; Berkeley, USA: 1960. A revision of the mites of the family of Spinturnicidae (Acarina) [Google Scholar]
- Seurat L.G. Litosoma filaria Bened., type d’une nouvelle section de Filaires opisthodelphes. Bull. Mus. Natl. Hist. Nat. 1921;27:103–106. [Google Scholar]
- Shriram A.N., Krishnamoorthy K., Vijayachari P. Diurnally subperiodic filariasis among the Nicobarese of Nicobar district - epidemiology, vector dynamics & prospects of elimination. Indian J. Med. Res. 2015;141:598–607. doi: 10.4103/0971-5916.159537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanyukovich M.K. Gamasid and argasid mites of bats in Baltic states and Leningrad region. Parazitologiya. 1990;24:193–200. [Google Scholar]
- Ughasi J., Bekard H.E., Coulibaly M., Adabie-Gomez D., Gyapong J., Appawu M., et al. Mansonia africana and Mansonia uniformis are vectors in the transmission of Wuchereria bancrofti lymphatic filariasis in Ghana. Parasites Vectors. 2012;5:89. doi: 10.1186/1756-3305-5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunker C.E., Chitwood M.B. Note on the occurrence of larval filariae in a mite parasitic on bats. Acarologia. 1972;14:530–532. [PubMed] [Google Scholar]
- Zahn A., Rupp D. Ectoparasite load in European vespertilionid bats. J. Zool. 2004;262:383–391. doi: 10.1017/S0952836903004722. [DOI] [Google Scholar]
- Zukal J., Bandouchova H., Brichta J., Cmokova A., Jaron K.S., Kolarik M., et al. White-nose syndrome without borders: Pseudogymnoascus destructans infection tolerated in Europe and palearctic Asia but not in North America. Sci. Rep. 2016;6 doi: 10.1038/srep19829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the article and its supplementary files. The newly generated sequences were deposited in GenBank under the accession numbers PV023858-PV023875.