Summary
Ambrosia beetles are social, fungal-farming insects that nest within tree xylem. Their close living conditions make them potentially vulnerable to microbial infectious diseases. We show that the insect pathogenic fungus Metarhizium anisopliae effectively infects and kills Xyleborus affinis adults, even within sawdust-based colony habitats. Healthy beetles did not avoid infected nestmates, and increased contact led to higher mortality and reduced offspring; however, larvae and pupae were still produced, even when colonies began with only infected beetles. Diseased individuals and Metarhizium CFUs were concentrated in the upper third of the nest, while surviving adults and brood were found in the middle/lower areas. A beetle symbiotic fungus, Neocosmospora sp. Xa1 was identified, which inhibited Metarhizium growth, potentially aiding in defense. Our findings suggest spatial structuring and microbial interactions within the nest help protect vulnerable brood to support colony persistence, revealing colony-level mechanisms that buffer against spread of infectious diseases, favoring offspring survival.
Subject areas: Biological sciences, Entomology, Microbiology
Graphical abstract

Highlights
-
•
Beetle colony structure limits spread of infectious disease, protecting offspring
-
•
Spatial bias of diseased/dead nestmates reduces infection risk in social colonies
-
•
Partner fungus Neocosmospora suppresses pathogenic Metarhizium growth
Biological sciences; Entomology; Microbiology
Introduction
Fungal farming has evolved in one tribe of ants and one subfamily of termites, but at least a dozen times in beetles.1,2,3 Ambrosia beetles (along with some bark beetles) actively culture fungi as a food source and represent a globally distributed group of wood-boring insects that include members that cause severe damage to host trees either directly and/or by vectoring plant disease-causing agents.4,5 Ambrosia beetles tunnel into the sapwood/xylem of host trees, excavating their nest galleries where they lay eggs, tend their brood, and cultivate obligate fungal symbionts that act as the sole source of nutrition for both larvae and adults. As a model of social organization, these beetles show high sociality, including division of labor, age polytheism, and sanitation behaviors related to their fungiculture lifestyle.6,7 Studies on the ambrosia beetle, Xyleborus affinis (sugarcane shot-hole borer) have shown defined social behaviors that include contributions of both adults and larvae to brood care, division of labor which includes delayed dispersal of adult daughters, and totipotent adult daughters laying eggs in the natal nest.8 Thus, similar to ants and termites, multiple generations can inhabit the same gallery as nestmates; however, unlike the former, they rarely, if ever, leave the nest, except for dispersal to new sites. Such restricted spatial constraints for ambrosia beetle nests can, in the absence of any countermeasures, facilitate the rapid spread of infectious disease agents. The cost of sociality in terms of infectious disease pressures is known to be impacted by group size, transmission (rates), and the nature of interactions within the social organizational framework of the colony. The spatial organization of insect nests has been viewed as a critical adaptation facilitating the evolution of the division of labor and possibly hindering the spread of disease.9 In many ant societies, worker caste/division of labor has been shown to be associated with worker age. In such age polytheism, young workers are associated with brood care, then subsequently move to more general nest tasks as they age, ultimately becoming foragers outside the nest in old age.10,11 These functional transitions are associated with a spatial correlation from the inside of the nest, where the brood is located, to the entrance, and finally to the outside of the nest. This spatial-social organization of the nest may also help prevent disease spread. Furthermore, evidence has indicated that highly hierarchical social network organization can reduce group-living infectious disease costs, particularly when confronted with highly transmissible pathogens, whereas prolonged epidemic outbreaks are seen in species displaying gregarious social networks.12,13 Modeling of infectious disease dynamics in insect societies has suggested the potential for ambivalent effects of high social interaction rates. Thus, although increased contact can facilitate disease transmission, compensating collective disease defenses (social immunity) and/or dilution of the pathogen, i.e., reduction of individual pathogen loads, in the colony by high contact may mitigate pathogen spread.14 The former, i.e., colony-level adaptations or social immunity, have been deemed major adaptations enhancing colony health and survival to infectious diseases, with mechanisms mediating social immunity linking olfaction, behavior, and innate immunity.15,16,17 Most such studies within invertebrates, however, have focused on ants and termites; little is known concerning the spread of microbial infectious agents within social beetles, and significant epi- or even enzootic infections within ambrosia beetle colonies and any resultant colony-level outcomes or effects have yet to be reported.
Such information can be critical not only as a social-immunological system to examine infectious disease transmission, but also because, due to their small size, haplodiploidy, and presence in wood substrates that are often transported over long distances via trade, several ambrosia species have emerged as being highly invasive.18,19,20,21 Furthermore, within this context, members of the Xyleborus genus include species invasive to the United States,22 which have brought their own fungal partners, e.g., Harringtonia lauricola (Ophiostomatales: Ophiostomataceae), which, in turn, is the causative agent of the devastating laurel wilt vascular disease affecting members of the Lauraceae tree family.23,24 Laurel wilt has killed over 500 million trees since its original invasion into the United States in the early 2000s, with the fungal pathogen able to switch hosts to indigenous beetle species (e.g., X. affinis), indicating the high likelihood for the continued geographic spread of the laurel wilt pathogen.4,25,26
Entomopathogenic fungi are capable of infecting and killing a range of ambrosia beetles.27 Isolates of Isaria (Hypocreales: Cordycipitaceae), Beauveria (Hypocreales: Cordycipitaceae), and/or Metarhizium (Hypocreales: Clavicipitaceae) have shown efficacy in laboratory settings against Xylosandrus germanus (Coleoptera: Curculionidae) and X. crassiusculus.28,29,30 Competition between biological control agents, e.g., Trichoderma harzianum (Hypocreales: Hypocreaceae), and the fungal symbionts of these beetles have also been shown to impact beetle brood production, with suppression of the beetle fungal mutualist by competing fungi and/or bacteria, e.g., Trichoderma and Bacillus (Caryophanales: Bacillaceae) species suppressing beetle (X. compactus) development.31,32 Similarly, entomopathogenic fungi can efficiently infect and kill Xyleborus species, including X. glabratus, X. crassiusculus, X. volvulus, X. bispanatus, and X. affinis, including reducing the number of eggs and larvae produced.33,34,35,36 However, although it has long been recognized that the wood-boring behavior and unique habitat of ambrosia beetles present a particular challenge, little is known concerning infectious disease dynamics within Xyleborus beetles, including the impacts of habitat, behaviors, mutualistic microbes, and other colony parameters on transmission, persistence, and effects on progeny. Early investigations indicated that Xyleborinus (note this is a distinct genus from Xyleborus above) saxesenii pushes diseased individuals into “death chambers” that are actively closed with sawdust.37 When Aspergillus spores were injected in laboratory nests of X. saxesenii, adult female workers increased their hygienic activity, including allogrooming and cannibalism, with such responses considered to significantly reduce pathogen prevalence in the nest.38 Furthermore, in these Xyleborinus beetles, both larvae and adults have been shown to participate in gallery enlargement and hygiene, as well as brood care, with diseased individuals either cannibalized or removed from the nest.7
Here, we investigated the transmission and impact of the entomopathogenic fungus M. anisopliae within colonies of the ambrosia beetle X. affinis. Specifically, we sought to determine: (1) whether horizontal transmission of M. anisopliae occurs within beetle colonies; (2) how transmission affects colony health, reproduction (larvae and pupae), and structure; (3) the spatial distribution patterns of infected individuals and fungal infectious cells within colony habitats; and (4) interactions between the pathogen and beetle fungal partners, identified here as Neocosmospora (previously Fusarium). Our results demonstrate significant pathogen transmission, which correlated with initial infection ratios, minimal behavioral avoidance of infected beetles, but distinct spatial segregation of diseased individuals, (fungal) pathogen cells, and progeny, which combined serve as robust protective strategies allowing for enhanced survival of the colony. In addition, we show the competitive suppression of M. anisopliae by the fungal mutualist Neocosmospora, suggesting its role in colony defense. These data provide a model for further exploration of infectious disease dynamics in a widespread group of social insects that inhabit and develop within specific habitats and spatial architectures, illustrating both behavioral and mutualistic microbial partner inputs in colony hygiene.
Results
Infection of X. affinis by Metarhizium sp.
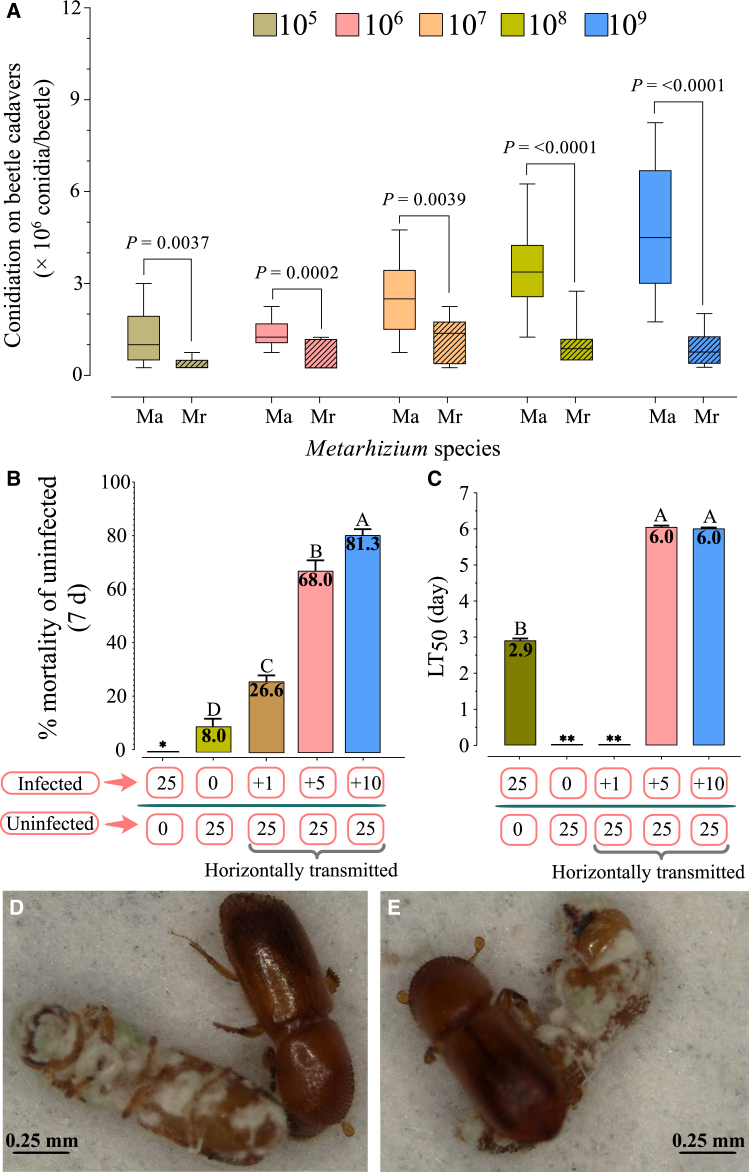
Two Metarhizium species, M. anisopliae, and M. robertsii, were initially tested for their efficiency in targeting adult X. affinis females in standard laboratory assays in which beetles were placed in Petri dishes containing moist sterile filter paper after infection (Figure S1). Both species were able to kill X. affinis adults, with M. anisopliae and M. robertsii displaying LT50 values of 4.3 ± 0.049 (±SE) d and 4.6 ± 0.124 days after infection with 107 conidia/ml (Figure S2), and LD50 values of 1.36 × 107 conidia/ml and 3.18 × 107 (1.63 × 105- 3.91 109) (CIs) conidia/ml 4 days after infection (Table S1), respectively. Control beetle mortality remained <10% up to 7 days but then increased dramatically on day 8, leading to a mean survival time of 8.5–9.0 days. Histological visualization of the infection process showed attachment and germination of conidia on the beetle surface, penetration of the cuticle, proliferation within the hemocoel/hemolymph, including interaction and escape from host hemocytes, invasion of insect tissues, host melanization/nodulation responses, and eventual host death and sporulation on the cadaver (Figure 1). Sporulation on host cadavers was quantified as a function of Metarhizium species and original inoculum, revealing significantly higher (3- to 6-fold, p < 0.01) sporulation of M. anisopliae (1-7×106 conidia/beetle) compared to M. robertsii (0.5–2×106 conidia/beetle, Figure 2A). Based on these experiments, the more virulent and higher cadaver spore-producing M. anisopliae strain was used for further studies.
Figure 1.
Histology of M. anisopliae infection of X. affinis
(A and B) Representative images of attachment of M. anisopliae (using a gfp-expressing strain) conidia to the beetle cuticle.
(C) Fungal appressoria formation on the cuticle surface.
(D) Penetration of the insect cuticle by M. anisopliae hyphae (outlined in yellow in box enlargement). Nuclei are stained (blue) with DAPI.
(E and F) Fungal hyphal bodies proliferate within the host hemocoel/body cavity. Arrows point to X. affinis fat body and muscle tissues.
(G) Interaction between X. affinis hemocytes and M. anisopliae hyphal bodies.
(H and I) Interaction between M. anisopliae hyphal bodies and X. affinis muscle tissue. Brightfield and GFP channel (green) illustrate M. anisopliae hyphal bodies and autofluorescence from insect tissue. DAPI channel (blue) shows nuclei staining, and the TRITC channel (red) shows actin filament staining of X. affinis muscle tissue.
(J) Host melanization/nodulation response to M. anisopliae infection.
(K and L) Fungal outgrowth and conidiation on X. affinis cadavers. Abbreviations: AP: appressoria and CO: conidia. Scale bars: 50 μm applies to panel A; 20 μm applies to panels B-J; and 200 μm applies to K-L.
Figure 2.
Conidiation of M. anisopliae and M. robertsii on X. affinis cadavers and horizontal transmission of M. anisopliae on X. affinis beetles within Petri plates (ex-gallery)
(A) Conidiation on cadavers was measured as a function of the initial infection dose, as detailed in the STAR Methods section.
(B) % mortality (7 days) of uninfected beetles when indicated numbers of directly infected beetles were added. Mortality of directly infected beetles is excluded from the numbers shown. (Note: ∗ = in all conditions, 100% mortality of the directly infected beetles was seen, but that data is not included as the axis refers to the originally uninfected population).
(C) LT50 values derived from the time course data of the experimental design as in (A). Note: Data for the cohort containing all directly infected beetles is included as reference. ∗∗ = LT50 could not be calculated as beetle mortality did not reach 50%.
(D and E) Behavioral observations indicated that healthy beetles did not avoid contact with infected beetles. All experiments were performed with three independent replicates. Error bars represent the standard error, and different letters represent significant differences at p < 0.05. Panel A shows results of pairwise comparisons between groups using unpaired t-tests. Panels B and C present post hoc statistical comparisons using the Tukey-Kramer Honest Significant Difference (HSD) test. Abbreviations: Ma: Metarhizium anisopliae, Mr: Metarhizium robertsii. Scale bars: 0.25 mm applies to panels D-E.
To determine consequences of availability of a gallery habitat on infection, a preliminary beetle survival experiment (in the absence of any infection) was performed in which beetles were placed in Petri dishes containing moist filter paper for either 4, 5, or 6 days, subsequently released into “habitat media” for 24 h, removed from the media and placed back in Petri dishes and mortality was recorded over time (experimental protocol outlined in Figure S3). The mean survival time for beetles under these conditions was 8.5–9.0 days, irrespective of the length of time spent on filter paper and allowing them to return to their habitat media, consistent with the in vitro infection experiments outlined above (Figure S4).
To test whether allowing freshly infected beetles the opportunity to burrow (and hence potentially remove conidia from the cuticle surface) would impact mortality, beetles were infected with either 107 or 108 conidia/ml of M. anisopliae, and then either immediately released into habitat media, or kept in Petri dishes with sterile moist filter for 4, 8, or 12 h before release into media. These time points represent initial conidial contact/attachment on the host cuticle (immediate release), consolidation of attachment (4 h), and germination (8–12 h) on the host surface. Beetles were then allowed to burrow for 24 h, after which they were removed from the media, placed back in Petri dishes, and mortality was measured over time (Figure S5). At the lower infection concentration (107 conidia/ml), the calculated LT50 was similar across all treatments ranging from 5.1–5.4 days, and similarly, at the higher infection concentration (108/conidia/ml), no significant differences were seen between treatments (LT50 from 4.3–4.5 days) (Figure S6).
Infectious disease transmission
Laboratory bioassays: To test whether horizontal transmission of M. anisopliae infection could occur, a set of beetles were infected with conidia (108/mL), marked with a yellow ink dot, incubated in a Petri plate containing moist sterile filter paper for 24 h, and subsequently mixed with healthy (unmarked) beetles, and mortality was measured over time (experimental protocol outlined in Figure S7). Controls included cohorts in which all beetles had been initially infected, and those to which no infected beetles were added. Test conditions included a range of infected: uninfected ratios, which included 1:25, 5:25, and 10:25 (Figures 2B–2E). Mortality of directly infected and (horizontal transmission to) healthy beetles was recorded separately, and only the latter is shown in the figure. All directly infected beetles died from the infection within 5 days, with an LT50 = 2.9 days. The % mortality of controls (ratio = 0:25 infected: uninfected) was <10% at 7 days, with the addition of one directly infected beetle (1:25), resulting in ∼30% mortality (of originally healthy beetles, again all initially infected beetles died within 3 days) (Figure 2B). Ratios of 5:25 and 10:25 (directly infected to uninfected) resulted in ∼68 and 81% mortality of healthy conspecifics, respectively, with calculated LT50 values of ∼6 days (Figure 2C, note an LT50 value could not be calculated for the 1:25 ratio as mortality did not reach >50%, and the LT50 value for the directly infected cohort is given as reference). In all cases, mortality due to fungal infection was confirmed by fungal outgrowth from cadavers. Behavioral observations indicated little to no specific avoidance of sick/dead beetles by healthy conspecifics, even when fungal sporulation was evident, and repeated instances of contact with such cadavers were seen (Figures 2D and 2E). In addition, no specific treatment of corpses under these conditions was noted (e.g., corpse movement, sanitation behavior).
Colony habitat bioassays: To examine the nature of disease transmission within the nest/gallery habitat, similar experiments were performed using different ratios of (directly) infected: uninfected (25:0, 0:25, 1:25, 5:25, and 10:25); however, in this instance, beetles were released into the media habitat (Experimental design illustrated in Figures S8A and S8B). As before, directly infected beetles were marked with a yellow dot, and at distinct time points (7, 14, and 25), the colonies were opened, and the number of dead beetles was recorded (Figure 3A). As before, all directly infected beetles (as marked by a yellow dot) had died by the 7-day time point (100% mortality; note: this mortality is excluded from the data shown in Figure 3A). Mortality in control colonies increased gradually from <10% to ∼18% over the 25 days. Addition of one directly infected beetle (to 25 healthy beetles) did not appreciably change overall mortality at any time point; however, addition of 5 infected beetles resulted in a gradual increase in cumulative mortality to 20, 30, and 40% at the 7, 14, and 25 days time points. Addition of 10 infected beetles increased initial mortality to ∼40% at 7 and 14 days, reaching 60% at the 25 days time point (Figure 3A). No significant differences were seen in beetle gallery formation between treatments (Figures 3B and 3C), although dead beetles exhibiting various stages of fungal outgrowth and sporulation were evident (Figures 3D–3G). The number of larvae and pupae produced in the colonies was also quantified (Figures 3H and 3I). No larvae were seen at the 7-day time point, with control uninfected colonies producing ∼86.33 ± 3.48 (±SE) larvae at 14 days and 11.33 ± 0.88 at 25 days (Figure 3H). Interestingly, even when all members of the colony had been directly infected, ∼24 ± 4.16 larvae were seen at 14 days, although only 2 ± 0.11 at 25 days. Addition of 1:25 or 5:25 (directly infected: healthy) decreased larval numbers down to 66.66 ± 4.4 and 78.33 ± 2.46 at 14 days (10–25% decrease, p < 0.01), but larval numbers for the 1:25 ratio were similar to controls for the 25 days (∼14.33 ± 1.85) time point, whereas they were significantly (p < 0.01) lower for the 5:25 ratio conditions (3.33 ± 1.45). At a ratio of 10:25 (directly infected: healthy), larval numbers were dramatically reduced at both the 14 and 25 days time points, declining to 15.33 ± 0.88 and 1.66 ± 0.68, respectively. No pupae were seen at the 7 or 14 days time points; however, 13.33 ± 0.89 were seen in control uninfected colonies, whereas only 2.66 ± 0.33 were seen in all (directly) infected colonies at the 25 days time point (p < 0.01, Figure 3I). Addition of one directly infected (to 25 healthy) decreased pupae number to 5.0 ± 0.57, with ratios of 5:25 and 10:25, resulting in pupae numbers similar to the “all infected” controls, namely, 2.66 ± 0.33. No apparent infection of larvae or pupae was noticed (Figures 3J and 3K), and although eggs could be seen under a microscope (Figures 3L and 3M), the total numbers could not be accurately determined and hence are not reported. Measurement of water content in the colony media indicated starting levels of 82.49 ± 1.65%, which decreased to 73.57 ± 0.92% and 63.83 ± 3.23% at 14 and 25 days, respectively (Figure S9).
Figure 3.
Transmission of M. anisopliae, analysis of larval and pupal presence within colony habitats
(A) % mortality at 7, 14, and 25 days post-introduction of directly infected beetles with indicated numbers of uninfected beetles. Mortality of directly infected beetles is excluded from the numbers shown. (Note: ∗ = in all conditions, 100% mortality of the directly infected beetles was seen, but that data is not included as the axis refers to the originally uninfected population).
(B and C) Representative images of beetle tunnels within the media habitat. The average tunnel diameter was ∼0.92 mm with an average length of 10 mm.
(D–G) Stages of beetle infection observed: dead uninfected beetles in the mycelial stage at 7 days (D), conidiation stage at 14 days (E), and 25 days (F-G).
(H) Larval numbers at 14 and 25 days post-introduction of directly infected beetles with indicated numbers of uninfected beetles. Larvae were not seen at the 7 days time point.
(I) Pupae numbers at 25 days post-introduction of directly infected beetles with indicated numbers of uninfected beetles. Pupae were not seen in the 7 and 14 days time points.
(J) Representative image of X. affinis larva. (K) Representative image of X. affinis pupae.
(L and M) Representative images of eggs. X. affinis eggs could be seen under a microscope, but could not be reproducibly counted. Panels A, H, and I were analyzed using the Tukey-Kramer Honest Significant Difference (HSD) test for multiple group comparisons. Error bars represent the standard error, and different letters represent significant differences at p < 0.05. Scale bars: 2.0 mm applies to panels B-C; 0.5 mm applies to panel E; and 0.25 mm applies to panels D-F-G.
Biased distribution of dead insects, larvae, pupae, and M. anisopliae colony-forming units (CFUs) as a function of infection ratio in beetle colonies
In examining infected colonies, although sporulation on the infected beetle cadavers could be seen, it was noted that colonies were not apparently overtaken by M. anisopliae, and no appreciable Metarhizium growth could be visually seen when colonies were dissected. In addition, dead beetles tended to be found in the upper regions of the colony, separate from larvae and pupae. To more systematically investigate this, colony habitat bioassays were performed as above via the introduction of different ratios of infected to uninfected beetles, but at each collection/analysis time point, the colony was separated into roughly three equal sections: upper (U), middle (M), and lower (L) (Figure S8C). Each section was examined separately for adult beetle mortality, number of living adults, and larval and pupal numbers. In addition, the number of M. anisopliae colony-forming units (CFUs) in each section was quantified as detailed in the STAR Methods section.
Since we aimed to examine distribution across different layers in these experiments, one concern was whether the orientation of the gallery habitat tubes, vertical or horizontal, would influence the results. Therefore, we conducted two sets of identical experiments with one set of tubes placed in a horizontal orientation and the other in a vertical orientation. All tubes were processed similarly, as detailed in the STAR Methods section. Data from each experiment were initially analyzed separately, revealing no significant differences (p > 0.0721) between horizontal and vertical orientations concerning the number of larvae, pupae, mortality rates, and Metarhizium CFUs. Consequently, data from both orientations were pooled for greater statistical analytic power and are presented below.
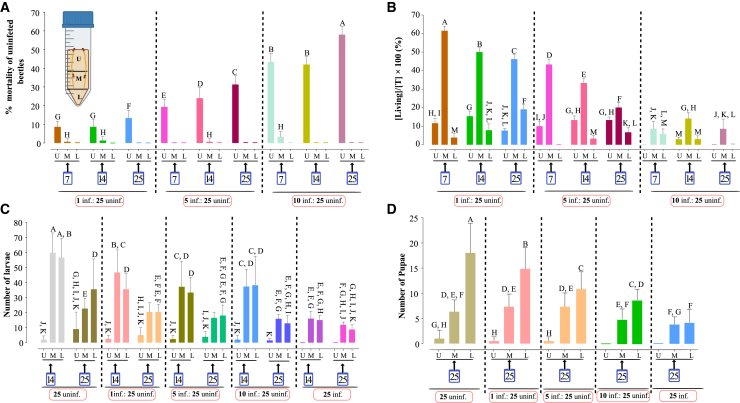
Almost all dead/mycosed adult beetles were found in the upper section of the colony, irrespective of infection ratio and across all time points (Figure 4A). Beetles that were directly infected (and released into the colonies) were similarly found almost exclusively in the upper colony section, independent of infection ratio and observation period (Figure S10). Similarly, beetles that died naturally, i.e., those that bore no obvious indication of fungal infection and did not show any sporulation on their cadavers, were also predominantly found in the upper section of the colony, irrespective of infection ratio and across all sampling time points, although overall levels of such individuals remained <10% (Figure S11).
Figure 4.
Spatial impact of transmission of M. anisopliae on beetle adults, larvae, and pupae under varying infection pressures over time
(A) Distribution of newly infected adult beetles was assessed at 7, 14, and 25 days post-inoculation across three sections (upper [U], middle [M], lower [L]) of sawdust media. Experiments were conducted at infection ratios of 1:25, 5:25, and 10:25 (infected: uninfected beetles). Bars represent infection percentages within each sawdust layer; different letters indicate statistically significant differences (p < 0.05).
(B) Distribution of healthy adult beetles at 7, 14, and 25 days post-inoculation (conditions as in A). Bars represent the percentage of living adults within each sawdust layer; different letters indicate statistically significant differences (p < 0.05).
(C) Larval distribution was recorded at 14 and 25 days post-inoculation. Experiments included infection ratios (1:25, 5:25, 10:25) and both negative (no infected beetles) and positive (all beetles infected) controls. Bars represent larval counts in each sawdust section (U, M, L); different letters indicate statistically significant differences (p < 0.05).
(D) Pupae distribution was evaluated at 25 days post-inoculation (conditions as in C). Panels A-D were analyzed using the Tukey-Kramer Honest Significant Difference (HSD) test. Bars indicate the number of pupae in each sawdust section; Error bars represent the standard error, and different letters denote statistically significant differences (p < 0.05). Abbreviations: [T]: total number of beetles in each colony.
In contrast, live adult beetles were found mainly in the middle section, with a minority found in the upper and lower sections (Figure 4B). For larvae, the majority were found in the middle or lower thirds of the colony across time (14 and 25 days) and infection ratio (from 1:25 to all infected), with the exception of the control colony (all non-infected) in which larvae were predominantly found in the middle and lower section at 14 days, but roughly equally distributed across all sections at the 25 days time point (Figure 4C). These data recapitulated the previous experiments described above, with decreased overall numbers of larvae seen as the infection ratio increased. Similarly, pupae were almost exclusively found in the middle and lower sections of the colony (25-day time point), irrespective of infection ratio, although total numbers decreased as a function of the ratio (Figure 4D). In contrast to the larval and pupal distributions, but consistent with the mycosed adults, M. anisopliae CFUs were found predominantly in the upper third section, with CFU levels correlating with infection ratio (Figure 5A). No M. anisopliae CFUs were detected in control colonies, with addition of 1 infected:25 uninfected showing a gradual increase from ∼100 to 400 CFU/g media in the upper third section from 7 to 25 days post-release into the colony, with few CFUs detected in the middle and lower sections. Higher levels of M. anisopliae CFUs were seen in the upper section as the infection ratio increased from 5:25 to all infected, peaking at ∼8,000 CFU/g media at 14 and 25 days in the “all infected” experimental condition. As a function of time and infected to uninfected ratio, M. anisopliae CFUs were detected in the middle and lower third sections, although always significantly lower than that found in the upper third section (p < 0.01). Not surprisingly, peak CFU levels in the middle and lower sections reached 300–1000 CFU/g media after 25 days in the 10:25 and all infected test conditions, with the upper sections in these samples reaching 1500–8000 CFU/g media.
Figure 5.
Spatial enumeration of Metarhizium CFUs within beetle colonies and interactions with Neocosmospora sp. Xa1: co-culture dynamics and impact of culture filtrates on colony-forming ability
(A) Metarhizium CFUs were quantified at 7, 14, and 25 days post-inoculation using semi-selective media from three sawdust sections: upper (U), middle (M), and lower (L). Experiments included infection ratios of 1:25, 5:25, and 10:25 (infected to uninfected beetles) and a positive control (all beetles infected). Bars indicate Metarhizium CFUs per gram of medium within each section. Letters denote statistically significant differences (p < 0.05).
(B) Co-culture interactions between Neocosmospora and Metarhizium anisopliae on different growth media. Fungal suspensions (4 μL at 104 conidia/mL) of each species were co-inoculated onto potato dextrose agar (PDA), Sabouraud dextrose agar (SDA), Czapek–Dox agar (CZA), and beetle sawdust medium. Colony morphology and fungal growth dynamics were photographed and documented over a 21-day incubation period. All treatments were performed in triplicate.
(C) Metarhizium CFU counts were assessed after exposure to filtrates obtained from Neocosmospora cultures grown in three media (CZB, PDB, and SDB) under either aerated (A) or static (S) conditions. The figure represents 80 μL of the conidial concentration of 103 conidia/mL. Panels A-C were analyzed using the Tukey-Kramer Honest Significant Difference (HSD) test. Error bars represent the standard error, and different letters indicate statistically significant differences among treatments (p < 0.05). Abbreviations: C: control, A: aerated condition, S: static condition, and CFU: colony-forming unit.
Identification of Neocosmospora sp. Xa1 as an ambrosia beetle partner fungus and interactions with M. anisopliae
To examine interactions between X. affinis ambrosia fungal partner and M. anisopliae, the fungal gardens from several control colonies were streaked, and one major isolate found in colonies across time points was single spore purified, and its identity was further characterized via PCR amplification and sequencing of the elongation factor-1 α (EF1α) genomic locus as detailed in the STAR Methods section. Phylogenetic analysis of the resultant sequence placed the isolate (Xa1) within the Neocosmospora (previously Fusarium solani species complex) clade, which includes known ambrosia beetle symbionts (Figure S12). Individual and co-culturing of the Neocosmospora sp. Xa1 isolate with M. anisopliae over a time course up to 21 days revealed that the former grew at an appreciably faster rate than the latter, with an antagonistic interface apparent between the two in standard mycological media (PDA, SDA, and CZA, Figures 5B and S13). Intriguingly, on plates made with beetle sawdust media (BSM), the Neocosmospora isolate was able to even more rapidly overtake M. anisopliae, surrounding the latter colony and significantly limiting its growth. A similar antagonistic pattern was observed when Neocosmospora was co-cultured with B. bassiana, with accelerated Neocosmospora growth and restricted expansion of the Beauveria colony across all media, including BSM (Figure S14).
To further probe the inhibitory effects of Neocosmospora sp. Xa1 on M. anisopliae, cell-free culture supernatants derived from Neocosmospora grown in CZB, PDB, and SDB with and without aeration were prepared as detailed in the STAR Methods section. All extracts significantly suppressed the recovery of M. anisopliae CFUs (p < 0.0001, Figure 5C). At a lower M. anisopliae conidial concentration (80 μL of 103 conidia/mL/plate, expected recovery of 80–90 CFUs/plate), suppression was 60–80% and at a higher initial conidial concentration (40 μL of 104 conidia/mL/plate, expected recovery of 400–500 CFUs/plate) (Figure S15), inhibition was 50–55%, with the exception of aerated culture supernatant from PBD, which showed only an ∼10–20% decrease in M. anisopliae CFU recovery. Exposure to Neocosmospora sp. Xa1 cell-free culture supernatants significantly (p = 0.0009) reduced B. bassiana CFUs compared to media-only controls. The degree of inhibition varied depending on the growth medium used for Neocosmospora cultivation. Supernatants from SDB and CZB media (especially under aeration) led to the most pronounced CFU reductions, over 40% in SDB treatments and ∼30% in CZB-A, whereas PDB-derived supernatants had a comparatively moderate effect (24–33% reduction), and CZB-S showed the least inhibition (∼9.6%) (Figure S16).
Discussion
Ambrosia beetles burrow into the sapwood of trees to elaborate their galleries. As social insects, within their habitat, they expand their galleries, tend their fungal gardens, lay eggs, and engage in brood care, among other behaviors.6 Little, however, is known concerning how these beetles and their cryptic habitats within trees affect the spread of infectious diseases, a phenomenon of particular importance in social animals that have networks of interactions. Spread of infections, particularly within the framework of social insects that often display low genetic diversity in colonies, can be especially problematic, and disease outbreaks mediated by evolutionary pressures as part of host-pathogen interactions have unique attributes when sociality is considered.9 Yet social insects show high resilience against infectious disease outbreaks, with behavioral, physiological, and organizational adaptations, which, as collective activities, have been termed social immunity, in conjunction with more canonical immunological defenses functioning together to protect colony members.
Our data confirm high mortality of X. affinis in “laboratory” bioassays, i.e., assays in which insects are infected with the agent and kept sequestered over the time course of the assay, using the insect pathogenic fungi, M. anisopliae and M. robertsii, although such experiments do not consider the nest/gallery habitat of these organisms. Release of directly infected insects into a colony media habitat did not affect ultimate mortality, even immediately after treatment (within 10 min), a time point before which the fungus has consolidated attachment (typically 1–4 h post-inoculation). These data suggest that burrowing does not have a significant effect on the removal of fungal cells (at least at the infection concentrations used). This timescale is consistent with what has been reported for infection of X. affinis by the insect pathogenic fungus B. bassiana; however, our data differ in that effects of burrowing were seen for B. bassiana infection.36 Attachment, consolidation, and initial germination on the host integument occur on the scale of 4–12 h and involves a complex series of steps, including appressoria formation (for Metarhizium but not all Beauveria sp.), secretion of cuticle degrading enzymes, both toxin production and scavenging/neutralization of host toxic molecules, leading to hyphal breaching of the cuticle.39,40,41,42 Once the cuticle has been penetrated, the fungus undergoes a dimorphic transition, producing free-floating hyphal bodies that circulate and infect the internal tissues of the host, successfully evading host immune systems.17,43,44,45 The fungus then works its way out, ultimately sporulating on the cadaver.46 In the experiments reported here, in all cases, mortality due to fungal infection was confirmed by fungal outgrowth and sporulation.
Although direct infection of beetles results in high mortality, the extent to which transmission of the disease can occur remains largely unexplored, particularly within the context of the colony habitat. In ex-gallery experiments, horizontal transmission resulted in a ratio (directly infected: uninfected) dependent infection of healthy conspecifics and their ultimate mortality. These experiments also showed an apparent lack of avoidance of infected/dead nestmates, with transmission to healthy nestmates also seen in a ratio-dependent manner. X. affinis constructs branched tunnel gallery systems and displays cooperative breeding coupled to division of labor between and among both adults and larvae.8 Interestingly, in gallery experiments, i.e., when infection was performed in colony media habitats, even when all members were originally directly infected (and died from the infection), a small number of larvae and pupae were produced. These results have important implications in terms of colony survival to epizootic diseases and even pest control, as they suggest that even if all members of a colony are infected, a small number of offspring can still be produced. As these offspring were apparently healthy and could develop into adults, this mechanism may help ensure the survival of the colony. To date, transmission of fungal insect pathogens within ambrosia beetle colonies has not been reported. By directly marking infected beetles (yellow dot), we could measure transmission, and our data indicate that such events of disease spread occur and are affected, not too surprisingly, by the ratio of infected to uninfected members of the colony. At a low infection ratio (1:25), little effects were seen in terms of overall colony “output”, i.e., larval and pupal numbers, under the conditions tested. At higher ratios (5:25 and 10:25), substantial reductions in adults, larvae, and pupae were seen, with mortality reaching 70% within 25 days, although in all cases, survivors were noted. In all instances, infection was considered positive only if fungal sporulation was seen on the cadaver, a phenomenon that we could directly observe in beetle galleries.
Further experiments examining the susceptibility of the surviving population to the fungal agent are warranted, although previous research has indicated that the development of resistance to entomopathogenic fungi by host insects is low/unlikely.47 It is possible that these beetles simply avoided infection or were infected with sub-lethal doses. As ambrosia beetles do not show a high degree of social hierarchy, and appear to organize more along gregarious lines, the potential for an epidemic outbreak is considered high.13 However, our data indicate an added variable of spatial segregation that can affect group interactions. In addition, the clear proportionality in disease spread dependent upon the number of initially diseased individuals can be explained by low contact rates, although high contact rates have been suggested to act to dilute pathogen loads on individuals.14 This does not seem to be the case for ambrosia beetles, because 100% mortality was seen for all initially infected individuals.
In examining colonies over the infection process, two phenomena were noted. First, although extensive M. anisopliae fungal outgrowth could be seen on (infected) cadavers and sometimes in the immediate surrounding gallery media, this was highly localized to the cadaver, and M. anisopliae did not apparently “take over” the colony. Second, cadavers tended to be found in the upper/top regions of the colony tubes, with surviving members found lower within the gallery habitat. To gain insight into these observations, we repeated the infection scenarios in colony habitats but separated the colony into thirds (upper, middle, and lower sections) and determined (1) adult mortality and the number of larvae and pupae in each section and (2) the distribution of M. anisopliae CFUs in each section. To remove any potential “geometry” bias in beetle tropism, i.e., movement upwards rather than side to side, separate experiments were performed, placing tubes in both vertical and horizontal orientations. No differences were seen between the datasets, indicating that tube orientation and/or any beetle movement preferences up-down rather than side to side, did not affect beetle population distribution and disease transmission. Overall, however, these data revealed a dramatic bias in the distribution of dead and living adults, as well as larval and pupal offspring. Dead insects were found almost exclusively in the top third layer (nearest to the surface of the media in the colony tube), whereas living adults, larvae, and pupae were mainly found in the middle and bottom layers.
These striking results open a major new question, namely, what is the mechanism leading to this biased distribution? One simple explanation is that dying insects seek to leave the nest. This is consistent with (apparently) non-infected beetle cadavers also being almost exclusively found in the upper section. Such a general mechanism would obviate the need for sensing infection per se, but does suggest a generalized tropism for dying beetles. It should be noted that we rarely found dead insects outside of the galleries themselves, although there was ample room for them to leave. Alternatively (or in conjunction), healthy nestmates may have moved sick or dying conspecifics (whether infected or not), a phenomenon well described in ants, e.g., necrophoretic behavior, with corpse movement and cannibalism shown for Xyleborinus saxesenii beetles.38,48 Although we did not explicitly look for sick/corpse removal, and we noted no particular avoidance of dead nestmates, nor did we observe actual removal. We further noted no clear evidence of cannibalism as all adult beetles could be accounted for, and no obvious signs of cannibalism/dismemberment were noted; however, further behavioral studies are warranted to examine these issues. It is entirely possible that the beetles in these two distinct genera have evolved differences in their responses to infections in general, and/or to specific infectious agents (Aspergillus versus Metarhizium). Concerning the distribution of M. anisopliae CFUs, correlating with the dead adults, the majority (>90%) of M. anisopliae CFUs were found in the upper section, with significantly lower numbers found in the middle, and little to no CFUs in the bottom sections. These data suggest that either due to intrinsic movement of dying conspecifics and/or extrinsic movement of such nestmates, infectious fungal cells can be at least partially sequestered away from healthy adults and offspring. However, such a mechanism, although contributing to limiting infectious disease spread, would not be sufficient. We noted that the humidity within the galleries ranged from 82.49–63.83% over the time course of the experiment; values within the range reported for optimal conditions for the beetle,49 but also particularly suitable for fungal (including M. anisopliae) growth. The extent to which horizontal transmission was solely mediated by direct contact between beetles or via contaminated media/aerosolized spore dispersal remains an open question. Our observations do not completely differentiate between the possibilities of direct contact, contaminated media, and/or other forms of spore dispersal. However, the following suggests that direct contact transmission is occurring: (1) in the in vitro experiments (and to a lesser extent within the colonies), we observed direct contact between healthy individuals and diseased/mycosed insects, (2) no apparent avoidance of diseased/mycosed beetles was seen by healthy individuals, (3) despite clearly mycosed individuals in the habitat-colonies, except immediately surrounding a dead insect, little to no Metarhizium fungal colonization of the habitat was seen, with this observation further supported by pathogen CFU counting of the media and the observed spatial distribution bias of the pathogen (within the habitat) noted. Finally, as no infection of (eggs) larvae and pupae was seen, at least significant areas of the nest were not contaminated with sufficient pathogen levels as to initiate infection. Overall, the opportunity for infection by contaminated media or some other form of spore dispersal would seem to be lower than via direct contact with a mycosed beetle.
The visual observation of the lack of M. anisopliae “taking over” the colony media and the confirmation of its uneven distribution within the beetle galleries raises two important points. First, this suggests that infection occurred mainly via horizontal transmission, i.e., contact with sick and/or dead nestmates, rather than infection by M. anisopliae growing in the media (as spread by sick/dead insects). Second, this opens the question as to why M. anisopliae does not proliferate within the media habitat. Two potential hypotheses are that the insect produces antimicrobial compounds to help keep galleries “clean” and/or that associated symbiotic microbes help suppress pathogens. To test the latter, we identified one partner fungus routinely found in our colonies as a Neocosmospora (formerly Fusarium solani) species. The Neocosmospora species complex is exceptionally large and includes both plant pathogens and ambrosia beetle symbionts.50,51,52 Presumably, this fungus can colonize the X. affinis mycangia (symbiotic organ),4,53,54 which acts as a reservoir for the fungus within the beetle body itself, although this requires further experimental verification. In vitro, plate antagonism assays revealed that the Neocosmospora isolate could out-compete M. anisopliae on most media but was particularly adept on the beetle habitat media. In addition, cell-free culture supernatants could suppress the growth of M. anisopliae and even that of another entomopathogenic fungus, B. bassiana, under a variety of conditions. These data demonstrate that partner beetle fungi can help suppress pathogenic microbial populations. However, both Neocosmospora and M. anisopliae (especially the former) can quite rapidly proliferate in gallery media (in vitro, i.e., in the absence of beetles). However, even in our (non-infected) beetle colonies, we do not see the rampant growth evident in vitro. This suggests important behavioral cultivation and gallery maintenance that limits even the growth of the mutualistic partner. Although far less studied than their ant and termite counterparts, bacteria in oral secretions of the spruce (bark) beetle, Dendroctonus rufipennis, have been shown to inhibit a range of pathogenic fungi that invade their galleries.55 Similarly, in the southern pine beetle, Dendroctonus frontalis, the fungal symbiotic mutualism is promoted by a bacterially produced compound (polyunsaturated peroxide), which is, however, toxic toward fungal antagonists.56 In addition, cycloheximide-producing Streptomyces have been shown to be associated with both X. saxesenii and X. affinis, which may help suppress unwanted fungi.57 Our data indicate that the fungal symbiotic partner itself may also contribute to the suppression of other microbes; however, our colonies likely contain a variety of other microbes (both bacteria and fungi) whose role(s) in these processes remain to be characterized. Finally, our data indicate an aspect of social immunity that may be particularly important for social (ambrosia) beetles, and that is the mechanisms that lead to offspring production and/or protection. While brood care, including sanitation/protection from disease-causing pathogens, is well known in other social insects (ants, bees, termites, and even wasps), socio-spatial dynamics within ambrosia beetle colonies may form a critical adaptation. Unlike other major social insects (e.g., most ants, termites, and bees), if these beetle females can survive the infection, they can simply leave the nest and act as foundresses for new colonies. This is particularly facilitated by the fact that these insects show haplo-diploidy, with females able to lay unfertilized eggs that develop into males with which they can mate to initiate a new colony. Indeed, the ability of a pathogen to infect within a colony with low genetic diversity may be one trade-off for the haplo-diploidy that significantly expands the survival potential of the species. Within this context, our data suggest a model where social immunity outcomes that favor, even simply spatially, but even more so in conjunction with the activity of beneficial microbes, the ability of eggs to develop into adults would be strongly adaptive.
In conclusion, we provide an expanded system for addressing questions concerning disease dynamics in social organisms. We infer the existence of mechanisms by which colony behavior outcomes vary in response to both low and high degrees of infection. Gallery maintenance and cultivation of partner fungi are programmed to help limit overgrowth of the symbiotic microbe, but can also be used to combat infectious fungal pathogens. Even under catastrophic conditions in which all members are infected (and ultimately) die, progeny are produced (in gallery areas away from the infectious agent) that can disperse and thus ensure survival to the next generation.
Limitations of the study
While our study demonstrates colony-level responses of X. affinis to fungal infection and highlights the spatial structuring and microbial interactions that mitigate pathogen spread, there are limitations to consider. First, our experimental conditions were conducted in artificial sawdust media under controlled laboratory settings, which may not fully capture the environmental complexity and microbial diversity of natural tree habitats. Second, although we observed spatial segregation of diseased individuals and pathogen CFUs, we did not directly quantify potential behavioral responses such as corpse removal, which may contribute to the observed patterns. Third, our study focused on a single fungal pathogen (M. anisopliae) and a single symbiotic partner (Neocosmospora sp. Xa1); additional pathogens or microbial interactions may yield different outcomes.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to Dr. Nemat O. Keyhani (keyhani@uic.edu).
Materials availability
This study did not generate new, unique reagents.
Data and code availability
-
•
The complete translation elongation factor 1-alpha (TEF1-α) locus sequence has been deposited at NCBI (GenBank: PV220985) and is publicly available as of the date of publication.
-
•
There is no code to report.
-
•
There are no other items to report.
Acknowledgments
The authors wish to thank Esther Tirmizi and Sourav Chakraborty for their assistance in rearing and maintenance of the beetle colonies. This research was funded in part by the US National Science Foundation (NSF), grant number IOS-2418026 to N.O.K.
Author contributions
A.M. conducted experiments, analyzed data, and drafted the manuscript. R.A.J. provided resources and materials, helped with microscopy, and revised the manuscript. N.O.K. supervised, conceptualized the content framework, analyzed data, and helped with the writing and revision of the manuscript.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Metarhizium anisopliae strain 549 | Gift from Dr. R. St. Leger (University of Maryland) | N/A |
| Metarhizium robertsii strain 2575 | Gift from Dr. R. St. Leger (University of Maryland) | N/A |
| Neocosmospora sp. Xa1 | Isolated in this study | NCBI: PV220985 |
| Chemicals, peptides, and recombinant proteins | ||
| Wood flour | System Three Resins, Inc., Auburn, WA, USA | NA |
| Coarse sweetgum sawdust | Greenville, South Carolina, USA | NA |
| Agar | ApeX BioResearch Products, Houston, TX, USA | Cat# 20273 |
| Sucrose | Sigma-Aldrich, St. Louis, MO, USA | Cat# 84097-1KG |
| Corn starch | ARGO, ACH Food Companies, Inc., Memphis, TN, USA | NA |
| Casein | Thermo Fisher Scientific Inc., Waltham, MA, USA | Cat# A137070.36 |
| Yeast extract | Fisher BioReagents™, Thermo Fisher Scientific, Waltham, MA, USA | Cat# 242415 |
| Wesson salt mixture | MP Biomedicals LLC, Solon, OH, USA | Cat# 902851 |
| Streptomycin sulfate | Thermo Fisher Scientific | Cat# BP910-5 |
| Ethanol (95%) | VWR or equivalent | CAS# 64-17-5 |
| Wheat germ oil | NOW® Foods | Cat# 1880 |
| Potato Dextrose Agar (PDA) | Criterion™, Hardy Diagnostics, USA | Cat# 6621 |
| Tween 20 | Promega | Cat# H5152 |
| Crystal violet | Sigma-Aldrich | Cat# C0775 |
| CTAB | Sigma-Aldrich | Cat# H6269 |
| Tetracycline | Sigma-Aldrich | Cat# T7660 |
| Chloramphenicol | Sigma-Aldrich | Cat# C0378 |
| Kanamycin sulfate | Sigma-Aldrich | Cat# K4000 |
| Non-toxic yellowish paint | MARSH®, Collinsville, IL, USA | Cat# M88FX-YP |
| Cetyl trimethyl ammonium bromide (CTAB) | Sigma-Aldrich | Cat# 57090 |
| Tissue-Tek® O.C.T. Compound | SAKURA | Cat#4583 |
| DAPI | Vector Laboratories Inc., Newark, CA, USA | Cat#H-1200-10 |
| Phalloidin | Vector Laboratories Inc., Newark, CA, USA | Cat#H-1600-10 |
| 2× Taq® PCR SuperMix | APExBIO, Houston, TX, USA | Cat#K1034 |
| Critical commercial assays | ||
| DNeasy Plant Mini Kit | Qiagen | Cat# 69106 |
| GeneJET PCR Purification Kit | Thermo Fisher Scientific | Cat# K0701 |
| Vectashield Hardset Antifade Mounting Medium with DAPI and Phalloidin | Vector Laboratories | Cat# H-1500-10 |
| Sabouraud dextrose agar (SDA) | Ward’s Science | Cat#470227-486 |
| Czapek–Dox agar (CZA) | Difco™ | Cat#233810 |
| Deposited data | ||
| Neocosmospora sp. Xa1 TEF1-α sequence | This paper | GenBank: PV220985 |
| Experimental models: organisms/strains | ||
| Xyleborus affinis beetles | Laboratory colony, University of Illinois Chicago | N/A |
| Oligonucleotides | ||
| 3′ side TEF1-α (983F) | 5′-GCY CCY GGH CAY CGT GAY TTY AT-3′ | See STAR Methods |
| 3′ side TEF1-α (2218R) | 5′-ATG ACA CCR ACR GCR ACR GTY TG-3′ | See STAR Methods |
| 5′ side TEF1-α (EFT1) | 5′-ATG GGT AAG GAR GAC AAG AC-3′ | See STAR Methods |
| 5′ side TEF1-α (1567R) | 5′-ACHGTRCCRATACCACCSATCTT-3′ | See STAR Methods |
| Software and algorithms | ||
| SAS JMP Pro version 18.0.1 | SAS Institute | https://www.jmp.com |
| OASIS version 2 survival analysis tool | Online tool | https://sbi.postech.ac.kr/oasis2/ |
| Topali version 2.5 | University of Edinburgh | http://www.topali.org/ |
| BioEdit | Ibis Biosciences | http://www.mbio.ncsu.edu/BioEdit/bioedit.html |
| MAFFT | Katoh Lab | https://mafft.cbrc.jp/alignment/software/ |
| MrBayes | Huelsenbeck Lab | http://mrbayes.sourceforge.net/ |
| FigTree | Institute of Evolutionary Biology, Univ. of Edinburgh | http://tree.bio.ed.ac.uk/software/figtree/ |
| BZ-X800 Analyzer software | Keyence Corporation | N/A |
| Other | ||
| Leica DM5000 B Microscope | Leica Microsystems | N/A |
| BZ-X810 Fluorescence Microscope | Keyence Corporation | N/A |
| Hemocytometer | Neubauer-improved, Lauda-Königshofen, Germany | N/A |
| FastPrep-24 homogenizer | MP Biomedicals, USA | N/A |
| NanoPhotometer® NP80 | Implen GmbH, Munich, Germany | N/A |
| C1000 Touch™ thermal cycler | Bio-Rad Laboratories, Hercules, CA, USA | N/A |
Experimental model and study participant details
The ambrosia beetle Xyleborus affinis colonies were maintained at 25°C on sterilized artificial diet blocks (see beetle rearing section). Adult beetles (mixed sexes) from 28 days post-adult eclosion were collected for all experiments. Sex stage was determined morphologically. All colony procedures received approval from the Institutional Biosafety Committee Policies of the University of Illinois, Chicago (IBC Number: 24-009).
Method details
Beetle rearing
Laboratory-reared colonies of the ambrosia beetle, X. affinis, were established following the methods described in our previous publication.4 In brief, 15 females with 3 male beetles were introduced into 50 mL polypropylene conical tubes (30 × 115 mm, FALCON®) containing 25 mL sawdust media. The sawdust media was prepared at a total volume of 1 liter, containing 120 g wood flour (System Three Resins, Inc., Auburn, WA, USA), 30 g coarse sweetgum sawdust (Greenville, South Carolina, USA), 40 g agar (ApeX BioResearch Products, Houston, TX, USA), 20 g sucrose (Sigma-Aldrich, St. Louis, MO, USA), 10 g corn starch (ARGO, ACH Food Companies, Inc., Memphis, TN, USA), 10 g casein (Thermo Fisher Scientific Inc., Waltham, MA, USA), 10 g yeast extract (Fisher BioReagents™, Thermo Fisher Scientific, Waltham, MA, USA), 2 g Wesson salt mixture (MP Biomedicals LLC, Solon, OH, USA), 0.7 g streptomycin sulfate (Thermo Fisher Scientific, Waltham, MA, USA), 10 mL of 95% ethanol, and 5 mL wheat germ oil (NOW® Foods, Bloomingdale, IL, USA). The beetles were allowed to excavate galleries and rear brood for 4-5 weeks, after which adult female and male beetles were collected to initiate fresh colony tubes.
Fungal growth and insect bioassays
Single-spore isolates of M. anisopliae strain 549 and M. robertsii strain 2575 (kind gift of R. St. Leger, U. of Maryland, Dept. of Entomology) were preserved in 20% sterilized glycerol (Thermo Fisher Scientific, Waltham, MA, USA) at -80°C and used to prepare cultures on a potato dextrose agar/broth (PDA/PDB) (Criterion™, Hardy Diagnostics, USA). Petri dishes were incubated unsealed in darkness at 25 ± 2°C for two weeks, followed by an additional week at 65% relative humidity with a 12-hour light/dark cycle. Conidia were collected using a spatula sterilized just before use, suspended in sterile 0.02% Tween 20 (Promega Corporation, Madison, USA), and vortexed for 2 minutes. Fungal suspensions were counted using a hemocytometer (Neubauer-improved, Lauda-Königshofen, Germany) observed under 20× magnification with an automated microscope (DM5000 B, Leica, Germany) and were diluted with sterilized double-distilled water (ddH2O) to desired concentrations (105-109 conidia/ml) as indicated. Viability and spore germination were confirmed for spore preparations by placing aliquots from harvested spores in PDB media for 12-18 h and monitoring germination. A conidium was considered germinated when the germ tube was equal in length to the diameter of the spore and, in all instances, was >95% before use. Adult female beetles were immersed in 200 μl of indicated fungal suspensions with constant agitation for 10 seconds. Control beetles were treated with sterile 0.02% Tween 20. After immersion, excess suspension was removed by placing the beetles on sterilized filter paper (Fisher Scientific Co., Ltd., Pennsylvania, USA). Seventy-five treated beetles, divided into three technical replicates (25/replicate), were transferred to 60 mm Petri plates containing moist, sterilized filter paper. Plates were maintained at 25 ± 2°C, 75 ± 7% relative humidity, with a 12-hour light/dark photoperiod. Mortality was recorded every 24 h and used to calculate the mean lethal time to 50% mortality (LT50) and the mean lethal dose (at a given time), resulting in 50% mortality (LD50).58 To quantify Metarhizium conidia on infected X. affinis, four infected beetles were randomly selected from each of the three technical replicates (12 infected beetles), and each beetle was placed into a 2 mL microcentrifuge tube containing 1 mL of 0.01% Tween 20 solution. The samples were then subjected to disruption using a FastPrep-24 homogenizer (MP Biomedicals, USA) for 2 minutes to dislodge the conidia from the beetle surface. The concentration of conidia in the suspension was determined using a hemocytometer. Bioassays were repeated at least twice using separate batches of conidia.
M. anisopliae bioassays
Petri plate assay-To measure horizontal transmission, a population of beetles was first treated with 1×108 conidia/ml of M. anisopliae and maintained in Petri plates with sterile, moist filter paper. Each infected beetle was marked with a tiny dot of non-toxic yellowish paint (MARSH®, Collinsville, IL, USA) on the elytra (hardened forewings) of the beetle to identify those directly infected. After 24 h, infected beetles were introduced to healthy conspecifics in Petri plates at different ratios, including 1:25, 5:25, and 10:25 infected: uninfected. Positive controls consisted of 25 infected beetles, and negative controls uninfected beetles (no added infected beetles). Each experiment consisted of three technical replicates, and the entire experiment was repeated three times using separate batches of conidia and beetles.
Release into colony media-These experiments were conducted as above, with several modifications: (1) infected beetles were released with healthy uninfected beetles into sawdust media, allowing for colony formation, and (2) mortality was monitored at discrete time points (7, 14, and 25 d post-release). At each time point, the sawdust media was carefully removed from the tubes and slowly dissected, and the number of dead and living adults was recorded. In addition, the number of larvae and pupae present at each time point was recorded. Beetles directly infected were discriminated from those acquiring infection via horizontal transmission by placement of a yellow ink dot on the former. Experiments were performed with three technical replicates (26-35 beetles/replicate) and repeated three times with independent batches of beetles and fungal conidia.
In a separate series of experiments, to examine the distribution of adults, larvae, pupae, and M. anisopliae in colonies, experiments were performed as above except at each time point, the sawdust media was removed, cut into three sections (upper, middle, lower), and the number of dead and living adults as well as larvae and pupae at each time point and section was recorded. M. anisopliae CFUs were determined in each section as follows: (i) each section was weighed (average ∼7.6 g), (ii) after adults, larvae, and pupae were counted and removed from the media, each section was transferred to a sterile Erlenmeyer flask, to which 30 mL of 0.01% sterile Tween-20 (Promega Corporation, Madison, USA) was added. The flask was shaken at 180 RPM for 6 hours to create a homogenous suspension. A 100-μL aliquot of the resulting suspension was then plated onto a semi-selective medium consisting of PDAY (10 g peptone/L, 40 g D-glucose anhydrous/L, 20 g agar/L, and 1 g yeast extract/L) supplemented with 0.6 g/L cetyl trimethyl ammonium bromide (CTAB), 0.6 g/L streptomycin sulfate, 0.3 g/L kanamycin, 0.05 g/L tetracycline, 1.0 g/L chloramphenicol, and 0.01 g/L crystal violet, with two replicates for each section. The plates were incubated in the dark at 25 ± 2°C for 14 days (RH ∼55 ± 5%), after which M. anisopliae colonies were enumerated. Data are given as colony-forming units (CFUs)/g of beetle media.
Microscopy
Xyleborus affinis beetles infected by M. anisopliae were cryo-sectioned and imaged using fluorescence microscopy as described previously4 with some modifications. Briefly, following infection at varying time points, beetles were embedded in molds containing Tissue-Tek optimal cutting temperature compound (OCT, Sakura) and rapidly frozen by immersion in liquid nitrogen-cooled isopentane. Once frozen, the OCT blocks were wrapped in aluminum foil, transferred to liquid nitrogen, and stored at -80°C until further processing. Before sectioning, the blocks were placed on dry ice for ∼30 min, followed by equilibration at -20°C in the cryostat chamber for 10-20 min. The blocks were then trimmed, mounted on cryostat pegs, sectioned at a thickness of 20 μm, and collected directly onto microscope slides. Where indicated, tissue sections were mounted in Vectashield Hardset antifade mounting medium containing DAPI and Phalloidin (Vector Laboratories Inc., Newark, CA, USA) for staining nuclei and actin filaments, respectively. Mounted samples were then coverslipped and allowed to harden for 24-48 h at 4°C prior to imaging. Samples were imaged using a BZ-X810 fluorescence microscope (Keyence Corporation, Osaka, Japan). Z-stacks of fluorescence channel images were assembled in the BZ-X800 Analyzer software and merged with single brightfield images to create overlays. Beetle hemolymph was collected and imaged for visualization of hemocytes and their interactions with M. anisopliae hyphal bodies as follows: X. affinis beetles infected by M. anisopliae for 24, 48, or 72 h were placed onto microscope slides, and their heads were removed under a dissecting microscope. Immediately following decapitation, gentle pressure was applied to the thorax using sterile dissecting pins to extrude hemolymph onto the microscope slide. Hemolymph was immediately mounted using a Vectashield Hardset antifade mounting medium with DAPI and Phalloidin. Slides were allowed to harden prior to imaging. Slides were analyzed using brightfield and fluorescence microscopy as above.
Determination of water content in sawdust media
To measure the water content of the sawdust media, the media in individual Falcon tubes was weighed and then allowed to dry in an oven at 105°C for 8 hours. After drying, the media were reweighed. The difference between the initial and final weights was calculated as the water content, which is expressed as a percentage of the total weight. For each time point (0, 14, and 25 d), three tubes were analyzed, and the average water content (%) was calculated.
Isolation of an X. affinis fungal partner
One fungal colony morphology was consistently seen from both extracts of beetle mycangia and plating of the nest gallery materials during colony maintenance. The isolate was a single colony purified and grown on potato dextrose agar (PDA) supplemented with 0.6 g L-1 streptomycin and 0.05 g L-1 tetracycline using a sterilized toothpick. Genomic DNA was extracted from freshly harvested mycelia using the DNeasy® Plant Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s protocol. DNA concentration was quantified using a NanoPhotometer® NP80 (Implen GmbH, Munich, Germany), and the extracted DNA was stored at −80°C until further use. The complete translation elongation factor 1-alpha (TEF1-α) locus was amplified using two primer sets. The first primer set, 983F/2218R, was used to amplify a ∼1,200 bp fragment spanning the 3′ region of TEF1-α (3′-TEF) using a touchdown PCR protocol. The reaction commenced with an initial denaturation at 94°C for 2 min, followed by 10 cycles in which the annealing temperature started at 66°C and decreased by 1°C per cycle. This was followed by 40 additional cycles consisting of denaturation at 94°C for 30 s, annealing at 56°C for 30 s, and extension at 72°C for 1 min. The amplification concluded with a final extension at 72°C for 10 min. The second set, EFT1/1567R, amplified a ∼1,162 bp fragment spanning the 5′ region of TEF1-α (5′-TEF). PCR conditions for the second primer set were as follows: initial denaturation at 94°C for 5 min, followed by 35 cycles of 94°C for 1 min, 55°C for 50 s, and 72°C for 3 min, with a final extension at 72°C for 10 min. Each PCR reaction was prepared in a 25 μL volume consisting of 6.5 μL 2× Taq® PCR SuperMix (APExBIO, Houston, TX, USA), 2.5 μL of each primer, 11.5 μL sterile distilled water, and 2 μL of DNA template. Amplifications were performed using a C1000 Touch™ thermal cycler (Bio-Rad Laboratories, Hercules, CA, USA). PCR amplicons were visualized on a 1% agarose gel, and positive PCR products were purified using the GeneJET PCR Purification Kit (Thermo Fisher Scientific, Waltham, MA, USA). The purified PCR products were submitted to Plasmidsaurus (www.plasmidsaurus.com) for Nanopore sequencing. The obtained sequences were assembled and edited with BioEdit (Ibis Biosciences, Carlsbad, CA, USA), and the resulting contig was deposited in NCBI under accession number PV220985.
Phylogenetic analysis of Neocosmospora
TEF1-α sequences for ambrosia Fusarium, and F. solani were obtained from,50,51,52 and downloaded from GenBank (National Center for Biotechnology Information, NCBI). Multiple sequence alignment was performed using MAFFT version 7 (https://doi.org/10.1093/molbev/mst010) using default settings. The optimal nucleotide substitution model for the TEF1-α locus was determined using FindModel (http://www.hiv.lanl.gov/content/sequence/findmodel/findmodel.html). The F81 model,59 incorporating gamma-distributed rate variation, was selected for EF1-α. Phylogenetic trees were inferred using RAxML and the Bayesian Hidden Markov Model (HMM-Bayes) approach as implemented in MrBayes. Both maximum likelihood (ML) and Bayesian inference (BI) analyses were conducted in Topali (tree TOPology-related analysis of Alignments Interface) version 2.5 for Windows. The RAxML tree was generated using 1,000 rapid bootstrap replicates, while BI was performed with two independent runs of 1,000,000 generations, sampling every 1,000 generations, with a 25% burn-in. Support values were considered significant at bootstrap values ≥70% for ML and posterior probabilities ≥0.7 for BI. The resulting phylogenetic trees were visualized using FigTree (http://tree.bio.ed.ac.uk/software/figtree/).
Co-culture of Neocosmospora and M. anisopliae on different media and effect of Neocosmospora sp. Xa1 extracts on M. anisopliae CFUs
To evaluate the interactions between Neocosmospora and Metarhizium, 4 μL of fungal suspensions (104 conidia/mL) were co-inoculated onto four different culture media: potato dextrose agar (PDA), Sabouraud dextrose agar (SDA), Czapek–Dox agar (CZA), and beetle sawdust medium, with three replicates for each medium. Fungal growth dynamics and colony morphology were monitored and documented through photographs taken over a 21-day incubation period. To assess whether similar interactions occur with other entomopathogenic fungi, the experiment was repeated using B. bassiana under identical conditions. Colony interactions and morphological changes were similarly monitored and documented over a 19-day incubation period.
To evaluate the impact of Neocosmospora sp. Xa1 on Metarhizium CFUs, Neocosmospora cultures were grown in PDB, CZB, and SDB +/- aeration (180 RPM) at 25 ± 2 °C for 4 d. Cell-free culture supernatants were prepared by filtering through 0.22 μm membrane filters (CellPro™ Premium Cell Strainers, Alkali Scientific, Fort Lauderdale, FL, USA) fitted on a 250 mL vacuum filtration flask to remove fungal biomass and obtain sterile filtrates. Aliquots (200 μl) of the cell-free culture supernatant were spread on PDA plates and allowed to dry overnight, after which Metarhizium conidial suspensions (40-80 μl of 103-104 conidia/mL) were spread on the plates, and the plates incubated in darkness at 25 ± 2 °C for 5 d, after which total CFUs counted. Control plates contained 100-200 μl of respective culture media. A total of six technical replicates were performed for each experimental condition, and the entire experiment was repeated twice. To address potential effects on other entomopathogenic fungi, a supplementary assay was conducted in which B. bassiana conidial suspensions (30 μl of 103 conidia/mL) were plated onto PDA pre-treated with 200 μl of the same Neocosmospora sp. Xa1 culture supernatants. CFUs were counted after 5d of incubation under identical conditions.
Quantification and statistical analysis
Three technical replicates were conducted for all experiments, with 25 beetles in each replicate, and the entire experiment was repeated at least twice with independent batches of beetles and fungal conidia. Data normality was assessed using the Shapiro-Wilk test, and homogeneity of variance was checked with Levene’s test (OriginLab, Northampton, MA) prior to the analysis of variance (ANOVA). Differences among means were analyzed using ANOVA and Tukey’s honestly significant difference (HSD) test at a 5% significance level. All statistical analyses were performed using SAS JMP Pro version 18.0.1 (SAS Institute Inc., Cary, NC, USA). Student’s two-sample t-tests were employed to assess differences in conidial production at a significance level of P < 0.05. Probit analysis was used to estimate the median lethal dose (LD50) and median lethal time (LT50), representing the dose and time at which 50% mortality occurred in the tested population, respectively. Mean survival times (MSTs) with Kaplan-Meier estimators (log-rank test) were calculated using the OASIS 2 online tool60 for assessing beetle survival and habitat effects in a controlled environment for gallery development on infection. For experiments repeated with independent batches, data from each batch were initially analyzed separately. Comparisons among corresponding groups across batches revealed no significant differences (P > 0.05); therefore, the data from replicate batches were pooled, and the combined results were presented.
Published: August 5, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2025.113281.
Supplemental information
References
- 1.Dzurenko M., Hulcr J. Ambrosia beetles. Curr. Biol. 2022;32:R61–R62. doi: 10.1016/j.cub.2021.11.043. [DOI] [PubMed] [Google Scholar]
- 2.Hulcr J., Stelinski L.L. The Ambrosia symbiosis: from evolutionary ecology to practical management. Annu. Rev. Entomol. 2017;62:285–303. doi: 10.1146/annurev-ento-031616-035105. [DOI] [PubMed] [Google Scholar]
- 3.Peris D., Delclòs X., Jordal B. Origin and evolution of fungus farming in wood-boring Coleoptera - a palaeontological perspective. Biol. Rev. Camb. Philos. Soc. 2021;96:2476–2488. doi: 10.1111/brv.12763. [DOI] [PubMed] [Google Scholar]
- 4.Joseph R., Bansal K., Keyhani N.O. Host switching by an ambrosia beetle fungal mutualist: Mycangial colonization of indigenous beetles by the invasive laurel wilt fungal pathogen. Environ. Microbiol. 2023;25:1894–1908. doi: 10.1111/1462-2920.16401. [DOI] [PubMed] [Google Scholar]
- 5.Osborn R.K., Castro J., Duong T.A., Hulcr J., Li Y., Martínez M., Cognato A.I. Symbiotic Fungi Associated With Xyleborine Ambrosia Beetles (Coleoptera: Curculionidae: Scolytinae) and the Imperative of Global Collaboration. Ann. Entomol. Soc. Am. 2023;116:51–71. doi: 10.1093/aesa/saac024. [DOI] [Google Scholar]
- 6.Biedermann P.H.W., Klepzig K.D., Taborsky M. Fungus Cultivation by Ambrosia Beetles: Behavior and Laboratory Breeding Success in Three Xyleborine Species. Environ. Entomol. 2009;38:1096–1105. doi: 10.1603/022.038.0417. [DOI] [PubMed] [Google Scholar]
- 7.Biedermann P.H.W., Taborsky M. Larval helpers and age polyethism in ambrosia beetles. Proc. Natl. Acad. Sci. USA. 2011;108:17064–17069. doi: 10.1073/pnas.1107758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biedermann P.H.W. Cooperative breeding in the ambrosia beetle Xyleborus affinis and management of its fungal symbionts. Front. Ecol. Evol. 2020;8 doi: 10.3389/fevo.2020.518954. [DOI] [Google Scholar]
- 9.Guo X., Chen J., Azizi A., Fewell J., Kang Y. Dynamics of social interactions, in the flow of information and disease spreading in social insects colonies: Effects of environmental events and spatial heterogeneity. J. Theor. Biol. 2020;492 doi: 10.1016/j.jtbi.2020.110191. [DOI] [PubMed] [Google Scholar]
- 10.Mersch D.P., Crespi A., Keller L. Tracking individuals shows spatial fidelity is a key regulator of ant social organization. Science. 2013;340:1090–1093. doi: 10.1126/science.1234316. [DOI] [PubMed] [Google Scholar]
- 11.Tschinkel W.R., Hanley N. Vertical organization of the division of labor within nests of the Florida harvester ant, Pogonomyrmex badius. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sah P., Leu S.T., Cross P.C., Hudson P.J., Bansal S. Unraveling the disease consequences and mechanisms of modular structure in animal social networks. Proc. Natl. Acad. Sci. USA. 2017;114:4165–4170. doi: 10.1073/pnas.1613616114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sah P., Mann J., Bansal S. Disease implications of animal social network structure: A synthesis across social systems. J. Anim. Ecol. 2018;87:546–558. doi: 10.1111/1365-2656.12786. [DOI] [PubMed] [Google Scholar]
- 14.Novak S., Cremer S. Fungal disease dynamics in insect societies: Optimal killing rates and the ambivalent effect of high social interaction rates. J. Theor. Biol. 2015;372:54–64. doi: 10.1016/j.jtbi.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Cremer S., Pull C., Furst M., Berenbaum M. Social Immunity: Emergence and Evolution of Colony-Level Disease Protection. Annu. Rev. Entomol. 2018;63:105–123. doi: 10.1146/annurev-ento-020117-043110. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W., Chen X., Tian J., Schal C., Mohamed A., Zang L.S., Xia Y., Keyhani N.O. An odorant-binding protein functions in fire ant social immunity interfacing with innate immunity. Open Biol. 2025;15 doi: 10.1098/rsob.240254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W., Chen X., Eleftherianos I., Mohamed A., Bastin A., Keyhani N.O. Crosstalk between immunity and behavior: insights from entomopathogenic fungi and their insect hosts. FEMS Microbiol. Rev. 2024;48 doi: 10.1093/femsre/fuae003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzurenko M., Ranger C.M., Hulcr J., Galko J., Kaňuch P. Origin of non-native Xylosandrus germanus, an invasive pest ambrosia beetle in Europe and North America. J. Pest. Sci. 2021;94:553–562. doi: 10.1007/s10340-020-01283-x. [DOI] [Google Scholar]
- 19.Vega F.E., Hofstetter R.W. Elsevier- Academic Press; 2015. Bark Beetles: Biology and Ecology of Native and Invasive Species. [Google Scholar]
- 20.Ploetz R.C., Hulcr J., Wingfield M.J., de Beer Z.W. Destructive Tree Diseases Associated with Ambrosia and Bark Beetles: Black Swan Events in Tree Pathology? Plant Dis. 2013;97:856–872. doi: 10.1094/PDIS-01-13-0056-FE. [DOI] [PubMed] [Google Scholar]
- 21.Joseph R., Keyhani N.O. Fungal mutualisms and pathosystems: life and death in the ambrosia beetle mycangia. Appl. Microbiol. Biotechnol. 2021;105:3393–3410. doi: 10.1007/s00253-021-11268-0. [DOI] [PubMed] [Google Scholar]
- 22.Harrington T.C., Yun H.Y., Lu S.S., Goto H., Aghayeva D.N., Fraedrich S.W. Isolations from the redbay ambrosia beetle, Xyleborus glabratus, confirm that the laurel wilt pathogen, Raffaelea lauricola, originated in Asia. Mycologia. 2011;103:1028–1036. doi: 10.3852/10-417. [DOI] [PubMed] [Google Scholar]
- 23.Harrington T.C., Fraedrich S.W., Aghayeva D.N. Raffaelea lauricola, a new ambrosia beetle symbiont and pathogen on the Lauraceae. Mycotaxon. 2008;104:399–404. [Google Scholar]
- 24.Joseph R., Lasa M., Zhou Y., Keyhani N.O. Unique Attributes of the Laurel Wilt Fungal Pathogen, Raffaelea lauricola, as Revealed by Metabolic Profiling. Pathogens. 2021;10:ARTN 528. doi: 10.3390/pathogens10050528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inch S., Ploetz R., Held B., Blanchette R. Histological and anatomical responses in avocado, Persea americana, induced by the vascular wilt pathogen, Raffaelea lauricola. Botany. 2012;90:627–635. doi: 10.1139/B2012-015. [DOI] [Google Scholar]
- 26.Ploetz R.C., Inch S.A., Perez Martinez J.M., White T.L. Systemic infection of avocado, Persea americana, by Raffaelea lauricola, does not progress into fruit pulp or seed. J. Phytopathol. 2012;160:491–495. doi: 10.1111/j.1439-0434.2012.01930.x. [DOI] [Google Scholar]
- 27.Reverchon F., Contreras-Ramos S.M., Eskalen A., Guerrero-Analco J.A., Quiñones-Aguilar E.E., Rios-Velasco C., Velázquez-Fernández J.B. Microbial biocontrol strategies for ambrosia beetles and thier associcated phytopathogenic fungi. Front. Sustain. Food Syst. 2021;5 doi: 10.3389/fsufs.2021.737977. [DOI] [Google Scholar]
- 28.Castrillo L.A., Griggs M.H., Ranger C.M., Reding M.E., Vandenberg J.D. Virulence of commercial strains of Beauveria bassiana and Metarhizium brunneum (Ascomycota: Hypocreales) against adult Xylosandrus germanus (Coleoptera: Curculionidae) and impact on brood. Biol. Control. 2011;58:121–126. doi: 10.1016/j.biocontrol.2011.04.010. [DOI] [Google Scholar]
- 29.Castrillo L.A., Griggs M.H., Vandenberg J.D. Granulate ambrosia beetle, Xylosandrus crassiusculus (Coleoptera: Curculionidae), survival and brood production following exposure to entomopathogenic and mycoparasitic fungi. Biol. Control. 2013;67:220–226. doi: 10.1016/j.biocontrol.2013.07.015. [DOI] [Google Scholar]
- 30.Kushiyev R., Tuncer C., Erper I., Ozdemir I.O., Saruhan I. Efficacy of native entomopathogenic funus, Isaria fumosorosea, against the bark and ambrosia beetles, Anisandrus dispar Fabricius and Xylosandrus germanus Blanford (Coleoptera: Curculionidae: Scolytinae) Egyptian Journal of Biological Pest Control. 2018;28 doi: 10.1186/s41938-018-0062-z. [DOI] [Google Scholar]
- 31.Castrillo L.A., Griggs M.H., Vandenberg J.D. Competition between biological control fungi and fungal symbionts of ambrosia beetles Xylosandrus crassiusculus and X. germanus (Coleoptera: Curculionidae): Mycelial interactions and impact on beetle brood production. Biol. Control. 2016;103:138–146. doi: 10.1016/j.biocontrol.2016.09.005. [DOI] [Google Scholar]
- 32.Gugliuzzo A., Aiello D., Biondi A., Giurdanella G., Siscaro G., Zappalà L., Vitale A., Garzia G.T., Polizzi G. Microbial mutualism supression by Trichoderma and Bacillus species for controlling the invasive ambrosia beetle Xylosandrus compactus. Biol. Control. 2022;170 doi: 10.1016/j.biocontrol.2022.104929. [DOI] [Google Scholar]
- 33.Avery P.B., Bojorque V., Gamez C., Duncan R.E., Carrillo D., Cave R.D. Spore acquisition and survival of ambrosia beetles associated with the laurel wilt pathogen in avocados after exposure to entomopathogenic fungi. Insects. 2018;9:49. doi: 10.3390/insects9020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castrejon-Antonio J.E., Tamez-Guerra P., Montesinos-Matias R., Ek-Ramos M.J., Garza-Lopez P.M., Arredondo-Bernal H.C. Selection of Beauveria bassiana (Hypocreales: Cordycipitaceae) strains to control Xyleborus affinis (Curculionidae: Scolytinae) females. PeerJ. 2020;8 doi: 10.7717/peerj.9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y., Avery P.B., Carrillo D., Duncan R.H., Lukowsky A., Cave R.D., Keyhani N.O. Identification of the Achilles heels of the laurel wilt pathogen and its beetle vector. Appl. Microbiol. Biotechnol. 2018;102:5673–5684. doi: 10.1007/s00253-018-9037-y. [DOI] [PubMed] [Google Scholar]
- 36.Castrejon-Antonio J.E., Tamez-Guerra P., Garcia-Ortiz N., Muniz-Paredes F., Sanchez-Rangel J.C., Montesinos-Matias R. Biocontrol of Xyleborus affinis (Curculionidae: Scolitinae) females and progeny by Beauveria bassiana (Hypocreales: Cordycipitaceae) in a sawdust artificial diet model. Insects. 2023;14:477. doi: 10.3390/insects14050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hubbard H.G. In: The ambrosia beetles of the United States. Howard L.O., editor. United States Government Printing Office; 1897. pp. 9–30. [Google Scholar]
- 38.Nuotcla J., Biedermann P., Taborsky M. Pathogen defence is a potential driver of social evolution in ambrosia beetles. Proc. Biol. Sci. 2019;286 doi: 10.1098/rspb.2019.2332. ARTN 20192332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X., Keyhani N.O., Liu H., Zhang Y., Xia Y., Cao Y. Glyoxal oxidase-mediated detoxification of reactive carbonyl species contributes to virulence, stress tolerance, and development in a pathogenic fungus. PLoS Pathog. 2024;20 doi: 10.1371/journal.ppat.1012431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y., Ren H., Wang F., Chen J., Ma L., Chen Y., Li X., Fan Y., Jin D., Hou L., et al. Cell detoxification of secondary metabolites by P4-ATPase-mediated vesicle transport. eLife. 2023;12 doi: 10.7554/eLife.79179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li G., Fan A., Peng G., Keyhani N.O., Xin J., Cao Y., Xia Y. A bifunctional catalase-peroxidase, MakatG1, contributes to virulence of Metarhizium acridum by overcoming oxidative stress on the host insect cuticle. Environ. Microbiol. 2017;19:4365–4378. doi: 10.1111/1462-2920.13932. [DOI] [PubMed] [Google Scholar]
- 42.Ortiz-Urquiza A., Keyhani N.O. Action on the surface: entomopathogenic fungi versus the insect cuticle. Insects. 2013;4:357–374. doi: 10.3390/insects4030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kan Y., He Z., Keyhani N.O., Li N., Huang S., Zhao X., Liu P., Zeng F., Li M., Luo Z., Zhang Y. A network of transcription factors in complex with a regulating cell cycle cyclin orchestrates fungal oxidative stress responses. BMC Biol. 2024;22:81. doi: 10.1186/s12915-024-01884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wanchoo A., Lewis M.W., Keyhani N.O. Lectin mapping reveals stage-specific display of surface carbohydrates in in vitro and haemolymph-derived cells of the entomopathogenic fungus Beauveria bassiana. Microbiology-Sgm. 2009;155:3121–3133. doi: 10.1099/mic.0.029157-0. [DOI] [PubMed] [Google Scholar]
- 45.Lewis M.W., Robalino I.V., Keyhani N.O. Uptake of the fluorescent probe FM4-64 by hyphae and haemolymph-derived hyphal bodies of the entomopathogenic fungus. Microbiology (Read.) 2009;155:3110–3120. doi: 10.1099/mic.0.029165-0. [DOI] [PubMed] [Google Scholar]
- 46.Fan Y., Liu X., Keyhani N.O., Tang G., Pei Y., Zhang W., Tong S. Regulatory cascade and biological activity of Beauveria bassiana oosporein that limits bacterial growth after host death. Proc. Natl. Acad. Sci. USA. 2017;114:E1578–E1586. doi: 10.1073/pnas.1616543114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao T., Wang Z., Huang Y., Keyhani N.O., Huang Z. Lack of resistance development in Bemisia tabaci to Isaria fumosorosea after multiple generations of selection. Sci. Rep. 2017;7 doi: 10.1038/srep42727. ARTN 42727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biedermann P.H.W., Klepzig K.D., Taborsky M. Costs of delayed dispersal and alloparental care in the fungus-cultivating ambrosia beetle Xyleborus affinis Eichhoff (Scolytinae: Curculionidae) Behav. Ecol. Sociobiol. 2011;65:1753–1761. doi: 10.1007/s00265-011-1183-5. [DOI] [Google Scholar]
- 49.Francke-Grossmann H. In: Symbiosis: Associations of invetebrates birds, ruminants and other biota. Henry S.M., editor. Academic Press; 1967. Ectosymbiosis in wood-inhabiting insects; pp. 142–206. [Google Scholar]
- 50.Carrillo J.D., Rugman-Jones P.F., Husein D., Stajich J.E., Kasson M.T., Carrillo D., Stouthamer R., Eskalen A. Members of the Euwallacea fornicatus species complex exhibit promiscuous mutualism with ambrosia fungi in Taiwan. Fungal Genet. Biol. 2019;133 doi: 10.1016/j.fgb.2019.103269. ARTN 103269. [DOI] [PubMed] [Google Scholar]
- 51.Lynn K.M.T., Wingfield M.J., Durán A., Oliveira L.S.S., de Beer Z.W., Barnes I. Novel Fusarium mutualists of two Euwallacea species infesting Acacia crassicarpa in Indonesia. Mycologia. 2021;113:536–558. doi: 10.1080/00275514.2021.1875708. [DOI] [PubMed] [Google Scholar]
- 52.Sandoval-Denis M., Lombard L., Crous P.W. Back to the roots: a reappraisal of Neocosmospora. Persoonia. 2019;43:90–185. doi: 10.3767/persoonia.2019.43.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joseph R.A., Tirmizi E., Masoudi A., Keyhani N.O. Cell structure of the preoral mycangia of Xyleborus (Coleoptera: Curculiondiae) ambrosia beetles. Insects. 2025;16 doi: 10.3390/insects16060644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joseph R.A., Bansal K., Nguyen J., Bielanski M., Tirmizi E., Masoudi A., Keyhani N.O. Fungi that live within animals: Application of cell cytometry to examine fungal colonization of ambrosia beetle (Xyleborus sp.) mycangia. J. Fungi. 2025;11 doi: 10.3390/jof11030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cardoza Y.J., Klepzig K.D., Raffa K.F. Bacteria in oral secretions of an endophytic insect inhibit antagonistic fungi. Ecol. Entomol. 2006;31:636–645. doi: 10.1111/j.1365-2311.2006.00829.x. [DOI] [Google Scholar]
- 56.Scott J.J., Oh D.C., Yuceer M.C., Klepzig K.D., Clardy J., Currie C.R. Bacterial protection of beetle-fungus mutualism. Science. 2008;322:63. doi: 10.1126/science.1160423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grubbs K.J., Surup F., Biedermann P.H.W., McDonald B.R., Klassen J.L., Carlson C.M., Clardy J., Currie C.R. Cycloheximide-producing Streptomyces associated with Xyleborinus saxesenii and Xyleborus affinis fungus-farming ambrosia beetles. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.562140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou T., Zhao Q., Li C., Ye L., Li Y., Keyhani N.O., Huang Z. Synergistic effects of the entomopathogenic fungus Isaria javanica and low doses of dinotefuran on the efficient control of the rice pest Sogatella furcifera. J. Integr. Agric. 2024;23:621–638. [Google Scholar]
- 59.Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 1981;17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 60.Han S.K., Lee D., Lee H., Kim D., Son H.G., Yang J.S., Lee S.J.V., Kim S. OASIS 2: online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget. 2016;7:56147–56152. doi: 10.18632/oncotarget.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The complete translation elongation factor 1-alpha (TEF1-α) locus sequence has been deposited at NCBI (GenBank: PV220985) and is publicly available as of the date of publication.
-
•
There is no code to report.
-
•
There are no other items to report.