Summary
Cardiovascular diseases (CVDs) remain one of the leading causes of death worldwide, imposing a significant societal burden and highlighting the urgent need for effective disease management strategies to reduce mortality and prevent disability. Recently, gut microbiota and its metabolites have been considered as an essential part of human physiology, emphasizing their contributions in CVD development and management. An increasing number of studies have shown that gut microbiota affects the process of CVDs either directly through changes in its composition and function or indirectly through its metabolites, and plays an important role in host physiology and disease development. This review provides a comprehensive overview of how gut microbes and their specific metabolites interact and contribute to the generation and development of CVDs. In addition, the therapeutic potential to treat CVDs by targeting the gut microbiota is also discussed, which may provide promising possibilities for the clinical treatment of CVDs in the future.
Subject areas: Cardiovascular medicine, Microbiome
Graphical abstract
Cardiovascular medicine; Microbiome
Introduction
Cardiovascular diseases (CVDs) rank as the primary global cause of morbidity and mortality.1 The World Health Organization reports that approximately 17.9 million individuals die of CVDs each year, representing 32 per cent of all deaths worldwide.2 CVDs encompass a range of non-infectious, and severe conditions, including heart failure, atherosclerosis, and hypertension. The complex interplay of genetic and environmental factors precludes attributing the disease to a single cause. Furthermore, inflammation, dyslipidemia, and diabetes mellitus are recognized as pivotal risk factors for CVDs, as they not only precipitate the disease but also significantly contribute to its progression and exacerbation.3,4,5
Throughout the past decade, investigations into the human gut microbiota have experienced rapid growth, establishing it as a burgeoning field of study.6 The human gut microbiota is a collection of microorganisms that colonize the gastrointestinal tract and consists of approximately 100 trillion microorganisms.7 These microorganisms achieve mutually beneficial coexistence with the host through a variety of physiological interactions, including nutrient absorption, digestion, energy provision, lipid metabolism, and immune system modulation, to maintain host homeostasis.8 At the beginning of the 21st century, the development of metagenomic and high-throughput sequencing technologies facilitated microbiome studies in several large population cohorts, which greatly advanced the understanding of the diversity of the gut microbiome and revealed many potential associations between the microbiome with health and disease.9 To date, an increasing number of studies have shown that gut microbiota perturbations contribute to disease pathogenesis, particularly cardiovascular disorders such as heart failure, hypertension, and atherosclerosis have become a prominent area of research.10,11,12,13 Compared with healthy controls, patients with heart failure show decreased abundance of gut microbiota, with major flora translocation and a reduced ratio of Firmicutes to Bacteroidetes.14 In addition, the gut microbiota also plays a crucial role in the development of hypertension15 and pulmonary hypertension.16 The gut microbiome in hypertensive individuals predominantly consists of Prevotella, and there is a marked reduction in both the richness and diversity of total microbial community.15 Atherosclerosis, the primary pathological basis of serious cardiovascular diseases and a leading cause of mortality,17 demonstrates established links to gut microbiota. Patients with atherosclerotic CVD exhibit microbial dysbiosis featuring decreased Bacteroides and Prevotella abundance with concomitant increases in Streptococcus and Escherichia.18 Furthermore, the opportunistic pathogen genus Collinsella has been observed to be elevated in patients with symptomatic atherosclerosis.
Altered gut microbial composition and function may influence host physiology and disease pathogenesis both locally and systemically through metabolites that communicate with distal organs.19,20,21,22 These metabolites mainly include trimethylamine N-oxide (TMAO),23 short-chain fatty acids (SCFAs),24 bile acids (BAs),25 lipopolysaccharide (LPS),26 amino acids27 and succinate.28 Recent research has extensively examined the role of gut microbiota-derived metabolites in CVDs, underscoring their potential as therapeutic targets for intervention in CVDs.
In this review, we discuss how gut microbes participate in and regulate the generation and development of CVDs. Moreover, we highlight an overview of several noteworthy gut microbiota-mediated metabolites and pathways associated with CVDs. Finally, potential therapeutic strategies for modulating gut microbes to influence CVD progression are considered.
Imbalance of gut microbiome
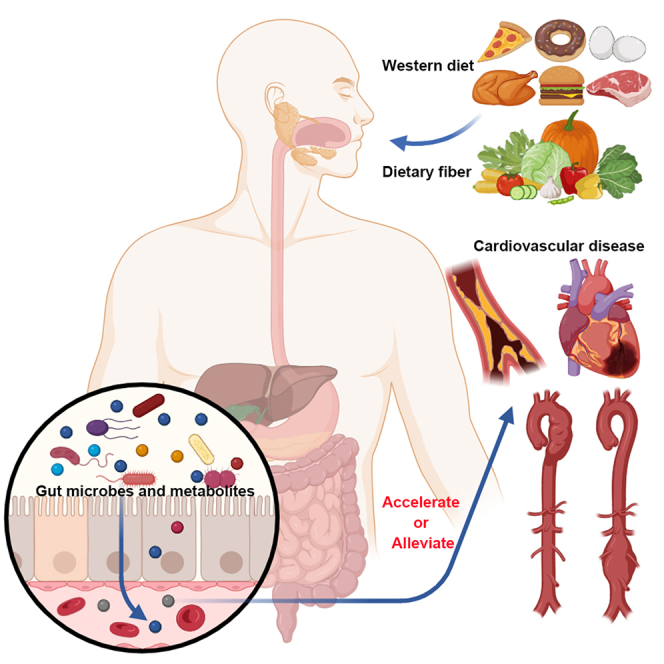
The human intestinal tract is home to trillions of microbial cells, which are integral to our healthy physiological ecosystem. This diverse community encompasses bacteria, fungi, archaea, and viruses, collectively known as the "microbiota," with their collective genome termed the "microbiome." The predominant bacterial constituents of the recognized intestinal microbial community include the phyla Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, and Verrucomicrobia.29 In healthy individuals, the "normal microbiome", encompassing the composition and function of the intestinal flora along with its related metabolites, remains relatively consistent, and the beneficial and harmful bacteria within the intestine sustain a fairly balanced state.30,31 However, the gut microbiome in the host may change considerably, especially in CVD pathological states (Figure 1).
Figure 1.
Diet can regulate the gut-heart axis and the gut-vascular axis through the gut microbiota
Disruption of gut microbiota, frequently induced by high-fat/high-sugar Western diets, contributes to CVD pathogenesis through key microbial metabolites. Dietary L-carnitine and choline are metabolized by gut microbiota to TMA, which hepatic FMOs oxidize into TMAO, a compound promoting CVD development. Conversely, gut microbiota-derived SCFAs produced from dietary fiber demonstrate protective effects against CVDs. TMA, trimethylamine; FMOs, flavin monooxygenases; TMAO, trimethylamine N-oxide; SCFAs, short-chain fatty acids; MI, myocardial infarction; TAD, thoracic aortic dissection; AAA, abdominal aortic aneurysm.
Alterations in the composition of intestinal microbiota: Dysbiosis
"Dysbiosis" denotes an imbalance within the body’s microbial community. Over the past decade, alterations in gut microbiota composition associated with multiple diseases and/or phenotypes have been the primary focus of most human microbiome research. Of these, Bacteroidetes and Firmicutes constitute the predominant portion of the gut microbiota in healthy adults, and the Firmicutes-to-Bacteroidetes ratio is regarded as an indicator of intestinal flora health.9 However, the human microbiome varies among individuals, populations, and environments and can influence human health and disease states. Research indicates that a decrease in the population of short-chain fatty acid-producing bacteria, including species such as Roseburia, Faecalibacterium, and Eubacterium rectale, along with an increase in the presence of opportunistic pathogens such as Klebsiella spp., Streptococcus spp., and Parabacteroides merdae, are linked to a heightened risk of CVDs.32 Hence, the type of microorganism as well as its relative abundance are elements that may influence the likelihood of developing CVDs.33
The field of microorganism research has undergone a transformation following the advent of bacterial genome analysis. Both 16S rRNA gene sequencing and metagenomic sequencing are extensively utilized methods for assessing changes in the composition and abundance of gut microorganisms. 16S sequencing, focusing on variable regions of the bacterial 16S rRNA gene, is cost-effective and suited for quick species composition and diversity assessment, but struggles with species-level identification. Conversely, metagenomic sequencing, analyzing entire metagenomic DNA, offers deep insights into gene functions and interspecies interactions. Together, they provide a more holistic perspective on the structure and functionality of the microbial community, with alterations in gut microbiota composition and function observed in cardiovascular diseases. For example, compared with the healthy control group, patients with symptomatic atherosclerosis showed increased abundance of Collinsella genus and decreased abundance of Roseburia and Eubacterium.13 A reanalysis of 25 Swedish and 385 Chinese fecal metagenomes revealed consistent depletion of key gut bacteria, including Bacteroides xylanisolvens, Odoribacter splanchnicus, Eubacterium eligens, Roseburia inulinivorans, and Roseburia intestinalis in patients with atherosclerosis . Functionally, healthy microbiomes exhibited enriched activity in starch degradation V (mediated by R inulinivorans and R intestinalis), glycolysis III, CDP-diacylglycerol biosynthesis (primarily from E eligens), and folate conversion (driven by Bacteroides spp.), while patients showed significant reduction in these pathways.34 Reduced microbial richness and diversity characterize the fecal microbiota in both hypertensive patients and mouse models. Studies have found that Prevotella and Klebsiella levels were elevated,15 whereas Roseburia spp. and Faecalibacterium prausnitzii abundance decreased in hypertensive patients.35 Additionally, gut microbiota dysbiosis is observed in patients with HF. Common alterations include increased Enterobacteriaceae, decreased Faecalibacterium prausnitzii, and elevated Bifidobacterium abundance.36 Patients with coronary artery disease (CAD) exhibited significant enrichment of Escherichia-Shigella and Enterococcus, alongside reductions in Faecalibacterium, Subdoligranulum, Roseburia, and Eubacterium rectale. Microbial network analysis further identified Faecalibacterium and Escherichia-Shigella as the keystone taxa in healthy controls and patients with CAD, respectively.37 Furthermore, Prevotella is associated with higher lifetime cardiovascular disease (CVD) risk, contrasting with Alloprevotella, which correlates with reduced risk.38 In this section, we enumerate the alterations in gut microbial composition linked to CVDs (Table 1).
Table 1.
Altered gut microbial compositions associated with CVDs in human and animal studies
| Species | Disease | Sample size | Technique | Altered gut microbial abundance |
References | |
|---|---|---|---|---|---|---|
| Increase | Decrease | |||||
| Human | Symptomatic atherosclerosis | 12 symptomatic atherosclerotic plaques, 13 controls | Metagenomic sequencing | Collinsella | Roseburia and Eubacterium | Karlsson et al.13 |
| Human | Atherosclerotic cardiovascular disease | 218 atherosclerotic cardiovascular disease (ACVD), 187 controls | Metagenomic sequencing | Streptococcus and Escherichia | Bacteroides and Prevotella | Jie et al.18 |
| Human | Coronary artery disease | 15 CAD, 5 controls | 16S sequencing | Prevotella | Clostridium and Faecalibacterium | Gózd-Barszczewska et al.39 |
| Human | Hypertension | 56 pre-hypertension, 99 primary hypertension, 41 controls | Metagenomic sequencing | Prevotella and Klebsiella | Li et al.15 | |
| Human | Hypertension | 60 primary hypertension, 60 controls | Metagenomic sequencing | Klebsiella spp., Streptococcus spp., and Parabacteroides merdae | Roseburia spp. and Faecalibacterium prausnitzii | Yan et al.35 |
| Human | Heart failure | 20 HF, 20 controls | 16S sequencing | Blautia, Collinsella, uncl. Erysipelotrichaceae and uncl. Ruminococcaceae | Luedde et al.40 | |
| Human | Chronic heart failure | 60 mild CHF, 30 moderate to severe CHF, 20 controls | Incubation using a selective agar | Campylobacter, Shigella, Salmonella, Yersinia enterocolitica, and Candida species | Luedde et al.40 | |
| Human | Chronic heart failure | 53 CHF, 41 controls | Metagenomic sequencing | Ruminococcus gnavus | Faecalibacterium prausnitzii | Cui et al.36 |
| Human | Atrial fibrillation | 50 atrial fibrillation, 50 controls | Metagenomic sequencing | Ruminococcus, Streptococcus, and Enterococcus | Faecalibacterium, Alistipes, Oscillibacter, and Bilophila | Zuo et al.41 |
| Human | Chronic Heart Failure | 22 CHF, 22 controls | Multicolor FISH analysis | Bacteroides/Prevotella, Eubacterium rectale, Fusobacterium prausnitzii (Fprau) | Sandek et al.42 | |
| Human | Heart failure | 84 HFrEF (40 discovery, and 44 validation), 266 controls | 16S sequencing | Uncultured bacterium, Prevotella, Hungatella, and Succiniclasticum | Lachnospiraceae family, Faecalibacterium and Bifidobacterium | Kummen et al.43 |
| Human | Cardiovascular disease | 76 type 2 diabetes and risk factors for CVDs | 16S sequencing | Roseburia, Eubacterium, and Faecalibacterium | Escherichia, Shigella, Bilophila, and Hungatella | Deng et al.44 |
| Human | Heart failure with preserved ejection fraction | 26 HFpEF, 67 controls | 16S sequencing | Firmicutes/Bacteroidetes | bacteria producing-SCFAs, particularly Ruminococcus | Beale et al.45 |
| Human | Hypertension | 6953 | Metagenomic sequencing | Firmicutes phylum change | Palmu et al.46 | |
| Human | Hypertension | 183 hypertension, 346 controls | 16S sequencing | Catabacter, Veillonella, Clostridium, Oscillibacter, Robinsoniella | Akkermansia, Ruminococcus, Anaerovorax, Sporobacter, Asaccharobacter | Palmu et al.47 |
| Human | Atherosclerosis | 226 atherosclerosis, 184 controls | Metagenomic sequencing | Streptococcus spp., Lactobacillus salivarius, Solobacterium moorei, and Atopobium parvulum | Bacteroides xylanisolvens, Odoribacter splanchnicus, Eubacterium eligens, Roseburia inulinivorans, and Roseburia intestinalis | Liu et al.34 |
| Human | Coronary artery disease | 70 coronary artery disease, 98 controls | 16S sequencing | Escherichia-Shigella and Enterococcus | Faecalibacterium, Subdoligranulum, Roseburia, and Eubacterium rectale | Zhu et al.37 |
| Human | Non-ischemic heart failure with reduced ejection fraction | 28 non-ischemic HFrEF, 19 controls | 16S sequencing | Streptococcus and Veillonella | SMB53 | Katsimichas et al.48 |
| Human | Heart failure | 12 HF, 12 controls | 16S sequencing | Eubacterium rectale and Dorea longicatena | Kamo et al.49 | |
| Human | Heart failure | 12 HF < 60 years, 10 HF > 60 years | 16S sequencing | Proteobacteria, Lactobacillus | Bacteroidetes, Faecalibacterium | Kamo et al.49 |
| Rabbits | Metabolic syndrome | 12 high-fructose high-fat diet (HFFD)-induced MetS, 9 controls | 16S sequencing | Firmicutes/Bacteroidetes ratio | Moughaizel et al.50 | |
| Rats | Hypertensive heart failure | 8 hypertensive heart failure (HHF) dahl salt-sensitive rat model, 8 controls | 16S sequencing | Firmicutes/Bacteroidetes | Muribaculaceae, Lachnospiraceae, and Lactobacillaceae | Li et al.51 |
| Rats | Hypertension | 7-8 per group SD rat | 16S sequencing | Verrucomicrobia phyla, Akkermansia genus | Hsu et al.52 | |
| Rats | Hypertension | 10 HSD, 10 controls | 16S sequencing | Erwinia genus, Christensenellaceae and Corynebacteriaceae families | Anaerostipes genus | Bier et al.53 |
| Mice | Atherosclerosis | 342 transgenes atherosclerotic mice | 16S sequencing | Roseburia sp. | Kasahara et al.54 | |
| Mice | Heart failure | 14 Thoracic aortic constriction (TAC) surgery, 8 controls | 16S sequencing | Helicobacter, Rikenellaceae, Colidextribacter, and Lactobacillus | Prevotellaceae, Muribaculum, and Rikenella | Bao et al.55 |
| Mice | Atherosclerosis | pro-atherogenic LDLr −/− ApoB100/100 mice either a low-fat/high-sucrose (LFHS) or a high-fat/low-sucrose (HFLS) diet | 16S sequencing | Lachnospiraceae, Rumino_Ruminococcus, Ruminococcaceae, Dorea, Oscillospira, Clostridiales, Coprococcus in LFHS-fed mice | Perazza et al.56 | |
Impairment of intestinal barrier
The intestinal barrier, primarily constituted by the mechanical attachment of the intestinal epithelium to their adjacent cell, exhibits selective permeability, which facilitates the uptake of nutrients, electrolytes, and water while barring the entry of toxins, antigens, and intestinal flora.57 The integrity of the intestinal mucosal barrier is essential for maintaining the balance between the intestinal internal environment and the host circulatory system. More and more studies have corroborated the “intestinal barrier of CVDs” validity in recent years. When the intestinal barrier is compromised, gut microbes or their components, such as lipopolysaccharide (LPS), can penetrate the mucosal layer and enter the bloodstream. As a powerful inflammation inducer, LPS triggers the immune system through its interaction with pattern recognition receptors, such as TLR4, present on immune cells. This interaction promotes the expression of inflammatory factors, contributing to chronic inflammation and worsening CVDs progression.58 Research indicates that myocardial ischemia/reperfusion injury (MI/RI) induces intestinal oxidative stress, resulting in the degradation of tight junction proteins (ZO-1, occludin), microvilli and mitochondrial structural damage, and mucus layer impairment, thereby compromising the intestinal barrier. Multi-omics analyses further identify the dysregulation of the complement and coagulation cascades, specifically downregulation of C3, Plg, and F13a1, as a central pathophysiological mechanism in the intestine during MI/RI. This dysregulation reflects immune exhaustion and impaired containment of translocated threats, directly exacerbating systemic inflammation and cardiac injury. And oral fullerene nanoscavenger (OFNS) treatment resolves systemic inflammation and prevents myocardial injury by regulating intestinal redox homeostasis, as validated through in vitro, in vivo, and multi-omics analyses.59
The gut microbiota helps maintain the intestinal barrier, contributing to immune activation and pathogen clearance. As early as 1997, Anker et al. found that in patients with CHF, systemic congestion and low cardiac output reduce intestinal perfusion, causing ischemia and damage to the selectively permeable intestinal epithelial barrier. This barrier dysfunction increases intestinal permeability, enabling bacterial translocation and endotoxin entry into the bloodstream thereby intensifying inflammation and contributing to HF progression.60 Subsequent studies have further demonstrated that altered intestinal permeability in patients with chronic heart failure during acute oedema exacerbation led to increased bacterial translocation, endotoxin, and cytokine concentrations.42,61,62 In addition to bacterial translocation and endotoxaemia, HF-associated congestion and ischemia alter gut microbiota composition and function, their metabolites such as TMAO exacerbate cardiac dysfunction, establishing a vicious cycle that worsens HF. Emerging evidence indicates myocardial ischemia/reperfusion (I/R) injury triggers gut dysbiosis and barrier impairment, enabling bacterial translocation and systemic lipopolysaccharide (LPS) release. This LPS influx activates innate immunity, mobilizes pro-inflammatory myeloid cells, including neutrophils and Ly6C-high monocytes from bone marrow and splenic reservoirs, thereby exacerbating cardiac inflammation and injury. Critically, glucagon-like peptide-2 (GLP-2)-mediated restoration of gut barrier integrity attenuates bacterial/LPS translocation, suppresses immunometabolic activation, and reduces myocardial damage, thus establishing the heart-gut-immune axis as a therapeutic target.63
Wu et al. reviewed that hypertension induced gut microbiota imbalance and gut barrier dysfunction, characterized by elevated harmful bacteria, hydrogen sulfide, and LPS levels, reduced beneficial bacteria and SCFAs, diminished intestinal tight junction proteins, and enhanced intestinal permeability.64 Another clinical study comparing intestinal barrier serum markers between hypertensive and non-hypertensive patients also demonstrated that the intestinal barrier dysfunction could potentially serve as a predictor for hypertension.65 On the other hand, a significant increase in plasma zonulin levels was observed in hypertensive patients relative to control subjects, as a regulator of tight junction proteins in the gut epithelium, these levels were discovered to be strongly correlated with systolic blood pressure, further supporting its potential involvement in gut barrier dysfunction observed in patients with high blood pressure.66
Changes in metabolites
In addition to direct interactions with host metabolism and the immune system, gut flora can influence cardiovascular development through their metabolites. A primary function of these microbes is to produce metabolites through diverse dietary fermentation, facilitating essential physiological processes.67 Research has shown that during the digestion of dietary components in the gut, they are co-metabolised by the microbiota and the host to produce a variety of metabolites, such as trimethylamine N-oxide (TMAO), short-chain fatty acids (SCFAs) and bile acids (BAs), some of which may be directly absorbed into the host’s bloodstream and function as "hormones" influencing distant organs. Alternatively, other metabolites are likely to be metabolized by host enzymes into downstream mediators or signaling molecules. The majority of microbial metabolites act synergistically in healthy individuals; however, others may exert harmful effects.68 In the next section, we provide detailed descriptions of several gut microbiota-derived metabolites and their driven metabolic pathways.
Gut microbiota-derived metabolites and metabolism
Trimethylamine N-oxide
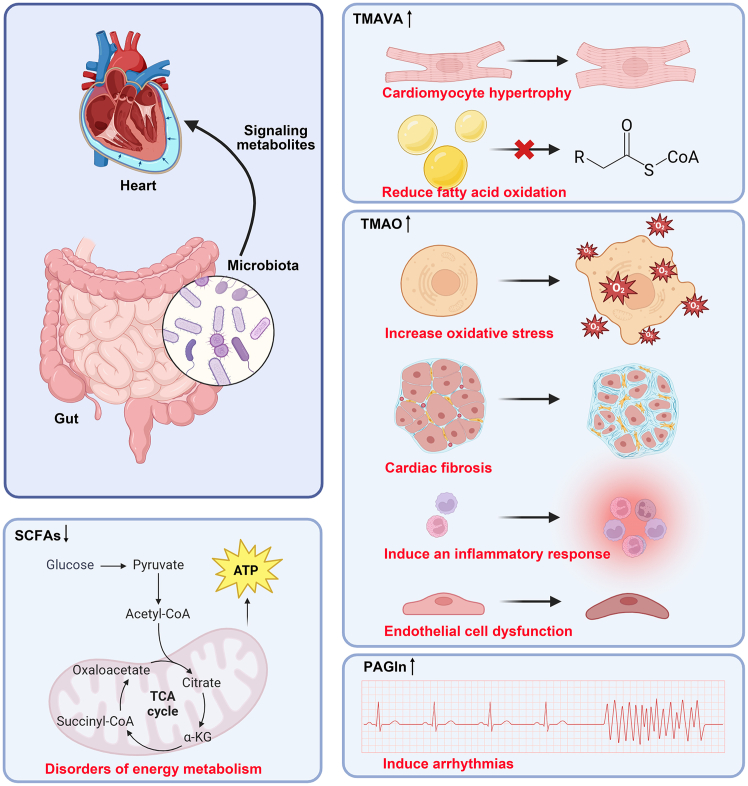
The gut microbiota produces a wide range of small-molecule metabolites, such as TMAO,23 SCFAs,24 BAs,25 and phenylacetylglutamine (PAGln).69 Dietary quaternary amines, such as phosphatidylcholine, choline, L-carnosine, betaine, γ-butyl betaine, crotonyl betaine, and glycerophosphorylcholine, are predominantly abundant in animal-based diets. These compounds are metabolized into trimethylamine (TMA) through the action of gut microbial enzymes, including choline TMA-lyase (encoded by the cutC/D genes) and carnitine oxygenase (encoded by cntA/B). Following microbial conversion in the intestinal lumen, TMA is absorbed by the intestinal epithelium and transported via the portal circulation to the liver. Within hepatocytes, flavin-containing monooxygenase 3 (FMO3) catalyzes the oxidation of TMA to trimethylamine N-oxide (TMAO). The resulting TMAO is then excreted primarily through renal clearance, with minor elimination occurring via transdermal pathways.23,70,71
Choline is converted to trimethylamine (TMA) by gut microbiota via two primary pathways: (1) direct cleavage by anaerobic microorganisms expressing the enzyme choline TMA-lyase (CutC), or (2) oxidation to betaine (catalyzed sequentially by choline dehydrogenase and betaine aldehyde dehydrogenase) followed by the reduction of betaine to TMA.71,72,73,74 Similarly, L-carnitine conversion to TMA occurs through two main routes: (1) direct conversion via the enzyme carnitine oxygenase and carnitine reductase, or (2) indirect conversion. In the indirect pathway, L-carnitine is first converted to γ-butyrobetaine by carnitine CoA-transferase (CaiB), and γ-butyrobetaine is then cleaved to TMA by γ-butyrobetaine TMA-lyase. Finally, betaine can be reduced directly to TMA via betaine reductase, or converted to L-carnitine (via a pathway involving levulinate dehydrogenase acting on an intermediate), and L-carnitine subsequently generates TMA as described above (Figure 1).70 TMAO contributes to CVDs by promoting vascular endothelial dysfunction and atherosclerosis. Mechanisms include inducing inflammatory responses and oxidative stress in endothelial cells,75,76 facilitating foam cell generation,77 disrupting cholesterol and bile metabolism,23,70 as well as enhancing platelet activation and thrombus formation.78 Studies in ApoE-deficient mice show TMAO supplementation exacerbates aortic lesions. Clinically, circulating TMAO levels correlate with CVD incidence and independently predict major adverse cardiac events (MACE).23,79 Furthermore, the TMAO precursors choline, carnitine, and betaine are also associated with CVD prevalence and predict MACE risk.79,80
Short-chain fatty acids
SCFAs are defined as fatty acids with a carbon chain length of six or fewer, primarily comprising three key components: acetate, propionate, and butyrate. These SCFAs are generated by anaerobic microorganisms in the colon that ferment indigestible dietary fibers and oligosaccharides, particularly from bacterial phyla such as Bacteroidetes and Firmicutes, including notable genera such as Lactobacillus, Faecalibacterium, and Ruminococcus. Among these, Faecalibacterium and Ruminococcus serve as the principal producers of short-chain fatty acids.81,82 Although not an SCFA, lactic acid can be metabolized by bacteria such as Fusobacterium hodgsonii into the SCFAs acetic acid, propionic acid, and butyric acid (Figure 1).83
Acetate constitutes the predominant short-chain fatty acid in the colon, comprising over half of the total SCFA detectable in feces. The majority of acetate originates from carbohydrate fermentation by intestinal bacteria, and the other approximately one-third is synthesized by acetate-producing bacteria. Unlike the acetic acid production pathway, which is widespread among various bacteria, the pathways for propionate and butyrate production are more conserved and substrate-specific. Propionate-producing microorganisms span several bacterial phyla but are prominent in a select number of genera, including the intestinal mucin-degrading Akkermansia muciniphila.84,85 Butyrate synthesis occurs via two primary pathways: the butyrate kinase pathway and the butyryl-CoA: acetate CoA-transferase pathway, which predominates among gut butyrate producers. The butyrate kinase pathway is observed in certain bacteria such as Enterococcus faecalis.86,87
As a metabolite produced by the intestinal microbiota, SCFAs are pivotal in sustaining human health through their functions in preserving the intestinal epithelial barrier integrity, diminishing inflammation, facilitating mucus production, and exerting direct influences on immune cells.81,88,89,90 An acute-phase randomized, controlled cross-over study revealed that a single-dose administration of 10 g inulin-propionate ester attenuates weight gain in overweight adults.91 Another 8-week randomized, double-blind, placebo-controlled trial showed that 500 mg propionate twice daily significantly reduced low-density lipoprotein cholesterol (LDL-c), total cholesterol, and non-high-density lipoprotein cholesterol levels in subjects with elevated baseline LDL-c.92
Mechanistically, SCFAs are capable of stimulating the secretion of the enteric hormone glucagon-like peptide (GLP)-1, thereby impacting the metabolic state and enhancing peripheral glucose clearance. This effect is predominantly mediated through its binding to G protein-coupled receptors, such as GPR43 (FFAR 2), GPR41 (FFAR 3), and GPR109A.93,94 Furthermore, SCFAs facilitate inflammatory processes as FFAR2 serves as a crucial receptor for the recruitment of neutrophils. Additionally, SCFAs can inhibit the proliferation of pathogenic microorganisms by creating acidic conditions. GPR109A, commonly referred to as hydroxycarboxylic acid receptor 2, specifically binds to butyric acid and β-hydroxybutyric acid, while it remains unresponsive to acetic acid and propionic acid.95 GPR109A is predominantly found in intestinal epithelial cells, immune cells, and adipocytes, suggesting its potential role in regulating immune cell homeostasis within the colon as well as lipid metabolism in adipose tissue.96 Additionally, another short-chain fatty acid receptor, Olfr78 is responsive to acetic acid and propionic acid, but not to butyric acid. It is primarily found in the kidney and blood vessels, where it plays a role in regulating renin secretion and antagonistically modulates blood pressure levels alongside GPR41 and GPR43.97,98,99 Pamela et al. found that butyric acid exerts anti-inflammatory effects by regulating intestinal macrophage function through the inhibition of histone deacetylase without affecting toll-like receptors, which are crucial protein molecules in nonspecific immune signaling and the activation of G protein-coupled receptors.100 In summary, SCFA receptors are widely distributed across tissues, reflecting both the systemic presence and cell type-specific functions of gut microbiota-derived SCFAs. These metabolites are consequently associated with multiple diseases, including obesity, type 2 diabetes (T2D), and CVDs.
Bile acids
BAs consist of primary and secondary bile acids. The liver is the only organ that synthesizes bile acids. Primary bile acids are synthesized directly from cholesterol within the liver, encompassing cholic acid (CA), chenodeoxycholic acid (CDCA), and their conjugates with glycine or taurine.101 Conversely, secondary bile acids form when intestinal microbiota 7α-dehydroxylate primary bile acids released into the gut, predominantly yielding deoxycholic acid (DCA) and lithocholic acid (LCA) along with their conjugates. Gut microorganisms are therefore essential for generating secondary bile acids, which subsequently modulate signaling pathways. Both primary and secondary bile acids serve as key regulators of host metabolism and immune responses.102,103
The microbiota plays a key role in the regulation of BA metabolism and transportation by engaging with several critical host bile acid receptors.104 Bile acids act as hormones by interacting with nuclear receptors such as the farnesoid X receptor (FXR), vitamin D receptor (VDR), pregnane X receptor (PXR), and constitutive androstane receptor (CAR), thus participating in the maintenance of lipid and glucose homeostasis, biometabolic processes, and immunomodulatory pathways.105,106 FXR is the predominant BA receptor and its agonists include free and bound bile acids, chenodeoxycholic acid (CDCA), lithocholic acid (LCA) and deoxycholic acid (DCA).107 Although conjugated and unconjugated primary/secondary BAs can activate FXR, their efficacy varies significantly. On the whole, CDCA serves as the most potent FXR agonist (EC50 = 10 μM), while DCA and LCA activate FXR with markedly lower potency (EC50 = 50 μM each). In contrast, hydrophilic bile acids such as ursodeoxycholic acid (UDCA) and muricholic acid (MCA) lack FXR-activating capability. Additionally, unconjugated BAs exhibit greater potency in activating FXR compared to their conjugated counterparts. For example, unconjugated BAs (CA, DCA, CDCA) enhance FXR expression, whereas conjugated forms (GCA, GDCA, GCDCA) suppress it.108,109 Except for FXR, various G protein-coupled receptors (GPCRs) are known to bind with bile acids, predominantly contributing to immunomodulatory functions and inhibiting NF-κB-mediated inflammation.105,110 Consistently, it has been noted that patients with CVDs exhibit reduced serum concentrations of primary bile acids, alongside an increased ratio of secondary to primary bile acids, when compared to healthy individuals.111 In turn, bile acids have the ability to modulate the composition of the gut microbiota in a species- and dose-dependent manner.112
Lipopolysaccharide
Unlike the metabolites derived from the gut microbiota previously mentioned, lipopolysaccharide (LPS), an endotoxin, is a major structural component of the outer membrane in Gram-negative bacteria. It consists of complex glycolipid molecules formed by covalently linked lipids and polysaccharides.113 LPS remains contained in bacteria under normal conditions but is released upon bacteriophage-induced membrane disruption, causing systemic inflammation and sepsis.114 Healthy individuals have an intestinal blood barrier that prevents LPS from entering the bloodstream, while ecological disturbances compromising this barrier and permitting bacterial translocation into the bloodstream.115 In addition, for those with periodontal disease, bacteria can directly infiltrate the systemic circulation, resulting in elevated levels of circulating LPS.116
LPS contributes to foam cell formation and LDL cholesterol accumulation, suggesting its proatherogenic properties.117 LPS upregulates CD14 and SR-AI expression in macrophages through the activation of JNK 1, which in turn increases oxidized LDL (oxLDL) uptake and foam cell production.118 Lipopolysaccharide-binding protein (LBP) participates in immune and inflammatory responses by binding to lipopolysaccharide (LPS) and facilitating the interaction of LPS with other pattern-recognition molecules in the plasma or cell membrane.119 Studies have shown that serum LBP levels are markedly elevated in individuals with coronary artery disease (CAD) compared to those without the condition, and LBP levels have emerged as an independent biomarker for predicting overall and cardiovascular mortality.120 Moreover, Toll-like receptor 4 (TLR4), a membrane-bound receptor for lipopolysaccharide, initiates NF-κB or JNK/SAPK signaling pathways and up-regulates chemokines and adhesion molecules upon activation.121 The inflammatory caspases, specifically caspase-4, -5, and -11, directly bind to bacterial lipopolysaccharide and activate pyroptosis, a process that is critical for innate defences.122 Mills et al. demonstrated that itaconate is a key anti-inflammatory metabolite that protects against LPS-induced mortality in vivo and reduces cytokine production. It activates Nrf2 signaling to suppress inflammation and regulate Type I interferons.123
Succinate
Succinate, a C4 dicarboxylic acid, is produced both in human cells and by the intestinal flora. It is mainly assisted by the sodium-dependent SLC13 transporter protein for transmembrane transport and significantly contributes to extracellular signaling by binding to the G protein-coupled succinate receptor (SUCNR1).124 Besides its production within human cells through the tricarboxylic acid (TCA) cycle, succinic acid is also recognized as a bacterial metabolite present in the intestinal lumen and feces. Research has demonstrated that germ-free mice lack detectable fecal succinate, highlighting the gut microbiota’s essential role in its production.125 Succinate is commonly synthesized via a branch of the TCA cycle that operates partially during the fermentation of microbial carbohydrates. Furthermore, succinate can also be generated via the glyoxylate shunt pathway and the 3-hydroxypropionate pathway.126
A variety of specific gut microbiota are involved in both the synthesis and utilization of succinate, with Bacteroidaceae, Parabacteroides, Veillonella, Ruminococcaceae and Prevotellaceae being the main succinate producers.126,127,128 Furthermore, specific Ruminococcus species, such as Ruminococcus albus and Ruminococcus flavefaciens, play a role in the synthesis of succinate. On the other hand, strains that utilize succinate encompass Clostridium, Acidaminococcaceae, Odoribacterium, and Phascolarctobacterium.86,129 Recent studies indicate the multifaceted roles of succinate in various physiological and pathological processes, including vascular endothelial damage, vascular smooth muscle cell proliferation and invasion, macrophage polarization, aortic aneurysm and dissection, ischemia-reperfusion injury, and cardiomyocyte hypertrophy.130 Specifically, succinate exacerbates endothelial dysfunction by elevating ROS levels, thereby undermining the vasodilatory effects of nitric oxide, triggering the renin-angiotensin system (RAS), and promoting thrombosis. Accumulated succinate can drive the growth and invasion of VSMC by activating RAS, accumulating HIF-1α, and stimulating the NF-κB pathway. In addition, it can also trigger the generation of ROS and secrete pro-inflammatory cytokines in macrophages, thereby fostering the development of aortic aneurysms and dissections (AAD) and atherosclerosis, respectively. Finally, the engagement of succinate with SUCNR1 can interfere with the negative feedback mechanism of angiotensin II, thereby fostering hypertension and advancing cardiac hypertrophy through PKA pathway activation, Ca2+ transients, and cardiomyocyte apoptosis (Figure 2).131
Figure 2.
Gut microbial dysbiosis contributes to IHD pathogenesis via key microbially derived metabolites
Gut microbiota-derived metabolites such as TMAO and succinic acid promote IHD and atherosclerosis. The pro-inflammatory microbial component LPS activates TLR4 and increases systemic inflammation and cardiac ischemia risk. On the other hand, dysbiosis diminishes SCFAs and compromises intestinal barrier integrity. In addition, microbially derived PAGln binds to adrenergic receptors and promotes platelet aggregation, thereby exacerbating CVDs. LPS, lipopolysaccharide; TMAO, trimethylamine N-oxide; SCFAs, short-chain fatty acids; TLR4, toll-like receptor 4; SUCNR1, succinate receptor 1; ROS, reactive oxygen species; TMA, trimethylamine; PAGln, phenylacetylgutamine.
Gut-heart axis
Alterations in gut microbial communities and the metabolites derived from these microorganisms may contribute to the initiation and worsening of CVDs, sparking extensive research into the underlying processes. This section and the subsequent section systematically illustrate the interconnections among specific bacterial species, metabolites, metabolic pathways, and associated cardiovascular diseases, as summarized in Table 2. Herein, we focus on elucidating the impact of gut microbiota and their derived metabolites on heart disease and the underlying molecular mechanisms involved.
Table 2.
Microbial metabolite production by gut bacteria and associated signaling pathways in CVDs
| Gut bacterial species | Microbial metabolites | Potential receptors and mechanistic pathways | Impact | Associated diseases | References |
|---|---|---|---|---|---|
| Increased Veillonella, Klebsiella, and Haemophilus | LPS↑ | TLR4 activation → pro-inflammatory cytokine release | Promotes plaque formation and inflammation | Ischemic heart disease (IHD) | Liu et al.132 |
| Decreased Roseburia and Eubacterium | SCFAs↓ | Reduced butyrate production → impaired immunomodulation | Attenuated protective effect | Ischemic heart disease (IHD) | Jie et al.18 |
| Increased Firmicutes/Proteobacteria | TMAO↑ | Dietary choline/carnitine → microbial CutC/D enzymes → TMA → hepatic FMO3 oxidation → TMAO | Promotes endothelial dysfunction and thrombosis | Ischemic heart disease (IHD) | Tang et al.79 |
| – | PAGln ↑ | Phenylalanine → microbial fermentation → phenylacetylglutamine | Activates adrenergic receptors → platelet hyperreactivity | Ischemic heart disease (IHD) | Nemet et al.69 |
| Increased Eubacterium rectale | TMAO ↑ | TMAO inhibits mitochondrial FAO → impaired energy metabolism | Exacerbates ventricular remodeling and cardiac dysfunction | Heart Failure (HF) | Tang et al.133; Organ et al.134 |
| Decreased Faecalibacterium | SCFAs (propionate/butyrate) ↓ | SCFA-GPCR signaling → blood pressure regulation and cardiac repair | Diminished cardioprotective effects | Heart Failure (HF) | Hu et al.135 |
| Increased Enterobacteriaceae | LPS ↑ | Impaired gut barrier → systemic LPS translocation → inflammation | Promotes myocardial inflammation and injury | Heart Failure (HF) | Sandek et al.42 |
| Increased Prevotella, and Klebsiella | TMAO ↑ | TMAO activates PERK/ROS/CaMKII pathway in VSMCs → enhanced vasoconstriction | Aggravates Ang II-induced hypertension | Hypertension | Jiang et al.136 |
| Decreased Roseburia, and Faecalibacterium | SCFAs (acetate) ↓ | Acetate → OLFR78 receptor activation → suppresses renin secretion | Impaired blood pressure regulation | Hypertension | Pluznick et al.98 |
| Increased Collinsella | TMAO ↑ | TMAO upregulates macrophage CD36/SR-A1 → foam cell formation | Accelerates plaque progression | Atherosclerosis | Wang et al.23 |
| Decreased Roseburia,and Eubacterium | SCFAs (propionate) ↓ | Propionate → Treg/IL-10 induction → suppresses NPC1L1 → reduced cholesterol absorption | Attenuated anti-atherogenic effect | Atherosclerosis | Haghikia et al.92 |
| Decreased Bacteroides, and Prevotella | Secondary bile acids ↑ | Microbial 7α-dehydroxylation → FXR receptor activation | Dysregulated lipid metabolism | Atherosclerosis | Jonsson et al.137 |
| – | TMAO ↑ | TMAO upregulates ER stress pathways | Promotes VSMC apoptosis | Thoracic Aortic Dissection (TAD) | Huang et al.138 |
| – | Indole-3-aldehyde (3-IAID) | Inhibits synthetic phenotype transition of VSMCs | Attenuates dissection progression | Thoracic Aortic Dissection (TAD) | Huang et al.139 |
| Decreased Roseburia intestinalis | SCFAs (butyrate) ↓ | Butyrate inhibits neutrophil NETosis → reduces inflammation | Diminished vascular protection | Abdominal Aortic Aneurysm (AAA) | Tian et al.140 |
| – | TMAO ↑ | Activates ER stress and apoptotic pathways in vascular wall | Promotes AAA formation | Abdominal Aortic Aneurysm (AAA) | Benson et al.141 |
| Decreased Lachnospiraceae | SCFAs (butyrate/valerate) ↓ | SCFAs modulate lymphocyte homeostasis → suppress ROS | Reduced cardioprotection | Doxorubicin Cardiotoxicity | Li et al.142 |
| Enterococcus faecalis | TMAVA ↑ | Inhibits BBOX → reduced carnitine synthesis → impaired mitochondrial FAO | Promotes hypertrophy | Cardiac Hypertrophy | Zhao et al.143 |
| – | Succinate ↑ | Activates cardiomyocyte GPR91 → MAPK/ERK pathway → pro-hypertrophic gene expression | Induces cardiomyocyte hypertrophy | Cardiac Hypertrophy | Aguiar et al.144 |
Role of gut microbiota and derived metabolites in ischemic heart disease
Ischemic heart disease (IHD) has become a major public health concern, imposing a considerable burden with its high morbidity and mortality.145,146,147 IHD is commonly also known as coronary heart disease (CHD) or coronary artery disease, referring to a cardiac condition marked by the narrowing or obstruction of the coronary arteries and affecting approximately one-third of the global population.
Dysbiosis of the gut microbiota is directly related to the pathogenesis of IHD.148 Patients with IHD often have intestinal microecological dysbiosis. At the phylum level, the diversity and compositions of the gut microbiota exhibited differences between individuals with CHD and those in the healthy control group, which were mainly reflected in the lower proportion of phylum Bacteroidetes and the higher proportion of Firmicutes in patients with CHD.149 Based on multi-omics analysis, including 16S rRNA sequencing analysis and metabolomics, Liu et al. found that both the gut microbiota composition and metabolite profiles underwent substantial alterations corresponding to the severity of CAD.132 Notably, analyses of disease subgroups suggested that the CAG17 abundance grew with worsening CAD severity. The CAG encompassed various gram-negative bacteria, including Veillonella, Haemophilus, and Klebsiella. Previous studies have shown that these bacteria can initiate the innate immune response through the production of LPS, leading to a subsequent inflammatory response mediated by the localized production of cytokines.150 In addition, a comprehensive clinical and multi-omic analysis of 199 patients with acute coronary syndromes also showed that alterations in gut microorganisms and their associated metabolites are present before the onset of clinical symptoms of IHD, and the abundance of butyrate-producing bacteria is reduced in patients with IHD, which may be an important reason for the reduced SCFAs in patients with IHD.151
Multiple intestinal bacterial metabolites are relevant in the pathophysiological progression of IHD, such as TMAO, SCFAs, BAs, LPS and PAGln (Figure 2). In 2013, Hazen et al.79 first reported that TMAO could be used as an independent risk factor for predicting IHD, and numerous studies have since confirmed this idea.133,152 Intestinal microorganisms are the main factor influencing the synthesis of TMAO, and several studies have reported that TMA metabolism-related species are mostly from the phylum Firmicutes and Proteobacteria.153,154 Jie et al. found that patients with IHD had reduced abundance of intestinal Roseburia and Eubacterium, both of which are clearly butyrate-producing organisms, suggesting that patients with IHD have a reduced potential for butyrate production.18 Another study also suggests that SCFAs and their production of associated gut bacteria are considered protective factors for IHD.12,13 On the other hand, gut microbes are important players in regulating the bile acid (BAs) cycle.25 Mayerhofer et al.111 found the rate of BAs is positively associated with the levels of circulating cholesterol in individuals with HF and IHD, nevertheless, there are few in-depth studies of the relevant strains of the gut microbiota responsible for BAs metabolism.
PAGln is generated when the intestinal microbiota ferments dietary phenylalanine, followed by conjugation with glutamine, and has been reported to be an independent predictor of IHD and other major adverse cardiovascular events.155 In patients with IHD, plasma PAGln levels correlate with coronary plaque burden. Elevated PAGln is associated with increased risk of obstructive disease and greater lesion complexity.156 Separately, mechanistic studies demonstrate that this gut microbiota-derived metabolite enhances cardiovascular risk by promoting platelet aggregation and thrombosis through G protein-coupled receptors (α2A, α2B, and β2-adrenergic receptors)).69
Role of gut microbiota and derived metabolites in heart failure
Heart failure (HF) is the end stage of all types of CVDs and the leading cause of cardiovascular death.157,158 An increasing number of studies have shown that intestinal flora and their metabolites play a role in the development of heart failure.14 Patients with HF have a different composition and reduced diversity of intestinal flora. For example, certain bacteria, such as Eubacterium rectale and Fusobacterium prausnitzii have been observed to be more prevalent among patients with HF, whereas the abundance of some beneficial bacteria, including Coriobacteriaceae, Erysipelotrichaceae, and Ruminococcaceae, was found to be reduced.40 These alterations can likewise affect systemic health and heighten vulnerability to Clostridium difficile infection.159 Similarly, studies have reported that patients with chronic heart failure (HF) exhibit a reduction in intestinal butyrate-producing bacterial communities, a substance that combats inflammation in the intestinal mucosa by boosting the production of regulatory T cells.160,161 Furthermore, there was a significant upregulation of microbial genes associated with the biosynthesis of bacterial LPS and the production of TMAO.11
Concomitant with alterations in gut microbiota composition, microbial metabolites exhibit significant changes in patients with HF. (Figure 3). TMAO levels were found to be associated with the development and poor prognosis in patients with HF.11,62 A study involving 720 patients followed over a 5-year period revealed that intestinal function was commonly disrupted in individuals with HF. Additionally, plasma TMAO levels were elevated in patients with HF compared to the control group, and a notable correlation was observed between TMAO concentrations, B-type natriuretic peptide levels, and an elevated mortality risk.162 Studies in animal models have found that mice fed diets supplemented with TMAO or choline develop pulmonary edema and cardiac hypertrophy, with significantly worse left ventricular ejection fractions and pronounced myocardial fibrosis, suggesting that the pathway of TMAO may directly play a role in the adverse ventricular remodeling and heart failure development.134 In addition to TMAO, LPS produced by intestinal flora has also been associated with the development of HF. Patients with chronic decompensated heart failure tend to have altered small intestinal function, with reduced active carrier-mediated intestinal transit and increased concentrations of LPS mediating the development of an intestinal inflammatory response, suggesting that intestinal wall oedema and intestinal epithelial dysfunction in patients with chronic decompensated heart failure correlate with the concentration of ectopic LPS.42,163
Figure 3.
Metabolite dysregulation significantly contributes to HF pathogenesis
TMAO exacerbates HF through multiple mechanisms, and reduced circulating SCFAs are associated with HF progression. Additional metabolites such as TMAVA and PAGln demonstrate adverse effects in HF. HF, heart failure; TMAO, trimethylamine N-oxide; SCFAs, short-chain fatty acids; TMAVA, N,N,N-trimethyl-5-aminovaleric acid; PAGln, phenylacetylgutamine.
Patients with heart failure (HF) exhibit reduced abundance of short-chain fatty acid (SCFA)-producing gut bacteria and consequently lower circulating SCFA levels. It was shown that the bacteria producing SCFAs were reduced in hypertensive heart failure rats, and the same was observed in patients with chronic heart failure (CHF).51 Specifically, patients with heart failure (HF) exhibited reduced levels of plasma propionate, butyrate, and isovalerate, while no significant difference was found in acetate and valerate levels.164 Notably, elevated concentrations of SCFAs, particularly propionate and butyrate, correlated with improved cardiac function in individuals with congestive heart failure.135 Other metabolites such as TMAVA and PAGln are also associated with HF. Mechanistically, SCFAs regulate blood pressure via renin secretion controlled by renal olfactory receptor 78 (Olfr78) within the juxtaglomerular apparatus,98 and promote post-myocardial infarction cardiac repair by enhancing CX3CR1+ monocyte infiltration in peri-infarct zones.165 These actions suggest SCFAs may mitigate inflammation-driven HF progression. Collectively, these results indicate that the gut microbiota significantly contributes to the pathophysiology of HF and holds promise as a potential therapeutic target.
Role of gut microbiota and derived metabolites in cardiotoxicity
Doxorubicin (DOX) is a potent anthracycline-based chemotherapeutic agent commonly used as first-line treatment for a range of malignancies. Nonetheless, its cardiotoxic side effects, such as progressive cardiac dilation, systolic dysfunction and eventual heart failure, constrain its clinical usage.166,167 Currently, the main protective interventions for preventing and treating chemotherapy-induced cardiotoxicity have not yet demonstrated satisfactory efficacy in clinical care.168
Increasing evidence suggests that changes in the composition and functional capacity of the gut microbiota are closely linked to the advancement of doxorubicin (DOX)-induced cardiotoxicity (DIC), with this progression being mediated through the gut-microbiota-cardiac axis.169 On the one hand, doxorubicin can induce significant alterations in the gut microbiota.170 Multiple research studies have shown that mice subjected to DOX treatment demonstrated gut dysbiosis compared with the control group.171,172 For example, Huang and his colleagues observed that mice injected with DOX exhibited a significant reduction in the abundances of Allobaculum, Muribaculum, and Lachnoclostridium, whereas there was a notable increase in the relative abundances of Faecalibaculum, Dubosiella, and Lachnospiraceae compared to the control group.173 Despite the significant role that lactobacilli and bifidobacteria play in maintaining the homeostasis of the host’s intestinal flora, which may be particularly suppressed by DOX.170 Notably, the fecal microbiota transplantation (FMT) from DOX-treated mice to germ-free (GF) mice induced cardiotoxicity, highlighting the critical role of gut microbiota dysbiosis in the development of DIC.171
On the other hand, studies have shown that metabolites generated by the gut microbiota, specifically SCFAs, are not only linked to energy metabolism but also contribute to the progression of DIC.174,175,176 Russo et al. demonstrated that the external administration of phenylalanine-butyramide (FAB) offered protection against DIC by averting mitochondrial dysfunction.176 Recent studies have developed a novel orally available and gut-targeted protein nanomedicine using the multifunctional outer membrane proteins of Akkermansia muciniphila, combining the fluorinated polyetherimide (FPEI) and hyaluronic acid (HA). By regulating intestinal microbiota diversity, increasing the proportion of short-chain fatty acid-producing bacteria Lachnospiraceae, and increasing the levels of butyric acid and valeric acid, this protein nanomedicine can further modulate splenic and cardiac lymphocyte homeostasis, attenuate inflammation and cardiac tissue ROS levels, and ultimately alleviate Dox-induced cardiotoxicity.142
Role of gut microbiota and derived metabolites in cardiac hypertrophy
Cardiac hypertrophy represents an early manifestation of heart failure progression. It not only increases risk for subsequent cardiac events and mortality but also contributes to adverse cardiovascular outcomes including heart failure, arrhythmias, and death.177 Advancing our understanding of the pathobiological mechanisms underlying cardiac hypertrophy and developing preventive strategies are critical for mitigating heart failure risk.178
The gut microbiota can impact cardiac function via its metabolites. Zhao et al. represent non-targeted metabolomics to identify the crucial role of TMAVA, a compound metabolized by gut microbiota such as Enterococcus faecalis, in cardiac hypertrophy.143 In summary, elevated plasma concentrations of TMAVA were linked to an increased risk of cardiac death and transplantation in patients. Moreover, TMAVA supplementation exacerbated cardiac hypertrophy and cardiac dysfunction in high-fat-fed and aortically constricted mice. Mechanistic investigations further revealed that TMAVA contributes to cardiac hypertrophy by inhibiting γ-butyl betaine hydroxylase (BBOX), thereby resulting in a lack of carnitine synthesis and uptake, which inhibited mitochondrial fatty acid oxidation and led to impaired cardiac energy metabolism and decreased cardiac function. Notably, TMAVA-induced cardiac hypertrophy in mice could be reversed by carnitine supplementation, suggesting that dietary control or intestinal flora intervention therapies to reduce TMAVA may be a viable strategy in future heart failure treatment studies.
In addition, succinate has the capacity to trigger cardiomyocyte hypertrophy through the direct activation of GPR91, a receptor extensively present throughout the body, within cardiomyocytes.179 The interaction of succinate with GPR91 in cardiomyocytes triggers intracellular signaling pathways that promote cardiac hypertrophy, one involving the activation of MAP/ERK kinase, leading to the phosphorylation of ERK1/2, which ultimately initiates the transcription of genes linked to cardiac hypertrophy.179 Alternatively, activating phospholipase C constitutes another pathway. Succinate binding to GPR91 increases the phosphorylation levels of Ca2+-treated proteins via a PKA-dependent mechanism, thereby enhancing the amplitude and rate of decay of the global Ca2+ transient and influencing hypertrophy.144 Beyond its role in activating GPR91 within cardiomyocytes, succinate further promotes cardiac hypertrophy by activating GPR91 in the kidney. This renal activation of GPR91 subsequently triggers the Renin-Angiotensin System (RAS), leading to an elevation in mean arterial blood pressure.180
Gut-vascular axis
Role of gut microbiota and derived metabolites in atherosclerosis
Atherosclerosis, a sophisticated and multi-factorial vascular ailment, not only contributes to the worldwide epidemic of CVDs but also stands as the predominant global cause of mortality.181,182
Mounting research indicates that the gut microbiota is a significant factor in the onset and advancement of atherosclerosis pathogenesis. The seminal study establishing the role of bacteria in the inflammatory response to atherosclerosis was based on the observation that bacterial DNA was detectable in human atherosclerotic plaques and that one of the most common bacteria was Chlamydia pneumoniae.183 Further investigations utilizing high-throughput sequencing and omics methodologies have uncovered a rise in inflammatory gut microbiota, such as Aminococcus, among those with carotid atherosclerosis, concurrently noting that levels of advantageous bacteria, including Anaerobic Bacillus spp., and butyrate-producing microorganisms are diminished.137
In addition to the gut microbiota itself, the metabolite TMAO derived from the microbiota contributes to atherosclerosis pathogenesis. Clinical studies consistently associate elevated plasma TMAO with increased CVD risk. Preclinical evidence demonstrates dietary choline or TMAO supplementation exacerbates atherosclerotic plaque formation and upregulates macrophage scavenger receptors (CD36, SR-A1).23 Human studies further correlate circulating TMAO with increased carotid intima-media thickness and atherosclerotic burden. Regardless of conventional cardiovascular risk, the intake of L-carnitine, a precursor to TMAO or the metabolization of L-carnitine by bacteria into an intermediate product of TMAO with γ-butyryl betaine, is adequate to foster atherosclerosis.70 In addition, the grape pomace polyphenol supplement Taurisolo reduces circulating TMAO and exerts anti-atherosclerotic effects in clinical trials.184 Collectively, these findings establish gut microbiota-derived TMAO as a significant contributor to atherosclerosis.185
On the contrary, microorganisms metabolize resistant starch and dietary fiber by fermentation and catabolism, and provide SCFAs to the host. One recent investigation demonstrated oral supplementation with propionic acid (PA), a type of SCFA, significantly reduced LDL, total and non-high-density lipoprotein cholesterol levels in hypercholesterolaemic humans. And in the HFD-fed Apoe−/− mice, PA not only diminished intestinal cholesterol absorption and the area of aortic atherosclerotic lesions but also enhanced the count of regulatory T cells and the concentration of interleukin IL-10 within the intestinal microenvironment, subsequently leading to the downregulation of Niemann-Pick C1-like 1 (Npc1l1) expression, a key intestinal cholesterol transporter. This study revealed for the first time the intestinal immunomodulatory impacts of PA, a metabolite derived from the microbiota, on the metabolism of intestinal cholesterol.92 In another study by Wang et al., the oral administration of a probiotic strain, Enterococcus faecalis NCIMB11508, to ApoE−/− mice altered the gut microbiota composition by elevating SCFA levels and reduced the levels of inflammatory cytokines in the aorta and serum, ultimately mitigating the progression of atherosclerosis.186 In general, metabolites of intestinal microbiota, such as TMAO and SCFAs, are also widely involved in the onset and progression of atherosclerosis, and the suppression of TMAO or an increase in SCFAs will effectively alleviate the development of atherosclerosis.
Role of gut microbiota and derived metabolites in hypertension
Hypertension ranks as one of the most widespread cardiovascular conditions globally. A variety of factors, including genetics, excessive salt intake, minimal physical activity, a sedentary mode of living, and environmental influences, can all play a role in elevating blood pressure levels.
Recently, there has been an increasing recognition of the intimate connection between the gut microbiota and hypertension. Cai et al. performed integrated metagenomic and metabolomic analyses of 41 healthy controls, 56 prehypertensive individuals, and 99 hypertensive patients, followed by fecal microbiota transplantation (FMT) into germ-free mice. The prehypertensive microbiome exhibited characteristics similar to hypertension, notably enriched Prevotella and Klebsiella. Additionally, both prehypertension and hypertension showed metabolomic alterations linked to gut dysbiosis. Critically, by transplanting feces from hypertensive human donors into germ-free mice, elevated blood pressure was observed to be transmutable via the microbiota, demonstrating direct microbiota influence on hypertension pathogenesis,15 showing that it is necessary to regulate the intestinal flora in the early stage of hypertension. On the other hand, hypertensive patients display elevated plasma TMAO. To investigate TMAO’s role in angiotensin II (Ang II)-induced vasoconstriction and hypertension, Zheng et al. infused Ang II (400 ng/kg/min) for 14 days in C57Bl/6 mice, administering 1% TMAO or antibiotics in drinking water. TMAO did increase systolic blood pressure and vasoconstriction, while antibiotics attenuated these effects. Mechanistically, TMAO activated the PERK/ROS/CaMKII/PLCβ3 pathway in vascular smooth muscle cells, enhancing Ca2+ release and potentiating Ang II-induced pressor responses.136
In addition, hypertension is associated with reduced abundance of SCFA-producing bacteria and concomitant intestinal alterations, including diminished gut hypoxia, dysbiosis, impaired epithelial barrier integrity, intestinal inflammation, and decreased plasma SCFA concentrations.187 These alterations further impair blood pressure regulation. Gut microbiota generates SCFAs through the fermentation of dietary fiber.188 These fiber-rich diets enhance the population of bacteria that produce acetic acid, thereby reducing deoxycorticosterone acetate (DOCA)-induced hypertension in mice, and administering acetic acid directly also exhibits a comparable influence on regulating blood pressure. Similarly, acetate levels elevated by prebiotics, probiotics, or acetate supplementation act to antihypertensive effects, suggesting that microbial acetate plays a pivotal role in blood pressure modulation.189 Moreover, the supplementation of propionate has been found to mitigate Ang II-induced hypertension in mouse models. Regarding butyrate, there is a negative correlation observed between plasma butyrate levels and portal hypertension, with the enhancement of butyrate-producing bacterial growth and the increase in butyrate production capacity contributing to improved blood pressure in pregnant females.190 Furthermore, some studies have shown that oral intake of butyrate can prevent the onset of high blood pressure.191 Collectively, these findings demonstrate the important role of microbial SCFAs in the regulation of blood pressure, although additional studies are needed to validate the clinical utility and underlying epigenetic mechanisms of SCFAs.
Role of gut microbiota and derived metabolites in thoracic aortic dissection
Thoracic aortic dissection (TAD) typically presents with an abrupt onset and a very high mortality rate.192 Currently, surgical intervention is the primary treatment modality. Pharmacological management of aortic dissection largely revolves around symptomatic relief, including the use of antihypertensive and vasodilatory agents.193,194 However, research on the gut-vascular axis has been limited. Du et al. collected plasma and fecal samples from patients with TAD and healthy individuals to assess TMAO levels and gut microbial composition, respectively. The study found that 253 aortic dissection (AD) patients had significantly elevated plasma TMAO levels compared to 98 healthy individuals, and that high plasma TMAO levels were positively correlated with AD severity.138 In another study, Ge and his team found that the gut microbiota-derived tryptophan metabolite, indole-3-aldehyde (3-IAID), exerts an effective therapeutic effect in a BAPN-induced mouse model of TAD by inhibiting the phenotypic transition of VSMCs from a contractile to a synthetic phenotype. Additionally, it also attenuates extracellular matrix degradation, diminishes macrophage infiltration, and inhibits the expression of inflammatory cytokines, collectively contributing to the mitigation of AD progression and providing valuable insights into the study of the gut-vascular axis in this field.139 In general, TAD is inextricably linked to the gut microbiota; however, the main research at present is focused on intestinal metabolites, such as TMAO and 3-IAID. The role of intestinal dysbiosis and intestinal barrier damage in aortic dissection needs to be further studied.
Role of gut microbiota and derived metabolites in abdominal aortic aneurysm
Abdominal aortic aneurysm (AAA) is a potential and fatal vascular disease that currently lacks effective non-surgical intervention. Similar to TAD, research on the mechanism of gut microbiota in AAA remains limited. Tang et al. examined TMAO and choline metabolites from plasma samples from 2 independent patient cohorts (total n = 2129) and found that elevated TMAO was associated with increased AAA incidence and growth. The research further demonstrated that TMAO produced by gut microbiota contributes to AAA development in mice by upregulating endoplasmic reticulum stress-related pathways within the aortic wall. Moreover, inhibiting TMAO was found to effectively alleviate the formation and progression of AAA.141 Another recent study showed that the composition of the gut microbiota in patients with AAA was significantly altered with a marked decrease in mainly R. intestinalis, and microbiota dysbiosis has also been confirmed to be an important factor in AAA. R. intestinalis and its metabolite butyrate profoundly decreased neutrophil infiltration, inhibited NADPH oxidase 2 (NOX2)-mediated neutrophil extracellular trap formation, reduced inflammation, and prevented the aberrant phenotypic conversion of VSMCs within the aortic wall, consequently leading to a significant attenuation in the progression of AAA.140 These results indicate that the intestinal microbiota is widely involved in the onset and progression of AAA, and that regulating the gut microbiota could offer an effective strategy for non-surgical AAA treatment.
Gut microbiota-targeted interventions/treatments
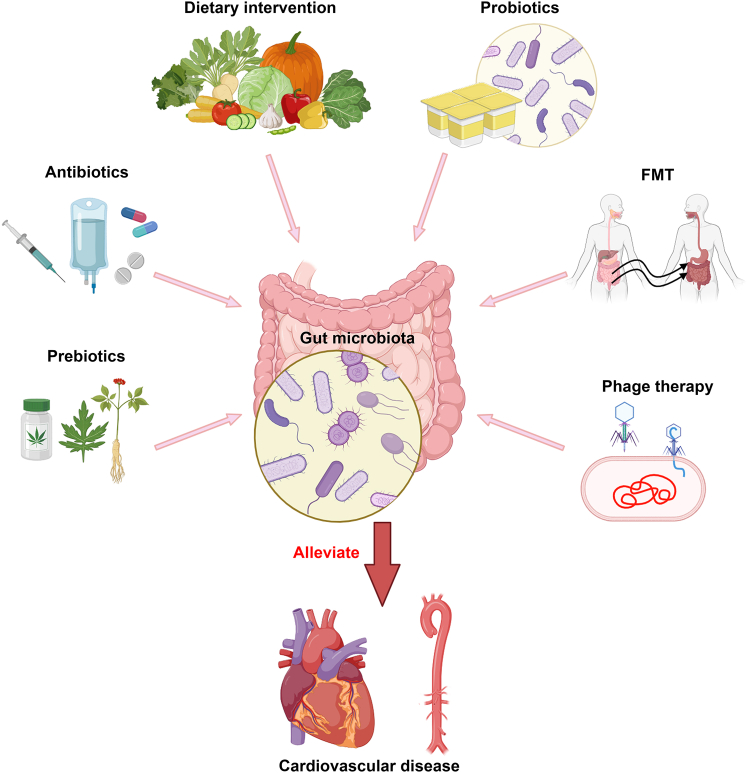
An expanding array of evidence indicates that the gut microbiota significantly contributes to the progression of CVDs. Consequently, the gut microbiota has emerged as an optimal target for the prophylaxis and management of diseases. This has led to the exploration of therapeutic strategies aimed at manipulating the composition of the gut microbiota and/or its metabolism, including dietary intervention, probiotics and prebiotics, fecal microbiota transplantation (FMT), live biotherapeutic products, and phage therapy. These strategies have restored lipid profiles, enhanced blood pressure control, and facilitated weight reduction in some patients with CVD. In this section, we will discuss the findings concerning the effects of these interventions on the gut microbiome in individuals with CVDs (Figure 4).
Figure 4.
Strategies for improving CVDs through the regulation of gut microbiota
The strategies discussed in the present review include the use of dietary interventions, probiotics and prebiotics, FMT, antibiotics, and phage therapy. FMT, fecal microbiota transplantation.
Dietary intervention
Dietary interventions have been extensively employed to mitigate chronic illnesses. For instance, heart-healthy diets abundant in vegetables and high in fiber are considered to have positive effects on the cardiovascular system.195 The formal recommendation adopted in the ACC/AHA guidelines is the Dietary Approaches to Stop Hypertension (DASH) diet, rich in vegetables, fruits, and unsaturated fats, and several observational studies have shown that the DASH diet reduces the incidence of CVDs.196,197,198,199 Over a median 22-year follow-up in a cohort of 35,004 participants, the DASH diet was linked to a lower risk of heart failure.199 In patients with HF, the DASH diet also improved 6-min walk test results, arterial adherence, exercise tolerance, and quality of life scores assessed 3 months after the intervention.200 Another systematic review and meta-analysis found that the DASH diet was associated with a reduced incidence of cardiovascular disease and improved blood pressure in individuals with and without diabetes.201 Emerging evidence demonstrated that the DASH diet combined with sodium restriction reduces myocardial damage and stress, and further reduces blood pressure.202 In addition, multiple randomized controlled trials have further demonstrated that the DASH diet improved risk factors for other metabolic diseases, including blood lipids, blood sugar control, and weight.
Similar to the DASH diet, the Mediterranean diet is similarly noted for other forms of grains, legumes, nuts, olive oil, seeds, legumes, and limited intake of red meat and small to moderate consumption of dairy products.203 Although most studies have shown that adhering to a Mediterranean dietary pattern reduces all-cause mortality in patients with CVD,204 Papadaki et al. reported that the Mediterranean diet failed to decrease the incidence of HF in the prespecified secondary analyses study.205 Possible reasons include the significant heterogeneity among patients with heart failure, stemming from distinct pathophysiological mechanisms underlying heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF). In HFrEF, overactivation of the neurohormonal system is the predominant pathophysiological driver. In contrast, HFpEF involves multiple overlapping mechanisms, including systemic inflammation, metabolic dysregulation, neuroendocrine activation, endothelial dysfunction, and autonomic dysregulation.206 Therefore, additional randomized controlled trials focusing on HF as the primary endpoint are required to more accurately evaluate the impact of health-conscious diets on the risk of HF.205,207
It is widely recognized that diet is one of the main factors shaping the composition of the gut microbiome. Different dietary patterns, such as a high-fiber plant-based diet or a high-fat animal-based diet, can lead to different types of microbes thriving or diminishing in the gut.208,209 For instance, dietary fiber may modify the host’s microbiota composition and promote the growth of microorganisms capable of degrading complex carbohydrates, such as Prevotella species, thereby improving cardiovascular health and function.210 High-fiber dietary interventions may also reduce the risk of obesity, type 2 diabetes, and cardiovascular disease by enhancing the production of beneficial metabolites like short-chain fatty acids, although efficacy depends on fiber type, dose, and individual microbiome characteristics.211 However, Western lifestyles with high salt consumption and low fiber content contribute to hypertension and CVDs. This diet diminishes microbial diversity, reduces the abundance of beneficial Lactobacillus murinus, Eubacterium, and Bifidobacterium, induces T helper 17 (TH17) cells activity, and leads to intestinal inflammation and reduced intestinal barrier integrity.212,213 In conclusion, dietary interventions are cost-effective and straightforward to implement in the prevention and treatment of CVDs. Nonetheless, given the variability in the composition and functions of gut microbes, individualized diets may be more appropriate than a universal diet.
Probiotics and prebiotics
Some evidence indicated that the utilization of specific probiotics or prebiotics might offer protective effects for the heart. Probiotics are described as beneficial bacteria that, when ingested in sufficient quantities, aim to establish a normal gut microbiological balance.214 Common probiotics commonly utilized in clinical practice include Lactobacillus and Bifidobacterium, which may be the most promising probiotic species.215 A meta-analysis suggested that consuming probiotics may improve blood pressure, reduce total and low-density lipoprotein (LDL) cholesterol levels, and may result in a decrease in risk factors associated with CVDs.216,217 In patients with chronic systolic heart failure, who received 3 months of Saccharomyces boulardii supplementation, improved left ventricular ejection fraction, reduced left atrial diameter, as well as cholesterol and uric acid levels were observed.218 In addition, other species of Lactobacillus within probiotics are recognized for their beneficial impacts on CVDs as well. Studies showed that Lactobacillus rhamnosus GR-1 yielded protective effects in a rat model of myocardial ischaemia reperfusion, significantly attenuated hypertrophy, and improved not only LV systolic function but also LV diastolic function.219 Lactobacillus rhamnosus GG (LGG) improved atherosclerosis in a GM-associated manner.220 Lam et al. also found that probiotic Lactobacillus plantarum 299v treatment led to a reduction in the area of myocardial infarction and an increase in cardiac function in rats with coronary artery ligation.221 Another animal model study has indicated that some probiotics such as Lactobacillus acidophilus ATCC 4356 can slow down atherosclerosis by inhibiting the absorption of intestinal cholesterol in ApoE−/− mice.222 Administration of probiotic mixtures, including L. coryniformis CECT5711 (K8) and L. gasseri CECT5714 (LC9), significantly attenuated cardiac and renal hypertrophy in spontaneously hypertensive rats, potentially owing to enhancements in the vascular pro-oxidant and pro-inflammatory balance.223 Nonetheless, the efficacy of relevant probiotics remains contradictory. Certain studies involving animal models and human clinical trials have also shown no benefit of probiotics on CVDs.224,225 This may be attributed to variations in the strains or dosages of probiotics administered, differences in experimental setups, and the diverse characteristics of the participants involved. An animal study showed that Lactobacillus reuteri ATCC significantly ameliorated high-fat diet-induced obesity but had no effect on the size or stability of atherosclerotic plaques. This may be because the bacteria treatment failed to reduce cholesterol or triglyceride levels or did not alter inflammation.224 Furthermore, clinical heterogeneity, including baseline health status and diet, may compromise result comparability. Genetic background and initial microbiome composition also strongly influence probiotic efficacy. Therefore, elucidating the complex interplay between host genetics and the gut microbiota is critical for advancing the development of targeted probiotic therapies. Since 2007, an increasing number of studies, particularly large-scale microbial genome-wide association studies (mbGWAS), have revealed the significant influence of host genetic factors on specific gut microbiota. For example, the LCT locus regulates Bifidobacterium,226 while blood type/FUT2 genes shape the abundance of Faecalibacterium prausnitzii.227 These findings lay the foundation for personalized probiotic treatment methods based on host genetics. Up to now, most probiotic studies remain predominantly focused on diseased populations, and further research is needed on the effects and impact of probiotic consumption on healthy individuals.228
Prebiotics are substances selectively utilized by host microorganisms and stimulate the activity of probiotics to promote health.229,230 While current prebiotics are primarily carbohydrate-based, other substances, including polyphenols and polyunsaturated fatty acids, may act as prebiotics.231 Polyphenols have been shown to greatly increase the production of Bifidobacteria, Lactobacilli, and butyrate in intestinal models.232 Most prebiotics induce an increase in SCFAs and improve metabolic health,233,234 for example, inulin mitigates atherosclerosis by promoting lipid metabolism, diminishing inflammation, and enhancing the integrity of the intestinal barrier.235 A randomized, double-blind controlled trial found that overweight and obese volunteers supplemented with 20g of inulin propionate per day had reduced pro-inflammatory interleukin-8 levels and altered intestinal bacterial flora at both the class level (increased Actinobacteria, decreased Clostridia) and the order level (decreased Clostridia) compared to volunteers receiving fiber supplements, whereas inulin had no effect on systemic inflammatory markers and changes at the intestinal bacterial species level were minimal.236 This discrepancy likely stems from inflammatory markers measured only in systemic circulation, while inulin’s effects could be localized to the gut mucosa. Moreover, in research involving elderly participants, a 10-week regimen of daily galacto-oligosaccharide (GOS) intake resulted in elevated immune function and higher counts of beneficial bacteria, particularly bifidobacteria, which significantly boosted phagocytic activity and the functioning of natural killer cells.237
Fecal microbiota transplantation
The process of transferring feces from a healthy donor into the gut of another recipient is called fecal microbiota transplantation (FMT).238 FMT represents the most basic intervention for gut flora and a well-established and broadly accepted treatment for recurrent Clostridium difficile infection.239,240 This strategy has recently proven successful in patients with cardiometabolic disorders as well.241 Although obese individuals with metabolic syndrome exhibit improved insulin sensitivity upon the initial infusion of intestinal microbes from lean donors,242 the results of the studies varied widely. Notably, FMT from a plant-based donor successfully shifted the gut microbiota composition of metabolic syndrome patients toward the donor profile, yet unexpectedly failed to modify their TMAO levels. This observed dissociation between microbial engraftment and changes in this key cardiometabolic biomarker underscores the necessity for sufficiently powered clinical trials with extended follow-up to achieve sustained efficacy. Furthermore, after treatment with FMT, extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli bacteremia occurred, and one patient died, which raised the safety concerns regarding the clinical application of FMT.243
Current applications of FMT in cardiovascular disease research rely predominantly on animal models. Li et al. employed both conventional FMT and gnotobiotic mice monocolonized with Prevotella copri to demonstrate that puerarin attenuated atherosclerosis. This protective effect occurred via the suppression of Prevotella copri abundance and consequent reduction in trimethylamine (TMA) production.244 In addition, aging is the main driver behind the rising incidence of heart failure. A recent study found that ferroptosis induced by the aged gut microbiota plays a critical role in heart failure pathogenesis. Transplanting fecal bacteria from elderly rats into young recipient rats leads to a decline in cardiac function, reduced exercise tolerance, exacerbated cardiac structural disorders, and increased expression of iron death markers.245 By transplanting the fecal microbiota from hypertensive patients with well-controlled (WC) blood pressure under ARB therapy into spontaneously hypertensive rats (SHRs) and concurrently administering ARB treatment, Zhong et al. found that the intestinal microbiota in the WC group potentiated valsartan’s antihypertensive efficacy. This finding indicates that the intestinal microbiota regulated by the RAAS inhibitor ARB can lower the systolic blood pressure levels of SHR and exert a protective effect on their hypertensive target organs.246 Nevertheless, there is currently insufficient data to support the correlation between fecal microbiota transplantation and the gut microbiota in human cardiovascular disease patients, so further research in this field is urgently needed. Significant safety concerns and practical obstacles further hinder the routine clinical application of FMT in CVDs. FMT carries inherent risks, including the potential transfer of pathogenic microorganisms or endotoxins, a risk that has been demonstrated in fatal cases involving the spread of drug-resistant Escherichia coli. Additionally, FMT indiscriminately alters the gut microbiota, potentially disrupting both beneficial commensal bacteria and harmful bacteria, leading to unforeseen side effects in recipients.247 Practical challenges include the difficulty of obtaining truly representative donor samples that reflect the complex biological characteristics of the gut microbiota, ensuring compatibility between donor and recipient microbiota, achieving stable survival and re-colonization of the transplanted microbiota, and addressing complex ethical and logistical issues such as donor screening, informed consent, and procedural safety monitoring. Therefore, although preliminary research results are encouraging, the role of FMT in cardiovascular disease management requires further evaluation and resolution of significant translational barriers before widespread clinical application can be considered.
Live biotherapeutic products
Distinct from FMT, live biotherapeutic products (LBPs) represent an emerging research field and are characterized by a class of biological therapeutic agents comprised of live microorganisms intended to prevent or treat disease by modulating the composition or function of the human microbiome.248 LBPs may comprise either a single microbial strain or a consortium of multiple microbial strains, and numerous studies have demonstrated that LBPs can modulate gut microbiota and mitigate a wide variety of diseases, including inflammatory,249,250 spanning infectious251 and metabolic diseases.21,252,253 Recently, several studies have also revealed the potential value of LBPs in CVD therapy. Liu et al. reported that the administration of live Parabacteroides merdae to male ApoE−/− mice fed a high-fat diet attenuated obesity-related atherosclerosis by enhancing branched-chain amino acid (BCAA) catabolism.254 In addition, prophylactic oral supplementation with Lactobacillus reuteri or its metabolite γ-aminobutyric acid (GABA) has been shown to exert a protective effect against acute myocardial ischemia-reperfusion injury. This protective effect is mediated by the reduction of inflammatory responses following cardiac injury, achieved through the inhibition of macrophage NLRP3 inflammasome activation.255 It is worth noting that LBPs may cause side effects such as gastrointestinal symptoms, risk of infection in immunocompromised individuals, bacterial translocation, and allergic or immune reactions. Given these potential risks, the development of LBPs should prioritize human and food-borne strains based on clinical need and evidence of efficacy.
Antibiotics
Improper antibiotic use can disrupt host microbiota and cause adverse effects. However, their administration is essential when indicated, for instance, to prevent bacterial translocation that could lead to life-threatening systemic infections. As far back as 2004, research conducted by Conraads and colleagues illustrated that broad-spectrum antibiotics decreased biomarkers of systemic inflammation in patients with HF, although it did not specifically target clinical symptoms.256 Similarly, a two-month dietary intervention in mice involving a broad-spectrum antibiotic cocktail was found to reverse the intestinal dysbiosis and vascular dysfunction induced by a Western diet, representing critical preclinical stages in the progression of CVDs.257 Nevertheless, in a recent study, Tang et al. discovered that mice treated with antibiotics experienced significant, dose-dependent mortality following myocardial infarction.165 Critically, antibiotics are a double-edged sword; antibiotic treatment may exacerbate the emergence of antibiotic-resistant bacteria, and their potential benefits and risks need to be carefully weighed.
Phage therapy
Bacteriophages (phages), viruses that infect bacteria, are critical determinants of bacterial community structure and function, and have been implicated in both gastrointestinal and systemic health, including cardiovascular-metabolic diseases.258,259,260 By infecting gut bacteria, phages can increase or decrease bacterial abundance, or alter the function of the bacterial host, and are therefore considered a potential therapeutic intervention for disease states where gut bacteria have a demonstrated role,261,262 primarily including fecal virome transplantation (FVT) and phage therapy. FVT involves transferring viral components from a healthy donor’s feces to a recipient’s gut to restore microbiome balance. Phage therapy, conversely, employs a targeted approach, isolating and transplanting phages specifically effective against the infecting bacterial strain.263,264 Phage therapy for cardiovascular disease is currently in the nascent stage of investigation, with main applications primarily focused on infective endocarditis and targeting prevalent antibiotic-resistant bacterial pathogens such as Escherichia coli, Enterococcus faecalis, Klebsiella pneumoniae, and Pseudomonas aeruginosa.265 Despite the safety, efficacy, and tolerability of phage therapy, its application still faces many challenges, including the possibility that in vivo phages may lose lysogenic activity during delivery, and current research remains limited to animal studies with insufficient clinical validation. Overall, intestinal phages modulate gut microbiota dynamics, influencing both gastrointestinal health and systemic conditions like cardiovascular disease, and phage therapy is a promising treatment for bacterial infections.
Conclusions and future perspectives
Over the past few decades, an accumulating body of evidence has highlighted a significant connection between the gut microbiome and CVDs. It is apparent that gut microbes interact with their host through various mechanisms. Abnormalities in the composition of gut microbiota, impaired gut barrier integrity, or changes in microbial metabolites are associated with an increased risk and pathological changes linked to CVDs. Studies have shown that metabolites of gut microbes, such as TMAO, are independent risk factors for CVDs, given their role in promoting inflammatory responses and increasing the atherosclerosis risk. Additionally, impaired gut barrier function and imbalanced microbial populations also lead to the translocation of bacteria and endotoxins, triggering a chronic inflammatory response and worsening the progression of CVDs.
Modulating the gut microbiota may provide new avenues for CVD prevention and treatment. Strategies such as dietary adjustments, the administration of probiotics and prebiotics, and even fecal microbiota transplantation could potentially improve cardiovascular health. In addition, new LBPs and phage therapy present innovative treatment possibilities for CVDs. With ongoing technological advancements and a more profound understanding of gut microbial mechanisms, modifying the gut microbiome holds considerable potential in combating CVDs.
Limitations of the study
Despite the understanding of the gut microbiota’s role in CVDs being progressively refined with ongoing research, and the gut microbiota is increasingly recognized as a potential therapeutic target for CVDs, further studies are crucial to fully grasp the underlying mechanisms connecting the gut microbiota to CVDs, evaluate the efficacy of these interventions, and ultimately improve the health of patients with CVD.
Acknowledgments
Youth Project of the National Natural Science Foundation of China (82400566); and the Youth Project of the Natural Science Foundation of Hubei Province (2023AFB127).
Author contributions
Jiaqi Yu, Chongming Wu, and Yuyu Li organized, conceived, and supervised the study. Jiaqi Yu and Yuyu Li performed literature searching and drafted the article. Ya-nan Yang, Weiyao Chen, Jiaxin Hu, and Zhechuan Jin revised the article. All authors read and approved the final article.
Declaration of interests
The authors have no conflicts of interest.
Contributor Information
Chongming Wu, Email: chomingwu@163.com.
Yuyu Li, Email: liyuyu0716@163.com.
References
- 1.Vos T., Abajobir A.A., Abate K.H., Abbafati C., Abbas K.M., Abd-Allah F., Abdulkader R.S., Abdulle A.M., Abebo T.A., Abera S.F. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/s0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattingly Q (2021). Cardiovascular Diseases. https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1.
- 3.Moschetti I., Brandt D., Perera R., Clarke M., Heneghan C. Adequacy of reporting monitoring regimens of risk factors for cardiovascular disease in clinical guidelines: systematic review. BMJ. 2011;342 doi: 10.1136/bmj.d1289. [DOI] [PubMed] [Google Scholar]
- 4.Xu W., Yu J., Yang Y., Li Z., Zhang Y., Zhang F., Wang Q., Xie Y., Zhao B., Wu C. Strain-level screening of human gut microbes identifies Blautia producta as a new anti-hyperlipidemic probiotic. Gut Microbes. 2023;15 doi: 10.1080/19490976.2023.2228045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Y., Rong L., Cui J., Cheng W., Fu L., Fan G., Wang H., Yu J., Li Z., Liu D., et al. Artificial lipids and macrophage membranes coassembled biomimetic nanovesicles for thoracic aortic dissection treatment. J. Control. Release. 2025;383 doi: 10.1016/j.jconrel.2025.113844. [DOI] [PubMed] [Google Scholar]
- 6.Zmora N., Suez J., Elinav E. You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019;16:35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 7.Sekirov I., Russell S.L., Antunes L.C.M., Finlay B.B. Gut microbiota in health and disease. Physiol. Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 8.Tang W.H.W., Kitai T., Hazen S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017;120:1183–1196. doi: 10.1161/circresaha.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J., Yang Y. Gut microbiome and its meta-omics perspectives: profound implications for cardiovascular diseases. Gut Microbes. 2021;13 doi: 10.1080/19490976.2021.1936379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang W.H.W., Li D.Y., Hazen S.L. Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol. 2019;16:137–154. doi: 10.1038/s41569-018-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang T., Santisteban M.M., Rodriguez V., Li E., Ahmari N., Carvajal J.M., Zadeh M., Gong M., Qi Y., Zubcevic J., et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/hypertensionaha.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlsson F.H., Fåk F., Nookaew I., Tremaroli V., Fagerberg B., Petranovic D., Bäckhed F., Nielsen J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012;3:1245. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marques F.Z., Nelson E., Chu P.Y., Horlock D., Fiedler A., Ziemann M., Tan J.K., Kuruppu S., Rajapakse N.W., El-Osta A., et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation. 2017;135:964–977. doi: 10.1161/circulationaha.116.024545. [DOI] [PubMed] [Google Scholar]
- 15.Li J., Zhao F., Wang Y., Chen J., Tao J., Tian G., Wu S., Liu W., Cui Q., Geng B., et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S., Rigatto K., Gazzana M.B., Knorst M.M., Richards E.M., Pepine C.J., Raizada M.K. Altered Gut Microbiome Profile in Patients With Pulmonary Arterial Hypertension. Hypertension. 2020;75:1063–1071. doi: 10.1161/hypertensionaha.119.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Wang Y., Xia Z., Xie Y., Ke D., Song B., Mu D., Yu R., Xie J. Noninvasive platelet membrane-coated Fe(3)O(4) nanoparticles identify vulnerable atherosclerotic plaques. Smart Med. 2024;3 doi: 10.1002/smmd.20240006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jie Z., Xia H., Zhong S.L., Feng Q., Li S., Liang S., Zhong H., Liu Z., Gao Y., Zhao H., et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017;8:845. doi: 10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang W.H.W., Hazen S.L. The Gut Microbiome and Its Role in Cardiovascular Diseases. Circulation. 2017;135:1008–1010. doi: 10.1161/circulationaha.116.024251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng X., Cai X., Hao H. Emerging targetome and signalome landscape of gut microbial metabolites. Cell Metab. 2022;34:35–58. doi: 10.1016/j.cmet.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Agus A., Clément K., Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70:1174–1182. doi: 10.1136/gutjnl-2020-323071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan Y., Pedersen O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021;19:55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.M., et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 25.Wahlström A., Sayin S.I., Marschall H.U., Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Marques F.Z., Mackay C.R., Kaye D.M. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat. Rev. Cardiol. 2018;15:20–32. doi: 10.1038/nrcardio.2017.120. [DOI] [PubMed] [Google Scholar]
- 27.Agus A., Planchais J., Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Xu J., Yang Y., Li X., Ding S., Zheng L., Xiong C., Yang Y. Pleiotropic activities of succinate: The interplay between gut microbiota and cardiovascular diseases. iMeta. 2023;2 doi: 10.1002/imt2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M., Gill S.R., Nelson K.E., Relman D.A. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lloyd-Price J., Abu-Ali G., Huttenhower C. The healthy human microbiome. Genome Med. 2016;8:51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi H., Liu Y., Qi X., Liang H., Chen H., Jiang P., Wang D. Dietary Recombinant Phycoerythrin Modulates the Gut Microbiota of H22 Tumor-Bearing Mice. Mar. Drugs. 2019;17 doi: 10.3390/md17120665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown J.M., Hazen S.L. Microbial modulation of cardiovascular disease. Nat. Rev. Microbiol. 2018;16:171–181. doi: 10.1038/nrmicro.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franzosa E.A., Hsu T., Sirota-Madi A., Shafquat A., Abu-Ali G., Morgan X.C., Huttenhower C. Sequencing and beyond: integrating molecular 'omics' for microbial community profiling. Nat. Rev. Microbiol. 2015;13:360–372. doi: 10.1038/nrmicro3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S., Zhao W., Liu X., Cheng L. Metagenomic analysis of the gut microbiome in atherosclerosis patients identify cross-cohort microbial signatures and potential therapeutic target. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020;34:14166–14181. doi: 10.1096/fj.202000622R. [DOI] [PubMed] [Google Scholar]
- 35.Yan Q., Gu Y., Li X., Yang W., Jia L., Chen C., Han X., Huang Y., Zhao L., Li P., et al. Alterations of the Gut Microbiome in Hypertension. Front. Cell. Infect. Microbiol. 2017;7:381. doi: 10.3389/fcimb.2017.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui X., Ye L., Li J., Jin L., Wang W., Li S., Bao M., Wu S., Li L., Geng B., et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci. Rep. 2018;8:635. doi: 10.1038/s41598-017-18756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Q., Gao R., Zhang Y., Pan D., Zhu Y., Zhang X., Yang R., Jiang R., Xu Y., Qin H. Dysbiosis signatures of gut microbiota in coronary artery disease. Physiol. Genomics. 2018;50:893–903. doi: 10.1152/physiolgenomics.00070.2018. [DOI] [PubMed] [Google Scholar]
- 38.Kelly T.N., Bazzano L.A., Ajami N.J., He H., Zhao J., Petrosino J.F., Correa A., He J. Gut Microbiome Associates With Lifetime Cardiovascular Disease Risk Profile Among Bogalusa Heart Study Participants. Circ. Res. 2016;119:956–964. doi: 10.1161/circresaha.116.309219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gózd-Barszczewska A., Kozioł-Montewka M., Barszczewski P., Młodzińska A., Humińska K. Gut microbiome as a biomarker of cardiometabolic disorders. Ann. Agric. Environ. Med. AAEM. 2017;24:416–422. doi: 10.26444/aaem/75456. [DOI] [PubMed] [Google Scholar]
- 40.Luedde M., Winkler T., Heinsen F.A., Rühlemann M.C., Spehlmann M.E., Bajrovic A., Lieb W., Franke A., Ott S.J., Frey N. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail. 2017;4:282–290. doi: 10.1002/ehf2.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zuo K., Li J., Li K., Hu C., Gao Y., Chen M., Hu R., Liu Y., Chi H., Wang H., et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. GigaScience. 2019;8:giz058. doi: 10.1093/gigascience/giz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandek A., Bauditz J., Swidsinski A., Buhner S., Weber-Eibel J., von Haehling S., Schroedl W., Karhausen T., Doehner W., Rauchhaus M., et al. Altered intestinal function in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007;50:1561–1569. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Kummen M., Mayerhofer C.C.K., Vestad B., Broch K., Awoyemi A., Storm-Larsen C., Ueland T., Yndestad A., Hov J.R., Trøseid M. Gut Microbiota Signature in Heart Failure Defined From Profiling of 2 Independent Cohorts. J. Am. Coll. Cardiol. 2018;71:1184–1186. doi: 10.1016/j.jacc.2017.12.057. [DOI] [PubMed] [Google Scholar]
- 44.Deng X., Zhang C., Wang P., Wei W., Shi X., Wang P., Yang J., Wang L., Tang S., Fang Y., et al. Cardiovascular Benefits of Empagliflozin Are Associated With Gut Microbiota and Plasma Metabolites in Type 2 Diabetes. J. Clin. Endocrinol. Metabol. 2022;107:1888–1896. doi: 10.1210/clinem/dgac210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beale A.L., O'Donnell J.A., Nakai M.E., Nanayakkara S., Vizi D., Carter K., Dean E., Ribeiro R.V., Yiallourou S., Carrington M.J., et al. The Gut Microbiome of Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2021;10 doi: 10.1161/jaha.120.020654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmu J., Salosensaari A., Havulinna A.S., Cheng S., Inouye M., Jain M., Salido R.A., Sanders K., Brennan C., Humphrey G.C., et al. Association Between the Gut Microbiota and Blood Pressure in a Population Cohort of 6953 Individuals. J. Am. Heart Assoc. 2020;9 doi: 10.1161/jaha.120.016641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun S., Lulla A., Sioda M., Winglee K., Wu M.C., Jacobs D.R., Jr., Shikany J.M., Lloyd-Jones D.M., Launer L.J., Fodor A.A., Meyer K.A. Gut Microbiota Composition and Blood Pressure. Hypertension. 2019;73:998–1006. doi: 10.1161/hypertensionaha.118.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katsimichas T., Ohtani T., Motooka D., Tsukamoto Y., Kioka H., Nakamoto K., Konishi S., Chimura M., Sengoku K., Miyawaki H., et al. Non-Ischemic Heart Failure With Reduced Ejection Fraction Is Associated With Altered Intestinal Microbiota. Circ. J. 2018;82:1640–1650. doi: 10.1253/circj.CJ-17-1285. [DOI] [PubMed] [Google Scholar]
- 49.Kamo T., Akazawa H., Suda W., Saga-Kamo A., Shimizu Y., Yagi H., Liu Q., Nomura S., Naito A.T., Takeda N., et al. Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moughaizel M., Dagher E., Jablaoui A., Thorin C., Rhimi M., Desfontis J.C., Mallem Y. Long-term high-fructose high-fat diet feeding elicits insulin resistance, exacerbates dyslipidemia and induces gut microbiota dysbiosis in WHHL rabbits. PLoS One. 2022;17 doi: 10.1371/journal.pone.0264215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L., Zhong S.J., Hu S.Y., Cheng B., Qiu H., Hu Z.X. Changes of gut microbiome composition and metabolites associated with hypertensive heart failure rats. BMC Microbiol. 2021;21:141. doi: 10.1186/s12866-021-02202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu C.N., Chang-Chien G.P., Lin S., Hou C.Y., Tain Y.L. Targeting on Gut Microbial Metabolite Trimethylamine-N-Oxide and Short-Chain Fatty Acid to Prevent Maternal High-Fructose-Diet-Induced Developmental Programming of Hypertension in Adult Male Offspring. Mol. Nutr. Food Res. 2019;63 doi: 10.1002/mnfr.201900073. [DOI] [PubMed] [Google Scholar]
- 53.Bier A., Braun T., Khasbab R., Di Segni A., Grossman E., Haberman Y., Leibowitz A. A High Salt Diet Modulates the Gut Microbiota and Short Chain Fatty Acids Production in a Salt-Sensitive Hypertension Rat Model. Nutrients. 2018;10:1154. doi: 10.3390/nu10091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kasahara K., Krautkramer K.A., Org E., Romano K.A., Kerby R.L., Vivas E.I., Mehrabian M., Denu J.M., Bäckhed F., Lusis A.J., Rey F.E. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat. Microbiol. 2018;3:1461–1471. doi: 10.1038/s41564-018-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bao P., Zhang Z., Liang Y., Yu Z., Xiao Z., Wang Y., Yu Y., Liu W., Chen X., Huang Z., et al. Role of the Gut Microbiota in Glucose Metabolism During Heart Failure. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.903316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perazza L.R., Mitchell P.L., Jensen B.A.H., Daniel N., Boyer M., Varin T.V., Bouchareb R., Nachbar R.T., Bouchard M., Blais M., et al. Dietary sucrose induces metabolic inflammation and atherosclerotic cardiovascular diseases more than dietary fat in LDLr(-/-)ApoB(100/100) mice. Atherosclerosis. 2020;304:9–21. doi: 10.1016/j.atherosclerosis.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Groschwitz K.R., Hogan S.P. Intestinal barrier function: molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009;124:3–22. doi: 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Violi F., Cammisotto V., Bartimoccia S., Pignatelli P., Carnevale R., Nocella C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat. Rev. Cardiol. 2023;20:24–37. doi: 10.1038/s41569-022-00737-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jia W., Sun J., Cao X., Xu Y., Wu Z., Zhou C., Huo J., Su S., Zhen M., Wang C., Bai C. Recovering intestinal redox homeostasis to resolve systemic inflammation for preventing remote myocardial injury by oral fullerenes. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2311673120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anker S.D., Egerer K.R., Volk H.D., Kox W.J., Poole-Wilson P.A., Coats A.J. Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am. J. Cardiol. 1997;79:1426–1430. doi: 10.1016/s0002-9149(97)00159-8. [DOI] [PubMed] [Google Scholar]
- 61.Niebauer J., Volk H.D., Kemp M., Dominguez M., Schumann R.R., Rauchhaus M., Poole-Wilson P.A., Coats A.J., Anker S.D. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353:1838–1842. doi: 10.1016/s0140-6736(98)09286-1. [DOI] [PubMed] [Google Scholar]
- 62.Nagatomo Y., Tang W.H.W. Intersections Between Microbiome and Heart Failure: Revisiting the Gut Hypothesis. J. Card. Fail. 2015;21:973–980. doi: 10.1016/j.cardfail.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao J., Zhang Q., Cheng W., Dai Q., Wei Z., Guo M., Chen F., Qiao S., Hu J., Wang J., et al. Heart-gut microbiota communication determines the severity of cardiac injury after myocardial ischaemia/reperfusion. Cardiovasc. Res. 2023;119:1390–1402. doi: 10.1093/cvr/cvad023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Z., Wang Q., Liu Y., Wang L., Ge Z., Li Z., Feng S., Wu C. Gut microbiota and hypertension: association, mechanisms and treatment. Clin. Exp. Hypertens. 2023;45:2195135. doi: 10.1080/10641963.2023.2195135. [DOI] [PubMed] [Google Scholar]
- 65.Li C., Xiao P., Lin D., Zhong H.J., Zhang R., Zhao Z.G., He X.X. Risk Factors for Intestinal Barrier Impairment in Patients With Essential Hypertension. Front. Med. 2020;7 doi: 10.3389/fmed.2020.543698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim S., Goel R., Kumar A., Qi Y., Lobaton G., Hosaka K., Mohammed M., Handberg E.M., Richards E.M., Pepine C.J., Raizada M.K. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin. Sci. 2018;132:701–718. doi: 10.1042/cs20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scott K.P., Gratz S.W., Sheridan P.O., Flint H.J., Duncan S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 68.Scalbert A., Brennan L., Manach C., Andres-Lacueva C., Dragsted L.O., Draper J., Rappaport S.M., van der Hooft J.J.J., Wishart D.S. The food metabolome: a window over dietary exposure. Am. J. Clin. Nutr. 2014;99:1286–1308. doi: 10.3945/ajcn.113.076133. [DOI] [PubMed] [Google Scholar]
- 69.Nemet I., Saha P.P., Gupta N., Zhu W., Romano K.A., Skye S.M., Cajka T., Mohan M.L., Li L., Wu Y., et al. A Cardiovascular Disease-Linked Gut Microbial Metabolite Acts via Adrenergic Receptors. Cell. 2020;180:862–877.e22. doi: 10.1016/j.cell.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Z., Roberts A.B., Buffa J.A., Levison B.S., Zhu W., Org E., Gu X., Huang Y., Zamanian-Daryoush M., Culley M.K., et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Craciun S., Balskus E.P. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc. Natl. Acad. Sci. USA. 2012;109:21307–21312. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai Y.Y., Huang F.Q., Lao X., Lu Y., Gao X., Alolga R.N., Yin K., Zhou X., Wang Y., Liu B., et al. Author Correction: Integrated metagenomics identifies a crucial role for trimethylamine-producing Lachnoclostridium in promoting atherosclerosis. NPJ Biofilms Microbiomes. 2022;8:40. doi: 10.1038/s41522-022-00303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramireddy L., Tsen H.Y., Chiang Y.C., Hung C.Y., Chen F.C., Yen H.T. The gene expression and bioinformatic analysis of choline trimethylamine-lyase (CutC) and its activating enzyme (CutD) for gut microbes and comparison with their TMA production levels. Curr. Res. Microb. Sci. 2021;2 doi: 10.1016/j.crmicr.2021.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brunt V.E., Gioscia-Ryan R.A., Casso A.G., VanDongen N.S., Ziemba B.P., Sapinsley Z.J., Richey J.J., Zigler M.C., Neilson A.P., Davy K.P., Seals D.R. Trimethylamine-N-Oxide Promotes Age-Related Vascular Oxidative Stress and Endothelial Dysfunction in Mice and Healthy Humans. Hypertension. 2020;76:101–112. doi: 10.1161/hypertensionaha.120.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma G., Pan B., Chen Y., Guo C., Zhao M., Zheng L., Chen B. Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci. Rep. 2017;37 doi: 10.1042/bsr20160244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang B., Qiu J., Lian J., Yang X., Zhou J. Gut Metabolite Trimethylamine-N-Oxide in Atherosclerosis: From Mechanism to Therapy. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.723886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu W., Gregory J.C., Org E., Buffa J.A., Gupta N., Wang Z., Li L., Fu X., Wu Y., Mehrabian M., et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang W.H.W., Wang Z., Levison B.S., Koeth R.A., Britt E.B., Fu X., Wu Y., Hazen S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Z., Tang W.H.W., Buffa J.A., Fu X., Britt E.B., Koeth R.A., Levison B.S., Fan Y., Wu Y., Hazen S.L. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 2014;35:904–910. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mann E.R., Lam Y.K., Uhlig H.H. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024;24:577–595. doi: 10.1038/s41577-024-01014-8. [DOI] [PubMed] [Google Scholar]
- 82.Cummings J.H., Pomare E.W., Branch W.J., Naylor C.P., Macfarlane G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scheiman J., Luber J.M., Chavkin T.A., MacDonald T., Tung A., Pham L.D., Wibowo M.C., Wurth R.C., Punthambaker S., Tierney B.T., et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019;25:1104–1109. doi: 10.1038/s41591-019-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yan J., Pan Y., Shao W., Wang C., Wang R., He Y., Zhang M., Wang Y., Li T., Wang Z., et al. Beneficial effect of the short-chain fatty acid propionate on vascular calcification through intestinal microbiota remodelling. Microbiome. 2022;10:195. doi: 10.1186/s40168-022-01390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duan C., Wu J., Wang Z., Tan C., Hou L., Qian W., Han C., Hou X. Fucose promotes intestinal stem cell-mediated intestinal epithelial development through promoting Akkermansia-related propanoate metabolism. Gut Microbes. 2023;15 doi: 10.1080/19490976.2023.2233149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Louis P., Hold G.L., Flint H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 87.Flint H.J., Duncan S.H., Scott K.P., Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015;74:13–22. doi: 10.1017/s0029665114001463. [DOI] [PubMed] [Google Scholar]
- 88.Hashemi Z., Fouhse J., Im H.S., Chan C.B., Willing B.P. Dietary Pea Fiber Supplementation Improves Glycemia and Induces Changes in the Composition of Gut Microbiota, Serum Short Chain Fatty Acid Profile and Expression of Mucins in Glucose Intolerant Rats. Nutrients. 2017;9 doi: 10.3390/nu9111236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Albillos A., de Gottardi A., Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 90.Bergman E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- 91.Chambers E.S., Viardot A., Psichas A., Morrison D.J., Murphy K.G., Zac-Varghese S.E.K., MacDougall K., Preston T., Tedford C., Finlayson G.S., et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haghikia A., Zimmermann F., Schumann P., Jasina A., Roessler J., Schmidt D., Heinze P., Kaisler J., Nageswaran V., Aigner A., et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur. Heart J. 2022;43:518–533. doi: 10.1093/eurheartj/ehab644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kimura I., Ichimura A., Ohue-Kitano R., Igarashi M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020;100:171–210. doi: 10.1152/physrev.00041.2018. [DOI] [PubMed] [Google Scholar]
- 94.Hamed S.A., Mohan A., Navaneetha Krishnan S., Wang A., Drikic M., Prince N.L., Lewis I.A., Shearer J., Keita Å.V., Söderholm J.D., et al. Butyrate reduces adherent-invasive E. coli-evoked disruption of epithelial mitochondrial morphology and barrier function: involvement of free fatty acid receptor 3. Gut Microbes. 2023;15 doi: 10.1080/19490976.2023.2281011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Offermanns S. Hydroxy-Carboxylic Acid Receptor Actions in Metabolism. Trends Endocrinol. Metab. 2017;28:227–236. doi: 10.1016/j.tem.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 96.Sivaprakasam S., Bhutia Y.D., Ramachandran S., Ganapathy V. Cell-Surface and Nuclear Receptors in the Colon as Targets for Bacterial Metabolites and Its Relevance to Colon Health. Nutrients. 2017;9 doi: 10.3390/nu9080856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bolognini D., Tobin A.B., Milligan G., Hodge D. The Pharmacology and Function of Receptors for Short-Chain Fatty Acids. Mol. Pharmacol. 2016;89:388–398. doi: 10.1124/mol.115.102301. [DOI] [PubMed] [Google Scholar]
- 98.Pluznick J.L., Protzko R.J., Gevorgyan H., Peterlin Z., Sipos A., Han J., Brunet I., Wan L.X., Rey F., Wang T., et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5:202–207. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang P.V., Hao L., Offermanns S., Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Collins S.L., Stine J.G., Bisanz J.E., Okafor C.D., Patterson A.D. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023;21:236–247. doi: 10.1038/s41579-022-00805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Song X., Sun X., Oh S.F., Wu M., Zhang Y., Zheng W., Geva-Zatorsky N., Jupp R., Mathis D., Benoist C., Kasper D.L. Microbial bile acid metabolites modulate gut RORγ(+) regulatory T cell homeostasis. Nature. 2020;577:410–415. doi: 10.1038/s41586-019-1865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tilg H., Adolph T.E., Trauner M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022;34:1700–1718. doi: 10.1016/j.cmet.2022.09.017. [DOI] [PubMed] [Google Scholar]
- 104.Sayin S.I., Wahlström A., Felin J., Jäntti S., Marschall H.U., Bamberg K., Angelin B., Hyötyläinen T., Orešič M., Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 105.Thibaut M.M., Bindels L.B. Crosstalk between bile acid-activated receptors and microbiome in entero-hepatic inflammation. Trends Mol. Med. 2022;28:223–236. doi: 10.1016/j.molmed.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 106.Molinaro A., Wahlström A., Marschall H.U. Role of Bile Acids in Metabolic Control. Trends Endocrinol. Metab. 2018;29:31–41. doi: 10.1016/j.tem.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 107.Makishima M., Okamoto A.Y., Repa J.J., Tu H., Learned R.M., Luk A., Hull M.V., Lustig K.D., Mangelsdorf D.J., Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 108.Yang Y., Wu C. Targeting gut microbial bile salt hydrolase (BSH) by diet supplements: new insights into dietary modulation of human health. Food Funct. 2022;13:7409–7422. doi: 10.1039/d2fo01252a. [DOI] [PubMed] [Google Scholar]
- 109.Appelman M.D., van der Veen S.W., van Mil S.W.C. Post-Translational Modifications of FXR; Implications for Cholestasis and Obesity-Related Disorders. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.729828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang X.X., Edelstein M.H., Gafter U., Qiu L., Luo Y., Dobrinskikh E., Lucia S., Adorini L., D'Agati V.D., Levi J., et al. G Protein-Coupled Bile Acid Receptor TGR5 Activation Inhibits Kidney Disease in Obesity and Diabetes. J. Am. Soc. Nephrol. 2016;27:1362–1378. doi: 10.1681/asn.2014121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mayerhofer C.C.K., Ueland T., Broch K., Vincent R.P., Cross G.F., Dahl C.P., Aukrust P., Gullestad L., Hov J.R., Trøseid M. Increased Secondary/Primary Bile Acid Ratio in Chronic Heart Failure. J. Card. Fail. 2017;23:666–671. doi: 10.1016/j.cardfail.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 112.Yokota A., Fukiya S., Islam K.B.M.S., Ooka T., Ogura Y., Hayashi T., Hagio M., Ishizuka S. Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microbes. 2012;3:455–459. doi: 10.4161/gmic.21216. [DOI] [PubMed] [Google Scholar]
- 113.Di Lorenzo F., Duda K.A., Lanzetta R., Silipo A., De Castro C., Molinaro A. A Journey from Structure to Function of Bacterial Lipopolysaccharides. Chem. Rev. 2022;122:15767–15821. doi: 10.1021/acs.chemrev.0c01321. [DOI] [PubMed] [Google Scholar]
- 114.Beutler B., Rietschel E.T. Innate immune sensing and its roots: the story of endotoxin. Nat. Rev. Immunol. 2003;3:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 115.Tulkens J., Vergauwen G., Van Deun J., Geeurickx E., Dhondt B., Lippens L., De Scheerder M.A., Miinalainen I., Rappu P., De Geest B.G., et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut. 2020;69:191–193. doi: 10.1136/gutjnl-2018-317726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lakio L., Lehto M., Tuomainen A.M., Jauhiainen M., Malle E., Asikainen S., Pussinen P.J. Pro-atherogenic properties of lipopolysaccharide from the periodontal pathogen Actinobacillus actinomycetemcomitans. J. Endotoxin Res. 2006;12:57–64. doi: 10.1179/096805106x89099. [DOI] [PubMed] [Google Scholar]
- 117.Funk J.L., Feingold K.R., Moser A.H., Grunfeld C. Lipopolysaccharide stimulation of RAW 264.7 macrophages induces lipid accumulation and foam cell formation. Atherosclerosis. 1993;98:67–82. doi: 10.1016/0021-9150(93)90224-i. [DOI] [PubMed] [Google Scholar]
- 118.Kapinsky M., Torzewski M., Büchler C., Duong C.Q., Rothe G., Schmitz G. Enzymatically degraded LDL preferentially binds to CD14(high) CD16(+) monocytes and induces foam cell formation mediated only in part by the class B scavenger-receptor CD36. Arterioscler. Thromb. Vasc. Biol. 2001;21:1004–1010. doi: 10.1161/01.atv.21.6.1004. [DOI] [PubMed] [Google Scholar]
- 119.Triantafilou M., Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23:301–304. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- 120.Lepper P.M., Kleber M.E., Grammer T.B., Hoffmann K., Dietz S., Winkelmann B.R., Boehm B.O., März W. Lipopolysaccharide-binding protein (LBP) is associated with total and cardiovascular mortality in individuals with or without stable coronary artery disease--results from the Ludwigshafen Risk and Cardiovascular Health Study (LURIC) Atherosclerosis. 2011;219:291–297. doi: 10.1016/j.atherosclerosis.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 121.Paik Y.H., Schwabe R.F., Bataller R., Russo M.P., Jobin C., Brenner D.A. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 122.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 123.Mills E.L., Ryan D.G., Prag H.A., Dikovskaya D., Menon D., Zaslona Z., Jedrychowski M.P., Costa A.S.H., Higgins M., Hams E., et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556:113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rajendran P., Rengarajan T., Thangavel J., Nishigaki Y., Sakthisekaran D., Sethi G., Nishigaki I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Faith J.J., Ahern P.P., Ridaura V.K., Cheng J., Gordon J.I. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng K.K., Wang G.Y., Zeng J., Zhang J.A. Improved succinate production by metabolic engineering. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/538790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.De Vadder F., Kovatcheva-Datchary P., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016;24:151–157. doi: 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 128.Ferreyra J.A., Wu K.J., Hryckowian A.J., Bouley D.M., Weimer B.C., Sonnenburg J.L. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe. 2014;16:770–777. doi: 10.1016/j.chom.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Serena C., Ceperuelo-Mallafré V., Keiran N., Queipo-Ortuño M.I., Bernal R., Gomez-Huelgas R., Urpi-Sarda M., Sabater M., Pérez-Brocal V., Andrés-Lacueva C., et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018;12:1642–1657. doi: 10.1038/s41396-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martínez-Reyes I., Chandel N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020;11:102. doi: 10.1038/s41467-019-13668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murphy M.P., O'Neill L.A.J. Krebs Cycle Reimagined: The Emerging Roles of Succinate and Itaconate as Signal Transducers. Cell. 2018;174:780–784. doi: 10.1016/j.cell.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 132.Liu H., Chen X., Hu X., Niu H., Tian R., Wang H., Pang H., Jiang L., Qiu B., Chen X., et al. Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome. 2019;7:68. doi: 10.1186/s40168-019-0683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tang W.H.W., Hazen S.L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Investig. 2014;124:4204–4211. doi: 10.1172/jci72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Organ C.L., Otsuka H., Bhushan S., Wang Z., Bradley J., Trivedi R., Polhemus D.J., Tang W.H.W., Wu Y., Hazen S.L., Lefer D.J. Choline Diet and Its Gut Microbe-Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload-Induced Heart Failure. Circ. Heart Fail. 2016;9 doi: 10.1161/circheartfailure.115.002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hu T., Wu Q., Yao Q., Jiang K., Yu J., Tang Q. Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res. Rev. 2022;81 doi: 10.1016/j.arr.2022.101706. [DOI] [PubMed] [Google Scholar]
- 136.Jiang S., Shui Y., Cui Y., Tang C., Wang X., Qiu X., Hu W., Fei L., Li Y., Zhang S., et al. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension. Redox Biol. 2021;46 doi: 10.1016/j.redox.2021.102115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jonsson A.L., Bäckhed F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017;14:79–87. doi: 10.1038/nrcardio.2016.183. [DOI] [PubMed] [Google Scholar]
- 138.Huang S., Gao S., Shao Y., Li P., Lu J., Xu K., Zhou Z., Li Y., Du J. Gut microbial metabolite trimethylamine N-oxide induces aortic dissection. J. Mol. Cell. Cardiol. 2024;189:25–37. doi: 10.1016/j.yjmcc.2024.02.007. [DOI] [PubMed] [Google Scholar]
- 139.Huang S.S., Liu R., Chang S., Li X., Weng X., Ge J. Gut Microbiota-Derived Tryptophan Metabolite Indole-3-aldehyde Ameliorates Aortic Dissection. Nutrients. 2023;15 doi: 10.3390/nu15194150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tian Z., Zhang Y., Zheng Z., Zhang M., Zhang T., Jin J., Zhang X., Yao G., Kong D., Zhang C., et al. Gut microbiome dysbiosis contributes to abdominal aortic aneurysm by promoting neutrophil extracellular trap formation. Cell Host Microbe. 2022;30:1450–1463.e8. doi: 10.1016/j.chom.2022.09.004. [DOI] [PubMed] [Google Scholar]
- 141.Benson T.W., Conrad K.A., Li X.S., Wang Z., Helsley R.N., Schugar R.C., Coughlin T.M., Wadding-Lee C., Fleifil S., Russell H.M., et al. Gut Microbiota-Derived Trimethylamine N-Oxide Contributes to Abdominal Aortic Aneurysm Through Inflammatory and Apoptotic Mechanisms. Circulation. 2023;147:1079–1096. doi: 10.1161/circulationaha.122.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li Z., Xing J., Ma X., Zhang W., Wang C., Wang Y., Qi X., Liu Y., Jian D., Cheng X., et al. An orally administered bacterial membrane protein nanodrug ameliorates doxorubicin cardiotoxicity through alleviating impaired intestinal barrier. Bioact. Mater. 2024;37:517–532. doi: 10.1016/j.bioactmat.2024.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao M., Wei H., Li C., Zhan R., Liu C., Gao J., Yi Y., Cui X., Shan W., Ji L., et al. Gut microbiota production of trimethyl-5-aminovaleric acid reduces fatty acid oxidation and accelerates cardiac hypertrophy. Nat. Commun. 2022;13:1757. doi: 10.1038/s41467-022-29060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aguiar C.J., Andrade V.L., Gomes E.R.M., Alves M.N.M., Ladeira M.S., Pinheiro A.C.N., Gomes D.A., Almeida A.P., Goes A.M., Resende R.R., et al. Succinate modulates Ca(2+) transient and cardiomyocyte viability through PKA-dependent pathway. Cell Calcium. 2010;47:37–46. doi: 10.1016/j.ceca.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 145.Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., de Ferranti S.D., Floyd J., Fornage M., Gillespie C., et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/cir.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mogensen U.M., Gong J., Jhund P.S., Shen L., Køber L., Desai A.S., Lefkowitz M.P., Packer M., Rouleau J.L., Solomon S.D., et al. Effect of sacubitril/valsartan on recurrent events in the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) Eur. J. Heart Fail. 2018;20:760–768. doi: 10.1002/ejhf.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li Y., Yu J., Wang Y. Mechanism of Coronary Microcirculation Obstruction after Acute Myocardial Infarction and Cardioprotective Strategies. Rev. Cardiovasc. Med. 2024;25:367. doi: 10.31083/j.rcm2510367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Piccioni A., de Cunzo T., Valletta F., Covino M., Rinninella E., Raoul P., Zanza C., Mele M.C., Franceschi F. Gut Microbiota and Environment in Coronary Artery Disease. Int. J. Environ. Res. Public Health. 2021;18 doi: 10.3390/ijerph18084242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cui L., Zhao T., Hu H., Zhang W., Hua X. Association Study of Gut Flora in Coronary Heart Disease through High-Throughput Sequencing. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/3796359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cybulsky M.I., Chan M.K., Movat H.Z. Acute inflammation and microthrombosis induced by endotoxin, interleukin-1, and tumor necrosis factor and their implication in gram-negative infection. Lab. Invest. 1988;58:365–378. [PubMed] [Google Scholar]
- 151.Talmor-Barkan Y., Bar N., Shaul A.A., Shahaf N., Godneva A., Bussi Y., Lotan-Pompan M., Weinberger A., Shechter A., Chezar-Azerrad C., et al. Metabolomic and microbiome profiling reveals personalized risk factors for coronary artery disease. Nat. Med. 2022;28:295–302. doi: 10.1038/s41591-022-01686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tang W.H.W., Wang Z., Kennedy D.J., Wu Y., Buffa J.A., Agatisa-Boyle B., Li X.S., Levison B.S., Hazen S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015;116:448–455. doi: 10.1161/circresaha.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu T.X., Niu H.T., Zhang S.Y. Intestinal Microbiota Metabolism and Atherosclerosis. Chin. Med. J. 2015;128:2805–2811. doi: 10.4103/0366-6999.167362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Falony G., Vieira-Silva S., Raes J. Microbiology Meets Big Data: The Case of Gut Microbiota-Derived Trimethylamine. Annu. Rev. Microbiol. 2015;69:305–321. doi: 10.1146/annurev-micro-091014-104422. [DOI] [PubMed] [Google Scholar]
- 155.Poesen R., Claes K., Evenepoel P., de Loor H., Augustijns P., Kuypers D., Meijers B. Microbiota-Derived Phenylacetylglutamine Associates with Overall Mortality and Cardiovascular Disease in Patients with CKD. J. Am. Soc. Nephrol. 2016;27:3479–3487. doi: 10.1681/asn.2015121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu Y., Liu S., Zhao Z., Song X., Qu H., Liu H. Phenylacetylglutamine is associated with the degree of coronary atherosclerotic severity assessed by coronary computed tomographic angiography in patients with suspected coronary artery disease. Atherosclerosis. 2021;333:75–82. doi: 10.1016/j.atherosclerosis.2021.08.029. [DOI] [PubMed] [Google Scholar]
- 157.Yu J., Li Y., Hu J., Wang Y. Interleukin-33 induces angiogenesis after myocardial infarction via AKT/eNOS signaling pathway. Int. Immunopharmacol. 2024;143 doi: 10.1016/j.intimp.2024.113433. [DOI] [PubMed] [Google Scholar]
- 158.Fan Y., Li F., Tan X., Ren L., Peng X., Yu J., Chen W., Jia L., Zhu F., Yin W., et al. Abnormal circulating steroids refine risk of progression to heart failure in ischemic heart disease. Eur. J. Clin. Invest. 2024;54 doi: 10.1111/eci.14156. [DOI] [PubMed] [Google Scholar]
- 159.Mamic P., Heidenreich P.A., Hedlin H., Tennakoon L., Staudenmayer K.L. Hospitalized Patients with Heart Failure and Common Bacterial Infections: A Nationwide Analysis of Concomitant Clostridium Difficile Infection Rates and In-Hospital Mortality. J. Card. Fail. 2016;22:891–900. doi: 10.1016/j.cardfail.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 160.Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., deRoos P., Liu H., Cross J.R., Pfeffer K., Coffer P.J., Rudensky A.Y. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Salzer U., Müller A., Zhou Q., Nieters A., Grundmann S., Wehr C. Susceptibility to infections and adaptive immunity in adults with heart failure. ESC Heart Fail. 2022;9:1195–1205. doi: 10.1002/ehf2.13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tang W.H.W., Wang Z., Fan Y., Levison B., Hazen J.E., Donahue L.M., Wu Y., Hazen S.L. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J. Am. Coll. Cardiol. 2014;64:1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sandek A., Bjarnason I., Volk H.D., Crane R., Meddings J.B., Niebauer J., Kalra P.R., Buhner S., Herrmann R., Springer J., et al. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int. J. Cardiol. 2012;157:80–85. doi: 10.1016/j.ijcard.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 164.Kirschner S.K., Deutz N.E.P., Rijnaarts I., Smit T.J., Larsen D.J., Engelen M.P.K.J. Impaired intestinal function is associated with lower muscle and cognitive health and well-being in patients with congestive heart failure. JPEN. J. Parenter. Enteral Nutr. 2022;46:660–670. doi: 10.1002/jpen.2193. [DOI] [PubMed] [Google Scholar]
- 165.Tang T.W.H., Chen H.C., Chen C.Y., Yen C.Y.T., Lin C.J., Prajnamitra R.P., Chen L.L., Ruan S.C., Lin J.H., Lin P.J., et al. Loss of Gut Microbiota Alters Immune System Composition and Cripples Postinfarction Cardiac Repair. Circulation. 2019;139:647–659. doi: 10.1161/circulationaha.118.035235. [DOI] [PubMed] [Google Scholar]
- 166.Jones D.N., Jordan J.H., Meléndez G.C., Lamar Z., Thomas A., Kitzman D.W., Suerken C., D'Agostino R.B., Jr., Hundley W.G. Frequency of Transition From Stage A to Stage B Heart Failure After Initiating Potentially Cardiotoxic Chemotherapy. JACC. Heart Fail. 2018;6:1023–1032. doi: 10.1016/j.jchf.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Beavers C.J., Rodgers J.E., Bagnola A.J., Beckie T.M., Campia U., Di Palo K.E., Okwuosa T.M., Przespolewski E.R., Dent S., American Heart Association Clinical Pharmacology Committee and Cardio-Oncology Committee of the Council on Clinical Cardiology and Council on Genomic and Precision Medicine; and the Council on Peripheral Vascular Disease Cardio-Oncology Drug Interactions: A Scientific Statement From the American Heart Association. Circulation. 2022;145:e811–e838. doi: 10.1161/cir.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 168.Gulati G., Heck S.L., Ree A.H., Hoffmann P., Schulz-Menger J., Fagerland M.W., Gravdehaug B., von Knobelsdorff-Brenkenhoff F., Bratland Å., Storås T.H., et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016;37:1671–1680. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huang C., Li X., Li H., Chen R., Li Z., Li D., Xu X., Zhang G., Qin L., Li B., Chu X.M. Role of gut microbiota in doxorubicin-induced cardiotoxicity: from pathogenesis to related interventions. J. Transl. Med. 2024;22:433. doi: 10.1186/s12967-024-05232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Flórez A.B., Sierra M., Ruas-Madiedo P., Mayo B. Susceptibility of lactic acid bacteria, bifidobacteria and other bacteria of intestinal origin to chemotherapeutic agents. Int. J. Antimicrob. Agents. 2016;48:547–550. doi: 10.1016/j.ijantimicag.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 171.An L., Wuri J., Zheng Z., Li W., Yan T. Microbiota modulate Doxorubicin induced cardiotoxicity. Eur. J. Pharm. Sci. 2021;166 doi: 10.1016/j.ejps.2021.105977. [DOI] [PubMed] [Google Scholar]
- 172.Huang K., Liu Y., Tang H., Qiu M., Li C., Duan C., Wang C., Yang J., Zhou X. Glabridin Prevents Doxorubicin-Induced Cardiotoxicity Through Gut Microbiota Modulation and Colonic Macrophage Polarization in Mice. Front. Pharmacol. 2019;10:107. doi: 10.3389/fphar.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Huang J., Wei S., Jiang C., Xiao Z., Liu J., Peng W., Zhang B., Li W. Involvement of Abnormal Gut Microbiota Composition and Function in Doxorubicin-Induced Cardiotoxicity. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.808837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Carley A.N., Maurya S.K., Fasano M., Wang Y., Selzman C.H., Drakos S.G., Lewandowski E.D. Short-Chain Fatty Acids Outpace Ketone Oxidation in the Failing Heart. Circulation. 2021;143:1797–1808. doi: 10.1161/circulationaha.120.052671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rephaeli A., Waks-Yona S., Nudelman A., Tarasenko I., Tarasenko N., Phillips D.R., Cutts S.M., Kessler-Icekson G. Anticancer prodrugs of butyric acid and formaldehyde protect against doxorubicin-induced cardiotoxicity. Br. J. Cancer. 2007;96:1667–1674. doi: 10.1038/sj.bjc.6603781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Russo M., Guida F., Paparo L., Trinchese G., Aitoro R., Avagliano C., Fiordelisi A., Napolitano F., Mercurio V., Sala V., et al. The novel butyrate derivative phenylalanine-butyramide protects from doxorubicin-induced cardiotoxicity. Eur. J. Heart Fail. 2019;21:519–528. doi: 10.1002/ejhf.1439. [DOI] [PubMed] [Google Scholar]
- 177.Hunter J.J., Chien K.R. Signaling pathways for cardiac hypertrophy and failure. N. Engl. J. Med. 1999;341:1276–1283. doi: 10.1056/nejm199910213411706. [DOI] [PubMed] [Google Scholar]
- 178.Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. doi: 10.1016/s0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 179.de Castro Fonseca M., Aguiar C.J., da Rocha Franco J.A., Gingold R.N., Leite M.F. GPR91: expanding the frontiers of Krebs cycle intermediates. Cell Commun. Signal. 2016;14 doi: 10.1186/s12964-016-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.He W., Miao F.J.P., Lin D.C.H., Schwandner R.T., Wang Z., Gao J., Chen J.L., Tian H., Ling L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- 181.Björkegren J.L.M., Lusis A.J. Atherosclerosis: Recent developments. Cell. 2022;185:1630–1645. doi: 10.1016/j.cell.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li Y., Zhang Y., Lu J., Yin Y., Xie J., Xu B. Anti-inflammatory mechanisms and research progress of colchicine in atherosclerotic therapy. J. Cell Mol. Med. 2021;25:8087–8094. doi: 10.1111/jcmm.16798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shor A., Kuo C.C., Patton D.L. Detection of Chlamydia pneumoniae in coronary arterial fatty streaks and atheromatous plaques. S. Afr. Med. J. 1992;82:158–161. [PubMed] [Google Scholar]
- 184.Annunziata G., Maisto M., Schisano C., Ciampaglia R., Narciso V., Tenore G.C., Novellino E. Effects of Grape Pomace Polyphenolic Extract (Taurisolo(®)) in Reducing TMAO Serum Levels in Humans: Preliminary Results from a Randomized, Placebo-Controlled, Cross-Over Study. Nutrients. 2019;11 doi: 10.3390/nu11010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ohira H., Tsutsui W., Fujioka Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J. Atheroscler. Thromb. 2017;24:660–672. doi: 10.5551/jat.RV17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhu Y., Yin C., Wang Y. Probiotic Enterococcus Faecium Attenuated Atherosclerosis by Improving SCFAs Associated with Gut Microbiota in ApoE(-/-) Mice. Bioengineering. 2024;11 doi: 10.3390/bioengineering11101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Felizardo R.J.F., Watanabe I.K.M., Dardi P., Rossoni L.V., Câmara N.O.S. The interplay among gut microbiota, hypertension and kidney diseases: The role of short-chain fatty acids. Pharmacol. Res. 2019;141:366–377. doi: 10.1016/j.phrs.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 188.Machate D.J., Figueiredo P.S., Marcelino G., Guimarães R.d.C.A., Hiane P.A., Bogo D., Pinheiro V.A.Z., Oliveira L.C.S.d., Pott A. Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21114093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.de la Cuesta-Zuluaga J., Mueller N.T., Álvarez-Quintero R., Velásquez-Mejía E.P., Sierra J.A., Corrales-Agudelo V., Carmona J.A., Abad J.M., Escobar J.S. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients. 2018;11:51. doi: 10.3390/nu11010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ziętek M., Celewicz Z., Szczuko M. Short-Chain Fatty Acids, Maternal Microbiota and Metabolism in Pregnancy. Nutrients. 2021;13:1244. doi: 10.3390/nu13041244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tain Y.L., Hou C.Y., Chang-Chien G.P., Lin S., Hsu C.N. Resveratrol Butyrate Ester Supplementation Blunts the Development of Offspring Hypertension in a Maternal Di-2-ethylhexyl Phthalate Exposure Rat Model. Nutrients. 2023;15 doi: 10.3390/nu15030697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hameed I., Cifu A.S., Vallabhajosyula P. Management of Thoracic Aortic Dissection. JAMA. 2023;329:756–757. doi: 10.1001/jama.2023.0265. [DOI] [PubMed] [Google Scholar]
- 193.Rylski B., Schilling O., Czerny M. Acute aortic dissection: evidence, uncertainties, and future therapies. Eur. Heart J. 2023;44:813–821. doi: 10.1093/eurheartj/ehac757. [DOI] [PubMed] [Google Scholar]
- 194.Goldfinger J.Z., Halperin J.L., Marin M.L., Stewart A.S., Eagle K.A., Fuster V. Thoracic aortic aneurysm and dissection. J. Am. Coll. Cardiol. 2014;64:1725–1739. doi: 10.1016/j.jacc.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 195.De Filippis F., Pellegrini N., Vannini L., Jeffery I.B., La Storia A., Laghi L., Serrazanetti D.I., Di Cagno R., Ferrocino I., Lazzi C., et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 196.Appel L.J., Moore T.J., Obarzanek E., Vollmer W.M., Svetkey L.P., Sacks F.M., Bray G.A., Vogt T.M., Cutler J.A., Windhauser M.M., et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997;336:1117–1124. doi: 10.1056/nejm199704173361601. [DOI] [PubMed] [Google Scholar]
- 197.Sacks F.M., Lichtenstein A.H., Wu J.H.Y., Appel L.J., Creager M.A., Kris-Etherton P.M., Miller M., Rimm E.B., Rudel L.L., Robinson J.G., et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation. 2017;136:e1–e23. doi: 10.1161/cir.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 198.Jones N.R.V., Forouhi N.G., Khaw K.T., Wareham N.J., Monsivais P. Accordance to the Dietary Approaches to Stop Hypertension diet pattern and cardiovascular disease in a British, population-based cohort. Eur. J. Epidemiol. 2018;33:235–244. doi: 10.1007/s10654-017-0354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ibsen D.B., Levitan E.B., Åkesson A., Gigante B., Wolk A. The DASH diet is associated with a lower risk of heart failure: a cohort study. Eur. J. Prev. Cardiol. 2022;29:1114–1123. doi: 10.1093/eurjpc/zwac003. [DOI] [PubMed] [Google Scholar]
- 200.Rifai L., Pisano C., Hayden J., Sulo S., Silver M.A. Impact of the DASH diet on endothelial function, exercise capacity, and quality of life in patients with heart failure. SAVE Proc. 2015;28:151–156. doi: 10.1080/08998280.2015.11929216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chiavaroli L., Viguiliouk E., Nishi S.K., Blanco Mejia S., Rahelić D., Kahleová H., Salas-Salvadó J., Kendall C.W., Sievenpiper J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients. 2019;11 doi: 10.3390/nu11020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Juraschek S.P., Kovell L.C., Appel L.J., Miller E.R., Sacks F.M., Chang A.R., Christenson R.H., Rebuck H., Mukamal K.J. Effects of Diet and Sodium Reduction on Cardiac Injury, Strain, and Inflammation: The DASH-Sodium Trial. J. Am. Coll. Cardiol. 2021;77:2625–2634. doi: 10.1016/j.jacc.2021.03.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kris-Etherton P., Eckel R.H., Howard B.V., St Jeor S., Bazzarre T.L., Nutrition Committee Population Science Committee and Clinical Science Committee of the American Heart Association AHA Science Advisory: Lyon Diet Heart Study. Benefits of a Mediterranean-style, National Cholesterol Education Program/American Heart Association Step I Dietary Pattern on Cardiovascular Disease. Circulation. 2001;103:1823–1825. doi: 10.1161/01.cir.103.13.1823. [DOI] [PubMed] [Google Scholar]
- 204.Lopez-Garcia E., Rodriguez-Artalejo F., Li T.Y., Fung T.T., Li S., Willett W.C., Rimm E.B., Hu F.B. The Mediterranean-style dietary pattern and mortality among men and women with cardiovascular disease. Am. J. Clin. Nutr. 2014;99:172–180. doi: 10.3945/ajcn.113.068106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Papadaki A., Martínez-González M.Á., Alonso-Gómez A., Rekondo J., Salas-Salvadó J., Corella D., Ros E., Fitó M., Estruch R., Lapetra J., et al. Mediterranean diet and risk of heart failure: results from the PREDIMED randomized controlled trial. Eur. J. Heart Fail. 2017;19:1179–1185. doi: 10.1002/ejhf.750. [DOI] [PubMed] [Google Scholar]
- 206.Borlaug B.A. The pathophysiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2014;11:507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 207.Sanches Machado d'Almeida K., Ronchi Spillere S., Zuchinali P., Corrêa Souza G. Mediterranean Diet and Other Dietary Patterns in Primary Prevention of Heart Failure and Changes in Cardiac Function Markers: A Systematic Review. Nutrients. 2018;10:58. doi: 10.3390/nu10010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xu Z., Knight R. Dietary effects on human gut microbiome diversity. Br. J. Nutr. 2015;113:S1–S5. doi: 10.1017/s0007114514004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang Y., Chen G.C., Wang Z., Luo K., Zhang Y., Li Y., McClain A.C., Jankowska M.M., Perreira K.M., Mattei J., et al. Dietary Acculturation Is Associated With Altered Gut Microbiome, Circulating Metabolites, and Cardiovascular Disease Risk in US Hispanics and Latinos: Results From HCHS/SOL. Circulation. 2024;150:215–229. doi: 10.1161/circulationaha.124.069824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ghosh T.S., Valdes A.M. Evidence for clinical interventions targeting the gut microbiome in cardiometabolic disease. BMJ. 2023;383 doi: 10.1136/bmj-2023-075180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Battson M.L., Lee D.M., Weir T.L., Gentile C.L. The gut microbiota as a novel regulator of cardiovascular function and disease. J. Nutr. Biochem. 2018;56:1–15. doi: 10.1016/j.jnutbio.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 213.Wilck N., Matus M.G., Kearney S.M., Olesen S.W., Forslund K., Bartolomaeus H., Haase S., Mähler A., Balogh A., Markó L., et al. Salt-responsive gut commensal modulates T(H)17 axis and disease. Nature. 2017;551:585–589. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 215.Patel R., DuPont H.L. New approaches for bacteriotherapy: prebiotics, new-generation probiotics, and synbiotics. Clin. Infect. Dis. 2015;60:S108–S121. doi: 10.1093/cid/civ177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Khalesi S., Sun J., Buys N., Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903. doi: 10.1161/hypertensionaha.114.03469. [DOI] [PubMed] [Google Scholar]
- 217.Shimizu M., Hashiguchi M., Shiga T., Tamura H.O., Mochizuki M. Meta-Analysis: Effects of Probiotic Supplementation on Lipid Profiles in Normal to Mildly Hypercholesterolemic Individuals. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Costanza A.C., Moscavitch S.D., Faria Neto H.C.C., Mesquita E.T. Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int. J. Cardiol. 2015;179:348–350. doi: 10.1016/j.ijcard.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 219.Gan X.T., Ettinger G., Huang C.X., Burton J.P., Haist J.V., Rajapurohitam V., Sidaway J.E., Martin G., Gloor G.B., Swann J.R., et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ. Heart Fail. 2014;7:491–499. doi: 10.1161/circheartfailure.113.000978. [DOI] [PubMed] [Google Scholar]
- 220.Chan Y.K., Brar M.S., Kirjavainen P.V., Chen Y., Peng J., Li D., Leung F.C.C., El-Nezami H. High fat diet induced atherosclerosis is accompanied with low colonic bacterial diversity and altered abundances that correlates with plaque size, plasma A-FABP and cholesterol: a pilot study of high fat diet and its intervention with Lactobacillus rhamnosus GG (LGG) or telmisartan in ApoE(-/-) mice. BMC Microbiol. 2016;16:264. doi: 10.1186/s12866-016-0883-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lam V., Su J., Koprowski S., Hsu A., Tweddell J.S., Rafiee P., Gross G.J., Salzman N.H., Baker J.E. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012;26:1727–1735. doi: 10.1096/fj.11-197921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Huang Y., Wang J., Quan G., Wang X., Yang L., Zhong L. Lactobacillus acidophilus ATCC 4356 prevents atherosclerosis via inhibition of intestinal cholesterol absorption in apolipoprotein E-knockout mice. Appl. Environ. Microbiol. 2014;80:7496–7504. doi: 10.1128/aem.02926-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gómez-Guzmán M., Toral M., Romero M., Jiménez R., Galindo P., Sánchez M., Zarzuelo M.J., Olivares M., Gálvez J., Duarte J. Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol. Nutr. Food Res. 2015;59:2326–2336. doi: 10.1002/mnfr.201500290. [DOI] [PubMed] [Google Scholar]
- 224.Fåk F., Bäckhed F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe-/- mice. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Vaghef-Mehrabany E., Vaghef-Mehrabany L., Asghari-Jafarabadi M., Homayouni-Rad A., Issazadeh K., Alipour B. Effects of probiotic supplementation on lipid profile of women with rheumatoid arthritis: A randomized placebo-controlled clinical trial. Health Promot. Perspect. 2017;7:95–101. doi: 10.15171/hpp.2017.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kurilshikov A., Medina-Gomez C., Bacigalupe R., Radjabzadeh D., Wang J., Demirkan A., Le Roy C.I., Raygoza Garay J.A., Finnicum C.T., Liu X., et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 2021;53:156–165. doi: 10.1038/s41588-020-00763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhernakova D.V., Wang D., Liu L., Andreu-Sánchez S., Zhang Y., Ruiz-Moreno A.J., Peng H., Plomp N., Del Castillo-Izquierdo Á., Gacesa R., et al. Host genetic regulation of human gut microbial structural variation. Nature. 2024;625:813–821. doi: 10.1038/s41586-023-06893-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Khalesi S., Bellissimo N., Vandelanotte C., Williams S., Stanley D., Irwin C. A review of probiotic supplementation in healthy adults: helpful or hype? Eur. J. Clin. Nutr. 2019;73:24–37. doi: 10.1038/s41430-018-0135-9. [DOI] [PubMed] [Google Scholar]
- 229.Dong C., Yu J., Yang Y., Zhang F., Su W., Fan Q., Wu C., Wu S. Berberine, a potential prebiotic to indirectly promote Akkermansia growth through stimulating gut mucin secretion. Biomed. Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111595. [DOI] [PubMed] [Google Scholar]
- 230.Yang Y.N., Wang Q.C., Xu W., Yu J., Zhang H., Wu C. The berberine-enriched gut commensal Blautia producta ameliorates high-fat diet (HFD)-induced hyperlipidemia and stimulates liver LDLR expression. Biomed. Pharmacother. 2022;155 doi: 10.1016/j.biopha.2022.113749. [DOI] [PubMed] [Google Scholar]
- 231.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 232.Fogliano V., Corollaro M.L., Vitaglione P., Napolitano A., Ferracane R., Travaglia F., Arlorio M., Costabile A., Klinder A., Gibson G. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol. Nutr. Food Res. 2011;55:S44–S55. doi: 10.1002/mnfr.201000360. [DOI] [PubMed] [Google Scholar]
- 233.Chambers E.S., Preston T., Frost G., Morrison D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018;7:198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Markowiak P., Śliżewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhang K., Zeng Y., Li J., Huang Y., Zhang N., Gong Y., Xiao K., Chen J., Chen T., Qiu H., et al. Inulin alleviates atherosclerosis through improving lipid metabolism, inflammation, and gut microbiota in ApoE-knockout mice: the short-chain is more efficacious. Front. Pharmacol. 2024;15 doi: 10.3389/fphar.2024.1445528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chambers E.S., Byrne C.S., Morrison D.J., Murphy K.G., Preston T., Tedford C., Garcia-Perez I., Fountana S., Serrano-Contreras J.I., Holmes E., et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: a randomised cross-over trial. Gut. 2019;68:1430–1438. doi: 10.1136/gutjnl-2019-318424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Vulevic J., Drakoularakou A., Yaqoob P., Tzortzis G., Gibson G.R. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 2008;88:1438–1446. doi: 10.3945/ajcn.2008.26242. [DOI] [PubMed] [Google Scholar]
- 238.Gupta A., Khanna S. Fecal Microbiota Transplantation. JAMA. 2017;318:102. doi: 10.1001/jama.2017.6466. [DOI] [PubMed] [Google Scholar]
- 239.van Nood E., Vrieze A., Nieuwdorp M., Fuentes S., Zoetendal E.G., de Vos W.M., Visser C.E., Kuijper E.J., Bartelsman J.F.W.M., Tijssen J.G.P., et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 240.Khan M.Y., Dirweesh A., Khurshid T., Siddiqui W.J. Comparing fecal microbiota transplantation to standard-of-care treatment for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018;30:1309–1317. doi: 10.1097/meg.0000000000001243. [DOI] [PubMed] [Google Scholar]
- 241.Vrieze A., Van Nood E., Holleman F., Salojärvi J., Kootte R.S., Bartelsman J.F.W.M., Dallinga-Thie G.M., Ackermans M.T., Serlie M.J., Oozeer R., et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916.e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 242.Kootte R.S., Levin E., Salojärvi J., Smits L.P., Hartstra A.V., Udayappan S.D., Hermes G., Bouter K.E., Koopen A.M., Holst J.J., et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017;26:611–619.e616. doi: 10.1016/j.cmet.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 243.DeFilipp Z., Bloom P.P., Torres Soto M., Mansour M.K., Sater M.R.A., Huntley M.H., Turbett S., Chung R.T., Chen Y.B., Hohmann E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019;381:2043–2050. doi: 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
- 244.Puerarin alleviates atherosclerosis via the inhibition of Prevotella copri and its trimethylamine production. Gut. 2024;73:1934–1943. doi: 10.1136/gutjnl-2024-331880. [DOI] [PubMed] [Google Scholar]
- 245.Zhang Y., Wei Y., Han X., Shi L., Yu H., Ji X., Gao Y., Gao Q., Zhang L., Duan Y., et al. Faecalibacterium prausnitzii prevents age-related heart failure by suppressing ferroptosis in cardiomyocytes through butyrate-mediated LCN2 regulation. Gut Microbes. 2025;17 doi: 10.1080/19490976.2025.2505119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Li J., Wang S.Y., Yan K.X., Wang P., Jiao J., Wang Y.D., Chen M.L., Dong Y., Zhong J.C. Intestinal microbiota by angiotensin receptor blocker therapy exerts protective effects against hypertensive damages. iMeta. 2024;3 doi: 10.1002/imt2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chehoud C., Dryga A., Hwang Y., Nagy-Szakal D., Hollister E.B., Luna R.A., Versalovic J., Kellermayer R., Bushman F.D. Transfer of Viral Communities between Human Individuals during Fecal Microbiota Transplantation. mBio. 2016;7 doi: 10.1128/mBio.00322-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tseng C.H., Wong S., Yu J., Lee Y.Y., Terauchi J., Lai H.C., Luo J.C., Kao C.Y., Yu S.L., Liou J.M., et al. Development of live biotherapeutic products: a position statement of Asia-Pacific Microbiota Consortium. Gut. 2025;74:706–713. doi: 10.1136/gutjnl-2024-334501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Button J.E., Cosetta C.M., Reens A.L., Brooker S.L., Rowan-Nash A.D., Lavin R.C., Saur R., Zheng S., Autran C.A., Lee M.L., et al. Precision modulation of dysbiotic adult microbiomes with a human-milk-derived synbiotic reshapes gut microbial composition and metabolites. Cell Host Microbe. 2023;31:1523–1538.e10. doi: 10.1016/j.chom.2023.08.004. [DOI] [PubMed] [Google Scholar]
- 250.van der Lelie D., Oka A., Taghavi S., Umeno J., Fan T.J., Merrell K.E., Watson S.D., Ouellette L., Liu B., Awoniyi M., et al. Rationally designed bacterial consortia to treat chronic immune-mediated colitis and restore intestinal homeostasis. Nat. Commun. 2021;12:3105. doi: 10.1038/s41467-021-23460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ducarmon Q.R., Kuijper E.J., Olle B. Opportunities and Challenges in Development of Live Biotherapeutic Products to Fight Infections. J. Infect. Dis. 2021;223:S283–S289. doi: 10.1093/infdis/jiaa779. [DOI] [PubMed] [Google Scholar]
- 252.Huang W., Zhu W., Lin Y., Chan F.K.L., Xu Z., Ng S.C. Roseburia hominis improves host metabolism in diet-induced obesity. Gut Microbes. 2025;17 doi: 10.1080/19490976.2025.2467193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Qiao S., Wang T., Sun J., Han J., Dai H., Du M., Yang L., Guo C.J., Liu C., Liu S.J., Liu H. Cross-feeding-based rational design of a probiotic combination of Bacterides xylanisolvens and Clostridium butyricum therapy for metabolic diseases. Gut Microbes. 2025;17 doi: 10.1080/19490976.2025.2489765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Qiao S., Liu C., Sun L., Wang T., Dai H., Wang K., Bao L., Li H., Wang W., Liu S.J., Liu H. Gut Parabacteroides merdae protects against cardiovascular damage by enhancing branched-chain amino acid catabolism. Nat. Metab. 2022;4:1271–1286. doi: 10.1038/s42255-022-00649-y. [DOI] [PubMed] [Google Scholar]
- 255.Wang J., Zhang H., Yuan H., Chen S., Yu Y., Zhang X., Gao Z., Du H., Li W., Wang Y., et al. Prophylactic Supplementation with Lactobacillus Reuteri or Its Metabolite GABA Protects Against Acute Ischemic Cardiac Injury. Adv. Sci. 2024;11 doi: 10.1002/advs.202307233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Conraads V.M., Jorens P.G., De Clerck L.S., Van Saene H.K., Ieven M.M., Bosmans J.M., Schuerwegh A., Bridts C.H., Wuyts F., Stevens W.J., et al. Selective intestinal decontamination in advanced chronic heart failure: a pilot trial. Eur. J. Heart Fail. 2004;6:483–491. doi: 10.1016/j.ejheart.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 257.Battson M.L., Lee D.M., Jarrell D.K., Hou S., Ecton K.E., Weir T.L., Gentile C.L. Suppression of gut dysbiosis reverses Western diet-induced vascular dysfunction. Am. J. Physiol. Endocrinol. Metab. 2018;314:E468–E477. doi: 10.1152/ajpendo.00187.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Cao Z., Sugimura N., Burgermeister E., Ebert M.P., Zuo T., Lan P. The gut virome: A new microbiome component in health and disease. EBioMedicine. 2022;81 doi: 10.1016/j.ebiom.2022.104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.de Jonge P.A., Wortelboer K., Scheithauer T.P.M., van den Born B.J.H., Zwinderman A.H., Nobrega F.L., Dutilh B.E., Nieuwdorp M., Herrema H. Gut virome profiling identifies a widespread bacteriophage family associated with metabolic syndrome. Nat. Commun. 2022;13:3594. doi: 10.1038/s41467-022-31390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yang K., Niu J., Zuo T., Sun Y., Xu Z., Tang W., Liu Q., Zhang J., Ng E.K.W., Wong S.K.H., et al. Alterations in the Gut Virome in Obesity and Type 2 Diabetes Mellitus. Gastroenterology. 2021;161:1257–1269.e13. doi: 10.1053/j.gastro.2021.06.056. [DOI] [PubMed] [Google Scholar]
- 261.Khan Mirzaei M., Deng L. New technologies for developing phage-based tools to manipulate the human microbiome. Trends Microbiol. 2022;30:131–142. doi: 10.1016/j.tim.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 262.Mirzaei M.K., Maurice C.F. Ménage à trois in the human gut: interactions between host, bacteria and phages. Nat. Rev. Microbiol. 2017;15:397–408. doi: 10.1038/nrmicro.2017.30. [DOI] [PubMed] [Google Scholar]
- 263.Biazzo M., Deidda G. Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases. J. Clin. Med. 2022;11 doi: 10.3390/jcm11144119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Gordillo Altamirano F.L., Barr J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019;32 doi: 10.1128/cmr.00066-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Strathdee S.A., Hatfull G.F., Mutalik V.K., Schooley R.T. Phage therapy: From biological mechanisms to future directions. Cell. 2023;186:17–31. doi: 10.1016/j.cell.2022.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]