Highlights
-
•
Cutaneous extranodal NK/T-cell lymphoma (ENKTL) is divided into primary (pc) or secondary (sc).
-
•
Interferon signaling, TCR signaling, and programmed cell death were enriched in pcENKTL.
-
•
Tumor microenvironment was different between pcENKTL and scENKTL.
-
•
Immune response and antigen presentation were increased in pcENKTL with complete remission.
-
•
scENKTL with short survival showed increased cancer-associated fibroblasts and BIRC5.
Keywords: lymphoma, Natural killer cell, Prognosis, Skin
Abstract
Introduction
Extranodal NK/T-cell lymphoma (ENKTL) with cutaneous involvement can be divided into primary cutaneous ENKTL (pcENKTL) and secondary cutaneous involvement (scENKTL). Despite different originations, the clinical and histopathological findings are indistinguishable. Moreover, the prognosis determinants in each entity have yet to be established. We investigated differences in spatially resolved transcriptome profiles between pcENKTL and scENKTL.
Materials and methods
Seven samples with 24 regions of interest were selected for pcENKTCL and scENKTL, using CD56 and CD3 morphology markers.
Results
In CD56-positive tumor cell areas, we detected 91 upregulated differentially expressed genes (DEGs) and 27 downregulated DEGs in pcENKTL compared with scENKTL. Protein–protein interaction network revealed significant enrichment of interferon signaling, T cell receptor signaling, and programmed cell death in pcENKTL. Moreover, significant enrichment of translation pathways and nonsense-mediated decay in scENKTL was observed. In immune cell areas, myeloid dendritic cell (p = 0.044) and M1 macrophage (p = 0.039) numbers were increased in pcENKTL. Conversely, neutrophil (p = 0.030) and M2 macrophage (p = 0.030) numbers were increased in scENKTL. We found an increased immune response and antigen presentation in pcENKTL with complete remission, while pcENKTL with progressive disease showed increased angiogenesis. Alternatively, scENKTL with long survival showed increased HLA expression and CD8 memory T cells and M1 macrophages, while scENKTL with short survival showed increased cancer-associated fibroblasts and BIRC5.
Conclusion
Overall, the differences in transcriptomic expression and tumor microenvironment between pcENKTL and scENKTL, as well as subgroups based on the prognosis could widen our understanding of the biological characteristics of ENKTL.
Graphical abstract
Introduction
Nasal extranodal NK/T-cell lymphoma (ENKTL) is a prototype of the Epstein–Barr virus (EBV)-driven T and NK cell lymphoproliferative disorder—a rare and aggressive lymphoma that is histopathologically characterized by prominent angioinvasion and angiodestruction [1]. Furthermore, nasal ENKTL has a high predilection for Asian and Latin American populations. It is related to the genetic background of this population rather than the endemic prevalence of EBV or specific EBV subtypes [1,2]. HLA-DPB1 was identified in a previous genome-wide association study as a susceptibility gene of ENKTL [3]. More recent research involving 700 cases of ENKTL discovered two novel loci, IL18RAP and HLA-DRB1 [4]. Both of two loci were significantly associated with increased risk of ENKTL [4]. This study emphasized that antigen binding and presentation and IL18 signals involving STAT pathways could be important for the carcinogenesis and progression of ENKTL [4]. Furthermore, several other studies have revealed several recurrent mutations associated with JAK–STAT signaling (STAT3, STAT5b, JAK3), tumor suppressors (TP53, MGA), RAS–MAPK signaling (NOTCH3, EPHA1, PTPRQ, PTPRK, GNAQ), and apoptosis (FAS) [[5], [6], [7], [8], [9], [10], [11], [12], [13]]. Moreover, gene expression analysis showed that several upregulated genes play a significant role in the pathogenesis of ENKTL, including BIRC5, MYC, PD-L1, RUNX3, AURKA, and PDGFRA [[14], [15], [16], [17], [18], [19], [20], [21]].
ENKTL with cutaneous involvement can be classified into two distinct subsets: primary cutaneous ENKTL (pcENKTL), which initially presents in the skin, or nasal ENKTL, which is accompanied by secondary cutaneous involvement (scENKTL) as a systematic disease [22]. A study by the International Peripheral T-cell Lymphoma Project revealed that 26 % of cases were extranasal in origin, most frequently in the intestine (37 %), followed by skin (26 %) [23]. Conversely, 25.1 % of nasal ENKTL developed distant metastasis after initial treatment, and the most frequent distant metastatic sites were skin and soft tissue (32.4 %) [24]. Interestingly, pcENKTL and scENKTL are clinically, histopathologically, and immunohistochemically indistinguishable despite their different originations [25,26]. Although a few studies showed that patients with pcENKTL seem to be associated with better survival than those with scENKTL, the median overall survival of pcENKTL and scENKTL ranged from 1 year to 3 years [26,27]. However, the reasons behind the aggressiveness of pcENKTL and scENKTL are largely unknown.
This study used spatial transcriptomic technology to select tumor and immune cell areas and compared transcriptomic expression and tumor microenvironment of pcENKTL and scENKTL. Moreover, we investigated the differences within each group based on the clinical course to identify prognoses-related factors.
Materials and methods
Patient selection and information
We found ENKTL patients with skin involvement who had received skin biopsy with immunohistochemical staining. We selected 7 pcENKTL patients and 7 scENKTL patients (Table S8). The clinical and follow-up data were extracted from medical records and photographs at the Department of Dermatology, Asan Medical Center, from January 2015 to December 2023. The histopathological data of these patients were retrieved from H&E and immunohistochemical slides. The staging was based on the Ann Arbor staging system. The clinicopathological features of the patients involved in this study are described in Supplementary Table 6. This study was approved by the institutional review board of Asan Medical Center (IRB no. 2021-0648).
Spatial transcriptome profiling
The spatial transcriptomic analysis workflow is described in Fig. S1. We performed spatial transcriptome profiling using formalin-fixed paraffin-embedded tumor tissues with 5 μm thicknesses, which were taken from 14 patients with ENKTL. The tissue samples were first stained with large panels of premixed biological probes, each containing a unique, UV-cleavable DNA oligonucleotide barcode. Three fluorescence-labeled morphological markers (CD56, CD3, and DNA) were used to visualize the tissue boundary. We selected 11 and 12 CD56-positive regions of interest (ROIs) (tumor cell areas) from pcENKTL samples and scENKTL samples, respectively. The selection of ROI was based on the expression of morphological markers and H&E slides (Fig. S2). In addition, we selected 13 and 12 CD56-negative/CD3-positive ROIs from pcENKTL samples and scENKTL samples, respectively (Fig. S2). Ultraviolet illumination of 48 defined ROIs led to the release of ROI-tagged DNA barcodes that were taken up by a microcapillary system. Subsequently, these were dispensed into a 96-well microtiter plate for further analysis using the GeoMx® DSP analysis software version 2.1 (NanoString Technologies, Seattle, WA).
We used 18,676 genes from GeoMx Human Whole Transcriptome Atlas Human RNA for Illumina Systems (NanoString Technologies, Seattle, WA). Sequencing quality for sufficient saturation to ensure low-expression sensitivity was conducted. Furthermore, normalization was performed using the third quartile (Q3) for differences in cellularity and ROI size. This processes were performed in and recommended by GeoMx® DSP analysis software.
ROI-based gene expression analysis
For dimensional reduction, principal component analysis and t-SNE were conducted using the DSP DA script (version 1.2) of the GeoMx® DSP analysis software (version 2.1) in both CD56-positive and CD56-negative/CD3-positive segments.
DEGs were screened out using the GeoMx® DSP analysis software with the criteria of the fold-change thresholds >2 or <0.5 and adjusted p < 0.05 in both CD56-positive and CD56-negative/CD3-positive segments. All DEGs are displayed as a volcano plot using ggplot2 in R version 4.3.3. Protein–protein interaction network analysis was performed using the Search Tool for the Retrieval of Interacting Genes (https://string-db.org/) to evaluate the main functions and correlation between the DEG protein products. Functional enrichments of the network were based on the Reactome pathway terms. Clustering was performed using the K-means clustering with the cluster number of 3. Database for annotation, visualization, and integrated discovery (DAVID) pathway analysis was also performed using the National Institutes of Health DAVID Bioinformatics (https://david.ncifcrf.gov/) to manifest the functions of DEGs.
Next, pathway analysis was conducted using the whole genes of CD56-positive ROIs of pcENKTL and scENKTL samples with the GeoMx® DSP analysis software, which applied the fast gene set enrichment analysis (fGSEA) package for R (available at https://github.com/ctlab/fgsea) from the Bioconductor and Reactome database.
Normalized expressions of representative genes involved in tumorigenesis or maintenance of oncogenic signal, JAK3, STAT3, PDGFRA, RUNX3, MYC, AURKA, EZH2, NOTCH1, NFKB1, NFKB2, CD274, HLA-DRB1, HLA-DPB1, IL18RAP, ATR, TP53, VEGFA, HGF, MET, CD38, FAS, BIRC5, and BCOR were analyzed in CD56-postive tumor ROIs. Immune checkpoint molecules, including PD-1, LAG3, TIM-3, CTLA-4, and TIGIT, were evaluated in CD56-negative/CD3-positive immune cell ROIs.
Immune cell deconvolution using the GeoMx® DSP analysis software with the gene set considered cell-type-specific was performed in CD56-negative/CD3-positive areas to identify the abundance of 14 immune cell populations (macrophages, mast cells, B cells, plasma cells, CD4+ T cells, CD8+ T cells, NK cells, plasmacytoid and myeloid dendritic cells, monocytes, neutrophils, regulatory T cells, endothelial cells, and fibroblasts). Moreover, we used CIBERSORT (https://cibersort.stanford.edu/) to determine the abundance score for macrophage subtypes.
Immunohistochemical staining
STAT1 and CXCL9 were selected from the DEGs between pcENKTL and scENKTL for IHC analysis. PD-L1 and CD163, a marker for M2 macrophage were also selected. Paraffin-embedded sections were immunostained with anti-STAT1 (1:3000, ab47425, Abcam, Cambridge, USA), anti-CXCL9 (1:400, ab202961, Abcam, Cambridge, USA), and anti-PD-L1 (1:1000, 315M-96, Cell marque, Rocklin, California, USA), anti-CD163 (1:400, NOVO, Newcastle, UK). IHC intensity was rated 0–3 based on expression using a semiquantitative method, as follows: 0, 1, 2, and 3, indicating <10 %, 10–30 %, 30–50 %, and >50 %, respectively, of the total area. The results of IHC staining were evaluated by two independent dermatologists (W.J.L. and M.E.C.) at × 100 magnification.
Statistical analysis
DEG analysis was conducted via an independent t-test using the GeoMx® DSP analysis software. Two-sided P-values <0.05 were considered statistically significant. The Mann–Whitney U test was used to compare normalized gene expression levels between two groups, and the rank biserial correlation coefficient (r) was calculated using the r = Z (z-statistic from the Mann–Whitney U test)/√N (sample size) formula. Multiple hypothesis testing in all analyses was controlled using the Benjamini–Hochberg procedure, and an FDR cutoff of <0.05 was considered statistically significant. When comparing the normalized gene expression in each group, we conducted the Mann–Whitney U test due to small sample sizes and satisfaction of normality assumption. Statistical analysis was performed using R version 4.3.3.
Results
Transcriptomic differences between pcENKTL and scENKTL in tumor cell areas
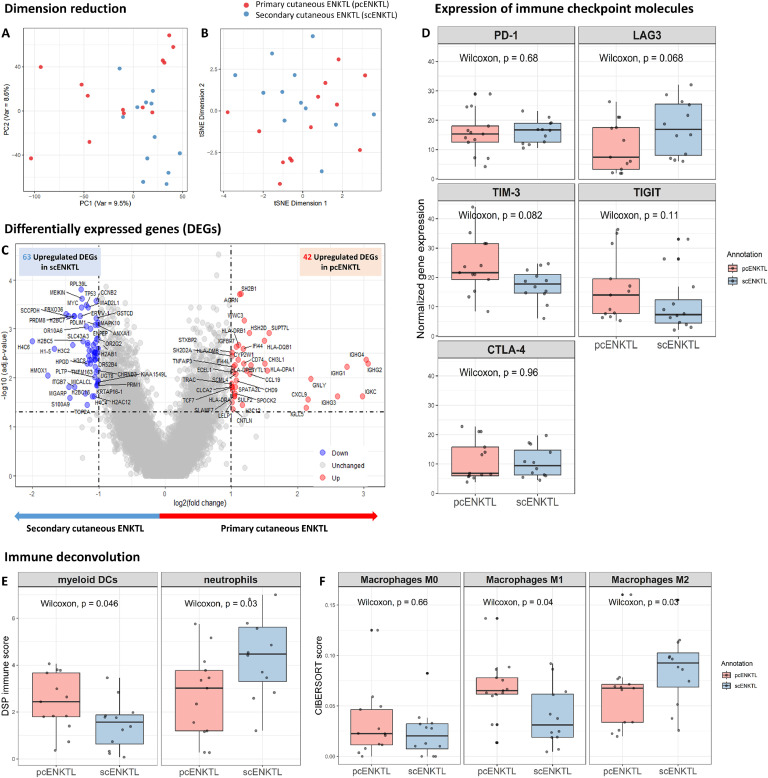
The principal component analysis and t-distributed stochastic neighbor embedding (t-SNE) results showed that pcENKTL and scENKTL formed distinct clusters in tumor cell segments (Fig. 1a and b). Further, differentially expressed genes (DEGs) analysis identified 91 upregulated DEGs and 27 downregulated DEGs in pcENKTL compared to scENKTL (Fig. 1c and Table S1) Protein–protein interaction network analysis revealed that upregulated DEGs in pcENKTL were associated with interferon-γ signaling regulation and ZAP-70 translocation to the immunological synapse (T cell receptor signaling) (Fig. 1d and 1e; Table S2). Pathway analysis showed 328 pathways were upregulated in pcENKTL compared to scENKTL (Table S3). Representative pathways were neutrophil degranulation (normalized enrichment score (NES) = 6.473, adjusted p = 0.001), interferon-γ signaling (NES = 6.410, adjusted p = 0.001), interferon signaling (NES = 6.140, adjusted p = 0.001), interferon-α/β signaling (NES = 5.396, adjusted p = 0.001), antigen processing-cross presentation (NES = 5.062, adjusted p = 0.001), T cell receptor signaling (NES = 3.945, adjusted p = 0.001), and programmed cell death (NES = 3.760, adjusted p = 0.001) Fig. 1f). Alternatively, 147 pathways were upregulated in scENKTL compared to pcENKTL (Table S3). Representative pathways were shown to include eukaryotic translation elongation (NES = 5.242, adjusted p = 0.001), viral mRNA translation (NES = 5.233, adjusted p = 0.001), peptide chain elongation (NES = 5.212, adjusted p = 0.001), eukaryotic translation termination (NES = 5.141, adjusted p = 0.001), and nonsense-mediated decay, independent of the exon junction complex (NES = 4.890, adjusted p = 0.001) (Fig. 1f). When comparing normalized expression of main genes contributing to malignant transformation in ENKTL, we found STAT3 (r = 0.462, p = 0.027), NOTCH3 (r = 0.449, p = 0.032), PD-L1 (r = 0.507, p = 0.016), IL18RAP (r = 0.552, p = 0.007), HLA-DRB1 (r = 0.680, p < 0.001), and HLA-DPB1 (r = 0.552, p = 0.007) were significantly upregulated, whereas TP53 (r = -0.462, p = 0.027) was significantly downregulated in pcENKTL compared to scENKTL (Fig. 1g).
Fig. 1.
Digital spatial transcriptome analysis of CD56-positive tumor cell areas revealed that transcriptomic expression differed between primary cutaneous NK/T cell lymphoma (pcENKTL) and secondary cutaneous NK/T cell lymphoma (scENKTL). A dimension reduction plot of principal component analysis (a) and t-distributed stochastic neighbor embedding (b) shows that pcENKTL (marked in red) and scENKTL (marked in blue) form distinct clusters in the CD56-positive area. (c) Volcano plot reveals differentially expressed genes with fold-changes >2 and p < 0.05 in CD56-positive tumor cell areas. Protein–protein interaction network analysis with clustering using upregulated DEGs in pcENKTL shows two main clusters, including a cluster of interferon-γ (d) and translocation of ZAP-70 to immunological synapse (e). (f) Pathway analysis shows significantly upregulated or downregulated pathways in pcENKTL compared to scENKTL. (g) Normalized expression of representative oncogenic genes in ENKTL.
Differences in tumor microenvironment and immune checkpoint inhibitors between pcENKTL and scENKTL
This study found that immune cell ROIs in pcENKTL and scENKTL did not form separate clusters in the principal component analysis and t-SNE analyses (Fig. 2a and b). DEG analysis of immune cell areas revealed that 42 genes were significantly upregulated, whereas 63 genes were significantly downregulated in pcENKTL compared to scENKTL (Fig. 2c). The expression of immune checkpoint molecules in immune cell areas was not significantly different between pcENKTL and scENKTL (Fig. 2d). Immune cell deconvolution from the DSP profiler found that myeloid dendritic cells (p = 0.046) were significantly increased, whereas neutrophils (p = 0.03) were significantly decreased in pcENKTL compared to scENKTL (Fig. 2e). We used the Cell-type Identification by Estimating Relative Subsets of RNA Transcripts (CIBERSORT) to uncover the differences in macrophage subtypes, which revealed that M1 macrophages (p = 0.04) were significantly increased. Comparatively, M2 macrophages (p = 0.03) were significantly decreased in pcENKTL compared to scENKTL (Fig. 2f).
Fig. 2.
Digital spatial transcriptome analysis of CD56-negative/CD3-positive immune cell areas reveals that the tumor microenvironment differed between primary cutaneous NK/T cell lymphoma (pcENKTL) and secondary cutaneous NK/T cell lymphoma (scENKTL). A dimension reduction plot of principal component analysis (a) and t-distributed stochastic neighbor embedding (b) reveal that pcENKTL (marked in red) and scENKTL (blue) did not form separate clusters in CD56-negative/CD3-positive area. (c) Volcano plot reveals differentially expressed genes with fold-changes >2 and p < 0.05 in CD56-negative/CD3-positive immune cell areas. (d) Immune checkpoint molecule expression did not differ between pcENKTL and scENKTL. (e) Immune cell deconvolution using a digital spatial profiler reveals that myeloid dendritic cells significantly increase while neutrophils significantly decrease in pcENKTL compared to scENKTL. (f) Cell-type identification by estimating relative subsets of RNA transcripts (CIBERSORT) shows that M1 macrophages significantly increase while M2 macrophages significantly decrease in pcENKTL compared to scENKTL.
Transcriptomic analysis of a pcENKTL patient in complete remission
We found a pcENKTL patient with very low blood EBV-DNA at the time of diagnosis (40 copies/mL) who underwent complete remission after standard treatment, with an overall survival of 228 months. Comparatively, most pcENKTL patients who exhibit high blood EBV-DNA at the time of diagnosis (median 6200 copies/mL) show progression after standard chemotherapy and, ultimately, die of the disease. In the pcENKTL with complete remission compared to pcENKTL with progressive disease, 153 upregulated DEGs and 151 downregulated DEGs were observed (Fig. 3a and Table S4). Moreover, DAVID pathway analysis using upregulated DEGs identified 28 significant pathways in pcENKTL with complete remission (Fig. 3b and Table S4). The top 5 pathways included the immune response (fold enrichment = 7.082, false discovery rate (FDR) <0.001), peptide antigen assembly with MHC class II protein complex (fold enrichment = 56.923, FDR <0.001), antigen processing and presentation (fold enrichment = 24.914, FDR <0.001), antigen processing and presentation of exogenous peptide antigen via MHC class II (fold enrichment = 33.576, FDR <0.001), and antigen processing and presentation of exogenous peptide antigen via MHC class II (fold enrichment = 45.538, FDR <0.001). On the other hand, DAVID analysis using DEGs found two significant upregulated pathways in pcENKTL with progressive disease compared to pcENKTL with complete remission. The two pathways were angiogenesis (fold enrichment = 7.045, FDR < 0.001) and cell adhesion (fold enrichment = 3.718, FDR = 0.028) (Table S5). We found that JAK–STAT pathway-related genes, JAK3 (r = 0.554, p = 0.012) and STAT5B (r = 0.185, p = 0.012), RUNX3 (r = 0.554, p = 0.024), TP53 (r = 0.369, p = 0.012) and HLA-DRB1 (r = 0.308, p = 0.024) were increased, whereas the tumor suppressor gene GNAQ (r = -0.062, p = 0.012) was significantly decreased in pcENKTL with complete remission compared to pcENKTL with progressive disease (Fig. 3c).
Fig. 3.
Digital spatial transcriptome analysis of primary cutaneous NK/T cell lymphoma (pcENKTL) with complete remission and pcENKTL with progressive disease. (a) A volcano plot shows differentially expressed genes (DEGs) in CD56-positive tumor areas comparing pcENKTL with complete remission and pcENKTL with progressive disease. (b) Pathway analysis using upregulated DEGs in pcENKTL with complete remission. (c) Normalized expression of representative oncogenic genes from CD56-positive tumor areas comparing pcENKTL with complete remission and pcENKTL with progressive disease.
Differences in transcriptomic expression and tumor microenvironment according to scENKTL survival outcomes
We divided the scENKTL patients into long-term scENKTL survival and short-term scENKTL survival based on an overall survival of 2 years. We found that 57 genes were significantly upregulated, and 53 were significantly downregulated in the short-term scENKTL cohort survival compared to the long-term survival cohort (Fig. 4a and Table S6). In the protein–protein interaction network analysis of 36 upregulated DEGs in the short-term scENKTL survival group, protein localization to CENP-A containing chromatin (Fig. S3a and Table S7) was the main cluster. In comparison, the main cluster of downregulated DEGs (9 DEGs) related to antigen processing and presentation of endogenous peptide antigen via MHC class Ⅱ (Fig. S3b and Table S7). HLA-DRB1 (r = -0.721, p = 0.009) and HLA-DPB1 (r = -0.667, p = 0.018) were significantly downregulated in relation to short-term survival from scENKTL, compared to long-term survival, whereas BIRC5 (r = 0.614, p = 0.036) was significantly upregulated (Fig. 4b). In addition, cancer-associated fibroblast marker, FAP (r = 0.680, p = 0.018) was significantly upregulated, while immune checkpoint molecules, LAG (r = -0.727, p = 0.01) and CTLA-4 (r = -0.820, p = 0.003) were significantly downregulated in immune cell areas of short-survival scENKTL compared to long-survival scENKTL (Fig. 4c and 4d). Immune cell composition of short-term scENKTL survival was different from long-survival from scENKTL (Fig. 4e). DSP immune cell analysis found that CD8 memory T cells and macrophages were significantly decreased in short-term survival of scENKTL compared to long-survival (Fig. 4f). In the CIBERSORT, M1 macrophages were significantly decreased in short-survival scENKTL compared to long-survival (Fig. 4g).
Fig. 4.
Digital spatial transcriptome analysis of secondary cutaneous NK/T cell lymphoma (scENKTL) with an overall survival of at least 2 years and scENLKTL with an overall survival of less than 2 years. (a) The volcano plot shows differentially expressed genes in CD56-positive tumor areas. (b) Normalized expression of representative oncogenic genes in CD56-positive tumor areas. Normalized expression of FAP, cancer-associated fibroblast (c), and immune checkpoint molecules (d) in CD56-negative/CD3-positive immune areas. Heatmap of immune cell deconvolution using digital spatial profiler (e) and pairwise box plot shows that CD8 memory T cells and macrophages are increased in scENKTL patients with overall survival of at least 2 years compared to scENLKTL with overall survival less than 2 years. (g) Cell-type identification by estimating relative subsets of RNA transcripts (CIBERSORT) shows that M1 macrophages significantly increase in scENKTL samples with an overall survival of at least 2 years compared to scENLKTL with an overall survival of less than 2 years.
Immunohistochemical staining confirms the differences between pcENKTL and scENKTL
We found that the top 10 hub DEGs between pcENKTL and scENKTL included CXCL9, STAT1, GBP1, CXCL11, TNFSF10, IFIH1, IRF1, CXCL10, MX1, and IFIT3. In the immunohistochemical staining study, the intense expressions of STAT1 and CXCL9 were more frequently observed in pcENKTL compared to scENKTL (p = 0.046 and p = 0.053, respectively) (Fig. S4a, b). Moreover, the intensity of PDL-1 expression was higher in pcENKTL than scENKTL (p = 0.096) (Fig. S4c). Given that CD163 is associated with M2 macrophage, we investigated the expression of CD163 in ENKTL patients. The staining intensity of CD163 was significantly higher in scENKTL than pcENKTL (p = 0.012) (Fig. S4d).
Discussion
ENKTL is a rare and aggressive lymphoma with poor survival outcomes. Additionally, based on a large-scale randomized controlled trial, there is currently no standard therapy owing to its rarity [28]. Moreover, the international prognostic index, widely used to predict prognosis in various non-Hodgkin lymphomas, could not discriminate between ENKTL risk groups. ENKTL is unique since it presents mostly as a localized disease (76 %) with a low rate of bone marrow involvement (6 %); however, it frequently manifests constitutional symptoms in localized disease (29 %) and shows rapid deterioration of general conditions [29,30]. Therefore, we suspected that pcENKTL and scENKTL adopted their unique oncogenic signaling and tumor microenvironment, resulting in aggressive biological features.
We used the protein–protein interaction network to identify that the main cluster of upregulated DEGs in pcENKTL, which was associated with regulating interferon-γ signaling. Previous studies revealed that ENKTL tumor cells secrete interferon-γ, leading to PD-L1 expression in either macrophages or tumor cells [20]. We also found that PD-L1 was significantly increased in tumor cell areas in pcENKTL compared to scENKTL; however, PD-1 expression in immune cell areas was not significantly different, consistent with previous research [18,31]. We found that JAK2, STAT1, and IRF-1 were included in the upregulated DEGs. These observations were consistent with an earlier report that interferon-γ induced PD-L1 overexpression through JAK2/STA1/IRF-1 signaling in EBV-positive gastric carcinoma [32]. A recent study showed that the JAK2/STAT5 pathway significantly increases PD-L1 in ENKTL patients [33]. However, the prognostic impact of PD-L1 is controversial since high expression was related to inferior treatment response in the early stage in contrast to better survival in the advanced stage [13,34]. Conversely, PD-L1 expression levels could offer a predictive value in various cancers for patient responses to immune checkpoint inhibitor treatment; thus, there is a possibility that pcENKTL patients may be more responsive to anti-PD-L1 immunotherapy [[35], [36], [37]]. Moreover, the secondary cluster of upregulated DEGs in pcENKTL related to antigen processing and presentation and included HLA-DRB1, HLA-DPB1, HLA-DQB1, HLA-DPA1, and HLA-DMA. Previous genome-wide association studies, including many ENKTL patients, revealed that HLA-DPB1 and HLA-DRB1 mutations were associated with ENKTL development. Furthermore, IL18RAP was significantly increased in pcENKTL compared to scENKTL. Previous studies implied IL18RAP could induce ENKTL cell proliferation by regulating cell cycle progression. Meanwhile, high-serum IL18 levels were positively correlated with high interferon-γ levels and poor prognosis [4,38]. Based on this observation, the adoption of IL18 inhibitors or IL18 binding protein agonists could be considered in the treatment of pcENKTL [39].
On the other hand, we found that translation pathways and nonsense-mediated decay pathways were significantly increased in scENKTL compared to pcENKTL. Notably, increased translation reflects the high proliferative status of cancers and has a direct role in tumorigenesis [40]. Nonsense-mediated decay pathways could play a protumorigenic role by selectively downregulating proteins involved in apoptosis, cell growth, and cell migration [41]. Since several inhibitors targeting nonsense-mediated decay are already available, adopting these compounds could provide a new therapeutic opportunity for scENKTL, ultimately increasing the immunogenicity of tumors [42].
In addition, we found that M1 macrophages and myeloid dendritic cells significantly increased in pcENKTL, while M2 macrophages and neutrophils significantly increased in scENKTL. Currently, limited studies have been conducted regarding the role of immune cells in ENKTL. Myeloid or classical-type dendritic cells exert their primary role as antigen presenters responsible for the induction of innate and adaptive immune responses against tumor cells [43]. A recent single-cell study revealed that increased expressions of the CD206 protein, a marker of M2 macrophage, were correlated with reduced overall survival [44]. A previous review reported that M2 macrophages secrete IL‑10, IL‑13, TGF‑β, VEGF, MMPs, and other growth factors to promote angiogenesis and extracellular matrix remodeling. In turn, it facilitates tumor growth and metastasis while suppressing immunity [45]. In addition, tumor-associated macrophages express PD‑L1, engaging PD‑1 on T cells to suppress their activation, as well as promoting regulatory T cells through IL-10 and TGF‑β [46,47]. Therefore, increased M2 macrophages in scENKTL could contribute to poor survival outcomes in scENKTL [48,49]. In addition, a study indicated that the neutrophil/lymphocyte ratio was associated with survival outcomes relating to overall survival and progression-free survival in early-stage ENKTL [50]. Lastly, we found several immunoglobulin genes upregulated in pcENKTL from both tumor and immune cell areas. A previous study revealed that high infiltration of CD20+ B cells in ENKTL was associated with improved survival outcomes [51].
Among the pcENKTL patients, we found a patient with an extraordinary clinical course with low EBV-DNA at the time of diagnosis who obtained complete remission after treatment. The upregulated DEGs in pcENKTL with complete remission were associated with antigen processing and presentation (MHC class Ⅱ) and innate and adaptive immune responses. Although several oncogenic genes were increasingly expressed (JAK3, STAT5B, RUNX3) in this group, active tumor antigen presentation and immune reaction against tumor cells may have more impact on the clinical course of pcENKTL patients. In addition, we found that angiogenesis was significantly upregulated in the DAVID analysis from DEGs in pcENKTL with progressive disease. In line with the angiocentric growth pattern, angiogenesis-related genes were overexpressed in ENKTL compared to other peripheral T cell lymphomas or normal NK cells [52,53]. This result suggests the potential for adopting anti-angiogenic agents, including anti-vascular endothelial growth factor (VEGF), as an effective therapeutic target, especially for progressive pcENKTL.
We discovered that scENKLT with overall survival under 2 years showed decreased HLA-associated genes and increased BIRC5, which encodes survivin and is expressed in most ENKTL patients; moreover, it is known to inhibit apoptosis and is associated with poor survival [54,55]. Moreover, in immune cell areas, FAP, a cancer-associated fibroblast maker, was significantly increased while LAG3 and CTLA4 immune checkpoint molecules were decreased. A recent study using PET/CT with radiolabeled FAP inhibitors showed that the standard uptake valuemax of ENKTL was relatively higher [56]. Further studies are required to elucidate the role of cancer-associated fibroblasts in the aggressiveness of ENKTL. If validated, cancer-associated fibroblasts could be a promising target of ENKTL treatments. Although studies regarding the expression of immune checkpoint molecules in ENKTL are limited, LAG-3 was expressed in 95% of ENKTL in previous analyses [57]. This study also unveiled that scENKTL, with overall survival of at least 2 years, had increased CD8 memory T cells and M1 macrophages in immune cell areas. This observation is consistent with previous studies that reported a correlation between elevated CD8 T cells in the tumor microenvironment and long-term disease control in numerous cancer types [[58], [59], [60]].
We acknowledge that this study harbors several limitations. Firstly, we only included a limited number of patients since we had to carefully select patients with rare ENKTL subtypes that included cutaneous involvement with definite diagnosis and origination; slides were meticulously reviewed for the selection of ROIs. Since every person has a different genetic background, small sample sizes can result in reduced statistical power. Moreover, small sample size could increase the risk of type 2 errors and increase variability, which is often influenced by outliers [61]. In addition, small samples may unintentionally omit important subgroups, potentially leading to an increased possibility of bias in subgroup analyses. Secondly, the pcENKTL and scENKTL patients were at different stages, which could influence the transcriptomic expression and tumor microenvironment. Moreover, patients received different treatments due to a lack of standard guidelines and various general health statuses and clinical features. In addition, we could not analyze the patients further based on the plasma EBV level due to the limited number of patients. Lastly, we could not definitely differentiate between malignant NK cells and benign NK cells despite utilizing morphology markers and reviewing histopathological slides.
In conclusion, we observed the differences between pcENKTL and scENKTL in terms of transcriptomic expression and tumor microenvironment (Fig. 5). Moreover, we found that ENKTL patients with poor prognosis had different immune reactions against tumors and oncogenic signals. Our findings might increase our understanding of the aggressive nature of ENKTL. Moreover, understanding the increased pathways and the expression levels of important therapeutic targets in each subgroup of ENKTL will be a very crucial initial step for additional clinical research and the development of customized treatments for ENKTL subgroups.
Fig. 5.
Schematic diagram of the transcriptomic expression and a tumor microenvironment that were significantly different between primary cutaneous NK/T cell lymphoma (pcENKTL) and secondary cutaneous NK/T cell lymphoma (scENKTL) as well as pcENLT and scENKTL subgroups, according to the clinical course.
CRediT authorship contribution statement
Myoung Eun Choi: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Hee Joo Yang: Writing – review & editing, Writing – original draft, Resources, Formal analysis, Conceptualization. Ji Hun Choi: Resources, Methodology, Investigation. Ik Jun Moon: Resources. Joon Min Jung: Validation. Chong Hyun Won: Software, Project administration. Sung Eun Chang: Software. Mi Woo Lee: Validation. Woo Jin Lee: Writing – review & editing, Validation, Supervision, Project administration, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The author is an Editorial Board Member/Editor-in-Chief/Associate Editor/Guest Editor for this journal and was not involved in the editorial review or the decision to publish this article.
Acknowledgments
Funding sources
This work was supported by grants from the National Research Foundation of Korea (NRF; grant number: NRF-2023R1A2C100730311), which are funded by the Ministry of Science and Information Technology (MSIT) of the Korean government. This study was also supported by the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea (Grant No. 2023IF0001-1)
IRB approval status
Reviewed and approved by the Institutional Review Board (IRB) of Asan Medical Center; approval no. 2021-0648
Data availability statement
The data that support these findings are available from the corresponding author upon reasonable request.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the authors did not use any generative AI and AI-assisted technologies
Acknowledgements
none
Footnotes
Short running title: Cutaneous extranodal NK/T cell lymphoma
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2025.102503.
Appendix. Supplementary materials
References
- 1.Harabuchi Y., Takahara M., Kishibe K., Nagato T., Kumai T. Extranodal natural killer/T-cell lymphoma, nasal type: basic science and clinical progress. Front. Pediatr. 2019;7:141. doi: 10.3389/fped.2019.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montes-Mojarro I.A., Fend F., Quintanilla-Martinez L. EBV and the pathogenesis of NK/T cell lymphoma. Cancers. 2021;13(6) doi: 10.3390/cancers13061414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z., Xia Y., Feng L.N., et al. Genetic risk of extranodal natural killer T-cell lymphoma: a genome-wide association study. Lancet Oncol. 2016;17(9):1240–1247. doi: 10.1016/S1470-2045(16)30148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin G.W., Xu C., Chen K., et al. Genetic risk of extranodal natural killer T-cell lymphoma: a genome-wide association study in multiple populations. Lancet Oncol. 2020;21(2):306–316. doi: 10.1016/S1470-2045(19)30799-5. [DOI] [PubMed] [Google Scholar]
- 5.Dobashi A., Tsuyama N., Asaka R., et al. Frequent BCOR aberrations in extranodal NK/T-Cell lymphoma, nasal type. Genes Chromosomes Cancer. 2016;55(5):460–471. doi: 10.1002/gcc.22348. [DOI] [PubMed] [Google Scholar]
- 6.Jiang L., Gu Z.H., Yan Z.X., et al. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat. Genet. 2015;47(9):1061–1066. doi: 10.1038/ng.3358. [DOI] [PubMed] [Google Scholar]
- 7.Kucuk C., Jiang B., Hu X., et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from gammadelta-T or NK cells. Nat. Commun. 2015;6:6025. doi: 10.1038/ncomms7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quintanilla-Martinez L., Kremer M., Keller G., et al. p53 Mutations in nasal natural killer/T-cell lymphoma from Mexico: association with large cell morphology and advanced disease. Am. J. Pathol. 2001;159(6):2095–2105. doi: 10.1016/S0002-9440(10)63061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen L., Liang A.C., Lu L., et al. Frequent deletion of Fas gene sequences encoding death and transmembrane domains in nasal natural killer/T-cell lymphoma. Am. J. Pathol. 2002;161(6):2123–2131. doi: 10.1016/S0002-9440(10)64490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sim S.H., Kim S., Kim T.M., et al. Novel JAK3-activating mutations in extranodal NK/T-cell lymphoma, nasal type. Am. J. Pathol. 2017;187(5):980–986. doi: 10.1016/j.ajpath.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Song T.L., Nairismagi M.L., Laurensia Y., et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood. 2018;132(11):1146–1158. doi: 10.1182/blood-2018-01-829424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takakuwa T., Dong Z., Nakatsuka S., et al. Frequent mutations of Fas gene in nasal NK/T cell lymphoma. Oncogene. 2002;21(30):4702–4705. doi: 10.1038/sj.onc.1205571. [DOI] [PubMed] [Google Scholar]
- 13.Xiong J., Cui B.W., Wang N., et al. Genomic and transcriptomic characterization of natural killer T cell lymphoma. Cancer Cell. 2020;37(3):403–419. doi: 10.1016/j.ccell.2020.02.005. e406. [DOI] [PubMed] [Google Scholar]
- 14.Dirmeier U., Hoffmann R., Kilger E., et al. Latent membrane protein 1 of Epstein-Barr virus coordinately regulates proliferation with control of apoptosis. Oncogene. 2005;24(10):1711–1717. doi: 10.1038/sj.onc.1208367. [DOI] [PubMed] [Google Scholar]
- 15.Sun L., Zhao Y., Shi H., Ma C., Wei L. LMP-1 induces survivin expression to inhibit cell apoptosis through the NF-kappaB and PI3K/akt signaling pathways in nasal NK/T-cell lymphoma. Oncol. Rep. 2015;33(5):2253–2260. doi: 10.3892/or.2015.3847. [DOI] [PubMed] [Google Scholar]
- 16.Selvarajan V., Osato M., Nah G.S.S., et al. RUNX3 is oncogenic in natural killer/T-cell lymphoma and is transcriptionally regulated by MYC. Leukemia. 2017;31(10):2219–2227. doi: 10.1038/leu.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iqbal J., Weisenburger D.D., Chowdhury A., et al. Natural killer cell lymphoma shares strikingly similar molecular features with a group of non-hepatosplenic γδ T-cell lymphoma and is highly sensitive to a novel aurora kinase A inhibitor in vitro. Leukemia. 2011;25(2):348–358. doi: 10.1038/leu.2010.255. [DOI] [PubMed] [Google Scholar]
- 18.Jo J.C., Kim M., Choi Y., et al. Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann. Hematol. 2017;96(1):25–31. doi: 10.1007/s00277-016-2818-4. [DOI] [PubMed] [Google Scholar]
- 19.Bi X.W., Wang H., Zhang W.W., et al. PD-L1 is upregulated by EBV-driven LMP1 through NF-κb pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J. Hematol. Oncol. 2016;9(1):109. doi: 10.1186/s13045-016-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagato T., Ohkuri T., Ohara K., et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol. Immunther. 2017;66(7):877–890. doi: 10.1007/s00262-017-1987-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y.P., Chang K.C., Su W.C., Chen TY. The expression and prognostic significance of platelet-derived growth factor receptor alpha in mature T- and natural killer-cell lymphomas. Ann. Hematol. 2008;87(12):985–990. doi: 10.1007/s00277-008-0539-z. [DOI] [PubMed] [Google Scholar]
- 22.Costa R.O., Pereira J., Lage L., Baiocchi OCG. Extranodal NK-/T-cell lymphoma, nasal type: what advances have been made in the last decade? Front. Oncol. 2023;13 doi: 10.3389/fonc.2023.1175545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Au W.Y., Weisenburger D.D., Intragumtornchai T., et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113(17):3931–3937. doi: 10.1182/blood-2008-10-185256. [DOI] [PubMed] [Google Scholar]
- 24.Zheng X., Qu B.L., Liu X., et al. Characteristics and prognosis of distant metastasis after primary treatment for early-stage extranodal nasal-type natural killer/T-cell lymphoma from the China Lymphoma Collaborative Group database. EJHaem. 2023;4(1):78–89. doi: 10.1002/jha2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi Y.L., Park J.H., Namkung J.H., et al. Extranodal NK/T-cell lymphoma with cutaneous involvement: 'nasal' vs. 'nasal-type' subgroups–a retrospective study of 18 patients. Br. J. Dermatol. 2009;160(2):333–337. doi: 10.1111/j.1365-2133.2008.08922.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee W.J., Jung J.M., Won C.H., et al. Cutaneous extranodal natural killer/T-cell lymphoma: A comparative clinicohistopathologic and survival outcome analysis of 45 cases according to the primary tumor site. J. Am. Acad. Dermatol. 2014;70(6):1002–1009. doi: 10.1016/j.jaad.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Jung J.M., Yang H.J., Won C.H., Chang S.E., Lee M.W., Lee WJ. Clinicopathological and prognostic study of primary cutaneous extranodal natural killer/T-cell lymphoma, nasal type: A systematic review. J. Dermatol. 2021;48(10):1499–1510. doi: 10.1111/1346-8138.15972. [DOI] [PubMed] [Google Scholar]
- 28.Major A., Porcu P., Haverkos BM. Rational targets of therapy in extranodal NK/T-cell lymphoma. Cancers. (Basel) 2023;15(5) doi: 10.3390/cancers15051366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J., Suh C., Park Y.H., et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J. Clin. Oncol. 2006;24(4):612–618. doi: 10.1200/JCO.2005.04.1384. [DOI] [PubMed] [Google Scholar]
- 30.Jeong SH. Extranodal NK/T cell lymphoma. Blood Res. 2020;55(S1):S63–s71. doi: 10.5045/br.2020.S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y.J., Won C.H., Chang S.E., Lee M.W., Choi J.H., Lee WJ. Expression of programmed death-1 in cutaneous extranodal natural killer/T-cell lymphoma and its effect on clinical findings and biological behaviour. J. Eur. Acad. Dermatol. Venereol. 2017;31(5):821–827. doi: 10.1111/jdv.14165. [DOI] [PubMed] [Google Scholar]
- 32.Moon J.W., Kong S.K., Kim B.S., et al. IFNgamma induces PD-L1 overexpression by JAK2/STAT1/IRF-1 signaling in EBV-positive gastric carcinoma. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-18132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rong Q.X., Wang F., Guo Z.X., et al. GM-CSF mediates immune evasion via upregulation of PD-L1 expression in extranodal natural killer/T cell lymphoma. Mol. Cancer. 2021;20(1):80. doi: 10.1186/s12943-021-01374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim W.Y., Jung H.Y., Nam S.J., et al. Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Arch. 2016;469(5):581–590. doi: 10.1007/s00428-016-2011-0. [DOI] [PubMed] [Google Scholar]
- 35.Ma K., Guo Y., Wang Y., Wang X., Xu Y., Sun C. P75.22 Efficacy of PD-1/PD-L1 immune checkpoint inhibitors for advanced NSCLC according to PD-L1 expression: A meta-analysis. J. Thorac. Oncol. 2021;16(3):S583. doi: 10.1016/j.jtho.2021.01.1657. [DOI] [Google Scholar]
- 36.Yoon H.H., Jin Z., Kour O., et al. Association of PD-L1 expression and other variables with benefit from Immune checkpoint inhibition in advanced gastroesophageal cancer: systematic review and meta-analysis of 17 phase 3 randomized clinical trials. JAMa Oncol. 2022;8(10):1456–1465. doi: 10.1001/jamaoncol.2022.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zouein J., Kesrouani C., Kourie HR. PD-L1 expression as a predictive biomarker for immune checkpoint inhibitors: between a dream and a nightmare. Immunotherapy. 2021;13(12):1053–1065. doi: 10.2217/imt-2020-0336. [DOI] [PubMed] [Google Scholar]
- 38.Lim S.W., Ryu K.J., Lee H., Ko Y.H., Kim W.S., Kim SJ. Serum IL18 is associated with hemophagocytosis and poor survival in extranodal natural killer/T-cell lymphoma. Leuk. Lymphoma. 2019;60(2):317–325. doi: 10.1080/10428194.2018.1480772. [DOI] [PubMed] [Google Scholar]
- 39.McInnes I.B., Liew F.Y., Gracie JA. Interleukin-18: a therapeutic target in rheumatoid arthritis? Arthritis Res. Ther. 2004;7(1):38. doi: 10.1186/ar1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silvera D., Formenti S.C., Schneider RJ. Translational control in cancer. Nat. Rev. Cancer. 2010;10(4):254–266. doi: 10.1038/nrc2824. [DOI] [PubMed] [Google Scholar]
- 41.Nogueira G., Fernandes R., García-Moreno J.F., Romão L. Nonsense-mediated RNA decay and its bipolar function in cancer. Mol. Cancer. 2021;20(1):72. doi: 10.1186/s12943-021-01364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bongiorno R., Colombo M.P., Lecis D. Deciphering the nonsense-mediated mRNA decay pathway to identify cancer cell vulnerabilities for effective cancer therapy. J. Exp. Clin. Cancer Res. 2021;40(1):376. doi: 10.1186/s13046-021-02192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ni X., Austin M., Langridge T., et al. CD209(+) monocyte-derived myeloid dendritic cells were increased in patients with leukemic cutaneous T-cell lymphoma undergoing extracorporeal photopheresis via the CELLEX(TM) system. Photodermatol. Photoimmunol. Photomed. 2020;36(4):290–298. doi: 10.1111/phpp.12552. [DOI] [PubMed] [Google Scholar]
- 44.Li Y.Q., Luo C.L., Jiang J.X., et al. Single-cell analysis reveals malignant cells reshape the cellular landscape and foster an immunosuppressive microenvironment of extranodal NK/T-cell lymphoma. Adv. Sci. 2023;10(36) doi: 10.1002/advs.202303913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L., Li Y., Li B. Interactions between cancer cells and tumor-associated macrophages in tumor microenvironment. Biochim. Biophys. Acta (BBA) - Rev. Cancer. 2025;1880(3) doi: 10.1016/j.bbcan.2025.189344. [DOI] [PubMed] [Google Scholar]
- 46.Wang B., Cheng D., Ma D., et al. Mutual regulation of PD-L1 immunosuppression between tumor-associated macrophages and tumor cells: a critical role for exosomes. Cell Commun. Signal. 2024;22(1):21. doi: 10.1186/s12964-024-01473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmad A. Epigenetic regulation of immunosuppressive tumor-associated macrophages through dysregulated microRNAs. Semin. Cell Dev. Biol. 2022;124:26–33. doi: 10.1016/j.semcdb.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Wang S., Wang J., Chen Z., et al. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. NPJ. Precis. Oncol. 2024;8(1):31. doi: 10.1038/s41698-024-00522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong X., Xie X., Wang Z., Zhang Y., Wang L. Tumor-associated macrophages in lymphoma: from mechanisms to therapy. Int. Immunopharmacol. 2022;112 doi: 10.1016/j.intimp.2022.109235. [DOI] [PubMed] [Google Scholar]
- 50.Wu W., Chen X., Li N., Luo Q., Zou L. A neutrophil/lymphocyte ratio as a significant predictor for patients with low-risk and early-stage extranodal NK-T-cell lymphoma. Indian J. Hematol. Blood Transfus. 2023;39(2):228–236. doi: 10.1007/s12288-022-01578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen M-M, Zeng G-P, Li J., et al. High infiltration of CD20+ B lymphocytes in extranodal natural killer/T-cell lymphoma is associated with better prognosis. Br. J. Haematol. 2020;191(5):e116–e120. doi: 10.1111/bjh.17069. [DOI] [PubMed] [Google Scholar]
- 52.Jorgensen J.M., Sorensen F.B., Bendix K., et al. Expression level, tissue distribution pattern, and prognostic impact of vascular endothelial growth factors VEGF and VEGF-C and their receptors flt-1, KDR, and flt-4 in different subtypes of non-hodgkin lymphomas. Leuk. Lymphoma. 2009;50(10):1647–1660. doi: 10.1080/10428190903156729. [DOI] [PubMed] [Google Scholar]
- 53.de Mel S., Hue S.S., Jeyasekharan A.D., Chng W.J., Ng SB. Molecular pathogenic pathways in extranodal NK/T cell lymphoma. J. Hematol. Oncol. 2019;12(1):33. doi: 10.1186/s13045-019-0716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ng S.B., Selvarajan V., Huang G., et al. Activated oncogenic pathways and therapeutic targets in extranodal nasal-type NK/T cell lymphoma revealed by gene expression profiling. J. Pathol. 2011;223(4):496–510. doi: 10.1002/path.2823. [DOI] [PubMed] [Google Scholar]
- 55.Andersen M.H., Svane I.M., Becker J.C., Straten PT. The universal character of the tumor-associated antigen survivin. Clin. Cancer Res. 2007;13(20):5991–5994. doi: 10.1158/1078-0432.CCR-07-0686. [DOI] [PubMed] [Google Scholar]
- 56.Jin X., Wei M., Wang S., et al. Detecting fibroblast activation proteins in lymphoma using (68)Ga-FAPI PET/CT. J. Nucl. Med. 2022;63(2):212–217. doi: 10.2967/jnumed.121.262134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng Y., Zhong M., Liu Y., Wang L., Tang Y. Expression of TIM-3 and LAG-3 in extranodal NK/T cell lymphoma, nasal type. Histol. Histopathol. 2018;33(3):307–315. doi: 10.14670/HH-11-931. [DOI] [PubMed] [Google Scholar]
- 58.Maimela N.R., Liu S., Zhang Y. Fates of CD8+ T cells in Tumor microenvironment. Comput. Struct. Biotechnol. J. 2019;17:1–13. doi: 10.1016/j.csbj.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han J., Khatwani N., Searles T.G., Turk M.J., Angeles CV. Memory CD8(+) T cell responses to cancer. Semin. Immunol. 2020;49 doi: 10.1016/j.smim.2020.101435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han J., Khatwani N., Searles T.G., Turk M.J., Angeles CV. Memory CD8+ T cell responses to cancer. Semin. Immunol. 2020;49 doi: 10.1016/j.smim.2020.101435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faber J., Fonseca LM. How sample size influences research outcomes. Dent. Press J Orthod. 2014;19(4):27–29. doi: 10.1590/2176-9451.19.4.027-029.ebo. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support these findings are available from the corresponding author upon reasonable request.