Abstract
The food-borne pathogen Listeria monocytogenes is notable for its ability to grow under osmotic stress and at low temperatures. It is known to accumulate the compatible solutes glycine betaine and carnitine from the medium in response to osmotic or chill stress, and this accumulation confers tolerance to these stresses. Two permeases that transport glycine betaine have been identified, both of which are activated by hyperosmotic stress and one of which is activated by low temperature. An osmotically activated transporter for carnitine, OpuC, has also been identified. We have isolated a Tn917-LTV3 insertional mutant that could not be rescued from hyperosmotic stress by exogenous carnitine. The mutant, LTS4a, grew indistinguishably from a control strain (DP-L1044) in the absence of stress or in the absence of carnitine, but DP-L1044 grew substantially faster under osmotic or chill stress in the presence of carnitine. LTS4a was found to be strongly impaired in KCl-activated as well as chill-activated carnitine transport. 13C nuclear magnetic resonance spectroscopy of perchloric acid extracts showed that accumulation of carnitine by LTS4a was negligible under all conditions tested. Direct sequencing of LTS4a genomic DNA with a primer based on Tn917-LTV3 yielded a 487-bp sequence, which allowed us to determine that the opuC operon had been interrupted by the transposon. It can be concluded that opuC encodes a carnitine transporter that can be activated by either hyperosmotic stress or chill and that the transport system plays a significant role in the tolerance of L. monocytogenes to both forms of environmental stress.
Listeria monocytogenes is a gram-positive, food-borne pathogen (15). It is widely distributed in the environment (28), and contamination of food is therefore common. Listeriosis occurs mainly in immunocompromised individuals, such as pregnant women, AIDS patients, and transplant recipients, and is approximately 25% fatal (19). L. monocytogenes has been shown to be tolerant to levels of osmotic stress as high as that afforded by 10% NaCl and grows at temperatures as low as −0.1°C (5). Thus, it is resistant to two major forms of food preservation.
A common response to hyperosmotic stress, after an initial loss of cellular water, is the intracellular accumulation of compatible solutes in order to restore osmotic pressure and turgor (29). The presence of the compatible solute glycine betaine or carnitine in the medium therefore confers a degree of osmotolerance (3, 13, 14, 22). We have also found that L. monocytogenes accumulates glycine betaine and carnitine in response to chill stress and that the presence of these compatible solutes in the medium confers cryotolerance (11, 13, 22). These osmolytes are not synthesized by L. monocytogenes and must be transported from the environment. The rate and the extent of their transport can be controlled by regulation of the synthesis of the transport systems and by their biochemical activation by osmotic gradients, temperature, or other factors.
In earlier work, we demonstrated that there are at least two transport systems for glycine betaine, which we referred to as porter I and porter II. Porter I, probably the product of the betL gene (21), is an Na+-glycine betaine symporter (8). It is activated by a hyperosmotic concentration gradient of salts or sucrose, but it exhibits normal Arrhenius temperature dependence and is therefore not activated by chill in membrane vesicles. Porter II, the product of the gbu genes (7, 12), is an ATP-binding cassette transporter which is activated by either an osmotic gradient or low temperature in membrane vesicles (7).
At least one carnitine transporter exists (3, 6, 22, 27) in L. monocytogenes. Fraser et al. (6) recently reported the sequence of an operon encoding a salt-activated transport system for carnitine. The operon was designated opuC because of the homology of its sequence to that of the compatible solute transporter OpuC of Bacillus subtilis.
In this contribution, we report that we have isolated a transposon insertion mutant that is impaired in both chill-activated and osmotically activated carnitine transport. We have characterized this mutant with respect to growth, transport, and solute accumulation under osmotic stress and chill stress, and we have shown that it is the opuCB gene that is disrupted by the transposon.
MATERIALS AND METHODS
Bacterial strains and media.
The L. monocytogenes strains used in this work were DP-L1044 (hly::Tn917-LTV3) (25) and LTS4a (this work). These strains are derived from the parent strain 10403S by insertion of Tn917-LTV3 at different loci. Cultures were kept on brain heart infusion (BHI) (Difco) agar plates containing 10 μg of chloramphenicol per ml at 4°C. Na+-deficient modified (lacking choline) Pine's medium (16) containing 0.5% glucose was used as the defined medium. Glycine betaine, carnitine, and KCl were purchased from Sigma Chemical Co., St. Louis, Mo.
Measurement of growth characteristics (generation time and lag phase).
To inoculate Pine's medium for growth rate determination, L. monocytogenes cultures were grown overnight at 30°C in BHI broth, and 1-ml aliquots were centrifuged in an Eppendorf minicentrifuge (11,750 × g, 10 min). The pellets were washed twice with 1-ml portions of Pine's medium and used to inoculate (0.5%) 125-ml Pyrex Nephelo flasks containing Pine's medium. These cultures were grown to cell densities of ca. 2 × 109 CFU/ml, diluted 10-fold in Pine's medium, and used to inoculate (1%) three sets of 125-ml Pyrex Nephelo flasks containing 15 ml of Pine's medium. These sets were incubated, stagnant, at 30, 7, or 4°C. The chill-stressed sets were incubated in the presence or absence of 1 mM carnitine. The set incubated at 30°C contained the same combination of osmolytes in the presence (8%) or absence (0%) of KCl.
Growth was monitored with a Klett-Summerson photoelectric colorimeter with a green (no. 54) filter. Each combination of strain, stress, and osmolyte was tested in triplicate. Specific growth rate constants (k) were calculated by plotting the natural logarithm of Klett units versus time and converted to their respective generation time values (g). For each culture, an approximate lag-phase value (h) was calculated; since cultures were inoculated to an initial density equal to the minimum detection level of the instrument, the lag phase was calculated as the midpoint between two successive measurements that displayed a detectable increase in optical density (OD) and after which all subsequent OD values were progressively higher.
Transport assays and steady-state cytoplasmic levels.
Carnitine transport rates were determined in triplicate by using [methyl-14C]carnitine (NEN, Boston, Mass.) as described previously for glycine betaine uptake (13). Uptake rates were normalized to total cellular protein by the bicinchoninic acid (BCA) method (24) (Pierce Chemical, Rockford, Ill.) and are reported as nanomoles of osmolyte per minute per milligram of cellular protein.
L. monocytogenes DP-L1044 and LTS4a were grown aerobically in modified Pine's medium containing 1 mM glycine betaine and carnitine in the presence of chill (7 and 4°C) and osmotic (8% KCl) stress. Unstressed cultures grown at 30°C with added osmolytes served as controls. Cultures were harvested at late log phase by centrifugation (4,080 × g, 10 min, 4°C). The supernatants were discarded, and pelleted cells were immediately washed with ice-cold 1% (control and chill-stressed cultures) or 9% (KCl-stressed cultures) KCl solution. Cytoplasmic contents were extracted with ice-cold 7% perchloric acid as described elsewhere (23).
Extracts were analyzed by natural-abundance 13C nuclear magnetic resonance (13C-NMR) spectroscopy with a Bruker Avance-500 NMR spectrometer operating at 125.8 MHz for 13C. Data were acquired using a 60° pulse and 3-s recycle time, which we have found to give accurate peak intensities. The GARP sequence (20) was used for proton decoupling and to obtain the nuclear Overhauser effect. The intensities of the trimethyl peaks of carnitine (54.6 ppm) and glycine betaine (53.9 ppm) and the Cβ and Cγ resonances of glutamate (28 and 34 ppm, respectively) were used to quantitate the levels of the osmolytes by comparison to the Cα and Cβ resonances for 50 mM alanine (at approximately 51 and 17 ppm, respectively), which was added as the internal standard. Total cellular protein at the time of harvest was used to normalize compatible solute concentrations.
Isolation of a carnitine transport-deficient mutant.
An l-carnitine transport-deficient mutant was isolated from a pool of Tn917-LTV3 insertion mutants of L. monocytogenes 10403S (4) (provided by B. Walsh, University of California, Davis). The selection procedure used was analogous to that described previously for glycine betaine (12). The pool was subjected to three rounds of penicillin enrichment in modified Pine's medium containing 8% NaCl, 1 mM carnitine, and 1 mg of penicillin per ml. Mutants were initially screened for growth on solid modified Pine's medium, for resistance to chloramphenicol (conferred by the transposon), and for inability to be rescued by 100 mM carnitine when stressed with 8% NaCl by replica plating. Putative transport mutants were subsequently analyzed for growth in liquid medium in the presence and absence of added NaCl and carnitine and for the uptake of [14C]carnitine. One mutant, strain LTS4a, that exhibited reduced osmotic tolerance and a markedly reduced rate of carnitine uptake was used for direct chromosomal sequencing and further studies.
DNA isolation and sequence analysis.
L. monocytogenes LTS4a was grown aerobically for 12 h at 30°C in BHI broth. Then 1 ml of this culture was centrifuged (11,750 × g, 10 min) in an Eppendorf minicentrifuge at room temperature. The supernatant was aspirated, leaving approximately 25 μl of liquid. The pellet was brought up in 150 μl of buffer (25 mM Tris-HCl, 50 mM EDTA [pH 8]), 2 μl of Ready-Lyse lysozyme solution (Epicentre Technologies, Madison, Wis.) was added to the resuspended cells, and the mixture was incubated for 8 h at 37°C. Chromosomal DNA was extracted and isolated using the MasterPure DNA purification kit (Epicentre Technologies) according to the manufacturer's instructions.
The chromosomal region flanking the transposon insertion was identified by direct chromosomal sequencing. The primer used for extension was 24 bp long (GTT AAA TGT ACA AAA TAA CAG CGA), derived from the sequence of Tn917-LTV3, on the proximal side of lacZ and about 70 bp from its end.
Direct genomic sequencing.
Chromosomal DNA was subjected to direct sequencing with the BigDye Terminator cycle sequencing kit (Applied Biosystems, Foster City, Calif.). Genomic DNA was sequenced in a 40-μl (2×) reaction mix containing 5 to 12 pmol of primer, 2.5 μg of L. monocytogenes LTS4a genomic DNA, and 16 μl of BigDye terminator mix.
Cycle sequencing was performed with the following program: 4 min at 95°C, followed by 60 cycles of 30 s at 95°C and 4 min at 60°C. Reaction products were purified using Centriflex gel filtration cartridges (Edge Biosystems, Gaithersburg, Md.), concentrated by ethanol precipitation, washed with 70% ethanol, and resuspended in 22 μl of template suppression reagent (Applied Biosystems). After denaturing at 95°C for 5 min, the samples were injected into an ABI 310 genetic analyzer (Applied Biosystems) and analyzed with ABI version 3.3 sequence analysis software. The genomic transposon insertion site was located by using the BLAST programs maintained at the NCBI web site of the National Library of Medicine (http://www.ncbi.nlm.nih.gov/BLAST/).
RESULTS
Mutant selection and isolation.
Mutants impaired in the ability to transport carnitine were isolated from a pool of Tn917-LTV3 L. monocytogenes mutants by screening for the loss of carnitine-dependent salt tolerance. Of approximately 3,000 mutants that were screened, 2 displayed both a reduction in growth under osmotic stress conditions and a substantial reduction in osmotically stimulated carnitine uptake. Of these, the mutant designated LTS4a was selected for sequence analysis.
Identification of L. monocytogenes opuC operon.
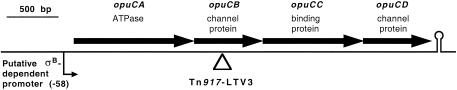
Direct sequencing of the chromosomal DNA of L. monocytogenes LTS4a with the 24-bp primer complementary to Tn917-LTV3 yielded a 487-bp sequence. This sequence was used in a BLASTn search (1) of the GenBank database. The only sequence showing significant homology (score, 250 bits; E value, 5e-64) was that of L. monocytogenes opuC (accession number AF249729), a putative carnitine transporter, submitted by O'Byrne as a direct submission. This sequence is that of an operon encoding four proteins, OpuCA (an ATPase), OpuCB (a membrane pore protein), OpuCC (a substrate binding protein), and OpuCD (a second membrane pore protein). The site of transposon insertion in the operon was found to be at nucleotide 1709, near the midpoint of the opuCB gene (Fig. 1). While this work was in preparation, an article appeared (6) describing the function of the gene product as an osmotically activated ABC transporter for l-carnitine.
FIG. 1.
Site of insertion of Tn917-LTV3 into the opuC operon, based on a 487-bp sequence obtained by direct sequencing of the genomic DNA of mutant LTS4a with a 24-bp primer derived from the sequence of the transposon.
Carnitine transport and growth characteristics under osmotic and cold stress.
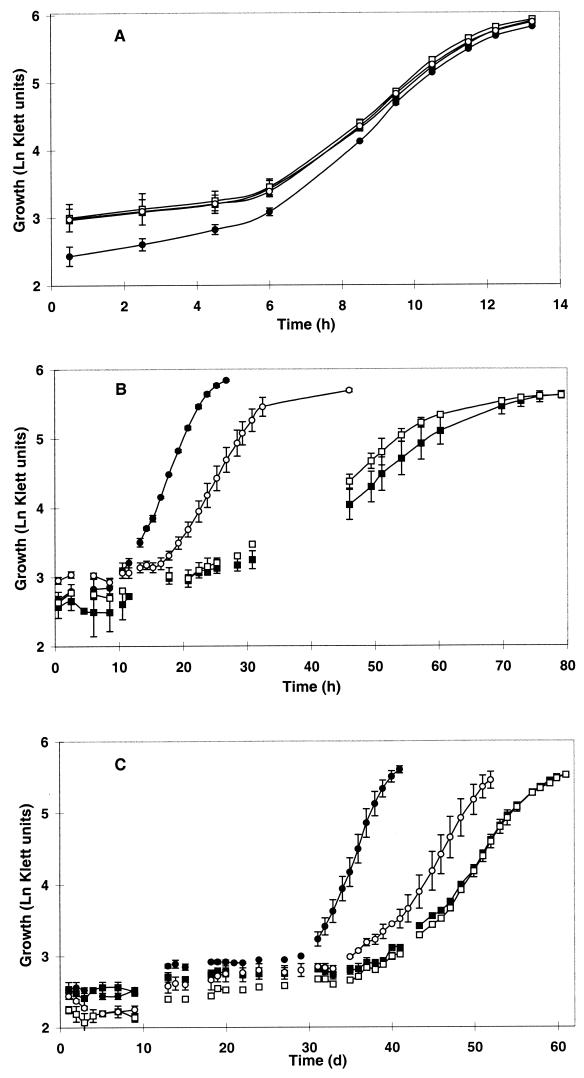
The growth characteristics of mutant LTS4a were investigated in defined liquid medium and compared to those of strain DP-L1044, a wild-type derivative containing the Tn917 transposon in the hly locus (25). No differences in growth characteristics were observed at 30°C in the absence of osmotic stress (Fig. 2A) or in the presence of 8% KCl in the absence of added carnitine (Fig. 2B). However, in the presence of 8% KCl and 1 mM carnitine, LTS4a displayed an almost twofold-longer lag phase and grew with a generation time (4.2 h) which was significantly longer than that of the wild type (3.0 h) (Fig. 2B).
FIG. 2.
Growth characteristics of L. monocytogenes LTS4a and DP-L1044. Cultures of DP-L1044 (solid symbols) and the carnitine transport mutant LTS4a (open symbols) were grown in BHI medium and inoculated into Na+-deficient modified Pine's medium (1% inoculum). These cultures were grown to late log phase and used to inoculate (1%) Na+-deficient modified Pine's medium containing 1 mM carnitine (circles) or no carnitine (squares). Cultures were grown at 30°C without added KCl (A), at 30°C with 8% KCl (B), or at 7°C without added KCl (C). Error bars indicate ±1 standard deviation of triplicate values. d, days.
Significant differences in generation time between strains were also observed in cultures grown under cold stress with 1 mM carnitine. At both 4°C (Fig. 2C) and 7°C (data not shown), the generation time of the mutant (78.8 and 27.5 h, respectively) was significantly and reproducibly (1.4-fold) higher than that of the wild type (55.6 and 19.7 h, respectively). Differences in lag phase (mutant longer) were also observed at both temperatures but were not statistically significant. Finally, no differences in lag phase or generation time were observed between strains grown at either temperature (4 or 7°C) in cultures without added carnitine.
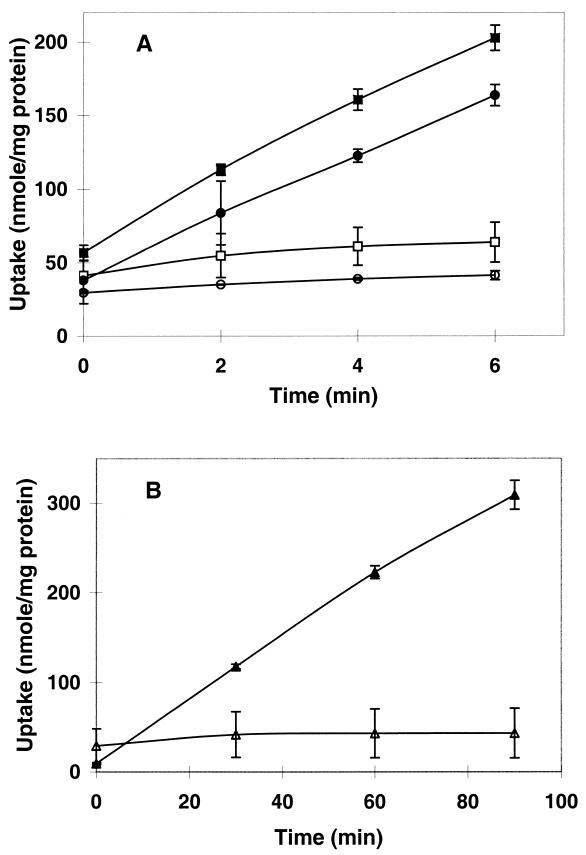
The rates of [14C]carnitine uptake by LTS4a and DP-L1044 were measured in Na+-deficient modified Pine's medium at 30°C with and without KCl (4 or 8%) (Fig. 3A) and at 7°C (with no added salt) (Fig. 3B). At 30°C in the absence of added salt, the rate of carnitine uptake was low for both DP-L1044 (0.8 nmol min−1 mg−1) and LTS4a (0.1 nmol min−1 mg−1). The nonzero rate of carnitine uptake under these conditions can be attributed to activation by the low baseline level of osmotic stress provided by the medium components. In the presence of external 4% KCl, the rate of carnitine uptake was drastically increased and was 10-fold higher in DP-L1044 (24 nmol min−1 mg−1) than in LTS4a (2.5 nmol min−1 mg−1). Increasing the medium osmolality to 8% KCl resulted in a further increase in the rate of transport (DP-L1044, 37.5 nmol min−1 mg−1; LTS4a, 9.4 nmol min−1 mg−1). At 7°C, the rate of carnitine transport was increased 4.5-fold in strain DP-L1044 (3.6 nmol min−1 mg−1) relative to that of cultures at 30°C, whereas the respective rates in mutant cultures were indistinguishable (0.1 nmol min−1 mg−1).
FIG. 3.
Carnitine transport activity of L. monocytogenes LTS4a and DP-L1044. Uptake of 100 mM [14C]carnitine was measured in DP-L1044 (solid symbols) and LTS4a (open symbols) grown to late log phase in Na+-deficient modified Pine's medium at 30°C with 4% KCl (circles) or 8% KCl (squares) (A) or at 7°C without added KCl (B). Transport was assayed as described in Materials and Methods. Error bars indicate ±1 standard deviation of triplicate values.
Compatible solute accumulation under stress.
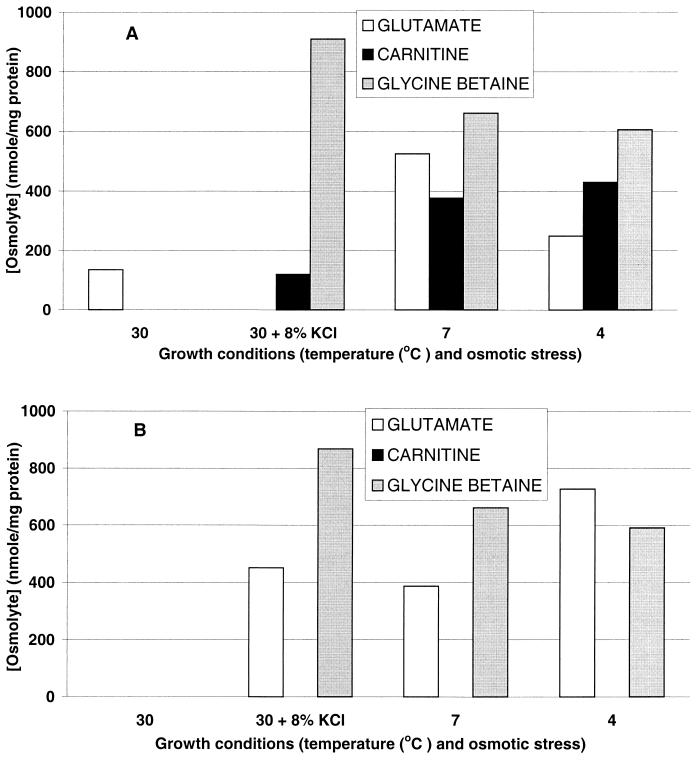
Cytoplasmic extracts of cultures (DP-L1044 and LTS4a) in balanced growth were subjected to 13C-NMR analysis. Both strains accumulated compatible solutes under both osmotic and chill stress, but with the exception of low levels of glutamate, no other osmolyte was present in cell extracts of either strain in the absence of stress (30°C cultures) (Fig. 4). When grown under stress, both strains accumulated glycine betaine at high levels (up to 910 nmol/mg of protein). Whereas carnitine was accumulated by DP-L1044 to high levels in response to chill stress and to low levels under KCl-mediated stress, no carnitine could be detected in extracts of LTS4a under either of these growth conditions.
FIG. 4.
Compatible solute accumulation by L. monocytogenes DP-L1044 (A) and mutant L. monocytogenes LTS4a (B) during balanced growth in modified Pine's medium under different conditions of osmotic and cold stress. Cultures were harvested and washed, and cytoplasmic contents were extracted with perchloric acid. Alanine (50 mM) was added to each extract as an internal standard, and compatible solutes were quantitated by natural-abundance 13C-NMR spectroscopy.
DISCUSSION
When the results of the growth characteristics and uptake experiments are viewed in conjunction, the following conclusions can be drawn. Transport of carnitine in L. monocytogenes is both osmotically and chill activated, and the accumulation of carnitine contributes to tolerance to both stresses. The mutant LTS4a is impaired in osmotically activated carnitine uptake and totally blocked in cold-activated uptake of this osmolyte. The compromised capacity to transport carnitine substantially decreases the ability of the organism to withstand elevated osmotic stress and, to a lesser extent, decreases its ability to proliferate under low temperatures.
The fact that carnitine transport in strain LTS4a under conditions of osmotic stress, although drastically reduced compared to the wild type, is not zero and is in fact osmotically activated suggests the existence of yet another osmotically activated carnitine transporter in L. monocytogenes. Alternatively, under these conditions, carnitine transport may proceed through one of the glycine betaine porters (Gbu or BetL) in addition to OpuC; the latter scenario is supported by current data from our laboratory. It is further supported by the observation that no detectable amounts of carnitine were evident in 13C-NMR spectra of cells grown in the presence of both glycine betaine and carnitine (Fig. 4). Under these conditions, the natural substrate glycine betaine should compete effectively with carnitine.
Our results demonstrate that a single transposon insertion in the opuCB gene of L. monocytogenes impairs both chill-activated and osmotically activated transport of the compatible solute carnitine. Hence, the salt-activated carnitine transporter identified by Fraser et al. and the chill-activated carnitine transporter that we have observed are one and the same. We have now shown that this transporter plays a significant role in the chill tolerance observed in L. monocytogenes, especially in the absence of glycine betaine (Fig. 2C).
Fraser et al. (6) originally identified the opuC operon as a group of open reading frames in L. monocytogenes Scott A. Their subsequent work revealed the presence of a homologous operon in strain EGD, a type strain employed in the genome sequencing effort, as well. The transporter encoded by this operon was characterized in strain EGD and found to be activated by 0.5 M NaCl. In our study, the operon was identified functionally, by enrichment and screening of a bank of transposon insertional mutants. In addition to the difference in experimental approach, we also used a different strain, 10403S. Strains 10403S and EGD are both of serotype 1/2a. It has been found that the diversity for specific genes between isolates of the same serotype is quite limited and that differences in nucleotide sequences tend to be silent (S. Kathariou, personal communication).
The serotypes of L. monocytogenes have been classified into two major divisions, designated I and II, on the basis of genetic clustering. Strain 10403S (serotype 1/2a) is a member of division I, whereas the best-known serotype, 4b, is a member of division II. It is therefore reasonable to expect that significant variation in sequence might be observed between strain 10403S and strains of serotype 4b, such as strain Scott A. A comparison of the nucleotide sequences of strain EGD and the serotype 4b strain reported in the TIGR database showed that the nucleotide sequences for opuCA, opuCB, opuCC, and opuCD were 94, 94, 94, and 96% identical, respectively. The proteins encoded by the operons of the two strains are essentially identical, with one conservative substitution in OpuCB and one substitution in OpuCD. This result is consistent with the report of Fraser et al. that the first two open reading frames of the EGD sequence were at least 94.5% identical to those of the Scott A sequence (6). This transporter is therefore highly conserved among members of the two most epidemiologically important serotypes, 1/2a and 4b.
OpuC is an ABC transporter composed of four types of subunit, one ATPase (presumably intracellular), one extracellular solute-binding protein, and two proteins comprising a 12-helix pore (6). The gbu operon encodes three types of protein, an ATPase, a binding protein, and a six-helix transmembrane protein (12); it is assumed that the pore is a homodimer, with a total of 12 transmembrane helices (10). Both Gbu and OpuC are activated by chill, whereas the monomeric BetL (21) is not (8). This observation suggests that activation by chill may involve a change in the organization of the subunits of multimeric transporters, perhaps mediated by changes in the physical state of the membrane, which, in recent work in Poolman's lab (26), has been implicated in the activation of an ABC transporter by osmotic strength as well.
By using direct genome sequencing, we were able to determine the point of insertion of Tn917-LTV3, identifying the interrupted gene, from a single sequencing run with only one primer, followed by a database search. The entire sequence of the genome of L. monocytogenes strain EGD is known (9); that of a serotype 4b strain is nearing completion, and the completed portions are now available for BLAST searches via the TIGR database (http://www.tigr.org/tdb/mdb/). Consequently, even unidentified genes that are not yet cataloged in GenBank can be identified with ease by using direct sequencing of genomic DNA from L. monocytogenes.
L. monocytogenes is a serious threat to the food supply, in part because of its ability to grow under stressful conditions (18). In order to predict the survival of L. monocytogenes in foods, models have been devised that relate growth to various environmental parameters, including pH, temperature, salinity, and inoculum level (17). This and other recent studies on stress-activated osmolyte accumulation by L. monocytogenes in foods (2, 22) suggest that levels of carnitine and glycine betaine should be included as variables in such predictive models. In addition, the depletion of compatible solutes from certain foods or the inclusion of inhibitors of osmolyte transport could attenuate the growth of L. monocytogenes in foods.
Acknowledgments
We thank Amy Arata for technical assistance.
This work was supported by grant 98-TSL-01 from Dairy Management, Inc., and by NSF grant NSF OSTI 97-24412 to the UCD NMR facility.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelidis, A., L. Smith, and G. Smith. 2002. Elevated carnitine accumulation by Listeria monocytogenes impaired in glycine betaine transport is insufficient to restore wild-type cryotolerance in milk whey. Int. J. Food Microbiol. 75:1-9. [DOI] [PubMed] [Google Scholar]
- 3.Beumer, R. R., M. C. Te Giffel, L. J. Cox, F. M. Rombouts, and T. Abee. 1994. Effect of exogenous proline, betaine, and carnitine on growth of Listeria monocytogenes in a minimal medium. Appl. Environ. Microbiol. 60:1359-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camilli, A., D. A. Portnoy, and P. Youngman. 1990. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J. Bacteriol. 172:3738-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole, M., M. Jones, and C. Holyoak. 1990. The effect of pH, salt concentration and temperature on the survival and growth of Listeria monocytogenes. J. Appl. Microbiol. 69:63-72. [DOI] [PubMed] [Google Scholar]
- 6.Fraser, K., D. Harvie, P. Coote, and C. O'Byrne. 2000. Identification and characterization of an ATP binding cassette l-carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 66:4696-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerhardt, P. N. M., L. T. Smith, and G. M. Smith. 2000. Osmotic and chill activation of glycine betaine porter II in Listeria monocytogenes membrane vesicles. J. Bacteriol. 182:2544-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerhardt, P. N. M., L. T. Smith, and G. M. Smith. 1996. Sodium-driven osmotically activated glycine betaine transport in Listeria monocytogenes membrane vesicles. J. Bacteriol. 178:6105-6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couvé, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K.-D. Entian, H. Fsihi, F. Garcia-Del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gómez-López, T. Hain, J. Hauf, D. Jackson, L.-M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueño, A. Maitournam, J. Mata Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J.-C. Pérez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J.-A. Vázquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 10.Higgins, C. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 11.Ko, R. 1996. Ph.D. thesis. University of California, Davis.
- 12.Ko, R., and L. T. Smith. 1999. Identification of an ATP-driven, osmoregulated glycine betaine transport system in Listeria monocytogenes. Appl. Environ. Microbiol. 65:4040-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko, R., L. T. Smith, and G. M. Smith. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bacteriol. 176:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patchett, R. A., A. F. Kelly, and R. G. Kroll. 1992. Effect of sodium chloride on the intracellular solute pools of Listeria monocytogenes. Appl. Environ. Microbiol. 58:3959-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson, L. J., and E. H. Marth. 1990. Listeria monocytogenes—threat to a safe food supply: a review. J. Dairy Sci. 73:912-928. [DOI] [PubMed] [Google Scholar]
- 16.Pine, L., M. Franzuz, and G. Malcolm. 1986. Guanine is a growth factor for Legionella species. J. Clin. Microbiol. 23:163-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razavilar, V., and C. Genigeorgis. 1998. Prediction of Listeria spp. growth as affected by various levels of chemicals, pH, temperature and storage time in a model broth. Int. J. Food Microbiol. 40:149-157. [DOI] [PubMed] [Google Scholar]
- 18.Ryser, E. P., and E. H. Marth. 1999. Listeria, listeriosis and food safety, 2nd ed. Marcel Dekker, New York, N.Y.
- 19.Schuchat, A., K. A. Deaver, J. D. Wenger, B. D. Plikaytis, L. Mascola, R. W. Pinner, A. L. Reingold, C. V. Broome, et al. 1992. Role of foods in sporadic listeriosis. I. Case-control study of dietary risk factors. JAMA 267:2041-2045. [PubMed] [Google Scholar]
- 20.Shaka, A. J., P. B. Barker, and R. J. Freeman. 1985. Computer-optimized decoupling scheme for wideband applications and low-level operation. J. Magn. Reson. 64:547-552. [Google Scholar]
- 21.Sleator, R. D., C. G. Gahan, T. Abee, and C. Hill. 1999. Identification and disruption of BetL, a secondary glycine betaine transport system linked to the salt tolerance of Listeria monocytogenes LO28. Appl. Environ. Microbiol. 65:2078-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith, L. T. 1996. Role of osmolytes in adaptation of osmotic and chill-stressed Listeria monocytogenes grown in liquid media and on processed meat surfaces. Appl. Environ. Microbiol. 62:3088-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, L. T., and G. M. Smith. 1989. An osmoregulated peptide found in stressed Rhizobium meliloti. J. Bacteriol. 171:4714-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 25.Sun, A. N., A. Camilli, and D. A. Portnoy. 1990. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect. Immun. 58:3770-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Heide, T., and B. Poolman. 2000. Osmoregulated ABC-transport system of Lactococcus lactis senses water stress via changes in the physical state of the membrane. Proc. Natl. Acad. Sci. USA 97:7102-7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verheul, A., F. M. Rombouts, R. R. Beumer, and T. Abee. 1995. An ATP-dependent l-carnitine transporter in Listeria monocytogenes Scott A is involved in osmoprotection. J. Bacteriol. 177:3205-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weis, J., and H. Seeliger. 1975. Incidence of Listeria monocytogenes in nature. Appl. Microbiol. 95:29-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yancey, P., M. Clark, S. Hand, R. Bowlus, and G. Somero. 1982. Living with water stress: evolution of osmolyte systems. Science 217:1214-1222. [DOI] [PubMed] [Google Scholar]