Abstract
Copper-silver (Cu-Ag) ionization has effectively controlled Legionella spp. in the hot water systems of numerous hospitals. However, it was ineffective at controlling Legionella in one Ohio hospital despite the confirmation of adequate total concentrations of copper and silver ions. The pH of the water at this hospital was found to be 8.5 to 9.0. The purpose of this study was to investigate the impact of pH and other water quality parameters, including alkalinity (HCO3−), hardness (Ca2+ and Mg2+), and amount of dissolved organic carbon (DOC), on the control of Legionella by Cu-Ag ionization. Initial concentrations of Legionella and copper and silver ions used in batch experiments were 3 × 106 CFU/ml and 0.4 and 0.08 mg/liter, respectively. Changes in bicarbonate ion concentration (50, 100, and 150 mg/liter), water hardness (Ca2+ at 50 and 100 mg/liter; Mg2+ at 40 and 80 mg/liter), and level of DOC (0.5 and 2 mg/liter) had no significant impact on the efficacy of copper and silver ions in killing Legionella at a neutral pH. When the pH was elevated to 9 in these experiments, copper ions achieved only a 10-fold reduction in the number of Legionella organisms in 24 h, compared to a millionfold decrease at pH 7.0. Silver ions were able to achieve a millionfold reduction in 24 h at all ranges of water quality parameters tested. Precipitation of insoluble copper complexes was observed at a pH above 6.0. These results suggest that pH may be an important factor in the efficacy of copper-silver ionization in controlling Legionella in water systems.
Copper-silver ionization is a disinfection technology that has been used with increasing frequency to control Legionella spp. in hospital hot water systems (8). Copper and silver ions are electrolytically generated and introduced into recirculating hot water lines. These positively charged metallic ions attach to the negatively charged bacterial cell wall and cause cell lysis and death (2, 4, 13). We have shown that copper and silver ions can act synergistically to kill Legionella (7). Ionization systems have now been installed in more than 75 hospitals in the United States (G. Lyslo and N. Silverberg, personal communication). The first 16 hospitals in the United States that installed these systems remain operational today, and each has reported efficacious results in controlling Legionella in its water distribution system after 5 to 11 years of operation (J. E. Stout, Y. E. Lin, and V. L. Yu, Survey of hospitals using copper-silver ionization for the control of Legionella, abstract from the 5th International Conference on Legionella, p. 80, 2000). The copper-silver ionization systems have outperformed hyperchlorination, superheating and flushing, and UV light systems because of the following advantages: (i) installation and maintenance are easy; (ii) efficacy is not affected by high water temperatures (unlike with chlorine and UV light systems); (iii) residual disinfectant protection occurs throughout the system (unlike with UV light); and (iv) recolonization is delayed because copper-silver ions kill rather than suppress Legionella (unlike with chlorine) (8). Disadvantages of copper-silver ionization systems have rarely been reported. Long-term treatment with copper and silver ions could theoretically result in the development of resistance to these ions. Although silver resistance has been demonstrated for other bacteria, resistance has been suggested but not demonstrated for Legionella (6, 11, 12).
One Ohio hospital replaced an existing hyperchlorination system with a copper-silver ionization system in an attempt to control the persistence of Legionella in its water system. However, Legionella persisted despite the documentation of adequate concentrations of copper and silver ions within the water; no malfunction of the system could be documented. Personnel from the Centers for Disease Control and Prevention conducted an on-site investigation and concluded that the installation of the ionization system neither reduced the number of positive samples nor terminated the transmission of the bacteria (3). The reason for the ionization system's inability to control Legionella remained unresolved.
The variation in water quality in this Ohio hospital was suspected to be the cause of the failure because an on-site measurement found the pH of the water to be as high as 8.9. The objective of this study was to investigate the impact of pH and other water quality parameters on the efficacy of copper and silver ions in killing Legionella.
MATERIALS AND METHODS
Direct observations.
One liter of hot water was obtained from an Ohio hospital whose copper-silver ionization system failed to control Legionella in the hospital water system. This water was sent to the Special Pathogens Laboratory, Pittsburgh, Pa., and the City of Pittsburgh Water Treatment Plant for analysis. Water quality analysis was conducted according to the procedures of Standard Methods for the Examination of Water and Wastewater and included analysis of hardness, alkalinity (as CaCO3), sulfate, chloride, iron, calcium, magnesium, total organic carbon (TOC) (all measured in milligrams per liter), and pH (1). Copper and silver analysis was performed by atomic absorption spectrophotometry. The bactericidal activity of the water was tested by the addition of Legionella pneumophila serogroup 1, as described below.
Test organism.
An environmental isolate of L. pneumophila serogroup 1 was used for this study. L. pneumophila was transferred from −20°C stock broth, inoculated onto buffered-charcoal-yeast extract agar medium, and incubated at 37°C. Inoculation was repeated after 48 h. After 24 h, the culture was removed from the medium and suspended in 30 ml of sterile deionized water. The cells were washed twice by centrifugation at 1,000 × g for 10 min. Two milliliters of the suspension was removed, and its cell content was standardized by comparison with the turbidity of McFarland standard no. 1 (approximate density of 3 × 108 CFU/ml). One milliliter of the standardized suspension was transferred to the test solution to achieve the initial concentration of 3 × 106 CFU/ml for each experiment.
Copper and silver ions.
Copper and silver ion stock solutions containing 10 and 1 mg/liter, respectively, were prepared by dissolving CuCl2(s) and AgCl(s) (Aldrich Chemical Co., Milwaukee, Wis.) in deionized water. Actual ion concentrations were confirmed at the beginning of each experiment by atomic absorption spectrophotometry (AAS). Teflon flasks (250 ml) were used for all batch experiments to prevent loss of Cu2+ and Ag+ from solution due to the adsorption of ions onto the walls of the container (5). All test solutions were sterilized by membrane filtration. The copper and silver ion concentrations tested were 0.4 and 0.08 mg/liter, respectively, because these ion concentrations have been shown to be effective in controlling Legionella in full-scale water distribution systems.
In order to determine the fate of copper ions at an elevated pH, the portions of soluble and precipitated copper ions were assessed as a function of pH. Copper ions were added to several flasks containing solution with a pH ranging from 2 to 9. Separation of the soluble and precipitated forms of copper in the solution at different pH values was performed by centrifuging 40 ml of the copper solution at 1,000 × g for 30 min. Thereafter, 35 ml of the supernatant containing soluble copper was removed from the solution and acidified with 0.05 N HNO3. The remaining 5 ml containing copper precipitates was also acidified with 0.05 N HNO3. The concentrations of soluble and precipitated copper were measured by AAS as described previously (7).
Water quality parameters. (i) pH.
The effect of pH on the efficacy of Cu-Ag ionization was tested by using solutions with pHs of 7 and 9. Test solutions were prepared by first adding 1 ml of 0.1 M NaHCO3 solution (pH 8.23) to 99 ml of deionized water. The pH was then adjusted to 7 and 9 by adding 1 M HCl and 1 M NaOH solutions, respectively.
(ii) Bicarbonate.
The impact of bicarbonate ions on the efficacy of ionization was tested at three concentrations: 50, 100, and 150 mg/liter as NaHCO3 (40, 80, and 120 mg/liter as CaCO3). A stock solution of 1,000 mg of NaHCO3 per liter was used to prepare the test solutions. The pH of the test solution was adjusted to 7 with 1 M HCl.
(iii) Hardness.
The influence of hardness on the efficacy of ionization was investigated at calcium ion concentrations of 0, 50, and 100 mg/liter and magnesium ion concentrations of 0, 40, and 80 mg/liter. Stock solutions containing 1,000 mg of calcium ions per liter and 1,000 mg of magnesium ions per liter were prepared by dissolving appropriate amounts of calcium chloride and magnesium chloride in deionized water and transferred to test solutions by using a dilution scheme.
(iv) DOC.
The influence of dissolved organic carbon (DOC) on the efficacy of ionization was investigated at DOC levels of 0, 0.5, 2, and 20 mg/liter. These concentrations were achieved by dissolving the appropriate amount of humic substance (International Humic Substances Society, St. Paul, Minn.) extracted from the Suwannee River in a 0.001 M KOH solution and diluting the solution to 1 liter with deionized water. The stock solution was stored in a brown bottle refrigerated at 4°C to prevent the decay of the organic carbon during storage. The final concentrations in the liquid phase were confirmed using a TOC analyzer (Dohrman DC 180 carbon analyzer; PerkinElmer, Norwalk, Conn.).
Batch disinfection experiments.
Test solutions buffered at pH 7 or 9 were seeded with an initial L. pneumophila serogroup 1 concentration of 3 × 106 CFU/ml. The actual bacterial concentration was determined by a plate count of organisms in the sample withdrawn at time zero. Teflon flasks were placed on a shaker, and temperature was controlled at 37°C. Upon the addition of disinfectant solution, the concentration of L. pneumophila serogroup 1 organisms was monitored over 72 h. Samples (1 ml) withdrawn from the batch reactor were immediately mixed with 10 μl of neutralizer solution (14.6% sodium thiosulfate and 10% sodium thioglycolate), serially diluted, and plated in duplicate onto buffered-charcoal-yeast extract agar culture medium. The culture plates were incubated for 72 h at 37°C, and the CFU were then enumerated (detection limit, 10 CFU/ml). Each disinfection experiment was performed in duplicate, and each data point on the time-kill curves represents an average from two experiments performed on different days.
Equilibrium calculations.
A chemical-equilibrium model (Visual MINTEQ, version 2.01b; KTH, Stockholm, Sweden) was used to calculate the changes in the chemical speciation of copper and silver ions under different water quality conditions and to study the impact of water quality parameters on the availability of dissolved metallic ions in solution. This model uses a thermodynamic database to determine the equilibrium distribution of various species in aqueous solution. It can also calculate pH titration curves for desired chemical species at fixed temperatures and in the presence of specified total concentrations of other chemicals, including DOC. The water quality parameters included in the simulations were pH; the levels of bicarbonate, calcium, magnesium, and chloride ions; and the level of DOC as humic acid. The pH range selected for the simulations was 6 to 11 to represent a variety of conditions that might be encountered in drinking water systems. The ranges of other parameters were also varied to reflect ranges typically found in drinking water systems in the United States. The baseline concentrations of HCO3−, Cl−, Ca2+, and Mg2+ in all modeling studies were 100, 40, 40, and 20 mg/liter, respectively.
Statistical analysis.
The software packages used for statistical analysis were Microsoft Excel (Microsoft Co., Seattle, Wash.) and Sigma Plot (SPSS Inc., Chicago, Ill.). All analyses were performed by using analysis of variance under 95% confidence intervals. A Significant difference among the samples was defined as an analysis of variance P value of less than 0.05.
RESULTS
Direct observations.
The copper and silver concentrations in a water sample from the Ohio hospital were 0.27 mg of copper per liter and 30 μg of silver per liter, concentrations known to be efficacious for Legionella disinfection. In addition, when 3 × 106 CFU of medium-grown Legionella organisms per ml was added to the hot water sample from the hospital, Legionella was killed within 24 h. Water quality analysis performed on this sample showed no significant differences from the water quality in Pittsburgh. The results were as follows: pH, 7.6; TOC, 1.89 mg/liter; hardness, 110 mg/liter; alkalinity, 40 mg/liter as CaCO3; chloride, 40 mg/liter; iron, 0.01 mg/liter; calcium, 31.2 mg/liter; magnesium 5.3 mg/liter; and sulfate, 72 mg/liter. Since the pH of water tends to reach a neutral pH over time due to the shift in the carbonate equilibrium in an open system, it was probable that measurement of pH after 3 days in transit and storage was not reflective of the pH in the hospital. Therefore, on-site measurement of water pH was performed at the Ohio hospital, and the pH was found to be 8.5 to 8.9.
Chemical-equilibrium model. (i) pH.
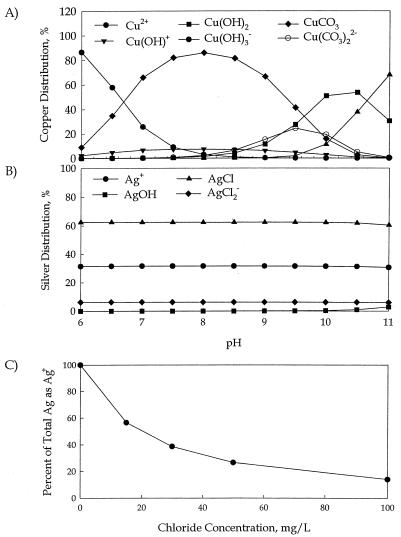
The predominant forms of copper and silver ions as a function of solution pH were determined under baseline conditions by using the Visual MINTEQ chemical-equilibrium model, and results are shown in Fig. 1A and B, respectively. The predominant forms of copper ions (Cu2+) at pHs 5, 7, and 9 were Cu(HCO3)+, Cu(CO3) (aqueous), and Cu(CO3)22−, respectively (Fig. 1A). In contrast, silver chloride was the predominant complex that determined the fate of silver ions in solution, while the solution pH played a secondary role (Fig. 1B). Silver hydroxide complexes became relevant only after the pH exceeded 10. Thus, the batch disinfection experiments were performed to evaluate the efficacy of copper and silver ions on L. pneumophila at pHs 7 and 9.
FIG. 1.
Impact of pH and chloride ions on copper and silver ion speciation in a chemical-equilibrium model. Higher pH favors copper anions in solution (A), while it has relatively little impact on silver ion distribution (B). (C) Higher chloride concentration leads to silver complexation.
(ii) Chloride ions.
Equilibrium modeling of the effect of chloride ions on the speciation of silver in solution at pH 7.5 showed that as the chloride concentration increased, silver complexation reduced the available silver cations (Fig. 1C) (other species were present at baseline values).
Batch disinfection experiments.
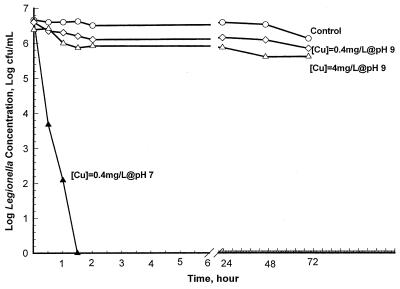
The bactericidal effect of copper ions on L. pneumophila at pHs 7 and 9 was initially tested by using a copper concentration of 0.4 mg/liter. At pH 9, copper failed to kill L. pneumophila (achieving a less than 10-fold reduction) in 72 h (Fig. 2). However, when the pH of the solution was reduced to 7.0, the 0.4 mg of copper per liter killed L. pneumophila (a millionfold reduction was observed) in 1.5 h. Testing of the efficacy of copper ions in killing L. pneumophila at pH 9 was performed with a concentration of 4 mg/liter, 10 times higher than the suggested concentration for adequate disinfection (7). Similarly, 4.0 mg of copper ions per liter failed to kill L. pneumophila in 72 h (Fig. 2). The influence of pH on the efficacy of silver ions in killing L. pneumophila was also tested. pH had no significant impact on silver ions in killing Legionella; 0.08 mg of silver ions per liter was able to achieve a millionfold reduction in the numbers of Legionella organisms in 24 h at all pH levels tested in this study (data not shown).
FIG. 2.
Impact of copper concentration on killing of Legionella at pH 9 in a batch disinfection model. At pH 9, copper concentrations of 4.0 and 0.4 mg/liter failed to kill L. pneumophila in 72 h. However, when the pH of the solution was reduced to 7.0, 0.4 mg of copper per liter achieved a millionfold reduction in the number of L. pneumophila organisms in 1.5 h.
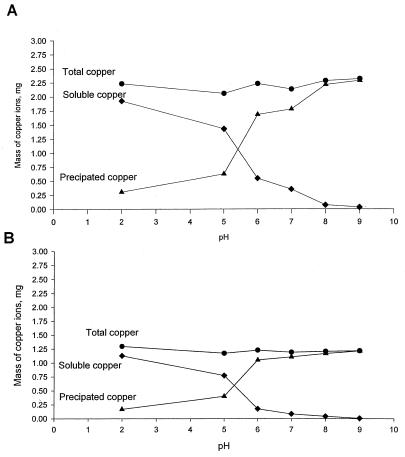
The change of copper complexes with pH variation was determined by measuring the concentration of dissolved copper at pH values from 2 to 9. The proportion of dissolved and precipitated copper ions was measured as a function of pH (from 2 to 9). Two initial copper ion concentrations, 1.26 and 2.30 mg/liter, were evaluated in this study. Only 16% of the copper ions added to buffered deionized water remained as soluble ions in the solution (Fig. 3); further reductions to 3 and 1% were observed at pHs 8 and 9, respectively. Thus, the solubility of copper decreased with increasing pH.
FIG. 3.
Impact of pH on total copper, soluble copper, and precipitated copper. Soluble (active) copper decreased with increasing pH, as determined by AAS. Total amounts of copper were 2.30 mg (A) and 1.26 mg (B).
(i) Bicarbonate ions.
Batch experiments were performed at pH 7 with NaHCO3 concentrations of 50, 100, and 150 mg/liter. No significant difference in the rates of Legionella inactivation was observed at the 95% confidence interval (P > 0.05). A copper ion concentration of 0.4 mg/liter achieved a millionfold reduction in the number of Legionella organisms in 1.5 h at all NaHCO3 concentrations tested in this study. This bicarbonate level also had no significant impact on the effectiveness of silver ions in killing L. pneumophila (data not shown).
(ii) Hardness ions.
The influence of calcium and magnesium on the efficacy of copper and silver ions in killing L. pneumophila was tested. Both copper (0.4 mg/liter) and silver (0.08 mg/liter) ions achieved millionfold reductions of L. pneumophila in 1.5 and 24 h, respectively, at all calcium and magnesium ion concentrations tested ([Ca2+] at 50 and 100 mg/liter and [Mg2+] at 40 and 80 mg/liter). No significant differences in the rates of L. pneumophila inactivation were observed for all hardness levels tested (data not shown).
(iii) DOC.
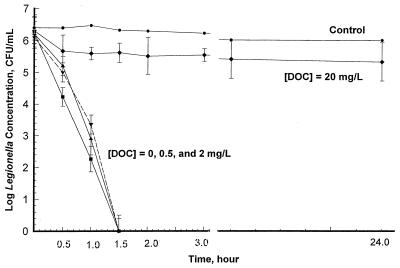
The impact of DOC on the efficacy of copper ions was tested using DOC levels of 0.5 and 2 mg/liter (concentrations that are representative of potable waters). A copper concentration of 0.4 mg/liter achieved a millionfold reduction in the number of L. pneumophila organisms in 1.5 h in the presence of 0.5 and 2 mg of DOC per liter (Fig. 4). However, at a DOC level of 20 mg/liter, a copper concentration of 0.4 mg/liter did not achieve a millionfold reduction in the number of L. pneumophila organisms in 24 h (Fig. 4). DOC levels had no significant impact on the effectiveness of silver ions in killing Legionella; 0.08 mg of silver ions per liter was able to achieve a millionfold Legionella reduction in 24 h regardless of the DOC concentration in the test solution (data not shown).
FIG. 4.
Impact of DOC on L. pneumophila inactivation with 0.4 mg of copper ions per liter (detection limit, 10 CFU/ml). DOC at a level of 20 mg/liter prevented a copper concentration of 0.4 mg/liter from killing L. pneumophila within 24 h.
DISCUSSION
Copper-silver ionization systems have been successful in controlling Legionella in more than 75 hospitals for observational periods ranging from 5 to 11 years. Copper concentrations of 0.2 to 0.4 mg/liter and silver concentrations of 0.02 to 0.04 mg/liter are recommended for efficient eradication, as documented by in vitro and field studies (7, 9, 10). The total copper and silver ion concentrations in one Ohio hospital were found to be within the acceptable range for efficacy; however, distal sites remained positive for Legionella. The pH of the water at this hospital was found to range from 8.5 to 8.9, which was higher than expected. It was hypothesized that the pH of the water of this hospital may have affected the efficacy of copper and silver ions in controlling Legionella.
Results from a chemical-equilibrium model showed that pH affected the ion speciation of copper and silver in solution. Higher pH values led to a shift in the predominant copper species from positively to negatively charged ions (Fig. 1A). It was therefore reasonable to expect that these negatively charged ions would be much less effective in eradicating Legionella due to repulsive electrostatic interactions with the cell wall. On the other hand, pH had little effect on silver. The predominant form of silver ions at all pH values used in the calculations was a silver chloride complex (Fig. 1B).
A series of batch disinfection experiments were conducted to determine the impact of pH and other water quality parameters on the efficacy of copper and silver ions in killing Legionella. A copper ion concentration of 0.4 mg/liter did not eradicate Legionella at pH 9 (Fig. 2). Moreover, the solubility of the copper complexes decreased as the pH increased (Fig. 3). The eradication of microorganisms by ionization is normally attributed to the attachment of the soluble (active) ion complex to bacterial cell walls. However, the active (soluble) form of copper ions may be insufficient to eradicate Legionella if the pH of the solution exceeds 9.
Thus, high pH levels may explain why ionization failed to control Legionella in this Ohio hospital. It should be noted that pH has a similar effect on chlorination because the most efficient chlorine compound, HOCl, is converted to the less efficacious OCl− at pH values of >7.0 (14). Thus, the high pH of the water may also have been the reason for the previous failure of hyperchlorination at the Ohio hospital. Alkalinity (HCO3−), hardness (CA2+, Mg2+), and DOC at concentrations commonly found in potable water did not have any significant impact on the efficacy copper and silver ions against Legionella.
pH had no significant impact on the predominant forms of silver ions in solution (Fig. 1B). Increasing the chloride ion concentration from 15 to 50 mg/liter may reduce the availability of positively charged silver ions from 56 to 26% of the total silver concentration (Fig. 1C). The chloride ion concentration in the water from the Ohio hospital was 40 mg/liter, which is not considered unusually high. It is possible that higher concentrations of chloride in water may decrease the availability of silver cations and reduce its biocidal potential.
The pH of the cold water delivered to hospitals may be elevated by water treatment plants, in compliance with U.S. Environmental Protection Agency guidelines to minimize copper and lead leaching from distribution piping. If the high pH of the incoming water is an issue, the pH can be reduced by the supplemental injection of acid into the hot water recirculating lines. For example, a Wisconsin hospital also experienced cultures consistently positive for Legionella at distal sampling sites despite installing a copper-silver ionization system (W. A. Bowler, Abstr. 9th Annu. Sci. Meet. Soc. Healthcare Epidemiol. Am., abstr. 53, 1999). The pH was also found to be between 8.5 and 8.8. Supplemental acid treatment of the hot water was implemented using a food-grade sulfuric acid. An acid injection system controlled by a timer was installed at the hot water recirculating line to inject acid into the hot water. Approximately 110 g of acid solution was used per day at a cost of $77 per 208-liter drum (G. Lyslo, personal communication). The pH of that water was then controlled at levels between 7 and 8. Tests for distal-site colonization by Legionella were negative after this intervention.
In conclusion, a high pH in water may compromise the efficacy of copper ions, and the biocidal efficacy of silver may be compromised by high concentrations of chloride.
REFERENCES
- 1.American Public Health Association. 1995. Standard methods for the examination of water and wastewater, 19th ed. American Public Health Association, Washington, D.C.
- 2.Britton, G., and V. Freihofer. 1978. Influence of extracellular polysaccharides on the toxicity of copper and cadmium towards Klebsiella ozenae. Microb. Ecol. 4:119-125. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1997. Sustained transmission of nosocomial Legionnaires' disease—Arizona and Ohio. Morb. Mortal. Wkly. Rep. 46:416-421. [PubMed] [Google Scholar]
- 4.Friedman, B. A., and P. R. Dugan. 1968. Concentration and accumulation of metallic ions by the bacterium Zoogloea. Dev. Ind. Microbiol. 9:381-387. [Google Scholar]
- 5.Landeen, L. K., M. T. Yahya, and C. P. Gerba. 1989. Efficacy of copper and silver ions and reduced levels of free chlorine in inactivation of Legionella pneumophila. Appl. Environ. Microbiol. 55:3045-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin, Y. E. 2000. Ionization failure not due to resistance. Clin. Infect. Dis. 31:1315-1316. [DOI] [PubMed] [Google Scholar]
- 7.Lin, Y. E., R. D. Vidic, J. E. Stout, and V. L. Yu. 1996. Individual and combined effects of copper and silver ions on inactivation of Legionella pneumophila. Water Res. 30:1905-1913. [Google Scholar]
- 8.Lin, Y. E., R. D. Vidic, J. E. Stout, and V. L. Yu. 1998. Legionella in water distribution systems. J. Am. Water Works Assoc. 90:112-121. [Google Scholar]
- 9.Liu, Z., J. E. Stout, M. Boldin, J. Rugh, W. F. Diven, and V. L. Yu. 1998. Intermittent use of copper-silver ionization for Legionella control in water distribution systems: a potential option in buildings housing individuals at low risk of infection. Clin. Infect. Dis. 26:138-140. [DOI] [PubMed] [Google Scholar]
- 10.Liu, Z., J. E. Stout, L. Tedesco, M. Boldin, C. Hwang, W. F. Diven, and V. L. Yu. 1994. Controlled evaluation of copper-silver ionization in eradicating Legionella pneumophila from a hospital water distribution system. J. Infect. Dis. 169:919-922. [DOI] [PubMed] [Google Scholar]
- 11.McHugh, S. L., R. C. Moellering, C. C. Hopkins, and M. N. Swartz. 1975. Salmonella typhimurium resistant to silver nitrate, chloramphenicol, and ampicillin. Lancet i:235-240. [DOI] [PubMed] [Google Scholar]
- 12.Rohr, U., M. Senger, F. Selenka, R. Turkey, and M. Wilhelm. 1999. Four years of experience with silver-copper ionization for control of Legionella in a German university hospital hot water plumbing system. Clin. Infect. Dis. 29:1507-1511. [DOI] [PubMed] [Google Scholar]
- 13.Slawson, R. M., H. Lee, and J. T. Trevors. 1990. Bacterial interactions with silver. Biol. Met. 3:151-154. [DOI] [PubMed] [Google Scholar]
- 14.Tchobanoglous, G., and F. L. Burton. 2002. Wastewater engineering—treatment, disposal and reuse, 3rd ed. Metcalf & Eddy, Inc., New York, N.Y.