Abstract
Fluorescent in situ hybridization with a 16S rRNA probe specific for Verrucomicrobia was used to (i) confirm the division-level identity of and (ii) study the behavior of the obligate intracellular verrucomicrobium “Candidatus Xiphinematobacter” in its nematode hosts. Endosymbionts in the egg move to the pole where the gut primordium arises; hence, they populate the intestinal epithelia of juvenile worms. During the host's molt to adult female, the endosymbionts concentrate around the developing ovaries to occupy the ovarian wall. Some bacteria are enclosed in the ripening oocytes for vertical transmission. Verrucomicrobia in males stay outside the testes because the tiny spermatozoids are not suitable for transmission of cytoplasmic bacteria.
Obligate intracellular symbioses of bacteria in Invertebrata are numerous (3, 4, 7, 8, 9, 10, 13, 21, 23) and constitute one of the fastest-growing fields in microbiology. This paper sheds more light on an endocytobiosis of a special kind of Verrucomicrobia in nematodes, “Candidatus Xiphinematobacter spp.” (24). Details on the taxonomic status “Candidatus” for incompletely described taxa can be found in the work of Murray and Schleifer (17) and Murray and Stackebrandt (18).
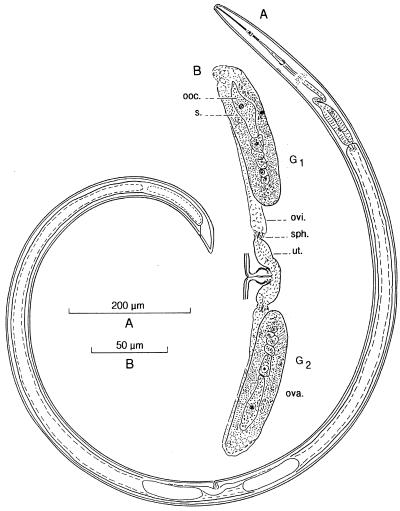
Xiphinema spp. (Dorylaimida, Longidoridae) are ectoparasitic nematodes found on economically important plants like vines and other fruit crops. The general morphology of a female is drawn in Fig. 1. Xiphinema nematodes possess an oral stylet (400 to 450 nm in diameter) with which they penetrate root hairs. Adults are 1 to 7 mm long and reproduce either sexually or by facultative thelytokous (mother-to-daughter) parthenogenesis. There are three or four juvenile stages (J1 through J4). Although two ovarian stem cells are present at J1, the ovaries do not develop before the molt from J3 or J4 to adulthood. Slightly later, yolk material synthesized by the adjacent gut epithelium is transferred to the ovarian epithelium, which then consists of nurse cells. These cells inject yolk and other maternal constituents into the growing oocytes as they pass by on their way down to the oviduct. The three incompletely described “Candidatus Xiphinematobacter species” are specifically associated with the predominantly parthenogenetic hosts Xiphinema brevicollum, Xiphinema americanum, and Xiphinema rivesi (24); more such symbioses probably exist within the so-called “X. americanum group.” The obligate intracellular bacteria belong to the division Verrucomicrobia (11, 15, 25), where they occupy a unique branch within subdivision 2 (according to reference 12).
FIG. 1.
General morphology (A) and reproductive system (B) of an entire X. americanum female. G1, anterior branch; G2, posterior branch; ooc., oocyte; ova., ovary; ovi., oviduct; s., symbiotic bacteria; sph., sphincter; ut., uterus.
It has been suggested that the incomplete description, based mainly on sequence characteristics, of new intracellular, inculturable, or uncultured “Candidatus species” be attended with in situ hybridization data as a supplementary indication of identity. rRNA-targeted oligonucleotide probe-based fluorescent in situ hybridization (FISH) allows for such cultivation-independent identification (2, 22). FISH has been used by Daims et al. (6) and Petroni et al. (19) to detect and identify Verrucomicrobia.
The aim of this work is twofold. First, in order to prove that the Xiphinema intracellular symbionts are indeed Verrucomicrobia, we provide FISH results obtained with a probe specific for Verrucomicrobia, EUB338-III (6). This probe, a specifically modified version of the older universal probe EUB338 (1), targets positions 338 to 355 (Escherichia coli numbering) in the 16S rRNA and is 100% complementary only to members of verrucomicrobial subdivisions 1 to 4. Second, we examined how the endosymbiont life cycle is tuned to that of the host at several crucial stages. We also report for the first time on the behavior of the microbes in the very rare Xiphinema males.
Nematodes.
X. brevicollum and X. americanum samples were collected in South Africa and stored at 4°C in their natural medium before being extracted from the soil as described by Vandekerckhove et al. (24).
Preparation for FISH.
Each preparation of nematodes was triplicated and comprised about 20 individuals covering most developmental stages (except males, which are extremely rare). All of the following steps were carried out in Eppendorf vessels with the simple replacement of fluids for each procedure. The specimens were surface sterilized for 1 min in 0.1% (wt/vol) benzalkonium chloride and rinsed twice for 2 min in sterile water with a physiological makeup (0.85% [wt/vol] NaCl) appropriate for nematodes. They were fixed for 10 min in a 1:1 blend of glacial acetic acid and ethanol, whereupon they were transferred into pure ethanol (twice for 5 min). Next, they were put for 10 min into a 1:1 mixture of methanol and phosphate-buffered Tween 20 (PBT; 150 mM NaCl, 10 mM Na3PO4, 0.1% [wt/vol] Tween 20 [pH 7.4]); they were then put for 30 min in 1% (vol/vol) formaldehyde in PBT, whereupon they were washed twice for 2 min each time in PBT.
FISH.
The 16S rRNA probes EUB338-III (5′-ACACCUACGGGUGGCAGC-3′) and Lax659 (5′-UGGUAGAGGGGGGUGGAA-3′) were covalently linked at their 5′ ends to the sulfoindocyanine chromophore Cy5 (carboxymethylindocyanin-succinimidylester; Amersham Life Science, Inc.). Lax659 is complementary to a variable 16S rRNA region of the chemoautotrophic, sulfur-oxidizing ectosymbiotic bacteria that grow on a Laxus sp. (Nematoda, Desmodoridae) (20) and served as a control for nonspecific binding of probe RNA and its fluorescent moiety under both high- and low-stringency conditions. Aliquotted stock solutions of 10 μM in TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 7.4]) were made; 10 μl thereof was used for each 100 μl of final hybridization mixture. The latter contained 20 mM Tris-HCl (pH 7.4), 0.02% (wt/vol) sodium dodecyl sulfate, 0.9 M NaCl, 5 mM EDTA, 60% (vol/vol) formamide, and a 1 μM concentration (±5 ng · μl−1) of the probe.
The hybridization conditions were largely adopted from the work of Manz et al. (16) and Daims et al. (6); high stringency was applied by keeping the closed vessels at 46°C for 3 h. This was followed by two 30-min washes at 48°C in a hybridization buffer containing no formamide; instead, equally stringent conditions were created by lowering the NaCl concentration to 0.008 M according to the formula of Lathe (14).
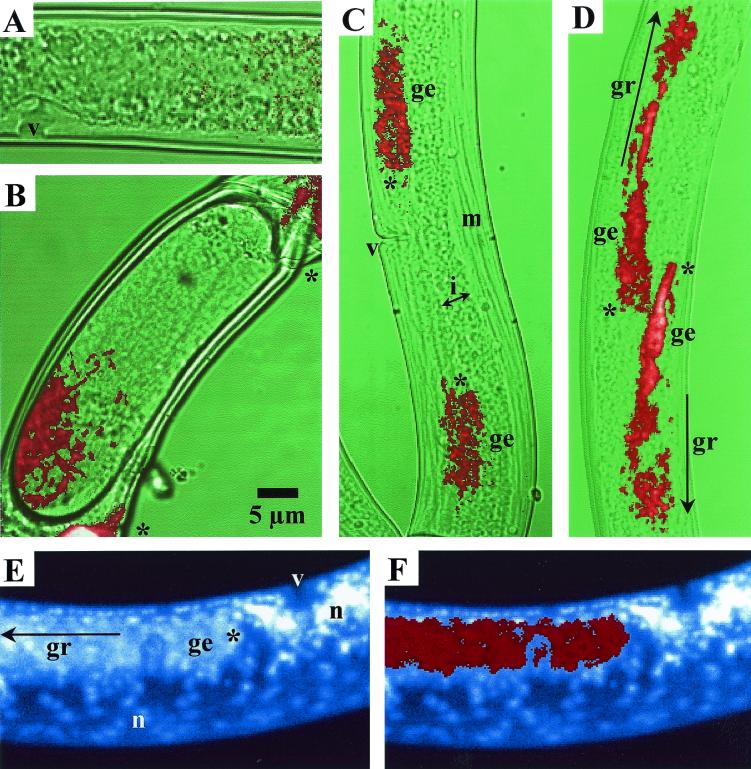
The images in Fig. 2A and 3 were taken under low-stringency conditions, with hybridization at 42°C and 0% formamide; washing also occurred at 42°C. This gave a roughly threefold-more-intense signal with better contrast on the confocal microscope.
FIG. 2.
FISH results after low-stringency hybridization with Lax659, a nonspecific probe (A), and high-stringency hybridization with EUB338-III, a verrucomicrobium-specific probe (B to F), to detect “Candidatus Xiphinematobacter brevicolli” (A, B, E, and F) and “Candidatus Xiphinematobacter americani” (C and D). (A) Newly molted female; (B) gravid female with endosymbionts at one egg pole; (C) newly molted female; (D) organism at a slightly later stage than that shown in panel C, with bacteria spreading downstream along the ovaries; (E) adult female showing endosymbionts and host cell nuclei after DAPI staining; (F) superimposed images at the same focal plane of the same female stained with DAPI and Cy5, with all endosymbionts being marked as Verrucomicrobia. ge, germinal zone of ovary; gr, growth zone of oocytes; i, intestine; m, body muscles; n, host cell nucleus; v, vulva. Single-headed arrows indicate directions of growth; ovarial apices are marked by asterisks.
FIG. 3.
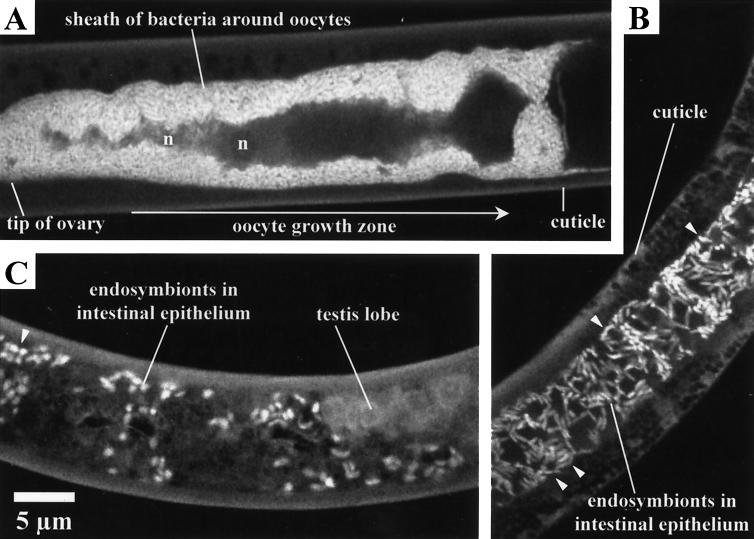
Low-stringency FISH images of “Candidatus Xiphinematobacter americani” organisms. (A and B) Adult females, with an ovary (A) and intestine (B) in the focal plane; (C) adult male. n, nucleus of growing oocyte. Arrowheads in panels B and C point to serial pairs of endosymbionts.
Mounting, DAPI counterstaining, and microscopy.
The specimens were pipetted out of the washing buffer onto microscope slides. A droplet of a DAPI (4′,6-diamidino-2-phenylindole) solution in distilled water (1 μg · ml−1) was added to stain the double-stranded DNA in any bacterium or host cell nucleus. The preparations were covered, sealed, and kept in the dark at 4°C until they could be viewed. Images were recorded with a Bio-Rad MRC-1024 confocal laser scanning system equipped with a krypton-argon laser (excitation filter wavelength, 647 nm; emission filter wavelength, 680 ± 32 nm) for use with Cy5, an argon ion laser (excitation filter wavelength, 357 ± 10 nm; emission filter wavelength, >460 nm) for use with DAPI, and a Nikon Eclipse TE300 microscope with apochromatic 60× or 40× oil immersion objectives (numerical aperture, 1.4 or 1.3, respectively). For image reconstruction, the software package Lasersharp (Bio-Rad) was used, as well as Confocal Assistant 4.02 and Adobe Photoshop 4.0.
Low-stringency hybridizations with the nonspecific Lax659 probe gave only a very weak background signal, with not a single bacterial cell being detected (Fig. 2A). Moreover, this background was completely absent from high-stringency-hybridization specimens, indicating that EUB338-III probe binding was highly specific. A comparison of corresponding images (Fig. 2E and F) also revealed that all endosymbiotic bacteria (DAPI stained) were Verrucomicrobia (Cy5 stained). On these grounds, the intracellular organisms first described and classified as three “Candidatus Xiphinematobacter species” (24) can be validly said to belong to the division Verrucomicrobia.
Endosymbionts were observed to heavily colonize the ovary wall epithelia of mature females (Fig. 2F and 3A). They were also readily apparent within the gut epithelium (Fig. 3B and C). It sometimes happens—though very rarely—that few symbionts are found, but not a single female has been found with none. These conclusions are based on observations of hundreds of females and the majority of the known males for each Xiphinema species.
“Candidatus Xiphinematobacter” organisms seem to have an amazing perception of their microhabitat. In the mononuclear intrauterine egg, these organisms migrate to one of the poles (Fig. 2B), and in the embryonated egg, they move further to surround one particular host nucleus: that of the intestinal stem cell (5). Logically, in juveniles, they occur only in the gut epithelial cells. But when a J4 organism molts into an adult female, the bacteria need to rearrange themselves in order to access the vertical transmission pathway: they must somehow invade the growing oocytes.
This decisive reshuffling can readily be visualized in newly molted and in young-adult females (exemplified by Fig. 2C and D). In newly molted organisms, the vast majority of the microbes are situated in the germinal zones of both ovaries, i.e., where the oogonia multiply. In young-adult females, the growing oocytes are provided with yolk from the nurse cells. This implies that the bacteria have been taken up by the ovarian wall epithelium prior to the provision of the yolk material. Yolk is primarily synthesized by gut cells and is secreted into adjacent ovarian wall (or nurse) cells soon after the transfer of the symbionts. Clearly, indirect symbiont transfer to ripening oocytes via nurse cells is the best warrant for vertical transmission. Nevertheless, not all bacteria migrate to the nurse cells: some may stay inside the gut cells (invisible on most confocal images because they are in a different focal plane), and a minority invade the oocytes directly (5).
Once inside the nurse cells, the bacteria enter a phase of intense proliferation (see, e.g., the difference in the occupation of space in Fig. 2C and D or 3A), but they never move farther than the growth zone, and the fluorescence can be seen to drop abruptly along the trajectory towards the oviduct (Fig. 3A, to the right). A secondary multiplication occurs inside the egg.
Interestingly, the endosymbionts also populate the male gut epithelium but not the ripe testes (Fig. 3C). Especially in male X. americanum organisms, they are less numerous and more coccus-like; they are more condensed than in females and are seldom found in serial pairs (compare Fig. 3B with C), as described by Vandekerckhove et al. (24). Such pairs are evidence of active division and consist of a mother cell from which a daughter cell is budding. The rareness of actively dividing cells in adult males is not surprising in light of the impossibility of vertical transmission: sperm cells do not offer enough space to harbor cytoplasmic symbionts. Instead, the symbionts in adult males appear somewhat latent, and they cannot be triggered to make the move to the testes, which are salient because of the complete absence of intracellular organisms.
All these findings point in the same direction: “Candidatus Xiphinematobacter species,” having coevolved with their nematode hosts for about 150 million years with no evidence of horizontal transmission or survival in the external environment (24), are totally reactive to and dependent on their female hosts—in almost every stage of their life cycles—for the ultimate purpose of each living organism: to maintain itself.
Acknowledgments
We thank A. J. Meyer (University of Stellenbosch, Stellenbosch, South Africa) for the X. americanum and X. brevicollum specimens used in this study. P. Van Oostveldt (Laboratory for Biochemistry and Molecular Cytology, Faculty of Agricultural and Applied Biological Sciences, Ghent University) is acknowledged for allowing us to use the confocal laser scanning microscope.
During the time this work was carried out, T. Vandekerckhove was a Research Assistant of the Fund for Scientific Research (F.W.O.), Flanders, Belgium. A. Coomans and M. Gillis are indebted to the F.W.O. for research and personnel grants. K. Cornelis was supported by a grant from the Interuniversity Poles of Attraction Program (grant P4/15).
REFERENCES
- 1.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R. I., W. Ludwig, and K.-H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann, P., L. Baumann, C.-Y. Lai, D. Rouhbakhsh, N. A. Moran, and M. A. Clark. 1995. Genetics, physiology, and evolutionary relationships of the genus Buchnera: intracellular symbionts of aphids. Annu. Rev. Microbiol. 49:55-94. [DOI] [PubMed] [Google Scholar]
- 4.Chang, K.-P., G. A. Dasch, and E. Weiss. 1984. Endosymbionts of fungi and invertebrates other than arthropods, p. 833-836. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, 1st ed., vol. 1. Williams and Wilkins, Baltimore, Md. [Google Scholar]
- 5.Coomans, A., T. T. M. Vandekerckhove, and M. Claeys. 2000. Transovarial transmission of symbionts in Xiphinema brevicollum (Nematoda: Longidoridae). Nematology 2:443-449. [Google Scholar]
- 6.Daims, H., A. Brühl, R. Amann, K.-H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 7.Dasch, G. A., E. Weiss, and K.-P. Chang. 1984. Endosymbionts of insects, p. 811-833. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, 1st ed., vol. 1. Williams and Wilkins, Baltimore, Md. [Google Scholar]
- 8.Felbeck, H., and D. L. Distel. 1992. Prokaryotic symbionts of marine invertebrates, p. 3891-3906. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed., vol. 4. Springer-Verlag, New York, N.Y. [Google Scholar]
- 9.Gutnick, D. L. 1992. Prokaryotic symbionts of the aphid, p. 3907-3913. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed., vol. 4. Springer-Verlag, New York, N.Y. [Google Scholar]
- 10.Heckmann, K., and H.-D. Görtz. 1992. Prokaryotic symbionts of ciliates, p. 3865-3890. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed., vol. 4. Springer-Verlag, New York, N.Y. [Google Scholar]
- 11.Hedlund, B. P., J. J. Gosink, and J. T. Staley. 1997. Verrucomicrobia div. nov., a new division of the bacteria containing three new species of Prosthecobacter. Antonie Leeuwenhoek 72:29-38. [DOI] [PubMed] [Google Scholar]
- 12.Hugenholtz, P., B. M. Goebel, and N. R. Pace. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 180:4765-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon, K. W. 1992. Prokaryotic symbionts of amoebae and flagellates, p. 3855-3864. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed., vol. 4. Springer-Verlag, New York, N.Y. [Google Scholar]
- 14.Lathe, R. 1985. Synthetic oligonucleotide probes deduced from amino acid sequence data: theoretical and practical considerations. J. Mol. Biol. 183:1-12. [DOI] [PubMed] [Google Scholar]
- 15.Liesack, W., and E. Stackebrandt. 1992. Occurrence of novel groups of the domain Bacteria as revealed by analysis of genetic material isolated from an Australian terrestrial environment. J. Bacteriol. 174:5072-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manz, W., R. Amann, W. Ludwig, M. Wagner, and K.-H. Schleifer. 1992. Phylogenetic oligodeoxynucleotide probes for the major subclasses of Proteobacteria: problems and solutions. Syst. Appl. Microbiol. 15:593-600. [Google Scholar]
- 17.Murray, R. G. E., and K. H. Schleifer. 1994. Taxonomic notes: a proposal for recording the properties of putative taxa of procaryotes. Int. J. Syst. Bacteriol. 44:174-176. [DOI] [PubMed] [Google Scholar]
- 18.Murray, R. G. E., and E. Stackebrandt. 1995. Taxonomic note: implementation of the provisional status Candidatus for incompletely described procaryotes. Int. J. Syst. Bacteriol. 45:186-187. [DOI] [PubMed] [Google Scholar]
- 19.Petroni, G., S. Spring, K.-H. Schleifer, F. Verni, and G. Rosati. 2000. Defensive extrusive ectosymbionts of Euplotidium (Ciliophora) that contain microtubule-like structures are bacteria related to Verrucomicrobia. Proc. Natl. Acad. Sci. USA 97:1813-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polz, M. F., D. L. Distel, B. Zarda, R. Amann, H. Felbeck, J. A. Ott, and C. M. Cavanaugh. 1994. Phylogenetic analysis of a highly specific association between ectosymbiotic, sulfur-oxidizing bacteria and a marine nematode. Appl. Environ. Microbiol. 60:4461-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preer, J. R., Jr., and L. B. Preer. 1984. Endosymbionts of protozoa, p. 795-811. In N. R. Krieg and J. G. Holt (ed.), Bergey’s manual of systematic bacteriology, 1st ed., vol. 1. Williams and Wilkins, Baltimore, Md. [Google Scholar]
- 22.Stahl, D. A., and R. Amann. 1991. Development and application of nucleic acid probes, p. 205-248. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley and Sons, Chichester, United Kingdom.
- 23.Stouthamer, R., J. A. J. Breeuwer, and G. D. D. Hurst. 1999. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 53:71-102. [DOI] [PubMed] [Google Scholar]
- 24.Vandekerckhove, T. T. M., A. Willems, M. Gillis, and A. Coomans. 2000. Occurrence of novel verrucomicrobial species, endosymbiotic in Xiphinema americanum-group species (Nematoda, Longidoridae) and associated with parthenogenesis. Int. J. Syst. Evol. Microbiol. 50:2197-2205. [DOI] [PubMed] [Google Scholar]
- 25.Ward-Rainey, N., F. A Rainey, H. Schlesner, and E. Stackebrandt. 1995. Assignment of hitherto unidentified 16S rDNA species to a main line of descent within the domain Bacteria. Microbiology 141:3247-3250. [Google Scholar]