Abstract
The gene DPP6 has been associated with behavioral phenotypes of alcohol use disorder (AUD) in recent human genome wide association studies. To further assess the role of this gene in ethanol-related traits, we tested Dpp6 knockout (KO) mice for ethanol conditioned place preference (CPP), locomotor activity, and ethanol-induced anxiolysis. Male homozygous KO mice (HOM) showed greater preference for the ethanol-paired context compared to wild type littermates (WT) and heterozygous KO mice (HET), while female mice showed no genotypic difference. HOM of both sexes exhibited greater novelty-induced hyperactivity in the CPP apparatus than HET and WT mice in the first two minutes. In a separate experiment, HOM mice showed enhanced locomotor activity following a 1.5 g/kg ethanol injection; however, they also displayed greater locomotor activity during habituation, suggesting basal locomotor differences. Following 1.5 and 2 g/kg injections, HOM mice exhibited EtOH-induced anxiolysis in the first 5 minutes, while the HET and WT mice did not. Lastly, HOM mice displayed a significant sedative response compared to WT animals following a 2 g/kg injection of ethanol. Ultimately, these findings validate a role for Dpp6 in modulating ethanol’s rewarding, anxiolytic, and sedative effects in a sex-dependent manner.
Keywords: Alcohol, Dpp6, knockouts, conditioned place preference, acute alcohol
Introduction
As of 2023, 10.2% of the United States population aged 12 and older met criteria for Alcohol Use Disorder (AUD) within the past year (1). AUD is a complex disorder with both genetic and environmental factors contributing to its etiology. In fact, genetics contribute roughly 50% (2), and emerging evidence from large human genome-wide association studies (GWAS) have identified several novel genes associated with AUD phenotypes. More recently, the dipeptidyl peptidase-like protein 6 (DPP6) gene has been associated with measures of alcohol consumption (e.g., number of drinks per week), problematic alcohol use, and alcohol use disorder (AUD) measurement (3–5), suggesting it may play a role in modulating alcohol-related behaviors.
DPP6 encodes an auxiliary subunit that modulates the biophysical properties and surface expression of A-type voltage-gated potassium channels, particularly Kv4.2. Notably, Dpp6 is most densely expressed in the hippocampus (HPC) and prefrontal cortex (PFC), regions that are both implicated in reward processing and learning (6–11). Behavioral phenotyping of Dpp6 knockout (KO) mice has revealed impairments in tasks related to recognition and spatial learning and memory, as demonstrated in paradigms such as the novel object recognition and Morris water maze tasks (12). Additionally, significant changes in locomotor behavior and weight have been reported in mice with a Dpp6 deletion. More specifically, KO mice tend to show increased locomotor activity and exhibit lower body and brain weights than wild types (WT) (12,13). However, no preclinical studies have assessed the role of Dpp6 in any alcohol-related phenotypes.
Ethanol (EtOH) conditioned place preference (CPP) is a well-established associative learning paradigm, whereby EtOH is paired with a specific context to elicit an unconditioned stimulus response. In this case, we can assess the rewarding/aversive properties of EtOH, which contributes to the development of AUD. Several brain regions have been implicated in CPP acquisition and expression, such as the bed nucleus of the stria terminalis (BNST), nucleus accumbens (NAc), PFC, and HPC (14–17). Beyond the behavioral differences, Dpp6 KO mice show changes in dendritic morphology and excitatory neurotransmission in the HPC (18), which could result in changes in EtOH CPP expression.
Here, we sought to functionally validate the role of Dpp6 in modulating sensitivity to ethanol’s rewarding and locomotor effects through two complementary experiments. Experiment 1 used an ethanol (EtOH) conditioned place preference (CPP) protocol to determine whether Dpp6 KO mice exhibit enhanced conditioned preference for an EtOH-paired context compared to WT controls. Experiment 2 assessed whether Dpp6 KO alters the acute locomotor and anxiolytic response to a stimulatory (1.5 g/kg) or mildly sedative (2 g/kg) dose of EtOH in each of the genotypes. Together these experiments highlight that the global knockout of Dpp6 alters behavioral responses to EtOH.
Methods
Animals
A total of 66 adult Dpp6 homozygous KO (HOM), heterozygous KO (HET), and wild type (WT) littermate mice were used in Experiment 1 (both sexes; postnatal day [PND] 57–152; n=4–17/sex/genotype). Experiment 2 used 20 adult mice of all three genotypes (both sexes; PND 74–101; n=4–8/sex/genotype). All animals came from an in-house breeding colony established from B6.Cg-Dpp6tm1.1Dahn/J cryorecovered breeders (Jax stock # 017972; Su et al. 2011) and maintained with HET x HET breeder pairs. All mice were genotyped via TransnetYX (Memphis, TN) using validated protocols. Mice were group-housed (2–5 per cage) in large Plexiglas cages equipped with a paper house and plastic upper-level compartment for enrichment and maintained on a 12:12 h light/dark cycle (lights on at 07:00). Food (PicoLab 5L0D) and water were available ad libitum. All procedures complied with institutional and national ethical standards and were approved by the University of New Mexico Health Sciences Center Institutional Animal Care and Use Committee.
Place conditioning and open field apparatus
CPP testing was conducted using a two-chambered apparatus (Med Associates, Inc., VT) fitted into a clear Plexiglas chamber (27.3 × 27.3 × 20.3 cm). The CPP insert consisted of two tactilely distinct compartments separated by a black wall and a removable guillotine door. One side featured a stainless-steel bar floor, and the other a grid floor; both were flanked by clear walls. The open field test used the same Plexiglas chamber without the CPP insert. Locomotor and positional data were recorded using the Med Associates Activity Monitor system. Chambers were cleaned with 10% isopropyl alcohol between animals to eliminate olfactory cues.
Drugs and solutions
0.9% sterile saline (UFC Bio, Amherst, NY, via Amazon) and 20% EtOH (v/v in saline) were administered intraperitoneally (i.p.) immediately prior to placement in the CPP chamber. Conditioning sessions alternated between 10 ml/kg saline or 2 g/kg EtOH injections. For the limited access drinking sessions, 200 proof EtOH (Decon Laboratories, King of Prussia, PA) was diluted in tap water to make a 20% (v/v) solution. During the open field experiment, mice received a 1.5 g/kg and 2 g/kg EtOH (in 20% v/v in saline) injection, and the saline volume equivalent of a 1.5 g/kg injection. All solutions were prepared daily prior to behavioral testing.
Experiment 1: Ethanol conditioned place preference
The CPP procedure consisted of a pretest phase, a conditioning phase, and a posttest phase. On the pretest day, mice were moved into the testing room, weighed, and allowed to acclimate for at least 60 min. They were then injected with saline and placed in the center of the apparatus and allowed to explore freely for 30 min to assess initial chamber preference. Due to an average baseline preference of 60.17% ± .012 for the grid floor chamber, a biased conditioning approach was used where each animal was assigned to receive ethanol in the initially non-preferred chamber. During conditioning days, the guillotine door was inserted, and animals were injected with the assigned drug and then placed in the corresponding chamber for five minutes. For example, on day 1, animals were all placed in the bar floor chamber following either 2 g/kg or 10 ml/kg saline injection depending on whether the bar floor type was their conditioned stimulus (CS+) or unconditioned stimulus (CS−), respectively. The following day animals were placed in the chamber with the grid floor type and received injections according to their conditioning group. Four days of conditioning trials were given each week, alternating between saline and ethanol trials. Finally, in the posttest phase (testing days 5, 12, and 19), all animals were injected with saline and placed in the center of the testing apparatus to assess preference (30-minute test). Time spent in each chamber and distance traveled were automatically recorded on each test day. We also computed a CPP change score for each test (% time in EtOH chamber on test day - % time in EtOH chamber on pretest). Two days following test 3, all mice underwent a two hour two-bottle choice homecage drinking procedure (see supplemental material).
Experiment 2: Open field activity
Locomotor activity was recorded on three consecutive days in 30-minute sessions. On days 1 and 2, mice received a saline injection prior to placement in the open field chamber. On day 3, mice were injected with 1.5 g/kg ethanol (20% v/v in saline). A follow-up test was conducted one week later following a 2 g/kg ethanol injection, during which activity was recorded over 60 minutes. Distance traveled (cm) and time spent in the center zone (14.45 cm × 14.45 cm) were automatically recorded.
Data Analysis
All data were analyzed using SPSS software (SPSS, Version 30, Chicago, IL) and graphed and analyzed with GraphPad Prism software (GraphPad Prism, v. 10.5.0, La Jolla, CA). Animals were excluded from analyses on certain days if they had a sipper leak or if the MedAssociates program did not track their behavior correctly. No animals were removed from the entire study as these issues were resolved for testing the following day. Repeated measure two-way or three-way analysis of variance (RM ANOVA) tests were used to assess test*genotype*sex or time*genotype*sex interactions. If there were no significant interactions with sex, data were collapsed. Significance values were set at p < 0.05. All data were assessed for normality using the Shapiro-Wilks Test, and if normality was violated, non-parametric tests were used. For repeated-measures data, violations of Mauchly’s test for sphericity were corrected using Greenhouse–Geisser estimates.
Results
HOM males significantly increased their time spent on the CS+, but not HOM females.
Experiment 1. This experiment tested whether the global homozygous or heterozygous knockout of Dpp6 would alter EtOH CPP. Figure 1 shows time spent on the ethanol-paired floor and CPP change score for each test. A three-way RM ANOVA of time spent on the EtOH-paired floor (test* sex* genotype; GGe ε = .74) revealed a significant main effect of test, [F(2.21, 132.84) = 9.5, p < .001], and significant test*sex, [F(2.21, 132.84) = 4.28, p = .01] and test*sex*genotype interactions, [F(4.43, 132.84) = 3.11, p = .01] (Figure 1A–B). To probe the three-way interaction, two-way RM ANOVAs of test*genotype for each sex were used. In females, no significant main effects or interactions emerged (p’s > .05; Figure 1A). In males, a two-way RM ANOVA (GGe corrected, ε = .75) revealed a significant main effect of test, [F(2.26, 81.23) = 14.68, p < .001], and a significant interaction of test*genotype, [F(6, 108) = 3.87, p = .01] (Figure 1B). Tukey’s multiple comparisons test indicated HOM males significantly increased their time spent on the EtOH-paired floor from pretest to tests 2 and 3, and from test 1 to tests 2 and 3 (p≤0.03 for all). At test 3, HOM males differed significantly from WT males (p = 0.02). Taken together, these data suggest that time spent on the CS+ was significantly increased in the male HOMs, but not in WT or HET males or females of any genotype.
Figure 1. CS+ preference and change scores for DPP6 WT, HET, and HOM mice.
A) Female mice did not show any significant changes in their preference for the CS+ after conditioning. B) Male HOM KO mice show significant increases in preference for CS+ on preference test 2 and 3, and on test 3 they show a significant increase as compared to WT males. C) Similar to preference scores, females did not show changes in their change score or in their ability to express CPP. D) Male KOs show significantly increased CPP change scores after conditioning on test 2 and test 3, both of which are significantly higher as compared to WTs. When assessing their CPP expression, only HET males on test 2 and HOM males on test 2 and 3 showed significant change from zero. WT Female (n = 8); HET Female (n=15); HOM Female (n= 4); WT Male (n= 14); HET Male (n= 17); HOM Male (n= 8) (*p < .05) (**p < .01) ($p < .05, change from zero) ($$p < .01, change from zero)
HOM Males Show Greater EtOH CPP Scores Compared to WTs, HETs, and HOM Females
To account for variations in baseline preference, we also analyzed CPP change scores (% time in CS+ on test N – % time in CS+ on pretest) using three-way RM ANOVA (GGe corrected, ε = .87). We found a significant main effect of test, [F(1.73, 103.91) = 11.2, p < .001], and significant interactions of test*sex, [F(2, 120) = 7.7, p = .001], and test*sex*genotype, [F(4, 120) = 5.47, p < .001] (Figure 1C–D). To probe the three-way interaction, we ran separate two-way RM ANOVAs of test and genotype for each sex. For females, there were no significant main effects or interactions (Figure 1C). In males, a two-way RM ANOVA (GGe corrected, ε = .79) revealed a significant main effect of test, [F(1.58, 56.83) = 19.43, p < .001], and a significant interaction of test*genotype, [F(4, 72) = 5.79, p < .001] (Figure 1D). Tukey’s multiple comparisons tests confirmed significant increases in CPP score in HOM males from test 1 to tests 2 and 3 (p ≤0.02), as well as between WT and HOM males on tests 2 and 3 (p ≤ 0.02). These data suggest that CPP change score was significantly increased in HOM males only, consistent with the raw CS+ preference scores.
Only Males Show Significant EtOH CPP Expression
One-sample t tests of CPP change scores (vs. zero) were used to assess the development of significant EtOH CPP expression. In females (Figure 1C), no group showed significant effects, though HETs showed trends at test 2 (p = .051) and test 3 (p = .077). In the males (Figure 1D), HETs showed significance at test 2 (M = 12.98%, SD = 24.199%), [t(16) = 2.2, p = .042], and a trend at test 3 (p = .068). HOM males showed significant effects at test 2 (M = 22.47%, SD = 12.87%), [t(7) = 4.94, p = .002], and test 3 (M = 25.84%, SD = 14.85%), [t(7) = 4.92, p = .002]. Taken together, we can confirm that the HOM males and the HET males showed significant changes in preference for the CS+ context after two and three rounds of conditioning.
HOM Mice Display Novelty-Induced Hyperactivity
Next, we assessed locomotor activity during the pretest (Figure 2A). A three-way RM ANOVA of time (30 minutes), sex, and genotype (GGe corrected, ε = .04) revealed significant main effects of time, [F(1.14, 68.40) = 931.95, p < .001], and sex, [F(1, 60) = 10.09, p = .002], and a significant interaction of time*sex, [F(29, 1740) = 9.5, p < .001]. When collapsed by genotype, a two-way RM ANOVA of time and sex revealed a significant main effect of time, [F (16.66, 1067) = 58.20, p < .001], and sex, [F (1, 64) = 11.97, p < .001], and no significant interactions.
Figure 2. Pretest locomotor activity in Dpp6 WT, HET, and HOM females and males.
A) pretest locomotor activity changed across the 30-minute session time and females moved around more than males. B) In the first 5 minutes of the session, both female and male HOM KOs exhibited greater locomotor activity in the first two minutes compared to WT and HET animals. Females also moved more than males in general. C) Total locomotor activity only showed a significant difference between females and males, suggesting that the genotypes move similarly. (*p < .05) (**p < .01) (##p < .01, ME sex) ($$$p < .001, HOM vs WT) (&&&p < .001, HOM vs HET)
A focused analysis of the first five minutes revealed significant main effects of time, [F(4, 240) = 20.13, p < .001], and sex, [F(1, 60) = 10.03, p = .002], and a significant interaction of time*genotype, [F(8, 240) = 4.01, p < .001] (Figure 2B). There was a trend towards a time*sex*genotype interaction (p = .06). Collapsing across sex, a two-way RM ANOVA, confirmed significant main effects of time [F (4, 252) = 122.8, p < .001] and genotype, [F (2, 63) = 5.264, p < .001], and a time*genotype interaction, [F (8, 252) = 11.35, p < .001]. Tukey’s multiple comparisons tests indicated the HOMs were significantly more active than WTs and HETs at minute 1 (both p’s < .001), and minute 2 (both p’s < .001). However, total locomotor activity showed only a main effect of sex, [F (1, 60) = 10.05, p = .002], suggesting that the locomotor differences are due to the initial novelty of the context (Figure 2C).
HOMs Show Greater Locomotor Activity on Saline Injection Days in Open Field Test
Figure 3 shows locomotor activity on each day of the open field test. On habituation day 1, a three-way RM ANOA of time (30 minutes), sex, and genotype (GGe corrected, ε = .34) revealed main effects of time, [F(9.99, 289.63) = 30.06, p < .001, ηp2 = .51], sex, [F(1, 29) = 8.14, p = .01], and genotype, [F(2, 29) = 13.68, p < .001], and significant interactions of time*sex, [F(9.99, 289.63) = 2.46], and time*genotype, [F(19.97, 289.63) = 2.08, p = .01] (Figure 3B). To probe the interactions, we collapsed on either sex or genotype. A two-way RM ANOVA of time and sex (GGe corrected, ε = .29) revealed a significant main effect of time, [F(8.5, 280.6) = 29.69, p < .001], sex, [F(1, 33) = 4.25, p = .047] and a time*sex interaction, [F(8.50, 280.6), p = .017]. Tukey’s multiple comparisons tests indicated that females were more active than males at minute 1, 3, 5, 13, 23, 24, and 30 (p’s < .05). Next, a two-way RM ANOVA of time and genotype (GGe corrected, ε = .32) revealed a significant main effect of time, [F(9.33, 298.47) = 28.94, p < .001], genotype, [F(2, 32) = 9.84, p < .001], and an interaction of time*genotype, [F(18.66, 298.47) = 1.86, p = .017]. Tukey’s multiple comparisons tests revealed elevated activity in HOMs vs. WTs at multiple time points: 1–3, 10, 11, 14, 18, 19, and 22 (p’s < .05) and HETs at minute 28 ( p = .02). Additionally, a two-way ANOVA of sex and genotype on total locomotor activity revealed a main effect of sex, [F (1, 29) = 8.144, p < .001], and genotype, [F (2, 29) = 13.68, p < .001] (Figure 3C), suggesting that females show greater locomotor activity than males and that HOMs show greater locomotor activity in a 30-minute test in a novel context.
Figure 3. Assessment of 30-minute locomotor activity each day.
A) A timeline of experiment 2. B) Habituation day 1 showed significant effects of sex, genotype, and time. C) Total locomotor activity showed main effects of sex and genotype. D) Habituation day 2 also showed significant effects of sex, genotype, and time. E) Total locomotor activity on day 2 shows a significant effect of genotype. F) An acute injection of 1.5 g/kg EtOH showed significant effects of sex, genotype, and time. G) Total locomotor on day 3 showed significant effects of sex and genotype. Lastly, H) an injection of 2 g/kg on day 10 showed significant effects of sex and time but not genotype. Similarly, I) shows a main effect of sex only. WT Female (n = 5); HET Female (n=8); HOM Female (n= 4); WT Male (n= 5); HET Male (n= 7); HOM Male (n= 6); (***p < .001) (##p < .01, ME of sex) (**p < .01 and ME genotype across time)
On habituation day 2, a three-way RM ANOVA revealed significant main effects of time, [F(29, 812) = 31.42, p < .001], sex, [F(1, 28) = 7.39, p = .01], and genotype, [F(2, 28) = 5.35, p = .01], with a trend toward a time*genotype interaction (p = .06). These data suggest that on the second day of the saline injection, females moved more than males and, overall, there were genotype differences across time (Figure 3D). Total distance travelled showed a genotype effect, [F (2, 28) = 5.48, p = .01], and a trend toward a main effect of sex (p = .06; Figure 3E). A post hoc Tukey’s multiple comparisons test revealed that HOM mice had significantly greater total distance traveled than WT mice (p = .007).
HOMs Show Greater Locomotor Activity Following a 1.5 g/kg EtOH Injection, but not a 2 g/kg EtOH Injection
On the first acute ethanol treatment day, a three-way RM ANOVA (GGe corrected, ε = .23) revealed significant main effects of time, [F(6.69, 194.09) = 31.98, p < .001], sex, [F(1, 29) = 8.17, p = .01], and genotype, [F(2, 29) = 5.58, p = .01]. These data suggest that an acute injection of 1.5 g/kg ethanol shows a significantly more stimulatory response in females compared to males and that there are significant differences between genotypes (Figure 3F). A two-way ANOVA of total locomotor activity showed similar effects of sex, [F (1, 29) = 8.17, p = .01], and genotype, [F (2, 29) = 5.58, p = .01] (Figure 3G), indicating enhanced EtOH-induced stimulation in HOMs compared to WTs and females compared to males.
Following a 2 g/kg injection of EtOH, a three-way RM ANOVA (GGe corrected, ε = .21) revealed significant main effects of time, [F(6.07, 176.06) = 50.95, p < .001], and sex, [F(1, 29) = 10.74, p < .01] (Figure 3H). In the first 30 minutes post-injection (Figure 3I), a two-way ANOVA revealed a main effect of sex, [F(1, 29) = 10.74, p < .01], and no other significant effects, suggesting that at a higher dose locomotor activity was similar across all genotypes.
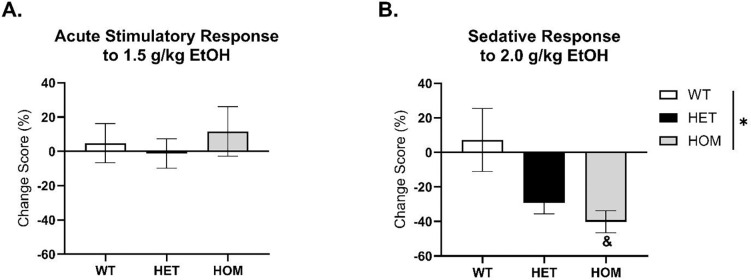
HOMs Show Sedative Response to 2 g/kg EtOH Injection
Change scores were calculated for each EtOH dose using day 2 (saline) as baseline. At the 1.5 g/kg EtOH dose, a two-way ANOVA of sex and genotype did not yield any significant effects, suggesting that the 1.5 g/kg dose did not induce a greater stimulatory response in the KOs (Figure 5A). At the 2 g/kg EtOH dose, a two-way ANOVA of sex and genotype revealed a significant main effect of genotype, [F (1, 28) = 0.2368, p = .02]. When collapsed by sex, a one-way ANOVA confirmed this genotype effect, [F (2, 31) = 4.702, p = .017]. Specifically, there was a significant difference between WTs and HOMs (p = .018), suggesting that HOMs display a greater sedative response in the first 30 minutes compared to WTs (Figure 5B).
Figure 5. Change scores of EtOH injection days from habituation day 2.
There were no main effects of sex, so groups were collapsed. A) 1.5 g/kg injection did not show any significant difference between sex or genotype. B) A 2 g/kg EtOH injection resulted in a main effect of genotype. Specifically, the HOMs showed a greater sedative response compared to WTs. (*p < .05) (&p < .05, different from WT)
Only HOMs Show EtOH-Induced Anxiolytic Behavior
Percent of time spent in the center of the Open Field Activity chambers across injection days can be seen in Figure 4. When collapsed by sex, a mixed-effects analysis (GGe corrected, ε = .64) revealed main effects of day, [F (1.917, 60.71) = 27.64, p < .001], genotype, [F (2, 32) = 8.868, p < .001], and a day*genotype interaction, [F (6, 95) = 3.942, p = .002] (Figure 4A). Tukey’s multiple comparisons test revealed HOMs spent significantly greater time in the center than WTs and HETs on day 3 (1.5 g/kg; p’s = .01 – .02) and day 10 (2 g/kg; p’s = .02 – .03), suggesting a genotypic-specific increase in anxiolytic response to ethanol. Within-genotype comparisons showed significant changes across days. In WTs, time spent in the center was lower on days 2, 3, and 10 compared to day 1 (p’s < .05). In the HETs, time spent in the center was lower on days 3 and 10 compared to both day 1 and day 2(p’s < .05). Lastly, HOMs spent significantly more time in the center on day 1 vs. day 2 and 3 (p’s < .05). These data indicate that the HOMs did not significantly differ from their second saline injection day on days where they received EtOH, when analyzing the entire 30-minute session (Figure 4A).
Figure 4. Anxiety-like behavior across test days.
A) The percent time spent in the center was significantly difference between genotypes across days. HOMs WTs and HETs showed decreased anxiolytic behavior compared to HOMs on the EtOH injection days. B) When we looked at the first five minutes, we found that genotypes were different across testing days. Similarly, WTs and HETs were significantly different from HOMs on days where EtOH was administered. C) Representative images of the locomotor activity seen in each genotype on day 3 (1.5 g/kg injection; #p < .05, different from Hab 1) ($p < .05, different from Hab 2) (*p < .05, different from HOMs in the same group).
Because of the genotypic differences in locomotor activity during the initial minutes of testing, we decided to look at the change in percent time spent in the center in the first five minutes (Figure 4B). A mixed effects analysis (GGe corrected, ε = .80) revealed significant main effects of day, [F (2.398, 75.95) = 12.54, p < .001], and genotype, [F (2, 32) = 15.96, p < .001], and a significant interaction of day*genotype, [F (6, 95) = 5.561, p < .001]. Tukey’s multiple comparisons test indicated significant findings between WTs and HOMs (p < .01; p < .001) and HETs and HOMs (p < .01; p < .001) at the 1.5 g/kg and 2 g/kg dose, respectively, with HOMs having greater center time than the other genotypes. There were also significant differences between days in each group. In WTs, there were differences between day 1 vs. day 2 and day 3 (p’s < .05). In HETs, there were differences between day 1 vs. day 2 and 3 (p’s < .05). Finally, in HOMs, there were differences between day 1 vs. day 10 (p < .05), day 2 vs. day 3 and 10 (p’s < .05), suggesting that in the first 5 minutes of the session HOMs exhibited an EtOH-induced anxiolytic response. Representative images of locomotor activity in the chamber on day 3 (1.5 g/kg EtOH injection) can be seen in Figure 4C.
Discussion
To our knowledge, this study is the first to validate the involvement of Dpp6 in alcohol-related phenotypes and reveals several key differences between WT and KO mice. In experiment 1, HOM males exhibited a greater CPP response than the other genotypes and HOM females. Additionally, HOM males and females exhibited significant increases in their initial locomotor activity during the pretest, suggesting novelty-induced hyperactivity. In experiment 2, we replicated novelty-induced hyperactivity in the HOMs, and we saw greater total locomotor activity across days. Interestingly, in HOM males and females, both a 1.5 g/kg and a 2 g/kg injection of EtOH significantly increased the percentage of time spent in the center of the open field test compared to the other genotypes, suggesting a greater anxiolytic response to EtOH in the first 5 minutes of the locomotor test, but not when analyzing the entire 30-minute session. However, when we compared the difference between each EtOH injection day and habituation day 2, we found that the higher dose produced a significant sedative response in the HOMs as compared to the WTs. Beyond behavioral differences, we assessed weights in these mice because a loss of Dpp6 is associated with lower body weight (12,13). Specifically, Lin et al. (2018) found that Dpp6 loss resulted in significantly lower body weights and brain weights at different developmental times. Here, we did not find significant differences between our WT and KO littermates (Figure S1 & S4); however, we did see a trend in the same direction.
First, we found a significant CPP response on test 2 and 3 in the male HOMs, and on test 2 in the male HETs (Figure 1D), suggesting that the male KOs are more sensitive to the rewarding properties of EtOH. A limitation of this study is that the WTs did not show preference for the EtOH-paired chamber, which could be explained by their C57BL6/J (B6) background. Several studies have highlighted that B6 mice are not as sensitive to the rewarding properties of EtOH as compared to DBA mice, and only modestly show EtOH CPP (19). Thus, regardless of the WT indifference, the male KOs were able to form stronger context-drug associations. Interestingly, we found that female KOs did not condition to the EtOH-paired chamber, which suggests that Dpp6 might be involved in a sex-dependent manner on associative or context-dependent learning; however, this remains a gap in the field.
There are several brain regions implicated in CPP, including the NAc, HPC, BNST, and the prelimbic cortex (20–22). Dpp6 is highly expressed in the HPC and is one of two auxiliary β-subunits that modulates the expression and function of A-type voltage-gated Kv4.2 channels, which are important for neuronal excitability (23,24). Thus, increased dendritic excitability in the HPC and, therefore, increased excitatory projections to the NAc could be driving the differences in the expression of EtOH CPP (25); however, other brain regions co-expressing Dpp6 and Kv4.2 channels, such as the striatum or cerebellar cortex should be considered (23). Additionally, in Dpp6 KO mice, Lin et al. (2018) found that KO mice displayed greater locomotor activity and deficits in several learning and memory paradigms. Similarly, Kiselycznyk, Hoffman, and Holmes (2012) found that Kv4.2 KO mice displayed greater locomotor activity and greater anxiolytic behavior, which is consistent with our results. Together, these findings support the notion that both Dpp6 and Kv4.2 channels are co-expressed (23), and suggests that both are also important for changes in locomotor activity.
Next, we found that HOM KO mice displayed greater novelty-induced hyperactivity in the CPP chamber and the open field chamber. This behavioral response involves the HPC, VTA, and locus coeruleus among other brain regions (26,27). Hippocampal circuits play an important role in novelty recognition (28), which further suggests that deletion of this gene disrupts hippocampal neurotransmission that may be important for this behavior. This is in line with Procaccini et al. (2011) who found more c-Fos expression in the hippocampus of GluA1-KO mice following novelty-induced hyperactivity.
Additionally, studies of both Dpp6 KO and Kv4.2 KO models have found that anxiety-like behavior is reduced measured by time spent in the open arms of the elevated plus maze(12,29). We did not significantly detect differences in anxiety-like behavior as measured by percent of the time spent in the center on habituation day 1 when mice received an injection of saline, which is in line with unpublished data from Lin et al. (2018). However, this study demonstrated that a 1.5 and 2 g/kg dose of EtOH significantly decreased anxiety-like behavior in male and female HOM KO mice compared to other genotypes, as measured by the percent of time they spent in the center of the open field chamber. The ventral HPC (vHPC) is implicated in anxiety-related behaviors (28). In fact, lesions and chemogenetic inhibition of the vHPC have shown to decrease anxiety-like behavior (30,31), suggesting that the reduction in anxiety-like behavior following EtOH seen in our experiment might be due to disruption of the ventral, but not dorsal, HPC. While EtOH has been shown to produce anxiolytic behavior independent of locomotor increases (32), we did not find this in our WT or HET animals, suggesting that full deletion of Dpp6 exacerbates the anxiolytic effect potentially produced by EtOH, and that this effect is present following both a 1.5 and 2 g/kg injection in the first 5 minutes.
Lastly, we found greater total locomotor activity in HOMs on all test days except following a 2 g/kg EtOH dose, suggesting that this dose was sufficient to blunt their hyperlocomotor activity. This is unexpected, given our findings in experiment 1; however, mice were only in the CPP chamber for 5 minutes, whereas they were in the open field chamber for 30 minutes to one hour. The sedative response found in the HOMs in the first 30 minutes in experiment 2 suggests that they may be more sensitive to the sedative properties of EtOH than WT or HET mice. While we did not see a stimulatory response at the 1.5 g/kg dose, it remains to be seen if the HOMs would have displayed an increased stimulatory response at a lower dose, given that CPP expression was greater at 2 g/kg. Additionally, these effects seem to be specific to systemic administration of EtOH. When mice self-administered EtOH, there were no significant differences in intake, nor did these intakes correlate with their CPP expression on test 3 (Figure S2). One limitation, though, is that these mice were given a two-hour drinking session in the regular light cycle to match the CPP experiment. Thus, future work should focus on intake patterns during their active cycle to see if Dpp6 has a role in EtOH consumption.
Conclusion
DPP6 is involved in several alcohol-related phenotypes, which warrants further investigation. This was the first study looking at the role of Dpp6 in the rewarding and sedative properties of alcohol. Overall, we found that male, but not female, HOM KOs are more sensitive to both the rewarding properties and sedative effects of 2.0 g/kg injection dose. Anxiolytic behavior following EtOH injections of both 1.5 and 2.0 g/kg was seen in both male and female HOM KO mice compared to HET KO and WT, and both sexes seemed to show a greater novelty-induced locomotor response than the other genotypes. Future work focusing on the underlying mechanisms are necessary to understand the behavioral differences seen here and their potential relationship to risk for AUD.
Supplementary Material
Acknowledgements
The authors wish to express sincere gratitude to Rebeka Sultana for maintaining the breeding colony for this research.
Funding statement
This work was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism to AMB-L (R00AA027835).
Footnotes
Conflict of interest disclosure
The authors declare no conflicts of interest.
Ethics approval statement
The animal studies protocols were approved by the Institutional Animal Care and Use Committee and the Animal Resource Facility at the University of New Mexico Health Sciences Center
Permission to reproduce material from other sources
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Reference
- 1.2023 NSDUH Detailed Tables | CBHSQ Data [Internet]. [cited 2025 Apr 30]. Available from: https://www.samhsa.gov/data/report/2023-nsduh-detailed-tables [Google Scholar]
- 2.Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med. 2015. Apr;45(5):1061–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hade AC, Philips MA, Reimann E, Jagomäe T, Eskla KL, Traks T, et al. Chronic Alcohol Use Induces Molecular Genetic Changes in the Dorsomedial Thalamus of People with Alcohol-Related Disorders. Brain Sci. 2021. Apr;11(4):435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou H, Sealock JM, Sanchez-Roige S, Clarke TK, Levey DF, Cheng Z, et al. Genome-wide meta-analysis of problematic alcohol use in 435,563 individuals yields insights into biology and relationships with other traits. Nat Neurosci. 2020. July;23(7):809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deak JD, Levey DF, Wendt FR, Zhou H, Galimberti M, Kranzler HR, et al. Genome-Wide Investigation of Maximum Habitual Alcohol Intake in US Veterans in Relation to Alcohol Consumption Traits and Alcohol Use Disorder. JAMA Netw Open. 2022. Oct 27;5(10):e2238880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigurdsson T, Duvarci S. Hippocampal-Prefrontal Interactions in Cognition, Behavior and Psychiatric Disease. Front Syst Neurosci [Internet]. 2016. Jan 26 [cited 2025 June 4];9. Available from: https://www.frontiersin.org/journals/systems-neuroscience/articles/10.3389/fnsys.2015.00190/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyer ES, Vitaro P, Wu S, Muir J, Tse YC, Cvetkovska V, et al. Reward integration in prefrontal-cortical and ventral-hippocampal nucleus accumbens inputs cooperatively modulates engagement. Nat Commun. 2025. Apr 15;16(1):3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience. 2010. Dec 15;171(3):830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeGates TA, Kvarta MD, Tooley JR, Francis TC, Lobo MK, Creed MC, et al. Reward behavior is regulated by the strength of hippocampus-nucleus accumbens synapses. Nature. 2018. Dec;564(7735):258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chau BKH, Jarvis H, Law CK, Chong TTJ. Dopamine and reward: a view from the prefrontal cortex. Behav Pharmacol. 2018. Oct;29(7):569. [DOI] [PubMed] [Google Scholar]
- 11.LeGates TA, Kvarta MD, Tooley JR, Francis TC, Lobo MK, Creed MC, et al. Reward behaviour is regulated by the strength of hippocampus–nucleus accumbens synapses. Nature. 2018. Dec;564(7735):258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin L, Murphy JG, Karlsson RM, Petralia RS, Gutzmann JJ, Abebe D, et al. DPP6 Loss Impacts Hippocampal Synaptic Development and Induces Behavioral Impairments in Recognition, Learning and Memory. Front Cell Neurosci. 2018. Mar 29;12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin L, Petralia RS, Holtzclaw L, Wang YX, Abebe D, Hoffman DA. Alzheimer’s disease/dementia-associated brain pathology in aging DPP6-KO mice. Neurobiol Dis. 2022. Nov;174:105887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pati D, Pina MM, Kash TL. Ethanol-induced conditioned place preference and aversion differentially alter plasticity in the bed nucleus of stria terminalis. Neuropsychopharmacology. 2019. Oct;44(11):1843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Z, Han H, Wang M, Xu L, Hao W, Cao J. Morphine conditioned place preference depends on glucocorticoid receptors in both hippocampus and nucleus accumbens. Hippocampus. 2006;16(10):809–13. [DOI] [PubMed] [Google Scholar]
- 16.Zavala AR, Weber SM, Rice HJ, Alleweireldt AT, Neisewander JL. Role of the prelimbic subregion of the medial prefrontal cortex in acquisition, extinction, and reinstatement of cocaine-conditioned place preference. Brain Res. 2003. Nov 14;990(1):157–64. [DOI] [PubMed] [Google Scholar]
- 17.Hitchcock LN, Lattal KM. Involvement of the dorsal hippocampus in expression and extinction of cocaine-induced conditioned place preference. Hippocampus. 2018;28(3):226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin L, Sun W, Throesch B, Kung F, Decoster JT, Berner CJ, et al. DPP6 regulation of dendritic morphogenesis impacts hippocampal synaptic development. Nat Commun. 2013;4:2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham CL, Shields CN. Effects of sex on ethanol conditioned place preference, activity and variability in C57BL/6J and DBA/2J mice. Pharmacol Biochem Behav. 2018. Oct;173:84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng R, Chen Y, Zhang J, Liu Q, Zheng Y, Wang Z. Prelimbic cortex is involved in the regulation of morphine-induced conditioned place preference in both resistant and sensitive mice. Sci Rep. 2025. Feb 15;15(1):5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pati D, Pina MM, Kash TL. Ethanol-induced conditioned place preference and aversion differentially alter plasticity in the bed nucleus of stria terminalis. Neuropsychopharmacology. 2019. Oct;44(11):1843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Zhu H, Liu Z, Chen X, Su X, Ma C, et al. A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat Neurosci. 2019. Dec;22(12):1986–99. [DOI] [PubMed] [Google Scholar]
- 23.Clark BD, Kwon E, Maffie J, Jeong HY, Nadal M, Strop P, et al. DPP6 localization in brain supports function as a Kv4 channel associated protein. Front Mol Neurosci [Internet]. 2008. Oct 23 [cited 2025 Apr 30];1. Available from: https://www.frontiersin.orghttps://www.frontiersin.org/journals/molecular-neuroscience/articles/10.3389/neuro.02.008.2008/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kise Y, Kasuya G, Okamoto HH, Yamanouchi D, Kobayashi K, Kusakizako T, et al. Structural basis of gating modulation of Kv4 channel complexes. Nature. 2021;599(7883):158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trouche S, Koren V, Doig NM, Ellender TJ, El-Gaby M, Lopes-dos-Santos V, et al. A Hippocampus-Accumbens Tripartite Neuronal Motif Guides Appetitive Memory in Space. Cell. 2019. Mar 7;176(6):1393–1406.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fois GR, Bosque-Cordero KY, Vazquez-Torres R, Miliano C, Nogues X, Jimenez-Rivera CA, et al. Locus coeruleus activation during environmental novelty gates cocaine-induced long-term hyperactivity of dopamine neurons. iScience. 2022. Apr 15;25(4):104154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Procaccini C, Aitta-aho T, Jaako-Movits K, Zharkovsky A, Panhelainen A, Sprengel R, et al. Excessive novelty-induced c-Fos expression and altered neurogenesis in the hippocampus of GluA1 knockout mice. Eur J Neurosci. 2011;33(1):161–74. [DOI] [PubMed] [Google Scholar]
- 28.Fanselow MS, Dong HW. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron. 2010. Jan;65(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiselycznyk C, Hoffman DA, Holmes A. Effects of genetic deletion of the Kv4.2 voltage-gated potassium channel on murine anxiety-, fear- and stress-related behaviors. Biol Mood Anxiety Disord. 2012. Mar 2;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C, Zhang Y, Shao S, Cui S, Wan Y, Yi M. Ventral Hippocampus Modulates Anxiety-Like Behavior in Male But Not Female C57BL/6 J Mice. Neuroscience. 2019. Oct 15;418:50–8. [DOI] [PubMed] [Google Scholar]
- 31.Parfitt GM, Nguyen R, Bang JY, Aqrabawi AJ, Tran MM, Seo DK, et al. Bidirectional Control of Anxiety-Related Behaviors in Mice: Role of Inputs Arising from the Ventral Hippocampus to the Lateral Septum and Medial Prefrontal Cortex. Neuropsychopharmacology. 2017. July;42(8):1715–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boerngen-Lacerda R, Souza-Formigoni MLO. Does the increase in locomotion induced by ethanol indicate its stimulant or anxiolytic properties? Pharmacol Biochem Behav. 2000. Oct;67(2):225–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.