Abstract
Malignant rhabdoid tumors (MRTs) are aggressive pediatric cancers with poor outcomes. MRTs exhibit low tumor mutational burden, yet recent studies reported immune cell infiltration. Here, we used spatial transcriptomics and multi-omic profiling to study immune cell infiltration in MRT samples. We observed a diverse repertoire of candidate tumor antigens (TAs), IRF1 signaling activity and elevated expression of antigen processing and presentation genes, strongly associated with a “hot” tumor immune microenvironment (TIME). Upregulation of factors involved in skeletal muscle development was observed in some MRTs with higher CD8 + T cell infiltration and the ratio of M1/M2 macrophages was positively correlated with cytotoxic lymphocyte infiltration. We identified genes, such as SPP1, preferentially expressed in tumor-associated macrophages (TAMs) and noted the MITF regulatory network appeared active in the M2-like TAMs.
Keywords: Malignant rhabdoid tumor, single-cell multiomics, spatial transcriptomics, tumor heterogeneity, tumor microenvironment, tumor antigens, tumor-associated macrophages
Introduction
Malignant rhabdoid tumors (MRTs) are rare but aggressive pediatric cancers that frequently arise in the kidney (renal MRTs), soft tissues (extracranial, extrarenal MRTs, eMRTs), and central nervous system (atypical teratoid rhabdoid tumors, AT/RTs)1–4. Treatment options vary depending on the site and stage, but often include local control through surgery or radiation therapy, in conjunction with chemotherapy (often vincristine, actinomycin, and doxorubicin)5, with additional consideration for EZH2-inhibitors6. The 5-year overall survival rate is 27%7, indicating the need for an improved understanding of MRT vulnerabilities and therapies to target these.
Nearly all MRTs exhibit biallelic mutations in SMARCB1 and, less commonly, biallelic mutations in SMARCA4, both of which encode core subunits of the switch/sucrose non-fermentable (SWI/SNF) chromatin-remodeling complex8,9. Similar to other pediatric malignancies, the somatic mutation rate in MRTs is low10–12. MRTs were thus initially believed to have minimal immune cell infiltration and be less likely to respond to immunotherapy13. However, studies using bulk and single-cell RNA-Seq have inferred the presence of tumor-infiltrating immune cells in MRT14,15. The levels of different infiltrating immune cells appeared correlated with different rhabdoid tumor molecular subgroups defined by DNA methylation and gene expression patterns14–19. Other studies investigated the mechanisms underlying the observed variation in immune cell infiltration levels. Leruste et al. reported that the immunogenicity of rhabdoid tumors, measured by the cytolytic activity (CYT) score20, is mediated by the re-expression of endogenous retroviruses and the activation of interferon signaling, arising from dysfunctional chromatin regulation as a result of SMARCB1 loss14, but previously we did not observe epigenetic derepression of retroviral elements15. Instead, we observed increased expression of immune-related genes, HOX genes, and mesoderm developmental regulators in MRT subgroups with higher levels of cytotoxic T cell infiltration15. We proposed that the increased T cell level observed in MRT “Group1 (ATRT-MYC-like)” was potentially attributable to the co-deletion of the immune modulator MIF along with SMARCB115.
Macrophages are the most abundant immune cell population in rhabdoid tumors and are a negative prognostic factor for AT/RT patient survival14–16, raising the possibility that the composition of the tumor immune microenvironment (TIME) may play an important role in regulating the antitumor response. Tumor-associated macrophages (TAMs) participate in various aspects of cancer progression, metastasis, and resistance to treatment. TAMs are not a homogeneous entity but rather exist in a spectrum of activation states and have been broadly classified into the M1 and M2 two subgroups, which respectively have anti-tumor or pro-tumor activities21,22. Single-cell RNA sequencing (scRNA-seq) of fluorescence-activated cell sorted myeloid cells from one peripheral blood mononuclear cell (PBMC) sample and three AT/RT samples revealed heterogeneous TAM subpopulations14. However, AT/RTs only represent the subset of MRTs that occur in the central nervous system, and extracranial MRTs may possess different TIME compositions. Analysis of extracranial MRT TIME thus has the potential to provide new insights into resident immune cell populations.
To study tumor cells in relation to TIME, we profiled primary MRT samples using spatial transcriptomic and multi-omic approaches. We found that MRTs express several candidate tumor antigens (TAs) but TA expression did not correlate with inferred cytotoxic lymphocyte levels. We observed that the immune cell composition varied between intra-tumor regions, as well as across different tumors and that the inferred activity of the IRF1 regulatory network and the expression of antigen processing and presentation genes were associated with cytotoxic lymphocyte infiltration. A subset of MRTs with high CD8 + T cell levels exhibited increased expression of a skeletal muscle development transcriptional program. M1/M2 macrophage ratios were positively correlated with cytotoxic lymphocyte infiltration, indicating the potential role of macrophages in modulating the immune response. We also identified genes and pathways preferentially associated with M2-like TAMs.
Results
Experimental Design
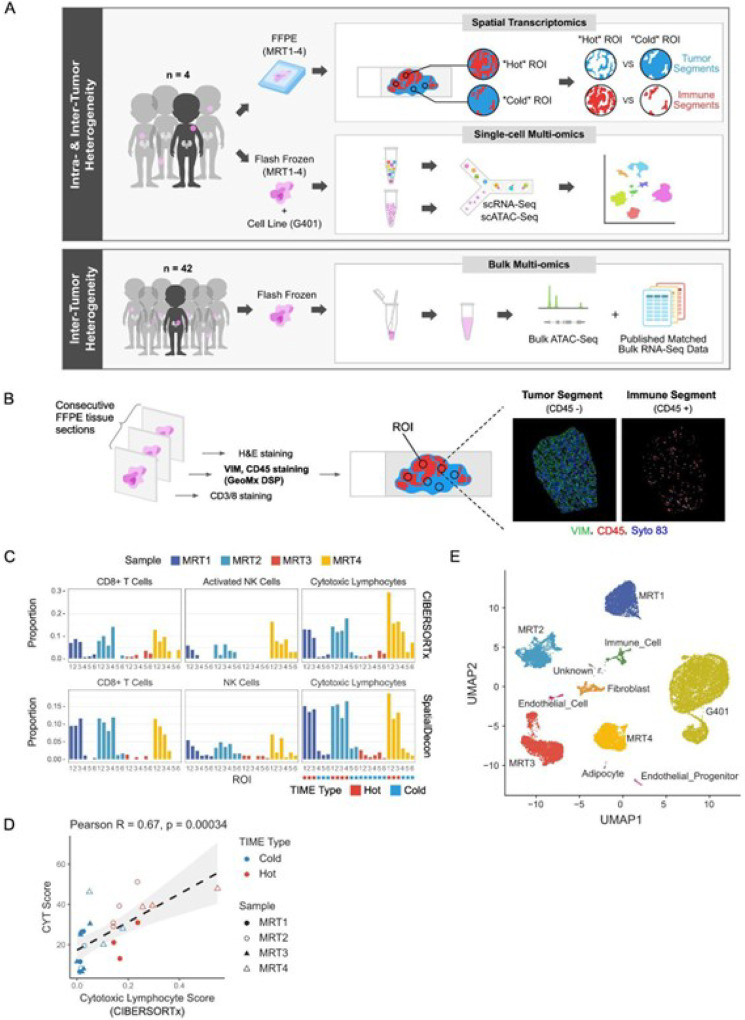
We profiled 4 MRT samples (MRT1– 4) using spatial transcriptomics and single-cell sequencing technologies (Methods; Fig. 1A–B). All four were extrarenal MRTs with either SMARCB1 loss (MRT1 and 2) or SMARCA4 loss (MRT3 and MRT4) (Fig. S2A; Table S1).
Figure 1. Experimental Design (See also Figures S1 and S2).
A) Summary of tumor samples and methods used in this study. Four MRT samples were profiled using spatial transcriptomics and single-cell technologies. To compare and validate findings in a larger cohort, bulk RNA-seq data from 65 MRT samples11 were obtained, and bulk ATAC-seq data were generated for 42 samples from the same cohort.
B) Overview of Bruker/NanoString GeoMX DSP spatial transcriptomics profiling strategy (Methods).
C) Assignment of tumor immune microenvironment (TIME) types based on cell type deconvolution of the immune segments from the 24 regions of interest (ROIs) profiled with spatial transcriptomics.
D) Scatterplot showing the correlation between CIBERSORTx-inferred cytotoxic lymphocyte infiltration and the cytolytic (CYT) score from spatial transcriptomic data. The grey band shows the 95% confidence interval.
E) UMAP visualization and annotation of clusters from single-cell multi-omic data (Methods).
Spatial transcriptomics
Six regions of interest (ROIs) per sample were selected based on T cell infiltration levels, with each ROI containing a CD45 + immune segment and a CD45- tumor segment (Fig. 1B; Methods). In total, 48 segments from 24 ROIs across MRT1–4 were profiled using the Bruker/NanoString GeoMx Digital Spatial Profiler (DSP) Human Whole Transcriptome Atlas (WTA) RNA Assay(Methods). The TIME types (“hot” or “cold”) of selected ROIs were defined based on the proportion of cytotoxic lymphocytes in the immune segments inferred by in silico deconvolution tools, CIBERSORTx23 and SpatialDecon23,24 (Fig. 1C; Table S4–5; Methods). The cytolytic activity (CYT) score20 (Methods), which serves as a measure of cytotoxic lymphocyte activity, was positively correlated with cytotoxic lymphocyte infiltration in immune segments (Pearson R = 0.67, p-value = 0.00034; Fig. 1D), supporting the TIME types defined by the deconvolution results.
Single-cell multi-omics
Using the 10X Genomics Chromium Single Cell Epi Multiome ATAC + Gene Expression (single-cell Multiome for short) assay, we profiled gene expression and chromatin accessibility in 22,922 nuclei isolated from MRT1–4 and an MRT cell line G401, which we used as a control for SMARCB1 loss when assigning cell types based on SMARCB1 expression (Methods). We combined scRNA and scATAC results from all samples to identify 11 cell clusters (Fig. S1A; Methods). Clusters 1–5 were each composed almost exclusively of cells (> 99.9% of cells) from one sample, while the other six clusters (clusters 6–11) contained cells from multiple samples (Fig. S1B). Notably, clusters 6–11 contained only cells from the four flash-frozen tumor tissues but not from the G401 cell line, compatible with the notion that these clusters were composed of cell types found only in primary tissues. We assigned cell type identity to clusters based on transcriptional and epigenetic features (Fig. S1C-G; Methods). Clusters 1–5 were identified as malignant cell clusters (G401 and MRT1–4), while clusters 6–11 were assigned to fibroblasts, immune cells, endothelial cells, endothelial progenitor cells, adipocytes, and an unidentifiable (“unknown”) cell type (Fig. 1E).
We compared our single cell results to bulk RNA-seq data we generated previously from 65 MRT samples11, and to bulk ATAC-seq data we generated in this study for 42 samples from the same cohort (Fig. 1A; Table S1; Methods).
MRTs express a wide variety of tumor antigens
Malignant rhabdoid tumors share a phylogenetic link with neural crest-derived Schwann cells and are arrested in their differentiation due to SMARCB1 loss25. Therefore, tissue-specific and developmental genes may be expressed abnormally in MRTs, triggering an immune response and stimulating T cell infiltration observed in the “hot” ROIs. To test this hypothesis, we compiled a list of 288 tumor antigens (TAs) (Table S326), including cancer-testis antigens and oncofetal genes, and examined their expression levels in MRTs.
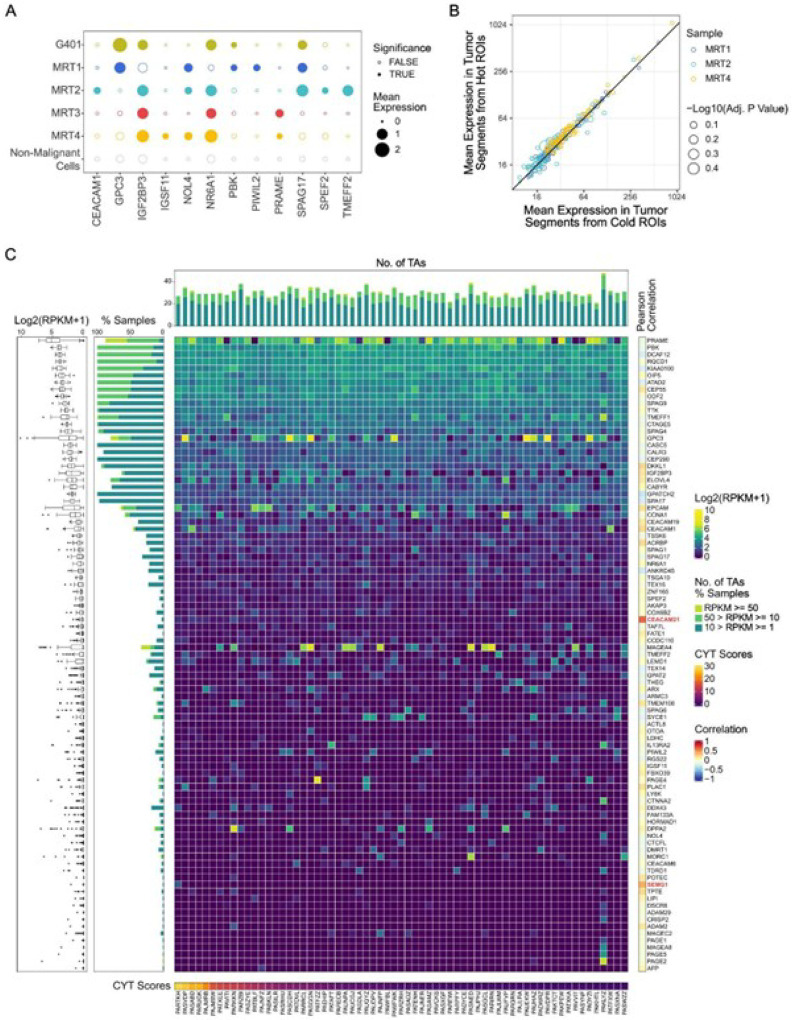
First, using single-cell multi-omic data, we compared the mean expression levels of TAs in malignant cell clusters with those in all non-malignant cells from all 4 MRTs (labeled as “non-malignant cells”) (Fig. 2A). We identified 12 TAs with mean expression levels that were 1) above 0.2 normalized counts in at least one tumor cluster and 2) significantly higher than in non-malignant cells (one-side Welch’s t-test, false discovery rate (FDR)-adjusted p-value < 1e-50). We found more than one TA was expressed per tumor, and seven TAs (GPC3, IGF2BP3, NOL4, NR6A1, PKB, PRAME, and SPAG17) were detected in multiple tumors. The expression of PRAME, a well-known TA frequently overexpressed in various cancers27–29, was confirmed using IHC (Fig. S2B).
Figure 2. MRTs express a wide variety of tumor antigens (TAs) (See also Figure S2).
A) Bubble heatmap depicting mean normalized single cell expression levels of selected TAs in tumor clusters (G401, MRT1–4 clusters) and a non-malignant cell cluster (combined non-malignant cells from MRT1–4). Dot size reflects mean expression levels, with solid dots indicating significantly higher expression in tumor clusters compared to non-malignant cells (one-sided Welch’s t-test, FDR-adjusted p-value < 1e-50).
B) Scatter plot showing mean TA expression levels in “hot” versus “cold” tumor segments in each sample profiled using spatial transcriptomics. Each circle represents a detected TA, with color indicating the sample and circle size representing −Log10(FDR-adjusted p-value). All adjusted p-values > 0.05.
C) Heatmap depicting expression levels of selected TAs (RPKM >= 1 in at least one MRT sample) across 65 MRTs profiled using bulk RNA-Seq. Columns represent tumor samples, ordered by CYT scores shown beneath the heatmap, while rows represent TAs, sorted by median expression. The boxplot on the left summarizes TA expression across the cohort, while the bar chart between the boxplot and heatmap illustrates the percentage of samples expressing each TA, grouped by expression levels (1–10 RPKM, 10–50 RPKM, and >=50 RPKM). The bar chart on top shows the number of TAs per tumor, categorized by expression levels (1–10 RPKM, 10–50 RPKM, >= 50 RPKM). Pearson correlations between each TA’s expression and CYT scores are shown on the right. TAs with expression levels significantly correlated with CYT scores (FDR-adjusted p < 0.05) are highlighted in red.
We then used spatial transcriptomics to compare the mean expression levels of TAs in tumor segments from “hot” and “cold” ROIs within the same sample. Transcripts from 91 TAs were detected above the limit of quantitation (Methods; Table S6) in at least one tumor segment. MRT3 was excluded as all ROIs were categorized as “cold”. Statistical tests indicated that none of these TAs were differentially expressed between “hot” and “cold” tumor segments (two-side Welch t-test, all FDR-adjusted p-values > 0.05; Fig. 2B).
We evaluated the expression levels of TAs in 65 MRT samples profiled using bulk RNA-seq11 (Fig. 2C; Methods). 91 TAs (Table S7) were detected with an RPKM (reads per kilobase per million mapped reads) > = 1 in at least one MRT sample. Twenty-three TAs seemed to be broadly expressed (RPKM > = 1 in more than 75% of the MRTs) while others (e.g., MAGEA4) showed prominent expression in only a subset of the tumor samples (Fig. 2C). We used the Pearson correlation coefficient to assess the relationship between TA expression and immune cytotoxic activity (measured by CYT score). The CYT score was strongly correlated with cytotoxic T cell markers in TCGA datasets20 and was also significantly positively correlated with cytotoxic lymphocyte infiltration (sum of CIBERSORT-inferred CD8 + T cell and activated NK cell fractions) in the MRT bulk RNA-Seq cohort (Pearson R = 0.82, FDR-adjusted p-value = 5.91e-16; Table S8). The expression levels of only two TAs were significantly positively correlated with CYT scores, namely CEACAM21 (Fig. S2C) and SEMG1 (Fig. S2D). Other TAs did not show a significant correlation (Pearson R range: −0.29 ~ 0.24). Despite the CYT score correlation, CEACAM21 was expressed at a low level in our cohort (median RPKM = 0.2054, RPKM > = 1 in 2 samples). Only one sample (PARTKH) had SEMG1 expression above 1 RPKM, which drove the aforementioned correlation, and when this outlier was removed, the correlation was no longer observed (Fig. S2D). The number of antigens expressed (RPKM > = 1) per tumor sample ranged from 21 to 48 (median 29), which did not correlate with the CYT score (Pearson R = 0.03, p-value = 0.8039).
These findings revealed that MRTs express a wide variety of potential TAs, as shown through single-cell, bulk, and spatial profiling approaches. However, TA expression levels were similar in tumor segments from “hot” and “cold” ROIs, and generally did not correlate with immune cytotoxic activity across MRTs.
Elevated expression of antigen processing and presentation genes in MRT is associated with a “hot” tumor immune microenvironment
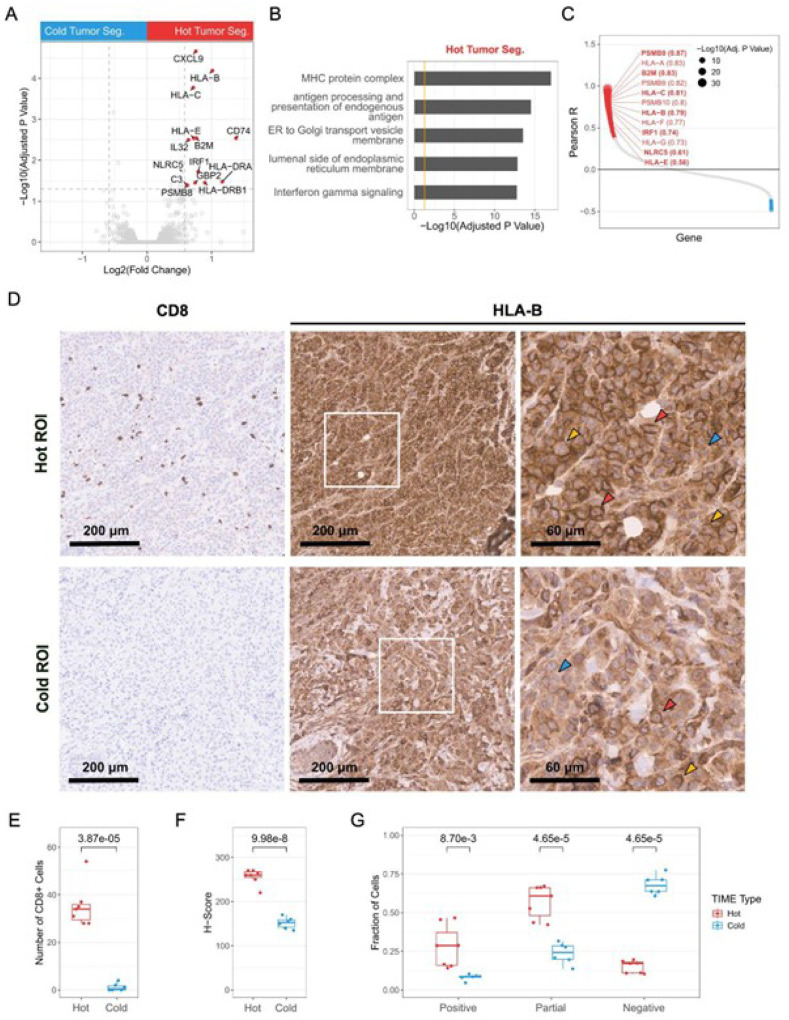
We next identified differentially expressed genes by comparing tumor segments occupying hot ROIs to tumor segments in cold ROIs (Fig. 3A; Table S9; Methods). Genes encoding components of the major histocompatibility complex (MHC) class I components (HLA-B, HLA-C, HLA-E, and B2M), MHC class I transactivators (IRF1 and NLRC5), and the immunoproteasome subunit PSMB8 (collectively referred to as “antigen processing and presentation genes”, APPGs) showed significantly higher expression levels in tumor segments within “hot” ROIs, compatible with processing and presentation of antigens. Gene ontology (GO) terms such as “MHC protein complex” and “antigen processing and presentation of endogenous antigen” were associated with the differentially expressed genes (DEGs; FDR-adjusted p-value < 0.05, fold-change > 1.5) in hot ROI tumor segments (Fig. 3B; Table S10). This finding aligns with a previous report that used bulk RNA-seq analysis to show that tumors with high CD8 + T cell populations exhibited elevated expression of antigen processing and presentation genes15. The expression of MHC class I genes and immunoproteasome subunits, including the seven APPGs mentioned above, were also significantly (FDR-adjusted p-value < 0.05) positively correlated with CYT scores across the 65 MRTs in the bulk RNA-seq cohort (Fig. 3C).
Figure 3. Elevated expression of antigen processing and presentation genes in MRT is associated with a “hot” tumor immune microenvironment.
A) Volcano plot showing differentially expressed genes between tumor segments from “hot” and “cold” ROIs in the spatial transcriptomic data. Red dots represent genes overexpressed in “hot” tumor segments, with gray dashed lines marking FDR-adjusted p-value = 0.05 and Log2(fold change) = ±Log2(1.5).
B) Functional enrichment analysis of genes preferentially expressed (FDR-adjusted p-value < 0.05 and fold-change > 1.5) in tumor segments from “hot” ROIs. The orange line indicates adjusted p-value = 0.05.
C) Pearson correlations between gene expression and CYT scores across 65 MRT samples profiled by bulk RNA-Seq. Dot size represents the FDR-adjusted p-value. Statistically significant correlations are shown in red (positive) and blue (negative). Genes involved in antigen processing and presentation are labeled with their Pearson correlation coefficients in parentheses, and APPGs identified in Figure 3A are highlighted in bold.
D) Representative IHC images showing the expression of CD8 (left panel) and HLA-B (middle and right panels) in “hot” (top panel) and “cold” (bottom panel) regions from MRT2. The right panels show the enlarged views of the highlighted regions (white squares) from the middle panels. Red, yellow, and blue arrowheads indicate examples of cells categorized as having “Positive” (red), “Partial” (yellow), and “Negative” (blue) membrane HLA-B.
E-F) Box plots depicting the number of CD8+ cells (E) and the H-Score for HLA-B staining (F) in the “hot” and “cold” regions of MRT2. P-values were calculated using two-sided Welch’s t-tests and are indicated above the plot.
G) Box plots showing manual quantification of “positive”, “partial”, and “negative” membrane HLA-B based on IHC images (examples in Fig. 3D). P-values were calculated using two-sided Welch’s t-tests and are indicated above the plot.
To confirm these findings, we stained two consecutive FFPE sections of MRT2 with anti-CD8A and anti-HLA-B antibodies (Methods). We selected 13 regions and examined the correlation between the level of infiltrating CD8 + T cells and the level of HLA-B expression in tumor cells across these regions (Fig. 3D and 3E). Tumor cells in regions with higher CD8 + T cell infiltration displayed elevated HLA-B expression, indicated by higher histochemical score (H-scores; Fig. 3F; Methods). These regions also showed a noticeable enrichment of the HLA-B signal at the cell membrane, presenting ring-like staining patterns (Fig. 3D and 3G; Methods). Conversely, tumor cells in regions with low or no CD8 + T cell infiltration exhibited reduced HLA-B levels and lacked enrichment at the cell surface in most cells (Fig. 3D, 3F, and 3G). Overall, these findings revealed an association between higher expression of antigen processing and presentation genes in tumor cells and a “hot” TIME type.
IRF1 signaling may regulate antigen processing and presentation genes and correlates with TIME types
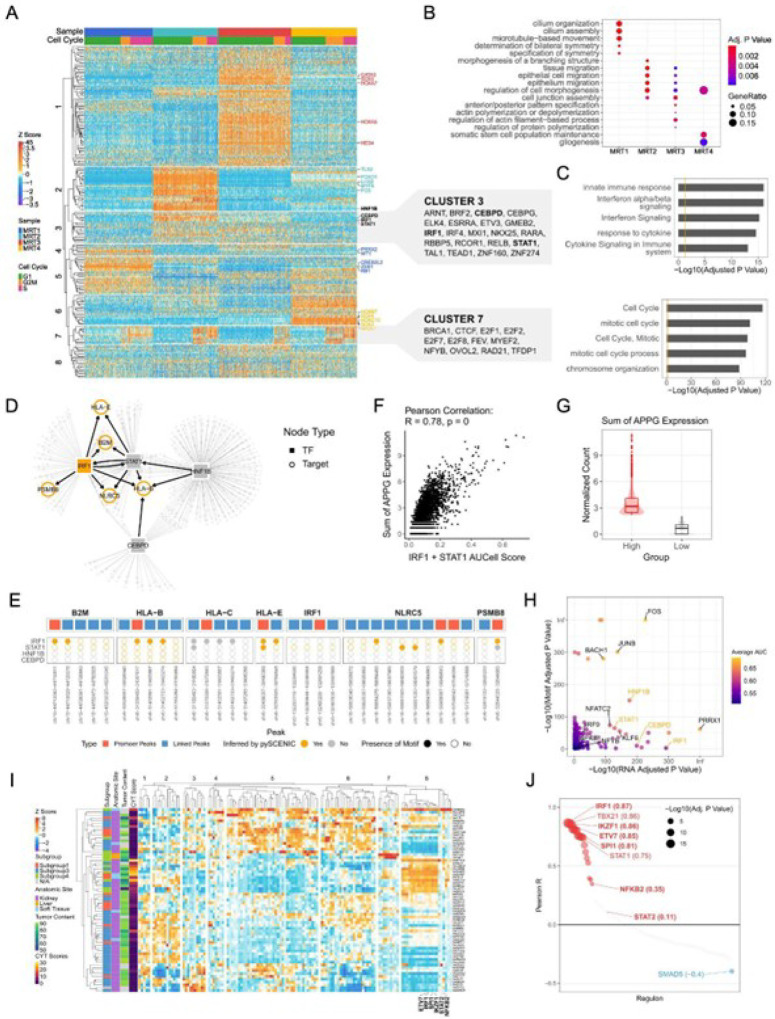
The observation that multiple genes involved in antigen processing and presentation were more highly expressed in the “hot” tumor segments motivated us to investigate the signaling networks regulating this pathway in tumor cells. We used pySCENIC30,31 and single cell expression data to infer “regulons”, defined as gene sets regulated by the same transcription factor (Methods). In total, 217 regulons were predicted to be active in at least a subset of tumor cells (Table S11). MRT1–4 each displayed different regulon activation patterns (Fig. 4A and S3A; Table S11) linked to different biological processes (Fig. 4B; Table S12). As expected, pySCENIC identified the activation of regulons associated with cell cycle regulation (cluster 7) in cells inferred to be in the G2M and S phases (Fig. 4A and 4C; Table S13; Methods). Notably, regulons in cluster 3 were associated with immune response pathways, such as “interferon signaling” and “antigen processing and presentation of endogenous peptide antigen via MHC class I” (Fig. 4A and 4C; Table S13).
Figure 4. IRF1 signaling is inferred to regulate antigen processing and presentation genes and correlate with TIME types in MRTs (See also Figure S3).
A) Heatmap displaying regulon activity scores (z-scores derived from AUCell scores) in malignant cells profiled using single-cell multi-omics. Columns represent individual cells, and rows represent regulons. Top annotations indicate cell sources and inferred cell cycle phases (based on the expression of G2/M and S phase markers from the scRNA-Seq data). The top 5 regulons with the highest regulon-specific score (RSS) in each tumor cluster (MRT1–4, Fig. S3A) are labeled on the right and colored by sample. Regulons inferred to regulate APPG expression are highlighted in bold.
B) Functional enrichment analysis of TFs and targets in regulons with RSS greater than 0.5 in each MRT.
C) Functional enrichment analysis of TFs and their targets within regulons in clusters 3 and 7 (orange line marks adjusted p-value = 0.05).
D) Transcriptional regulatory network based on regulons inferred from single-cell multi-omic data using pySCENIC. TFs and targets are shown as solid squares and open circles, respectively. The APPGs are highlighted in orange.
E) Visualization of TF motifs within the promoters and inferred linked peaks of APPG. The top panel shows the promoter (red) and linked peaks (blue). In the bottom panel, solid and open circles represent the presence or absence of motifs in each peak, respectively. Circles are colored orange if the APPGs are inferred to be regulated by the TFs by pySCENIC.
F) Scatter plot showing the correlation between the combined IRF1 and STAT1 regulon activities (sum of their AUCell scores) and the total APPG expression levels.
G) Box and violin plots showing the distribution of total APPG expression in “APPG high” and “APPG low” groups.
H) Scatter plot showing TFs with higher expression and enriched motif accessibility in the “APPG high” group compared to the “APPG low” group. The x- and y-axes represent the adjusted p-values for gene expression and motif accessibility enrichment, respectively. Only TFs with adjusted p-values < 0.05 for both are shown. Dots are colored by the mean of AUC (area under the curve) values calculated from gene expression and motif accessibility data, reflecting the strength as markers.
I) Heatmap displaying regulon activity scores (z-scores derived from AUCell scores) derived from the bulk RNA-Seq data. Each row represents an MRT sample, and each column represents a regulon. Left annotations indicate methylation-based subgroups15, anatomic sites, tumor contents, and CYT scores. Regulons inferred to regulate the expression of HLA-A/B/C/E/F/G, B2M, IRF1, NLRC5, and PSMB8/9/10 are labeled.
J) Pearson correlations between regulon regulon activity (AUCell) scores and CYT scores across 65 MRT samples profiled by bulk RNA-Seq. Dot size represents the FDR-adjusted p-value. Statistically significant correlations are shown in red (positive) and blue (negative). Regulons inferred to regulate the expression of HLA-A/B/C/E/F/G, B2M, IRF1, NLRC5, and PSMB8/9/10 are highlighted in bold, with the Pearson correlation coefficients labeled in parentheses.
Four regulons (IRF1, STAT1, HNF1B, and CEBPD regulons) were inferred to control the expression of at least one APPG (Fig. 4D; Table S14). Analysis of gene expression and chromatin accessibility data coprofiled from the same nuclei revealed open chromatin regions correlated with the expression of each APPG (referred to as “linked peaks”, Methods). IRF1 and STAT1 motifs were present in the promoter and/or linked peaks of several APPGs, indicating they may directly regulate these APPGs (Fig. 4E). In contrast, HNF1B and CEBPD motifs were not found in any peaks (Fig. 4E). The inferred activity of IRF1 and STAT1 regulons were positively correlated with the sum of normalized APPG expression in tumor cells (Pearson R = 0.78, p-value = 0; Fig. 4F). When cells were divided into “APPG high” and “APPG low” groups based on total APPG expression (Fig. 4G; Methods), both IRF1 and STAT1 showed greater inferred activity (higher transcription factor (TF) expression and greater motif enrichment) in the “APPG high” group (Fig. 4H, Table S15; Methods). Other TFs, for example, IRF9, NFKB1, and NFYB, which are known to regulate the expression of MHC class I genes and immunoproteasome subunits32, also exhibited greater inferred activity in the “APPG high” group, further supporting enhanced antigen processing and presentation in these cells.
To validate these findings in a larger cohort and examine the correlation between regulon activity and TIME types (indicated by CYT scores) across MRT samples, we performed regulon analysis using the 65 bulk RNA-seq datasets. We identified six TFs (IRF1, STAT2, SPI1, IKZF1, ETV7, and NFKB2) potentially regulating genes involved in antigen processing and presentation (HLA-A/B/C/E/F/G, B2M, IRF1, NLRC5, and PSMB8/9/10) (Fig. 4I, S3B; Table S16). Hierarchical clustering grouped these regulons into cluster 8, co-activated in a subset of MRTs (Fig. 4I). Activities of all but the STAT2 regulons were significantly positively correlated with the CYT scores (Fig. 4J). IRF1 was again inferred to regulate APPG expression, confirming our single-cell results, and showed the highest correlation with CYT scores. Functional enrichment analysis revealed that regulons in cluster 8 were involved in interferon-gamma signaling and antigen processing and presentation (Fig. S3C; Table S17). By integrating bulk ATAC-seq data, available for 42 samples, we identified promoter and linked peaks for each target gene and examined TF motifs within these peaks for genes inferred to be regulated by the corresponding TF (Methods). Motifs of all TFs were confirmed in the promoter and/or linked peaks of several predicted targets (Fig. S3D).
Together, our analysis indicated higher IRF1 expression in tumor segments in “hot” ROIs and a correlation between IRF1 signaling and CYT scores across MRTs. Such variation in IRF1 signaling could potentially influence APPG expression levels in tumor cells, affect their antigen processing and presentation capabilities and ultimately impact tumor immunity and TIME type.
The skeletal muscle development program is upregulated in a subset of MRTs with high CD8 + T cell infiltration
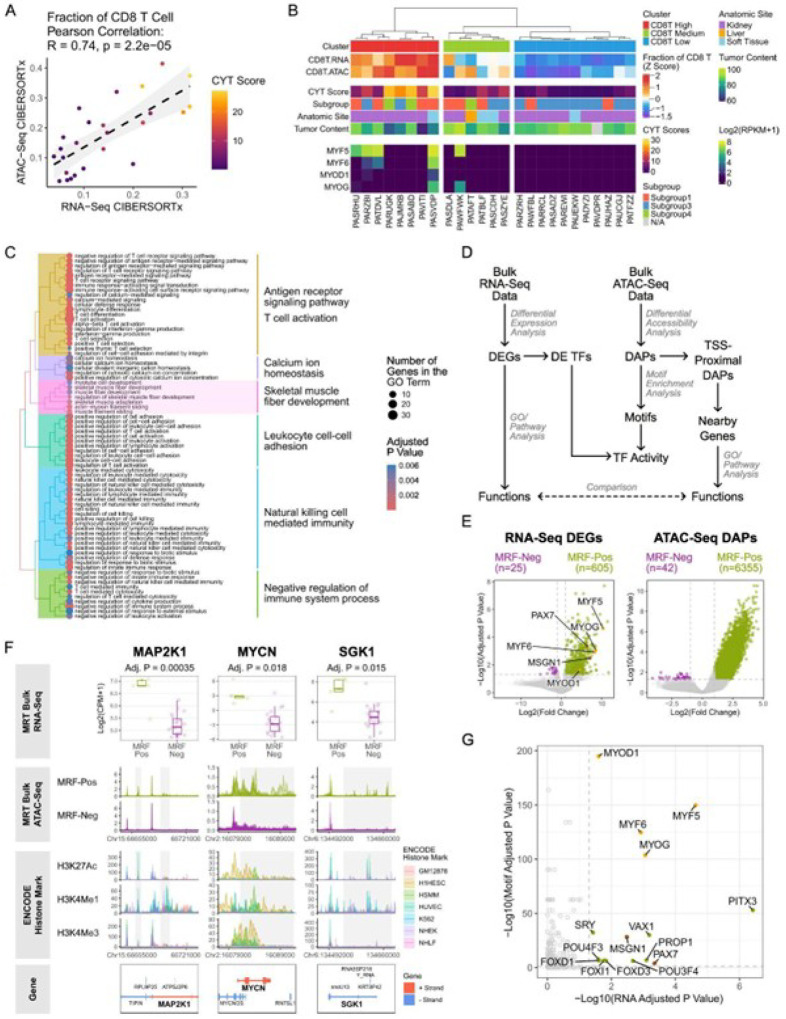
To further investigate transcriptional programs upregulated in tumors with “hot” TIME, we used CIBERSORTx to infer immune cell compositions for the 42 MRTs with matched bulk RNA-seq and ATAC-seq data (Table S18–19; Methods). We focused on samples where ATAC and RNA-seq both estimated a similar proportion of CD8 + T cells (Fig. 1A; Methods). Twenty-five samples met these criteria and were grouped into “CD8T High,” “CD8T Medium,” and “CD8T Low” clusters using hierarchical clustering based on the inferred CD8 + T cell fractions (Fig. 5B; Methods). Differential expression analysis identified 189 DEGs (FDR-adjusted p-value < 0.05, fold-change > 2) enriched in the “CD8T High” compared to “CD8T Low” groups (Table S20). In addition to immune response-related genes, the “CD8T High” group preferentially expressed skeletal muscle development genes (Fig. 5C; Table S21), including myogenic regulatory factors (MRFs) such as MYF6, MYOD1, and MYOG33 (Table S20). This was attributed to a greater proportion of MRF-positive samples (samples have at least one MRF with > = 1 RPKM) in the “CD8T High” group (one-sided Fisher’s exact test, p-value = 0.02345; Fig. 5B). Compared to the MRF-negative MRTs (n = 20), the MRF-positive MRTs (n = 5) exhibited 605 DEGs and 6,355 differentially accessible peaks (DAPs), including 333 peaks within 1,000 bp of transcription start sites (TSS-proximal DAPs) (Fig. 5D–E; Table S22–23). Both the DEGs and genes with TSS-proximal DAPs in the MRF-positive samples were associated with skeletal muscle development (Table S24). Seventy-eight DEGs in MRF-positive samples had at least one TSS-proximal DAP (Table S25). Examples include MAP2K1, a key component of mitogen-activated protein kinase (MAPK) pathway, which showed higher expression with additional open chromatin regions at potential regulatory sites in MRF-positive MRTs (Fig. 5F). Inhibition of MAP2K1/2 has been shown to reduce proliferation and promote apoptosis in AT/RT cell lines34. While MYC expression remains similar between MRF-positive and negative groups (FDR-adjusted p-value = 0.52), MYCN, which plays a critical role in promoting cell proliferation during embryonic development35, appears to be more highly expressed in MRF-positive MRTs with expanded chromatin accessibility (Fig. 5F). Interestingly, the human embryonic stem cell line (H1HESC) and human skeletal muscle myoblasts (HSMM) exhibit stronger H3K27Ac36 and H3K4Me336 signals across the MYCN region compared to more differentiated cell lines (Fig. 5F). SGK1, a serine/threonine kinase in the PI3K signaling pathway, is also upregulated in MRF-positive MRTs, accompanied by increased chromatin accessibility at potential enhancer regions (Fig. 5F). All four MRFs (MYF5, MYF6, MYOG, and MYOD1) showed higher expression levels and enriched motifs in the MRF-positive samples, suggesting generally increased MRF activity (Fig. 5D, 5G; Table S26). Additionally, PAX7, which participates in embryonic skeletal muscle development and is required for the specification of satellite cells (muscle stem cells)37,38, and MSGN1, a key regulator in the development of the paraxial mesoderm that gives rise to skeletal muscles39, were inferred to be active in the MRF positive samples (Fig. 5D, 5G; Table S26). Using single-cell multi-omic data from MRT1–4, we mapped MRF expression to malignant cells (Methods). MYF6 exhibited elevated expression levels and greater motif enrichment in MRT2 tumor cells (Fig. S3E), consistent with the higher MYF6 regulon specificity scores (RSS, representing how specific a regulon is to a particular cell population31) observed in these cells (Fig. S3A). These findings revealed that a subset of MRTs with “hot” TIME display a distinct transcriptional profile characterized by enhanced skeletal muscle development programs.
Figure 5. The skeletal muscle development program is upregulated in a subset of MRTs with high CD8 T cell infiltration (See also Figure S3).
A) Scatter plot showing the CIBERSORTx-inferred CD8+ T cell fractions based on bulk RNA-Seq and bulk ATAC-Seq data. Dots are colored by CYT scores. The grey band shows the 95% confidence interval.
B) The top panel shows hierarchical clustering based on CIBERSORTx-inferred CD8+ T cell fractions from bulk RNA-seq and ATAC-seq data (shown as z scores). The middle panel includes annotations for CYT scores, methylation-based subgroups15, anatomic sites, and tumor content. The bottom panel displays the expression levels of the four myogenic regulatory factors (MRFs).
C) Hierarchical clustering of the top 75 enriched GO terms (ranked by adjusted p-values) associated with genes preferentially expressed (FDR-adjusted p-value < 0.05, fold-change > 2; Table S16) in the “CD8T High” group (Fig. 5B). Dot size indicates the number of genes preferentially expressed in the ‘“CD8T High” group within each GO term, and dot color represents the adjusted p-values.
D) Integrative analysis pipeline for matched bulk RNA-seq and ATAC-seq data. DEGs: differentially expressed genes; DAPs: differentially accessible peaks, DE TFs: differentially expressed transcription factors. TSS: transcription start site.
E) Volcano plots showing DEGs (left plot) and DAPs (right plot) between MRF-positive (green dots) and MRF-negative MRTs (purple dots). Gray dashed lines mark FDR-adjusted p-value = 0.05 and Log2(fold change) = ±Log2(2). The four MRFs are highlighted in orange. PAX7 and MSGN1 are highlighted in brown.
F) Examples of DEGs with TSS-proximal DAPs. The top panel shows box plots of normalized gene expression between MRF-positive and negative groups. Below, normalized ATAC-seq signals and ENCODE histone marks36,82–84 (H3K27Ac, H3K4Me1, and H3K4Me3) from seven cell lines (GM12878, a lymphoblastoid cell line; H1HESC, H1 human embryonic stem cell line; HSMM, human skeletal muscle myoblasts; HUVEC, human umbilical vein endothelial cells; K562, a human erythroleukemic cell line; NHEK, normal human epidermal keratinocytes; and NHLF, normal human lung fibroblasts) are displayed. Gray boxes highlight regions with increased chromatin accessibility in MRF-positive samples. Gene models are shown in the bottom panel.
G) Scatter plot showing TFs with higher expression and enriched motif accessibility in the MRF-positive MRTs compared to MRF-negative MRTs. The x- and y-axes represent the FDR-adjusted p-values for gene expression and motif accessibility enrichment, respectively. Gray dashed lines mark adjusted p-value = 0.05. The four MRFs are colored in orange. PAX7 and MSGN1 are colored in brown.
The M1/M2 macrophage ratio is correlated with cytotoxic lymphocyte infiltration
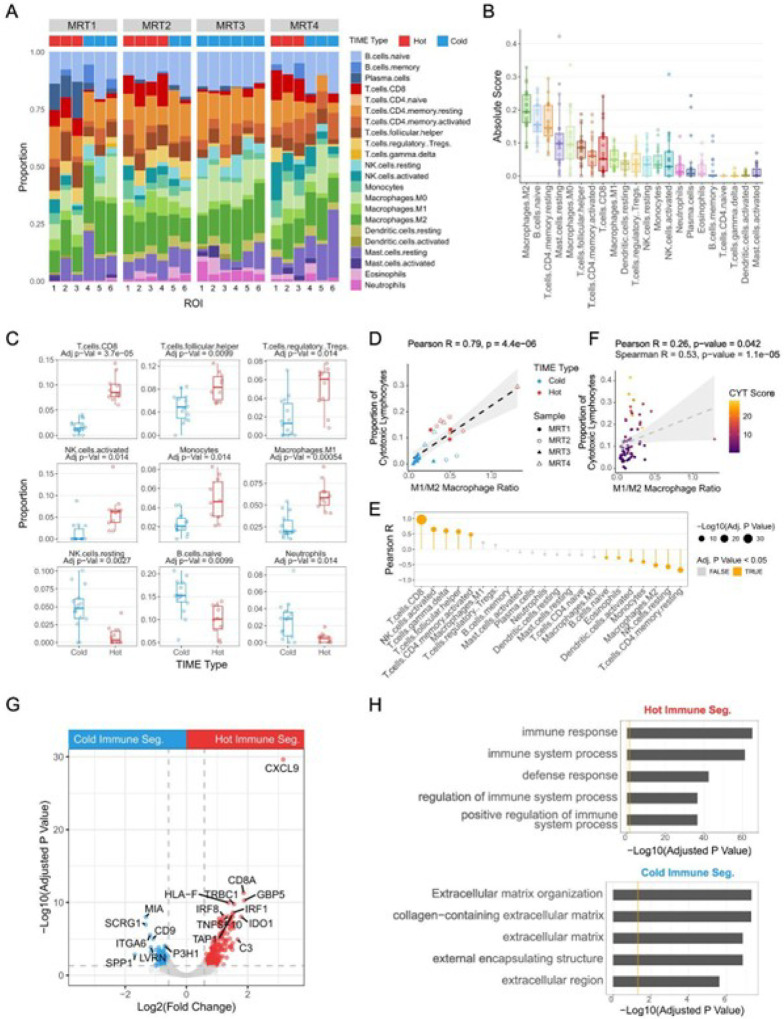
Next, we characterized non-malignant cells in MRT, using spatial transcriptomic data and CIBERSORTx to infer and compare immune cell populations in “hot” and “cold” immune segments (Methods; Fig. 6A; Table S4). As reported from analysis of bulk RNA-seq data14, M2-like macrophages were the most abundant population, followed by resting memory CD4 + T cells and naive B cells (Fig. 6B). We then identified cell types enriched in “hot” and “cold” immune segments (two-sided Welch t-tests, Methods; Fig. 6C and S4A). In addition to CD8 + T cells and activated NK cells, monocytes, M1 macrophages, T follicular helper cells (Tfh), and regulatory T cells (Treg) were significantly enriched in the “hot” immune segments. In contrast, higher proportions of resting NK cells, naive B cells, and neutrophils were found in immune segments from “cold” ROIs. M2-like macrophages showed a non-significant trend toward higher abundance in “cold” regions (Fig. S4A), but the M1/M2 macrophage ratio correlated significantly with cytotoxic lymphocyte infiltration (Pearson R = 0.79, p-value = 4.433e-06; Fig. 6D).
Figure 6. The M1/M2 macrophage ratio is positively correlated with cytotoxic lymphocyte infiltration (See also Figure S4).
A) Stacked bar plot showing the CIBERSORTx-inferred relative abundance of immune cell populations in the immune segments from the 24 ROIs profiled by spatial transcriptomics, with the TIME type of each ROI labeled above.
B) Box plot showing the absolute scores of immune cell types inferred by CIBERSORTx from immune segments in the spatial transcriptomic data. Each dot represents one of the 24 immune segments from the 24 ROIs across the four MRT samples.
C) Box plots showing differences in immune cell types inferred by CIBERSORTx between immune segments from “hot” and “cold” ROIs. P-values were calculated using two-sided Welch t-tests, with FDR correction for multiple comparisons.
D) Scatterplot showing the correlation between M1/M2 macrophage ratio and the proportion of cytotoxic lymphocytes in the tumor microenvironment. The grey band shows the 95% confidence interval.
E) Lollipop plot showing correlations between the fraction of cytotoxic lymphocytes (the sum of CD8 T cell and activated NK cell fractions) and the fraction of other immune cell types inferred by CIBERSORTx using bulk RNA-Seq data. The FDR was used to correct for multiple comparisons.
F) Scatterplot showing the correlation between M1/M2 macrophage ratio and proportion of cytotoxic lymphocytes inferred by CIBERSORTx using bulk RNA-Seq data. The grey band shows the 95% confidence interval.
G) Volcano plot showing differentially expressed genes between immune segments from “hot” and “cold” ROIs in the spatial transcriptomic data. Red and blue dots represent differentially expressed genes in “hot” and “cold” immune segments, respectively, with gray dashed lines marking FDR-adjusted p-value = 0.05 and Log2(fold change) = ±Log2(1.5).
H) Functional enrichment analysis of genes preferentially expressed (FDR-adjusted p-value < 0.05 and fold-change > 1.5) in “hot” (top) and “cold” (bottom) immune segments. The orange line indicates adjusted p-value = 0.05.
To validate our observations in a larger cohort, we performed in silico cell type deconvolution using CIBERSORTx and 65 bulk RNA-seq datasets11 (Table S18; Methods) and calculated the Pearson correlation coefficients between the fraction of each cell type and the fraction of cytotoxic lymphocytes (sum of CD8 + T cell and activated NK cell fractions; Fig. 6E, Table S27). In addition to CD8 + T cells and activated NK cells, gamma delta T cells (γδ T cells), T follicular helper cells (Tfh), and activated memory CD4 + T cells were significantly positively correlated with cytotoxic lymphocyte fractions across patients. M1 macrophages and regulatory T cells (Treg) also showed positive, though not significant, correlations. The cell types significantly negatively correlated with the cytotoxic lymphocyte fraction included resting memory CD4 + T cells, resting NK cells, M2 macrophages, monocytes, activated dendritic cells, eosinophils, and naive B cells. Furthermore, the M1/M2 macrophage ratio was significantly positively correlated with cytotoxic lymphocyte levels (Pearson R = 0.26, p-value = 0.042 with outlier, Fig. 6F; Pearson R = 0.45, p-value = 0.00023 without outlier, Fig. S4B). Overall, these findings reveal distinct immune cellular compositions associated with different TIME types and support a relationship between the M1/M2 macrophage ratio and cytotoxic lymphocyte levels observed in the spatial transcriptomic data.
Next, we performed differential gene expression analyses using spatial transcriptomic data to identify genes and pathways associated with different TIME types. This revealed 245 and 126 DEGs (FDR-adjusted p < 0.05, fold-change > 1.5) in immune segments from “hot” and “cold” ROIs, respectively (Fig. 6G; Table S9; Methods). “Hot” immune segments exhibited higher expression of genes encoding T cell receptors (CD8A, CD3E, TRAC, and TRBC1), chemokines (CXCL9, CXCL10, CXCL13, CCL5, and CCL19), interferon signaling factors (IRF1, IRF8, STAT1, and GBP1), immunoproteasomes (PSMB8/9/10), MHC complexes (HLA-A/B/C/E/F/DQA1/DQA2/DQB1/DPA1/DPB1/DRA/DRB1), and MHC transactivators (NLRC5 and CIITA). LYZ, the gene encoding lysozyme, showed a positive correlation with M1-like macrophage and cytotoxic T cell numbers40. It was more highly expressed in the “hot” immune segments, aligning with the higher M1/M2 ratio and T cell levels in these regions (Table S9). Pathway analysis revealed that genes overrepresented in “hot” immune segments were involved in immune response and lymphocyte activation (Fig. 6H; Table S10; Methods). In contrast, genes overexpressed in “cold” immune segments were associated with extracellular matrix organization (Fig. 6H; Table S10). Several genes in cold segments (e.g. SPP1, CD9, and SCRG1) were previously implicated in M2-like macrophage polarization and anti-inflammatory activities41–44 (Fig. 6G; Table S9).
Multi-omic characterization of MRT-associated macrophages revealed genes and pathways underlying M2-like phenotype
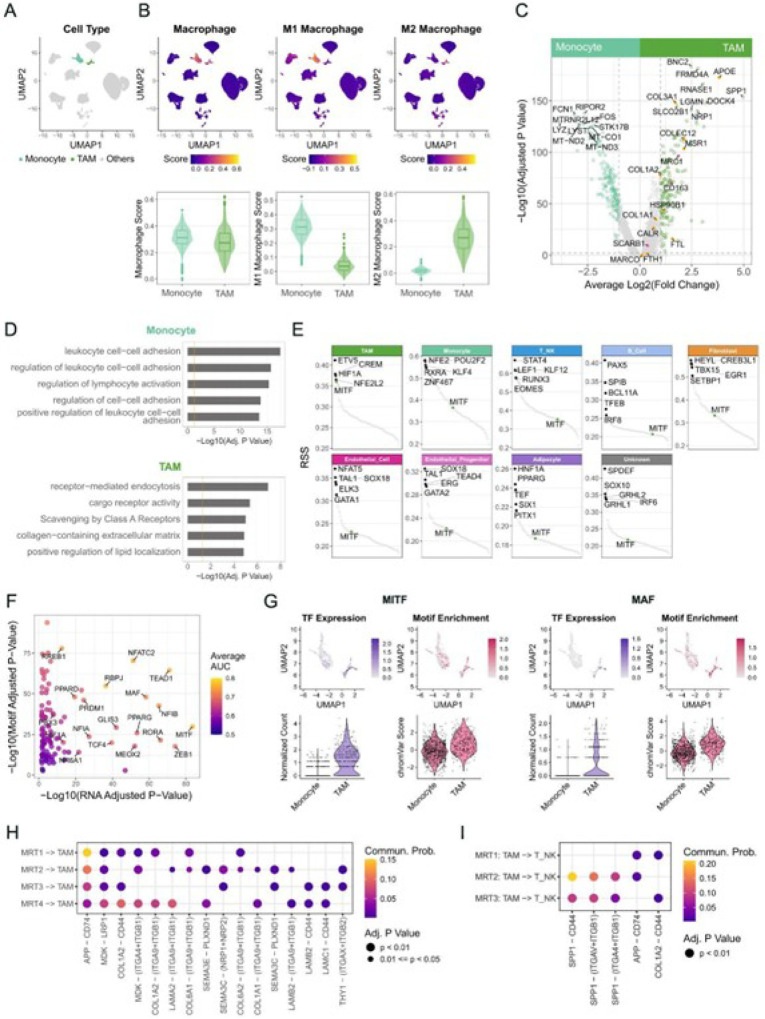
To identify genes and signaling pathways up-regulated in the TAMs, we compared TAM single-cell profiles to single-cell profiles from peripheral blood mononuclear cells (PBMCs) of a healthy donor (Methods). We merged the MRT and the PBMC single-cell gene expression and ATAC datasets and identified 14 clusters (Fig. S5; Methods). These included a “TAM” cluster with 342 cells and a “monocyte” cluster containing 32 cells from MRT1–4, and 815 cells from the PBMC dataset (Fig. 7A). Using macrophage signature genes (Table S2), we found that monocyte cluster cells exhibited an M1-like phenotype, while the TAM cluster cells showed higher M2 macrophage signature scores (Fig. 7B).
Figure 7. Multi-omic characterization of MRT-associated macrophages revealed genes and pathways underlying M2-like phenotype (See also Figures S5 and S6).
A) UMAP visualizations of PBMC and MRT clusters profiled by single-cell multi-omic assay (Fig. S5B). The monocyte and TAM clusters are colored.
B) The overall expression scores of macrophage, M1 macrophage, and M2 macrophage signatures are colored on UMAP (top) and presented as box and violin plots (bottom).
C) Volcano plot showing differentially expressed genes between monocyte and TAM clusters profiled by single-cell multi-omic assay. The light teal and green dots represent differentially expressed genes in monocytes and TAM clusters, respectively, with gray dashed lines marking FDR-adjusted p-value = 0.05 and Log2(fold change) = ±Log2(1.5). Orange dots indicate genes in the gene set “Scavenging by Class A Receptors” (Reactome: R-HSA-3000480), while pink dots indicate additional scavenger receptor genes.
D) Functional enrichment analysis of genes preferentially expressed (FDR-adjusted p-value < 0.05 and fold-change > 1.5) in monocyte (top) and TAM (bottom) clusters. The orange line indicates adjusted p-value = 0.05.
E) Regulon-specific score (RSS) plot depicting the specificity of regulons in each cell type. MITF regulon is highlighted in green in all subplots.
F) Scatter plot illustrating TFs with higher expression and enriched motif accessibility in the TAM cluster compared to the monocyte cluster. The x- and y-axes represent the adjusted p-values for gene expression and motif accessibility enrichment, respectively. Only TFs with adjusted p-values < 0.05 for both are shown. Dots are colored by the mean of AUC values calculated from gene expression and motif accessibility data, reflecting the strength as markers
G) Comparing the gene expression (colored in purple) and motif accessibility (colored in pink) of MITF and MAF in the monocyte and TAM clusters, visualized on UMAP (top) and as violin plots (bottom).
H-I) Dot heatmaps showing inferred cell-cell communications between tumor cells and TAMs (H) and between TAMs and T cells (I) using single-cell multi-omic data. MRT4 was excluded from the TAM-T cell interaction analysis due to insufficient T cells. Each row of dots represents the inferred interactions between the indicated cell types. Each column represents a ligand-receptor pair. Only ligand-receptor pairs identified in multiple samples were displayed. The dot color shows communication probabilities reported by CellChat.
Differential gene expression comparisons of these two clusters revealed the monocyte cluster expressed mitochondrial respiratory chain genes (e.g. MT-CO1/2/3, MT-ND1/2/3/4/5, MT-CYB, and MT-ATP6/8) and genes involved in regulating multiple aspects of the immune system, such as leukocyte cell-cell adhesion, regulation of T cell activation, and lymphocyte proliferation (Fig. 7C–D; Table S28–29). LYZ also showed higher expression in the monocyte cluster (expressed in 92.8% of cells in the monocyte cluster and 15.5% of cells in the TAM cluster; Table S28). In contrast, the TAM cluster differentially expressed genes involved in endocytosis and scavenger receptor pathways (Fig. 7C–D; Table S28–29). Notably, SPP1, an immune checkpoint gene suppressing T cell activation41, was strongly expressed in the TAM cluster (71.1% of cells) compared to the monocyte cluster (0.7% of cells) (Fig. 7C; Table S28). As noted earlier, SPP1 was more highly expressed in “cold” immune segments in the spatial transcriptomics data, consistent with the notion that these regions tend to have a higher proportion of M2-like macrophages.
We next sought to identify transcriptional regulators potentially underpinning these TAM gene expression patterns. Regulon analysis identified cell-type-specific regulatory networks for each non-malignant cell type, including those previously reported, for example, PPARG in adipocytes45; PAX5 regulon in B cells46,47; EOMES, and RUNX3 regulons in T cells and NK cells48–53; and SOX18 in endothelial cells54 (Fig. 7E; Table S30). CREM and NFE2L2 have been linked with the anti-inflammatory phenotype of M2-like macrophages55,56, and their regulons indeed displayed high RSS in M2-like TAMs. A HIF1A regulon also showed high RSS, consistent with lower expression levels of mitochondrial respiratory chain genes in the TAM cluster compared to the monocyte cluster (Fig. 7C; Table S28). Interestingly, the MITF regulon showed high specificity in TAMs but was low in other non-malignant cell types. MITF, a master regulator of melanogenesis and a melanoma oncogene, has also been reported to regulate the immunosuppressive functions of myeloid-derived suppressor cells (MDSCs) in a mouse model57. We identified 106 TFs with elevated inferred activity (higher gene expression and greater motif enrichment) in the TAM cluster compared to the monocyte cluster (Fig. 7F, Table S31). These included TFs known to promote an M2-like phenotype and are critical for TAM-mediated immunosuppressive functions, such as MAF58 (Fig. 7G). Notably, MITF also exhibited greater inferred activity in the TAM cluster (Fig. 7G), consistent with the MITF regulon’s higher specificity in TAMs (Fig. 7E).
Taken together, the single-cell multi-omic data confirmed a stronger M2 signature in MRT TAMs and revealed regulatory networks (e.g. the MITF regulon) that potentially play roles in their functions. Our analyses also revealed the upregulation of SPP1 in M2-like TAMs, which may suppress T cell activation41.
Inference of cell-cell interaction identified potential crosstalk that may shape the tumor immune microenvironment
To explore potential interactions in tumors, we used CellChat59 (Methods) and single-cell multi-omics data to infer cell-cell interactions in MRT1–4 (Fig. S6A-B). We identified 360 significant (p-value < 0.05) ligand-receptor pairs from 72 pathways, including 198 pairs between tumor and non-malignant cells that span 44 pathways (Fig. S6C; Table S32–33). Focusing on the potential influence of tumor cells on macrophages, we identified 37 ligand-receptor interactions, with APP-CD74 and MDK-LRP1 consistently observed across all four samples (Fig. 7H). Interestingly, a study has shown that MDK is upregulated in gall bladder cancer with ErbB pathway mutations and promotes the differentiation of immunosuppressive macrophages by interacting with LRP1 on TAM60. Several interactions between TAMs and T cells were also predicted, including the SPP1-CD44 interaction in MRT2 and MRT3 (Fig. 7I). Overall, these findings may reveal potential tumor-macrophage and macrophage-T cell interactions that could lead to an immunosuppressive TIME and complicate the development of effective treatment approaches.
Discussion
MRT biology must be better understood to create more effective therapeutic strategies. Chromatin modifiers, kinases, cell cycle regulators, and signaling pathways have emerged as potential therapeutic targets in both preclinical studies and clinical trials5,61. Despite the low tumor mutational burden, the observed heterogeneity in immune cell infiltration in rhabdoid tumors has sparked interest in investigating immunotherapy as a potential treatment avenue5,61. An ongoing Phase II trial (NCT04416568) is evaluating the safety and effectiveness of combined PD-1 and CTLA-4 blockade in SMARCB1-negative tumors, including MRT. Motivated by these developments, we sought to explore the heterogeneity in TIME types, which is relevant for developing effective therapeutic strategies.
Our study revealed a wide range of TA expression profiles in MRT, lending support to the investigation of TA-targeted immunotherapies as a treatment option. For example, the cancer-testis antigen encoded by PRAME was expressed in a substantial proportion of MRT. Overexpression of PRAME has been reported in many pediatric and adult cancers27–29, which has ignited research focused on immune-based interventions that specifically target PRAME in several cancer contexts62. Another gene, MAGEA4, exhibited elevated expression in some MRTs. Currently, a targeted treatment using genetically modified autologous T cells for multiple MAGEA4-positive solid cancers is undergoing phase I clinical trials (NCT03132922) and has shown promising results63.
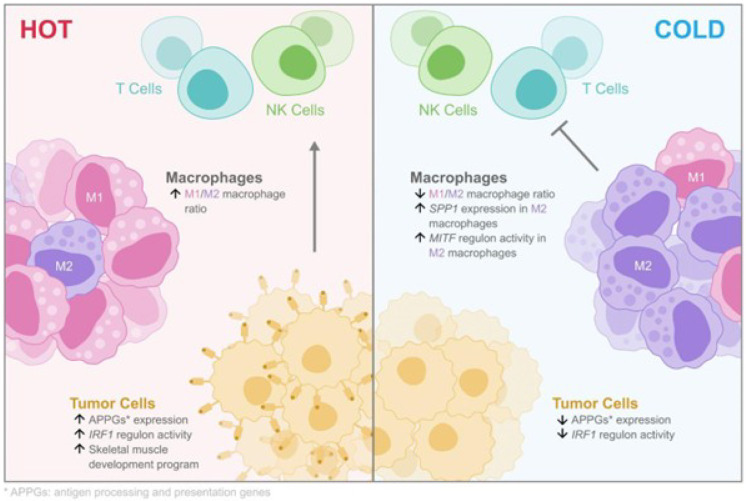
Interestingly, the expression of most TAs did not correlate with the TIME types, consistent with previous observations14, indicating the possible presence of additional factors influencing immune response. Our analyses uncovered intra- and inter-tumor heterogeneity in the expression of genes involved in antigen processing and presentation pathways (Fig. 8). We also observed variability in the inferred activity of the IRF1 regulatory network, which was predicted to regulate these APPGs (Fig. 8). Both APPGs and inferred IRF1 regulon activity strongly correlated with cytotoxic lymphocyte infiltration and/or activity. Therefore, APPG expression levels and IRF1 signaling activity may help stratify MRT patients for immunotherapy.
Figure 8.
A simplified model of genes and pathways associated with different TIME types in MRT
Notably, IFNγ treatment can enhance the expression of HLA genes in MRT cell lines64. Several clinical trials are underway to evaluate the efficacy of IFNγ as a standalone treatment or in combination with other anticancer drugs65 highlighting the potential of cytokines as an intervention for promoting immune recognition and response.
The upregulation of skeletal muscle developmental programs were observed in five MRTs and appeared correlated with higher CD8 + T cell infiltration levels (Fig. 8). This is consistent with a recent study that revealed that overexpression of muscle development-related genes are associated with better survival outcomes in a subgroup of MRTs66. Additionally, we identified genes that may be involved in tumor growth in MRF-positive MRTs and may present novel therapeutic potential. For example, the MAPK pathway is frequently activated in AT/RTs67. Binimetinib (MEK162), a MAP2K1/2 inhibitor, has been shown to reduce the growth of AT/RT cell lines in vitro and in flank xenografts34. This drug is FDA-approved for treating BRAF-mutant melanoma68 and non-small cell lung cancer69 in combination with the BRAF inhibitor encorafenib and is undergoing Phase II clinical trials for pediatric low-grade gliomas and other Ras/Raf/ERK pathway-activated tumors (NCT02285439).
Our analyses revealed immune cell populations associated with distinct TIME phenotypes both within and across tumors, and identified a significant positive correlation between the M1/M2 macrophage ratio and the infiltration of cytotoxic lymphocytes in MRTs (Fig. 8). Studies have reported that the subtypes of TAMs, particularly the M1/M2 macrophage ratio, is a better prognostic predictor compared to overall TAM densities70–74. Furthermore, emerging evidence reveals the key roles of macrophage differentiation in treatment resistance and improved responses with macrophage manipulations. M2-like TAMs also contribute to immune checkpoint blockade (ICB) therapy failure and are associated with suboptimal outcomes of CAR-T cell therapy in mouse models75–77. These findings underscore the potential of targeting TAMs as a promising adjunct to conventional immunotherapies. Encouragingly, agents targeting macrophages are undergoing clinical trials for cancer treatment78.
By employing a suite of genomics approaches, our study discovered pathways and regulatory networks that exhibit greater specificity and enhanced activity in M2-like macrophages compared to M1-like monocytes (Fig. 8). For example, our results highlighted MITF as an important TF gene in TAMs from MRT patients. A recent study used mouse models to demonstrate that MITF is upregulated in tumor-associated monocytic MDSCs and contributes to their immunosuppressive function57. Remarkably, suppression of MITF expression led to reduced tumor size and enhanced T-cell activity57. Compounds such as ML329 and CH5552074 have been reported to inhibit the activity of the MITF pathway79,80, offering exciting possibilities for the development of innovative therapeutic strategies that target MITF-expressing TAMs. We also found that SPP1 is upregulated in M2-like TAMs, which may suppress T cell activation41 through the inferred SPP1-CD44 interaction between TAMs and T cells. This suggests that SPP1 could be a potential target for modulating the TIME in MRT.
Limitations of the study
Our study has limitations that should be acknowledged. First, 4 samples were analyzed using single-cell multi-omic and spatial transcriptomic technologies, all from soft tissue, due to the scarcity of primary MRT samples (in the US, the annual incidence rates among children under 15 are 0.89 per million for AT/RT, 0.19 per million for renal MRTs, and 0.32 per million for extrarenal MRTs81). We addressed this limitation in part by validating our observations in a larger MRT cohort profiled using bulk sequencing, seeking to enhance the robustness and generalizability of our observations. Secondly, our study relied predominantly on bioinformatic analysis, and further experimental studies are necessary to confirm the functional implications of these observations. Even so, our results provide insights into the complex factors at play in shaping the MRT TIME and serve as a foundation for future studies.
Supplementary Material
Supplementary Files
This is a list of supplementary files associated with this preprint. Click to download.
Acknowledgments
The authors wish to acknowledge the participation of patients and their families in research. We thank the Children’s Oncology Group (COG, NCTN Operations Center Grant U10CA180886, COG Biospecimen Bank Grant U24CA196173, NCTN Statistics & Data Center Grant U10CA180899, and St Baldrick’s Foundation) for providing samples, and the Sequencing, Bioinformatics, and Project Management teams at Canada’s Michael Smith Genome Sciences Centre for expert technical contributions. We extend our gratitude to Dr. Keegan Korthauer and Giuliano Netto Flores Cruz for insightful discussions, Michelle Dittrick for her role as the data guardian and her assistance with the REB application, Dr. Liuliu Pan and David Kroeppler from the Bruker/NanoString Technology Access Program (TAP) for conducting the spatial transcriptomics assay, Diane L. Trinh for generously sharing her expertise in the 10X Genomics Chromium Single Cell Multiome ATAC + Gene Expression assay and members of the Marra lab for helpful discussions. M.A.M. gratefully acknowledges the support of BC Cancer, the BC Cancer Foundation, the Canada Research Chairs program, the Canada Foundation for Innovation (projects 20070, 30981, and 33408), Genome BC, Genome Canada and the University of British Columbia (UBC). This study was supported by the Canadian Institutes of Health Research (CIHR; FDN-143288), and a Dr. Donald B. Rix Pathology & Laboratory Medicine Research Grant. This study was conducted with the financial support of the Terry Fox Research Institute and the Terry Fox Foundation. Disclaimer: The views expressed in the publication are the views of the authors and do not necessarily reflect those of the Terry Fox Research Institute or the Terry Fox Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of interests
M.A.M. is the Terry Fox Leader in Cancer Genome Science and serves on Terry Fox Research Institute’s (TFRI) Research Council, Network Council, Canadian Spectrum Working Group, Technology Working Group (Co-chair), Open Accrual Working Group, Digital Health Discovery Platform (Co-chair), and as Co-lead of the BC Cancer Consortium, which receives and allocates TFRI funding.
TFRI provides funding support to M.A.M.’s research program and trainees.
Contributor Information
Marco Marra, University of British Columbia.
Dan Jin, University of British Columbia.
Joshua Dubland, BC Children’s and Women’s Hospital.
Elizabeth Mullen, Dana-Farber Cancer Institute.
James Geller, Peckham Center for Cancer and Blood Disorders, Rady Children’s Hospital, San Diego, CA University of California San Diego, La Jolla, CA.
Jonathan Bush, University of British Columbia.
References
- 1.Haas J. E., Palmer N. F., Weinberg A. G. & Beckwith J. B. Ultrastructure of malignant rhabdoid tumor of the kidney. A distinctive renal tumor of children. Hum Pathol 12, 646–657 (1981). [DOI] [PubMed] [Google Scholar]
- 2.Rorke L. B., Packer R. J. & Biegel J. A. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg 85, 56–65 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Frühwald M. C., Bourdeaut F. & Furtwängler R. Extracranial Rhabdoid Tumours. in Pediatric Oncology 429–447 (Springer International Publishing, Cham, 2022). [Google Scholar]
- 4.Beckwith J. B. & Palmer N. F. Histopathology and prognosis of Wilms tumors: results from the First National Wilms’ Tumor Study. Cancer 41, 1937–1948 (1978). [DOI] [PubMed] [Google Scholar]
- 5.Nemes K. et al. Current and Emerging Therapeutic Approaches for Extracranial Malignant Rhabdoid Tumors. Cancer Manag. Res. 14, 479–498 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chi S. N. et al. Tazemetostat for tumors harboring SMARCB1/SMARCA4 or EZH2 alterations: results from NCI-COG pediatric MATCH APEC1621C. J Natl Cancer Inst 115, 1355–1363 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinhard H. et al. Rhabdoid tumors in children: prognostic factors in 70 patients diagnosed in Germany. Oncol. Rep. 19, 819–823 (2008). [PubMed] [Google Scholar]
- 8.Versteege I. et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature 394, 203–206 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Schneppenheim R. et al. Germline nonsense mutation and somatic inactivation of SMARCA4/BRG1 in a family with rhabdoid tumor predisposition syndrome. Am J Hum Genet 86, 279–284 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun H.-J. E. et al. Genome-wide profiles of extra-cranial malignant rhabdoid tumors reveal heterogeneity and dysregulated developmental pathways. Cancer Cell 29, 394–406 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee R. S. et al. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J. Clin. Invest. 122, 2983–2988 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majzner R. G., Heitzeneder S. & Mackall C. L. Harnessing the Immunotherapy Revolution for the Treatment of Childhood Cancers. Cancer Cell 31, 476–485 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Leruste A. et al. Clonally Expanded T Cells Reveal Immunogenicity of Rhabdoid Tumors. Cancer Cell 36, 597–612.e8 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Chun H.-J. E. et al. Identification and Analyses of Extra-Cranial and Cranial Rhabdoid Tumor Molecular Subgroups Reveal Tumors with Cytotoxic T Cell Infiltration. Cell Rep. 29, 2338–2354.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melcher V. et al. Macrophage-tumor cell interaction promotes ATRT progression and chemoresistance. Acta Neuropathol. 139, 913–936 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Johann P. D. et al. Atypical Teratoid/Rhabdoid Tumors Are Comprised of Three Epigenetic Subgroups with Distinct Enhancer Landscapes. Cancer Cell 29, 379–393 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Torchia J. et al. Integrated (epi)-Genomic Analyses Identify Subgroup-Specific Therapeutic Targets in CNS Rhabdoid Tumors. Cancer Cell 30, 891–908 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho B. et al. Molecular subgrouping of atypical teratoid/rhabdoid tumors-a reinvestigation and current consensus. Neuro. Oncol. 22, 613–624 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rooney M. S., Shukla S. A., Wu C. J., Getz G. & Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao J., Liang Y. & Wang L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy. Front Immunol 13, 888713 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills C. D., Kincaid K., Alt J. M., Heilman M. J. & Hill A. M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164, 6166–6173 (2000).10843666 [Google Scholar]
- 23.Newman A. M. et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 37, 773–782 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danaher P. et al. Advances in mixed cell deconvolution enable quantification of cell types in spatial transcriptomic data. Nat. Commun. 13, 385 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Custers L. et al. Somatic mutations and single-cell transcriptomes reveal the root of malignant rhabdoid tumours. Nat Commun 12, 1407 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almeida L. G. et al. CTdatabase: a knowledge-base of high-throughput and curated data on cancer-testis antigens. Nucleic Acids Res. 37, D816–9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brohl A. S. et al. Immuno-transcriptomic profiling of extracranial pediatric solid malignancies. Cell Rep. 37, 110047 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda H. et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity 6, 199–208 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Kaczorowski M. et al. PRAME Expression in Cancer. A Systematic Immunohistochemical Study of >5800 Epithelial and Nonepithelial Tumors. Am. J. Surg. Pathol. 46, 1467–1476 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aibar S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van de Sande B. et al. A scalable SCENIC workflow for single-cell gene regulatory network analysis. Nat. Protoc. 15, 2247–2276 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Jongsma M. L. M., Guarda G. & Spaapen R. M. The regulatory network behind MHC class I expression. Mol. Immunol. 113, 16–21 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Buckingham M. & Rigby P. W. J. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev Cell 28, 225–238 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Shahab S. et al. MEK Inhibition Suppresses Growth of Atypical Teratoid/Rhabdoid Tumors. J Neuropathol Exp Neurol 79, 746–753 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charron J. et al. Embryonic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes Dev 6, 2248–2257 (1992). [DOI] [PubMed] [Google Scholar]
- 36.Ernst J. et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473, 43–49 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seale P. et al. Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777–786 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Relaix F., Rocancourt D., Mansouri A. & Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435, 948–953 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Yoon J. K., Moon R. T. & Wold B. The bHLH class protein pMesogenin1 can specify paraxial mesoderm phenotypes. Dev Biol 222, 376–391 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Barros M. H. M., Segges P., Vera-Lozada G., Hassan R. & Niedobitek G. Macrophage polarization reflects T cell composition of tumor microenvironment in pediatric classical Hodgkin lymphoma and has impact on survival. PLoS One 10, e0124531 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klement J. D. et al. An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J Clin Invest 128, 5549–5560 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tippett E., Cameron P. U., Marsh M. & Crowe S. M. Characterization of tetraspanins CD9, CD53, CD63, and CD81 in monocytes and macrophages in HIV-1 infection. J. Leukoc. Biol. 93, 913–920 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Suzuki M. et al. Tetraspanin CD9 negatively regulates lipopolysaccharide-induced macrophage activation and lung inflammation. J. Immunol. 182, 6485–6493 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Inoue M. et al. SCRG1 suppresses LPS-induced CCL22 production through ERK1/2 activation in mouse macrophage Raw264.7 cells. Mol. Med. Rep. 15, 4069–4076 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosen E. D. et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4, 611–617 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Nutt S. L., Heavey B., Rolink A. G. & Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature 401, 556–562 (1999). [DOI] [PubMed] [Google Scholar]
- 47.Rolink A. G., Nutt S. L., Melchers F. & Busslinger M. Long-term in vivo reconstitution of T-cell development by Pax5-deficient B-cell progenitors. Nature 401, 603–606 (1999). [DOI] [PubMed] [Google Scholar]
- 48.Taniuchi I. et al. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell 111, 621–633 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Woolf E. et al. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci U S A 100, 7731–7736 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearce E. L. et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302, 1041–1043 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Ohno S.-I. et al. Runx proteins are involved in regulation of CD122, Ly49 family and IFN-gamma expression during NK cell differentiation. Int Immunol 20, 71–79 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Townsend M. J. et al. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity 20, 477–494 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Gordon S. M. et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity 36, 55–67 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pennisi D. et al. Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat Genet 24, 434–437 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi E. H. et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 7, 11624 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luan B. et al. CREB pathway links PGE2 signaling with macrophage polarization. Proc. Natl. Acad. Sci. U. S. A. 112, 15642–15647 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee A. et al. Novel role of microphthalmia-associated transcription factor in modulating the differentiation and immunosuppressive functions of myeloid-derived suppressor cells. J Immunother Cancer 11, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu M. et al. Transcription factor c-Maf is a checkpoint that programs macrophages in lung cancer. J. Clin. Invest. 130, 2081–2096 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y. et al. Single-cell RNA-sequencing atlas reveals an MDK-dependent immunosuppressive environment in ErbB pathway-mutated gallbladder cancer. J. Hepatol. 75, 1128–1141 (2021). [DOI] [PubMed] [Google Scholar]
- 61.Nemes K. & Frühwald M. C. Emerging therapeutic targets for the treatment of malignant rhabdoid tumors. Expert Opin. Ther. Targets 22, 365–379 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Al-Khadairi G. & Decock J. Cancer Testis Antigens and Immunotherapy: Where Do We Stand in the Targeting of PRAME? Cancers 11, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hong D. S. et al. Autologous T cell therapy for MAGE-A4 solid cancers in HLA-A*02 patients: a phase 1 trial. Nat. Med. 29, 104–114 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haworth K. B. et al. Characterization of MHC Class I and β−2-Microglobulin Expression in Pediatric Solid Malignancies to Guide Selection of Immune-Based Therapeutic Trials. Pediatr. Blood Cancer 63, 618–626 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Ni L. & Lu J. Interferon gamma in cancer immunotherapy. Cancer Med. 7, 4509–4516 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fincke V. E. et al. Clinical and Molecular Risk Factors in Extracranial Malignant Rhabdoid Tumors: Toward an Integrated Model of High-Risk Tumors. Clin Cancer Res 30, 4667–4680 (2024). [DOI] [PubMed] [Google Scholar]
- 67.Weingart M. F. et al. Disrupting LIN28 in atypical teratoid rhabdoid tumors reveals the importance of the mitogen activated protein kinase pathway as a therapeutic target. Oncotarget 6, 3165–3177 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.FDA approves encorafenib and binimetinib in combination for unresectable or metastatic melanoma with BRAF mutations. U.S. Food and Drug Administration; https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-encorafenib-and-binimetinib-combination-unresectable-or-metastatic-melanoma-braf (2018). [Google Scholar]
- 69.FDA approves encorafenib with binimetinib for metastatic non-small cell lung cancer with a BRAF V600E mutation. U.S. Food and Drug Administration; https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-encorafenib-binimetinib-metastatic-non-small-cell-lung-cancer-braf-v600e-mutation (2023). [Google Scholar]
- 70.Cui Y.-L., Li H.-K., Zhou H.-Y., Zhang T. & Li Q. Correlations of tumor-associated macrophage subtypes with liver metastases of colorectal cancer. Asian Pac. J. Cancer Prev. 14, 1003–1007 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Pantano F. et al. The role of macrophages polarization in predicting prognosis of radically resected gastric cancer patients. J. Cell. Mol. Med. 17, 1415–1421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Väyrynen J. P. et al. The Prognostic Role of Macrophage Polarization in the Colorectal Cancer Microenvironment. Cancer Immunol Res 9, 8–19 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yin S. et al. The Prognostic and Clinicopathological Significance of Tumor-Associated Macrophages in Patients with Gastric Cancer: A Meta-Analysis. PLoS One 12, e0170042 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mei J. et al. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: A systemic review and meta-analysis. Oncotarget 7, 34217–34228 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodriguez-Garcia A. et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat. Commun. 12, 877 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang M. et al. Interferon gamma inhibits CXCL8-CXCR2 axis mediated tumor-associated macrophages tumor trafficking and enhances anti-PD1 efficacy in pancreatic cancer. J Immunother Cancer 8, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang H. et al. CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis. Mol. Cancer 19, 41 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duan Z. & Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther 6, 127 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aida S. et al. MITF suppression by CH5552074 inhibits cell growth in melanoma cells. Cancer Chemother. Pharmacol. 79, 1187–1193 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Faloon P. W. et al. A Small Molecule Inhibitor of the MITF Molecular Pathway. in Probe Reports from the NIH Molecular Libraries Program (National Center for Biotechnology Information (US), Bethesda (MD), 2012). [PubMed] [Google Scholar]
- 81.Heck J. E. et al. Epidemiology of rhabdoid tumors of early childhood. Pediatr. Blood Cancer 60, 77–81 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo Y. et al. New developments on the Encyclopedia of DNA Elements (ENCODE) data portal. Nucleic Acids Res 48, D882–D889 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perez G. et al. The UCSC Genome Browser database: 2025 update. Nucleic Acids Res 53, D1243–D1249 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]