Abstract
Background:
Chronic obstructive pulmonary disease (COPD) is characterized by progressive lung function decline, commonly measured by forced expiratory volume in one second (FEV1). Uncovering the genetic basis of FEV1 decline is essential for understanding COPD pathophysiology and for developing therapies. We hypothesized that gene expression patterns in inflammatory pathways are associated with FEV1 decline.
Methods:
We analyzed whole blood RNA-sequencing data from the 5 (n = 4,147) and 10 year visits (n = 435) in the COPDGene Study. Gene expression was assessed in three analyses: cross-sectional associations with FEV1 at two separate time points, association between year 5 gene expression and FEV1 changes from year 5–10, and longitudinal changes in both gene expression and FEV1. A gene signature derived from the 5-year visit was linked to FEV1 decline across three intervals (baseline to 5 years, 5 to 10 years, and baseline to 10 years) and tested for validation in the ECLIPSE study.
Results:
Distinct gene sets emerged in the three analyses (Cross-sectional: 961 genes; FEV1 Change: 179; Longitudinal: 532). Only two genes (NOV and AC009404.2) overlapped across all analyses, while unique genes (e.g., MMP9, IL1RL1, and CHI3L1) were context-specific. Pathway analysis of genes from the longitudinal analysis highlighted oxidative stress and immune processes. A 20-gene signature was derived, including 17 genes positively and three negatively associated with FEV1. These signatures were significantly associated with FEV1-related traits in COPDGene and ECLIPSE.
Conclusions:
These findings reveal molecular markers of FEV1 decline, offering insights into COPD pathophysiology and potential therapeutic targets.
Keywords: COPD, Pulmonary function tests, RNA-sequencing, Longitudinal analysis, Biomarkers
Background
Chronic Obstructive Pulmonary Disease (COPD) is a heterogeneous disease characterized by persistent respiratory symptoms and airflow limitation. Major causes include environmental factors such as exposure to cigarette smoke and pollutants, together with molecular factors such as alpha-1 antitrypsin deficiency [1]. The forced expiratory volume in 1 second (FEV1) on spirometry is a widely recognized and critical marker used for diagnosing and monitoring the severity of COPD [2]. Studies show that FEV1 decline correlates with exacerbations and mortality risk [3]. Longitudinal analyses have shown that an accelerated decline in FEV1 is a key factor in COPD development, with research indicating that up to 50% of cases arise from this progressive decline [4]. Thus, understanding factors that contribute to FEV1 decline is vital for the effective management of COPD [5].
While lifestyle and environmental exposures, such as continued smoking and repeated exacerbations, are significant contributors, the mechanisms driving COPD progression are still poorly understood. Growing evidence suggests that molecular factors play a crucial role in disease progression, particularly through their effect on lung function decline [6]. Utilizing large longitudinal studies like the Genetic Epidemiology of COPD Study (COPDGene) with clinical and RNA-sequencing data, provides an opportunity to explore these mechanisms more deeply.
We hypothesized that gene expression patterns are associated with subsequent FEV1 decline and may serve as early biomarkers of COPD progression. To test this, we used models assessing the association between gene expression and future FEV1 decline, as well as within-subject changes over time. Combining insights from these approaches provides a more comprehensive understanding of COPD progression, enabling the identification of molecular underpinnings and potential therapeutic targets [6].
Methods
COPDGene Study
COPDGene is a longitudinal prospective study that recruited 10,192 smokers with at least a 10-pack-year smoking history, aged between 45 and 80 years, including subjects with and without COPD at enrollment [7]. Clinical assessments and questionnaires were collected at enrollment, with follow-up visits occurring 5 (Phase 2) and 10 years (Phase 3) after enrollment. Blood samples were collected during the second and third visits for RNA sequencing. Additional details are provided in the online supplement.
Gene Expression:
COPDGene RNA-sequencing methods were reported previously [8, 9]. RNA-seq data from whole blood samples of 4,147 participants in Phase 2 and 511 participants in Phase 3 were retained for analysis after quality control.
Data Processing and Linear Regression Analysis
We performed linear regression analyses to evaluate the relationship between gene expression and lung function decline, focusing primarily on FEV1 change and longitudinal models (Fig. 1, Supplemental Table 1). The FEV1 change analysis used gene expression data from Phase 2 along with the change in FEV1 for three intervals: Phase1-Phase2, Phase2-Phase3, and Phase1-Phase3. For the longitudinal analysis, we assessed the changes of both Phase 2 and Phase 3 FEV1 and gene expression data.
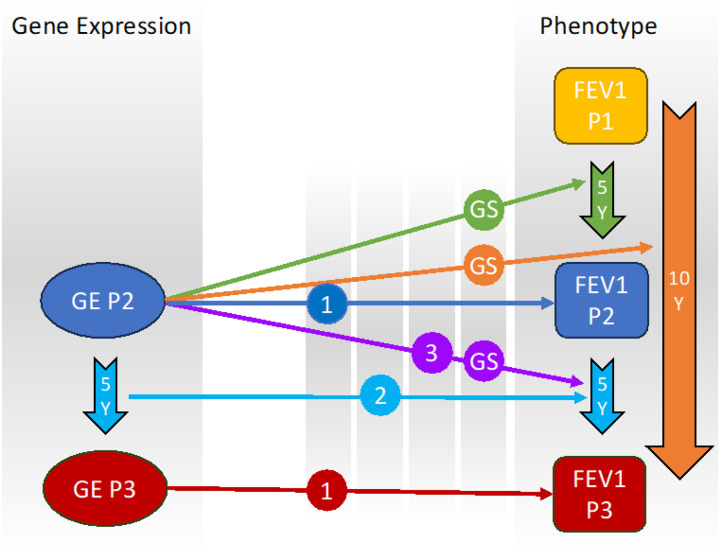
Figure 1. Multi-phase analysis of FEV1 in COPDGene.
(1) Cross-sectional analysis: Association between gene expression (GE) in Phase 2 and Phase 3 with FEV1 in Phase 2 and Phase 3, respectively. (2) Longitudinal gene expression change analysis: Association between the change in gene expression from Phase 2 to Phase 3 and the change in FEV1 from Phase 2 to Phase 3. (3) FEV1 change analysis: Association between gene expression in Phase 2 and the change in FEV1 from Phase 2 to Phase 3. (GS) Gene signature generation: Combining the three analyses to create a gene signature associated with FEV1 change by examining gene expression in Phase 2 and FEV1 change across all three intervals. FEV1, forced expiratory volume in one second; P, Phase; Y, Years.
For the longitudinal analysis, we combined gene expression data from both phases, ensuring that only samples present in both phases were included. Batch effects were adjusted using ComBat-seq [10]. Samples belonging to unique batches were excluded since the batch correction method does not accept single-batch samples. We retained genes where at least 80% of samples had counts per million (CPM) > 1. We then normalized the data using the TMM method and applied the voom transformation. In the longitudinal analysis, we calculated the difference in gene expression between the two-time points. Finally, we performed a linear model in Limma [11] which enables precise variance estimation and covariate adjustment in large sample sizes, adjusting for age, race, sex, smoking status (current or former), and white blood cell count and differential percentages.
Pathway Enrichment Analysis
We performed pathway enrichment analysis using the 532 genes identified with p < 0.05 in the longitudinal analysis. These genes were submitted to g:Profiler (https://biit.cs.ut.ee/gprofiler). The data sources used for this analysis included Gene Ontology (Biological Process), KEGG, Reactome, and WikiPathways. To identify significant pathways, we applied the g:SCS threshold, which adjusts for multiple testing to control the family-wise error rate. The resulting pathways were ranked based on their adjusted p-values.
FEV Change Gene Signature
To reduce multiple testing burden for association with other COPD phenotypes, generated a gene signature associated with FEV1 change by performing linear regression analysis. We included all subjects with FEV1 readings across three intervals (Phase 1 to Phase 2, Phase 2 to Phase 3, and Phase 1 to Phase 3) and identified genes with a nominal p-value < 0.05 for each interval. The gene signature was determined by identifying the overlapping genes across all three intervals. Genes were separated based on the direction of their effect into a positive and negative gene signature. We then performed Gene Set Variation Analysis (GSVA)[12] to assign each subject a score based on these gene sets. We then assessed the association between the gene signature and COPD-related traits, using linear or logistic regression analysis, adjusting for age, race, sex, and smoking status, as well as white blood cell counts and differential percentages.
Validation in ECLIPSE Study
The ECLIPSE Study was a longitudinal prospective study involving 2,747 participants with at least a 10-pack-year smoking history [13], aged between 40–75 years. Study visits occurred at enrollment, three months, and every six months until 3 years. Data collected included spirometry, questionnaires, and other clinical assessments. Gene expression microarray (Affymetrix Human Gene 1.1 ST array) data are available for 627 peripheral blood samples from both COPD and control subjects[13].
Results
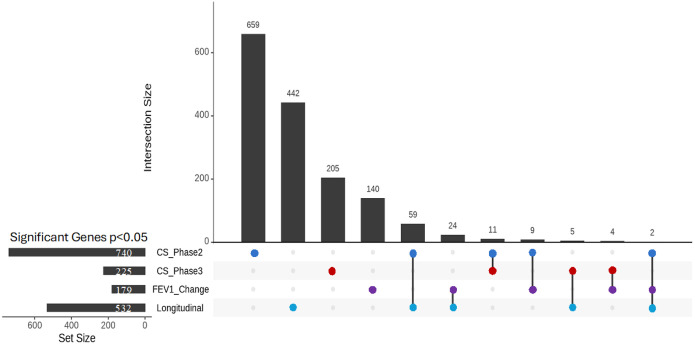
We selected 435 subjects who had both RNA-sequencing and phenotype data in Phase 2 and Phase 3 (Table 1). While the primary focus was on longitudinal and FEV1 change analyses, we also conducted cross-sectional analyses at Phases 2 and 3 for context; full results are reported in the Supplementary Materials (Supplemental Tables 2–3). For the FEV1 change analysis, where we tested the association between gene expression in Phase 2 and the change in FEV1 from Phase 2 to Phase 3, we identified 179 genes at p < 0.05 (Supplemental Table 4). Finally, in the longitudinal analysis, where we assessed the association between the change in gene expression and the change in FEV1, we found 532 genes at p < 0.05 (Supplemental Table 5). To explore the overlap between these analyses, we examined the intersections of genes identified in FEV1 change, longitudinal, and supplementary cross-sectional analyses. Interestingly, only two genes (NOV and AC009404.2) were shared across all three analyses, suggesting a limited but potentially critical set of common molecular determinants (Fig. 2). Additionally, each analysis revealed unique sets of genes previously related to COPD (Supplemental Table 6), cross-sectional (TNF, TLR4, IL6R), FEV1 change (CHI3L1, VEGFA, FOXO3), and longitudinal (MMP9, IL1RL1, ALOX5AP), highlighting context-specific markers of lung function decline. These results demonstrate the importance of combining cross-sectional and longitudinal approaches to capture dynamic changes in gene expression associated with lung function decline.
Table 1.
Characteristics of the study population
| Phase 2 | Phase 3 | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | ||
| Age | Years | 64.52 | 8.17 | 48.1–84.6 | 69.19 | 8.28 | 53–89.4 |
| FEV1 post-BD | Liters | 2.31 | 0.76 | 0.4–4.44 | 2.12 | 0.75 | 0.32–4.36 |
| FEV1% predicted | % | 84.26 | 22.59 | 14.7–137.6 | 82.34 | 24.24 | 12.7–144.2 |
| Neutrophils | % | 58.26 | 9.56 | 25–91 | 60.02 | 9.64 | 19.3–93.9 |
| Lymphocytes | % | 30.5 | 9.03 | 7.0–59 | 28.25 | 9.27 | 2.6–78.9 |
| Eosinophils | % | 2.57 | 1.98 | 0–25 | 2.5 | 1.65 | 0–8.8 |
| Monocytes | % | 8.04 | 2.53 | 1–22.0 | 8.43 | 2.39 | 0–22 |
| WBC | K/uL | 6.84 | 1.98 | 2.8–18.9 | 6.83 | 1.97 | 2.3–22.6 |
| ΔFEV1(Phase2–3) | ml/year | −40.98 | 56.46 | −406–179 | |||
| Pack Years of smoking | 44 | 23.69 | 10–145 | 44.94 | 23.95 | 10–145 | |
| Phase2 | Phase3 | ||||||
| N = 435 | Units | N = 435 | Units | ||||
| Sex (Male) | % | 49 | Sex (Male) | % | 49 | ||
| Former Smokers | % | 67 | Former Smokers | % | 70 | ||
| Race (non-Hispanic White) | % | 74 | Race (White) | % | 74 | ||
| Race (African American) | % | 26 | Race (African American) | % | 26 | ||
| Smoking status in phase 2–3 | FF | FC | CF | CC | |||
| 278 | 12 | 25 | 120 | ||||
FEV1 post-BD: FEV1 post bronchodilator; FF: former smoker in Phase2 and Phase3; CC: Current smoker un Phase2 and Phase3; CF: Current smoker in Phase2 and Former smoker in Phase3; FC: Former smoker in Phase2 and Current smoker in Phase3
Figure 2. Upset plot of Overlapping Genes Across Cross-Sectional and Longitudinal Analyses.
See Figure 1 for descriptions of analyses. CS, Cross-Sectional; FEV1, forced expiratory volume in one second.
We then conducted a pathway enrichment analysis on the 532 genes identified at p < 0.05 in the longitudinal gene-expression analysis (Supplemental Table 7). This analysis identified significantly associated pathways related to hemoglobin production and erythrocyte function, including heme biosynthesis, hemoglobin metabolic process, and erythrocyte differentiation. Additionally, pathways such as myeloid cell differentiation and immune system processes were enriched.
We conducted linear regression analyses to assess the relationship between gene expression in Phase 2 and FEV1 change across three intervals. In the Phase 1 to Phase 2 interval, 3,805 subjects with both phenotype and RNA-seq data were analyzed, resulting in 1,545 genes with p < 0.05 (Supplemental Table 8). For the Phase 2 to Phase 3 interval, 2,045 subjects were included, resulting in 608 genes with p < 0.05(Supplemental Table 9). Lastly, in the Phase 1 to Phase 3 interval, 2,037 subjects were analyzed, resulting in 521 genes with p < 0.05(Supplemental Table 10). To generate a gene signature associated with FEV1 change, we identified all overlapping genes with a nominal p-value < 0.05 across the three intervals, taking into account their fold change direction. This approach resulted in 17 genes positively associated with FEV1 (Positive Gene Signature) and 3 genes negatively associated with FEV1 (Negative Gene Signature). We then assigned each patient a score based on the expression of these two gene signatures using GSVA (Fig. 3).
Figure 3. Heatmap of FEV1 change Gene Signatures.
Subjects are sorted based on their GSVA scores for the gene signatures, with the top 10% of subjects from each end of the GSVA score distribution selected for the figure. Non-linear LOESS regression was applied to the mean FEV1 change values across three intervals—Phase1-Phase2, Phase2-Phase3, and Phase1-Phase3—for each subject. The heatmap represents the normalized gene expression data for each subject, with values scaled by row to highlight relative gene expression differences across the cohort. ΔFEV1, forced expiratory volume in one second change.
Instead of testing individual genes, we tested the association of the two composite gene signatures with phenotypes related to lung function, finding significant associations with FEV1 in Phase 2 (the same year as gene expression collection) and Phase 3 (five years later), rapid FEV1 decline (defined as more than 40 ml/yr) [14], and COPD case-control status (Table 2). The positive and negative gene signatures were associated with other COPD-related traits, including exacerbation frequency in the previous year, severe exacerbations requiring hospitalization or emergency department visits, and chest CT measurements of percent emphysema (LAA 950) and airway wall thickness (Pi10)[15]. Both signatures showed a direction of association aligning with the known association between FEV1 and the respective trait.
Table 2.
FEV1 decline gene signature association with COPD-related traits
| Positive Gene Signature | Negative Gene Signature | ||||
|---|---|---|---|---|---|
| Beta | P.value | Beta | P.value | ||
| FEV1 at Phase 2 (ml) | 310.82 | 1.47E-18 | −170.17 | 4.79E-13 | Linear |
| FEV1 at Phase 3 (ml) | 310.35 | 2.13E-11 | −161.43 | 1.91E-07 | Linear |
| FEV1 Rapid Decline Phase1_Phase2 (< 40ml/yr) | −0.27 | 1.17E-02 | 0.23 | 1.20E-03 | Binary |
| FEV1 Rapid Decline Phase2- Phase 3 (< 40ml/yr) | −0.38 | 8.92E-03 | 0.29 | 2.64E-03 | Binary |
| FEV1 Rapid Decline Phase1- Phase3 (< 40ml/yr) | −0.69 | 3.02E-06 | 0.36 | 2.68E-04 | Binary |
| COPD case-control (Gold Stage 0 VS 2–4) | −1.06 | 3.41E-16 | 0.75 | 1.03E-17 | Binary |
| COPD related Traits | |||||
| Airway Wall Thickness (Pi10) | −0.15 | 1.50E-06 | 0.13 | 1.16E-10 | Linear |
| Severe Exacerbations | −1.18 | 9.94E-10 | 0.33 | 8.17E-03 | Binary |
| Exacerbation Frequency | −0.26 | 2.76E-10 | 0.08 | 4.01E-03 | Linear |
| % emphysema (−950HU) | −2.78 | 1.84E-09 | 1.8 | 4.01E-09 | Linear |
| Change P1 to P2: Emphysema | −0.77 | 2.12E-04 | 0.47 | 6.34E-04 | Linear |
| Validation (ECLIPSE) | |||||
| FEV1 at baseline | 360.24 | 4.89E-05 | −352.33 | 3.71E-08 | Linear |
| FEV1 at 1 year | 333.5 | 1.60E-04 | −300.8 | 3.09E-06 | Linear |
| FEV1 at 2 years | 329.82 | 1.98E-04 | −312.88 | 1.20E-06 | Linear |
| FEV1 at 3 years | 294.17 | 1.54E-03 | −327.72 | 1.35E-06 | Linear |
| FEV1 Change ml/yr | 4.68 | 6.52E-01 | −2.5 | 7.43E-01 | Linear |
| % emphysema (−950HU) | −4.49 | 7.49E-03 | 4.28 | 3.59E-04 | Linear |
| Exacerbation Frequency | −0.73 | 3.10E-04 | 0.03 | 8.54E-01 | Linear |
| Airway Wall Thickness (Pi10) | −0.018 | 2.89E-01 | 0.018 | 4.39E-01 | Linear |
GOLD, global initiative for chronic Obstructive lung Disease; FEV1, forced expiratory volume in 1 second. Pi10, The square root of the wall area of a hypothetical airway with a 10-mm internal perimeter
Finally, we aimed to validate our findings in the ECLIPSE study by testing the association between the two gene signatures and FEV1 measurements at baseline and follow-up visits, FEV1 decline, exacerbation frequency, percent emphysema, and Pi10. Both signatures showed significant associations with FEV1 at baseline and follow-up, as well as with the percent emphysema and exacerbation frequency (Table 2). However, we were unable to find a significant association between the gene signatures and the change in FEV1 (ml/yr).
Discussion
We performed RNA-sequencing on whole blood samples collected at Phase 2 (5-year) and Phase 3 (10-year) visits in the COPDGene Study to identify gene expression signatures associated with FEV1 decline. Our primary analyses included FEV1 change and longitudinal models; cross-sectional findings are presented in the Supplementary Materials to provide additional context. Each analysis revealed a unique set of genes, and there was a limited overlap between the results, highlighting the complexity of gene expression changes over time in COPD. In addition, we generated a gene signature relevant to lung function decline that is significantly associated with key clinical traits related to COPD. Finally, we were able to replicate most of the gene signature associations in the ECLIPSE study, demonstrating the robustness of the composite signatures.
We compared the outcomes of the FEV1 change analysis with the cross-sectional analysis. We found that 15 genes (p < 0.05) overlapped between the FEV1 change analyses and the cross-sectional analysis. Additionally, 137 genes were uniquely associated with FEV1 change and not identified in the other analyses. These findings suggest that while some genes are consistently associated with FEV1 in both types of analysis, other genes may be more relevant for changes in lung function over time.
Finally, when we compared these previous analyses with the longitudinal linear regression analysis, which examined the association between changes in gene expression from Phase 2 to Phase 3 and the change in FEV1 over the same period, we observed several overlaps. Two genes were common to both the cross-sectional and FEV1 change analyses, 64 genes overlapped only with the cross-sectional analysis, 24 genes were shared exclusively with the FEV1 Change analysis, and 442 genes were unique to the longitudinal analysis. These results suggest that the longitudinal analysis uncovers additional gene associations that may be missed by cross-sectional or FEV1 change approaches, emphasizing the value of longitudinal methods in identifying genes related to changes in lung function.
Among the genes identified in the longitudinal analysis, several have previously been associated with FEV1 and COPD. MMP9 encodes the enzyme Matrix metalloproteinase-9 (MMP-9) which is implicated in the development of emphysema, mediating inflammation through extracellular matrix degradation and neutrophil recruitment [16]. IL1RL1 encodes Interleukin 1 receptor-like 1 (IL1RL1), also known as suppression of tumorigenicity 2 (ST2), is a receptor for interleukin 33 (IL-33) and is part of the interleukin 1 receptor family. IL-33 and its receptor ST2 have a role in mediating immune responses and alveolar damage[17], and this pathway is a target for novel COPD therapies including itepekimab and astegolimab [18, 19]. ALOX5AP gene encodes arachidonate 5-lipoxygenase activating protein (ALOX5AP) that is essential in producing leukotriene B4 (LTB4), a molecule that drives inflammation by recruiting neutrophils to the lungs in COPD [20]. Elevated LTB4 levels in sputum are associated with worsened lung function and disease progression in COPD [21].
Only two genes (NOV and AC009404.2) overlapped across all three analyses, highlighting their potential significance in COPD and lung function regulation. NOV, encoding the CCN3 protein, is involved in inflammation and apoptosis. It has been identified as a biomarker for acute lung injury[22], suggesting its potential role in lung repair and disease pathogenesis.
The gene signature we generated for FEV1 change includes several genes previously associated with lung function, COPD, and related traits. AKAP6, which encodes A-kinase anchoring protein 6 (AKAP6), has been implicated in both COPD and lung function (FEV1/FVC ratio) through genome-wide association studies [23, 24]. Similarly, GPR15, which encodes G protein-coupled receptor 15, has emerged as a key gene associated with smoking status and more severe COPD [25, 26]. It acts as a chemoattractant receptor regulating immunity and T-cell migration, and its increased expression in smokers highlights its role in inflammation and smoking-related lung damage [27]. Single nucleotide polymorphisms in SLC4A10 and LAMA2, which encode solute carrier family 4 member 10 (SLC4A10) and laminin subunit alpha 2 (LAMA2) respectively, have also been associated with smoking status and lung function (forced vital capacity) [28–30], further supporting their relevance in COPD pathophysiology. Other genes in the signature, such as Chromosome 12 Open Reading Frame 42 (C12orf42) and phosphatidylinositol glycan anchor biosynthesis, class L (PIGL), have been associated with pulmonary arterial hypertension [31], smoking initiation [29], and FVC [24].
In addition to the genes previously associated with lung function and COPD, our gene signature was also associated with important COPD clinical traits related to FEV1 decline, such as severe exacerbations, exacerbation frequency, percent emphysema, and airway wall thickness. These associations suggest that the molecular factors influencing FEV1 decline may also be relevant to structural changes in the lungs and the severity of disease manifestations.
Interestingly, we were able to replicate most of the gene signature associations in another longitudinal COPD study, demonstrating consistent associations with FEV1 on a cross-sectional level, as well as with traits like airway wall thickness and exacerbation frequency. However, we were unable to replicate the association with FEV1 change. A possible reason is the differences in the time scale for lung function testing. In COPDGene, spirometry was performed every five years, whereas, in ECLIPSE, the measurements were taken annually, with a maximum follow-up of three years, which may have reduced the power of the analysis. This shorter follow-up may not have allowed enough time for a significant change in FEV1 to be detected.
Our study has several limitations. RNA-seq data were taken from blood samples, which may not perfectly capture the lung-specific aspects of COPD, However, they are well-suited for clinical applications and epidemiological research. This approach is particularly useful given that COPD has significant systemic comorbidities, including muscle loss, cardiovascular disease, and osteoporosis [32], highlighting the need for broader biomarker analysis beyond the lungs. The gene signature was generated using RNA-seq data from COPDGene, but the validation study used microarray data. Despite the differing technologies, the signature was replicated for many of the traits, which may imply better transferability of a gene signature biomarker as opposed to the expression of single genes. The sample size of Phase 3 RNA-sequencing was relatively small, which may have reduced our statistical power. Additionally, the longitudinal analysis was performed on only two-time points five years apart, which does not provide a full trajectory of FEV1 decline. Future studies will include larger sample sizes and more time points to improve the prediction of gene expression associations with lung function decline.
In conclusion, we identified genes associated with FEV1 decline in COPD, providing both previously known and novel insights into the molecular contributors to lung function decline. Our findings suggest that the identified genes may serve as potential biomarkers for COPD progression and targets for therapeutic intervention. Further studies are needed to validate these findings in larger cohorts, with more frequent longitudinal assessments, and in diverse populations, to better understand the clinical utility of these gene signatures in predicting disease trajectory for COPD patients.
Supplementary Material
Supplementary Files
This is a list of supplementary files associated with this preprint. Click to download.
Conflict of Interest Statement:
Zaid W. Elhusseini, Omar Rafique, Min Hyung Ryu, and Ingo Ruczinski declare no conflicts of interest. Peter Castaldi reports grant support from NIH to his institution related to this work, and outside the submitted work, consultancy fees from Verona Pharma and Genentech, and grants pending from Sanofi. Don D. Sin has received honoraria from GSK, AZ, and BI for giving COPD-related talks to physicians and scientists, and serves as chair of a DSMB for an NHLBI-sponsored trial. Craig P. Hersh reports grants to his institution from NHLBI, Alpha-1 Foundation, and Bayer, and consulting fees from Apogee Therapeutics, Chiesi, Genentech, Ono Pharma, Sanofi, Takeda, and Verona Pharma.
Funding
This work was supported by NHLBI grants R01HL166231, K24HL173667, R01HL157879, U01HL089897 and U01HL089856 and by NIH contract 75N92023D00011. The COPDGene study (NCT00608764) has also been supported by the COPD Foundation through contributions made to an Industry Advisory Committee that has included AstraZeneca, Bayer Pharmaceuticals, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, and Sunovion.
Abbreviations
- COPD
Chronic Obstructive Pulmonary Disease
- FEV1
Forced Expiratory Volume in 1 Second
- FVC
Forced Vital Capacity
- CPM
Counts Per Million
- GSVA
Gene Set Variation Analysis
- CS
Cross-Sectional
- Pi10
Airway Wall Thickness
- BD
Before Bronchodilator
- WBC
White Blood Cells
Funding Statement
This work was supported by NHLBI grants R01HL166231, K24HL173667, R01HL157879, U01HL089897 and U01HL089856 and by NIH contract 75N92023D00011. The COPDGene study (NCT00608764) has also been supported by the COPD Foundation through contributions made to an Industry Advisory Committee that has included AstraZeneca, Bayer Pharmaceuticals, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, and Sunovion.
Footnotes
Additional Declarations: No competing interests reported.
Ethics approval and consent to participate
The study was approved by the institutional review boards (IRBs) of participating centers in the COPDGene study, and all participants provided written informed consent. The research was conducted in accordance with the Belmont Report.
Contributor Information
Zaid W. Elhusseini, Harvard Medical School
Omar Rafique, Harvard Medical School.
Min Hyung Ryu, St. Paul’s Hospital, University of British Columbia.
Peter Castaldi, Harvard Medical School.
Don D. Sin, St. Paul’s Hospital, University of British Columbia
Ingo Ruczinski, Johns Hopkins Bloomberg School of Public Health.
Craig P. Hersh, Harvard Medical School
Data Availability
The phenotype and RNA-seq data used in this study are available through the COPDGene Study under controlled access via dbGaP (accession numbers: phs000179.v6.p2, phs000765.v3.p2).
References
- 1.Chronic obstructive pulmonary disease (COPD). https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed 5 September 2024)
- 2.GOLD Report - Global Initiative for Chronic Obstructive Lung Disease - GOLD. https://goldcopd.org/2024-gold-report/ (accessed 5 September 2024)
- 3.Rennard SI, Drummond MB. Early chronic obstructive pulmonary disease: definition, assessment, and prevention. Lancet. 2015;385:1778. doi: 10.1016/S0140-6736(15)60647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lange P, Celli B, Agustí A, et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med. 2015;373:111–22. doi: 10.1056/NEJMOA1411532 [DOI] [PubMed] [Google Scholar]
- 5.Hobbs BD, Hersh CP. Integrative genomics of chronic obstructive pulmonary disease. Biochem Biophys Res Commun. 2014;452:276–86. doi: 10.1016/J.BBRC.2014.07.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shrine N, Guyatt AL, Erzurumluoglu AM, et al. New genetic signals for lung function highlight pathways and chronic obstructive pulmonary disease associations across multiple ancestries. Nat Genet. 2019;51:481–93. doi: 10.1038/S41588-018-0321-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryu MH, Yun JH, Morrow JD, et al. Blood Gene Expression and Immune Cell Subtypes Associated with Chronic Obstructive Pulmonary Disease Exacerbations. Am J Respir Crit Care Med. 2023;208:247–55. doi: 10.1164/RCCM.202301-0085OC/SUPPL_FILE/DISCLOSURES.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obeidat M, Nie Y, Chen V, et al. Network-based analysis reveals novel gene signatures in peripheral blood of patients with chronic obstructive pulmonary disease. Respir Res. 2017;18:1–11. doi: 10.1186/S12931-017-0558-1/FIGURES/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Parmigiani G, Johnson WE. ComBat-seq: batch effect adjustment for RNA-seq count data. NAR Genom Bioinform. 2020;2. doi: 10.1093/NARGAB/LQAA078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/NAR/GKV007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hänzelmann S, Castelo R, Guinney J. GSVA: Gene set variation analysis for microarray and RNA-Seq data. BMC Bioinformatics. 2013;14:1–15. doi: 10.1186/1471-2105-14-7/FIGURES/7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Eur Respir J. 2008;31:869–73. doi: 10.1183/09031936.00111707 [DOI] [PubMed] [Google Scholar]
- 14.Vestbo J, Edwards LD, Scanlon PD, et al. Changes in Forced Expiratory Volume in 1 Second over Time in COPD. New England Journal of Medicine. 2011;365:1184–92. doi: 10.1056/NEJMOA1105482/SUPPL_FILE/NEJMOA1105482_DISCLOSURES.PDF [DOI] [PubMed] [Google Scholar]
- 15.Boueiz A, Chang Y, Cho MH, et al. Lobar Emphysema Distribution Is Associated With 5-Year Radiological Disease Progression. Chest. 2018;153:65–76. doi: 10.1016/j.chest.2017.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Churg A, Zhou S, Wright JL. Series ‘matrix metalloproteinases in lung health and disease’: Matrix metalloproteinases in COPD. Eur Respir J. 2012;39:197–209. doi: 10.1183/09031936.00121611 [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Hu Y, Chen Y, et al. IL-33 induces production of autoantibody against autologous respiratory epithelial cells: a potential mechanism for the pathogenesis of COPD. Immunology. 2019;157:137–50. doi: 10.1111/IMM.13054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabe KF, Celli BR, Wechsler ME, et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med. 2021;9:1288–98. doi: 10.1016/S2213-2600(21)00167-3 [DOI] [PubMed] [Google Scholar]
- 19.Yousuf AJ, Mohammed S, Carr L, et al. Astegolimab, an anti-ST2, in chronic obstructive pulmonary disease (COPD-ST2OP): a phase 2a, placebo-controlled trial. Lancet Respir Med. 2022;10:469–77. doi: 10.1016/S2213-2600(21)00556-7 [DOI] [PubMed] [Google Scholar]
- 20.Kostikas K, Gaga M, Papatheodorou G, et al. Leukotriene B4 in exhaled breath condensate and sputum supernatant in patients with COPD and asthma. Chest. 2005;127:1553–9. doi: 10.1378/CHEST.127.5.1553 [DOI] [PubMed] [Google Scholar]
- 21.Woolhouse IS, Bayley DL, Stockley RA. Sputum chemotactic activity in chronic obstructive pulmonary disease: effect of alpha(1)-antitrypsin deficiency and the role of leukotriene B(4) and interleukin 8. Thorax. 2002;57:709–14. doi: 10.1136/THORAX.57.8.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu HP, Huang HY, Wu DM, et al. Regulatory mechanism of NOV/CCN3 in the inflammation and apoptosis of lung epithelial alveolar cells upon lipopolysaccharide stimulation. Mol Med Rep. 2020;21:1872–80. doi: 10.3892/MMR.2019.10655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosentino J, Behsaz B, Alipanahi B, et al. Inference of chronic obstructive pulmonary disease with deep learning on raw spirograms identifies new genetic loci and improves risk models. Nat Genet. 2023;55:787–95. doi: 10.1038/S41588-023-01372-4 [DOI] [PubMed] [Google Scholar]
- 24.Kichaev G, Bhatia G, Loh PR, et al. Leveraging Polygenic Functional Enrichment to Improve GWAS Power. Am J Hum Genet. 2019;104:65–75. doi: 10.1016/J.AJHG.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker MM, Chase RP, Lamb A, et al. RNA sequencing identifies novel non-coding RNA and exon-specific effects associated with cigarette smoking. BMC Med Genomics. 2017;10. doi: 10.1186/S12920-017-0295-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh AJ, Saferali A, Lee S, et al. Blood RNA sequencing shows overlapping gene expression across COPD phenotype domains. Thorax. 2022;77:115–22. doi: 10.1136/THORAXJNL-2020-216401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersen AM, Lei MK, Beach SRH, et al. Inflammatory biomarker relationships with helper T cell GPR15 expression and cannabis and tobacco smoking. J Psychosom Res. 2021;141. doi: 10.1016/J.JPSYCHORES.2020.110326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlsson Linnér R, Biroli P, Kong E, et al. Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat Genet. 2019;51:245–57. doi: 10.1038/S41588-018-0309-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu M, Jiang Y, Wedow R, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51:237–44. doi: 10.1038/S41588-018-0307-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saunders GRB, Wang X, Chen F, et al. Genetic diversity fuels gene discovery for tobacco and alcohol use. Nature. 2022;612:720–4. doi: 10.1038/S41586-022-05477-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pu A, Ramani G, Chen Y-J, et al. Identification of novel genetic variants, including PIM1 and LINC01491, with ICD-10 based diagnosis of pulmonary arterial hypertension in the UK Biobank cohort. Frontiers in drug discovery. 2023;3. doi: 10.3389/FDDSV.2023.1127736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agusti À, Soriano JB. COPD as a systemic disease. COPD. 2008;5:133–8. doi: 10.1080/15412550801941349 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The phenotype and RNA-seq data used in this study are available through the COPDGene Study under controlled access via dbGaP (accession numbers: phs000179.v6.p2, phs000765.v3.p2).