Abstract
Pet dogs spontaneously develop a form of diffuse large B cell lymphoma (DLBCL) that recapitulates many of the features of double hit (MYC/BCL2) human DLBCL. We recently completed a clinical trial in dogs with DLBCL using a combination of canine anti-CD20 antibody and low dose doxorubicin followed by one of three small molecule immune-modulating agents (KPT-9274, TAK-981 or RV1001). Clinical outcomes and tumor specific biomarkers of response from these dogs have been previously reported. In this study, we used the NanoString Canine IO panel to assess dynamic changes in gene counts from peripheral blood mononuclear cells (PBMCs) collected longitudinally from these from dogs over the course of their treatment to identify immune correlates associated with early relapse versus long-term survivorship. Increases in interferon-stimulated gene (ISG) signatures and immune skewing genes [CCR9, CD209 (DC-SIGN), CMKLR and DDX58 (RIG-I)] were associated with shorter (<400 day) survival times and early relapse. In contrast, CD1Eand CCL14 were elevated post-immunotherapy in long-term (>400 day) survivors, suggesting that these may be associated with protective immune signatures. Examining genes that were expressed in short- versus long-term survivors early on in the treatment regimen identified TBHD, NPNT and ISG20 as elevated in dogs with shorter survival times at day 7. To facilitate point-of-care PBMC gene expression testing that could be used to distinguish those dogs likely to require more intensive treatment regimens in advance of relapse, we developed qPCR assays for TBHD, NPNT and ISG20. Together these data provide proof of principle that biomarker interrogation in PBMCs can help predict early relapse and poor responders to inform clinical management of DLBCL.
Keywords: Diffuse large B cell lymphoma (DLBCL), canine (dog), cancer immunotherapy, interferon (IFN), liquid/blood biopsy, immune landscape
Introduction
Diffuse large B cell lymphoma (DLBCL) represents one of the most common forms of non-Hodgkin lymphoma in humans1. The current standard therapy for human DLBCL integrates B cell depletion (rituximab) with multi-agent chemotherapy (CHOP), which has significantly enhanced efficacy compared to protocols that contain only cytotoxic agents2,3,4. While this results in long-term survivorship for over 70% of affected patients, challenges remain to effectively treat those who fail to respond or experience early relapse. The intensive regimen is also associated with cardiotoxicity and may not be well tolerated by elderly patients5–7. Consequently, there have been recent efforts to develop treatment regimens that are less dose-intense, or incorporate small molecule inhibitor/immunotherapy combinations without the inclusion of cytotoxic agents8–10. However, no consistent biomarkers exist to predict early relapse in elderly/frail patients and as such, significant challenges remain with respect to identifying those patients likely to benefit from non-CHOP based regimens.
Dogs serve as a spontaneous large animal model of DLBCL11, and have been instrumental in testing of novel small molecule inhibitors such as ibrutinib12 and acalabrutinib13, as well as chimeric antigen receptor (CAR) T cell based therapies14. We leveraged DLBCL in pet dogs as a spontaneous large animal model to determine whether a modified low dose chemo-immunotherapy regimen could achieve outcomes similar to CHOP without the associated toxicities15–17. Specifically, we treated dogs with three different protocols including a caninized anti-CD20 antibody (rituximab equivalent), low dose doxorubicin and one of 3 small molecule immune modulating drugs (TAK981, KPT9274 and RV100115,16). We showed that all three regimens were extremely well tolerated, resulting in median survival times that were equivalent or superior to those achieved with CHOP alone15,16. However, within each arm there were dogs that did not respond well to the therapy, experiencing early relapse and short survival times. In the current study, we utilized longitudinal collections of peripheral blood mononuclear cells (PBMCs) from dogs treated in this study to understand whether long-term survivorship could be predicted based on the immune landscape as assessed by PBMC gene expression signatures. Ultimately, such a non-invasive approach has the potential to provide clinical meaningful information relevant for informing treatment strategies in both dogs and humans.
Materials & methods
Study Design
This study utilized PBMCs from pet dogs enrolled in a non-randomized clinical trial aimed at evaluating the effectiveness of combining a canine anti-CD20 monoclonal antibody with doxorubicin and specific targeted small molecule inhibitors (RV1001, KPT-9274, TAK-981) for the treatment of diffuse large B-cell lymphoma (DLBCL). The trial was conducted following protocol #G2017–110, approved by the Tufts University Institutional Animal Care and Use Committee (IACUC), and studies were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Informed consent was obtained from all participating dog owners; all potential risks involved in the study were discussed with owners prior to enrollment. The protocol and outcomes have been previously described15,16; an overview of the study is provided in Fig. 1. Briefly, KPT-9274 is a dual inhibitor of PAK4 (p21-activated kinase 4) and NAMPT; PAK4 is a serine/threonine protein kinase that promotes tumor cell survival and proliferation, and NAMPT is a critical enzyme for NAD + salvage pathway synthesis18,19. TAK-981 is a SUMO-activating enzyme inhibitor and induces genes associated with Type I interferon responses20. RV1001 is a PI3Kδ inhibitor, a pathway known to be enriched in human and canine DLBCL21. Each dog received a combination treatment regimen comprising doxorubicin, anti-CD20 antibodies, and a small molecule inhibitor specific to their study group (Table 1).
Figure 1.
Study overview. Dogs analyzed in this study all received anti-CD20 antibody and doxorubicin induction therapy and were separated into 3 treatment arms with small molecule inhibitors TAK-981, KPT-9274 and RV1001. Weekly blood draws were performed. PBMCs were isolated and RNA was extracted for NanoString analysis with the goal of identifying peripheral biomarkers associated with long-term survival.
Table 1.
Study arms from DLBCL veterinary trial.
| Treatment Arm | Long-term survivors- Yes (LTS-Y) N; median days survived ± standard deviation |
Long-term survivors - No (LTS-N) N; median days survived ± standard deviation |
|---|---|---|
| TAK981 | 2; 612 ± 288 | 3; 292 ± 124 |
| KPT9274 | 4; 688 ± 119 | 3; 62 ± 50 |
| RV1001 | 4; 751 ± 146 | 2; 55 ± 52 |
Blood collection was performed at every study visit, using CPT tubes (BD Biosciences) for PBMC purification22. PBMCs were isolated from the CPT tubes according to the manufacturer’s instructions and cryopreserved for RNA isolation and downstream analyses. We confirm the study design is reported in accordance with ARRIVE guidelines.
Inclusion & Exclusion Criteria:
Dogs were eligible for inclusion if they met the following criteria: a diagnosis of DLBCL established via lymph node biopsy, a minimum weight of 10 kg, an age of at least one year, the presence of at least two peripheral lymph nodes measuring 2 cm or greater in diameter, and adequate organ function confirmed through standard laboratory assessments. Dogs were excluded if they presented with uncontrolled autoimmune conditions, severe cardiovascular disease, recent major surgery, pregnancy, lactation, central nervous system involvement, or were receiving concurrent medications that could potentially confound study outcomes.
RNA isolation & NanoString Analysis
RNA from canine PBMCs collected at Days 0 (pre-treatment), D7, D21/28 (depending on treatment arm) and at relapse were extracted using QIAGEN RNeasy mini kits with gDNA eliminator tubes per the manufacturer’s protocol. RNA concentrations were quantified using a Nanodrop spectrophotometer. The nCounter Canine IO Panel was employed for hybridization, using 100ng of RNA (MAX machine) per sample incubated over a 19.5-hour period. Microarrays were analyzed on the NanoString nCounter Sprint system according to the manufacturer’s guidelines.
Microarray Data & Statistical Analysis:
Data analysis was conducted using nSolver software and NanoString partner analysis ROSALIND® (https://rosalind.bio/) nCounter software. Housekeeping probes for normalization were chosen by analyzing common selected housekeeping genes across all 3 treatment arms using nSolver software. These genes were then selected, and normalized count files were generated in nSolver and imported into ROSALIND software. Comparisons across arms with correction for time were performed in ROSALIND, and two-way comparisons for long-term survivorship were performed in nSolver software using an overall survival time (OST) cutoff of 400 days. Significant genes were chosen as log2fold change less than or greater than 1.5, and p < 0.05. To help ensure stringency in biomarker identification, we also incorporated P-value adjustment was performed using the Benjamini-Hochberg method. Data were graphed in GraphPad Prism software version 10 or newer, and two-way ANOVAs for long-term survivorship and time were analyzed with Dunnett’s post hoc tests comparing to baseline time point.
Realtime PCR & Statistical Analysis
RNA from canine PBMCs was isolated using RNeasy kits (Qiagen) in accordance with the manufacturer’s instructions. Complementary DNA (cDNA) synthesis was performed using iScript kits (BioRad). Quantitative PCR was carried out using SYBR green kits (BioRad) on a CFX96 instrument (BioRad), running 40 cycles at 56°C. Primer sequences are listed in Table 2. The ΔΔCT method was used for calculating copy numbers in Microsoft Excel. Data were graphed in GraphPad prism software and student’s t tests were used to compare long-term survivorship yes versus no groups.
Table 2.
qPCR primers used for this study.
| Gene name | Forward Primer | Reverse Primer |
|---|---|---|
| TBHD | CTC GTG CCA TAA ACT GTG CG | ACT CAC ACT TGC TCC CGA AG |
| NPNT | GAA GCC TCG GCC CTG CAA G | AGC ATA TAT CCG TTG AGA CAG TA |
| ISG20 | CCC GCT GCA GCC TCG TGG A | TGG GTC CTG TAG TCA GTG ATC TC |
| GAPDH | GAT GGG CGT GAA CCA TGA G | TCA TGA GGC CCT CCA CGA T |
Results
The primary goal of this work was to determine whether gene expression signatures in PBMCs could predict long-term survivorship (defined as overall survival time greater than 400 days) across the three treatment cohorts (Fig. 1). With respect to progression free survival (PFS), there were roughly equal numbers of dogs with long (n = 10) and short (n = 8) PFS (defined as ≤ 90 days or > 90 days, respectively based on our prior study endpoints16). Importantly, there was an equal distribution of dogs with short and long term PFS in each of the three cohorts (Table 1). Using the NanoString Canine IO Panel, we evaluated PBMC gene expression signatures across all dogs at 4 key time points: baseline (D0), post-induction therapy/B cell depletion (D7), post-immunotherapy (D21/28 based on the treatment regimen) and relapse. Loss of B cell gene signatures (PAX5, MS4A1, CD19) was noted in nearly all dogs at D7 and D21/28 (Fig. 2A–C), concordant with B cell depletion secondary to use of the canine anti-CD20 mAb15. At relapse, the B cell signatures recovered (Fig. 2D–F), supporting the loss of clinical remission. Importantly, the kinetics of B cell specific gene expression signatures were consistent across the three different treatment regimens (Fig. 2G–I).
Figure 2.
Examination of PBMC gene signatures in DLBCL trial patients across all arms confirms loss of B cell signatures. A. Post-B cell depletion, B. post-immunotherapy and C. relapse gene expression signatures compared to baseline. Comparison of B cell gene signatures in long-term survivors (LTS) yes versus no reveals similar levels of peripheral B cell depletion as assessed by D. CD19, E. MS4A1 (CD20) and F. PAX5 expression. n=10 LTS Y, n=8 LTS N. G. CD19, H. MS4A1 (CD20) and I. PAX5 expression by treatment arm. n=5 TAK-981, 7 KPT-9274 and 6 RV1001 recipients. (two-way ANOVA with Dunnet’s post hoc tests significant as indicated).
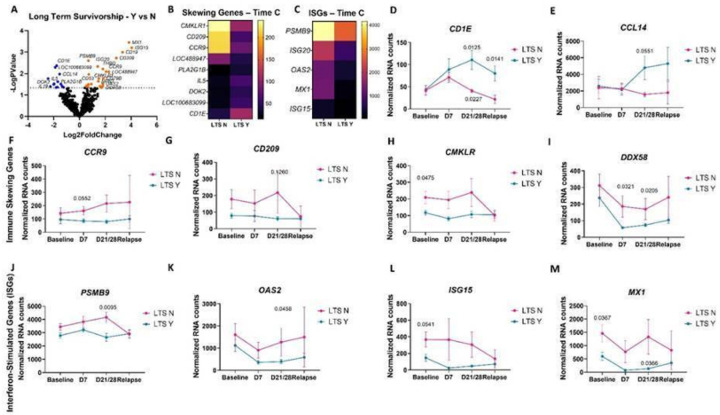
Unbiased gene expression analysis of PBMCs from all dogs at all time points, examining long versus short term survivorship, revealed several differentially expressed genes (DEGs) that could serve as candidate biomarkers (Fig. 3A–C). Next, we compared expression of these DEGs singly over time. CD1E and CCL14 predicted long-term survivorship post-chemo-immunotherapy (Fig. 3D & E). DEGs that mediate immune skewing were differentially associated with survivorship. Specifically, CCR9, which is associated with mucosal homing T cells, was increased in dogs with short survival (Fig. 3F). CD209, which is a marker of M2 macrophages, was also elevated in those with poor outcomes (Fig. 3G). Two additional genes CMKLR, an atypical chemokine receptor, and DDX58, a nucleic acid sensor, were also elevated in dogs with poor survival across all time points (Fig. 3H&I). Interestingly, interferon stimulated genes (ISGs) including MX1, ISG15, ISG20 and PSMB9 were associated with a worse outcome at all time points (Fig. 3J–M).
Figure 3.
Examining PBMC gene expression by survivorship identifies immune skewing genes and interferon-stimulated genes (ISGs) are associated with lower survival time, whereas CD1E and CCL14 are associated with long-term survivorship. A. Unbiased gene expression analysis in long-term survivors yes versus no (n=10 LTS Y, 8 LTS N). B. Heatmap of immune skewing genes and C. ISGs at time point C by survivorship. D. CD1E and E. CCL14 counts over time have a statistically significant increase postimmunotherapy (time point C) in long-term survivors. Immune skewing genes including F. CCR9, G. CD209, H. CMKLR and I. DDX58 were elevated or trending higher in dogs with shorter overall survival times. ISGs including J. PSMB9, K. OAS2, L. ISG15 and M. MX1 are increased in poor responders compared to long-term survivors. (two-way ANOVA with Dunnet’s post hoc tests significant as indicated).
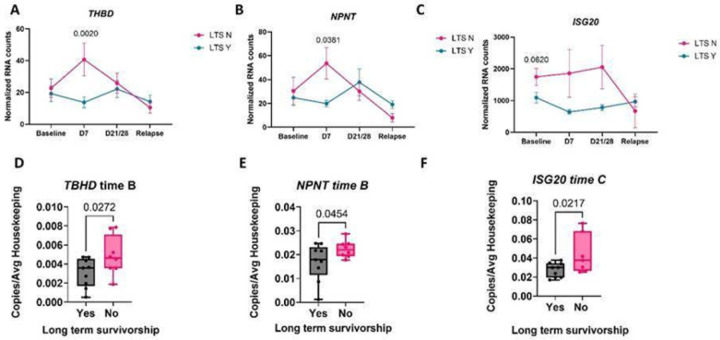
Next we specifically examined genes that could be used to predict outcome at early time points post treatment initiation (D7 and D21/28) as these mark a critical point during treatment initiation that defines induction of remission and depletion of B cells. Elevated TBHD, which encodes thrombomodulin, and NPNT, or the nephronectin gene, predicted poor survivorship at D7 post-treatment (Fig. 4A & B). Additionally, elevated ISGs such as ISG20 predicted poor survivorship at baseline, D7 and D21/28 post-treatment (Fig. 4C). To validate these findings, we used qPCR to confirm that TBHD, NPNT and ISG20 expression were significantly elevated in dogs with worse outcomes at D7 post chemo-immunotherapy (Fig. 4D–F).
Figure 4.
Identifying genes associated with poor survivorship post-B cell depletion and post-immunotherapy for catching patients who need more aggressive treatment. A. TBHD, B. NPNT and C. ISG20 counts over time. D. TBHD, E. NPNT and F. ISG20 qPCR validation at the indicated time points. (two-way ANOVA with Dunnet’s post hoc tests significant as indicated).
Discussion
Over the past decade there has been rapid development of several non-invasive methodologies capable of predicting outcomes and/or directing therapy in cancer patients. Collectively known as liquid biopsy, this typically involves serial blood collection to analyze circulating elements (i.e., DNA, RNA, exosomes) released by tumor cells. We reasoned that a similar approach could be used in the context of immunotherapy studies in which PBMC gene signatures could serve as a surrogate biomarker associated with treatment response and patient outcomes. We leveraged a previous study in dogs with DLBCL in which they were randomized to receive one of three different chemo-immunotherapy regimens15,16 and analyzed gene expression signatures at various time points over the course of therapy. Using the NanoString canine IO panel, we found that the expression of two genes, CD1E and CCL14, represent potential biomarkers of long-term survivorship post-treatment.
CD1E is a member of the immunoglobulin supergene family which delivers lipid and glycolipid antigens to T cells23. Several studies have evaluated the preclinical efficacy of CD1 restricted T cell responses in anti-tumor immunity24,25. Harnessing CD1 restricted responses may therefore represent a novel immunotherapy based approach for canine DLBCL. CCL14 is a small cytokine within the CC chemokine family. It is expressed in several tissues including the spleen, bone marrow, liver, muscle, and gut, promoting the activation and migration of immune cells. Because CCL14 can recruit tumor infiltrating immune cells (TIIC) such as CD4 + and CD8 + T cells, neutrophils, and B cells, it has been associated with amplifying anti-tumor immunity in hepatocellular carcinoma26 and lung adenocarcinoma27. However, CCL14 has a negative association with prognosis in gastric28 and thyroid cancer29. The precise roles of CD1E and CCL14 in DLBCL have not yet been explored as most of the prior studies have been in the context of solid tumors.
We also identified immune skewing genes linked to a poor outcome across all time points assessed, including CCR9, CD209, CMKLR and DDX58. Typically associated with mucosal-homing T cells, CCR9 is a chemokine receptor that binds the ligand CCL25. CCR9 is also linked to less activated and less differentiated dendritic cell (DC) populations30 that can induce Tregs31. Interestingly, CCR9 expression in human DLBCL is highly correlated with gut involvement32. Given that we demonstrated robust B cell depletion in all dogs, however, it is more likely that CCR9 is associated with poor T cell/DC responses to DLBCL in the context of the canine clinical trial. Another gene associated with poor immune skewing is CD209 (also called DC-SIGN). In human DLBCL, malignant B cells recruit monocytes and DCs to support tumor cell survival33. Interestingly, a similar situation has been described in human peripheral T cell lymphoma in which lymphoma associated macrophages are intimately linked to disease progression34. CMKLR is a G-protein coupled receptor with homology to many other chemokine receptors35. Its role in cancer is controversial, with some studies supporting an anti-tumor role and others a pro-tumorigenic role (reviewed in36). DDX58 encodes RIG-I and acts as an immune response factor and mediator of IFN antiviral responses. RIG-1 can promote protective anti-tumor immune responses in colon cancer37, and it can prevent breast cancer metastasis38, possibly through its ability to upregulate type 1 IFN as well as other cytokines39. It has been shown that lenalidomide, an agent now used to treat DLBCL in elderly/frail patients, acts in part by activating RIG-1 domains to inhibit tumor cell proliferation40. Consequently, it is not yet clear why upregulation of DDX58 would be linked to poor outcomes in the context of chemoimmunotherapy.
Interestingly, we found that interferon-stimulated gene (ISG) signatures were associated with poor prognosis in this study. While historically it was believed that IFN responses were integral to anti-tumor immunity, it has become increasingly clear that they are highly contextual and can actually be pro-tumorigenic in specific tumor settings41. For example, in glioblastoma, IFN signatures are associated with a worse prognosis42. Additionally, in breast cancer, IFN signatures are associated with metastatic disease in specific tumor phenotypes: ESR1+/ERBB2 − tumor metastasis is associated with IFN expression whereas ERBB2 + is not43. Here we show that the ISGs PSMB9, ISG15, ISG20, OAS2 and MX1 and the DDX58 sensor which promotes IFN expression are associated with shorter overall survival time in canine DLBCL, regardless of the immunotherapeutic regimen used.
In the current study we found that two genes, TBHD and NPNT, were elevated in the PBMCs from dogs with poor outcomes post-B cell depletion. TBHD encodes thrombomodulin (TM), an endothelial cell receptor that binds thrombin converting it from a procoagulant enzyme to an anti-coagulant. High levels of plasma TM have been linked to outcome across several different tumor types including those of embryonal, epithelial and lymphatic origin44,45. Its role in lymphoma has not been previously described, however, high levels of TM and von Willebrand factor were associated with a worse outcome in children with acute lymphoblastic leukemia46. NPNT, also known as nephronectin, is an extracellular matrix protein in the epidermal growth factor (EGF) family. NPNT plays a large role in cell mobility, structure, and signaling as it binds integrin α8β1. Previous studies showed that it is overexpressed in breast cancer tissue and is associated with metastasis and poor prognosis47–49. It is interesting to note that blood levels of NPNT are elevated in autoimmune experimental autoimmune encephalitis (EAE) mice and that it influences disease pathology by modulating the Th17/Treg balance50. Additionally, NPNT expression is upregulated in mouse models of acute and chronic hepatitis, supporting recruitment of CD4 + T cells into the liver51. Its potential role in DLBCL has not been previously described.
Lastly, we demonstrated that a subset of genes identified in the nCounter analyses, TBHD, NPNT and ISG20, could be used to predict response to outcome using qRT-PCR. These have potential use for the development of a point-of-care assay (i.e., digital droplet PCR) to help predict which dogs with DLBCL may require more intense follow-up or an altered treatment regimen, based on the change in gene expression between baseline (day 0) and day 7 of the treatment protocol.
In summary, data generated from this clinical trial show that biomarkers correlating with outcome in the context of novel chemo-immunotherapy treatments for DLBCL can be discerned from longitudinal analysis of PBMCs, providing a mechanism for rapid assessment of treatment efficacy and subsequent therapeutic intervention as needed.
Acknowledgements
We thank all the canine patients and their human companions for participating in this study.
Funding:
This work was supported by NIH U01CA224153-01. H.L.G. was supported by the National Institutes of Health under Award Number K01OD028268-01A1. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest:
JMR is an inventor on a use patent filed for “Diagnosis of skin conditions in veterinary and human patients” for CTCL; and on use patents for targeting CXCR3 (0#15/851,651) and IL15 (# 62489191) for the treatment of vitiligo. CAL received Grant/Research Support and Consulting Engagements from Fidocure. The other authors have no conflicts of interest to disclose.
Abbreviations
- DLBCL
Diffuse large B cell lymphoma
- DC
Dendritic cell
- CMKLR
Chemokine like receptor
- IFN
Interferon
- ISG
Interferon stimulated gene
- LTS
Long-term survivorship
- NPNT
Nephronectin
- TBHD
Thrombomodulin
Funding Statement
This work was supported by NIH U01CA224153–01. H.L.G. was supported by the National Institutes of Health under Award Number K01OD028268–01A1. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Kirthana Rao, UMass Chan Medical School.
Zechuan Rao, UMass Chan Medical School.
Angelina Huang, UMass Chan Medical School.
Scott Heston, UMass Chan Medical School.
Max Wang, UMass Chan Medical School.
Ümmügülsüm Yildiz-Altay, UMass Chan Medical School.
Fatima Qutab, UMass Chan Medical School.
Danny A. Kwong, UMass Chan Medical School.
Heather L. Gardner, Tufts University.
Jillian M. Richmond, Tufts University.
Cheryl A. London, Tufts University.
Data availability statement:
Study data are provided within the manuscript, and have been deposited on the Gene Expression Omnibus (GEO) Database under accession GSE302459 at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE302459.
References
- 1.Ito D., Frantz A. M. & Modiano J. F. Canine lymphoma as a comparative model for human non-Hodgkin lymphoma: recent progress and applications. Vet. Immunol. Immunopathol. 159, 192–201 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Susanibar-Adaniya S. & Barta S. K. 2021 Update on Diffuse large B cell lymphoma: A review of current data and potential applications on risk stratification and management. Am. J. Hematol. 96, 617–629 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavlasova G. & Mraz M. The regulation and function of CD20: an ‘enigma’ of B-cell biology and targeted therapy. Haematologica 105, 1494–1506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stegemann M., Denker S. & Schmitt C. A. DLBCL 1L-What to Expect beyond R-CHOP? Cancers 14, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di M., Huntington S. F. & Olszewski A. J. Challenges and opportunities in the management of diffuse large B-cell lymphoma in older patients. Oncologist 26, 120–132 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmittlutz K. & Marks R. Current treatment options for aggressive non-Hodgkin lymphoma in elderly and frail patients: practical considerations for the hematologist. Ther. Adv. Hematol. 12, 2040620721996484 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balough E., Ariza A., Asnani A. & Hoeger C. W. Cardiotoxicity of anthracyclines. Cardiol. Clin. 43, 111–127 (2025). [DOI] [PubMed] [Google Scholar]
- 8.Gini G. et al. Lenalidomide plus rituximab for the initial treatment of frail older patients with DLBCL: the FIL_ReRi phase 2 study. Blood 142, 1438–1447 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Arcari A., Cavallo F., Puccini B. & Vallisa D. New treatment options in elderly patients with Diffuse Large B-cell Lymphoma. Front. Oncol. 13, 1214026 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren Y. et al. Polatuzumab Vedotin, zanubrutinib and rituximab (Pola-ZR) achieved rapid and deep response in untreated frail and elderly DLBCL. Ann. Hematol. 104, 2823–2830 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Będkowska D., Al-Ameri S., Wieczorek A., Bubak J. & Miszczak M. What we know and do not yet know about the canine model of lymphoma in human medicine-the current state of knowledge. Cancers (Basel) 17, 596 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honigberg L. A. et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. U. S. A. 107, 13075–13080 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrington B. K. et al. Preclinical evaluation of the novel BTK inhibitor acalabrutinib in canine models of B-cell non-Hodgkin lymphoma. PLoS One 11, e0159607 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panjwani M. K. et al. Establishing a model system for evaluating CAR T cell therapy using dogs with spontaneous diffuse large B cell lymphoma. Oncoimmunology 9, 1676615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLinden G. P. et al. Safety and biologic activity of a canine anti-CD20 monoclonal antibody in dogs with diffuse large B-cell lymphoma. J. Vet. Intern. Med. 38, 1666–1674 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dittrich K. et al. Baseline tumor gene expression signatures correlate with chemoimmunotherapy treatment responsiveness in canine B cell lymphoma. PLoS One 18, e0290428 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Heiden A. D. et al. Characterization of the genomic landscape of canine diffuse large B-cell lymphoma reveals recurrent H3K27M mutations linked to progression-free survival. Sci. Rep. 15, 4724 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takao S. et al. Targeting the vulnerability to NAD+ depletion in B-cell acute lymphoblastic leukemia. Leukemia 32, 616–625 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Li N. et al. Dual PAK4-NAMPT inhibition impacts growth and survival, and increases sensitivity to DNA-damaging agents in Waldenström macroglobulinemia. Clin. Cancer Res. 25, 369–377 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patra-Kneuer M. et al. The sumoylation inhibitor TAK-981 in combination with the CD19-targeting antibody tafasitamab shows enhanced anti-tumor activity in preclinical B-cell lymphoma models. Blood 138, 2268–2268 (2021). [Google Scholar]
- 21.Zamani-Ahmadmahmudi M., Najafi A. & Nassiri S. M. Reconstruction of canine diffuse large B-cell lymphoma gene regulatory network: detection of functional modules and hub genes. J. Comp. Pathol. 152, 119–130 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Chen H. et al. Functional comparison of PBMCs isolated by Cell Preparation Tubes (CPT) vs. Lymphoprep Tubes. BMC Immunol. 21, 15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barral D. C. & Brenner M. B. CD1 antigen presentation: how it works. Nat. Rev. Immunol. 7, 929–941 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Consonni M., de Lalla C., Bigi A., Dellabona P. & Casorati G. Harnessing the CD1 restricted T cell response for leukemia adoptive immunotherapy. Cytokine Growth Factor Rev. 36, 117–123 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Bagchi S., Li S. & Wang C.-R. CD1b-autoreactive T cells recognize phospholipid antigens and contribute to antitumor immunity against a CD1b+ T cell lymphoma. Oncoimmunology 5, e1213932 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu Y. et al. CCL14 is a prognostic biomarker and correlates with immune infiltrates in hepatocellular carcinoma. Aging 12, 784–807 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun B.-E. et al. The chemokine CCL14 is a potential biomarker associated with immune cell infiltration in lung adenocarcinoma. Discover Oncology 15, 293 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu B. et al. Chemokine ligand 14 correlates with immune cell infiltration in the gastric cancer microenvironment in predicting unfavorable prognosis. Front. Pharmacol. 15, 1397656 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M.-M., Zhao Y.-D., Li Q. & He Y.-J. Chemokine CCL14 affected the clinical outcome and correlated with immune infiltrates in thyroid carcinoma. Histol. Histopathol. 38, 695–707 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Drakes M. L., Stiff P. J. & Blanchard T. G. Inverse relationship between dendritic cell CCR9 expression and maturation state. Immunology 127, 466–476 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pathak M. et al. CCR9 signaling in dendritic cells drives the differentiation of Foxp3+ Tregs and suppresses the allergic IgE response in the gut. Eur. J. Immunol. 50, 404–417 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Wu W., Doan N., Said J., Karunasiri D. & Pullarkat S. T. Strong expression of chemokine receptor CCR9 in diffuse large B-cell lymphoma and follicular lymphoma strongly correlates with gastrointestinal involvement. Hum. Pathol. 45, 1451–1458 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Mueller C. G. et al. Critical role of monocytes to support normal B cell and diffuse large B cell lymphoma survival and proliferation. J. Leukoc. Biol. 82, 567–575 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Gao X. et al. Targeting lymphoma-associated macrophage expansion via CSF1R/JAK inhibition is a therapeutic vulnerability in peripheral T-cell lymphomas. Cancer Res. Commun. 2, 1727–1737 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gantz I. et al. Molecular cloning of a novel receptor (CMKLR1) with homology to the chemotactic factor receptors. Cytogenet. Cell Genet. 74, 286–290 (1996). [DOI] [PubMed] [Google Scholar]
- 36.Umar M. I. et al. The Adipokine Component in the Molecular Regulation of Cancer Cell Survival, Proliferation and Metastasis. Pathol. Oncol. Res. 27, 1609828 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng Y. et al. Activation of DDX58/RIG-I suppresses the growth of tumor cells by inhibiting STAT3/CSE signaling in colon cancer. Int. J. Oncol. 61, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang Y.-C. et al. Glucose transporter 4 promotes head and neck squamous cell carcinoma metastasis through the TRIM24-DDX58 axis. J. Hematol. Oncol. 10, 11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu H., Feng Y. & He M.-L. Targeting type I interferon induction and signaling: How Zika virus escapes from host innate immunity. Int. J. Biol. Sci. 19, 3015–3028 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tong K. I. et al. Combined EZH2 inhibition and IKAROS degradation leads to enhanced antitumor activity in diffuse large B-cell lymphoma. Clin. Cancer Res. 27, 5401–5414 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Budhwani M., Mazzieri R. & Dolcetti R. Plasticity of Type I Interferon-Mediated Responses in Cancer Therapy: From Anti-tumor Immunity to Resistance. Front. Oncol. 8, 322 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duarte C. W. et al. Expression signature of IFN/STAT1 signaling genes predicts poor survival outcome in glioblastoma multiforme in a subtype-specific manner. PLoS One 7, e29653 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Callari M. et al. Subtype-dependent prognostic relevance of an interferon-induced pathway metagene in node-negative breast cancer. Mol. Oncol. 8, 1278–1289 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanly A. M. & Winter D. C. The role of thrombomodulin in malignancy. Semin. Thromb. Hemost. 33, 673–679 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Hanly A. M., Hayanga A., Winter D. C. & Bouchier-Hayes D. J. Thrombomodulin: tumour biology and prognostic implications. Eur. J. Surg. Oncol. 31, 217–220 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Hagag A. A. E., Abdel-Lateef A. E. & Aly R. Prognostic value of plasma levels of thrombomodulin and von Willebrand factor in Egyptian children with acute lymphoblastic leukemia. J. Oncol. Pharm. Pract. 20, 356–361 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Steigedal T. S. et al. Nephronectin is Correlated with Poor Prognosis in Breast Cancer and Promotes Metastasis via its Integrin-Binding Motifs. Neoplasia 20, 387–400 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magnussen S. N. et al. Nephronectin promotes breast cancer brain metastatic colonization via its integrin-binding domains. Sci. Rep. 10, 12237 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D. et al. NPNT promotes early-stage bone metastases in breast cancer by regulation of the osteogenic niche. J Bone Oncol 13, 91–96 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Honda M. et al. Nephronectin influences EAE development by regulating the Th17/Treg balance via reactive oxygen species. Am. J. Physiol. Cell Physiol. 322, C699–C711 (2022). [DOI] [PubMed] [Google Scholar]
- 51.Inagaki F. F. et al. Nephronectin is upregulated in acute and chronic hepatitis and aggravates liver injury by recruiting CD4 positive cells. Biochem. Biophys. Res. Commun. 430, 751–756 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Study data are provided within the manuscript, and have been deposited on the Gene Expression Omnibus (GEO) Database under accession GSE302459 at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE302459.