Abstract
Culture-dependent and -independent techniques were combined to characterize the physiological properties and the ecological impacts of culture-resistant phylotypes of thermophiles within the order Aquificales from a subsurface hot aquifer of a Japanese gold mine. Thermophilic bacteria phylogenetically associated with previously uncultured phylotypes of Aquificales were successfully isolated. 16S ribosomal DNA clone analysis of the entire microbial DNA assemblage and fluorescence in situ whole-cell hybridization analysis indicated that the isolates dominated the microbial population in the subsurface aquifer. The isolates were facultatively anaerobic, hydrogen- or sulfur/thiosulfate-oxidizing, thermophilic chemolithoautotrophs utilizing molecular oxygen, nitrate, ferric iron, arsenate, selenate, and selenite as electron acceptors. Their versatile energy-generating systems may reflect the geochemical conditions of their habitat in the geothermally active subsurface gold mine.
Culture-resistant phylotypes of thermophiles within the order Aquificales are prevalent in microbial mats or filamentous communities that often form in a certain temperature range (50 to 90°C) of habitats in global terrestrial hot spring environments such as Yellowstone National Park (12, 26), Iceland (29, 32), and Japan (40), in subterranean hot springs (23), and even in deep-sea hydrothermal vent systems (25, 27). In some microbial communities, relatively well-identified members closely related to the genera Hydrogenobacter and Thermocrinis are dominant, whereas the predominant components of other communities are uncultivated phylotypes genetically distinct from cultured members of the Aquificales (11, 12, 25-27, 29, 32, 40). Their resistance to cultivation has prevented characterization of their physiological properties and ecological impacts on microbial communities.
The Hishikari gold mine is located in Kagoshima Prefecture, Japan, approximately 20 km northwest of the active volcanoes of Mt. Kirishima. With increasing depth, the mine tunnels first penetrate the Pleistocene andesites (1.0 to 1.8 million years [Ma]) up to 200 m below the land surface (13). The andesites overlie the Shimanto-Supergroup shale and sandstone stratum (>650 Ma) dominated by geothermally heated fluid flow along subvertical fractures (13). Quartz veins form along the fractures (0.84 to 1.01 Ma), which contain extremely high concentrations of gold (50 g of Au per metric ton) (13). From the lower level of the hot aquifer zone, which is the deepest level of the gold mine at 320 m below the land surface, a number of slightly inclined drill holes equipped with valved pipes have been placed to explore undiscovered gold veins and control the aquifer level (dewatering station).
In the course of the culture-independent, molecular phylogenetic survey of the microbial communities in the hot aquifer water samples in this study, it was found that ribosomal DNA (rDNA) signatures closely related to previously uncultivated phylotypes of members of the Aquificales were predominantly recovered from the whole microbial DNA assemblages in the hot aquifer water. Based on the culture-independent molecular survey, we sought to cultivate and isolate these unidentified Aquificales and determine their physiological properties associated with ecological roles and geochemical processes in their habitats. The predominance of previously culture-resistant Aquificales phylotypes, their successful cultivation, and characterization of their novel metabolic and physiological properties are described here. In addition, their versatile energy-generating system is discussed in relation to the geochemical setting in the geothermally active subsurface gold mine.
MATERIALS AND METHODS
Sample collection, processing, and physical measurements.
For nucleic acid extraction experiments, hot aquifer water samples (30 liters each) were collected in sterile plastic bags with gas directly from the valved pipes placed in each of the inclined drill holes (AW-S and AW-D) at the deepest level of the mine. The hot aquifer water samples were transported to the laboratory at ambient temperature and within 24 h. Approximately 20 liters of water was filtered through cellulose-acetate filters (Advantec, Tokyo, Japan) with a pore size of 0.22 μm and a diameter of 47 mm. The microbial particles with the filters were stored at −80°C prior to DNA extraction.
For chemical analysis of the water samples, hot aquifer water samples (500 ml each) were collected in 1-liter sterile glass bottles (Schott Glaswerke) directly from the valved pipes of AW-S and AW-D, the gas phase was replaced with 100% nitrogen, and then the bottles were tightly sealed with butyl rubber stoppers. The water samples were stored at 4°C in the dark prior to chemical analysis. Gas (500 ml) was collected by using a gas sampling bag equipped with a triple-cocked glass funnel that was connected to the outlet pipe with a specified rate of aquifer water flow. The gas components were also sampled with syringes from the hot aquifer water flow and immediately stored in 100-ml butyl rubber-capped glass bottles (Schott Glaswerke) balanced with either 100% He or 100% Ar.
For whole-cell fluorescent in situ hybridization (FISH) analysis, the microbial particles from the hot aquifer flow were concentrated through the filtration system directly equipped with the valved pipe (AW-S), which has naturally high backing pressure, by using a cellulose-acetate filter with a pore size of 0.22 μm and a diameter of 47 mm. A total of 10 liters of water was filtered, and the filter with the microbial particles was immediately stored in 10 ml of mj water (36) that had been filtered through a 0.22-μm-pore filter and autoclaved with 3.7% formaldehyde.
Physical properties such as temperature, pH, conductivity, dissolved oxygen (DO) content, salinity, total dissolved solids, and oxidation-reduction potential were measured at the time of sampling with a digital thermometer and multiprobe U-21 (Horiba, Kyoto, Japan).
Chemical analyses.
The gas composition was measured by using gas chromatograph model Micro GC CP2002 (GL Sciences, Tokyo, Japan). Cation (Na, K, Mg, and Ca) concentrations were obtained from Sumitomo Metal Mining Co. Ltd. Anion samples were analyzed by using ion chromatography on a Shim-pack IC column (Shimadzu, Kyoto, Japan). The concentration of silica in the water was determined by the molybdenum-yellow method (41). The diazotization method was employed to determine the concentration of nitrate or nitrite (24) in addition to ion chromatography, and Nessler's reagent was employed to measure the ammonia concentration (1). The concentrations of soluble ferrous and ferric iron forms were measured by using ferrozine (20). Sulfide was analyzed quantitatively by the methylene blue method (9).
Determination of arsenate, arsenite, and phosphate concentrations was performed as previously described (15). The thiosulfate concentration was measured by iodine titration by using a spectrophotometer independently of ion chromatography. These analytic procedures were used not only for chemical analysis of the hot aquifer water samples but also to determine changes in inorganic substrates during bacterial growth.
Microscopic observation.
The hot aquifer water samples in plastic sampling bags were filtered through polycarbonate filters (Advantec) with a pore size of 0.22 μm and a diameter of 13 mm. The filters were fixed for 20 min in 3.7% formaldehyde, then rinsed twice in deionized, distilled water (DDW), and stained by treatment with DDW containing 4′,6′-diamidino-2-phenylindole (DAPI) (10 μg ml−1) at room temperature for 20 min. The filters were briefly rinsed in DDW and examined under epifluorescence by using an Olympus BX51 microscope with the Olympus Camedia C3030 digital camera system.
Transmission electron microscopy (TEM) of negatively stained cells was carried out as described by Zillig et al. (42). Approximately 1 liter of the hot aquifer water samples in plastic sampling bags was filtered through cellulose-acetate filters (Advantec) with a pore size of 0.22 μm and a diameter of 13 mm. The filters were resuspended with 500 μl of DDW containing 3.7% formaldehyde. The resuspended microbial particles or the cultured cells were negatively stained with 2% (wt/vol) uranyl acetate and observed under a JEOL JEM-1210 electron microscope at an accelerating voltage of 80 kV. The elemental composition of the particles accumulated during bacterial growth in the presence of selenate and selenite was analyzed by scanning electron microcopy (SEM) and energy-dispersive X-ray spectroscopy, as previously described (36).
Extraction of DNA and rDNA clone analysis.
DNA was extracted from preserved filters by using the Soil DNA kit Mega Prep (Mo Bio Laboratories, Inc., Solana Beach, Calif.) according to the manufacturer's recommended protocol. As a routine control to check for experimental contamination, a blank (no-filter) sample was extracted in the same manner. Microbial rDNA was amplified from DNA extracts from samples and controls by the PCR by using LA Taq polymerase with GC buffer (TaKaRa, Kyoto, Japan). The oligonucleotide primers used were Bac27F (18) and 1492R (18) for the bacterial rDNA and Arch21F (8) and 1492R (18). Reaction mixtures were prepared in which the concentration of each oligonucleotide primer was 0.1 μM and that of the DNA template was 1 ng μl−1. Thermal cycling was performed by using the GeneAmp 9600 (Perkin-Elmer, Foster City, Calif.) under the following conditions: denaturation at 96°C for 25 s; annealing at 50°C for 45 s; and extension at 72°C for 120 s, for a total of 30 cycles.
Amplified rDNA from five separate reactions was pooled and purified as previously described (37). Cloning and sequencing were followed by the previously described procedure (37). The Bac27F (18) or Arch21F (8) primer was used for partial sequencing analysis.
Phylogenetic analyses.
Single-strand sequences approximately 400 nucleotides in length were analyzed. The sequence similarity was analyzed by using the FASTA component program of DNASIS (Hitachi Software, Tokyo, Japan). rDNA sequences with ≥98% similarity by FASTA were assigned to the same clone type. A representative sequence of each clone type was subjected to sequence similarity analysis against the prokaryotic small-subunit (SSU) rRNA database and the nonredundant nucleotide sequence databases of GenBank, EMBL, and DDBJ by using the gapped-BLAST search algorithm (2, 6).
Approximately 0.9 kb of the sequence of each representative rDNA clone was determined from both strands. The sequences were manually aligned with the prokaryotic SSU rDNA data from the Ribosomal Data Project II (RDP-II) (21), and the rDNA sequences were determined from the new isolates based on primary and secondary structure considerations. Phylogenetic analyses were restricted to nucleotide positions that could be unambiguously aligned (33). Evolutionary distance matrix analysis (by using the Kimura two-parameter method, the least-squares distance method, and a transition-transversion rate of 2.0) and neighbor-joining analysis were performed by using the PHYLIP package (version 3.5; obtained from J. Felsenstein, University of Washington, Seattle). Bootstrap analysis was used to provide confidence estimates for phylogenetic tree topologies.
Quantification of the archaeal rDNA population.
Quantification of the archaeal rDNA population in whole microbial DNA assemblages was performed by using a quantitative fluorescent PCR method as previously described (35). An archaeal rDNA mixture containing equal amounts of three types of archaeal rDNA (Haloarcula japonica, Palaeococcus ferrophilus, and Sulfurisphaera sp.) was used as a standard (35).
Whole-cell FISH analysis.
An rRNA-targeted oligonucleotide probe was designed for the detection of new isolates that were potentially predominant in the subsurface hot aquifer waters. The AF175 probe (5′-GGGCTTTGGAGTCCCCTCTT-3′) corresponded to positions 182 to 208 in Escherichia coli 16S rDNA. The probe sequence was analyzed by using Check Probe analysis from the RDP-II (21), and the gapped-BLAST search algorithm (2, 6) was used to confirm the specificity of the probe for the rDNA sequences of new isolates. In addition, dot hybridization analysis was carried out with representative bacterial rDNA clones obtained from the hot aquifer water samples.
The purified rDNA samples (100 ng) amplified from the representative bacterial rDNA clones were blotted onto positively charged nylon membranes (Roche Diagnostics) and cross-linked to the membranes by exposure to 120 mJ of UV light energy by using a UV Stratalinker 1800 (Strategene, Torrey Pines, Calif.). The membrane was hybridized with the AF175 probe (5 ng μl−1), which was labeled at the 5′ end with digoxigenin and purified by high-performance liquid chromatography (HPLC) (Amersham Pharmacia Biotech, Amersham, United Kingdom). After hybridization, the membrane was washed, and the digoxigenin-labeled oligonucleotide probe hybridized with the targeted rDNA sequence was detected by using a digoxigenin luminescent detection kit for nucleic acids (Roche Diagnostics). All hybridization and washing procedures were carried out under the conditions described below.
For the whole-cell hybridization experiments, the fixed microbial particles suspended on the filter with 10 ml of mj water containing 3.7% formaldehyde were immobilized on 3-aminopropyltriepoxysilane-coated slides. Hybridization was performed at 59°C for 5 h in hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl [pH 7.4], 0.1% sodium dodecyl sulfate [SDS], Denhardt's solution) containing 15% formamide and the fluorescein isothiocyanate-labeled AF175 probe (5 μg ml−1). After hybridization, the slides were washed at 44°C for 20 min with hybridization buffer containing 50% formamide. The fluorescence signal from the probe was intensified by using an Alexa Fluor 488 signal amplification kit for fluorescein-conjugated probes (Molecular Probes) following the manufacturer's instructions. Finally, the slides were stained with DDW containing DAPI (10 μg ml−1) at 4°C for 20 min and examined under epifluorescence by using the Olympus BX51 microscope with the Olympus Camedia C3030 digital camera system.
Enrichment and isolation.
To cultivate previously uncultivated Aquificales strains, enrichment was undertaken at a wide range of temperatures (45 to 80°C) under a variety of chemotrophic conditions. The media used contained different electron donors, electron acceptors, and carbon sources and were designed for the growth of microorganisms with metabolic activities predicted by the physical and geochemical features of the subsurface hot aquifer. A total of 900 enrichment culture conditions were tested.
Inoculation was performed at the site of the dewatering station. A 1-ml syringe was used to sample the hot aquifer water (AW-S) immediately after vigorous emission from the valve of the borehole, and samples were inoculated into 3 ml of medium in test tubes. The inoculated tubes were incubated at the designated temperatures in the laboratory within 7 h after sampling.
Three of the media, mjANHOX-NO3, mjANHOX-As, and mjAESOX, were found to be conducive to the growth of the predominant phylotype of Aquificales. The media contained 25 mM NaHCO3, 5 mM Na2SiO3, 1 mM NH4Cl, and 1 ml of vitamin mixture (5) per liter of mj water (36). In addition, mjANHOX-NO3 and mjANHOX-As media contained 10 mM NaNO3 and 2 mM Na2HAsO4, respectively, and were supplied with a gas mixture of H2-CO2 (80:20; 300 kPa). For the mjAESOX medium, 0.25 mM Na2S and 10 mM Na2S2O3 were added, and a gas mixture of N2-CO2-O2 (75:20:5; 100 kPa) was supplied. To prepare the media, 5 mM Na2SiO3 and 1 mM NH4Cl were dissolved in mj water, and the pH was adjusted to around 7.5 with HCl before autoclaving. After autoclaving, concentrated solutions of vitamins (5), NaHCO3, and other inorganic or organic substrates were added to the prepared media. These solutions were separately sterilized by autoclaving except for the vitamins, which were filter sterilized. The pH of the media was adjusted to 7.5 with H2SO4 or NaOH in an anaerobic chamber under a 95% N2-5% H2 atmosphere. The medium was dispensed at 20% of the total bottle or tube volume and tightly sealed with a butyl rubber stopper under the designated gas phase and pressure.
Positive enrichment cultures were obtained from tubes incubated at 50, 55, 60, 65, and 70°C. The dilution-to-extinction technique was used to obtain pure cultures from the enrichment cultures at 65°C (38). The purified strains from mjANHOX-NO3, mjANHOX-As, and mjAESOX were designated HGM-K1, HGM-K2, and HGM-K3, respectively.
Physiological and molecular characterization of isolates.
All experiments described below were conducted in duplicate, mainly with strain HGM-K1. In an attempt to examine whether the potential electron donors and acceptors supported growth, various inorganic materials [H2, O2 (20, 5, or 1%, vol/vol), sodium thiosulfate (10 mM), elemental sulfur (3%, wt/vol), sodium sulfide (5 mM), cysteine-HCl (5 mM), sodium sulfite (5 mM), sodium sulfate (10 mM), sodium nitrate (10 mM), ammonium chloride (10 mM), sodium nitrite (5 mM), ferric citrate (5 mM), ferrihydrite (10 mM), vernadite (10 mM), sodium arsenate (2 mM), sodium arsenite (2 mM), sodium selenite (2 mM), and sodium selenate (2 mM)] and organic compounds such as yeast extract (0.02%, wt/vol), peptone (0.02%, wt/vol), tryptone (0.02%, wt/vol), Casamino Acids (0.02%, wt/vol), starch (0.02%, wt/vol), and sodium fumarate (10 mM) were tested instead of the combination of H2-NO3−, H2-HAsO42−, and S2O32−-O2 in mjANHOX-NO3, mjANHOX-As, and mjAESOX, respectively. Ferric citrate, ferrihydrite [Fe(OH)3], and vernadite (MnO2) were prepared as described by Kostka and Nealson (16).
Heterotrophic growth was tested by using organic compounds [yeast extract (0.02%, wt/vol), peptone (0.02%, wt/vol), tryptone (0.02%, wt/vol), Casamino Acids (0.02%, wt/vol), and starch (0.02%, wt/vol)] as potential carbon sources in the absence of CO2 and HCO3−. Bacterial growth was evaluated by direct counting of DAPI-stained cells. When positive growth was observed on a given set of electron donor and acceptor, shifts in the cell count and the concentration of substrates and end products during growth were determined by ion chromatography, colorimetric assays, and gas chromatography as described above. These experiments were performed under the optimum growth conditions of 63°C and pH 7.5.
To determine the effects of pH on growth, the pH of mjANHOX-NO3 medium was adjusted to various levels with 10 mM acetate-acetic acid buffer (pH 4 to 5), MES (morpholineethanesulfonic acid, pH 5 to 6), piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES, pH 6 to 7), HEPES (pH 7 to 7.5), and Tris (pH 8 to 9.5). After autoclaving, the pH of the medium was confirmed and readjusted with HCl or NaOH to the desired pH at room temperature. The pH was found to be stable during the cultivation period. Media were prepared with various dilutions of MJ (−N) synthetic seawater (24) [1× MJ (−N) synthetic seawater containing 30 g of NaCl liter−1] to determine the effect of sea salt concentration on growth.
DNA was prepared from strain HGM-K1, -K2, and -K3 cells grown in mjANHOX-NO3 medium as described by Marmur and Doty (22). The 16S rRNA gene was amplified by PCR with the Bac27F and 1492R primers. The 1.5-kb PCR product was directly sequenced by the dideoxynucleotide chain termination method. DNA-DNA hybridization analysis was performed in the newly isolated strains by using their genomic DNAs (36).
Nucleotide sequence accession number.
The 16S rDNA sequences of strains HGM-K1 and -K2 and those of pHAuB-D and -J were deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases under accession numbers AB071324, AB071325, AB071326, and AB071327, respectively.
RESULTS AND DISCUSSION
Physical and chemical properties of samples.
The physical and chemical properties of the subsurface hot aquifer water samples (AW-S and AW-D) are summarized in Table 1. Hot aquifer water samples from the two inclined drill holes had similar physical and geochemical features. This may be because the two boreholes have similar aquifer sources. Relatively high concentrations of methane in the gas components and ammonium ions in the water samples were noted. Although the oxidation-reduction potential represented the reductive state of the hot aquifer water, some DO was detected.
TABLE 1.
Physical and chemical properties of hot subsurface aquifer liquids and gases from the Hishikari gold mine
| Property | AW-S | AW-D |
|---|---|---|
| Chemical | ||
| Na+ (mg liter−1) | 510 | 530 |
| K+ (mg liter−1) | 23 | 27 |
| Mg2+ (mg liter−1) | 16 | 18 |
| Ca2+ (mg liter−1) | 85 | 75 |
| Cl− (mg liter−1) | 270 | 350 |
| HCO3− (mM) | 15.3 | 16.9 |
| SiO2 (mM) | 1.6 | 1.8 |
| SO42− (μM) | 514 | 508 |
| S2−/HS− (μM) | 94 | 110 |
| NH4+ (μM) | 93.9 | 98.6 |
| NO3− (μM) | 27.3 | 25.5 |
| NO2− (μM) | <2.0 | <2.0 |
| Fe(III) (μM) | 79 | 69 |
| Fe(II) (μM) | 72.3 | 73 |
| As(V) (μM) | 10.7 | 9.2 |
| As(III) (μM) | <1.0 | <1.0 |
| Se(VI) (μM) | 12.5 | 11.7 |
| Se(IV) (μM) | <3.0 | <3.0 |
| PO43− (μM) | 5.4 | 5.1 |
| S2O32− (μM) | 12.8 | 14.4 |
| Gas phase | ||
| H2 (ppm) | 1,520 | 1,480 |
| CH4 (ppm) | 27,600 | 32,400 |
| CO2 (ppm) | 736,000 | 744,000 |
| H2S (ppm) | <10 | <10 |
| Physical property | ||
| Temperature (°C) | 70.4 | 72.6 |
| pH | 6.25 | 6.12 |
| Conductivity (Sm−1) | 0.16 | 0.2 |
| Dissolved oxygen (mg liter−1) | 0.15 | 0.14 |
| Salinity (%) | 0.1 | 0.1 |
| Total dissolved solids (g liter−1) | 1.1 | 1.3 |
| Oxidation-reduction potential (mV) | −104 | −115 |
Izawa et al. (13) pointed out that the formation of extremely high-grade gold-bearing quartz veins was strongly affected by the unconformity between the Cretaceous sedimentary and the Pleistocene volcanic strata and between the sulfide-rich, reductive, superheated steam and the oxygenated, cooler groundwater in the Hishikari gold mine. The relatively high concentrations of methane and ammonium ion may be derived from the thermodegradation of organic materials deposited in the sedimentary rocks. In addition, the detectable DO in the water probably suggests mixing with oxygenated groundwater. These environmental settings might have a major impact on the formation and function of the microbial communities in the hot subsurface aquifer waters.
Culture-independent molecular analyses.
The microbial community structure in the hot subsurface aquifer beneath the Hishikari gold mine was determined by rDNA clone analysis based on the entire microbial DNA assemblage extracted directly from the hot aquifer water. The microbial community density determined by direct counting of DAPI-stained cells was 1.2 × 104 cells liter−1 for AW-S and 1.0 × 104 cells liter−1 for AW-D. Since the aquifer water samples were filtered directly through 0.22-μm pores, solid particles larger than 0.22 μm in the aquifer waters were also included. The microbial populations in the hot aquifer waters may represent both the free-living microbial population and the population attached to the particles.
Based on quantitative fluorogenic PCR (35), the bacterial rDNA population dominated the archaeal rDNA population (0.08% and 0.05% in AW-S and AW-D, respectively) in the whole microbial rDNA populations obtained from hot aquifer water samples. The bacterial rDNA community structures retrieved from the bacterial rDNA clone libraries of the two aquifer water samples were similar, and two bacterial rDNA phylotypes predominated in the entire microbial DNA assemblages (phylotypes pHAuB-D and pHAuB-J) (Fig. 1). The pHAuB-D phylotype was the most abundant (20 of 41 clones in AW-S and 18 of 37 clones in AW-D) and was phylogenetically associated with a culture-resistant group of microorganisms within the Aquificales, such as SRI-40 (97.5%) (29) and NAK-14 (97.5%) (40), obtained from Icelandic and Japanese hot spring microbial mats, respectively (Fig. 1). The other predominant phylotype, pHAuB-J (15 of 41 clones in AW-S and 14 of 37 clones in AW-D), was closely related to the uncultivated beta subclass of Proteobacteria OPB37 (96.1%) (12), recovered from Yellowstone National Park hot spring sediments (Fig. 1).
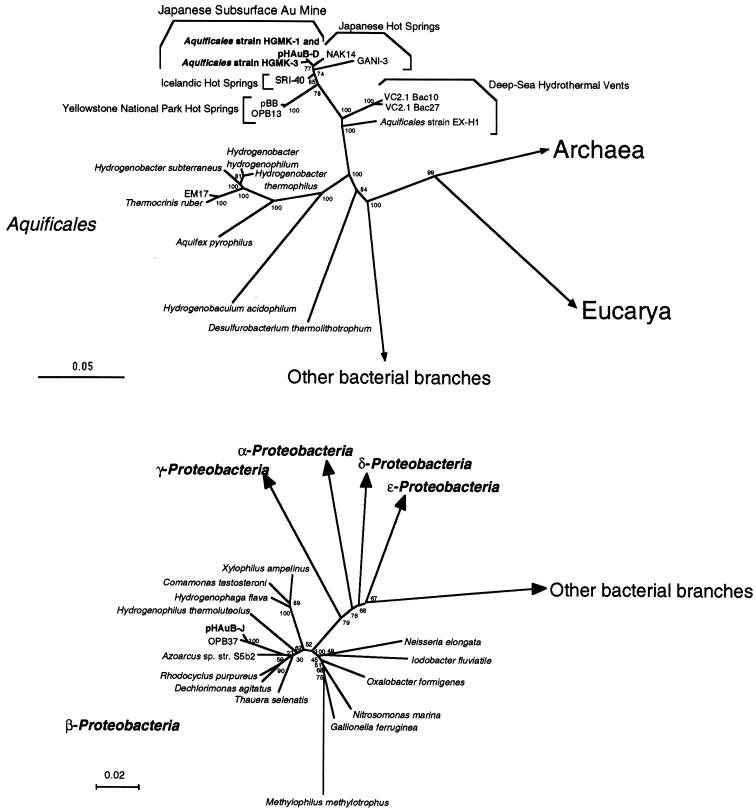
FIG. 1.
Phylogenetic trees of representative Aquificales strains and phylotypes (upper panel) and of beta subdivision Proteobacteria strains and phylotypes (lower panel). Each of the trees was inferred by neighbor-joining analysis of approximately 600 homologous positions of the rDNA sequence. Environmental clones VC2.1 Bac10 and VC2.1 Bac27 were obtained from deep-sea hydrothermal vent environments (25, 27), and EM17, pBB, OPB13, and OPB37 were from Yellowstone National Park hot spring communities (12, 26, 28). The clones SRI-40, NAK14, and GANI-3 were recovered from Icelandic and Japanese hot spring microbial mats (29, 32, 40). Bootstrap analysis was performed with 100 resampled data sets. Bar, 0.05 (upper panel) or 0.02 (lower panel) change per nucleotide. The accession numbers of the 16S rDNA sequences in this figure are: Desulfurobacterium thermolithotrophicum, AJ001049; Hydrogenobaculum acidophilus, D16296; Aquifex pyrophilus, M83548; Thermocrinis ruber, AJ005640; EM17, U05661; Hydrogenobacter subterraneus, AB026268; Hydrogenobacter thermophilus, Z30214; Hydrogenobacter hydrogenophilum, Z30242; Aquificales sp. strain EX-H1, AF188332; VC2.1 Bac10, AF068791; VC2.1 Bac27, AF068801; pBB, AF113542; OPB13, AF027098; SRI-40, AF255598; NAK14, AB005738; GANI-3, AB005735; Aquificales sp. strain HGM-K1, AB071324; strain HGM-K3, AB071325; pHAuB-D, AB071326; pHAuB-J, AB071327; OPB37, AF026985; Xylophilus ampelinus, AF078758; Comamonas testosteroni, M11224; Hydrogenophaga flava, AF078771; Hydrogenophilus thermoluteolus, AB009828; Azoarcus sp. strain S5b2, L15532; Rhodocyclus purpureus, M34132; Dechlorimonas agitatus, AF047462; Thauera selenatis, X68491; Methylophilus methylotrophus, M29021; Gallionella ferruginea, L07897; Nitrosomonas marina, Z46990; Oxalobacter fornigenes, U49757; Iodobacter fluviatile, M22511; and Neisseria elongata, L06171.
These microbial rDNA community structures were different from those observed in other subsurface hot-water environments, such as the deeper and hotter subsurface geothermal water in the Hacchoubaru geothermal electric plant (34) and subterranean hot springs in Iceland (23). The results suggest that these bacterial phylotypes were predominant, active, indigenous microbial populations in the subsurface aquifer environments of the Hishikari gold mine.
Enrichment and isolation of previously uncultivated Aquificales.
To determine the physiological properties and ecological impacts of the most abundant phylotype of Aquificales in the hot subsurface aquifer water, enrichment culture was undertaken at a wide range of temperatures (45 to 80°C) under a variety of chemotrophic conditions. A total of 900 enrichment culture conditions were tested, and three of the media were conducive to the growth of the predominant phylotype of Aquificales. After successful enrichment, the dilution-to-extinction technique was used to obtain pure cultures designated HGM-K1, -K2, and -K3. DNA from these new isolates was extracted, and amplification and direct sequence determination of SSU rRNA genes (16S rDNAs) were performed by PCR. The purity of the isolates was confirmed by microscopic observation, and repeated sequencing of 16S rDNA and whole-cell FISH were performed with a specific oligonucleotide probe, as described below.
Among the three isolates, strains HGM-K1 and HGM-K2 had identical rDNA sequences, and the sequence of strain HGM-K3 was 99.6% similar to that of HGM-K1. In addition, the rDNA sequences of HGM-K1 and -K3 were nearly identical (100% and 99.6%, respectively) to that of the environmental rDNA clones represented by pHAuB-D. Strains HGM-K1, -K2, and -K3 were all found to belong to genetically identical species based on the DNA-DNA hybridization test (>80%) (36). Phylogenetic analysis of 16S rDNA sequences revealed that the new isolates were most closely associated with the environmental rDNA clones obtained from Japanese hot spring microbial mats (40) (Fig. 1). In addition, the phylogenetic tree reflected a relationship consistent with the biogeographical distribution of the poorly defined lineage of Aquificales obtained from global geothermal environments (Fig. 1).
FISH analysis.
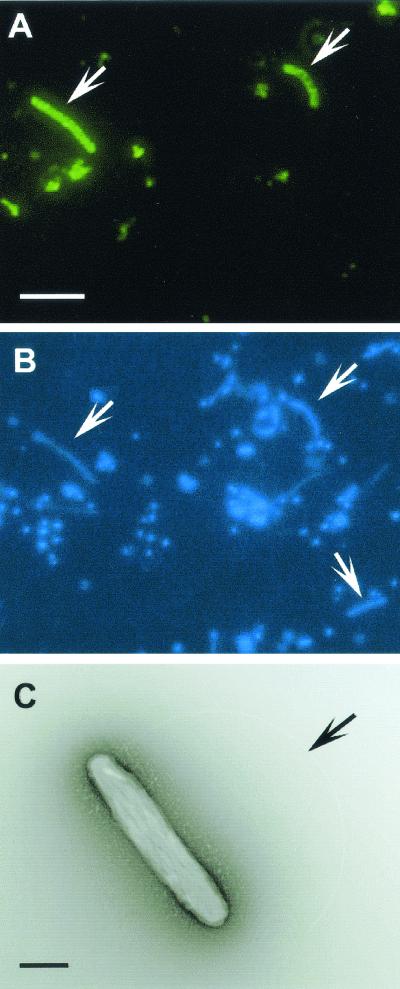
By using the newly obtained 16S rDNA sequences of the isolates and the rDNA clone, an oligonucleotide probe specifically binding rDNA sequences of the isolates and the clone was designed. Based on in silico analyses, the probe had two or more bases different from those in any other microbial rDNA sequences, including Aquificales strains and clones. In addition, dot hybridization analysis revealed that the probe was specific for the targeted sequence (pHAuB-D) among the representative bacterial rDNA clones obtained from the hot aquifer water samples under the same conditions as for the whole-cell FISH experiment. Approximately one-third (140 of 421 cells) of the microbial cells stained with DAPI revealed a fluorescent signal derived from the specific probe targeting strains HGM-K1, -K2, and -K3 and the pHAuB-D phylotype (Fig. 2A and 2B). The result clearly indicated that the bacterial phylotype within Aquificales predominantly occurring in the hot subsurface aquifer was successfully cultivated and isolated in the laboratory.
FIG. 2.
Whole-cell FISH analysis of novel lineages of Aquificales in subsurface hot aquifer water (A and B) and a transmission electron micrograph of negatively stained cells of new isolate strain HGM-K1 (C). Microorganisms in the hot aquifer water were concentrated by filtration and fixed with 3.7% paraformaldehyde at the sampling time. Filtered microorganisms were hybridized with a fluorescein-labeled AF175 probe as described in Materials and Methods. Arrows indicate cells with Alexa Fluor 488 (A) and DAPI (B) signals and the polar flagellum of strain HGM-K1 (C). Scale bars: 5 μm (A and B); 0.4 μm (C).
Physiological and metabolic properties of isolates.
The cells of the new isolates were motile, gram-negative rods approximately 1.5 to 2.5 μm long and 0.3 to 0.5 μm wide, with a polar flagellum (Fig. 2C). The cellular surface was covered with a wavy structure often observed in Aquificales strains (Fig. 2C) (28, 36). Approximately one-third of the microbial cells negatively stained with uranyl acetate were recognized to have a similar wavy structure under TEM observation of a sample directly filtered from the hot aquifer, although all cells had a similar straight to slightly curved, rod-shaped morphology. Direct observation of the water samples under TEM also supported the predominant occurrence of the new isolates in the subsurface aquifer.
Strain HGM-K1 grew optimally at a temperature range of 60 to 65°C, in the neutral pH range, and at a salt concentration of 6 g liter−1 when cultured in the mjANHOX-NO3 medium. These conditions appeared to be consistent with the in situ conditions of the hot aquifer waters.
The ability of isolate HGM-K1 to utilize various electron donors, electron acceptors, and carbon sources was investigated by using a range of substrates (Table 2). Strain HGM-K1 grew solely on molecular hydrogen, thiosulfate, or elemental sulfur as a possible electron donor and on carbon dioxide as a carbon source in a chemolithoautotrophic manner (Table 2). Other reduced sulfur compounds such as sulfide and cysteine-HCl did not serve as electron donors. No complex organic substrate (yeast extract, peptone, tryptone, Casamino Acids, or starch) supported growth. When either hydrogen or thiosulfate was provided as an electron donor, the isolate was able to utilize molecular oxygen, nitrate, soluble (ferric citrate) and insoluble (ferrihydrite) iron(III), arsenate, selenate, and selenite. Nitrite, manganese(IV), arsenite, sulfite, sulfate, and fumarate were unable to support growth. When strain HGM-K1 was grown on hydrogen as an electron donor, fully aerobic conditions (H2-CO2-O2, 65:15:20; 100 kPa) completely inhibited growth, while no inhibitory effect was observed during growth with thiosulfate or elemental sulfur as an electron donor under fully aerobic conditions (N2-CO2-O2, 65:15:20; 100 kPa). A similar electron donor-acceptor profile was obtained when strain HGM-K3 was examined.
TABLE 2.
Effect of potential electron donors, electron acceptors, and carbon sources on growth of strain HGM-K1a
| Electron donorb | Electron acceptor (concn) | Carbon source | Gas phase | Maximum cell yieldc |
|---|---|---|---|---|
| H2 | NO3− (10 mM) | CO2 + HCO3− | H2/CO2, 80/20; 300 kPa | ++ |
| O2 (20%) | CO2 + HCO3− | H2/CO2/O2, 65/15/20; 100 kPa | NG | |
| O2 (5%) | CO2 + HCO3− | H2/CO2/O2, 75/20/5; 100 kPa | ++ | |
| O2 (1%) | CO2 + HCO3− | H2/CO2/O2, 79/20/1; 100 kPa | ++ | |
| Ferric citrate (5 mM) | CO2 + HCO3− | H2/CO2, 80/20; 300 kPa | ++ | |
| Ferrihydrite (10 mM) | CO2 + HCO3− | H2/CO2, 80/20; 300 kPa | ++ | |
| HAsO42− (2 mM) | CO2 + HCO3− | H2/CO2, 80/20; 300 kPa | ++ | |
| SeO42− (2 mM) | CO2 + HCO3− | H2/CO2, 80/20; 300 kPa | ++ | |
| SeO32− (2 mM) | CO2 + HCO3− | H2/CO2, 80/20; 300 kPa | ++ | |
| None | CO2 + HCO3− | H2/CO2, 80/20; 300 kPa | NG | |
| None | Any shown above | CO2 + HCO3− | N2/CO2, 80/20; 300 kPa | NG |
| Organic substrates | Any shown above | Organic substrates | N2; 300 kPa | NG |
| H2 | Any shown above | Organic substrates | H2; 300 kPa | NG |
| Any shown above | CO2 + HCO3− + organic substrates | H2/CO2, 80/20; 300 kPa | ++ | |
| S0 or S2O32− | O2 (20%) | CO2 + HCO3− | N2/CO2/O2, 65/15/20; 100 kPa | ++ |
| O2 (5%) | CO2 + HCO3− | N2/CO2/O2, 75/20/5; 100 kPa | ++ | |
| O2 (1%) | CO2 + HCO3− | N2/CO2/O2, 79/20/1; 100 kPa | ++ | |
| NO3− (10 mM) | CO2 + HCO3− | Ar/CO2, 80/20; 300 kPa | ++ | |
| Ferric citrate (5 mM) | CO2 + HCO3− | N2/CO2, 80/20; 300 kPa | + | |
| Ferrihydrite (10 mM) | CO2 + HCO3− | N2/CO2, 80/20; 300 kPa | + | |
| HAsO42− (2 mM) | CO2 + HCO3− | N2/CO2, 80/20; 300 kPa | + | |
| SeO42− (2 mM) | CO2 + HCO3− | N2/CO2, 80/20; 300 kPa | + | |
| SeO32− (2 mM) | CO2 + HCO3− | N2/CO2, 80/20; 300 kPa | + | |
| Any shown above | Organic substrates | N2; 300 kPa | NG | |
| None | CO2 + HCO3− | N2/CO2, 80/20; 300 kPa | NG |
These experiments were conducted with media containing various combinations of electron donors, electron acceptors, carbon sources, and gas phases instead of those in the mjANHOX-NO3 medium.
Yeast extract (0.02%), tryptone (0.02%), peptone (0.02%), Casamino Acids (0.02%), and starch (0.02%) were tested as organic substrates. The concentrations of elemental sulfur and thiosulfate added were 3% and 10 mM, respectively.
Maximum cell yield is indicated as follows: ++, >2.0 × 108 cells ml−1; +, <2.0 × 108 cells ml−1 but >7.0 × 107 cells ml−1; NG, no growth.
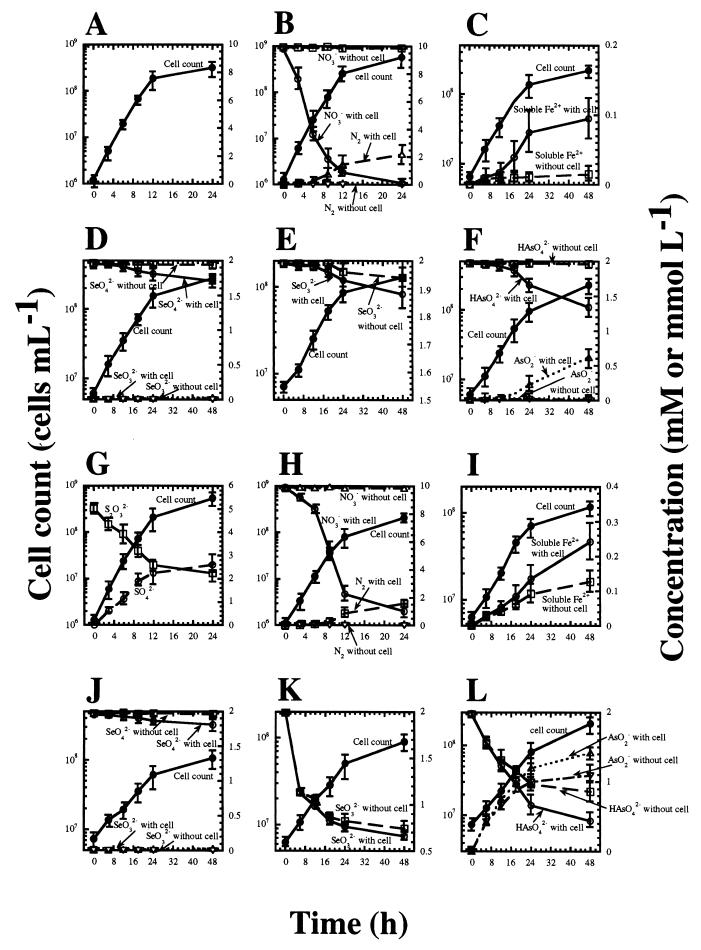
To confirm that strain HGM-K1 metabolized inorganic substrates during growth, shifts in the cell count and the concentration of substrates and end products during growth were determined (Fig. 3). When an inorganic material was provided as an electron acceptor in the presence of thiosulfate, the concentration of thiosulfate decreased and the concentration of sulfate increased during growth (Fig. 3G). In media without inocula, no oxidation of thiosulfate to sulfate was observed. No potential intermediate products such as elemental sulfur and sulfite by oxidation of thiosulfate were detected. These results indicate that thiosulfate served as an electron donor and was metabolized to sulfate by the isolate. Although the oxidation of H2 was not quantified, the concentration of H2 in the gas phase decreased during growth (data not shown).
FIG. 3.
Time course of oxidation of H2 (A to F) and thiosulfate (G to L) and reduction of various electron acceptors such as O2 (5%, vol/vol) (A, G), nitrate (10 mM) (B, H), ferrihydrite (10 mM) (C, I), selenate (2 mM) (D, J), selenite (2 mM) (E, K), or arsenate (2 mM) (F, L) and concomitant bacterial growth of strain HGM-K1. The bars indicate the 95% confidence intervals (n = 4). Either H2 (H2-CO2, 80:20; 300 kPa) or thiosulfate (10 mM) was provided as an electron donor. The oxidation of thiosulfate to sulfate is indicated only in panel G, while it was observed during bacterial growth with any electron acceptor. Although the oxidation of H2 was not quantified, the concentration of H2 in the gas phase was decreased during growth (data not shown).
When either H2 or thiosulfate was provided as the sole energy source, O2, nitrate, ferric ion, arsenate, selenate, and selenite concentrations were reduced during growth. The concentration of N2 in the gas phase increased with the decrease in nitrate concentration during growth, and no apparent reduction of nitrate occurred in the absence of inocula (Fig. 3B and 3H). No accumulation of potential end products such as nitrite, N2O, and ammonium ions was detected. When insoluble iron(III) (ferrihydrite) was provided as an electron acceptor, accumulation of the soluble iron(II) form was detected in media containing either H2 or thiosulfate with inocula (Fig. 3C and 3I). Although iron reduction was observed in both the presence and absence of bacterial growth with thiosulfate as the electron donor, more of the soluble iron(II) form was detected in the medium accompanying bacterial growth (Fig. 3I). This implies that bacterial iron reduction occurs during growth.
The concentration of selenate also decreased during growth with either H2 or thiosulfate as the energy source (Fig. 3D and 3J). However, no apparent accumulation of selenite was observed (Fig. 3D and 3J). Microscopic observation of the cultures with selenate or selenite indicated that a considerable proportion of bacterial cells had reddish particles covering or adhering to them, and these reddish particles also accumulated in the medium containing thiosulfate and selenite in both the presence and absence of inocula. SEM-energy-dispersive X-ray spectroscopy analysis indicated that the particles consisted entirely of selenium atoms and were elemental selenium (Se0). These elemental selenium particles may be produced by the chemical or microbial reduction of selenate and selenite.
The concentration of arsenite increased with the decrease in arsenate concentration during growth (Fig. 3F and 3L). Since the new isolates were unable to utilize arsenite instead of arsenate (Table 2), arsenite was probably an end product of arsenate reduction. On the basis of these results, the isolates were facultatively anaerobic, hydrogen- or sulfur/thiosulfate-oxidizing chemolithoautotrophs utilizing molecular oxygen, nitrate, ferric iron(III), arsenate, selenate, and selenite as electron acceptors.
The energy metabolism of the new isolates capable of utilizing various combinations of electron donors and acceptors, coupled with their autotrophy, was unique compared with that of other Aquificales members and compared with any other form of microbial energy metabolism. Most members of the order Aquificales are autotrophs and able to obtain energy by oxidation of molecular hydrogen and reduced sulfur compounds (29, 32). To our knowledge, except for the possible Aquificales member Desulfurobacterium thermolithotrophum (19), molecular oxygen is recognized as the primary electron acceptor, although the nitrate-reducing growth of Hydrogenobacter thermophilus TK-6 has recently been reported (31).
D. thermolithotrophum is a strictly anaerobic chemolithoautotroph that oxidizes hydrogen and reduces thiosulfate, elemental sulfur, and sulfite. A similar anoxic hydrogen-oxidizing autotroph with elemental sulfur as an electron acceptor was recently found in isolates belonging to the epsilon subdivision of the Proteobacteria from deep-sea hydrothermal vents (7). Only members of homoacetogenic or sulfate-reducing bacteria are known to be autotrophic, anoxic, hydrogen-oxidizing bacteria with carbon dioxide or sulfate, while a diversity of anoxic, hydrogen-oxidizing chemolithoautotrophs are found among the hyperthermophilic Archaea within the orders Thermoproteales (utilizing nitrate, elemental sulfur, thiosulfate, ferric iron, arsenate, selenate, and selenite as electron acceptors), Desulfurococcales (nitrate, elemental sulfur, and thiosulfate), Sulfolobales (elemental sulfur), and Archaeoglobales (nitrate) and within the methanogenic Archaea (carbon dioxide) (10). Many fewer autotrophic, anoxic sulfur-thiosulfate oxidizers are known.
Several bacterial species of the genera Thiobacillus, Thiomicrospira, Paracoccus, and Thioalkalivibrio are able to grow chemolithoautotrophically with oxidation of elemental sulfur and thiosulfate coupled with denitrification (17, 30). In addition, anoxic sulfur/thiosulfate-disproportionating, chemolithoautotrophic bacteria mainly belonging to the delta subdivision of the Proteobacteria have been discovered in freshwater lake and marine sediments (3, 4, 14, 39). Some of those strains coupled the disproportionation with manganese and iron reduction (39). Considering the efficient sulfur-thiosulfate oxidation with O2 and nitrate and the absolute requirement for both an electron donor and acceptor, the new isolates may unidirectionally oxidize sulfur or thiosulfate. The new isolates are the first microorganisms known to obtain energy through anoxic sulfur-thiosulfate oxidation by ferric iron, arsenate, selenate, and selenite and to possess versatile energy-generating systems.
The versatile energy metabolism may provide a key insight into the reason for the prevalent occurrence of the isolates in hot subsurface aquifer water and their impact on microbial ecosystems and geochemical processes. As shown in Table 1, the hot subsurface aquifer water underlying the Hishikari gold mine contains abundant amounts of chemicals potentially serving as electron donors or acceptors and carbon sources for novel lineages of microorganisms. The environmental conditions may therefore provide a suitable subsurface hot-water habitat for Aquificales. Further characterization of the isolates and their metabolisms is now under way.
Acknowledgments
We are grateful to the management of the Sumitomo Metal Mining Co. Ltd. for its cooperation in and understanding of our research. Special thanks go to Katsuyuki Uematsu for his assistance in TEM analysis and Cynthia Yenches for checking the English in the manuscript.
REFERENCES
- 1.Allen, S. E., H. M. Grimshaw, J. A. Parkinson, and C. Quarmby. 1974. Inorganic constituents: nitrogen, p. 184-206. In S. E. Allen (ed.), Chemical analysis of ecological materials, Blackwell Scientific Publications, London, England.
- 2.Altschul, S. F., T. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bak, F., and H. Cypionka. 1987. A novel type of energy metabolism involving fermentation of inorganic sulphur compounds. Nature 326:891-892. [DOI] [PubMed] [Google Scholar]
- 4.Bak, F., and N. Pfennig. 1987. Chemolithotrophic growth of Desulfovibrio sulfodismutans sp. nov. by disproportionation of inorganic sulfur compounds. Arch. Microbiol. 147:184-189. [Google Scholar]
- 5.Balch, W. E., G. E. Fox, L. J. Magrum, C. R. Woese, and R. S. Wolfe. 1979. Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 43:260-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson, D. A., M. S. Boguski, D. J. Lipman, J. Ostell, and B. F. F. Ouellette. 1998. Genbank. Nucleic Acids Res. 26:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell, B. J., C. Jeanthon, J. E. Kostka, G. W. Luther III, and S. C. Cary. 2001. Growth and phylogenetic properties of novel bacteria belonging to the epsilon subdivision of the Proteobacteria enriched from Alvinella pompejana and deep-sea hydrothermal vents. Appl. Environ. Microbiol. 67:4566-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delong, E. F. 1992. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 89:5685-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonselius, S. H. 1983. Determination of hydrogen sulfide, p. 73-80. In K. Grasshoff, M. Ehrhardt, and K. Kremling (ed.), Sea water analysis. Verlag Chemie, Weinheim, Germany.
- 10.Garrity, G. M., and J. G. Holt. 2001. Domain Archaea, p. 167-355. In D. R. Boone and R. W. Castenholz (ed.), Bergey's manual of systematic bacteriology. Springer-Verlag, New York, N.Y.
- 11.Huber, R., W. Eder, S. Heldwein, G. Wanner, H. Huber, R. Rachel, and K. O. Stetter. 1998. Thermocrinis ruber gen. nov., sp. nov., a pink-filament-forming hyperthermophilic bacterium isolated from Yellowstone National Park. Appl. Environ. Microbiol. 64:3576-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hugenholtz, P., C. Pitulle, K. Hershberger, and N. R. Pace. 1998. Novel division-level bacterial diversity in a Yellowstone hot spring. J. Bacteriol. 180:366-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izawa, E., Y. Urashima, K. Ibaraki, R. Suzuki, T. Yokoyama, K. Kawasaki, A. Koga, and S. Taguchi. 1990. The Hishikari gold deposit: high-grade epithermal veins in Quaternary volcanics of southern Kyushu, Japan. J. Geochem. Explor. 36:1-56. [Google Scholar]
- 14.Janssen, P. H., A. Schuhmann, F. Bak, and W. Liesack. 1996. Disproportionation of inorganic sulfur compounds by the sulfate-reducing bacterium Desulfocapsa thiozymogenes gen. nov., sp. nov. Arch. Microbiol. 166:184-192. [Google Scholar]
- 15.Johnson, D. L., and M. E. Q. Pilson. 1972. Spectrophotometric determination of arsenite, arsenate, and phosphate in natural water. Anal. Chim. Acta 58:289-299. [Google Scholar]
- 16.Kostka, J., and K. H. Nealson. 1998. Isolation, cultivation and characterization of iron- and manganese-reducing bacteria, p. 58-78. In R. S. Burlage, R. Atlas, D. Stahl, G. Geesey, and G. Sayler (ed.), Techniques in microbial ecology. Oxford University Press, New York, N.Y.
- 17.Kuenen, J. G., L. A. Robertson, and O. H. Tuovinen. 1990. The genera Thiobacillus, Thiomicrospira, and Thiosphaera, p. 2638-2657. In A. Barlows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes. Springer-Verlag, New York, N.Y.
- 18.Lane, D. J. 1985. 16S/23S sequencing, p. 115-176. In E. Stackbrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, New York, N.Y.
- 19.L'Haridon, S., A.-L. Reysenbach, G. Glenat, D. Prieur, and C. Jeanthon. 1995. Desulfurobacterium thermolithotrophum gen. nov., sp. nov., a novel autotrophic, sulfur-reducing bacterium isolated from a deep-sea hydrothermal vent. Int. J. Syst. Bacteriol. 48:701-711. [DOI] [PubMed] [Google Scholar]
- 20.Lovley, D. R., and E. J. P. Phillips. 1986. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 51:683-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, J. M. Stredwick, G. M. Garrity, B. Li, G. J. Olsen, S. Pramanik, T. M. Schmidt, and J. M. Tiedje. 2000. The RDP (Ribosomal Database Project) continues. Nucleic Acids Res. 28:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marmur, J., and P. Doty. 1962. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J. Mol. Biol. 5:109-118. [DOI] [PubMed] [Google Scholar]
- 23.Marteinsson, V. T., S. Hauksdottir, C. F. V. Hobel, H. Kristmannsdottir, G. O. Hreggvidsson, and J. K. Kristjansson. 2001. Phylogenetic diversity analysis of subterranean hot springs in Iceland. Appl. Environ. Microbiol. 67:4242-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsunaga, K., and M. Nishimura. 1969. Determination of nitrate in sea water. Anal. Chim. Acta 43:350-353. [DOI] [PubMed] [Google Scholar]
- 25.Reysenbach, A.-L., A. B. Banta, D. R. Boone, S. C. Cary, and G. W. Luther. 2000. Microbial essentials at hydrothermal vents. Nature 404:835. [DOI] [PubMed] [Google Scholar]
- 26.Reysenbach, A.-L., M. Ehringer, and K. Hershberger. 2000. Microbial diversity at 83°C in calcite springs, Yellowstone National Park: another environment where Aquificales and “Korarchaeota” coexist. Extremophiles 4:61-67. [DOI] [PubMed] [Google Scholar]
- 27.Reysenbach, A.-L., K. Longnecker, and J. Kirshtein. 2000. Novel bacterial and archaeal lineages from an in situ growth chamber deployed at a Mid-Atlantic Ridge hydrothermal vent. Appl. Environ. Microbiol. 66:3798-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reysenbach, A.-L., G. S. Wickham, and N. R. Pace. 1994. Phylogenetic analysis of the hyperthermophilic pink filament community in Octopus spring, Yellowstone National Park. Appl. Environ. Microbiol. 60:2113-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skirnisdottir, S., G. O. Hreggvidsson, S. Hjorleifsdottir, V. T. Marteinsson, S. K. Petursdottir, O. Holst, and J. K. Kristjansson. 2000. Influence of sulfide and temperature on species composition and community structure of hot spring microbial mats. Appl. Environ. Microbiol. 66:2835-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorokin, D. Y., J. G. Kuenen, and M. S. M. Jetten. 2001. Denitrification at extremely high pH values by the alkaliphilic, obligately chemolithoautotrophic, sulfur-oxidizing bacterium Thioalkalivibrio denitrificans strain ALJD. Arch. Microbiol. 175:94-101. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki, M., Z. J. Cui, M. Ishii, and Y. Igarashi. 2001. Nitrate respiratory metabolism in an obligately autotrophic hydrogen-oxidizing bacterium, Hydrogenobacter thermophilus TK-6. Arch. Microbiol. 175:75-78. [DOI] [PubMed] [Google Scholar]
- 32.Takacs, C. D., M. Ehringer, R. Favre, M. Cermola, G. Eggertsson, A. Palsdottir, and A.-L. Reysenbach. 2001. Phylogenetic characterization of the blue filamentous bacterial community from an Icelandic geothermal spring. FEMS Microbiol. Ecol. 35:123-128. [DOI] [PubMed] [Google Scholar]
- 33.Takai, K., and K. Horikoshi. 1999. Genetic diversity of archaea in deep-sea hydrothermal vent environments. Genetics 152:1285-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takai, K., and K. Horikoshi. 1999. Molecular phylogenetic analysis of archaeal intron-containing genes coding for rRNA obtained from a deep-subsurface geothermal water pool. Appl. Environ. Microbiol. 65:5586-5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takai, K., and K. Horikoshi. 2000. Rapid detection and quantification of members of the archaeal community by quantitative PCR by using fluorogenic probes. Appl. Environ. Microbiol. 66:5066-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takai, K., T. Komatsu, and K. Horikoshi. 2001. Hydrogenobacter subterraneus sp. nov., an extremely thermophilic, heterotrophic bacterium unable to grow on hydrogen gas, from deep subsurface geothermal water. Int. J. Syst. E vol. Microbiol. 51:1425-1435. [DOI] [PubMed] [Google Scholar]
- 37.Takai, K., D. P. Moser, M. Deflaun, T. C. Onstott, and J. K. Fredrickson. 2001. Archaeal diversity in waters from deep South African gold mines. Appl. Environ. Microbiol. 67:5750-5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takai, K., A. Sugai, T. Itoh, and K. Horikoshi. 2000. Palaeococcus ferrophilus gen. nov., sp. nov., a barophilic, hyperthermophilic archaeon from a deep-sea hydrothermal vent chimney. Int. J. Syst. E vol. Microbiol. 50:489-500. [DOI] [PubMed] [Google Scholar]
- 39.Thamdrup, B., K. Finster, J. W. Hansen, and F. Bak. 1993. Bacterial disproportionation of elemental sulfur coupled to chemical reduction of iron and manganese. Appl. Environ. Microbiol. 59:101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto, H., A. Hiraishi, K. Kato, H. X. Chiura, Y. Maki, and A. Shimizu. 1998. Phylogenetic evidence for the existence of novel thermophilic bacteria in hot spring sulfur-turf microbial mats in Japan. Appl. Environ. Microbiol. 64:1680-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokoyama, T., Y. Sato, Y. Maeda, T. Tarutani, and P. Maeda. 1993. Siliceous deposits formed from geothermal water. I. The major constituents and the existing states of iron and aluminum. Geochem. J. 27:375-384. [Google Scholar]
- 42.Zillig, W., I. Holz, D. Janekovic, H. P. Klenk, E. Imsel, J. Trent, S. Wunderl, V. H. Forjaz, R. Coutinho, and T. Ferreira. 1990. Hyperthermus butylicus, a hyperthermophilic sulfur-reducing archaebacterium that ferments peptides. J. Bacteriol. 172:3959-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]