Abstract
A 67-year-old woman underwent a screening colonoscopy, which revealed a 7-mm rectal subepithelial lesion. Endoscopic submucosal dissection (ESD) confirmed a grade 1 rectal neuroendocrine neoplasm (NEN), and no further treatment was administered. One year after ESD, she was diagnosed with lung cancer, which recurred 4 years later and required chemotherapy. Nine years after ESD, multiple liver metastases from the rectal NEN were found, which were well controlled with radiofrequency ablation. The patient died 13 years after ESD due to the progression of lung cancer. This case highlights the need for long-term follow-up in patients with small rectal NENs treated with endoscopic resection.
Keywords: endoscopic submucosal dissection, liver metastasis, rectal neuroendocrine neoplasm
Introduction
Rectal neuroendocrine neoplasms (NENs) are relatively rare, with an incidence of approximately 1.04 per 100,000 individuals, although this incidence has increased in recent years (1). With the increasing use of screening colonoscopy for the early detection of colorectal cancers, 93.3-100% of NENs are currently diagnosed when they are <10 mm in diameter (2). Tumors of this size have a low prevalence of metastasis (<3%) (3). Therefore, the guidelines of the European Neuroendocrine Tumor Society, North American Neuroendocrine Tumor Society, and Japanese Neuroendocrine Tumor Society recommend endoscopic resection of rectal NENs <10 mm in diameter, provided there is no evidence of muscular invasion (4,5).
Although rare, metastasis of rectal NENs <10 mm in diameter has been reported, with the liver being the most common metastatic site (6). A few case reports have documented liver metastasis emerging >5 years after the resection of rectal NENs of <10 mm in diameter in cases that were initially considered recurrence-free (6).
We herein present a case of metachronous liver metastasis identified during long-term follow-up after endoscopic submucosal dissection (ESD) of a rectal NEN of <10 mm in diameter.
Case Report
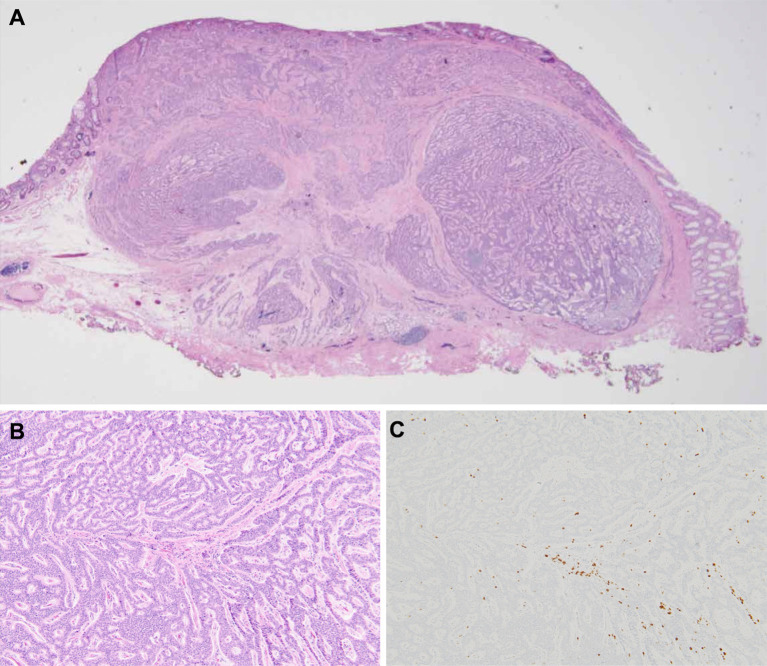
A 67-year-old woman with a medical history of hypertension and hyperlipidemia underwent a screening colonoscopy, which revealed a 7-mm yellowish subepithelial lesion in the lower rectum (Fig. 1A). Endoscopic ultrasonography revealed a 7-mm hypoechoic mass confined to the submucosa (Fig. 1B). Her biopsy results suggested rectal NEN. According to the existing guidelines, endoscopic resection was selected with curative intent. The tumor was successfully resected via ESD. Histopathological examination of the resected specimen with hematoxylin and eosin staining (Fig. 2A) revealed that the tumor cells were arranged in a trabecular pattern and were confined to the submucosa without evidence of lymphovascular invasion (Fig. 2B). Immunohistochemical staining revealed that the tumor cells were positive for synaptophysin and CD56. The Ki-67 labeling index was 0.8% (Fig. 2C). The mitotic count was <1 per 10 high-power fields. Based on these findings, the patient was diagnosed with grade 1 rectal NEN. The horizontal margin was negative; however, the evaluation of the vertical margin was inconclusive owing to thermal damage caused by ESD. After discussing the options with the patient, no additional treatment was administered.
Figure 1.
(A) Colonoscopy image revealing a 7-mm yellowish subepithelial lesion in the lower rectum. (B) Endoscopic ultrasonography image revealing a 7-mm hypoechoic mass confined to the submucosa.
Figure 2.
Pathological findings of the resected specimen. Hematoxylin and Eosin staining: (A) panoramic view (×20) and (B) tumor cells arranged in a trabecular pattern (×100). (C) MIB-1 staining: the Ki-67 labeling index is 0.8% (×100).
Postoperatively, the patient was monitored annually using contrast-enhanced computed tomography (CT). Additionally, colonoscopy was performed annually, biopsy specimens were obtained from the ESD scar, and no evidence of local recurrence was observed. One year after ESD, CT revealed a mass in the right lung. The patient underwent partial lobectomy, which revealed stage IB lung cancer (adenocarcinoma). However, the lung cancer recurred 4 years after ESD; hence, systemic chemotherapy was initiated. After lung cancer recurrence, the patient was monitored every six months using plain computed tomography. Nine years after ESD, a 30-mm hypovascular nodule was detected in segment 6 of the liver (Fig. 3). Fat-suppressed magnetic resonance imaging revealed a 30-mm nodule in segment 6 (Fig. 4A), a 9-mm nodule in segment 2 (Fig. 4), and a 10-mm nodule in segment 4 (Fig. 4C), all of which showed high signal intensity on T2-weighted images. Immunohistochemical staining of the fine-needle biopsy specimen of the hepatic nodule in segment 6 revealed that the tumor cells, arranged in a trabecular pattern (Fig. 5A), were positive for synaptophysin (Fig. 5B) and CD56. The Ki-67 labeling index was 10% (Fig. 5C). The mitotic count was 2 per 10 high-power fields. Based on these findings, we diagnosed the hepatic nodules as grade 2 NENs. A systemic evaluation was conducted using somatostatin receptor scintigraphy, esophagogastroduodenoscopy, and colonoscopy, and no NENs in other organs were observed outside the liver lesions. We diagnosed the liver lesions as metastases from the rectal NEN. The patient underwent radiofrequency ablation (RFA) for multiple liver metastases, which successfully controlled the metastatic lesions. However, the patient died 13 years after ESD due to lung cancer progression.
Figure 3.
Computed tomography image revealing liver metastasis (arrowheads). (A) Non-contrast. (B) Arterial phase. (C) Portal venous phase. (D) Delayed phase.
Figure 4.
Fat-suppressed T2-weighted magnetic resonance image revealing a 30-mm nodule in segment 6 (A, arrowhead), a 9-mm nodule in segment 2 (B, arrowhead), and a 10-mm nodule in segment 4 (C, arrowhead).
Figure 5.
Pathological findings of the fine needle biopsy of the hepatic nodule in segment 6 (×100). (A) Hematoxylin and Eosin staining. (B) Synaptophysin staining. (C) MIB-1 staining: the Ki-67 labeling index is 10.0% (×100).
Discussion
We present a case of metachronous liver metastasis that was identified during long-term follow-up after ESD for a rectal NEN of <10 mm in diameter.
Endoscopic treatment is generally considered safe and effective for rectal NENs of <10 mm in diameter because of their low risk of metastasis (7,8). Various endoscopic techniques are available, including modified endoscopic mucosal resection, ESD, and endoscopic submucosal resection with band ligation (9). Among these, endoscopic submucosal resection with band ligation has is associated with higher complete resection rates and shorter procedure times than ESD, making it the preferred first-line treatment for rectal NENs of <10 mm in diameter (10). Nevertheless, in practice, the choice of treatment technique is based on factors such as tumor size, endoscopic characteristics, and the expertise available at each medical center (7). Long-term outcomes for patients who have undergone endoscopic treatment have generally been favorable, with no recurrence or metastasis reported, irrespective of the resection margin status (11).
As the risk of metastasis from rectal NENs of <10 mm in diameter is exceedingly low (4,5), the situation in the present case raises the question of whether the liver lesions were primary or metastatic. Liver nodules were diagnosed as metastases from rectal NEN for the following reasons: (i) primary hepatic NENs are exceedingly rare (12), whereas the liver is the most common site of metastasis from rectal NENs (13); (ii) primary hepatic NENs are typically hypervascular (14), whereas the hepatic nodules in the present case were hypovascular; and (iii) the Ki-67 labeling index tends to be higher in metastatic lesions in the liver than in primary lesions (15).
RFA is recognized as a valuable palliative treatment option because of its favorable safety profile and capacity to relieve symptoms in patients with metastatic liver NENs. A previous systematic review reported a 5-year survival rate of 57-80% after RFA. A large liver metastasis volume, presence of symptoms, male sex, presence of extrahepatic disease, and a high Ki-67 proliferative index are poor prognostic factors (16). In addition, our patient had recurrent lung cancer with a poor prognosis. Given these factors, we considered RFA to be a good treatment option in the present case.
The optimal follow-up period and imaging modality after endoscopic resection of rectal NENs of <10 mm in diameter vary among guidelines (4,5), and no clear consensus exists, even within facilities in the same country. Therefore, post-treatment follow-up plans are usually determined at the clinician's discretion (8). NENs tend to grow slowly and recurrence may only be detected after a prolonged period, as demonstrated in the present case. Therefore, long-term follow-up of more than 5 years is recommended after the endoscopic resection of rectal NENs. However, monitoring all patients for a long period after endoscopic resection of rectal NENs may be cost-prohibitive, and further evidence is warranted to establish an optimal follow-up strategy for patients with NENs of <10 mm in diameter.
This case report had several limitations. Immunohistochemical staining was not performed to evaluate lymphovascular invasion in the present case. Previous studies have indicated that lymphovascular invasion is a strong risk factor for metastasis in rectal NENs (17,18). However, identifying lymphovascular invasion in rectal NENs is often challenging owing to the minimal cytological atypia exhibited by tumor cells. In response to this challenge, the use of immunohistochemical staining has been recommended to diagnose lymphovascular invasion in rectal NENs (19-21). In this regard, Sekiguchi et al. reported that re-evaluation of lymphovascular invasion in 90 rectal NENs from 86 patients treated with endoscopic resection using immunohistochemical staining revealed a surprisingly high proportion of cases testing positive for lymphovascular invasion (22). However, despite this finding, these patients did not experience recurrence without secondary treatment during the median follow-up period of >5 years. These observations raise questions regarding the clinical significance of lymphovascular invasion in small rectal NENs detected using immunohistochemical staining. Therefore, while immunohistochemical staining can provide valuable insights, its routine use for diagnosing lymphovascular invasion in rectal NENs is not essential. Further studies are required to establish their clinical relevance (23).
In conclusion, long-term follow-up may be necessary after endoscopic resection of rectal NENs, even those of <10 mm in diameter. No consensus has been reached regarding the optimal follow-up period or appropriate imaging modality for use after endoscopic resection of rectal NENs; therefore, additional evidence is needed. This case report highlights the need to determine optimal follow-up strategies after the endoscopic resection of small rectal NENs.
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 3: 1335-1342, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scherübl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy 41: 162-165, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Ngamruengphong S, Kamal A, Akshintala V, et al. Prevalence of metastasis and survival of 788 patients with T1 rectal carcinoid tumors. Gastrointest Endosc 89: 602-606, 2019. [DOI] [PubMed] [Google Scholar]
- 4.Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas 42: 557-577, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramage JK, De Herder WW, Delle Fave G, et al. ENETS Consensus Guidelines Update for Colorectal Neuroendocrine Neoplasms. Neuroendocrinology 103: 139-143, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Kwaan MR, Goldberg JE, Bleday R. Rectal carcinoid tumors: review of results after endoscopic and surgical therapy. Arch Surg 143: 471-475, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Hong SM, Baek DH. Endoscopic treatment for rectal neuroendocrine tumor: which method is better? Clin Endosc 55: 496-506, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma XX, Wang LS, Wang LL, Long T, Xu ZL. Endoscopic treatment and management of rectal neuroendocrine tumors less than 10 mm in diameter. World J Gastrointest Endosc 15: 19-31, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bang BW, Park JS, Kim HK, Shin YW, Kwon KS, Kim JM. Endoscopic resection for small rectal neuroendocrine tumors: comparison of endoscopic submucosal resection with band ligation and endoscopic submucosal dissection. Gastroenterol Res Pract 2016: 6198927, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamada Y, Tanaka K, Mukai K, et al. Efficacy of endoscopic resection for rectal neuroendocrine tumors smaller than 15 mm. Dig Dis Sci 68: 3148-3157, 2023. [DOI] [PubMed] [Google Scholar]
- 11.Moon CM, Huh KC, Jung SA, et al. Long-term clinical outcomes of rectal neuroendocrine tumors according to the pathologic status after initial endoscopic resection: a KASID multicenter study. Am J Gastroenterol 111: 1276-1285, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 97: 934-959, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Knox CD, Anderson CD, Lamps LW, Adkins RB, Pinson CW. Long-term survival after resection for primary hepatic carcinoid tumor. Ann Surg Oncol 10: 1171-1175, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Takayasu K, Muramatsu Y, Sakamoto M, et al. Findings in primary hepatic carcinoid tumor: US, CT, MRI, and angiography. J Comput Assist Tomogr 16: 99-102, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Richards-Taylor S, Tilley C, Jaynes E, et al. Clinically significant differences in Ki-67 proliferation index between primary and metastases in resected pancreatic neuroendocrine tumors. Pancreas 46: 1354-1358, 2017. [DOI] [PubMed] [Google Scholar]
- 16.Mohan H, Nicholson P, Winter DC, et al. Radiofrequency ablation for neuroendocrine liver metastases: a systematic review. J Vasc Interv Radiol 26: 935-942.e1, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Konishi T, Watanabe T, Kishimoto J, et al. Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut 56: 863-868, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park CH, Cheon JH, Kim JO, et al. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy 43: 790-795, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Fogt F, Zimmerman RL, Ross HM, et al. Identification of lymphatic vessels in malignant, adenomatous and normal colonic mucosa using the novel immunostain D2-40. Oncol Rep 11: 47-50, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Inoue T, Mori M, Shimono R, et al. Vascular invasion of colorectal carcinoma readily visible with certain stains. Dis Colon Rectum 35: 34-39, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Vass DG, Ainsworth R, Anderson JH, et al. The value of an elastic tissue stain in detecting venous invasion in colorectal cancer. J Clin Pathol 57: 769-772, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekiguchi M, Sekine S, Sakamoto T, et al. Excellent prognosis following endoscopic resection of patients with rectal neuroendocrine tumors despite the frequent presence of lymphovascular invasion. J Gastroenterol 50: 1184-1189, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Sekiguchi M, Matsuda T, Saito Y. Treatment strategy and post-treatment management of colorectal neuroendocrine tumor. DEN Open 4: e254, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]