Abstract
Background
For patients diagnosed with primary aldosteronism (PA) accompanied by bilateral adrenal lesions, identifying optimal candidates for surgical intervention remains a significant clinical challenge. Although adrenal venous sampling (AVS) is currently the gold standard for lateralizing aldosterone hypersecretion, its technical complexity, invasiveness, and interpretive difficulties restrict its widespread adoption. In this study, we aimed to investigate the clinical application of 68Ga-pentixafor positron emission tomography/computed tomography (PET/CT) as a non-invasive imaging modality in AVS-free surgical decision-making for PA patients with bilateral adrenal lesions.
Results
Among the 51 patients who underwent 68Ga-pentixafor PET/CT, 36 patients had adrenalectomy, with the surgical side determined by PET/CT lateralization. The postoperative complete biochemical and clinical success rates for these patients were 91.67% and 100%, respectively. Additionally, receiver operating characteristic curve analysis indicated that PET/CT results were favorable predictors of postoperative outcomes in surgical patients. Postoperative pathological evaluation of 68Ga-pentixafor PET/CT-guided surgical patients revealed that 86.11% had adrenocortical adenomas with positive CYP11B2 and CXCR4 expression.
Conclusion
CXCR4-targeted 68Ga-pentixafor PET/CT can be effectively utilized in surgery decision-making for PA patients with bilateral adrenal lesions, offering a potential alternative to AVS and maybe applied to predict postoperative biochemical and clinical success.
Trial registration
68Ga-Pentixafor PET/CT for Guiding Surgical Treatment of Primary Aldosteronism With Bilateral Adrenal Lesions; Trial registration number: NCT06247566; Date of registration: 2021-11-01; URL of trial registry record: https://clinicaltrials.gov/study/NCT06247566.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13550-025-01309-4.
Keywords: 68Ga-pentixafor PET/CT, CXCR4, Primary aldosteronism, Surgery decision-making, Bilateral adrenal lesions
Introduction
Primary aldosteronism (PA) is characterized by excessive production of renin-independent aldosterone, which increases blood volume, potassium excretion, and sodium storage in the body, accompanied by inhibition of renin-angiotensin system activity. Clinically, patients with PA often present with hypertension and hypokalemia [1]. Furthermore, patients with PA face a significantly higher risk of cardiovascular and cerebrovascular complications compared to those with primary hypertension, even when matched for blood pressure [2]. PA is mainly classified into two subtypes: unilateral aldosterone hypersecretion with contralateral aldosterone non-secretion (most commonly due to unilateral aldosterone-producing adenoma [APA]) and bilateral aldosterone hypersecretion (most often resulting from idiopathic hyperaldosteronism [IHA] or bilateral APA). Accurate differentiation of these subtypes is critical, as patients with unilateral or lateralized adrenal aldosterone hypersecretion may benefit from surgical removal of the adrenal lesion. For patients without lateralization, mineralocorticoid receptor antagonists (MRAs) are the recommended treatment. PA with bilateral adrenal lesions is a more complicated situation. A small percentage of APAs that appear as bilateral nodules on computed tomography (CT) are likely to be misdiagnosed as IHA, while cases of IHA that appear as adrenal microadenomas on CT may be mistaken for APAs, resulting in unnecessary unilateral adrenalectomy.
Adrenal vein sampling (AVS), which determines the lateralization of aldosterone hypersecretion, has long been considered the gold standard for identifying PA subtypes in patients with bilateral adrenal lesions and for guiding treatment decisions [3–5]. However, the feasibility of AVS remains controversial because of its substantial cost, invasiveness with potential risks, and complicated technique with a relatively high failure rate [6, 7]. In regions where AVS is not feasible, patients with bilateral lesions associated with PA may be treated with MRAs as a first-line therapy. Alternatively, in certain cases, suspected aldosterone-producing lesions may be surgically resected based on imaging characteristics observed in adrenal CT scans [8]. In these cases, larger lesions or those exhibiting radiological features typical of cortical adenomas are typically targeted for removal. However, there is still the possibility that these lesions could be non-functional, making this empirical approach prone to treatment failure. As a result, considerable efforts have been directed towards finding a cost-effective, convenient, and non-invasive alternative to AVS.
The C-X-C chemokine receptor 4 (CXCR4), a G protein-coupled transmembrane receptor, is highly expressed in aldosterone-producing tissues [9]. Its expression is strongly correlated with the expression of aldosterone synthase (CYP11B2) [9, 10]. The radiolabeled ligand 68Ga-pentixafor, which selectively binds to CXCR4 receptors on cell membranes, enables the visualization of tissues with elevated CXCR4 expression using PET/CT imaging [11]. The feasibility and accuracy of 68Ga-pentixafor PET/CT for in vivo imaging of CXCR4 receptors have been well-documented in diagnosing, staging, and evaluating treatment responses in various malignancies, including lung cancer, multiple myeloma, glioma, and sarcoma [12–15]. Consequently, there is increasing interest in utilizing this non-invasive imaging technique for the diagnosis and subtyping PA [10, 16–21].
Studies have demonstrated significant differences in 68Ga-pentixafor uptake between APA and non-functional adrenal adenomas (NFA) on PET/CT scans [10, 16, 21]. In a previous study, it was reported 68Ga-pentixafor PET/CT could enhance the diagnosis of PA subtypes, with the maximum standardized uptake value (SUVmax) of IHA lesions and NFAs being significantly lower than that of APAs [21]. APA patients with a nodule greater than 1 cm in diameter, when SUVmax was 7.3 or greater, the specificity was 100% [21]. Furthermore, accumulating evidence suggests that 68Ga-pentixafor PET/CT can facilitate non-invasive diagnosis in most PA cases, identify surgically curable PA, and demonstrate strong consistency with AVS [17, 22–25]. These findings indicate that 68Ga-pentixafor PET/CT may serve as a first-line test for PA classification. However, limited research has explored the value of 68Ga-pentixafor PET/CT in predicting surgical outcomes of PA patients, especially those with bilateral adrenal lesions. Herein, in the complicated situation of PA with bilateral adrenal lesions and the limited application of AVS, we initially employed 68Ga-pentixafor PET/CT imaging as a non-invasive alternative to AVS, for evaluating PA patients with bilateral adrenal lesions who may benefit from surgery and monitored their prognosis to assess the clinical significance of this imaging modality in guiding surgery decision-making and treatment strategies.
Methods
Study population
This retrospective study was approved by the Institutional Review Board of our institution (2023111517). Medical records of all patients (n = 102) diagnosed with PA and bilateral adrenal lesions at a tertiary medical center from January 1, 2020, to July 1, 2024, were reviewed. The study was registered with ClinicalTrials.gov (NCT06247566). The inclusion criteria were as follows: (1) patient has hypertension (blood pressure ≥ 140/90 mmHg or taking antihypertensive medications) and/or hypokalemia; (2) positive confirmatory test (captopril test and/or saline infusion test); (3) adrenal CT scan showing bilateral adrenal lesions. Patients with other common secondary hypertension (n = 4) and severe comorbidity potentially interfering with treatment (n = 5) were excluded. Besides, 38 patients were excluded because only CT imaging was performed, and 4 patients were excluded due to missing postoperative medical records. Ultimately, 51 patients were included in the final analysis (Fig. 1). Otherwise, all enrolled patients have completed a 1-mg overnight dexamethasone suppression test with negative result to exclude concurrent Cushing syndrome.
Fig. 1.
Flow chart of patient cohort selection. PET/CT, positron emission tomography/computer tomography; CT, computed tomography; MRAs, mineralocorticoid-receptor antagonists
Baseline information for all included patients were collected and included the following variables: age, sex, height, weight, body mass index (BMI), comorbidities, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), number of antihypertensive medications, serum potassium level, plasma aldosterone concentration (PAC), direct renin concentration (DRC), aldosterone-to-renin ratio (ARR), lesion size on CT, and pathological data. The PAC, DRC and ARR were all detected under lying condition. In addition, the max nodule diameter was utilized to present the lesion size for PA with multiple nodules.
68Ga-pentixafor PET/CT and image analysis
The 68Ga-pentixafor was prepared as previously described [21]. Examinations were conducted at our institution using a specialized PET/CT scanner (Discovery 690 Elite scanner; GE Healthcare). A committee-certified nuclear medicine physician (T.L.), blinded to the patient’s clinical records, evaluated the PET/CT data. Positive lesions were identified by visual analysis when there was a significant difference in uptake between one side of the adrenal gland and the surrounding normal adrenal tissue. Negative lesions showed similar or lower uptake compared to the surrounding adrenal tissue. The lesion’s SUVmax, lesion-to-liver uptake ratio (LLR), and dominant lesion-to-inferior lesion ratio (DIR) were quantified. In patients undergoing PET/CT, the dominant side of bilateral adrenal lesions was determined by an experienced radiologist through visual analysis. The SUV values of our center are calculated through standardized methods based on the patient’s body weight.
AVS interpretation
AVS examination was performed in some confirmed PA patients with bilateral adrenal lesions. Moreover, AVS was conducted to attain a definitive diagnosis in 12 PA patients with bilateral adrenal lesions, with 5 patients successfully undergoing the procedure [4].
Surgery decision-making
The basis for selecting surgical lateralization is mainly determined by the multidisciplinary diagnosis and treatment (MDT) team’s discussion based on the patient’s examination results. Although we have repeatedly emphasized the necessity for AVS, the percentage of patients undergoing AVS remains low. The main consideration is the information from 68Ga-pentixafor PET/CT. The patient’s willingness is also taken into consideration to make the decision. When 68Ga-pentixafor PET/CT indicated clear positive lesion and the patient was willing to proceed with surgery, we would remove the positive lesion. If bilateral adrenal lesions were positive and the patient was willing to undertake surgery, we would tend to remove the side with higher SUVmax. All the patients who underwent surgery had unilateral adrenalectomy.
Follow-up
All PA patients who consented to surgical treatment were followed up for three months post-surgery in the outpatient department of our institution. The Primary Aldosteronism Surgical Outcome Study (PASO) was used to assess outcomes [26]. Consensus criteria were applied to evaluate the surgical outcome of patients into complete, partial, or absent clinical and biochemical success. The outcomes were strictly defined based on PASO criteria, with complete clinical success requiring normotension (blood pressure < 140/90 mmHg) without antihypertensives, and complete biochemical success necessitating normalized ARR and potassium levels. We provided detailed definitions in Supplemental File 1.
Immunohistochemistry
All postoperative paraffin-embedded specimens from surgical patients were subjected to immunohistochemical analysis. The antibodies used and their dilutions were as follows: CXCR4 (1:1000, 60042-1-Ig, Proteintech) and CYP11B2 (1:200 MABS1251, Millipore). Immunohistochemistry was used to assess the expression of aldosterone synthase CYP11B2 and CXCR4. The score was rated as follows: 0 points for 0%, 1 point for 0–10%, 2 points for 10–50%, 3 points for 50–75%, and 4 points for 75–100%. A score of 0–1 was considered negative, and a score of 2–4 was considered positive. All immunohistochemical results were independently evaluated by a pathologist (X.F.) who was blinded to the patient’s clinical characteristics and imaging data before the test.
Statistical analysis
Data analysis was performed using IBM SPSS 26.0. The Shapiro-Wilk test was conducted to test for normality of the data. Normally distributed continuous variables were analyzed using independent t-tests, whereas the Mann-Whitney test was employed to evaluate non-normally distributed variables. Count data were expressed as frequencies (%) and compared using the chi-square test or Fisher’s exact test. Data were expressed as mean ± standard deviation (χ ± S) or median (interquartile range). The receiver operating characteristic (ROC) curves were drawn to determine the predictive thresholds of the SUVmax, LLR, and DIR of 68Ga-pentixafor PET/CT in surgical outcomes. GraphPad Prism 8.0 was used for data visualization. A p value of < 0.05 was considered statistically significant.
Results
Patient clinical characteristics
The medical records of 102 patients diagnosed with PA and bilateral adrenal lesions were reviewed. Although all patients were advised to undergo AVS, the majority declined this invasive procedure. Following the application of various exclusion criteria, a total of 55 patients (55/93, 59.14%) completed the 68Ga-pentixafor PET/CT and 51 were deemed eligible for inclusion in the final analysis because 4 patients were excluded due to missing postoperative medical records. The performance of PET/CT is illustrated in Fig. 2. Among the included patients, 36 (36/51, 70.59%) underwent adrenalectomy based on PET/CT results, while 15 (15/51, 29.41%) received MRAs due to non-lateralized aldosterone secretion indicated by PET/CT. The patient clinical characteristics are detailed in Table 1. The overall cohort had a mean age of 52.63 ± 10.72 years, with 58.82% (30/51) males and 41.18% (21/51) females. No significant differences were observed between groups in age, gender distribution, or BMI. The surgical group showed higher median plasma aldosterone concentration (PAC [lying condition]: 268.0 pg/ml vs. 202.0 pg/ml) and lower serum potassium levels (median: 2.87 mmol/L vs. 3.16 mmol/L), consistent with more pronounced aldosterone hypersecretion (Table 1).
Fig. 2.
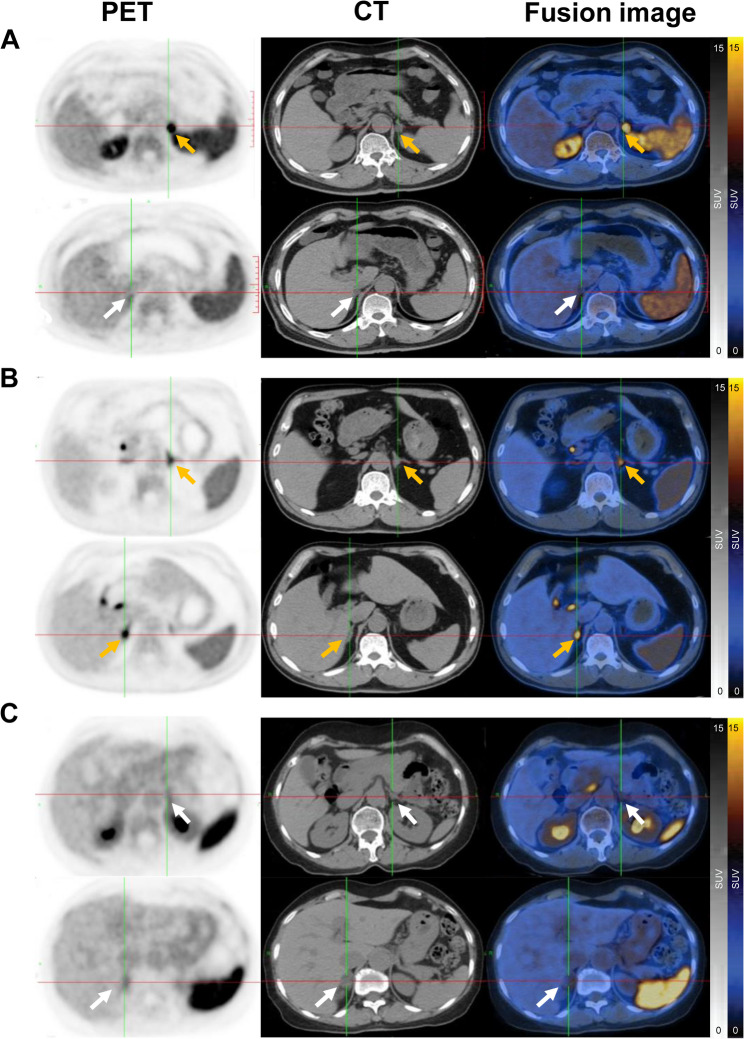
68Ga-pentixafor PET/CT imaging findings in PA patients with bilateral adrenal lesions. (A) 68Ga-pentixafor PET/CT images demonstrating positive lesions on one side and negative lesions on the other. A 52-year-old man, presented with persistent hypertension and hypokalemia for 10 years. ARR exceeding 30, peak blood pressure of 180/110 mmHg and minimum blood potassium level of 2.93mmol/l. CT scans revealed bilateral lesions in the adrenal glands (indicated by arrows, the white arrows mean negative lesions, and yellow arrows mean positive), with PET/CT showing a notably higher SUVmax in the left adrenal gland lesion (SUVmax = 26.8, yellow arrows) compared to the right (SUVmax = 4.6, white arrows). (B) Images showing positive lesions on both sides in a 53-year-old-man with 20 years hypertension and hypokalemia, ARR > 30, maximum blood pressure of 210/110 mmHg, and minimum blood potassium of 1.98 mmol/l, CT showed bilateral adrenal lesions (yellow arrows), and PET/CT exhibited positive lesions on both side (SUVmax = 9.8 vs.7.2, yellow arrows). (C) Images displaying negative lesions on both sides in a 69-year-old-woman with 30 years of hypertension and hypokalemia. ARR > 30, maximum blood pressure of 190/120 mmHg, and minimum blood potassium of 3.28 mmol/l. CT showed bilateral adrenal lesions (white arrows), and PET/CT exhibited similar values of bilateral adrenal uptake (SUVmax = 3.4 vs. 3.5, white arrows). PET/CT, positron emission tomography/computer tomography; PA, primary aldosteronism; CT, computed tomography; ARR, aldosterone-to-renin ratio; SUVmax, the maximum standardized uptake value
Table 1.
Demographic and clinical characteristics of study population
| Clinical characteristics | Total n = 51 |
Surgical group n = 36 |
MRA group n = 15 |
p value |
|---|---|---|---|---|
| Age (year) | 52.63 ± 10.72 | 52.61 ± 9.65 | 52.67 ± 13.34 | 0.083 |
| Gender | ||||
| Male | 30 (58.82%) | 19 (52.80%) | 11 (73.33%) | 0.517 |
| Female | 21 (41.18%) | 17 (47.20%) | 4 (26.67%) | |
| BMI (kg/m2) | 24.86 ± 3.54 | 24.94 ± 3.55 | 24.67 ± 3.63 | 0.722 |
| Duration of hypertension (year) | 9.00 (2.00, 10.50) | 10.00 (2.25, 10.00) | 10.00 (6.00, 20.00) | 0.031 |
| Systolic pressure (mmHg) | 180.0 (165.0, 200.0) | 180.0 (161.3, 197.5) | 180.0 (170.0, 200.0) | 0.440 |
| Diastolic pressure (mmHg) | 105.0 (100.0, 110.0) | 107.5 (100.0, 110.0) | 105.0 (100.0, 120.0) | 0.876 |
| Mean arterial pressure (mmHg) | 145.0 (135.0, 150.0) | 145.0 (135.0, 150.0) | 142.5 (135.0, 155.0) | 0.480 |
| Number of antihypertension medications | 2.00 (1.00, 2.00) | 2.00 (1.00, 2.00) | 3.00 (2.00, 3.00) | 0.018 |
| Creatinine (mmol/L) | 80.5 (62.7, 103.0) | 84.0 (63.50, 107.43) | 54.00 (37.00, 63.00) | 0.787 |
| Serum potassium (mmol/L) | 3.03 (2.74, 3.30) | 2.87 (2.34, 3.23) | 3.16 (2.80, 3.45) | 1.000 |
| PAC (lying condition) (pg/ml) | 266.0 (148.0, 547.0) | 268.0 (155.3, 637.8) | 202.0 (115.0, 475.0) | 0.235 |
| DRC (lying condition) (uIU/ml) | 1.51 (1.20, 2.17) | 1.41 (0.63, 2.61) | 2.17 (1.19, 2.80) | 0.358 |
| ARR (lying condition) (pg/ml/uIU/ml) | 181.42 (54.0, 537.70) | 220.5 (78.0, 627.2) | 71.82 (47.8, 221.5) | 0.136 |
| Nodule diameter (mm) | 12.0 (9.00, 17.00) | 13.00 (10.25, 19.00) | 11.00 (7.00, 12.000) | 0.011 |
| Opposite nodule diameter | 7.00 (6.00, 10.00) | 7.00 (6.00, 10.00) | 7.00 (6.00, 9.00) | 0.427 |
| Ratio of nodule diameter | 1.57 (1.20, 2.17) | 1.73 (1.22, 2.32) | 1.28 (1.17, 1.78) | 0.063 |
Abbreviations: BMI, body mass index; PAC, plasma aldosterone concentration; DRC, direct renin concentration; ARR, aldosterone/renin ratio
Of the 36 patients who underwent functional PET/CT and surgery, the SUVmax value of lesions on the surgically treated side was significantly higher than that of non-excised lesions (15.30 ± 7.18 vs. 4.91 ± 1.42, p < 0.001). As anticipated, the SUVmax value of both sides of the adrenal glands in the 15 patients treated with MRAs was comparable (5.01 ± 1.45 vs. 4.63 ± 1.51, p = 0.424) (Fig. 3), which is more subtyping into IHA based on previous study [21].
Fig. 3.
68Ga-pentixafor PET/CT imaging findings in PA patients with bilateral adrenal lesions. ***p < 0.001
Preoperative clinical features and follow-up results
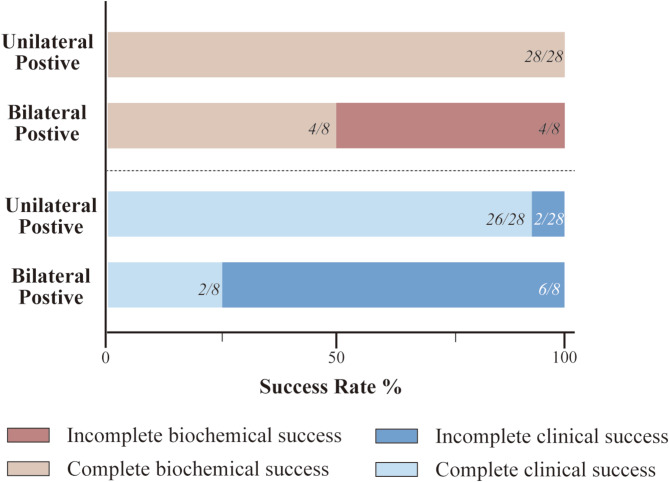
Preoperative and postoperative outcomes for patients who underwent adrenalectomy are detailed in Table 2. Postoperative indicators, including blood pressure, number of antihypertensive medications, serum potassium levels, and biochemical markers, were significantly lower compared to preoperative values (Table 2). The complete biochemical success rate was 88.89% (32/36), and the complete clinical success rate was 77.78% (28/36) (Fig. 4; Table 3). Visual analysis revealed unilateral positive lesions in 28 patients (28/36, 77.78%). All 28 patients (100%) achieved complete biochemical success, and 26 (26/28, 92.86%) achieved complete clinical success. The remaining 8 patients (8/36, 29.03%) exhibited bilateral positive lesions, with surgery performed on the side showing higher uptake. Of these, 2 patients (2/8, 25%) achieved complete clinical success, while 6 (6/8, 75%) had incomplete clinical success. Regarding biochemical outcomes, 4 patients (4/8, 50%) achieved complete success, 4 patient (4/8, 50%) experienced incomplete success (Fig. 4).
Table 2.
Preoperative and postoperative clinical features
| Postoperative clinical features | After Surgery | Before Surgery | p value |
|---|---|---|---|
| Systolic pressure (mmHg) | 130.0 (127.0-133.8) | 180.0 (161.3, 197.5) | < 0.001 |
| Diastolic pressure (mmHg) | 80.0 (74.8–88.8) | 107.5 (100.0, 110.0) | < 0.001 |
| Mean arterial pressure (mmHg) | 105.0 (100.0-110.0) | 145.0 (135.0, 150.0) | < 0.001 |
| Number of antihypertension medications | 0.00 (0.00–0.00) | 2.00 (1.00, 2.00) | < 0.001 |
| Serum potassium (mmol/L) | 3.90 ± 0.34 | 2.87 (2.34, 3.23) | < 0.001 |
| PAC (lying condition) (pg/ml) | 59.5 (40.1–82.2) | 268.0 (155.3, 637.8) | < 0.001 |
| DRC (lying condition) (uIU/ml) | 4.23 (3.11–7.56) | 1.41 (0.63, 2.61) | < 0.001 |
| ARR (lying condition) (pg/ml/uIU/ml) | 11.88 (6.91–18.83) | 220.5 (78.0, 627.2) | < 0.001 |
Abbreviations: PAC, plasma aldosterone concentration; DRC, direct renin concentration; ARR, aldosterone/renin ratio
Fig. 4.
Rate of biochemical and clinical success of patients underwent surgery
Table 3.
Imaging characteristics of 68Ga-pentixafor PET/CT in different prognosis of PA patients
| Total n = 36 |
Clinical success | p value | Biochemical success | p value | ||||
|---|---|---|---|---|---|---|---|---|
| Complete | Incomplete | Complete | Incomplete | |||||
| Visual analysis | ||||||||
| Unilateral positive lesions and contralateral negative lesions |
28 (77.78%) |
26 (72.22%) |
2 (5.56%) |
< 0.001 |
28 (77.78%) |
0 (0%) |
0.001 | |
| Bilateral positive lesions |
8 (22.22%) |
2 (5.56%) |
6 (16.67%) |
4 (11.11%) |
4 (11.11%) |
|||
| SUVmax of the lesions | 15.29 ± 7.18 | 17.67 ± 6.27 | 6.96 ± 2.01 | < 0.001 | 15.73 ± 6.62 | 6.13 ± 1.80 | 0.012 | |
| SUVmax of the opposite lesions | 4.89 ± 1.42 | 4.85 ± 1.47 | 5.05 ± 1.31 | 0.726 | 5.14 ± 1.41 | 4.13 ± 1.10 | 0.291 | |
| SUVmax of the liver | 2.30 ± 0.55 | 2.27 ± 0.59 | 2.41 ± 0.41 | 0.529 | 2.39 ± 0.54 | 2.00 ± 0.61 | 0.562 | |
| DIR | 3.35 ± 1.80 | 3.91 ± 1.65 | 1.38 ± 0.22 | < 0.001 | 3.32 ± 1.72 | 1.48 ± 0.10 | < 0.001 | |
| LLR | 6.97 ± 3.61 | 8.13 ± 3.23 | 2.90 ± 0.67 | < 0.001 | 6.84 ± 3.03 | 3.16 ± 0.76 | < 0.001 | |
Abbreviations: SUVmax, the maximum standardized uptake value; DIR, dominant/inferior side uptake ratio; LLR, lesion/liver uptake ratio
Efficacy of 68Ga-pentixafor PET/CT for predicting surgical outcomes
The prognostic value of 68Ga-pentixafor PET/CT was evaluated in the 36 surgically treated patients. Comparative analyse demonstrated that patients with complete clinical success had significantly higher SUVmax values than those with incomplete success (17.67 ± 6.27 vs. 6.96 ± 2.01). Similarly, patients with complete biochemical success exhibited higher SUVmax values than those with incomplete success (15.73 ± 6.62 vs. 6.13 ± 1.80). Additionally, the levels of DIR (3.91 ± 1.65 vs. 1.38 ± 0.22, p < 0.001) and LLR (8.13 ± 3.23 vs. 2.90 ± 0.67, p < 0.001) were significantly higher in the complete clinical success group compared to the incomplete success group. Similar trends were observed for biochemical success (DIR: 3.32 ± 1.72 vs. 1.48 ± 0.10, p < 0.001; LLR: 6.84 ± 3.03 vs. 3.16 ± 0.76, p < 0.001) (Table 3).
Predictive performance of 68Ga-pentixafor PET/CT indicators
The sensitivity, specificity, and accuracy of 68Ga-pentixafor PET/CT visual analysis for predicting complete clinical success were 92.86%, 75.00%, and 88.89%, respectively (Table 4; Fig. 5A). ROC curve analysis identified an optimal SUVmax cut-off value of 10.00 for complete clinical success, with an area under the curve (AUC) of 0.9063 (95% confidence interval [CI], 0.7949–1.000, p = 0.0005). For DIR, the optimal cut-off was 1.613, with an AUC of 0.9598 (95% CI, 0.8873–1.000, p < 0.0001), sensitivity of 90.32%, specificity of 87.50%, and accuracy of 90.32%. For LLR, the optimal cut-off was 4.575, with an AUC of 0.9777 (95% CI, 0.9352–1.000, p < 0.0001), sensitivity of 85.71%, specificity of 100%, and accuracy of 88.89% (Table 4; Fig. 5A).
Table 4.
The predictive efficacy of 68Ga-pentixafor PET/CT based on visual and semi-quantitative analysis
| Sensitivity | Specificity | Accuracy | AUC | 95%CI | p value | |
|---|---|---|---|---|---|---|
| Clinical success | ||||||
| Visual analysis | 92.86% | 75.00% | 88.89% | 0.8393 | 0.6510-1.000 | 0.0038 |
| SUVmax=10.00 | 85.71% | 87.50% | 86.11% | 0.9063 | 0.7949-1.0000 | 0.0005 |
| DIR = 1.613 | 96.43% | 87.50% | 90.32% | 0.9598 | 0.8873-1.0000 | < 0.0001 |
| LLR = 4.575 | 85.71% | 100% | 88.89% | 0.9777 | 0.9352-1.0000 | < 0.0001 |
| Biochemical success | ||||||
| Visual analysis | 87.50% | 100% | 88.89% | 0.9375 | 0.8577-1.0000 | 0.0048 |
| SUVmax=8.30 | 90.63% | 100% | 91.67% | 0.9453 | 0.8705-1.0000 | 0.0041 |
| DIR = 2.031 | 75.00% | 100% | 77.78% | 0.8203 | 0.6875–0.9531 | 0.0390 |
| LLR = 4.062 | 81.25% | 100% | 86.11% | 0.8984 | 0.7921-1.0000 | 0.0103 |
Abbreviations: SUVmax, the maximum standardized uptake value; DIR, dominant/inferior side uptake ratio; LLR, lesion/liver uptake ratio
Fig. 5.
The ROC curve of SUVmax, DIR, and LLR for predicting outcomes. (A) The ROC curve of SUVmax, DIR, and LLR for predicting clinical success; (B) The ROC curve of SUVmax, DIR, and LLR for predicting biochemical success. ROC, receiver-operating characteristic; AUC, area under the ROC curve; LLR, lesion/liver uptake ratio; DIR, dominant/inferior side uptake ratio
When complete biochemical success was used as the endpoint, the sensitivity, specificity, and accuracy of visual analysis were 87.50%, 100%, and 88.89%, respectively (Table 4; Fig. 5B). The optimal SUVmax cut-off for complete biochemical success was 8.30, with an AUC of 0.9453 (95% CI, 0.8577–1.000, p = 0.0048), sensitivity of 90.63%, specificity of 100%, and accuracy of 91.67%. For DIR, the optimal cut-off was 2.031, with an AUC of 0.8203 (95% CI, 0.6875–0.9531, p = 0.0390), sensitivity of 75%, specificity of 100%, and accuracy of 77.78%. For LLR, the optimal cut-off was 4.062, with an AUC of 0.8984 (95% CI, 0.7921–1.000, p = 0.0103), sensitivity of 81.25%, specificity of 100%, and accuracy of 86.11% (Table 4; Fig. 5B).
Postoperative pathological results
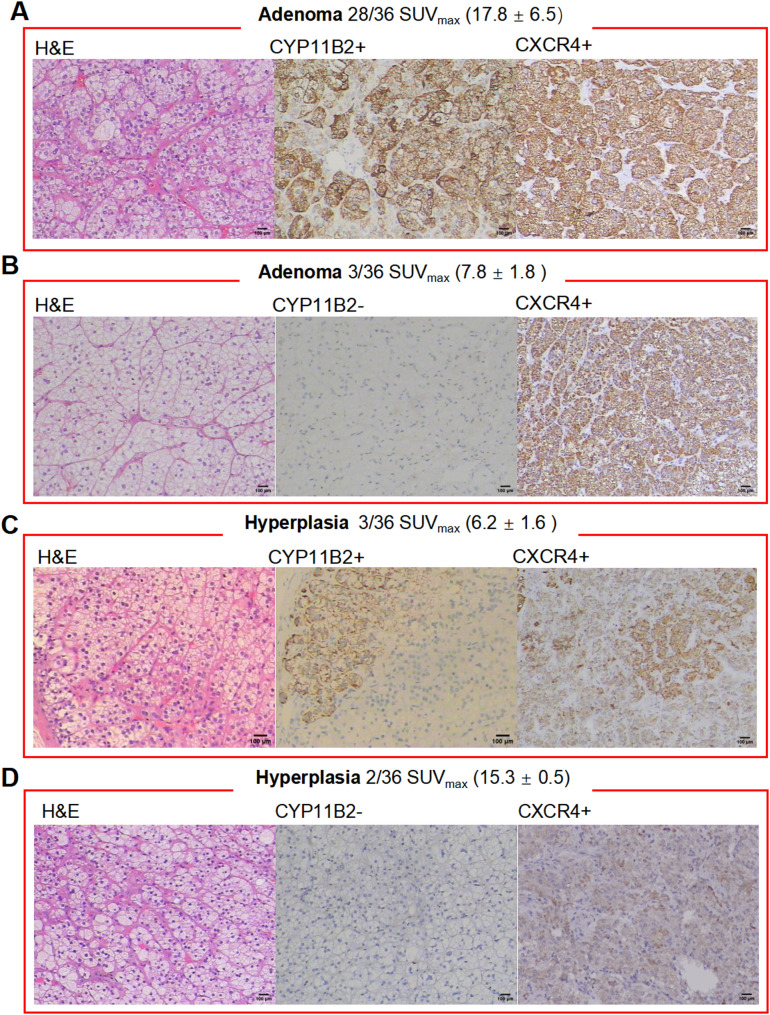
Postoperative pathological results were analyzed to explore factors contributing to better surgical outcomes in patients undergoing 68Ga-pentixafor PET/CT. All these patients exhibited prominently positive CXCR4 staining. Among the 36 patients, 77.78% (28/36) were diagnosed with adrenocortical adenoma, showing positive CYP11B2 expression (Fig. 6A). Additionally, 8.33% (3/36) had adrenocortical adenomas without CYP11B2 expression but with significant CXCR4 expression (Fig. 6B). Conversely, 13.89% (5/36) were diagnosed with adrenocortical hyperplasia, with 3 showing positive CYP11B2 expression and 2 showing negative staining. All hyperplastic lesions exhibited mild CXCR4 expression (Fig. 6C-D). Overall, the consistency between CXCR4 and CYP11B2 expression was 86.11% (31/36).
Fig. 6.
Pathological subtypes of PA patients and their postoperative immunohistochemical results. (A) Adrenal cortical adenoma with positive CYP11B2 and positive CXCR4 expression; (B) Adrenal cortical adenoma with negative CYP11B2 expression and positive CXCR4 expression; (C) Adrenal cortical adenoma-like hyperplasia with positive CYP11B2 and positive CXCR4 expression; (D) Adrenal cortical adenoma-like hyperplasia with negative CYP11B2 and positive CXCR4 expression. (magnification 100×, bar = 100 μm)
Comparison between AVS and 68Ga-pentixafor PET/CT
In our cohort, 5 patients underwent both successful AVS and 68Ga-pentixafor PET/CT. The consistency between the two methods was 80% (4/5). Two patients showed concordant lateralization on both tests and achieved complete clinical and biochemical success, with pathological findings confirming CYP11B2-positive cortical adenomas. However, one patient exhibited unilateral lateralization on PET/CT but no dominant secretion on AVS. And unilateral adrenalectomy resulted in clinical and biochemical success, with pathological confirmation of a CYP11B2-positive cortical adenoma. The remaining two patients showed no lateralization on either AVS or 68Ga-pentixafor PET/CT test, resulting in MRAs (Table 5).
Table 5.
Comparison of AVS and 68Ga-pentixafor PET/CT imaging results
| Patient | AVS | 68Ga-pentixafor PET/CT | Pathological result | CYP11B2 | Biochemical success | Clinical success | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lateralization | LI | Lateralization | SUVmax | LLR | DIR | |||||
| 1 | L | 13.79 | L | 14.9 | 8.28 | 5.14 | Adenoma | Positive | Complete | Complete |
| 2 | L | 2.78 | L | 14.8 | 7.40 | 3.36 | Adenoma | Positive | Complete | Complete |
| 3 | B | 1.35 | R | 15.8 | 6.58 | 2.26 | Adenoma | Positive | Complete | Complete |
| 4 | B | 1.75 | B | 5.3 | 2.21 | 1.33 | Medical treatment | |||
| 5 | B | 1.41 | B | 4.3 | 2.35 | 1.02 | Medical treatment | |||
Abbreviations: PET/CT, positron emission tomography/computed tomography; AVS, adrenal vein sampling; LI, lateralization index; L, left; B, bilateral; R, right
Discussion
This retrospective analysis demonstrates that surgical intervention guided by CXCR4-targeted 68Ga-pentixafor PET/CT achieves favorable clinical and biochemical outcomes in PA patients with bilateral adrenal lesions. Parameters including visual analysis, SUVmax, LLR, and DIR effectively identified surgical candidates, suggesting CXCR4-based functional imaging could circumvent the need for AVS in this complex subgroup. Precision and noninvasiveness diagnostics of disease is currently an emerging trend in the clinic. Our study proposes a paradigm shift toward CXCR4 PET/CT-guided surgery decision-making in AVS-free way for PA patients with bilateral adrenal lesions.
Current guidelines recommend using AVS as the gold standard for PA lateralization and treatment selection [4, 27–29]. However, technical limitations, inconsistent criteria use, and high failure rates hinder widespread adoption of AVS [30, 31]. Despite the introduction of AVS at our center in 2020, only 12 out of 51 (23.53%) PA patients with bilateral adrenal lesions successfully underwent AVS in this study. Notably, the 80% concordance between 68Ga-pentixafor PET/CT and AVS lateralization in our study, coupled with superior surgical outcome prediction (100% vs. 67%), indicated the potential to replace AVS. Our results echo the reports from other investigators, in which the tests showed a 66.7% − 90% concordance rate and 68Ga-pentixafor PET/CT was equal to or even better than AVS in predicting outcomes after surgery [17–19, 23, 25]. Previous studies predominantly focused on unilateral PA patients, with only a small subset of patients with bilateral lesions included. Discrepancies among these studies could be attributed to variations in sample size and characteristics. Before considering revisions to the guidelines, further investigation is warranted to determine whether 68Ga-pentixafor PET/CT can serve as a cost-effective, convenient, and non-invasive alternative to AVS.
Multiple studies have demonstrated the reliability of CXCR4-targeted functional imaging in identifying aldosterone-secreting nodules and classifying PA [9, 10, 16–19, 21]. For instance, Ding et al. reported that the SUVmax value of APAs (15.3 ± 7.7) was significantly higher than that of suspected unilateral adrenal hyperplasia (UAH) (9.1 ± 2.7), IHA (4.3 ± 1.3), and NFA (4.4 ± 1.7) [18]. Similarly, a previous study found a median SUVmax value of 14.5 in APAs, which was notably higher than that of IHA (4.8) and NFA (4.65) [21]. In our study, the average SUVmax value of adrenal lesions selected for surgical removal was 15.30 ± 7.18, underscoring the significant utility of 68Ga-pentixafor PET/CT in guiding surgical decision-making for bilateral adrenal lesions by effectively identifying aldosterone-producing nodules such as APAs. This finding was further corroborated by postoperative pathological analysis. Notably, 68Ga-pentixafor PET/CT demonstrated superior performance in identifying APAs with positive CYP11B2 expression (77.8%, 28/36). Our results support the high concordance rate between CXCR4 and CYP11B2 expression, as previously reported [10, 16, 21]. However, two patients with CYP11B2-negative/CXCR4-positive adenomas still achieved biochemical remission, suggesting alternative aldosterone-secreting mechanisms linked to somatic mutations [32–34], which this phenomenon warranted investigation into whether CXCR4 overexpression reflects latent autonomous secretion despite negative CYP11B2 staining.
Evidence from various studies has demonstrated the utility of several indicators of CXCR4-targeted 68Ga-pentixafor PET/CT imaging—such as visualization, LLR, and lesion-to-contralateral adrenal gland ratio (LCR)—in distinguishing and predicting APAs [10, 16–19, 21]. However, most of these studies prioritized pathological diagnosis and provided limited insights into prognosis. Recently, Ding et al. reported that 68Ga-pentixafor PET/CT could surgically differentiate eligible from ineligible micronodules, primarily based on surgical pathology and outcomes [19]. Notably, all adrenal lesions analyzed in their study were smaller than 1 cm, with the majority being unilateral. To our knowledge, this is the first study to evaluate the efficacy of 68Ga-pentixafor PET/CT in predicting surgical outcomes for PA patients with bilateral adrenal lesions, a scenario that poses greater complexity and challenges in clinical practice. Our findings suggest that visual assessment of 68Ga-pentixafor PET/CT images and semiquantitative analysis of PET data may be sufficient to predict adrenalectomy outcomes in PA patients with bilateral adrenal lesions with high accuracy. Specifically, patients diagnosed with lateralized disease, characterized by unilateral positive or asymmetrically bilateral positive lesions, may benefit from the removal of the affected adrenal gland. Interestingly, unlike previous studies focused on identifying APAs, the application of semiquantitative analysis of 68Ga-pentixafor PET data to predict surgical outcomes may require a relatively higher SUVmax cut-off value. This discrepancy may be partially attributed to the complexity of predicting PA prognosis. In fact, surgical outcomes in PA patients are influenced not only by pathological classification but also by confounding factors such as primary hypertension and comorbidities like chronic renal failure, which needs further investigation [8, 35].
This study has several limitations. First, its retrospective nature and single-center design may have introduced selection bias and influenced the findings. Second, the limited sample size, resulting from our focus on bilateral adrenal lesions, might have led to insufficient statistical significance and biased outcomes. Third, although high-resolution adrenal CT was used to initially detect bilateral adrenal lesions, some microadenomas or smaller lesions might have been missed. Additionally, most tumor lesions in our study were ≥ 1 cm in diameter, which obstructed the analysis of the diagnostic efficacy of PET/CT for nodules smaller than 1 cm, potentially introducing uncertainty into our results. Importantly, since most patients declined the AVS test, we could not assess the potential of false negative results of 68Ga-pentixafor PET/CT, which may influence treatment decisions. Furthermore, the majority of treatment decisions in this study were based on 68Ga-pentixafor PET/CT without AVS, which could represent another potential source of bias. Therefore, we emphasize the need for a large-scale, prospective, randomized, controlled, multicenter trial to further validate the applicability of 68Ga-pentixafor PET/CT as an alternative to AVS in guiding surgical treatment for PA patients.
Conclusion
In summary, this retrospective study demonstrates that CXCR4-targeted 68Ga-pentixafor PET/CT can be effectively utilized for surgery decision-making in PA patients with bilateral adrenal lesions, providing a potential alternative to AVS for prognostic prediction. This study lays a solid foundation for future randomized controlled trials comparing 68Ga-pentixafor PET/CT with AVS in patients with PA and bilateral adrenal lesions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the MJ Language Editing Services (https://www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Author contributions
ZS has contributed to the data collection and analysis and was a major contributor in writing the manuscript. YH has contributed to the data collection and analysis, wrote the original draft, and provided funding. TL has contributed to the PET/CT imaging collection and analysis. MG and ZX have contributed to the data collection and AVS analysis. XF has contributed to immunohistochemical results evaluation and data collection. BL, BZ, YY and JC have assisted the follow-up data collection and imaging acquisition. TJ and XC have contributed to the revision of the article. KC has contributed to the conception of the study, assisted in the data analysis and the revision of the article. LL and YG have contributed to the conception of the study, the revision of the article, and funding acquisition. All authors read and approved the final manuscript.
Funding
This study was funded by National Natural Science Foundation of China (82273121), Hunan Natural Science Foundation (2022JJ20096, 2024JJ5612), and National Research Center for Clinical Medicine of Geriatric Diseases Clinical Research Fund (2022LNJJ12).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of the Xiangya Hospital, Central South University (2023111517). This article does not contain any experiments with animals. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Written informed consent was obtained from the patient for publication of this study and accompanying images.
Competing interests
The authors have no competing interests to disclose in relation with this article.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhiwei Shu and Yao He contributed equally to this work as co-first authors.
Contributor Information
Kai Cheng, Email: kai_cheng@csu.edu.cn.
Longfei Liu, Email: longfei_liu@csu.edu.cn.
Yu Gan, Email: ganyu@csu.edu.cn.
References
- 1.Choy KW, Fuller PJ, Russell G, Li Q, Leenaerts M, Yang J. Primary aldosteronism. BMJ (Clinical Res ed). 2022;377:e065250. [DOI] [PubMed] [Google Scholar]
- 2.Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50. [DOI] [PubMed] [Google Scholar]
- 3.Kempers MJ, Lenders JW, van Outheusden L, van der Wilt GJ, Schultze Kool LJ, Hermus AR, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151:329–37. [DOI] [PubMed] [Google Scholar]
- 4.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–916. [DOI] [PubMed] [Google Scholar]
- 5.Burrello J, Burrello A, Pieroni J, Sconfienza E, Forestiero V, Amongero M, et al. Prediction of hyperaldosteronism subtypes when adrenal vein sampling is unilaterally successful. Eur J Endocrinol. 2020;183:657–67. [DOI] [PubMed] [Google Scholar]
- 6.Rossi GP, Barisa M, Allolio B, Auchus RJ, Amar L, Cohen D, et al. The adrenal vein sampling international study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2012;97:1606–14. [DOI] [PubMed] [Google Scholar]
- 7.Rossi GP. Primary aldosteronism: JACC State-of-the-Art review. J Am Coll Cardiol. 2019;74:2799–811. [DOI] [PubMed] [Google Scholar]
- 8.Williams TA, Gong S, Tsurutani Y, Tezuka Y, Thuzar M, Burrello J, et al. Adrenal surgery for bilateral primary aldosteronism: an international retrospective cohort study. Lancet Diabetes Endocrinol. 2022;10:769–71. [DOI] [PubMed] [Google Scholar]
- 9.Heinze B, Fuss CT, Mulatero P, Beuschlein F, Reincke M, Mustafa M, et al. Targeting CXCR4 (CXC chemokine receptor type 4) for molecular imaging of Aldosterone-Producing adenoma. Hypertension. 2018;71:317–25. [DOI] [PubMed] [Google Scholar]
- 10.Ding J, Zhang Y, Wen J, Zhang H, Wang H, Luo Y, et al. Imaging CXCR4 expression in patients with suspected primary hyperaldosteronism. Eur J Nucl Med Mol Imaging. 2020;47:2656–65. [DOI] [PubMed] [Google Scholar]
- 11.Mayerhoefer ME, Raderer M, Weber M, Lamm W, Kiesewetter B, Hacker M, et al. 68Ga-Pentixafor PET/MRI for treatment response assessment in mantle cell lymphoma: comparison between changes in lesion CXCR4 expression on PET and lesion size and diffusivity on MRI. Clin Nucl Med. 2023;48:557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waheed A, Singh B, Watts A, Kaur H, Singh H, Dhingra K, et al. 68 Ga-Pentixafor PET/CT for in vivo imaging of CXCR4 receptors in glioma demonstrating a potential for response assessment to radiochemotherapy: preliminary results. Clin Nucl Med. 2024;49:e141–8. [DOI] [PubMed] [Google Scholar]
- 13.Watts A, Singh B, Basher R, Singh H, Bal A, Kapoor R, et al. 68Ga-Pentixafor PET/CT demonstrating higher CXCR4 density in small cell lung carcinoma than in non-small cell variant. Eur J Nucl Med Mol Imaging. 2017;44:909–10. [DOI] [PubMed] [Google Scholar]
- 14.Watts A, Singh B, Singh H, Bal A, Kaur H, Dhanota N, et al. [(68)Ga]Ga-Pentixafor PET/CT imaging for in vivo CXCR4 receptor mapping in different lung cancer histologic sub-types: correlation with quantitative receptors’ density by immunochemistry techniques. Eur J Nucl Med Mol Imaging. 2023;50:1216–27. [DOI] [PubMed] [Google Scholar]
- 15.Shekhawat AS, Singh B, Malhotra P, Watts A, Basher R, Kaur H, et al. Imaging CXCR4 receptors expression for staging multiple myeloma by using (68)Ga-Pentixafor PET/CT: comparison with (18)F-FDG PET/CT. Br J Radiol. 2022;95:20211272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Y, Ding J, Cui Y, Li T, Sun H, Zhao D, et al. Functional nodules in primary aldosteronism: identification of CXCR4 expression with (68)Ga-pentixafor PET/CT. Eur Radiol. 2023;33:996–1003. [DOI] [PubMed] [Google Scholar]
- 17.Hu J, Xu T, Shen H, Song Y, Yang J, Zhang A, et al. Accuracy of Gallium-68 pentixafor positron emission tomography-Computed tomography for subtyping diagnosis of primary aldosteronism. JAMA Netw Open. 2023;6:e2255609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding J, Tong A, Zhang Y, Wen J, Zhang H, Hacker M, et al. Functional characterization of adrenocortical masses in nononcologic patients using (68)Ga-Pentixafor. J Nucl Med. 2022;63:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding J, Li X, Liu S, Gao Y, Zheng G, Hacker M, et al. Clinical value of (68)Ga-Pentixafor PET/CT in subtype diagnosis of primary aldosteronism patients with adrenal micronodules. Journal of nuclear medicine: official publication. Soc Nuclear Med. 2024;65:117–24. [DOI] [PubMed] [Google Scholar]
- 20.Ding J, Tong A, Zhang Y, Wen J, Huo L. Intense 68Ga-Pentixafor activity in Aldosterone-Producing adrenal adenomas. Clin Nucl Med. 2020;45:336–9. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y, Long T, Peng N, Zhen M, Ye Q, Zhang Z, et al. The value of targeting CXCR4 with 68Ga-Pentixafor PET/CT for subtyping primary aldosteronism. J Clin Endocrinol Metab. 2023;109:171–82. [DOI] [PubMed] [Google Scholar]
- 22.Yin X, Ai K, Luo J, Liu W, Ma X, Zhou L, et al. A comparison of the performance of (68)Ga-Pentixafor PET/CT versus adrenal vein sampling for subtype diagnosis in primary aldosteronism. Front Endocrinol. 2024;15:1291775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi T, Lu D, Cui Y, Zhang Z, Yang X, Zhang J et al. (68)Ga-pentixafor PET/CT Is a Supplementary Method for Primary Aldosteronism Subtyping Compared with Adrenal Vein Sampling. Molecular imaging and biology. 2024;27:142–150. [DOI] [PMC free article] [PubMed]
- 24.Zuo R, Liu S, Ren X, Li W, Xia Z, Xu L, et al. Typing diagnostic value of (68)Ga-pentixafor PET/CT for patients with primary aldosteronism and unilateral nodules. Endocrine. 2025;87:314–24. [DOI] [PubMed] [Google Scholar]
- 25.Zuo R, Liu S, Li W, Xia Z, Xu L, Pang H. Clinical value of (68)Ga-pentixafor PET/CT in patients with primary aldosteronism and bilateral lesions: preliminary results of a single-centre study. EJNMMI Res. 2024;14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5:689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, Tian W, Zhang L, Zhang J, Zhou B. Assessing the quality of guidelines for primary aldosteronism: which guidelines are worth applying in diverse settings? J Hypertens. 2019;37:1500–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulatero P, Bertello C, Rossato D, Mengozzi G, Milan A, Garrone C, et al. Roles of clinical criteria, computed tomography scan, and adrenal vein sampling in differential diagnosis of primary aldosteronism subtypes. J Clin Endocrinol Metab. 2008;93:1366–71. [DOI] [PubMed] [Google Scholar]
- 29.Lim V, Guo Q, Grant CS, Thompson GB, Richards ML, Farley DR, et al. Accuracy of adrenal imaging and adrenal venous sampling in predicting surgical cure of primary aldosteronism. J Clin Endocrinol Metab. 2014;99:2712–9. [DOI] [PubMed] [Google Scholar]
- 30.Dekkers T, Prejbisz A, Kool LJS, Groenewoud H, Velema M, Spiering W, et al. Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol. 2016;4:739–46. [DOI] [PubMed] [Google Scholar]
- 31.Lee SE, Park SW, Choi MS, Kim G, Yoo JH, Ahn J, et al. Primary aldosteronism subtyping in the setting of partially successful adrenal vein sampling. Therapeutic Adv Endocrinol Metabolism. 2021;12:2042018821989239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Sousa K, Boulkroun S, Baron S, Nanba K, Wack M, Rainey WE, Genetic, Cellular, and Molecular Heterogeneity in Adrenals With Aldosterone-Producing Adenoma., Hypertension et al. Dallas, Tex: (1979). 2020;75:1034-44. [DOI] [PMC free article] [PubMed]
- 33.Monticone S, Castellano I, Versace K, Lucatello B, Veglio F, Gomez-Sanchez CE, et al. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol. 2015;411:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dekkers T, ter Meer M, Lenders JW, Hermus AR, Schultze Kool L, Langenhuijsen JF, et al. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? J Clin Endocrinol Metab. 2014;99:E1341–51. [DOI] [PubMed] [Google Scholar]
- 35.Burrello J, Burrello A, Stowasser M, Nishikawa T, Quinkler M, Prejbisz A, et al. The primary aldosteronism surgical outcome score for the prediction of clinical outcomes after adrenalectomy for unilateral primary aldosteronism. Ann Surg. 2020;272:1125–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.