Abstract
The unfolded protein response pathway is an evolutionarily conserved cytoprotective signaling cascade, essential for cell function and survival. Unfolded protein response signaling is tightly integrated with bone cell differentiation and function, and chronic unfolded protein response activation has been identified in bone disease. The unfolded protein response has been found to promote oncogenesis and drug resistance, raising the possibility that unfolded protein response modulators may have activity as anti-cancer agents. Cancer-associated bone disease remains a major cause of morbidity for patients with multiple myeloma or bone-metastatic disease. Understanding the critical role of unfolded protein response signaling in cancer development and metastasis, as well as its role in bone homeostasis, may lead to novel mechanisms by which to target cancer-associated bone disease. In this review, we summarize the current research delineating the roles of the unfolded protein response in bone biology and pathophysiology, and furthermore, review unfolded protein response modulating agents in the contexts of cancer and cancer-associated bone disease.
Subject terms: Bone cancer, Bone
Introduction
The endoplasmic reticulum (ER) is a distinct, membrane-bound organelle recognized for its role in protein synthesis, processing, and transport. As unfolded polypeptides enter the ER lumen, they undergo protein folding and disulfide bond formation via a multitude of enzymes and chaperone proteins.1 This ER microenvironment is specifically tailored for these processes, therefore physiological disturbances such as change in pH or lack of chaperone proteins, disrupts protein processing, increasing misfolded protein accumulation, ultimately inducing ER stress.2,3 To address this, the eukaryotic cell developed a cytoprotective signaling cascade termed the unfolded protein response (UPR) pathway.4 Upon sensing of ER stress, UPR signaling halts global protein translation while selectively promoting translation of ER chaperone proteins and removal processes such as ER-associated degradation (ERAD) and autophagy, to overcome misfolded protein loads.5 However, if UPR activation cannot restore proteostasis, the pro-apoptotic, terminal UPR signaling cascade is activated.
Due to the pro-survival role of the UPR, cancer cells commonly upregulate this signaling cascade in response to the hypoxic, nutrient deprived conditions of the tumor niche. This ultimately incurs a multitude of oncogenic benefits such as premetastatic niche formation, metastasis, and treatment resistance in the process.6–8 In fact, identification of elevated ER stress proteins in tumors corresponds with poor prognosis and adverse treatment outcomes in osteosarcoma (OS), multiple myeloma (MM), breast, prostate, and kidney cancer, all of which are cancers localized to (or which commonly metastasize to) the bone.9–15 The incidence of primary or metastatic bone cancer is relatively low (suspected to be about 5% of all cancer cases) with ~35 000 people in the United States dying each year with bone metastases.16 Whether bone-derived [OS, Ewing sarcoma (ES)] or bone-metastasizing, cancer cells drastically change the bone marrow microenvironment to promote their selective growth and survival, ultimately disrupting other essential processes occurring in the medullar cavity, such as bone remodeling.17
The homeostatic process of bone remodeling which occurs throughout adulthood is a tightly regulated process mediated by osteoclast bone-resorbing, osteoblast bone-forming, and osteocyte mechanosensory cells. Disruption of the remodeling cycle can result in bone loss or increased bone density, however in both cases the bone is brittle and can easily fracture. As a result, skeletal structure and integrity is compromised, significantly contributing to disease morbidity and negatively impacting overall patient survival in cancer-induced bone disease.18,19 The current treatment options for cancer-induced bone disease consist primarily of anti-osteoclastic agents such as nitrogen bisphosphonates (NBP) or tumor necrosis factor (TNF) superfamily member 11 (TNFSF11, also known as RANKL) monoclonal antibody denosumab.20,21 However, while these therapies show efficacy in reducing patient risk of further skeletal related events (SRE), whether they exhibit direct anti-neoplastic benefits has not been fully established.20,22,23 In recent years, chronic UPR activation has been identified in bone disease due to UPR signaling being tightly integrated with bone cell differentiation and function. Understanding the critical role of UPR signaling in cancer development and metastasis, as well as its role in bone homeostasis, suggests potential application for UPR-modulating therapeutics in cancer-associated bone disease. In this review, we summarize the current findings supporting the role of the UPR in bone biology and pathophysiology. Furthermore, we review UPR modulating agents, either FDA approved or currently undergoing clinical trial investigation, and discuss their dual anti-neoplastic and bone-related therapeutic potential.
The UPR
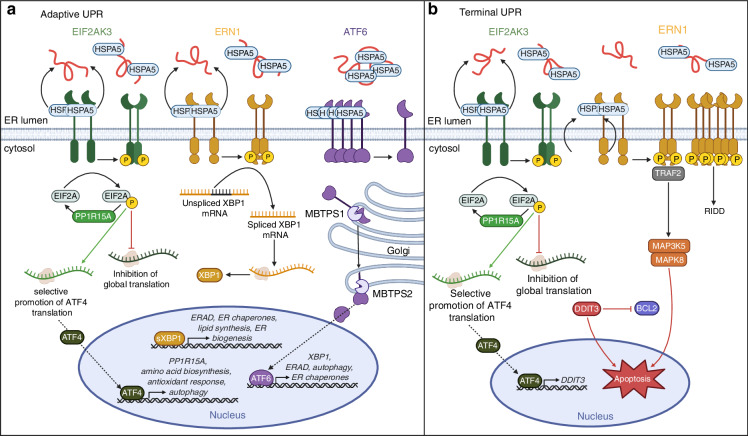
The mammalian UPR consists of three, distinct signaling cascades, mediated by ER transmembrane proteins: eukaryotic translation initiation factor 2 alpha kinase 3 (EIF2AK3, also known as PERK), activating transcription factor 6 (ATF6), and ER to nucleus signaling 1 (ERN1, also known as IRE1) (Fig. 1a).24 Under basal conditions, ER chaperone, heat shock protein family (HSP70) member 5 (HSPA5, also known as GRP78 or BiP), occupies the luminal domains of EIF2AK3, ATF6, and ERN1, respectively, sterically hindering their activation. In the presence of misfolded proteins, HSPA5 dissociates allowing for receptor homodimerization and autophosphorylation [i.e., EIF2AK3, ERN1 or translocation to the Golgi (i.e., ATF6) for proteolytic processing]. EIF2AK3 activation initiates phosphorylation of eukaryotic translation initiation factor 2A (EIF2A), inhibiting global protein translation excluding activating transcription factor 4 (ATF4).25 ATF4 then activates downstream processes such as the antioxidant response, amino acid biosynthesis, and autophagy to ameliorate ER stress.26 ATF4 also induces expression of protein phosphatase 1 regulatory subunit 15A (PP1R15A, also known as GADD34) which promotes the dephosphorylation of phosphorylated EIF2A (p-EIF2A), attenuating ATF4 expression, and restoring protein synthesis.27 Alternatively, ERN1 activation mediates splicing of X-box binding protein 1 (XBP1) mRNA, yielding the active XBP1 splice variant (sXBP1). Active sXBP1 then translocates to the nucleus where it initiates transcription of genes involved in protein folding, secretion, and degradation.28 The ATF6 arm of the UPR is unique in that HSPA5 dissociation allows for translocation of ATF6 to the Golgi for proteolytic processing by both membrane bound transcription factor peptidase, site-1 (MBTPS1) and site-2 (MBTPS2). Transcriptionally active ATF6 then translocates to the nucleus where it stimulates expression of XBP1 along with other ER chaperones and reinforces ERAD and autophagic removal of misfolded proteins.29
Fig. 1.
Signaling cascades of the UPR. a Adaptive UPR signaling consists of three major signaling cascades (EIF2AK3, ERN1, ATF6) and prioritizes absolution of ER stress in order to restore normal protein processing and cell function. EIF2AK3 is activated upon HSPA5 dissociation and binding of misfolded proteins in the ER lumen, promoting receptor dimerization and autophosphorylation. Activated EIF2AK3 phosphorylates EIF2A leading to global inhibition of protein translation with the exception of selective translation of ATF4 mRNA. ATF4 then mediates transcription of UPR target genes to promote amino acid biosynthesis, the antioxidant response, and autophagy to relieve ER stress. ATF4 also promotes transcription of phosphatase PP1R15A to dephosphorylate EIF2A and restore normal protein translation. HSPA5 dissociation from ERN1 leads to receptor dimerization and autophosphorylation. Activated ERN1 RNase splices XBP1 mRNA with translation generating transcriptionally active sXBP1. sXBP1 promotes transcription of genes associated with ERAD, lipid synthesis, ER biogenesis and ER chaperone expression in attempt to alleviate ER stress. Dissociation of HSPA5 from ATF6 allows for receptor translocation to the Golgi apparatus where it is proteolytically cleaved by MBTPS1 and MBTPS2 yielding its active transcriptional form. Transcriptionally active ATF6 then translocates to the nucleus where it promotes continuation of UPR signaling via induction of XBP1, ERAD, autophagy, and ER chaperone expression. b Terminal UPR, which is activated in response to chronic, prolonged ER stress. Persistent EIF2AK3-EIF2A translation of ATF4 activates expression of pro-apoptotic protein DDIT3 which inhibits BCL2, allowing for induction of apoptosis. Prolonged ERN1 activation can promote interactions with adaptor protein TRAF2 mediating downstream MAP3K5 and MAPK8-mediated apoptosis. Prolonged phosphorylation also favors higher order assembly of ERN1 oligomers, preventing XBP1 splicing and alternatively promoting regulated ERN1-dependent decay (RIDD). (Abbreviations: EIF2AK3 eukaryotic translation initiation factor 2 alpha kinase 3, ATF6 activating transcription factor 6, ERN1 ER to nucleus signaling 1, HSP70 HSPA5 heat shock protein family A member 5, EIF2A eukaryotic translation initiation factor 2 subunit alpha, p-EIF2A phosphorylated EIF2A, ATF4 activating transcription factor 4, XBP1 X-box-binding protein-1, sXBP1 spliced XBP1, MBTPS1 membrane bound transcription factor peptidase, site 1, MBTPS2 membrane bound transcription factor peptidase, site 2, DDIT3 DNA damage inducible transcript 3, Bcl-2 B-cell lymphoma 2, TRAF2 TNF receptor associate factor 2, MAP3K5 mitogen-activated protein kinase kinase kinase 5, MAPK8 mitogen-activated protein kinase 8)
These signaling cascades mediate the adaptive UPR, where pathway activation is focused on survival (Fig. 1a). However, when the adaptive signaling cascade cannot restore normal protein processing and folding, causing irremediable ER stress, terminal UPR signaling is activated (Fig. 1b). The terminal UPR is primarily mediated by ERN1 and EIF2AK3 signaling axes.30 Sustained EIF2AK3 activation leads to ATF4-mediated expression of pro-apoptotic transcription factor DNA damage-inducible transcript 3 (DDIT3, also known as CHOP) which inhibits anti-apoptotic protein B-cell lymphoma-2 (BCL2) for induction of BCL2 like 11 (BCL2L11) and apoptosis.31 Alternatively, prolonged ERN1 phosphorylation promotes higher order assembly of oligomers, attenuating XBP1 splicing, and alternatively favoring regulated ERN1-dependent decay (RIDD) for selective degradation of mRNA substrates.32 This leads to a downregulation in XBP1-mediated expression of ER chaperones, dampening the adaptive pro-survival axis. Furthermore, prolonged activation of ERN1 can promote assembly of adaptor protein TNF receptor associate factor 2 (TRAF2) with mitogen-activated protein kinase (MAPK) kinase kinase 5 (MAP3K5) activating downstream MAPK8-mediated apoptosis (Fig. 1b).33,34
Genetic evidence for the UPR in skeletal disease
Chronic ER stress and resulting UPR-induced apoptosis is associated with a multitude of human diseases such as neurodegeneration, diabetes and cancer.35–39 Loss of UPR signaling components is associated with a variety of human skeletal dysplasias, therefore suggesting a functional role for UPR signaling in bone cell homeostasis, and insinuating a role for ER stress in bone disease.4,40–43 Of note, mutations in UPR proteins EIF2AK3, ATF4, and ATF6-like cAMP responsive element binding (CREB) transcription factors, have been shown to affect chondrocyte growth and overall bone health. However, due to this review highlighting the application of UPR treatment in adult bone remodeling, which does not directly involve chondrocytes, effects in chondrocytes will not be discussed. The following publications are recommended for more information on the UPR in chondrocytes.40,44–46
Wolcott-Rallison syndrome (WRS) is a rare, autosomal recessive disorder resulting from mutation in EIF2AK3. While the specific mutational event varies across families, these patients lack either partial or complete EIF2AK3 catalytic activity resulting in neonatal or early infancy type 1 diabetes, epiphyseal dysplasia, growth retardation, and osteoporosis.47–49 Liu et al. conducted a study analyzing how different single nucleotide polymorphisms (SNP) in the EIF2AK3 gene impact bone mineral density (BMD) in two distinct populations. They discovered one nonsynonymous SNP to have borderline associations with decreased forearm BMD in both cohorts, and functional effects were revealed to be haplotype-specific with low-BMD associated with elevated levels of EIF2A, overall indicating a potential connection between BMD and EIF2AK3 functionality.
Coffin-Lowry Syndrome (CLS) is an X-lined condition characterized by severe intellectual disability, delayed development, craniofacial and skeletal abnormalities.50,51 CLS is caused by a mutation in RPS6KA3, encoding ribosomal S6 kinase A3, which is known to activate ATF4.52 In normal osteoblasts RPS6KA3 is thought to phosphorylate ATF4, inducing osteoblast specific gene expression and posttranslational regulation of type I collagen synthesis.52 Therefore, UPR dysfunction may contribute to the skeletal defects seen in CLS as theorized by lack of RPS6KA3 function.
Mutations in other UPR pathway components such as MBTPS1 and MBTPS2 are also associated with skeletal abnormalities. MBTPS1 and MBTPS2 mediate the cleavage and activation of various ER bound transcription factors like ATF6.53 An X-linked recessive form of osteogenesis imperfecta (OI), also referred to as brittle bone disease, has been attributed to defects in MBTPS2.54 Individuals with this mutation experience moderate to severe OI due to impaired MBTPS2-regulated intramembrane proteolysis of transcription factors ATF6, cAMP responsive element binding protein 3 like 1 (CREB3L1, also known as OASIS), and sterol regulatory element binding protein transcription factor 1 (SREBF1).54 Furthermore, multiple severe recessive forms of OI are associated with a mutations in CREB3L1, and ER stress inducible gene, cystine rich with EGF like domains 2 (CRELD2), is implicated in the pathogenesis of many skeletal dysplasias.55,56
Altogether, these genetic aberrations and resulting skeletal phenotypes suggest a critical role for UPR signaling in bone biology and begin to clarify its potential as a therapeutic target.
The cycle of bone remodeling
Bone physiology and structure is constantly adapting throughout development with “bone modeling” (marrow formation and bone elongation) occurring during embryonic development, adolescence, and early adulthood, and “bone remodeling” (focal bone maintenance) occurring throughout the remainder of adulthood.57 The bone remodeling cycle consists of the characteristic “basic multicellular unit” (BMU) comprising osteoclasts, osteoblasts, osteocytes, and designated precursors.58 Various stimuli can activate the cycle of bone remodeling with the main initiators being mechanical stress, hormone secretion, and/or lack of bioavailable calcium or vitamin D. The cycle initiates when stimuli trigger expression of osteoclast differentiation factor TNFSF11 from osteocyte and/or osteoblast cells.59,60 TNFSF11 binds TNF receptor superfamily member 11a (TNFRSF11A, also known as RANK) on pre-osteoclast cells, promoting precursor migration and fusion.61 Osteoclast arrival and binding to the indicated bone site initiates sealing zone formation and acidification of the resorptive compartment.62–64 As old bone is resorbed, growth factors are released from the matrix which bind receptors on tissue-resident, mesenchymal type cells, referred to as skeletal stem cells (SSC), stimulating commitment to the osteoblast lineage.65 Fully differentiated osteoblasts then secrete new osteoid and promote matrix mineralization while dually signaling for osteoclast apoptosis.66,67 Once all osteoclasts have been removed and the osteoblasts have secreted sufficient new osteoid, Osteoblasts either embed themselves into the bone matrix becoming terminally differentiated osteocytes or become quiescent bone-lining cells (BLC), awaiting reactivation of the next bone remodeling cycle.68
The foundation for physiological bone maintenance is the controlled balance between bone resorption and bone formation, which starts with the number of mature osteoclast and osteoblast cells. Generation of these highly specialized cell types from their precursors requires a multitude of intracellular signaling cascades which have been elegantly described in a various publications.69–75 For the purpose of this review, we will briefly describe the main signaling cascades involved in osteoclast and osteoblast differentiation and function, which will later be re-examined with a UPR-integrated perspective.
Osteoclast differentiation
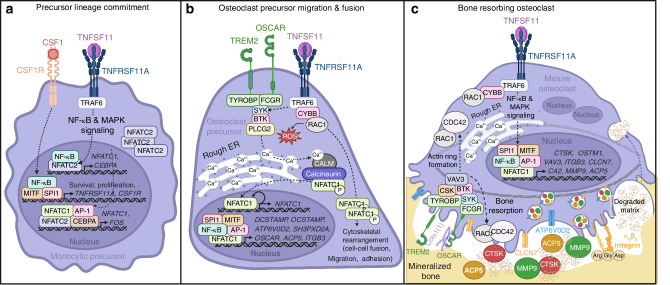
Osteoclasts are derived from either erythromyeloid progenitors from the yolk sac (fetal osteoclasts—bone modeling) or circulating monocytes and bone marrow resident myeloid precursors (adult osteoclasts—bone remodeling).76,77 Differentiation is initiated upon binding of colony stimulating factor 1 (CSF1), produced primarily by MSCs and mature osteoblasts, to macrophage colony stimulating factor 1 receptor (CSF1R) on osteoclast precursors (Fig. 2a).78,79 Downstream CSF1 signaling promotes precursor survival and proliferation through activation of transcription factors Spi-1 proto-oncogene (SPI1, also known as PU.1) and melanocyte inducing transcription factor (MITF), in addition to surface expression of TNFRSF11A and CSF1R.80,81 Binding of TNFSF11, expressed either in its membrane-bound or free form, to TNFRSF11A on differentiating precursors recruits TNF receptor associated factor 6 (TRAF6) and mediates nuclear factor kappa B (NF-κB) and MAPK signaling.79,82,83 Active NF-κB localizes to the promoter of nuclear factor of activated T cells 1 (NFATC1) where it cooperates with transcription factor NFATC2 to induce NFATC1 expression.84,85 CCAAT/enhancer binding protein alpha (CEBPA), also thought to be a target of NF-κB signaling, promotes expression of essential osteoclast transcription factor, FOS.86,87 FOS forms a dimeric transcriptional complex with JUN family proteins creating activator protein 1 (AP-1) which cooperates with CEBPA to induce NFATC1 expression (Fig. 2a).69,86
Fig. 2.
Signaling cascades during osteoclast differentiation. a CSF1 binds CSF1R on monocytic precursors activating transcription factors MITF and SPI1, promoting precursor survival and proliferation, and expression of TNFSF11 receptor (TNFRSF11A) and CSF1R. Surface expression of TNFRSF11A allows for TNFSF11 binding and activation of TRAF6 mediated NF-κB and MAPK signaling. Active NF-κB translocates to the nucleus where it promotes CEBPA expression and binds the NFATC1 promoter where it cooperates with NFATC2 to induce NFATC1 expression. CEBPA promotes expression of FOS which dimerizes with JUN to form active transcriptional complex AP-1. CEBPA and AP-1 in combination with NF-κB, NFATC2, and NFATC1 promote continued expression of FOS and NFATC1. b During TNFSF11-TNFRSF11A signaling, TRAF6 interacts with CYBB and RAC1 generating ROS and promoting cytoskeletal rearrangement necessary for precursor cell fusion, migration, and adhesion. TNFRSF11A signaling also mediates phosphorylation of adaptor proteins TYROBP and FCRG which activate downstream kinases, SYK, BTK, and PLCG2, triggering ER calcium release and activation of calmodulin (CALM)-dependent phosphatase calcineurin. Active calcineurin in turn dephosphorylates cytosolic NFATC1, allowing its translocation to the nucleus where it cooperates with transcription factors SPI1, MITF, AP-1, and NF-κB to promote osteoclast-specific gene expression. Additionally, NFATC1 binds its own promoter auto-amplifying its expression. c TNFRSF11A signaling continues to promote expression of proteins necessary for osteoclast resorption via NF-κB and MAPK signaling. αVβ3 integrin or TREM2/OSCAR can bind RGD sequences on ECM proteins in the bone matrix initiating the resorptive signaling cascade mediated by TYROBP, CSK, SYK, RAC1, and CDC42 to regulate actin ring formation and bone resorption. Endosomal trafficking mediates acidification of the sealing zone via transport of proton pump ATP6V0D2 and chloride channel CLCN7 and release of proteases such as CTSK, MMP9, and ACP5. Degraded bone matrix is removed via transcytosis and released into the local microenvironment. (Abbreviations: CSF1 colony stimulating factor 1, CSF1R CSF1 receptor, TNFRSF11A tumor necrosis factor receptor family member 11A, TNFSF11 tumor necrosis factor soluble factor 11, MITF melanocyte-inducing transcription factor, TRAF6 tumor necrosis factor receptor-associated factor 6, MAPK mitogen-activated protein kinase, NFATC1 nuclear factor of activated T cells 1, CEBPA CCAAT enhancer binding protein alpha, AP-1 activator protein 1, TREM2 triggering receptor expressed on myeloid cells 2, OSCAR osteoclast-associated Ig-like receptor, TYROBP transmembrane immune signaling adaptor, FCRG Fc gamma receptor, SYK spleen associated tyrosine kinase, BTK Bruton’s tyrosine kinase, PLCG2 phospholipase C gamma 2, CALM calmodulin, CYBB cytochrome b-245 beta chain, ROS reactive oxygen species, DCSTAMP dendrocyte expressed seven transmembrane protein, OCSTAMP osteoclast stimulatory transmembrane protein, ATP6V0D2 ATPase H+ transporter V0 subunit d2, SH3PXD2A SH3 and PX domains 2A, ITGB3 integrin subunit beta 3, CLCN7 chloride voltage-gated channel 7, OSTM1 osteoclastogenesis associated transmembrane protein1, CTSK cathepsin K, ACP5 tartrate-resistant acid phosphatase 5, VAV3 vav guanine nucleotide exchange factor 3, CA2 carbonic anhydrase 2, MMP9 matrix metallopeptidase 9
As myeloid lineage cells, differentiating osteoclasts express immunoglobulin-like receptors, triggering receptor expressed on myeloid cells 2 (TREM2) and osteoclast-associated Ig-like receptor (OSCAR), with associated transmembrane immune signaling adaptor (TYROBP) and Fc gamma receptor (FCGR), respectively (Fig. 2b).88 TYROBP and FCRG contain immunoreceptor tyrosine-based activation motifs (ITAM) which mediate phosphorylation of spleen associated tyrosine kinase (SYK), Bruton’s tyrosine kinase (BTK), and phospholipase C gamma 2 (PLCG2) in cooperation with TNFRSF11A signaling.89 Phosphorylation of PLCG2 triggers intracellular calcium release, which then binds calcium-dependent kinase, calmodulin, which activates the downstream phosphatase calcineurin.90 Active calcineurin can then dephosphorylate cytosolic inactive NFATC1 therefore increasing levels of transcriptionally active protein.90 ER calcium release is also stimulated by cytochrome b-245 beta chain (CYBB, also known as NOX2) and cytosolic component RAC1, association with TRAF6.91–93 CYBB and Rac1 promote formation of reactive oxygen species (ROS) which stimulates ER calcium release, further promoting NFATC1 activation.94 NFATC1 cooperates with SPI1, MITF, and AP-1 to foster expression of osteoclast-specific genes to mediate precursor fusion (i.e., dendrocyte expressed seven transmembrane protein (DCSTAMP), osteoclast stimulatory transmembrane protein (OCSTAMP), ATPase H+ transporter V0 subunit d2 (ATP6V0D2), SH3 and PX domains 2A (SH3PXD2A), OSCAR, integrin subunit beta 3 (ITGB3) and bone resorption (i.e., chloride voltage-gated channel 7 (CLCN7), osteoclastogenesis associated transmembrane protein1 (OSTM1), cathepsin K (CTSK), tartrate-resistant acid phosphatase 5 (ACP5), vav guanine nucleotide exchange factor 3 (VAV3), carbonic anhydrase 2 (CA2), and matrix metallopeptidase 9 (MMP9)) (Fig. 2b).95–102
Once all necessary structural and functional components are present, αVβ3 integrin expressing osteoclasts can adhere to the bone surface initiating intracellular organization of podosome clusters and formation of the actin ring (Fig. 2c). Integrin engagement of Arg-Gly-Asp (RGD) sequences in ECM proteins (i.e., fibronectin, vitronectin, and osteopontin) activates the resorptive signaling cascade via TYROBP, nonreceptor tyrosine kinase SRC, SYK and downstream activation of RAC1 and CDC42.103–106 RAC1 and CDC42, Rho family small GTPases, regulate cytoskeletal rearrangement such as actin ring formation and apposition to the bone surface, in addition to facilitating resorptive processes at the ruffled boarder.62 Furthermore, transcytosis of degraded bone products mediates local release of growth factors, and endosomal trafficking of transporters and resorptive enzymes to the basolateral membrane facilitates sealing zone acidification and bone resorption (Fig. 2c).
Osteoblast differentiation
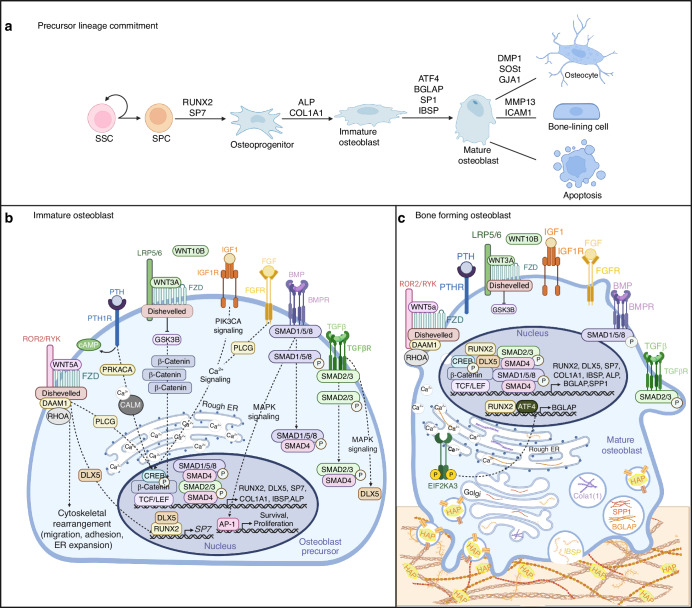
Osteoblasts are derived from resident SSCs and multipotent progenitors (SSPC) which can differentiate into myoblasts, chrondrocytes, adipocytes, or osteoblasts.107,108 Early signaling events which favor osteoblast lineage commitment initiate the de-repression of runt-related transcription factor-2 (RUNX2) and downstream target osterix (SP7) (Fig. 3a).109–114 RUNX2+ and SP7+ osteoprogenitors continue to proliferate while promoting expression of collagen type 1 alpha chain 1 (COL1A1) and alkaline phosphatase (ALP) (Fig. 3a, b).115 At the end of this stage, immature osteoblasts are highly expressing ALP and COL1A1, and their proliferative potential is limited (Fig. 3b). RUNX2 induces expression of ATF4 and immature osteoblasts then begin to promote intracellular processing and secretion of bone matrix proteins, signaling the transition to osteoblast maturity (Fig. 3c).52,115 ATF4 interacts with RUNX2 and promotes expression of target genes bone gamma-carboxyglutamate protein (BGLAP, also known as osteocalcin) resulting in mature osteoblasts expressing secreted phosphoprotein 1 (SP1, also known as osteopontin), integrin binding sialoprotein (IBSP), ALP, and COL1A1. As osteoblasts lay down new osteoid, they dually mediate matrix maturation through secretion of matrix vesicles containing various transporters and enzymes that promote utilization of phosphate and calcium for matrix mineralization (Fig. 3c).116 From this point, terminally differentiated osteoblasts can either undergo apoptosis, become BLCs, or embed themselves into the bone matrix as terminally differentiated osteocytes. The specific molecular pathways which mediate the osteoblast to osteocyte or BLC transition are less understood, however elevated expression of dentin matrix acidic phosphoprotein 1, sclerostin, and gap junction protein alpha 1 (GJA1, also known as connexin 43) is characteristic of osteocytes while intercellular adhesion molecule 1 (ICAM1) and matrix metallopeptidase 13 (MMP13) expression is specific to BLCs (Fig. 3a).117–119
Fig. 3.
Signaling cascades during osteoblast differentiation. a Stepwise changes in gene expression throughout osteoblast lineage commitment and differentiation. b Multiple signaling cascades mediate the continued differentiation of immature osteoblast cells. TGFβ binds TGFβR and mediates RUNX2 and SP7 expression via induction of SMAD2/3 and SMAD4 transcriptional activation, and MAPK-mediated induction of DLX5. BMPs mediate precursor survival and proliferation via MAPK activation of AP-1 and promote osteoblast-specific gene expression via SMAD1/5/8 interactions with SMAD4. Canonical WNT signaling is activated by WNT3A or WNT10B binding FZD receptor allowing for binding to co-receptor LRP5/6. Binding inhibits GSK3B kinase activity, allowing for β-catenin to translocate to the nucleus where it interacts with TCF/LEF transcription factors to promote RUNX2 expression. Non-canonical WNT signaling is mediated by WNT5A binding FZD and interacting with co-receptor ROR2/RYK. Adaptor protein disheveled activates DAAM1 and RHOA mediating precursor migration, adhesion and ER expansion while also promoting DLX5-mediated induction of SP7 via MAPK signaling. PTH binding to PTH1R promotes production of cAMP, activating PRKACA, inducing calcium oscillation and activating transcription factor CREB to promote osteoblast-specific gene expression. Binding of growth factors IGF1 and FGF to cognate receptors activate PIK3CA signaling to induce PLCG2, further promoting calcium signaling and CREB mediated osteoblast gene expression. c Mature osteoblasts continue to promote osteoblast-specific gene expression via BMP, TGFβ, WNT, PTH, and growth factor signaling. RUNX2 and EIF2AK3 activation mediate induction of ATF4 which promotes expression of BGLAP. Mature osteoblasts begin to secrete matrix proteins such as BGLAP, SP1, IBSP, and COL1A1 to facilitate new bone formation, and secrete vesicles containing HAP to promote matrix maturation. (Abbreviations: SSC skeletal stem cell, SPC skeletal progenitor cell, RUNX2 runt-related transcription factor-2, SP7 Sp7 transcription factor, DMP1 dentin matrix acidic phosphoprotein 1, SOST sclerostin, GJA1 gap junction protein alpha 1, MMP13 matrix metallopeptidase 13, ICAM1 intracellular adhesion molecule 1, TGFβ transforming growth factor β, TGFβR TGFβ receptor, MAPK mitogen-activated protein kinase, DLX5 distal-less homeobox 5, BMP bone morphogenic protein, BMPR BMP receptor, AP-1 activator protein 1, LRP5/6 low-density lipoprotein receptor-related protein 5 or 6, FZD frizzled, TCF/LEF T cell factor/lymphoid enhancer factor, GSK3B glycogen synthase kinase 3 beta, FGF fibroblast growth factor, FGFR FGF receptor, IGF1 insulin-like growth factor 1, IGF1R IGF1 receptor, PIK3CA phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha, PLCG phospholipase C gamma, PTH parathyroid hormone, PTH1R PTH1 receptor, PRKACA protein kinase cAMP-activated catalytic subunit alpha, CREB cAMP-response element binding protein, CALM calmodulin, ROR2/RYK receptor tyrosine kinase of the ROR-2 and Ryk families, DAAM1 disheveled associated activator of morphogenesis 1, EIF2AK3 eukaryotic initiation factor 2 alpha kinase 3, ATF4 activating transcription factor 4, BGLAP bone gamma-carboxyglutamate protein, SP1 secreted phosphoprotein 1, IBSP integrin binding sialoprotein, COL1A1 collagen type 1 alpha 1 chain, HAP hydroxyapatite)
Other growth factors and cytokines, either circulatory or from the local microenvironment, further promote RUNX2, SP7, and other osteoblast-specific genes to promote differentiation (Fig. 3b, c). Fibroblast growth factor (FGF) and insulin like growth factor 1 (IGF1) bind receptor tyrosine kinases on osteoblast precursors and initiate MAPK, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), and PLCG2 signaling cascades to promote proliferation, survival, and continued RUNX2 expression.120,121 Bone morphogenic proteins 2, 4, 7 (BMP) or transforming growth factor β (TGFβ), bind cognate receptors on osteoblast precursors and initiate SMAD1/5/8 or SMAD2/3 signaling respectively, which cooperate with SMAD4 and promote expression of RUNX2 and SP7.109,122 BMP signaling also regulates osteogenesis through a SMAD-independent mechanism, in which transcription factor, distal-less homeobox 5 (DLX5) mediates RUNX2 and SP7 expression through MAPK signaling.123,124 Parathyroid hormone (PTH), known to exhibit anabolic effects on the skeleton, binds PTH-1 receptor (PTH1R) on pre-osteoblasts activating heterotrimeric G protein stimulation, production of cAMP, induction of protein kinase cAMP-activated catalytic subunit alpha (PRKACA), calcium oscillation, and activating transcription factor cAMP-response element binding protein (CREB) to promote osteoblast-specific gene expression.125 Interestingly, PTH binding to PTH1R can form a signaling complex with WNT pathway protein low-density lipoprotein receptor related protein 5 or 6 (LRP5/6), activating canonical WNT signaling in the absence of a WNT ligand.126,127 Alternatively, canonical WNT signaling is activated primarily by ligands WNT3A or WNT10B with noncanonical signaling induced by WNT5A.128,129 Canonical signaling follows WNT binding receptor frizzled (FZD) and co-receptor LRP5/6 on pre-osteoblasts, promoting β-catenin translocation to the nucleus where interactions with transcription factors T cell factor/lymphoid enhancer factor (TCF/LEF) increase RUNX2 levels to inhibit adipogenesis (i.e., CEBPA, peroxisome proliferator activated receptor gamma (PPARG)).126,130–132 Alternatively, non-canonical WNT signaling promotes calcium oscillation to induce SP7 expression, in addition to activating RHOA for cytoskeletal rearrangement and MAPK8 signaling to promote DLX5 expression (Fig. 3b, c).133,134
Osteoblasts and the UPR
Osteoblasts rely on intracellular secretory processes to facilitate collagen production and secretion. As a result, differentiating osteoblasts require ER expansion and upregulation of UPR proteins to proactively mitigate proteotoxic stress. Skeletal dysplasias such as osteogenesis imperfecta (OI), characterized by mutations in the type I collagen gene, display abnormal osteoblasts possessing misfolded collagen proteins indicative of chronic ER stress. Due to accumulation of mutated collagen and inability to restore normal collagen folding, ER stress may mediate osteoblast apoptosis seen in OI and other osteoblast-related pathologies.135
The critical role of EIF2AK3 in osteoblast biology was confirmed by global knockout of EIF2AK3 (EIF2AK3−/−) in mice resulting in a similar phenotype to that in WRS, such as growth retardation and severe osteopenia.136–139 Specifically, osteoblast defects were determined to result from impaired collagen processing and secretion, and decreased mineralization capacity.137 Expression of genes indicating osteoblast maturity such as ALP, COL1A1, BGLAP, and IBSP were also found to be reduced in the skeletal tissue of EIF2AK3−/− mice.136 This could be due to loss of PTH-mediated ATF4 expression and promotion of osteogenesis. Systemic knockout of EIF2AK3 downstream target ATF4−/− or its regulatory kinase, RPS6KA3−/−, results in severe osteopenia and phenotypes similar to WRS and CLS.52,139 With phenotypes of ATF4−/−, RPS6KA3−/−, and EIF2AK3 −/− mice suggesting a functional role of EIF2AK3/ATF4 signaling in bone development, it is important to note that secondary effects on bone due to gene inactivation in non-skeletal tissues cannot be excluded. Evidence that ATF4 is at least partially dispensable for early SSPC differentiation led to the discovery that ATF4 and its downstream target RPS6KA3, post-transcriptionally mediate COLα1(1) synthesis and expression of IBSP and BGLAP, indicating a critical role in late-stage osteoblast differentiation and function.140 This is supported by in vitro data utilizing the small molecule salubrinal, which inhibits the dephosphorylation of EIF2A, allowing for prolonged EIF2A inhibition of protein translation and selective ATF4 expression. Studies looking at the effects of salubrinal treatment on MC3T3-E1 osteoblast-like cells showed increased ATF4 expression and mineralization capacity, indicating a potential bone-forming benefit.141 Further investigation revealed PTH induces ER stress activating HSP90-dependent EIF2AK3/EIF2A-mediated ATF4 expression.142,143 Furthermore, salubrinal was found to mediate osteoblast expression of autophagy marker ATG7, alleviating ER stress.144 Additional in vitro studies utilizing pro-osteogenic cytokine BMP2 found BMP2 induced mild ER stress and EIF2AK3-mediated ATF4 activation, ultimately regulating expression of osteoblast genes BGLAP and IBSP.139
Tohmonda et al. have extensively studied the role of ERN1/XBP1 signaling in osteoblast biology by utilizing immortalized mouse embryonic fibroblasts (mEF). Due to global ERN1 knockout being embryonically lethal, mEFs derived from ERN1−/− embryos were used.145 Utilizing BMP-2 to stimulate mEF differentiation, they found ERN1−/− mEFs failed to mature into osteoblasts, also seen with ERN1 and XBP1-siRNA-transfected mEFs.145 Through stimulation of ER stress, they found mEFs and MC3T3-E1 cells to induce SP7 expression in an XBP1-dependent and TRAF2-independent manner.145 Further investigation into ERN1/XBP1 pathway targets revealed BMP2 induced XBP1 cleavage which upregulated PTH1R expression on the osteoblast cell surface.146 However, this could indicate an indirect effect on osteoclast differentiation with PTH binding of PTH1R inducing osteoblast TNFSF11 expression. This was confirmed by findings from Iyer et al. which found that the ER stress inducer tunicamycin induced TNFSF11 expression in primary osteoblast cultures. Tunicamycin inhibits N-linked glycosylation in the ER, resulting in misfolded proteins and activation of all 3 UPR pathways in osteoblast cells via increased levels of p-EIF2A cleaved ATF6, and sXBP1.147 Administration of tunicamycin to adult mice caused ER enlargement in osteoblasts and osteocytes, indicating ER stress, as well as increased TNFSF11 expression and osteoclast number.147 However overall changes in bone mass were not reported.147 These studies suggest aberrant ER stress can promote a vicious osteolytic cycle through UPR-mediated induction of osteoblast TNFSF11 expression.
ATF6−/− mice with systemic inactivation of the protein, demonstrate no apparent defects, but the mice are more sensitive to chemically-induced ER stress compared to wild-type.29 Interestingly, ATF6 also contains the osteoblast-specific cis-acting element 2 (OSE2) motif, and through various loss and gain of function studies utilizing MC3T3-E1 cells, Jang et al. found ATF6 positively regulates BGLAP expression and matrix mineralization.148 However, ATF6−/− mice lacked an overt phenotype suggesting ATF6 as dispensable for bone development and maintenance.29 Global knockout of structurally homologous protein, CREB3L1−/−, resulted in osteoporotic mice exhibiting a similar phenotype to CREB3L1-deficiency in humans.149,150 Selective reintroduction of CREB3L1 into osteoblast cells of CREB3L1−/− mice restored bone density suggesting a direct regulatory role of CREB3L1 in osteoblast function.150 Importantly, osteoblasts of CREB3L1−/− mice exhibited enlarged rough ERs with accumulation of bone matrix proteins such as BGLAP and procollagen.149,150 Reintroduction of CREB3L1 rescued ER morphology suggesting CREB3L1 modulates osteoblast intracellular processing and secretion of bone matrix proteins.150 Furthermore, CREB3L1 was found to bind the osteoblast-specific COL1A1 promoter via a UPR element (UPRE)-like sequence, implicating CREB3L1 as a mediator of UPR-mediated COL1A1 expression and processing in osteoblast cells.149 Interestingly, cAMP response element binding protein 3 like 3 (CREB3L3), also structurally homologous to ATF6, negatively regulates BMP2-mediated osteogenic signaling, suggesting a role in preventing overactivation of osteogenesis (Fig. 4a).151
Fig. 4.
Integration of UPR signaling cascades in bone cell differentiation. a BMP-2 SMAD1/5/8 signaling and non-canonical WNT signaling promote ER stress and UPR pathway activation. EIF2AK3 activation induces ATF4 which promotes expression of SP7 and interacts with RPS6KA3 to post-transcriptionally regulate COLα1(1) processing. ERN1 splices XBP1 with sXBP1 promoting expression of SP7 and PTH1R. Activated ATF6 and CREB3L1 translocate to the Golgi where they are proteolytically processed to produce their active transcriptional form. In the nucleus, ATF6 promotes BGLAP expression and CREB3L1 promotes COL1A1 expression. CREB3L3 is also activated upon ER stress with its active transcriptional form negatively regulating osteoblast signaling. b TNFRSF11A-TNFSF11 signaling stimulates calcium signaling and ROS production which induce ER stress and UPR activation. ERN1 and EIF2AK3 activation mediates ATF4 and sXBP1 promotion of NFATC1 and other osteoclast-specific genes. ATF4 also mediates CSF1 induced surface expression of TNFRSF11A. Activated CREB3, CREB3L3, and CRELD2 translocate to the Golgi where they are proteolytically processed to produce their active transcriptional form. In the nucleus, CREB3L3 promotes NFATC1 expression and CREB3 promotes expression of other osteoclast-specific genes. Alternatively, CRELD2 negatively regulates osteoclast differentiation via inhibition of ER calcium release. (Abbreviations: EIF2AK3 eukaryotic initiation factor 2 alpha kinase 3, ERN1 ER to nucleus signaling 1, XBP1 X-box-binding protein-1, sXBP1 spliced XBP1, MBTPS1 membrane bound transcription factor peptidase, site 1, MBTPS2 membrane bound transcription factor peptidase, site 2, RUNX2 runt-related transcription factor-2, SP7 Sp7 transcription factor, TGFβ transforming growth factor β, TGFβR TGFβ receptor, MAPK mitogen-activated protein kinase, DLX5 distal-less homeobox 5, BMP bone morphogenic protein, BMPR BMP receptor, AP-1 activator protein 1, LRP5/6 low-density lipoprotein receptor-related protein 5 or 6, FZD frizzled, TCF/LEF T cell factor/lymphoid enhancer factor, GSK3B glycogen synthase kinase 3 beta, FGF fibroblast growth factor, FGFR FGF receptor, IGF1 insulin-like growth factor 1, IGF1R IGF1 receptor, PIK3CA phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha, PLCG phospholipase C gamma, PTH parathyroid hormone, PTH1R PTH1 receptor, PRKACA protein kinase cAMP-activated catalytic subunit alpha, CREB cAMP-response element binding protein, CALM calmodulin, ROR2/RYK receptor tyrosine kinase of the ROR-2 and Ryk families, DAAM1 disheveled associated activator of morphogenesis 1, ATF4 activating transcription factor 4, BGLAP bone gamma-carboxyglutamate protein, SP1 secreted phosphoprotein 1, IBSP integrin binding sialoprotein, COL1A1 collagen type 1 alpha 1 chain, RPS6KA3 ribosomal S6 kinase A3, CREB3L1 cAMP responsive element binding protein 3 like 1, CREB3L3 cAMP responsive element binding protein 3 like 3, CSF1 colony stimulating factor 1, CSF1R CSF1 receptor, TNFRSF11A tumor necrosis factor receptor family member 11A, TNFSF11 tumor necrosis factor soluble factor 11, MITF melanocyte-induced transcription factor, TRAF6 tumor necrosis factor receptor-associated factor 6, MAPK mitogen-activated protein kinase, NFATC1 nuclear factor of activated T cells 1, CEBPA CCAAT enhancer binding protein alpha, AP-1 activator protein 1, TREM2 triggering receptor expressed on myeloid cells 2, OSCAR osteoclast-associated Ig-like receptor, TYROBP transmembrane immune signaling adaptor, FCRG Fc gamma receptor, SYK spleen associated tyrosine kinase, BTK Bruton’s tyrosine kinase, PLCG2 phospholipase C gamma 2, CALM calmodulin, CYBB cytochrome b-245 beta chain, ROS reactive oxygen species, DCSTAMP dendrocyte expressed seven transmembrane protein, OCSTAMP osteoclast stimulatory transmembrane protein, ATP6V0D2 ATPase H+ transporter V0 subunit d2, SH3PXD2A SH3 and PX domains 2A, ITGB3 integrin subunit beta 3, CLCN7 chloride voltage-gated channel 7, OSTM1 osteoclastogenesis associated transmembrane protein1, CTSK cathepsin K, ACP5 tartrate-resistant acid phosphatase 5, VAV3 vav guanine nucleotide exchange factor 3, CA2 carbonic anhydrase 2, MMP9 matrix metallopeptidase 9, CREB3 cAMP responsive element binding protein 3, CRELD2 cystine rich with EGF like domains 2
Osteoclasts and the UPR
Having determined that ERN1/XBP1 signaling influences osteoblast biology, Tohmonda et al. confirmed an additional impact on osteoclast biology. UPR activation was found to occur during early osteoclastogenesis with TNFSF11-treated bone marrow-derived macrophages (BMM) exhibiting a transient increase in spliced XBP1 transcripts.152 This was confirmed to result from ERN1/XBP1 signaling and found it to be essential for osteoclastogenesis in vitro with siRNA silencing of ERN1 in wild-type (WT) BMMs found to diminish differentiation and resorption. To evaluate the role of ERN1 in vivo they generated ERN1flox/flox/Mx1-Cre mice (ERN1Mx1) which depleted ERN1 from the BMM cells. ERN1Mx1 mice exhibited significantly increased bone mass and decreased osteoclast numbers compared to WT.152 To confirm these effects were due to defective osteoclastogenesis, ERN1 transcript levels in ERN1Mx1 osteoblasts were evaluated and while a moderate reduction (~27%) was confirmed, no significant changes in osteoblast marker expression were evident. To further validate that decreased bone mass results from osteoclasts lacking ERN1, lethally irradiated WT mice were injected with ERN1Mx1 BMMs and maintained for 12 weeks. Mice reconstituted with ERN1Mx1 BMs exhibited a higher bone mass with decreased osteoclast number and resorption compared to mice reconstituted with WT BMMs.152 With luciferase reporter assays confirming potential XBP1 binding sites in the NFATC1 promoter, decreased osteoclastogenesis in vivo may be the result of decreased NFATC1 expression. To make the final connection between the UPR and osteoclastogenesis they determined TNFSF11-TNFRSF11A signaling to induce transient ER stress partially through oscillation of intracellular calcium levels promoting ERN1 endonuclease activity, XBP1 splicing, and NFATC1 expression.152
As previously mentioned, Wei et al. found global loss of EIF2AK3 significantly decreased osteoblast number and functional capacity, but effects on osteoclast number and functionality was not reported.136 However, it was reported that ACP5 and CTSK expression in the long bones of EIF2AK3 −/− mice was depleted by 55% compared to WT.136 ACP5 enzyme activity in the serum and bone extracts was also reduced and total bone protein levels of CTSK were depleted.136 Interestingly, impaired osteoclast activity in EIF2AK3 −/− mice was partially correlated to reduced bone TNFSF11 expression. In aggregate these studies suggest that overall bone loss in EIF2AK3−/− mice is due to reduction in bone formation and depletion of TNFSF11-expressing osteoblasts, suggesting reduced osteoblast-mediated activation of osteoclast differentiation and resorption. Therefore, whether reduced resorption was a direct result of EIF2AK3 loss in osteoclast cells is unclear. A direct role was confirmed with use of EIF2AK3 inhibitor GSK2606414 in vitro which revealed significant inhibitory effects on osteoclast differentiation and resorption through disruption of TNFSF11-induced MAPK and NF-κB signaling.153 Additionally, evidence of EIF2AK3 activation in fluoride-induced bone resorption suggests a potential role for EIF2AK3 in osteoclast activation.154
Cao et al. set out to establish whether ATF4 played a direct role in osteoclast biology utilizing global knockout ATF4 (ATF4−/−) mice.155 Initial evaluations found ATF4−/− mice exhibited decreased osteoclast number and ACP5 activity in tibiae compared to WT. Furthermore, analysis of BMMs from ATF4−/− mice in vitro revealed a dramatic reduction in differentiation potential and bone resorption. Utilizing the ACP5 promoter they generated an osteoclast-targeted transgenic mouse model where they overexpressed ATF4 (ACP5-ATF4-tg). ACP5-ATF4-tg mice displayed a severe osteopenic phenotype with a significant reduction in bone mass and trabecular number. Furthermore, evidence of significantly increased ACP5 activity and osteoclast number in ACP5-ATF4-tg tibiae compared to control confirmed that ATF4 overexpression increased osteoclast differentiation and resorption in vivo.155 Ultimately, ATF4 was determined to be a critical downstream target of CSF1-PIK3CA signaling during early osteoclast differentiation, and found to be required for CSF1 induction of TNFRSF11A expression in BMMs.155 Furthermore, ATF4 was associated with NFATC1 in a TNFSF11-dependent manner.155 However, in vitro studies looking at the effects of salubrinal, therefore prolonging ATF4 expression, on TNFSF11-stimulated osteoclastogenesis revealed an inhibitory effect on NFATC1 expression and RAC1 GTPase activity, suggesting a potential negative feedback loop mediated by ATF4.156–160 This inhibitory effect on osteoclastogenesis could explain why salubrinal treatment was found to ameliorate bone loss in models of OVX-induced, TNFSF11-induced, and disuse osteoporosis and even accelerated bone healing after injury in vivo.141,160–162
The role of ATF6 in osteoclastogenesis is less well known but seems to be mostly unaffected by UPR-induced osteoclastogenesis.163 Structurally homologous ER bound transcription factors CREB3L3, CREB3L1, and cAMP response element-binding protein 3 (CREB3 or LUMAN) have been suggested to play a role in UPR-mediated bone effects.164,165 CREB3 expression was found to be induced during osteoclastogenesis directly stimulating cell fusion through induction of DCSTAMP.166 However, CREB3 activation was determined ultimately independent of ER stress signaling in that CREB3 was only detectable upon TNFSF11 stimulation and not upon induction of physiological ER stress.166 Further investigation emphasized the role of calcium signaling and generation of reactive oxygen species (ROS) during osteoclast differentiation which may mediate ER stress and UPR activation. For example, ROS production and calcium flux during osteoclast differentiation were found to induce ERN1-mediated promotion of osteoclast markers, ACP5 and CTSK.167–169 Similarly, TNFSF11-induced ROS generation was found to activate CREB3L3 promotion of NFATC1 expression, with inhibition of ROS production using N-acetylcysteine (NAC) significantly reducing TNFSF11-induced CREB3L3 activation and NFATC1 expression.170 Furthermore, NADPH oxidase 4 (NOX4), known to mediate TNFSF11-induced autophagy and osteoclastogenesis, was found to activate EIF2AK3/EIF2A/ATF4 signaling.171 EIF2AK3 signaling activation was determined to result from elevated levels of non-mitochondrial ROS from TNFSF11-mediated induction of autophagy.171 Use of the selective ER calcium-dependent ATPase inhibitor thapsigargin, which depletes intracellular calcium stores activating UPR signaling, revealed thapsigargin to have a biphasic effect on osteoclastogenesis with mild stress levels promoting osteoclast signaling and drastic, prolonged calcium depletion leading to caspase-3 cleavage and apoptosis.168 Thapsigargin has been shown to upregulate EIF2A, EIF2AK3, HSPA5, and ERN1 during osteoclast differentiation promoting TNFSF11-induced NF-κB activity.153,163,172 Alternatively, CRELD2, was found to negatively regulate osteoclast differentiation through inhibition of ER calcium release and impaired calcium-dependent activation of NFATC1 (Fig. 4b).55
UPR-modulators and potential applications in cancer-associated bone disease
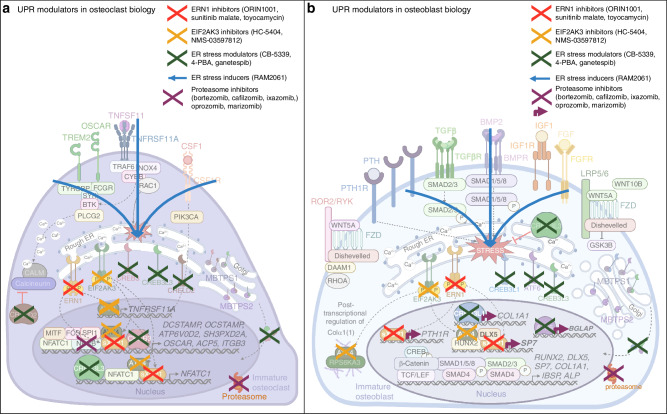
While UPR modulators have demonstrated potential bone related benefits in vivo, translation of these agents to clinical use in bone disease has yet to be achieved. Current treatment options for cancer-induced bone disease consist primarily of anti-osteoclastic agents such as NBPs or the monoclonal antibody denosumab. However, while these therapies show efficacy in reducing patient risk of further SRE, whether they exhibit an additional anti-neoplastic benefit has not been confirmed. Understanding the critical role of UPR signaling in cancer development and metastasis, as well as its role in bone homeostasis, suggests potential application for UPR-modulating therapeutics in cancer-induced bone disease (Fig. 5).
Fig. 5.
Potential effects of UPR modulating agents on bone cell differentiation. a Inhibition of UPR signaling with ERN1 inhibitors, EIF2AK3 inhibitors, or ER stress modulators disrupts osteoclast-specific gene expression mediated by ATF4, sXBP1, CREB3, and CREB3L3. Alternatively, ER stress inducers may promote osteoclast differentiation through induction of the UPR and induction of osteoclast genes. Proteasome inhibitors disrupt proteasomal degradation preventing NF-κB-mediated signaling and gene expression. b Inhibition of UPR signaling with ERN1 inhibitors, EIF2AK3 inhibitors, or ER stress modulators disrupts osteoblast-specific gene expression mediated by ATF4, sXBP1, ATF6, and CREB3L1. ER stress inducers may promote osteoblast differentiation through induction of the UPR and induction of osteoblast genes. Proteasome inhibitors disrupt proteasomal degradation inducing the UPR and promoting osteoblast-specific gene expression by ATF4, sXBP1, ATF6, and CREB3L1 and ATF4 mediated COLα1(1) processing. (Abbreviations: EIF2AK3 eukaryotic initiation factor 2 alpha kinase 3, ERN1 ER to nucleus signaling 1, XBP1 X-box-binding protein-1, sXBP1 spliced XBP1, MBTPS1 membrane bound transcription factor peptidase, site 1, MBTPS2 membrane bound transcription factor peptidase, site 2, CSF1 colony stimulating factor 1, CSF1R CSF1 receptor, TNFRSF11A tumor necrosis factor receptor family member 11A, TNFSF11 tumor necrosis factor soluble factor 11, MITF melanocyte-induced transcription factor, TRAF6 tumor necrosis factor receptor-associated factor 6, MAPK mitogen-activated protein kinase, NFATC1 nuclear factor of activated T cells 1, AP-1 activator protein 1, TREM2 triggering receptor expressed on myeloid cells 2, OSCAR osteoclast-associated Ig-like receptor, TYROBP transmembrane immune signaling adaptor, FCRG Fc gamma receptor, SYK spleen associated tyrosine kinase, BTK Bruton’s tyrosine kinase, PLCG2 phospholipase C gamma 2, ACP5 tartrate resistant acid phosphatase 5, CREB3 cAMP responsive element binding protein 3, CRELD2 cystine rich with EGF like domains 2, DCSTAMP dendrocyte expressed seven transmembrane protein, OCSTAMP osteoclast stimulatory transmembrane protein, ATP6V0D2 ATPase H+ transporter V0 subunit d2, SH3PXD2A SH3 and PX domains 2A, ITGB3 integrin subunit beta 3, ACP5 tartrate-resistant acid phosphatase 5, RUNX2 runt-related transcription factor-2, SP7 Sp7 transcription factor, TGFβ transforming growth factor β, TGFβR TGFβ receptor, MAPK mitogen-activated protein kinase, DLX5 distal-less homeobox 5, BMP bone morphogenic protein, BMPR BMP receptor, AP-1 activator protein 1, LRP5/6 low-density lipoprotein receptor-related protein 5 or 6, FZD frizzled, TCF/LEF T cell factor/lymphoid enhancer factor, GSK3B glycogen synthase kinase 3 beta, FGF fibroblast growth factor, FGFR FGF receptor, IGF1 insulin-like growth factor 1, IGF1R IGF1 receptor, PLCG phospholipase C gamma, PTH parathyroid hormone, PTH1R PTH1 receptor, CREB cAMP-response element binding protein, ROR2/RYK receptor tyrosine kinase of the ROR-2 and Ryk families, DAAM1 disheveled associated activator of morphogenesis 1, ATF4 eukaryotic activating transcription factor 4, BGLAP bone gamma-carboxyglutamate protein, SP1 secreted phosphoprotein 1, IBSP integrin binding sialoprotein, COL1A1 collagen type 1 alpha 1 chain, RPS6KA3 ribosomal S6 kinase A3, CREB3L1 cAMP responsive element binding protein 3 like 1, CREB3L3 cAMP responsive element binding protein 3 like 3)
The UPR has been widely recognized for its role in oncogenesis, conferring a pro-survival and drug resistance benefit in many cancers.173 Due to the hypoxic, nutrient-deficient environment of the tumor niche, cancer cells often require activation of survival pathways like the UPR to combat these conditions.6 Therefore, it is no surprise that tumors exhibiting increased ER stress signaling correspond with poor prognosis and adverse treatment outcomes.174–179 This has fostered the development of UPR-targeting therapeutics for cancer, such as ERN1 inhibitors, EIF2AK3 inhibitors, along with agents targeting other UPR components, such as ERAD and chemical chaperones. Most of these inhibitors have only been evaluated in the realm of anti-tumor activity, without any studies on bone remodeling. This next section will summarize the efficacy of these therapies in bone-associated cancer, such as MM, OS, and bone-metastatic breast and prostate cancer, and review any preclinical studies which may inform the application of these therapies in the realm of bone health.
EIF2AK3 inhibitors
EIF2AK3 plays multiple roles in oncogenesis such as promoting tumor angiogenesis and mediating hypoxia- and chemoresistance; therefore indicating potential for EIF2AK3-specific targeting for cancer treatment.180–184 Evaluation of first-generation EIF2AK3 inhibitor (GSK2656157) confirmed an anti-neoplastic benefit in various tumor xenograft models promoting the application of EIF2AK3 inhibitors for cancer treatment.185,186 Furthermore, investigation of a second-generation EIF2AK3 inhibitor (GSK2606414) revealed inhibitory effects on osteoclast precursor differentiation and autophagy.153 To elucidate any significant effects on bone remodeling, GSK2606414 was evaluated in ovariectomized (OVX) mice where it was found to restore bone density. Specifically, treatment was found to significantly increase trabecular bone density and reduce ACP5+ osteoclast numbers suggesting anti-resorptive effects in vivo.153 While these studies were not conducted in a setting of cancer-induced bone disease, the anti-tumor effects noted with the first generation EIF2AK3 inhibitor, suggests potential application in cancer-induced osteolytic bone disease.185,186
Translation of early generation EIF2AK3 inhibitors to the clinical setting has been difficult due to overt toxicity to pancreatic β-cells in humans, however next generation inhibitors with greater selectivity for EIF2AK3 are currently undergoing clinical trial development.187,188 While these inhibitors are not currently under investigation for cancer-associated bone disease, relevant clinical trials and potential bone-related effects are summarized in Table 1. Considering the effects of EIF2AK3 signaling on osteoclast/osteoblast differentiation, EIF2AK3 inhibition exhibits potential for dual therapeutic anti-neoplastic and bone-related benefits.
Table 1.
Preclinical and clinical data summarizing the effects of promising UPR modulators in cancer
| Drug | Mechanism of action | Preclinical data | Clinical data |
|---|---|---|---|
| HC-5404 | Selective EIF2AK3 inhibitor | Anti-tumor activity in mouse models of renal cell carcinoma with evidence of synergy in combination with anti-angiogenic agents.269 | Potential efficacy in heavily pretreated patients with solid tumors.188 |
| NMS-03597812 | ATP-competitive EIF2AK3 and GCN2 inhibitor | Anti-tumor activity in a mouse model of MM with evidence of synergy in combination with standard of care agents.270 | Under investigation for treatment of RR acute myeloid leukemia (NCT06549790) |
| ORIN1001 (MKC-8866) | Selective ERN1 RNase inhibitor | Anti-tumor effects in mouse models of triple negative breast and prostate cancer with evidence of synergy when used in combination with current approved chemotherapeutics.192,193,198 | Potential efficacy with single-agent use in a subset of patients with advanced solid tumors who exhibited disease stabilization or partial response271 Recently evaluated for treatment of RR breast cancer in combination with Abraxane (NCT03950570) but results are unavailable at this time |
| Sunitinib malate | Multi-targeted tyrosine kinase inhibitor, including ATP-competitive ERN1 kinase inhibitor | Anti-tumor activity in mouse models of OS.199,200 | Approved for use in renal cell carcinoma, gastrointestinal stromal tumor, pancreatic neuroendocrine tumor |
| Toyocamycin | ERN1 ATP-dependent RNase inhibitor | Anti-tumor activity in a mouse model of MM.201 | Lacked efficacy in patients with advanced solid tumors202 |
| CB-5083, CB-5339 | ATP-competitive VCP inhibitor | Anti-tumor activity in mouse models of MM and lung carcinoma.272,273 | Under investigation for single agent and combination use with standard of care treatment for acute myeloid leukemia (NCT04372641, NCT04402541) |
| 4-PBA | Histone deacetylase inhibitor that also acts as a chemical chaperone - binds exposed hydrophobic regions of misfolded proteins in the ER | Restored anti-tumor immunity in mice with ER stress induced tumor growth.274 | Lacked efficacy as single agent and in combination for treatment of solid tumors and hematological malignancies.275,276 |
| RAM2061 | GGDPS inhibitor | Anti-tumor activity in mouse models of MM and ES.223,225 Reduced osteoclast number in CD-1 mice.227 | None |
MM multiple myeloma, RR relapsed/refractory, OS osteosarcoma, EW Ewing sarcoma, GGDPS geranylgeranyl diphosphate synthase
ERN1 inhibitors
ERN1/XBP1 signaling is highly associated with cancer progression as elevated sXBP1 levels correlate with advanced disease stage and poor prognosis in MM, OS, breast and prostate cancer.189–193 Specifically in breast cancer, ERN1 signaling is activated as a result of hypoxia, with signaling inducing expression of hypoxia inducible factor 1 subunit alpha (HIF1A) and downstream targets, solute carrier family 2 member 1 (SLC2A1) and lactate dehydrogenase A (LDHA), incurring hypoxia-resistance and tumor growth.191,194 Anti-tumor effects in various cancer models have confirmed the anti-neoplastic potential of ERN1 inhibitors, with one inhibitor currently being evaluated in the clinical setting (ORIN1001) but bone-specific effects have yet to be evaluated (Table 1).192,193,195–198
Interestingly, the FDA approved, multi-targeted tyrosine kinase inhibitor, sunitinib malate, was found to also inhibit ERN1 autophosphorylation and RNase splicing activity. Evaluation of sunitinib treatment in a human xenograft OS mouse model indicated slowed tumor growth, reduced primary tumor vascularization, and inhibition of lung metastasis.199 Sunitinib single-agent treatment in a mouse model of bone metastatic breast cancer significantly reduced tumor growth and also decreased ACP5+ osteoclast numbers at the tumor-bone interface. However reduction in osteoclast number was not statistically significant.200 Toyocamycin, another ERN1 RNase inhibitor, has been shown to exhibit anti-MM activity in vivo due to inhibition of ERN1XBP1 signaling.201 However while phase I clinical trial (NSC-63701) investigation demonstrated an acceptable safety profile in humans, the lack of anti-tumor efficacy halted further development of toyocamycin.202 Considering the confirmed anti-neoplastic effect of ERN1 inhibitors and the role of ERN1 signaling in osteoclast differentiation (i.e., NFATC1 signaling), there is reason to believe that this class of drugs may have anti-resorptive benefits in the setting of osteolytic cancer-associated bone disease (Table 1).
ER stress modulators
Valosin-containing protein (VCP) is an ERAD associated protein with known pathogenic mutations in bone disease like Paget’s and upregulation in cancer.203,204 VCP primarily functions by extracting misfolded polyubiquitinated proteins from the ER and delivering them to the proteasome for degradation. Therefore, VCP inhibition would increase misfolded protein load, inducing ER stress and potentially apoptosis. A VCP-specific inhibitor, VCP20, was found to prolong survival and improve bone destruction in a murine model of MM via disruption of NF-κB signaling in MM and osteoclast cells.205 VCP20 has yet to be evaluated in humans, however, other VCP inhibitors (CB-5083, CB-5339) have been clinically investigated over the years (Table 1). Unfortunately, due to issues with off-target toxicity the development of these compounds today has slowed.
Heat shock proteins (HSP), such as HSPA5, are molecular chaperones facilitating protein folding and UPR induction. Considering HSPs such as HSP90 are overexpressed and associated with poor prognosis in cancer, HSP90-selective targeting has become a popular area of interest with a total of 18 HSP90 inhibitors entering clinical trials.206 Unfortunately, due to dose-limiting toxicity and poor bioavailability, none have been approved for clinical use. Second-generation HSP90 inhibitor, ganetespib (STA-9090), exhibited potent anti-tumor effects and superior solubility in vivo suggesting greater potential for translation to the clinic. In vitro studies found HSP90 to mediate PTH activation of EIF2AK3 in osteoblast cells, with use of HSP90 inhibitor, geldanamycin, being found to decrease osteoblast EIF2AK3 protein levels in response to PTH treatment.142,207 Understanding PTH-induced EIF2AK3 signaling to mediate osteoblast TNFSF11 expression indicates a potential regulatory role for HSP90 in osteoblast-mediated TNFSF11 osteoclast activation. Understanding that ganetespib demonstrated safety and tolerability in phase I trials, supports further investigation into its potential application in cancer-induced bone disease.206,208
Sodium phenylbutyrate, or 4-phenylbutyrate (4-PBA), is a histone deacetylase inhibitor which also acts as a chemical chaperone, promoting protein folding and alleviation of ER stress. Evaluation of 4-PBA in a murine hindlimb suspension (HLS) model of disuse osteoporosis revealed bone-protective, and potentially anabolic, effects.209 This model utilized skeletally mature mice that underwent 3 weeks of HLS promoting generation of ROS in the bone, causing ER stress and significant loss of cortical and trabecular bone density.209 A subset of HLS mice receiving 4-PBA treatment for 21 days exhibited a significant degree of bone restoration, even more so than control mice which did not undergo HLS.209 The anti-osteoclastic effects of 4-PBA were attributed to downregulation of CTSK and ACP5 which were upregulated in HLS mice; however, the overall bone restorative effect is more likely attributed to alleviation of osteoblast ER stress.209 This is supported by enhanced mineral-to-matrix ratio, collagen maturity, and protein crosslinking following 4-PBA treatment, suggesting alleviation of ER stress in HLS osteoblasts, and therefore restoration of bone matrix protein processing and trafficking.
The osteoblast-restorative effect of 4-PBA has also been confirmed in other ER stress mediated pathologies. In a murine model of OI, osteoblasts were found to undergo ER stress-mediated apoptosis from inadequate collagen processing and secretion, but 4-PBA treatment alternatively promoted postnatal bone growth and reduced facture incidence.210 Furthermore, generation of osteoblast-specific ATG7 conditional knockout mice revealed reduced bone mass resulted from impaired osteoblast formation and matrix mineralization.211 Treatment with 4-PBA was found to ameliorate ER stress triggered in ATG7-deficient osteoblasts and restored function similarly to that seen in OI defective osteoblasts.210,211
4-PBA is currently approved for use in humans, albeit for the indication of urea cycle disorders. Multiple studies have failed to exhibit single-agent anti-tumor efficacy of 4-PBA in humans; however, it is still under investigation for treatment of solid tumors in combination with other chemotherapeutic agents (Table 1).
Isoprenoid biosynthesis pathway inhibitors
Bisphosphonates, specifically the nitrogen-containing bisphosphonate zoledronic acid (ZA), are the frontline treatment option for patients with bone metastases or myeloma bone disease (MBD).212 NBPs exert their anti-osteoclastic effects by inhibiting enzyme farnesyl diphosphate synthase (FDPS) which mediates the formation of isoprenoid donors (farnesyl pyrophosphate (FPP), geranylgeranyl pyrophosphate (GGPP)) necessary for small GTPase post-translational modification (farnesylation, geranylgeranylation).213,214 Inhibition of Rho GTPase geranylgeranylation, is the recognized anti-osteoclastic mechanism behind NBPs, due to the role of Rho-regulated cytoskeletal processes in osteoclast differentiation and resorption.213,215 Aside from this direct anti-osteoclastic effect, ZA is thought to impart an anti-neoplastic benefit through direct and selective killing of tumor cells.23 In vitro studies confirmed anti-tumor properties such as ability to inhibit proliferation, adhesion, invasion, and induce cancer cell apoptosis, with clinical studies suggesting a potential survival benefit in MM, OS, and bone-metastatic breast or prostate cancer.23,216–220 Significant anti-tumoral effects of ZA in cancers associated with elevated UPR expression, suggests UPR-mediated apoptosis to potentially mediate ZA’s anti-neoplastic effects.221 While this could indicate application of ZA beyond the skeleton, potential is limited due to inability to reproduce anti-tumor effects in vivo. ZA has a 95% or greater affinity for hydroxyapatite (HAP; primary component of the bone matrix), therefore ZA distributes selectively to the bone, with remaining compound excreted unmodified by the kidneys.212,222 Thus, the lack of systemic distribution is the primary roadblock for application of NBPs for cancers outside of the bone.
The enzyme geranylgeranyl diphosphate synthase (GGDPS) directly follows FDPS in the isoprenoid biosynthesis pathway such that GGDPS inhibition prevents formation of only isoprenoid GGPP, without depleting FPP levels. Therefore, GGDPS inhibition presented a targeted strategy for selectively inhibiting protein geranylgeranylation without impacting farnesylation.223 GGDPS inhibitors (GGSIs) have demonstrated anti-neoplastic activity in malignancies characterized by aberrant protein production such as MM, by disrupting Rab-mediated protein trafficking, inducing ER stress, and UPR-mediated apoptosis.223–225 An α-methylated triazole bisphosphonate, RAM2061, was found to inhibit tumor growth and prolong survival in mouse models of MM and ES, and also, importantly, exhibits key drug-like features such as prolonged half-life, metabolic stability, and systemic distribution.223,225 Furthermore, RAM2061 in combination with proteasome inhibitor (PI) bortezomib, significantly reduced tumor growth in a MM xenograft model compared to either agent alone.226 While these studies confirmed anti-tumor effects, flank xenograft models did not allow for assessment of changes in bone remodeling, therefore, subsequent studies evaluated RAM2061 treatment on osteoclasts in vitro. RAM2061 exhibited anti-osteoclastic effects comparable to ZA, confirmed to result from disruption of Rho GTPase gernaylgeranylation.227 Evaluation of short course RAM2061 treatment in young CD-1 mice revealed no significant effects on bone morphology, volume, or serum levels of bone-formation or -turnover markers (CTX, P1NP, TRACP-5b).227 However, a decrease in ACP5+ osteoclasts was observed.227 HAP binding experiments determined RAM2061 to bind HAP with 60% affinity, which in conjunction with previously published distribution studies, raises the possibility that this agent could have both direct anti-tumor and anti-osteoclastic activity.
Novel thienopyrimidine-based GGSIs have also demonstrated anti-MM activity in mouse models of MM.228–230 While the investigators did not evaluate any bone parameters in these studies, they did evaluate the HAP affinity of their lead compound and found it bound HAP with affinity equivalent to ZA.229 While this suggests potential for potent bone-restoring effects, it likely limits the anti-tumor activity of this compound against cancer outside of the skeleton.
Proteasome inhibitors
PIs are one of the most well-studied classes of UPR modulators. The ubiquitin-proteasome system (UPS) mediates the degradation of more than 80% of cellular proteins.231 Therefore, UPS inhibition significantly increases misfolded protein load, triggering prolonged UPR signaling, and subsequent apoptosis.232 Bortezomib was the first-in-class, boronate-based reversible PI to receive FDA approval after it was found to improve progression-free survival and overall survival of patients with MM.233,234 While emerging as one of the most effective treatments for MM, it was quickly recognized for its additional bone-restorative benefits.235–237 At the cellular level, bortezomib was found to promote bone formation through activation of ERN1/XBP1 signaling and ATF4 expression in osteoblasts, promoting differentiation and maturation. This pro-osteoblastic effect was confirmed in various clinical trials where bortezomib was found to elevate serum levels of bone formation markers such as bone ALP (bALP) and BGLAP and decreased levels of osteoblast inhibitor DKK1.238–241 Importantly, bone anabolic effects were specifically attributed to PIs, with inclusion or exclusion of bortezomib from combination treatment regimens differentially impacting bone disease.242,243 Bortezomib was also found to reduce serum levels of bone resorption markers (CTX, TRACP-5b) and osteoclast activator, soluble TNFSF11 (sTNFSF11), suggesting an anti-resorptive benefit.244,245 This was confirmed with bortezomib found to inhibit osteoclast differentiation through disruption of UPS mediated degradation of NF-κB inhibitor, IκB, and inhibition of MAPK14 (also known as p38) signaling during osteoclast differnetaition.246,247 This supported further investigation into the bone-specific effects of bortezomib, including an observational study (NCT01026701) evaluating the effects of intravenous bortezomib on osteoblast activity in patients with MM. While the study has been completed, results are not available at this time.
Due to development of drug resistance and dose-limiting side effects (e.g., peripheral neuropathy), second-generation PIs were developed. Carfilzomib, an epoxyketone-based irreversible PI, demonstrated safety, tolerability, and anti-tumor activity in humans leading to fast-track FDA approval for single agent use in patients with RR MM.248–252 The bone anabolic effects of carfilzomib were demonstrated in vivo with non-tumor bearing mice and mice with disseminated MM exhibiting increased trabecular bone volume, decreased bone resorption, and enhanced bone formation.253 Bone-forming effects were confirmed to result from ERN1/XBP1 signaling in osteoblasts as expected, but promotion of osteogenesis was attributed primarily to activation of WNT-dependent β-catenin signaling.254 Interestingly, carfilzomib was also found to exhibit anti-osteoclastic effects unseen with bortezomib, such as disrupting TNFSF11-induced NF-κB signaling and PTH-induced osteoclastogenesis.255 The specific bone metabolic effects of carfilzomib were confirmed in a phase II study which found single agent use in patients with RR MM to decrease serum markers of bone resorption (CTX, TRACP-5b).256 Significant changes in bone formation markers (P1NP, OCN) were generally not observed, except in patients who achieved hematological partial response or better.256 Similarly, a prospective study evaluating the impact of carfilzomib with dexamethasone on bone metabolism in RR MM found significantly decreased serum levels of bone resorption markers, CTX and TRACP-5b.254 Importantly, an increase in serum levels of bone formation markers P1NP, OCN, bALP was also noted.254 Whether this is due to a direct bone-anabolic effect or indirect due to reduced MM disease burden requires clarification.
Ixazomib was the first oral PI to receive FDA approval following confirmation that the addition of ixazomib to lenalidomide and dexamethasone significantly increased PFS compared to lenalidomide and dexamethasone alone.257 Ixazomib is similar to bortezomib in that it is a boronate-based, reversible PI. However, evaluation of ixazomib and bortezomib in vivo revealed ixazomib to be superior in reducing tumor burden and preventing bone loss in MM.258 Ixazomib bone anabolic effects were confirmed to result from ERN1/XBP1 signaling activation and β-catenin signaling in osteoblasts, with concurrent inhibition of NF-κB signaling and osteoclast formation.258 An open label phase II study (NCT04028115) found 3-month long treatment to impart a net bone-anabolic effect in patients with MM with evidence of decreased CTX and TRACP-5b serum levels, however P1NP and bALP bone formation markers seemed unaffected.259 Evaluation of ixazomib treatment in other indications such as OS showed in vivo treatment enhanced overall survival by slowing primary tumor metastasis and inhibiting growth of pulmonary and abdominal metastases; while bortezomib only inhibited the growth of pulmonary metastases. This study revealed the potential application of ixazomib treatment in OS and suggested that ixazomib has greater ability to penetrate tumors due to superior pharmacokinetic and pharmacodynamic properties.258,260,261
Due to issues of off-target toxicities and drug resistance with early generation PIs, third-generation PIs were developed. Oprozomib, an orally administered derivative of carfilzomib, exhibited significantly greater anti-osteoclastic activity in vitro compared to bortezomib, and conferred an anti-tumor benefit in MM in vivo.253 Furthermore, oprozomib dose-dependently increased ALP activity and mineralization of osteoblasts in vitro which was a direct result of ERN1 activation, due to lack of mineralization with oprozomib treatment in ERN1 knockout cells.253 Phase I studies (NCT01129349, NCT01365559) revealed tolerability and efficacy in patients with solid tumors and hematological malignancies, however gastrointestinal (GI) related toxicities were common suggesting the need for a formulation change.262 GI toxicity from oprozomib was ameliorated when used in combination with dexamethasone (NCT01832727) supporting the continued investigation of two different oprozomib formulations in combination with dexamethasone and pomalidomide for RR MM (NCT02939183).263,264
Marizomib is unique from other PIs in that it is a naturally occurring compound derived from marine bacterium. Mechanistically, it irreversibly binds and inhibits all three active sites on the proteasome, suggesting prolonged pharmacodynamic activity. Marizomib was found to be superior in its ability to inhibit NF-κB signaling and inhibiting osteoclastogenesis compared to bortezomib.265 Furthermore, it was found to induce apoptosis in bortezomib-resistant MM cells due to a slightly different apoptotic mechanism leading to inhibition of tumor growth and prolonged survival in MM in vivo.266 Phase I and II (NCT00396864, NCT00629473, NCT00461045) investigation in patients with solid tumors or hematological malignancies revealed lack of severe peripheral neuropathy or hematological toxicity seen with first-generation PIs, and demonstrated efficacy in patients with bortezomib-resistance.267,268 Application has continued to be pursued in combination use with pomalidomide and dexamethasone in relapsed/refractory MM (NCT02103335, NCT05050305), and due to its unique ability to cross the blood-brain-barrier, marizomib is undergoing investigation in central nervous system (CNS) involved MM (NCT05050305) and glioblastoma (NCT02330562, NCT03345095).
Conclusions
The UPR is tightly integrated with bone cell differentiation and function and plays a salient role in oncogenesis. Bone is the third most common site of metastasis and localization of cancer cells to the medullar cavity results in disruption of normal homeostatic processes, including bone remodeling, leading to secondary bone disease. Understanding the connection between the UPR and cancer-associated bone disease presents a unique opportunity to dually target cancer cells and promote restoration of bone remodeling. While the FDA-approved agents sunitinib malate and 4-PBA have UPR modulatory activities, the effects on bone parameters in humans have not been reported. Development of next generation EIF2AK3 inhibitors or ERN1 inhibitors may yield selective agents which can directly modulate the UPR in cancer-associated bone disease. Considering that tumor cell metabolism varies based on primary vs metastatic sites, future studies should aim to clarify whether UPR modulation and targeting is altered by tumor location. PIs are widely used for treatment of MM and MBD, and PI treatment was found to incur significant bone anabolic benefits. Further clarification on whether PIs abrogate bone disease in the setting of other primary-bone or metastatic-bone cancers remains to be determined. While current agents for cancer-induced bone disease such as ZA and denosumab prevent further incidence of SREs, the magnitude of effect on the tumor itself has yet to be clarified. Emerging therapeutic classes such as the GGSIs hold promise due to their anti-osteoclastic and anti-tumor effects, but further studies are required to determine the extent to which these agents can abrogate cancer-induced bone disease in vivo. Ultimately, the successful application of UPR modulators for cancer-induced bone disease will be dependent on agents having the requisite pharmacokinetic and biodistribution properties to enable sufficient bone exposure as well as specific on-target effects on bone and tumor while minimizing off-target toxicities.
Acknowledgements
All figures were created on Biorender.com. This work was supported by the National Institutes of Health (Grants P30 CA036727 and R01 CA258621) and funding from the University of Nebraska Medical Center Graduate Studies Assistantship.
Author contributions
M.E.M. performed the literature review and wrote the manuscript. S.A.H. contributed to manuscript writing and editing.
Competing interests
The authors declare no competing interests.
References
- 1.Hebert, D. N. & Molinari, M. In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol. Rev.87, 1377–1408 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Hetz, C., Zhang, K. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol.21, 421–438 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiseman, R. L., Mesgarzadeh, J. S. & Hendershot, L. M. Reshaping endoplasmic reticulum quality control through the unfolded protein response. Mol. Cell.82, 1477–1491 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hetz, C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol.13, 89–102 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Wu, J. & Kaufman, R. J. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ.13, 374–384 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Chen, X. & Cubillos-Ruiz, J. R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer21, 71–88 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raines, L. N. & Huang, S. C. How the unfolded protein response is a boon for tumors and a bane for the immune system. Immunohorizons7, 256–264 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu, L., Zhang, W., Zhang, X. H. & Chen, X. Endoplasmic reticulum stress in bone metastases. Front Oncol.10, 1100 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi, C. et al. A novel prognostic signature in osteosarcoma characterised from the perspective of unfolded protein response. Clin. Transl. Med.12, e750 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang, D. et al. Comprehensive analysis of the unfolded protein response in breast cancer subtypes. JCO Precis Oncol1, 1-9 (2017). [DOI] [PMC free article] [PubMed]
- 11.Yarapureddy, S. et al. ATF6α activation enhances survival against chemotherapy and serves as a prognostic indicator in osteosarcoma. Neoplasia21, 516–532 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagratuni, T. et al. XBP1s levels are implicated in the biology and outcome of myeloma mediating different clinical outcomes to thalidomide-based treatments. Blood116, 250–253 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Luk, J. M. et al. Proteomic profiling of hepatocellular carcinoma in Chinese cohort reveals heat-shock proteins (Hsp27, Hsp70, GRP78) up-regulation and their associated prognostic values. Proteomics6, 1049–1057 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Tan, S. S. et al. GRP78 up-regulation is associated with androgen receptor status, Hsp70-Hsp90 client proteins and castrate-resistant prostate cancer. J. Pathol.223, 81–87 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Kuroda, K. et al. Glucose-regulated protein 78 positivity as a predictor of poor survival in patients with renal cell carcinoma. Urol. Int.87, 450–456 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Ryan, C. et al. Epidemiology of bone metastases. Bone158, 115783 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Ingangi, V. et al. Role of microenvironment on the fate of disseminating cancer stem cells. Front. Oncol.9, 82 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, J. F. et al. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: a large population-based study. Ann. Transl. Med.8, 482 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizzoli, R. et al. Cancer-associated bone disease. Osteoporos. Int.24, 2929–2953 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Moos, R. et al. Management of bone health in solid tumours: From bisphosphonates to a monoclonal antibody. Cancer Treat. Rev.76, 57–67 (2019). [DOI] [PubMed] [Google Scholar]
- 21.D’Oronzo, S., Coleman, R., Brown, J. & Silvestris, F. Metastatic bone disease: pathogenesis and therapeutic options: up-date on bone metastasis management. J. Bone Oncol.15, 004–004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Groot, A. F., Appelman-Dijkstra, N. M., van der Burg, S. H. & Kroep, J. R. The anti-tumor effect of RANKL inhibition in malignant solid tumors—a systematic review. Cancer Treat. Rev.62, 18–28 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Zekri, J., Mansour, M. & Karim, S. M. The anti-tumour effects of zoledronic acid. J. Bone Oncol.3, 25–35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Read, A. & Schroder, M. The unfolded protein response: an overview. Biology10, 348 (2021). [DOI] [PMC free article] [PubMed]
- 25.Harding, H. P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature397, 271–274 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Harding, H. P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell.11, 619–633 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Brush, M. H., Weiser, D. C. & Shenolikar, S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol. Cell Biol.23, 1292–1303 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida, H. et al. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell107, 881–891 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Wu, J. et al. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell.13, 351–364 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Zinszner, H. et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev.12, 982–995 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCullough, K. D. et al. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell Biol.21, 1249–1259 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollien, J. & Weissman, J. S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science313, 104–107 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Urano, F. et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science287, 664–666 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Yoneda, T. et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J. Biol. Chem.276, 13935–13940 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Khanna, M., Agrawal, N., Chandra, R. & Dhawan, G. Targeting unfolded protein response: a new horizon for disease control. Expert Rev. Mol. Med.23, e1 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Maurel, M. et al. Controlling the unfolded protein response-mediated life and death decisions in cancer. Semin Cancer Biol.33, 57–66 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Wang, M. & Kaufman, R. J. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer14, 581–597 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Hetz, C., Chevet, E. & Harding, H. P. Targeting the unfolded protein response in disease. Nat. Rev. Drug Discov.12, 703–719 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Wang, S. & Kaufman, R. J. The impact of the unfolded protein response on human disease. J. Cell Biol.197, 857–867 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horiuchi, K., Tohmonda, T. & Morioka, H. The unfolded protein response in skeletal development and homeostasis. Cell Mol. Life Sci.73, 2851–2869 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang, W., Gong, Y. & Yan, L. ER stress, the unfolded protein response and osteoclastogenesis: a review. Biomolecules. 13, 1050 (2023). [DOI] [PMC free article] [PubMed]
- 42.Iyer, S. & Adams, D. J. Bone and the unfolded protein response: in sickness and in health. Calcif. Tissue Int.113, 96–109 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lv, W. et al. The Role of XBP1 in bone metabolism. Front Endocrinol.14, 1217579 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes, A. et al. Endoplasmic reticulum stress and unfolded protein response in cartilage pathophysiology: contributing factors to apoptosis and osteoarthritis. Int. J. Mol. Sci. 18, 665 (2017). [DOI] [PMC free article] [PubMed]
- 45.Rellmann, Y., Eidhof, E. & Dreier, R. Review: ER stress-induced cell death in osteoarthritic cartilage. Cell Signal.78, 109880 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Pan, Y. et al. Perspective study of upr signaling molecules as potential biomarkers in bone metabolism. Cell Signal.1, 14–21 (2023). [Google Scholar]
- 47.Biason-Lauber, A., Lang-Muritano, M., Vaccaro, T. & Schoenle, E. J. Loss of kinase activity in a patient with Wolcott-Rallison syndrome caused by a novel mutation in the EIF2AK3 gene. Diabetes51, 2301–2305 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Delépine, M. et al. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat. Genet.25, 406–409 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Brickwood, S. et al. Wolcott-Rallison syndrome: pathogenic insights into neonatal diabetes from new mutation and expression studies of EIF2AK3. J. Med. Genet.40, 685–689 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coffin, R., Phillips, J. L., Staples, W. I. & Spector, S. Treatment of lead encephalopathy in children. J. Pediatr.69, 198–206 (1966). [DOI] [PubMed] [Google Scholar]
- 51.Lowry, B., Miller, J. R. & Fraser, F. C. A new dominant gene mental retardation syndrome. Association with small stature, tapering fingers, characteristic facies, and possible hydrocephalus. Am. J. Dis. Child.121, 496–500 (1971). [PubMed] [Google Scholar]
- 52.Yang, X. et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell117, 387–398 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Haze, K. et al. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell.10, 3787–3799 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindert, U. et al. MBTPS2 mutations cause defective regulated intramembrane proteolysis in X-linked osteogenesis imperfecta. Nat. Commun.7, 11920 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duxfield, A., Munkley, J., Briggs, M. D. & Dennis, E. P. CRELD2 is a novel modulator of calcium release and calcineurin-NFAT signalling during osteoclast differentiation. Sci. Rep.12, 13884 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hartley, C. L. et al. Armet/Manf and Creld2 are components of a specialized ER stress response provoked by inappropriate formation of disulphide bonds: implications for genetic skeletal diseases. Hum. Mol. Genet.22, 5262–5275 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yahara, Y. et al. The origins and roles of osteoclasts in bone development, homeostasis and repair. Development. 149, dev199908 (2022). [DOI] [PMC free article] [PubMed]
- 58.Jilka, R. L. Biology of the basic multicellular unit and the pathophysiology of osteoporosis. Med. Pediatr. Oncol.41, 182–185 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Nakashima, T. et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med.17, 1231–1234 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Xiong, J. et al. Matrix-embedded cells control osteoclast formation. Nat. Med.17, 1235–1241 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takito, J. & Nakamura, M. Heterogeneity and actin cytoskeleton in osteoclast and macrophage multinucleation. Int. J. Mol. Sci. 21, 6629 (2020). [DOI] [PMC free article] [PubMed]
- 62.Teitelbaum, S. L. The osteoclast and its unique cytoskeleton. Ann. N. Y. Acad. Sci.1240, 14–17 (2011). [DOI] [PubMed] [Google Scholar]
- 63.Ng, P. Y., Brigitte Patricia Ribet, A. & Pavlos, N. J. Membrane trafficking in osteoclasts and implications for osteoporosis. Biochem Soc. Trans.47, 639–650 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Minoshima, M. et al. In vivo multicolor imaging with fluorescent probes revealed the dynamics and function of osteoclast proton pumps. ACS Cent. Sci.5, 1059–1066 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang, W., Yang, S., Shao, J. & Li, Y. P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci.12, 3068–3092 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murshed, M. et al. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev.19, 1093–1104 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang, L. et al. Osteoblast-induced osteoclast apoptosis by Fas ligand/FAS pathway is required for maintenance of bone mass. Cell Death Differ.22, 1654–1664 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Franz-Odendaal, T. A., Hall, B. K. & Witten, P. E. Buried alive: how osteoblasts become osteocytes. Dev. Dyn.235, 176–190 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Park, J. H., Lee, N. K. & Lee, S. Y. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol. Cells40, 706–713 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Asagiri, M. & Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone40, 251–264 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Takayanagi, H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J. Mol. Med.83, 170–179 (2005). [DOI] [PubMed] [Google Scholar]
- 72.Ponzetti, M. & Rucci, N. Osteoblast differentiation and signaling: established concepts and emerging topics. Int. J. Mol. Sci. 22, 6651 (2021). [DOI] [PMC free article] [PubMed]
- 73.Zhu, S., Chen, W., Masson, A. & Li, Y. P. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov.10, 71 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomas, S. & Jaganathan, B. G. Signaling network regulating osteogenesis in mesenchymal stem cells. J. Cell Commun. Signal.16, 47–61 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Veis, D. J. & O’Brien, C. A. Osteoclasts, master sculptors of bone. Annu. Rev. Pathol.18, 257–281 (2023). [DOI] [PubMed] [Google Scholar]
- 76.Yahara, Y. et al. Erythromyeloid progenitors give rise to a population of osteoclasts that contribute to bone homeostasis and repair. Nat. Cell Biol.22, 49–59 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacome-Galarza, C. E. et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature568, 541–545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mun, S. H., Park, P. S. U. & Park-Min, K. H. The M-CSF receptor in osteoclasts and beyond. Exp. Mol. Med.52, 1239–1254 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yao, G. Q. et al. Targeted overexpression of the two colony-stimulating factor-1 isoforms in osteoblasts differentially affects bone loss in ovariectomized mice. Am. J. Physiol. Endocrinol. Metab.296, E714–E720 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun, Y. et al. Macrophage-osteoclast associations: origin, polarization, and subgroups. Front Immunol.12, 778078 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsukasaki, M. et al. Stepwise cell fate decision pathways during osteoclastogenesis at single-cell resolution. Nat. Metab.2, 1382–1390 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Kobayashi, N. et al. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J.20, 1271–1280 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lomaga, M. A. et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev.13, 1015–1024 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Asagiri, M. et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med.202, 1261–1269 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takatsuna, H. et al. Inhibition of RANKL-induced osteoclastogenesis by (-)-DHMEQ, a novel NF-kappaB inhibitor, through downregulation of NFATc1. J. Bone Min. Res.20, 653–662 (2005). [DOI] [PubMed] [Google Scholar]
- 86.Chen, W. et al. C/EBPalpha regulates osteoclast lineage commitment. Proc. Natl. Acad. Sci. USA110, 7294–7299 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grigoriadis, A. E. et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science266, 443–448 (1994). [DOI] [PubMed] [Google Scholar]
- 88.Koga, T. et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature428, 758–763 (2004). [DOI] [PubMed] [Google Scholar]
- 89.Shinohara, M. et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell132, 794–806 (2008). [DOI] [PubMed] [Google Scholar]
- 90.Hogan, P. G., Chen, L., Nardone, J. & Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev.17, 2205–2232 (2003). [DOI] [PubMed] [Google Scholar]
- 91.Ha, H. et al. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp. Cell Res.301, 119–127 (2004). [DOI] [PubMed] [Google Scholar]
- 92.Lee, N. K. et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood106, 852–859 (2005). [DOI] [PubMed] [Google Scholar]
- 93.Lee, N. K., Choi, H. K., Kim, D. K. & Lee, S. Y. Rac1 GTPase regulates osteoclast differentiation through TRANCE-induced NF-kappa B activation. Mol. Cell. Biochem.281, 55–61 (2006). [DOI] [PubMed] [Google Scholar]
- 94.Liu, Y. et al. Loureirin B suppresses RANKL-induced osteoclastogenesis and ovariectomized osteoporosis via attenuating NFATc1 and ROS activities. Theranostics9, 4648–4662 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matsumoto, M. et al. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J. Biol. Chem.279, 45969–45979 (2004). [DOI] [PubMed] [Google Scholar]
- 96.Takayanagi, H. et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell.3, 889–901 (2002). [DOI] [PubMed] [Google Scholar]
- 97.Väänänen, H. K. et al. Evidence for the presence of a proton pump of the vacuolar H(+)-ATPase type in the ruffled borders of osteoclasts. J. Cell Biol.111, 1305–1311 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim, K. et al. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol. Endocrinol.22, 176–185 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim, K. et al. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J. Biol. Chem.280, 35209–35216 (2005). [DOI] [PubMed] [Google Scholar]
- 100.Oikawa, T. et al. Tks5-dependent formation of circumferential podosomes/invadopodia mediates cell-cell fusion. J. Cell Biol.197, 553–568 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crotti, T. N. et al. NFATc1 regulation of the human beta3 integrin promoter in osteoclast differentiation. Gene372, 92–102 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.So, H. et al. Microphthalmia transcription factor and PU.1 synergistically induce the leukocyte receptor osteoclast-associated receptor gene expression. J. Biol. Chem.278, 24209–24216 (2003). [DOI] [PubMed] [Google Scholar]
- 103.Nakamura, I. et al. Role of alpha(v)beta(3) integrin in osteoclast migration and formation of the sealing zone. J. Cell Sci.112, 3985–3993 (1999). [DOI] [PubMed] [Google Scholar]
- 104.Faccio, R. et al. Vav3 regulates osteoclast function and bone mass. Nat. Med.11, 284–290 (2005). [DOI] [PubMed] [Google Scholar]
- 105.Chambers, T. J. & Fuller, K. How are osteoclasts induced to resorb bone? Ann. N. Y. Acad. Sci.1240, 1–6 (2011). [DOI] [PubMed] [Google Scholar]
- 106.Zou, W. et al. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J. Cell Biol.176, 877–888 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pittenger, M. F. et al. Multilineage potential of adult human mesenchymal stem cells. Science284, 143–147 (1999). [DOI] [PubMed] [Google Scholar]
- 108.Melis, S., Trompet, D., Chagin, A. S. & Maes, C. Skeletal stem and progenitor cells in bone physiology, ageing and disease. Nat. Rev. Endocrinol.21, 135–153 (2025). [DOI] [PubMed] [Google Scholar]
- 109.Lee, K. S. et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell Biol.20, 8783–8792 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Akiyama, H. et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc. Natl. Acad. Sci. USA102, 14665–14670 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ducy, P. et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell89, 747–754 (1997). [DOI] [PubMed] [Google Scholar]
- 112.Nakashima, K. et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell108, 17–29 (2002). [DOI] [PubMed] [Google Scholar]
- 113.Joeng, K. S. & Long, F. The Gli2 transcriptional activator is a crucial effector for Ihh signaling in osteoblast development and cartilage vascularization. Development136, 4177–4185 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tu, X., Joeng, K. S. & Long, F. Indian hedgehog requires additional effectors besides Runx2 to induce osteoblast differentiation. Dev. Biol.362, 76–82 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kalajzic, I. et al. Expression profile of osteoblast lineage at defined stages of differentiation. J. Biol. Chem.280, 24618–24626 (2005). [DOI] [PubMed] [Google Scholar]
- 116.Hasegawa, T. et al. Matrix vesicle-mediated mineralization and osteocytic regulation of bone mineralization. Int. J. Mol. Sci. 23, 9941 (2022). [DOI] [PMC free article] [PubMed]
- 117.Bonewald, L. F. The amazing osteocyte. J. Bone Min. Res.26, 229–238 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Everts, V. et al. The bone lining cell: its role in cleaning Howship’s lacunae and initiating bone formation. J. Bone Min. Res.17, 77–90 (2002). [DOI] [PubMed] [Google Scholar]
- 119.Matic, I. et al. Quiescent bone lining cells are a major source of osteoblasts during adulthood. Stem Cells34, 2930–2942 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ornitz, D. M. & Marie, P. J. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev.29, 1463–1486 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xian, L. et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med.18, 1095–1101 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen, G., Deng, C. & Li, Y. P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci.8, 272–288 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee, M. H. et al. BMP-2-induced Osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem. Biophys. Res. Commun.309, 689–694 (2003). [DOI] [PubMed] [Google Scholar]
- 124.Lee, K. S., Hong, S. H. & Bae, S. C. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene21, 7156–7163 (2002). [DOI] [PubMed] [Google Scholar]
- 125.Vilardaga, J. P. et al. Molecular mechanisms of PTH/PTHrP Class B GPCR signaling and pharmacological implications. Endocr. Rev.44, 474–491 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Westendorf, J. J., Kahler, R. A. & Schroeder, T. M. Wnt signaling in osteoblasts and bone diseases. Gene341, 19–39 (2004). [DOI] [PubMed] [Google Scholar]
- 127.Wan, M. et al. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev.22, 2968–2979 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bennett, C. N. et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl Acad. Sci. USA102, 3324–3329 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kang, S. et al. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J. Biol. Chem.282, 14515–14524 (2007). [DOI] [PubMed] [Google Scholar]
- 130.Bennett, C. N. et al. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J. Bone Min. Res.22, 1924–1932 (2007). [DOI] [PubMed] [Google Scholar]
- 131.Hu, H. et al. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development132, 49–60 (2005). [DOI] [PubMed] [Google Scholar]
- 132.Bilkovski, R. et al. Role of WNT-5a in the determination of human mesenchymal stem cells into preadipocytes. J. Biol. Chem.285, 6170–6178 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arnsdorf, E. J., Tummala, P. & Jacobs, C. R. Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS ONE4, e5388 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tu, X. et al. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev. Cell.12, 113–127 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Besio, R. et al. Cellular stress due to impairment of collagen prolyl hydroxylation complex is rescued by the chaperone 4-phenylbutyrate. Dis. Model. Mech. 12, dmm038521 (2019). [DOI] [PMC free article] [PubMed]
- 136.Wei, J. et al. PERK is essential for neonatal skeletal development to regulate osteoblast proliferation and differentiation. J. Cell Physiol.217, 693–707 (2008). [DOI] [PubMed] [Google Scholar]
- 137.Oslowski, C. M. & Urano, F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol.490, 71–92 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang, P. et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell Biol.22, 3864–3874 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Saito, A. et al. Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J. Biol. Chem.286, 4809–4818 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang, X. & Karsenty, G. ATF4, the osteoblast accumulation of which is determined post-translationally, can induce osteoblast-specific gene expression in non-osteoblastic cells. J. Biol. Chem.279, 47109–47114 (2004). [DOI] [PubMed] [Google Scholar]
- 141.He, L. et al. Osteoporosis regulation by salubrinal through eIF2alpha mediated differentiation of osteoclast and osteoblast. Cell Signal.25, 552–560 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang, K. et al. The PERK-EIF2alpha-ATF4 signaling branch regulates osteoblast differentiation and proliferation by PTH. Am. J. Physiol. Endocrinol. Metab.316, E590–E604 (2019). [DOI] [PubMed] [Google Scholar]
- 143.Pawlak, K., Sieklucka, B. & Pawlak, D. Paracrine kynurenic pathway activation in the bone of young uremic rats can antagonize anabolic effects of PTH on bone turnover and strength through the disruption of PTH-dependent molecular signaling. Int. J. Mol. Sci. 22, 6563 (2021). [DOI] [PMC free article] [PubMed]
- 144.Li, J. et al. eIF2α signaling regulates autophagy of osteoblasts and the development of osteoclasts in OVX mice. Cell Death Dis.10, 921 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tohmonda, T. et al. The IRE1α-XBP1 pathway is essential for osteoblast differentiation through promoting transcription of Osterix. EMBO Rep.12, 451–457 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tohmonda, T. et al. The IRE1alpha-XBP1 pathway positively regulates parathyroid hormone (PTH)/PTH-related peptide receptor expression and is involved in pth-induced osteoclastogenesis. J. Biol. Chem.288, 1691–1695 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Iyer, S. et al. Elevation of the unfolded protein response increases RANKL expression. FASEB Bioadvance2, 207–218 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jang, W. G. et al. BMP2 protein regulates osteocalcin expression via Runx2-mediated Atf6 gene transcription. J. Biol. Chem.287, 905–915 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Murakami, T. et al. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat. Cell Biol.11, 1205–1211 (2009). [DOI] [PubMed] [Google Scholar]
- 150.Murakami, T. et al. Distinct mechanisms are responsible for osteopenia and growth retardation in OASIS-deficient mice. Bone48, 514–523 (2011). [DOI] [PubMed] [Google Scholar]
- 151.Jang, W. G., Kim, E. J. & Koh, J. T. Tunicamycin negatively regulates BMP2-induced osteoblast differentiation through CREBH expression in MC3T3E1 cells. BMB Rep.44, 735–740 (2011). [DOI] [PubMed] [Google Scholar]
- 152.Tohmonda, T. et al. IRE1α/XBP1-mediated branch of the unfolded protein response regulates osteoclastogenesis. J. Clin. Investig.125, 3269–3279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guo, J. et al. PERK controls bone homeostasis through the regulation of osteoclast differentiation and function. Cell Death Dis.11, 847 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sun, F. et al. A role for PERK in the mechanism underlying fluoride-induced bone turnover. Toxicology325, 52–66 (2014). [DOI] [PubMed] [Google Scholar]
- 155.Cao, H. et al. Activating transcription factor 4 regulates osteoclast differentiation in mice. J. Clin. Investig.120, 2755–2766 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hamamura, K., Tanjung, N. & Yokota, H. Suppression of osteoclastogenesis through phosphorylation of eukaryotic translation initiation factor 2 alpha. J. Bone Min. Metab.31, 618–628 (2013). [DOI] [PubMed] [Google Scholar]
- 157.Yokota, H. et al. Effects of salubrinal on development of osteoclasts and osteoblasts from bone marrow-derived cells. BMC Musculoskelet. Disord.14, 197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.He, L. et al. Osteoporosis regulation by salubrinal through eIF2α mediated differentiation of osteoclast and osteoblast. Cell Signal.25, 552–560 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hamamura, K. et al. In vitro and in silico analysis of an inhibitory mechanism of osteoclastogenesis by salubrinal and guanabenz. Cell Signal.27, 353–362 (2015). [DOI] [PubMed] [Google Scholar]
- 160.Li, J. et al. eIF2alpha signaling regulates autophagy of osteoblasts and the development of osteoclasts in OVX mice. Cell Death Dis.10, 921 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang, P. et al. Salubrinal promotes healing of surgical wounds in rat femurs. J. Bone Min. Metab.30, 568–579 (2012). [DOI] [PubMed] [Google Scholar]
- 162.Li, J. et al. Role of endoplasmic reticulum stress in disuse osteoporosis. Bone97, 2–14 (2017). [DOI] [PubMed] [Google Scholar]
- 163.Lee, E. G. et al. Increased RANKL-mediated osteoclastogenesis by interleukin-1β and endoplasmic reticulum stress. Jt. Bone Spine81, 520–526 (2014). [DOI] [PubMed] [Google Scholar]
- 164.Saito, A. et al. Regulation of endoplasmic reticulum stress response by a BBF2H7-mediated Sec23a pathway is essential for chondrogenesis. Nat. Cell Biol.11, 1197–1204 (2009). [DOI] [PubMed] [Google Scholar]
- 165.Nikaido, T. et al. Expression of the novel transcription factor OASIS, which belongs to the CREB/ATF family, in mouse embryo with special reference to bone development. Histochem. Cell Biol.116, 141–148 (2001). [DOI] [PubMed] [Google Scholar]
- 166.Kanemoto, S. et al. Luman is involved in osteoclastogenesis through the regulation of DC-STAMP expression, stability and localization. J. Cell Sci.128, 4353–4365 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Komarova, S. V. et al. RANK ligand-induced elevation of cytosolic Ca2+ accelerates nuclear translocation of nuclear factor kappa B in osteoclasts. J. Biol. Chem.278, 8286–8293 (2003). [DOI] [PubMed] [Google Scholar]
- 168.Yip, K. H. et al. Thapsigargin modulates osteoclastogenesis through the regulation of RANKL-induced signaling pathways and reactive oxygen species production. J. Bone Min. Res.20, 1462–1471 (2005). [DOI] [PubMed] [Google Scholar]
- 169.Wang, K., Niu, J., Kim, H. & Kolattukudy, P. E. Osteoclast precursor differentiation by MCPIP via oxidative stress, endoplasmic reticulum stress, and autophagy. J. Mol. Cell Biol.3, 360–368 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim, J. H. et al. Endoplasmic reticulum-bound transcription factor CREBH stimulates RANKL-induced osteoclastogenesis. J. Immunol.200, 1661–1670 (2018). [DOI] [PubMed] [Google Scholar]
- 171.Sun, J. et al. Nox4 promotes RANKL-induced autophagy and osteoclastogenesis via activating ROS/PERK/eIF-2alpha/ATF4 pathway. Front Pharmacol.12, 751845 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee, W. S. et al. Tacrolimus regulates endoplasmic reticulum stress-mediated osteoclastogenesis and inflammation: in vitro and collagen-induced arthritis mouse model. Cell Biol. Int.42, 393–402 (2018). [DOI] [PubMed] [Google Scholar]
- 173.Wang, G., Yang, Z. Q. & Zhang, K. Endoplasmic reticulum stress response in cancer: molecular mechanism and therapeutic potential. Am. J. Transl. Res.2, 65–74 (2010). [PMC free article] [PubMed] [Google Scholar]
- 174.Zhao, Y. et al. XBP1 regulates the protumoral function of tumor-associated macrophages in human colorectal cancer. Signal Transduct. Target. Ther.6, 357 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gifford, J. B. et al. Expression of GRP78, master regulator of the unfolded protein response, increases chemoresistance in pancreatic ductal adenocarcinoma. Mol. Cancer Ther.15, 1043–1052 (2016). [DOI] [PubMed] [Google Scholar]
- 176.Lee, E. et al. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res.66, 7849–7853 (2006). [DOI] [PubMed] [Google Scholar]
- 177.Shuda, M. et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J. Hepatol.38, 605–614 (2003). [DOI] [PubMed] [Google Scholar]
- 178.Zhang, J. et al. Association of elevated GRP78 expression with increased lymph node metastasis and poor prognosis in patients with gastric cancer. Clin. Exp. Metastasis.23, 401–410 (2006). [DOI] [PubMed] [Google Scholar]
- 179.Daneshmand, S. et al. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum. Pathol.38, 1547–1552 (2007). [DOI] [PubMed] [Google Scholar]
- 180.Blais, J. D. et al. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol. Cell Biol.26, 9517–9532 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang, Y. et al. The unfolded protein response induces the angiogenic switch in human tumor cells through the PERK/ATF4 pathway. Cancer Res.72, 5396–5406 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hart, L. S. et al. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J. Clin. Investig.122, 4621–4634 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cullinan, S. B. et al. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell Biol.23, 7198–7209 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dey, S. et al. ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. J. Clin. Investig.125, 2592–2608 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Atkins, C. et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res.73, 1993–2002 (2013). [DOI] [PubMed] [Google Scholar]
- 186.Axten, J. M. et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J. Med. Chem. 55, 7193–7207 (2012). . [DOI] [PubMed]
- 187.Yu, Q. et al. Type I interferons mediate pancreatic toxicities of PERK inhibition. Proc. Natl. Acad. Sci. USA112, 15420–15425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dudek, A. Z. et al. A multicenter, open-label, phase 1a study of HC-5404 in patients with advanced solid tumors. J. Clin. Oncol.42, e15118 (2024). [Google Scholar]
- 189.Yang, J. et al. Overexpression of X-box binding protein 1 (XBP1) correlates to poor prognosis and up-regulation of PI3K/mTOR in human osteosarcoma. Int. J. Mol. Sci.16, 28635–28646 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gambella, M. et al. High XBP1 expression is a marker of better outcome in multiple myeloma patients treated with bortezomib. Haematologica99, e14–e16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen, X. et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature508, 103–107 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sheng, X. et al. IRE1α-XBP1s pathway promotes prostate cancer by activating c-MYC signaling. Nat. Commun.10, 323 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Unal, B. et al. Targeting IRE1alpha reprograms the tumor microenvironment and enhances anti-tumor immunity in prostate cancer. Nat. Commun.15, 8895 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Romero-Ramirez, L. et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res.64, 5943–5947 (2004). [DOI] [PubMed] [Google Scholar]
- 195.Papandreou, I. et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood117, 1311–1314 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cross, B. C. et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc. Natl. Acad. Sci. USA109, E869–E878 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mimura, N. et al. Blockade of XBP1 splicing by inhibition of IRE1alpha is a promising therapeutic option in multiple myeloma. Blood119, 5772–5781 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Logue, S. E. et al. Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nat. Commun.9, 3267 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kumar, R. M. et al. Sunitinib malate (SU-11248) reduces tumour burden and lung metastasis in an intratibial human xenograft osteosarcoma mouse model. Am. J. Cancer Res.5, 2156–2168 (2015). [PMC free article] [PubMed] [Google Scholar]
- 200.Schem, C. et al. Preclinical evaluation of sunitinib as a single agent in the prophylactic setting in a mouse model of bone metastases. BMC Cancer13, 32 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ri, M. et al. Identification of Toyocamycin, an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA splicing. Blood Cancer J.2, e79 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wilson, W. L. Phase I study with toyocamycin (NSC-63701). Cancer Chemother. Rep.52, 301–303 (1968). [PubMed] [Google Scholar]
- 203.Fessart, D. et al. P97/CDC-48: proteostasis control in tumor cell biology. Cancer Lett.337, 26–34 (2013). [DOI] [PubMed] [Google Scholar]
- 204.Weihl, C. C., Pestronk, A. & Kimonis, V. E. Valosin-containing protein disease: inclusion body myopathy with Paget’s disease of the bone and fronto-temporal dementia. Neuromuscul. Disord.19, 308–315 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wei, R. et al. Inhibition of VCP modulates NF-kappaB signaling pathway to suppress multiple myeloma cell proliferation and osteoclast differentiation. Aging (Albany NY).15, 8220–8236 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li, Z. N. & Luo, Y. HSP90 inhibitors and cancer: prospects for use in targeted therapies. Oncol. Rep. 49, 6 (2023). [DOI] [PMC free article] [PubMed]
- 207.Mak, K. K. et al. Hedgehog signaling in mature osteoblasts regulates bone formation and resorption by controlling PTHrP and RANKL expression. Dev. Cell.14, 674–688 (2008). [DOI] [PubMed] [Google Scholar]
- 208.Wang, Y., Trepel, J. B., Neckers, L. M. & Giaccone, G. STA-9090, a small-molecule Hsp90 inhibitor for the potential treatment of cancer. Curr. Opin. Investig. Drugs11, 1466–1476 (2010). [PubMed] [Google Scholar]
- 209.Al-Daghestani, H. et al. Pharmacological inhibition of endoplasmic reticulum stress mitigates osteoporosis in a mouse model of hindlimb suspension. Sci. Rep.14, 4719 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Duran, I. et al. 4-PBA treatment improves bone phenotypes in the Aga2 mouse model of osteogenesis imperfecta. J. Bone Min. Res.37, 675–686 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li, H. et al. Defective autophagy in osteoblasts induces endoplasmic reticulum stress and causes remarkable bone loss. Autophagy14, 1726–1741 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rogers, M. J., Mönkkönen, J. & Munoz, M. A. Molecular mechanisms of action of bisphosphonates and new insights into their effects outside the skeleton. Bone139, 115493 (2020). [DOI] [PubMed] [Google Scholar]
- 213.Fisher, J. E. et al. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc. Natl. Acad. Sci. USA96, 133–138 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.van Beek, E. et al. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem. Biophys. Res. Commun.264, 108–111 (1999). [DOI] [PubMed] [Google Scholar]
- 215.Coxon, F. P. et al. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J. Bone Min. Res.15, 1467–1476 (2000). [DOI] [PubMed] [Google Scholar]
- 216.Christou, A., Ferreira, N. & Sophocleous, A. Effects of zoledronic acid on osteosarcoma progression and metastasis: systematic review and meta-analysis. Clin. Exp. Med.23, 3041–3051 (2023). [DOI] [PubMed] [Google Scholar]
- 217.Morgan, G. J. et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet376, 1989–1999 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Brown, J. et al. Prolonged bone health benefits for breast cancer patients following adjuvant bisphosphonate therapy: the BoHFAB study. J. Bone Min. Res.39, 8–16 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhou, W. et al. Novel therapeutic targets on the horizon: an analysis of clinical trials on therapies for bone metastasis in prostate cancer. Cancers16, 627 (2024). [DOI] [PMC free article] [PubMed]
- 220.Early Breast Cancer Trialists' Collaborative Group. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 386, 1353–1361 (2015). [DOI] [PubMed]
- 221.LAN, Y.-C. et al. Zoledronic acid-induced cytotoxicity through endoplasmic reticulum stress triggered REDD1-mTOR pathway in breast cancer cells. Anticancer Res.33, 3807–3814 (2013). [PubMed] [Google Scholar]
- 222.Rondeau, J. M. et al. Structural basis for the exceptional in vivo efficacy of bisphosphonate drugs. ChemMedChem1, 267–273 (2006). [DOI] [PubMed] [Google Scholar]
- 223.Haney, S. L. et al. In vivo evaluation of isoprenoid triazole bisphosphonate inhibitors of geranylgeranyl diphosphate synthase: impact of olefin stereochemistry on toxicity and biodistribution. J. Pharmacol. Exp. Ther.371, 327–338 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Holstein, S. A. & Hohl, R. J. Isoprenoid biosynthetic pathway inhibition disrupts monoclonal protein secretion and induces the unfolded protein response pathway in multiple myeloma cells. Leuk. Res.35, 551–559 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Haney, S. L. et al. Evaluation of geranylgeranyl diphosphate synthase inhibition as a novel strategy for the treatment of osteosarcoma and Ewing sarcoma. Drug Dev. Res.84, 62–74 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Haney, S. L. et al. Geranylgeranyl diphosphate synthase inhibitor and proteasome inhibitor combination therapy in multiple myeloma. Exp. Hematol. Oncol.11, 5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Muehlebach, M. E. et al. Geranylgeranyl diphosphate synthase inhibition impairs osteoclast differentiation, morphology, and resorptive activity. JBMR .9, ziae133 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lacbay, C. M. et al. Unraveling the prenylation-cancer paradox in multiple myeloma with novel geranylgeranyl pyrophosphate synthase (GGPPS) inhibitors. J. Med. Chem.61, 6904–6917 (2018). [DOI] [PubMed] [Google Scholar]
- 229.Lee, H. F. et al. Synthesis and evaluation of structurally diverse C-2-substituted thienopyrimidine-based inhibitors of the human geranylgeranyl pyrophosphate synthase. J. Med. Chem.65, 2471–2496 (2022). [DOI] [PubMed] [Google Scholar]
- 230.Boutin, R. et al. Discovery and evaluation of C6-substituted pyrazolopyrimidine-based bisphosphonate inhibitors of the human geranylgeranyl pyrophosphate synthase and evaluation of their antitumor efficacy in multiple myeloma, pancreatic ductal adenocarcinoma, and colorectal cancer. J. Med. Chem.66, 15776–15800 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hershko, A. & Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem.67, 425–479 (1998). [DOI] [PubMed] [Google Scholar]
- 232.Obeng, E. A. et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood107, 4907–4916 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kane, R. C., Bross, P. F., Farrell, A. T. & Pazdur, R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist8, 508–513 (2003). [DOI] [PubMed] [Google Scholar]
- 234.Hambley, B., Caimi, P. F. & William, B. M. Bortezomib for the treatment of mantle cell lymphoma: an update. Ther. Adv. Hematol.7, 196–208 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Richardson, P. G. et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N. Engl. J. Med.352, 2487–2498 (2005). [DOI] [PubMed] [Google Scholar]
- 236.Pennisi, A. et al. The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. Am. J. Hematol.84, 6–14 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mohty, M. et al. The effects of bortezomib on bone disease in patients with multiple myeloma. Cancer120, 618–623 (2014). [DOI] [PubMed] [Google Scholar]
- 238.Zhang, D. et al. ER stress arm XBP1s plays a pivotal role in proteasome inhibition-induced bone formation. Stem Cell Res. Ther.11, 516 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zangari, M. et al. A prospective evaluation of the biochemical, metabolic, hormonal and structural bone changes associated with bortezomib response in multiple myeloma patients. Haematologica96, 333–336 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zangari, M. et al. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br. J. Haematol.131, 71–73 (2005). [DOI] [PubMed] [Google Scholar]
- 241.Heider, U. et al. Serum concentrations of DKK-1 decrease in patients with multiple myeloma responding to anti-myeloma treatment. Eur. J. Haematol.82, 31–38 (2009). [DOI] [PubMed] [Google Scholar]
- 242.Delforge, M. et al. Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trial in multiple myeloma. Eur. J. Haematol.86, 372–384 (2011). [DOI] [PubMed] [Google Scholar]
- 243.Terpos, E. et al. VTD consolidation, without bisphosphonates, reduces bone resorption and is associated with a very low incidence of skeletal-related events in myeloma patients post ASCT. Leukemia28, 928–934 (2014). [DOI] [PubMed] [Google Scholar]
- 244.Terpos, E. et al. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br. J. Haematol.135, 688–692 (2006). [DOI] [PubMed] [Google Scholar]
- 245.Uy, G. L. et al. Bortezomib inhibits osteoclast activity in patients with multiple myeloma. Clin. Lymphoma Myeloma7, 587–589 (2007). [DOI] [PubMed] [Google Scholar]
- 246.von Metzler, I. et al. Bortezomib inhibits human osteoclastogenesis. Leukemia21, 2025–2034 (2007). [DOI] [PubMed] [Google Scholar]
- 247.Zavrski, I. et al. Proteasome inhibitors abrogate osteoclast differentiation and osteoclast function. Biochem. Biophys. Res. Commun.333, 200–205 (2005). [DOI] [PubMed] [Google Scholar]
- 248.Herndon, T. M. et al. U.S. Food and Drug Administration approval: carfilzomib for the treatment of multiple myeloma. Clin. Cancer Res.19, 4559–4563 (2013). [DOI] [PubMed] [Google Scholar]
- 249.O’Connor, O. A. et al. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin. Cancer Res.15, 7085–7091 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Alsina, M. et al. A phase I single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma. Clin. Cancer Res.18, 4830–4840 (2012). [DOI] [PubMed] [Google Scholar]
- 251.Vij, R. et al. An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. Br. J. Haematol.158, 739–748 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Vij, R. et al. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood119, 5661–5670 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hurchla, M. A. et al. The epoxyketone-based proteasome inhibitors carfilzomib and orally bioavailable oprozomib have anti-resorptive and bone-anabolic activity in addition to anti-myeloma effects. Leukemia27, 430–440 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Terpos, E. et al. Carfilzomib improves bone metabolism in patients with advanced relapsed/refractory multiple myeloma: results of the CarMMa study. Cancers13, 1257 (2021). [DOI] [PMC free article] [PubMed]
- 255.Yang, Y., Blair, H. C., Shapiro, I. M. & Wang, B. The proteasome inhibitor carfilzomib suppresses parathyroid hormone-induced osteoclastogenesis through a RANKL-mediated signaling pathway. J. Biol. Chem.290, 16918–16928 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Suvannasankha, A. et al. Phase 2 study of carfilzomib and bone metabolism in patients with relapsed multiple myeloma. Blood130, 1826–1826 (2017). [Google Scholar]
- 257.Moreau, P. et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N. Engl. J. Med.374, 1621–1634 (2016). [DOI] [PubMed] [Google Scholar]
- 258.Garcia-Gomez, A. et al. Preclinical activity of the oral proteasome inhibitor MLN9708 in Myeloma bone disease. Clin. Cancer Res.20, 1542–1554 (2014). [DOI] [PubMed] [Google Scholar]
- 259.Diaz-delCastillo, M. et al. Increased bone volume by ixazomib in multiple myeloma: 3-month results from an open label phase 2 study. J. Bone Min. Res.38, 639–649 (2023). [DOI] [PubMed] [Google Scholar]
- 260.Harris, M. A. et al. The proteasome inhibitor ixazomib inhibits the formation and growth of pulmonary and abdominal osteosarcoma metastases in mice. Cancers12, 1207 (2020). [DOI] [PMC free article] [PubMed]
- 261.Gupta, N. et al. A phase I study to assess the mass balance, excretion, and pharmacokinetics of [(14)C]-ixazomib, an oral proteasome inhibitor, in patients with advanced solid tumors. Investig. New Drugs36, 407–415 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Infante, J. R. et al. A first-in-human dose-escalation study of the oral proteasome inhibitor oprozomib in patients with advanced solid tumors. Investig. New Drugs34, 216–224 (2016). [DOI] [PubMed] [Google Scholar]
- 263.Hari, P. et al. Efficacy and safety results from a phase 1b/2, multicenter, open-label study of oprozomib and dexamethasone in patients with relapsed and/or refractory multiple myeloma. Leuk. Res.83, 106172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Shah, J. et al. Oprozomib, pomalidomide, and dexamethasone in patients with relapsed and/or refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk.19, 570–578 e1 (2019). [DOI] [PubMed] [Google Scholar]
- 265.Ahn, K. S. et al. Salinosporamide A (NPI-0052) potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through down-modulation of NF-kappaB regulated gene products. Blood110, 2286–2295 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Chauhan, D. et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell.8, 407–419 (2005). [DOI] [PubMed] [Google Scholar]
- 267.Harrison, S. J. et al. Phase I clinical trial of marizomib (NPI-0052) in patients with advanced malignancies including multiple myeloma: study NPI-0052-102 final results. Clin. Cancer Res.22, 4559–4566 (2016). [DOI] [PubMed] [Google Scholar]
- 268.Richardson, P. G. et al. Phase 1 study of marizomib in relapsed or relapsed and refractory multiple myeloma: NPI-0052-101 Part 1. Blood127, 2693–2700 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Stokes, M. E. et al. PERK inhibition by HC-5404 sensitizes renal cell carcinoma tumor models to antiangiogenic tyrosine kinase inhibitors. Clin. Cancer Res.29, 4870–4882 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Perrera, C. et al. Abstract 1615: NMS-812, a novel potent PERK inhibitor that also inhibits GCN2, exhibits strong anti-tumor activity as single agent and in combination in preclinical models. Cancer Res.83, 1615–1615 (2023). [Google Scholar]
- 271.Gabrail, N. Y. et al. A phase 1/2 trial of ORIN1001, a first-in-class IRE1 inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 39, 3080–3080 (2021).
- 272.Zhou, H. J. et al. Discovery of a first-in-class, potent, selective, and orally bioavailable inhibitor of the p97 AAA ATPase (CB-5083). J. Med. Chem.58, 9480–9497 (2015). [DOI] [PubMed] [Google Scholar]
- 273.Le Moigne, R. et al. The p97 inhibitor CB-5083 is a unique disrupter of protein homeostasis in models of multiple myeloma. Mol. Cancer Ther.16, 2375–2386 (2017). [DOI] [PubMed] [Google Scholar]
- 274.Lee, B. R. et al. Elevated endoplasmic reticulum stress reinforced immunosuppression in the tumor microenvironment via myeloid-derived suppressor cells. Oncotarget5, 12331–12345 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Gilbert, J. et al. A phase I dose escalation and bioavailability study of oral sodium phenylbutyrate in patients with refractory solid tumor malignancies. Clin. Cancer Res.7, 2292–2300 (2001). [PubMed] [Google Scholar]
- 276.Camacho, L. H. et al. Phase I dose escalation clinical trial of phenylbutyrate sodium administered twice daily to patients with advanced solid tumors. Investig. New Drugs25, 131–138 (2007). [DOI] [PubMed] [Google Scholar]