Abstract
Glioblastoma multiforme (GBM) is the most common and aggressive malignant primary brain tumor. Current therapies (temozolomide/radiotherapy) often encounter resistance, necessitating novel molecular targets. Bioinformatics analysis was performed for the data obtained from TCGA, COSMIC, cbioPortal, and MethSurv databases. Tools such as GO, KEGG, GSEA, ROC, and protein–protein interactions (PPI) were employed to investigate the role that UPP1 has on GBM. The effects of UPP1 were further validated using RT-PCR and Western blotting(WB). UPP1 overexpression correlated with poor survival (P < 0.05) and immunosuppression, showing positive associations with immune infiltrates (Tregs, DCs, Th1/Th17 cells) and inflammation-related pathway activation. Silencing UPP1 suppressed proliferation (P < 0.001) in glioma cells. Several DNA methylation patterns of UPP1(8 CpG sites, e.g., cg07703017) were identified as having significant prognostic value. UPP1 drives immunosuppression and serves as a dual biomarker: expression levels stratify prognosis, while methylation profiles offer therapeutic insights. Its immunometabolic regulation positions UPP1 as a promising target for GBM precision therapy.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-16907-4.
Keywords: UPP1, Glioma, Immune infiltration, Biomarker
Subject terms: Prognostic markers, Oncology
Introduction
The most recent WHO classification update for central nervous system (CNS) tumors has reclassified glioblastoma (GBM), originally categorized under the 2007 WHO criteria and TCGA framework, into two distinct entities: IDH-wildtype glioblastoma (IDH-wt GBM) and IDH-mutant astrocytoma, grade 4 (IDH-mt Astrocytoma, CNS WHO grade 4)1. For clarity, the term GBM in this study exclusively refers to IDH-wildtype glioblastoma, in accordance with the latest WHO CNS5 nomenclature.
GBM is characterized by pronounced heterogeneity, rapid proliferation, aggressive invasion, and resistance to treatment, often exhibiting diffuse infiltrative growth that complicates complete surgical resection2. In spite of the remarkable survival advantages brought by conventional therapies, namely surgery, radiotherapy, and chemotherapy, the prognosis remains poor, as the median survival time is merely 14 months3. Contemporary strides in molecular subtyping along with genomic research have deepened our understanding of GBM pathogenesis, offering new avenues for targeted and personalized therapies4.
Over the past few years, cancer immunotherapy has emerged as a promising approach for the therapeutic management of GBM5. Immunotherapies based on checkpoint inhibitors, particularly those targeting CTLA-4, PD-1, and PD-L1, have shown significant progress in certain solid malignancies like melanoma as well as non-small cell lung carcinoma. However, their efficacy in GBM remains limited. Immune cells that infiltrate tumors, especially tumor-associated macrophages (TAMs) and regulatory T cells (Tregs), hold a pivotal position in facilitating immune evasion within GBM, and they exert a profound influence on patient prognosis and response to immunotherapy. The complex tumor microenvironment, high heterogeneity, and immune-suppressive characteristics of GBM present substantial challenges for immunotherapy6. Although many studies have explored the potential of combination therapies, vaccines, and adoptive cell therapies, no highly effective immunotherapeutic strategies have been widely applied in clinical practice. Thus, further exploring the immune underpinnings of GBM and the developing novel immunotherapeutic strategies remain vital research directions.
Pyrimidine metabolism plays a critical role in tumor development and progression7. In pyrimidine metabolism, Uridine Phosphorylase 1 (UPP1) functions as a key enzyme, catalyzing the conversion of uridine or deoxyuridine to uracil and either ribose-1-phosphate or deoxyribose-1-phosphate. This process plays a crucial role in nucleotide metabolism and nucleic acid synthesis8. While UPP1 is typically expressed at low levels in normal tissues, it is markedly upregulated in various cancers, such as colorectal, breast, and liver cancers, and is closely linked to tumor cell proliferation and metabolic reprogramming9. Additionally, UPP1 is involved in the metabolism and mechanism of action of certain antimetabolite drugs, such as 5-fluorouracil, which renders it a prospective target for cancer therapeutic interventions10,11. Recent studies have identified UPP1 as a critical oncogene in gliomas, playing a role in tumorigenesis and immune evasion. Its abnormal expression is associated with the invasive growth of gliomas and may promote immune evasion by affecting the function of tumor-infiltrating immune cells, such as regulatory T cells and tumor-associated macrophages12. However, the specific role of UPP1 in GBM, the most aggressive subtype of glioma, and its relationship to prognosis and immune regulation remain to be fully explored. The observations highlight the prospective value of UPP1 as a characteristic prognostic and immune-associated biomarker for GBM.
Results
High expression of UPP1 in GBM
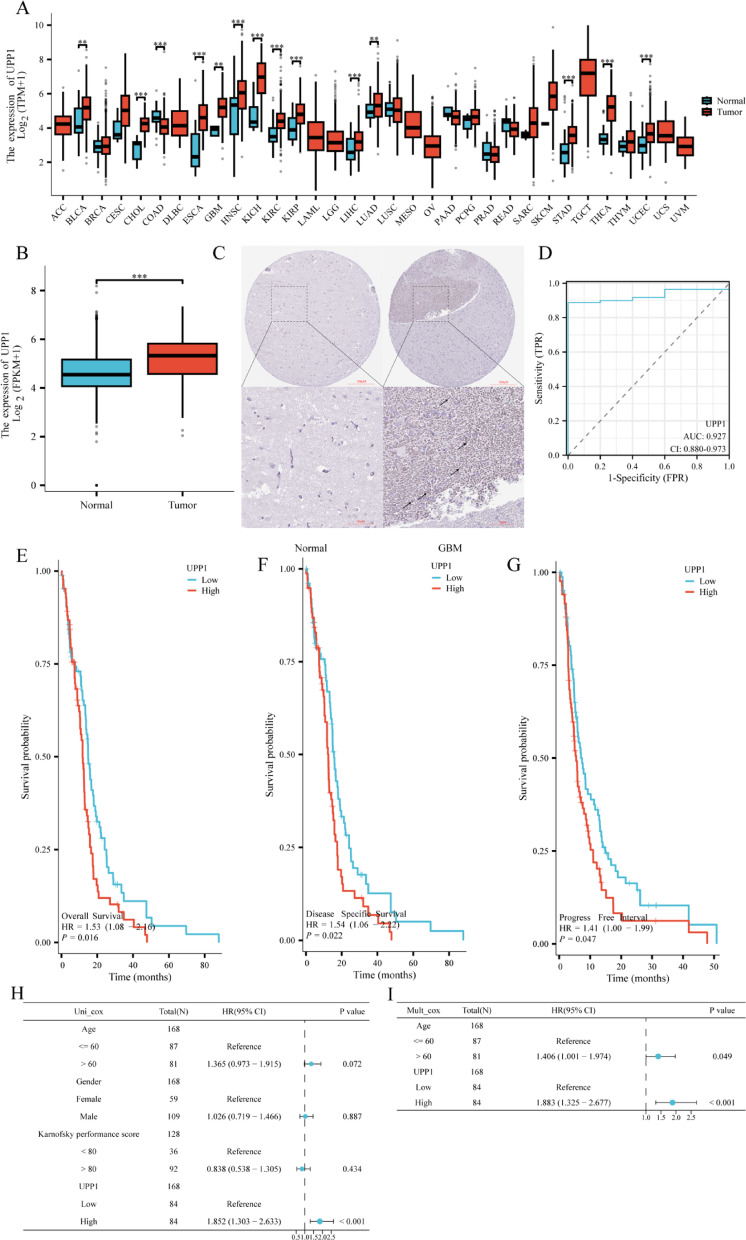
To investigate the expression levels of UPP1 in normal and tumor tissues, we analyzed UPP1 mRNA expression data from the TCGA database using the R package. The results showed that UPP1 expression was significantly higher in several tumor types, including BLCA, CHOL, ESCA, GBM, HNSC, KICH, KIRC, LIHC, LUAD, STAD, TGCT, and UCEC, compared to normal tissues. However, UPP1 expression in COAD tumor tissues was lower than in normal tissues (Fig. 1A). We further evaluated UPP1 mRNA expression in GBM patients using TCGA data and compared it with adjacent normal tissues. The results revealed that UPP1 expression was significantly higher in GBM tissues compared to the adjacent normal tissues (Fig. 1B). The comparative immunohistochemical image of UPP1 in normal tissues and brain tissues of GBM patients also confirms this view (Fig. 1C). Similarly, UPP1 may serve as a potential diagnostic biomarker, with an AUC of 0.927 (Fig. 1D). Survival analysis based on OS, DSS, and PFI indicated that GBM patients with high UPP1 expression had poorer survival outcomes (Fig. 1E–G).To assess the prognostic value of UPP1 in GBM, we performed univariate and multivariate Cox regression analyses based on TCGA clinical data. In the univariate analysis, UPP1 expression, age, sex, and Karnofsky Performance Status (KPS) were included as variables. The results indicated that high UPP1 expression was significantly associated with worse overall survival (P < 0.01; HR > 1). In the multivariate model, UPP1 expression and age were incorporated. UPP1 remained an independent prognostic factor for poor survival after adjusting for age (P < 0.05), suggesting its potential as a clinically relevant biomarker in GBM (Fig. 1H, I).
Fig. 1.
Expression of UPP1 in GBM Tissue. A UPP1 expression levels across various human cancers in the TCGA database. The figure shows the increased or decreased expression of UPP1 in different cancer types compared to normal tissues. B UPP1 expression in GBM tissues from the TCGA database. C Representative Immunohistochemistry (IHC) images showing UPP1 protein expression in normal brain and glioma tissues.
(source: https://www.proteinatlas.org, accessed November 19, 2024) and the upper and lower scale bar are 200 μm and 50 μm respectively. D Receiver Operating Characteristic (ROC) curve for UPP1. E–G Overall survival (OS), progression-free interval (PFI), and disease-specific survival (DSS) survival curves for UPP1 from the TCGA database. H, I Univariate and multivariate Cox regression analyses of overall survival in GBM patients based on TCGA dataset. UPP1 expression and selected clinical variables were included. HRs, 95% CIs, and P values are indicated. *P < 0.05; **P < 0.01; ***P < 0.001.
DNA methylation analysis of UPP1 in GBM
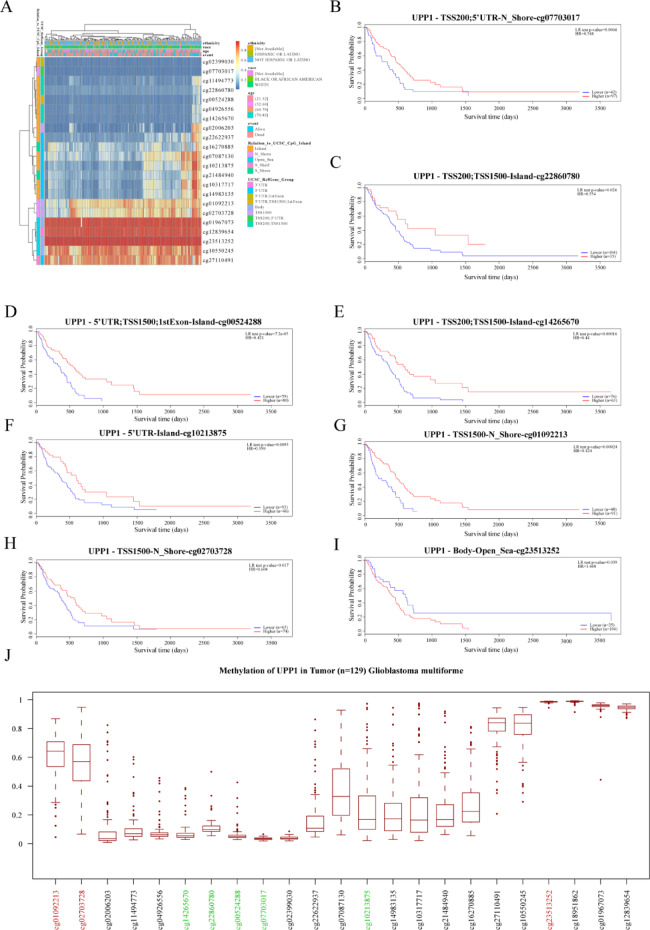
DNA methylation is an epigenetic modification associated with tumorigenesis and progression. DNA methyltransferases on CpG island methylation can act as transcription factors that either suppress or promote cell growth, and this process is reversible. We present a heatmap illustrating the DNA methylation patterns of the UPP1 gene and its expression levels in GBM. Figure 2A shows these patterns, and the analysis also identifies significant prognostic DNA methylation markers for UPP1, including cg07703017, cg22860780, cg00524288, cg14265670, cg10213875, cg01092213, cg02703728, and cg23513252 (Fig. 2B-I + Table 1). Among the aforementioned DNA methylation markers, the average β-values of cg07703017, cg22860780, cg00524288, cg14265670 and cg10213875 were all ≤ 0.2, indicating complete unmethylation. In contrast, cg01092213 and cg23513252 had average β-values ≥ 0.6, suggesting complete methylation (Fig. 2J).
Fig. 2.
DNA Methylation of UPP1 in GBM from TCGA. A Heatmap showing the DNA methylation expression levels of the UPP1 gene in GBM, generated using the MethSurv platform. B–I Prognostic value of individual CpG sites of the UPP1 gene in GBM. Significance threshold is based on LR test p-value < 0.05. The CpG sites cg07703017, cg22860780, cg00524288, cg14265670, cg10213875, cg01092213, ccg02703728, and cg23513252 exhibit significant DNA methylation levels in GBM. J DNA methylation levels (β values) of UPP1-associated CpG sites in glioblastoma multiforme (GBM) tumor samples (n = 129) from the TCGA dataset.
Table 1.
Prognostic value of single CpG of the UPP1 gene family in GBM by MethSurv platform.
| Gene-CpG | HR | LR Test p-Value |
|---|---|---|
| UPP1-5′UTR;1stExon-island-cg02399030 | 0.647 | 0.057 |
| UPP1-TSS200; 5′UTR-N_Shore-cg07703017 | 0.548 | 0.0064* |
| UPP1-TSS200;TSS1500-Island-cg11494773 | 0.727 | 0.13 |
| UPP1-TSS200;TSS1500-Island-cg22860780 | 0.554 | 0.024* |
| UPP1-5′UTR; TSS1500;1stExon-Island-cg00524288 | 0.421 | 0.000072* |
| UPP1-TSS200;TSS1500-Island-cg04926556 | 0.712 | 0.17 |
| UPP1-TSS200;TSS1500-Island-cg14265670 | 0.44 | 0.00016* |
| UPP1-TSS1500-island-cg02006203 | 0.724 | 0.12 |
| UPP1-5′UTR-Island-cg22622937 | 0.641 | 0.06 |
| UPP1-5′UTR-S_Shore-cg16270885 | 1.46 | 0.094 |
| UPP1-5′UTR-Island-cg07087130 | 0.698 | 0.14 |
| UPP1-5′UTR-Island-cg10213875 | 0.559 | 0.0095* |
| UPP1-5′UTR-S_Shore-cg21484940 | 0.79 | 0.34 |
| UPP1-5′UTR-Island-cg10317717 | 0.808 | 0.38 |
| UPP1-5′UTR-Island-cg14983135 | 0.641 | 0.065 |
| UPP1-TSS1500-N_Shore-cg01092213 | 0.424 | 0.00024* |
| UPP1-TSS1500-N_Shore-cg02703728 | 0.604 | 0.017* |
| UPP1-Body-Open_Sea-cg01967073 | 1.385 | 0.12 |
| UPP1-3’UTR-Open_Sea-cg12839654 | 0.827 | 0.46 |
| UPP1-Body-Open_Sea-cg23513252 | 1.668 | 0.039* |
| UPP1-Body-Open_Sea-cg10550245 | 0.67 | 0.076 |
| UPP1-5′UTR-S_Shelf-cg27110491 | 0.812 | 0.39 |
*p<0.050.
Enrichment analysis of UPP1 expression phenotypes
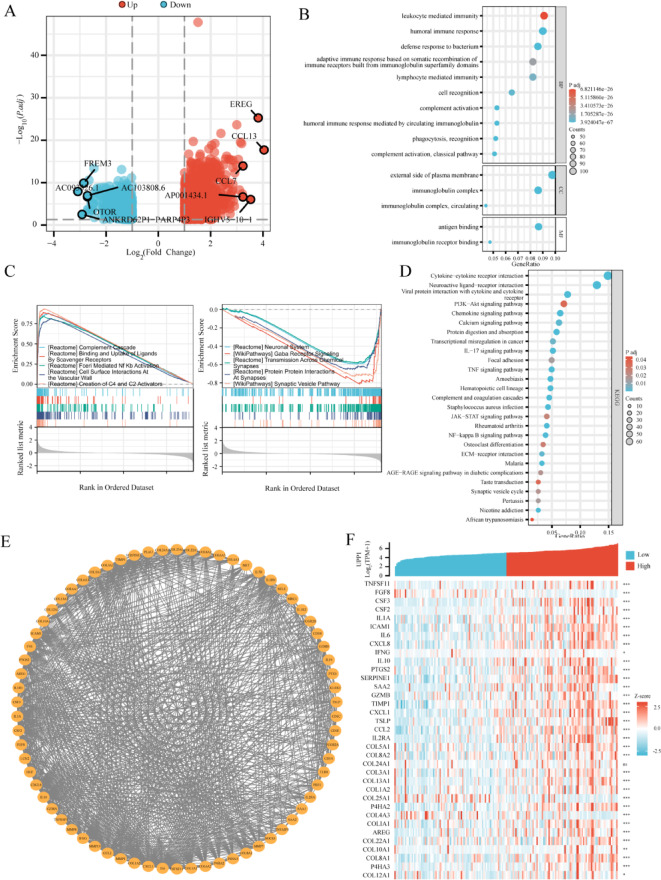
Differential gene expression (DEG) analysis based on the TCGA database identified 1996 DEGs (|LogFC| > 1, adjusted p-value < 0.05) between high and low UPP1 expression GBM samples. In this analysis, UPP1 low-expression samples were treated as the control group. As shown in the volcano plot, 1008 genes (LogFC > 1, adjusted p-value < 0.05) were identified as upregulated. Among them, UPP1 has the highest correlation with CCL13, showing a strong positive correlation (Supplement material 2). While 988 genes (LogFC < -1, adjusted p-value < 0.05) were downregulated in GBM samples with high UPP1 expression (Fig. 3A). Gene Ontology (GO) analysis identified key functional categories, including molecular functions (MF), cellular components (CC), and biological processes (BP), with significant associations to antigen binding, humoral immunity, and complement activation (Fig. 3B). Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis revealed that UPP1 and its co-expressed mRNAs are enriched in 27 signaling pathways, including cytokine–cytokine receptor interactions, inflammation, and calcium ion signaling (Fig. 3C). Gene Set Enrichment Analysis (GSEA) was performed using the data from the 1996 DEGs to further explore pathways associated with UPP1 in GBM. The results indicated that high UPP1 expression was associated with the regulation of complement cascade, binding and uptake of ligands by scavenger receptors, NFκB activation, and vascular wall cell interactions. Conversely, pathways such as neuronal regulation, GABA receptor signaling, trans-synaptic transport, synaptic protein interactions, and synaptic vesicles were suppressed in high UPP1 expression samples (Fig. 3D). (Supplementary material 3). The MCODE plugin was used to identify 70 hub genes related to UPP1 in the protein–protein interaction (PPI) network (Fig. 3E). Correlation analysis between UPP1 mRNA expression and the top 30 hub genes is shown (Fig. 3F).
Fig. 3.
Enrichment analysis of UPP1 expression phenotype. A Volcano plot of differential gene expression (DEG) analysis based on the TCGA database for UPP1, highlighting the top 10 most significantly different genes. B GO functional enrichment analysis of UPP1 and its co-expressed mRNAs, showing the top 15 enriched functions. C GSEA enrichment analysis of DEGs. D All KEGG signaling pathways associated with UPP1 and its co-expressed mRNAs. Pathway image adapted from the KEGG database (https://www.kegg.jp/kegg/kegg1.html) with permission from Kanehisa Laboratories. E Protein–protein interactions (PPI) network analysis of DEGs. F Heatmap showing the top 30 genes associated with UPP1.
Correlation between UPP1 expression and GBM immunity
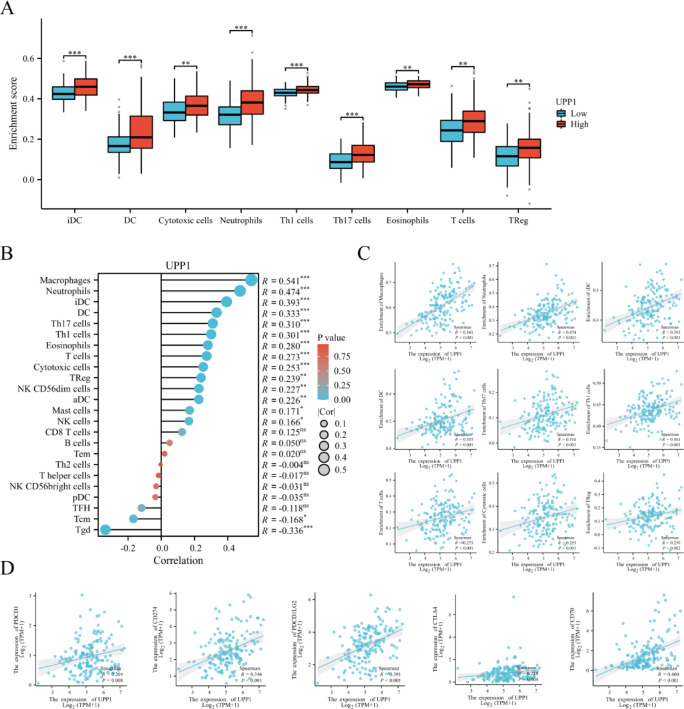
To examine the impact of UPP1 expression on the tumor microenvironment, immune infiltration analysis was conducted using the ssGSEA method. As shown in the figure and corresponding Excel data, the high UPP1 expression group exhibited significantly higher proportions of Immature Dendritic Cells (iDC), Dendritic Cells (DC), Cytotoxic Cells, Neutrophils, T Helper 1 Cells (Th1), T Helper 17 Cells (Th17), Eosinophils, T Cells, and Regulatory T Cells (Tregs) compared to the low expression group (Fig. 4A). Additionally, the ssGSEA algorithm was employed to evaluate the relationship between UPP1 expression and the relative abundance of 24 immune cell types ain GBM (Fig. 4B). UPP1 expression was significantly correlated with various types of immune cells, including Macrophages (P < 0.001, R = 0.541), Neutrophils (P < 0.001, R = 0.474), Immature Dendritic Cells (iDC; P < 0.001, R = 0.393), Dendritic Cells (DC; P < 0.001, R = 0.333), T Helper 17 Cells (Th17; P < 0.001, R = 0.310), T Helper 1 Cells (Th1; P < 0.001, R = 0.301), T Cells (P < 0.001, R = 0.273), Cytotoxic Cells (P < 0.001, R = 0.253), and T Regulatory Cells (Tregs; P = 0.002, R = 0.239) (Fig. 4C). Spearman correlation analysis further revealed a positive association between UPP1 expression and the expression of immune checkpoint molecules, including PD-1 (PDCD1; P < 0.001, R = 0.204), PD-L1 (CD274; P < 0.001, R = 0.346), PDCD1LG2 (P < 0.001, R = 0.391), CTLA4 (P < 0.001, R = 0.218), and CD70 (P < 0.001, R = 0.460) (Fig. 4D).
Fig. 4.
Correlation between UPP1 expression and GBM immunity. A Correlation between UPP1 expression and immune cell populations. Compared to the low UPP1 expression group, the high UPP1 expression group in GBM showed significantly higher proportions of iDC, DC, Cytotoxic Cells, Neutrophils, Th1 Cells, Th17 Cells, Eosinophils, T Cells, and Tregs. **P < 0.01, ***P < 0.001. B Bar chart showing the correlation between UPP1 expression and 24 immune-infiltrating cell types. The x-axis represents correlation coefficients, while the y-axis represents immune cell types. C Positive correlations between UPP1 expression and immune cell types, including Macrophages, Neutrophils, iDC, DC, Th17 Cells, Th1 Cells, T Cells, Cytotoxic Cells, and Tregs. D Correlation between UPP1 expression and tumor immune checkpoints. UPP1 expression is significantly associated with PDCD1 (PD-1), CD274 (PD-L1), CD273 (PDCD1LG2), CTLA4, and CD70.
In vitro and vivo validation of UPP1
The migratory capacity of tumor cells serves as a critical parameter for in vitro assessment of malignant cancer progression. To evaluate the role of UPP1 in regulating glioblastoma (GBM) cell migration in vitro, we conducted Transwell migration assays. Prior to functional evaluation, the transfection efficiency of LV-shUPP1 was first verified through quantitative PCR (qPCR) and Western blot (WB) analyses, which demonstrated effective knockdown of UPP1 expression (Fig. 5A–C). Subsequent Transwell experiments revealed that UPP1 knockdown significantly attenuated the migratory capacity of U87 cells (Fig. 5D, E). Consistent with these findings, in vivo experiments confirmed substantial upregulation of UPP1 expression in tumor-bearing models (Fig. 5F, G).
Fig. 5.
In vitro and vivo validation on UPP1. A Assessment of shUPP1 knockdown efficiency by quantitative PCR (qPCR). n = 5. Representative Western blot (WB) images (B) and corresponding quantitative analysis (C) validating shUPP1-mediated UPP1 silencing, from left to right: Untreat, Scrambled shRNA, 10MOI LV-shUPP1. n = 8. Transwell migration assay images (D) and statistical quantification (E) demonstrating attenuated cell migration upon UPP1 knockdown. n = 6. F, G Representative WB and quantification of UPP1 protein expression in normal and tumor-bearing brain tissues. n = 4. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
To place our findings in the context of existing literature, we compared our study with previous work on UPP1, particularly the study by Wang et al.12which first proposed UPP1 as an immune-related biomarker in gliomas. While Wang et al. identified UPP1 as a pan-glioma immune biomarker through computational analysis, our study extends this foundational work by providing experimentally validated insights specific to IDH-wildtype glioblastoma (GBM). We demonstrate, for the first time, UPP1’s functional role in promoting tumor cell migration via Transwell assays, and confirm its upregulation at the protein level in orthotopic models, highlighting its clinical relevance. Moreover, we discover eight novel UPP1 CpG methylation sites (e.g., cg07703017), providing new prognostic biomarkers that enhance survival stratification beyond transcriptomic signatures. Importantly, our comprehensive systems analysis reveals novel mechanistic insights: UPP1 activation of immunosuppressive NF-κB and complement signaling pathways, suppression of synaptic transmission, and interaction with key immune modulators (TNFSF11, CSF3). Collectively, these findings establish UPP1 as a mechanistically defined and therapeutically actionable target in IDH-wildtype GBM.
To better understand the functional relevance of UPP1 in glioblastoma biology, we further investigated its role in nucleotide metabolism and immune regulation. Uridine, also known as uracil nucleoside, is one of the four nucleosides that form RNA and serves as a precursor for uracil. Targeting uridine metabolic pathways has the potential to drive the creation of new treatment approaches for cancer and metabolic disorders, as well as aid in modulating immune responses13. UPP1 is pivotal in pyrimidine metabolism as it controls uridine homeostasis and boosts the pyrimidine salvage pathway14. Its relationship with immune-related pathways and its prognostic significance in GBM warrant further investigation, particularly against the backdrop of the immunosuppressive tumor microenvironment. This environment is characterized by tumor-associated macrophages, regulatory T cells, and other immune evasion mechanisms. Understanding the interplay between UPP1 expression, immune infiltration, and patient prognosis could provide new insights into GBM biology and therapeutic strategies. Under glucose-deprived tumor conditions, UPP1 mediates the metabolic recycling of uridine-derived ribose to maintain neoplastic cell bioenergetics, ultimately driving tumorigenesis and improving cellular viability13. The functional crosstalk between UPP1 and immune checkpoints in TME reveals a novel mechanism through which UPP1 may orchestrate tumor-immune interactions12. 5-Fluorouracil (5-FU), a commonly employed chemotherapeutic drug for diverse cancers, has its clinical use restricted because of dose—related toxicity. Clinical trial reports indicate that certain small—molecule inhibitors of UPP1, like BAU, are capable of protecting normal tissues from the harm caused by 5-FU toxicity15. Chen et al. demonstrated that UPP1 plays a critical role in regulating macrophage activity through potential mechanisms, including the ubiquitin-proteasome pathway, regulation of precursor proteins, and modulation of the expression of phagocytosis-associated surface receptors. Moreover, their findings highlight the importance of UPP1 in modulating fundamental tumorigenic processes, like cell proliferation, migration, and immune evasion16.Given these roles, it is plausible that UPP1 may also be involved in promoting epithelial-to-mesenchymal transition (EMT), a key process associated with tumor invasiveness. Although our current study focused on migration, we acknowledge that further validation of EMT-related features—such as invasion capacity and the expression of canonical EMT markers (e.g., Vimentin, N-cadherin)—would help delineate UPP1’s mechanistic role more clearly.In vivo validation in animal models is essential to elucidating the role of UPP1 in glioma progression. In this study, we utilized xenograft models to demonstrate that UPP1 expression is upregulated in tumor tissues. While these results support the oncogenic role of UPP1, a more comprehensive evaluation—including longitudinal monitoring of tumor growth dynamics, histopathological assessment of xenograft tissues, and survival analysis—would offer deeper mechanistic insights and translational relevance.
During this research, we looked into the expression levels of UPP1 mRNA in GBM and normal tissues with the help of the TCGA database. Our discoveries show a marked elevation in both UPP1 mRNA and protein levels within GBM samples. Notably, GBM patients with lower UPP1 expression demonstrated better prognoses, suggesting that UPP1 serves as a valuable prognostic marker. Analysis of the receiver operating characteristic (ROC) curve backed up the possibility of UPP1 being used as a diagnostic biomarker. Moreover, DNA methylation serves as a pivotal epigenetic mechanism in modulating clinical outcomes and tumorigenesis17. Specific DNA methylation patterns of UPP1, including sites cg07703017, cg22860780, cg00524288, cg14265670, cg10213875, cg01092213, cg02703728, and cg23513252, were found to have significant prognostic value. Previous studies have demonstrated that the CpG island methylator phenotype (G-CIMP) in gliomas is associated with global DNA hypermethylation, low immune infiltration, and favorable prognosis, primarily observed in IDH-mutant tumors18. In contrast, IDH-wildtype glioblastomas are typically non-G-CIMP, characterized by hypomethylation, immunological activation, and poor outcomes. In our study, we identified multiple hypomethylated CpG sites within the UPP1 gene (e.g., cg07703017, cg22860780), which were associated with increased UPP1 expression and poor prognosis. This hypomethylation-driven overexpression of UPP1 may contribute to an immunosuppressive tumor microenvironment by modulating the recruitment and function of immune cells. Indeed, we observed that high UPP1 expression correlated with increased infiltration of dendritic cells, regulatory T cells, and cytotoxic cells. These findings suggest a potential regulatory axis in which DNA hypomethylation of UPP1 enhances its transcription, thereby influencing immune cell behavior in IDH-wildtype gliomas. Further functional studies are needed to clarify the direct epigenetic regulation of UPP1 and its immunomodulatory effects. In summary, our results suggest that UPP1 is a promising prognostic factor in GBM, with potential applications in both diagnosis and prognosis.
Immune infiltration in GBM is a prominent area of research. Infiltrating immune cells represent vital constituents of the tumor immune microenvironment and have the potential to either promote or impede tumor progression19. In our study, we discovered a notable association between the expression of UPP1 and the degree of immune cell infiltration in GBM. We observed that elevated UPP1 expression levels had a positive correlation with the infiltration of diverse immune cells, such as immature dendritic cells (iDC), dendritic cells (DC), cytotoxic cells, neutrophils, Th1 cells, Th17 cells, eosinophils, T cells, and regulatory T cells (Treg) in GBM. Cytotoxic T cells serve a vital part in tumor immune surveillance by pinpointing and annihilating tumor cells20. Elevated UPP1 expression could potentially enable the aggregation of these cells in the tumor microenvironment, which in turn encourages the immune - mediated elimination of the tumor. However, UPP1 is also associated with immunosuppressive cells like regulatory T cells, suggesting that it may help tumors evade immune detection by modulating the activity of these suppressive cells. This dual role indicates that UPP1 may have complex immune regulatory functions within the tumor microenvironment, potentially enhancing anti-tumor immune responses while also supporting immune tolerance and escape mechanisms that allow tumor growth and metastasis. Integrated Analysis of Immune Dynamics reveals that the UPP1-high microenvironment exhibits a characteristic immunosuppressive signature. Macrophage-dominated suppression is evidenced by the strongest immune correlation observed with macrophages (R = 0.541, P < 0.001; Fig. 4C), which secrete IL-10 and TGF-β to inhibit T-cell function and promote PD-L1 expression. PD-L1-mediated immune evasion results from tumor cell-intrinsic PD-L1 upregulation (R = 0.346, P < 0.001; Fig. 4D), directly suppressing cytotoxic T-cell activity and explaining the disconnection between cytotoxic cell infiltration (R = 0.253) and anti-tumor efficacy. Ancillary suppression by Tregs further consolidates immunosuppression through CTLA-4 signaling and metabolic competition (R = 0.239). The convergence of this macrophage-driven suppression (R = 0.541) and PD-L1 elevation (R = 0.346) creates a “double shield” against immune attack. Consequently, the modest cytotoxic infiltration (R = 0.253) proves clinically ineffective. This mechanistic synergy underpins the poor prognosis associated with UPP1-high GBM.
Furthermore, the connection between UPP1 and immune cells implies that UPP1 has a key part in the immune microenvironment of GBM. To delve deeper into the function of UPP1 in GBM, we performed GSEA using the TCGA dataset. The results revealed that pathways primarily associated with immune and inflammatory responses were significantly enriched in cases with high UPP1 expression. In contrast, pathways related to neurotransmission and synaptic activity were notably enriched in cases with low UPP1 expression. These findings collectively highlight the significant impact of UPP1 on immune infiltration in GBM. Among the UPP1-related genes in GBM patients, genes such as TNFSF1121, CSF322, CSF223, and IL1A are closely linked to immune and inflammatory responses, playing vital roles in the activation, recruitment, and regulation of immune cell functions. Additionally, genes like ICAM124, PTGS225, and SERPINE126 are involved in tumor cell migration, invasion, and modulation of the tumor microenvironment. FGF827 and CSF228 may contribute to metabolic reprogramming in tumors, promoting tumor cell growth and metastasis. Based on these data, UPP1 not only serves as a potential target for immunotherapy but also may provide new biomarkers for early tumor diagnosis and prognosis assessment. Moreover, the dual regulatory role of UPP1 suggests that therapeutic strategies targeting UPP1 must consider the diversity of immune cells and the complexity of the tumor microenvironment. In addition, in the future, the combined application of UPP1 inhibitors and standard treatment can be explored in IDH wild-type glioblastoma, while patients with IDH mutations may benefit from other targeted strategies.Future studies should aim to further dissect the mechanisms by which UPP1 modulates immune evasion in gliomas. In particular, elucidating the causal role of UPP1 in shaping the recruitment, activation, and suppressive function of regulatory T cells and macrophages will be critical. Moreover, the interplay between UPP1 and immune checkpoint pathways such as PD-1/PD-L1 or CTLA-4 may provide actionable insights into immunotherapeutic resistance. These potential interactions raise the prospect of UPP1 not only as a biomarker but also as a therapeutic co-target to improve immune reactivation. Combination strategies involving UPP1 inhibitors and immune checkpoint blockade warrant exploration in preclinical models, especially in IDH-wildtype glioblastoma.
A limitation of this study stems from incomplete IDH status validation in historical datasets such as TCGA, where IDH-wildtype designation was presumed for enrolled GBM cases. Although prior studies indicate that IDH-wildtype glioblastoma accounts for over 95% of cases in WHO CNS3 (2007)-defined GBM cohorts29,30residual IDH-mutant samples may persist due to incomplete molecular stratification in historical datasets, necessitating cautious interpretation of subtype-specific analyses. Future validation in prospectively curated cohorts adhering to WHO CNS5 standards is essential to confirm these findings. While UPP1 presents itself as a promising therapeutic target, effectively inhibiting or modulating its expression to alter the tumor microenvironment remains a complex challenge.
Materials and methods
This retrospective analysis leveraged the TCGA repository, focusing on glioblastoma (GBM) cohorts classified under the WHO 2007 (CNS3) criteria. While IDH-mutant cases were not systematically excluded, prior evidence confirms that 80–95% of TCGA-GBM samples exhibit IDH-wildtype status31. Consequently, our findings predominantly capture the dominant biological characteristics of the IDH-wildtype subgroup, which exhibit substantial alignment with the molecularly refined GBM entity defined by the WHO CNS5 classification (2021).
Analysis of UPP1 expression profile
Data sourced from the TCGA database (https://portal.gdc.cancer.gov) was utilized to analyze UPP1 expression. The “ggplot2” package served the purpose of visualizing UPP1 expression, while the “pROC” package was applied to conduct the analysis of the ROC curve.
Functional enrichment analysis
To probe into the function of UPP1, multiple analyses were carried out, namely Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG)32,33 enrichment analysis, as well as Gene Set Enrichment Analysis (GSEA). The “clusterProfiler” package was used to automate the GO and KEGG term analysis process34. Patients were stratified into low- and high-UPP1 expression groups based on the median expression value (50th percentile)derived from TCGA RNA-seq data (FPKM normalized values). Specifically: low-expression group: Samples with UPP1 expression ≤ median value (bottom 50%) and high-expression group: Samples with UPP1 expression > median value (top 50%). Differential gene expression analysis was performed using raw RNA-seq counts from the same TCGA cohort. The DESeq2 package (1.36.0) was employed to identify significantly dysregulated genes between the two groups, with thresholds set at |log2 fold change| > 1 and adjusted p-value (FDR) < 0.0535.
Human protein atlas database
The Human Protein Atlas (https://www.proteinatlas.org, accessed October 7, 2024) is a Swedish-based project aimed at mapping all human proteins in cells, tissues, and organs using integrated omics technologies. These include antibody-based imaging, mass spectrometry-based proteomics, transcriptomics, and systems biology. The database was used to compare UPP1 protein expression in GBM tissues and normal brain tissues.
Immune-related analysis of UPP1
The ssGSEA algorithm was used to evaluate the infiltration of 24 immune cell types in GBM tissue samples [GSVA: Gene Set Variation Analysis for microarray and RNA-seq data]. Spearman correlation analysis was performed to assess the relationship between UPP1 expression and immune cells, as well as immune checkpoint molecules, including PD-1 (PDCD1), PD-L1 (CD274), PDCD1LG2 (PD-L2), CTLA4, and TNFSF7 (CD70). Correlation results were visualized using the “ggplot2” package. The Wilcoxon rank-sum test was applied to evaluate the enrichment of immune-infiltrating cells in GBM samples with high versus low UPP1 expression.
Differential and correlation analysis of UPP1 at the gene level
Differential expression and correlation analysis of UPP1 in GBM samples were performed using the DESeq2 and STAT packages, based on data from the TCGA database. GBM samples were divided into high- and low-expression groups based on the median UPP1 expression level. Volca no plots were generated using the “ggplot2” package, with |LogFC| > 1 and an adjusted p-value < 0.05 considered as the threshold for differentially expressed genes (DEGs). DEGs and their protein–protein interactions (PPI) were visualized using the STRING database and analyzed with Cytoscape software. The MCODE plugin was used to identify hub genes. Finally, based on the gene correlation analysis, genes were ranked in descending order by Pearson correlation values, and the top 30 correlated genes were extracted.
Correlation analysis of UPP1 expression and GBM patient prognosis
Survival data for GBM patients from the TCGA database were used to analyze the relationship between UPP1 expression and patient prognosis. The “survminer” package was employed to generate survival curves for overall survival (OS), progression-free interval (PFI), and disease-specific survival (DSS). Univariate Cox regression was applied to identify risk factors for OS based on clinical features and UPP1 expression levels. Multivariate Cox proportional hazards regression was subsequently performed to evaluate whether UPP1 expression serves as an independent prognostic factor36. Clinical covariates such as age were included in the model. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated, and statistical significance was determined using the Wald test.
DNA methylation of the UPP1 gene
DNA methylation plays a critical role in prognosis and tumor progression and serves as a potential biomarker. To explore the methylation patterns of UPP1 in GBM, we used the integrated online tool MethSurv (https://biit.cs.ut.ee/methsurv/, accessed August 12, 2024). The methylation status of individual CpG sites in the UPP1 gene was analyzed, and survival analysis was conducted using the MethSurv platform. The differential DNA methylation results were further screened using the publicly available Wanderer database (http://maplab.imppc.org/wanderer/, accessed on June 20, 2025). CpG sites with a methylation β-value ≤ 0.2 were defined as completely unmethylated, while those with β-values ≥ 0.6 were considered fully methylated37.
In vitro validation of UPP1
The U87 human glioblastoma cell line was procured from MeisenCTCC (China). U87 cells (2*107 cells/ml) suspended in 5µL PBS were stereotaxically implanted into BALB/c nude mice (6-week-old, male, purchased from BK company, Shanghai, China). ShUPP1 plasmids and control plasmids for silencing UPP1 expression were purchased from Brainvta Technology (Wuhan). TRIzol reagent was employed to extract total RNA from glioblastoma cells transfected with shRNA. Subsequently, PrimeScript™ RT Master Mix (Takara) was utilized to reverse-transcribe the RNA into cDNA. To gauge transcript abundance, quantitative real-time PCR (qPCR) was carried out on a LightCycler® 480 System (Roche) by means of gene-specific primers. Real-time monitoring of reaction fluorescence was conducted to achieve accurate mRNA quantification. The relative expression levels were computed via the 2−ΔΔCt method and then standardized against the endogenous reference gene β-actin. The primer sequence was synthesized by Sangon Biotech(Shanghai, China) and listed in Supplementary material 1. For protein analysis, treated U-87MG cells were lysed and centrifuged to collect supernatants. The Western blot (WB) method was used to detect protein levels. The specific procedure was as follows: the BCA assay (Thermo Scientific) was employed to measure protein concentrations. Equivalent quantities of protein were resolved via SDS - PAGE and then transferred onto PVDF membranes (Millipore). Once the membranes were blocked using 5% non-fat milk for 1 h, they were incubated with primary antibodies overnight at 4 °C: UPP1 (Proteintech, 14186-1-AP, 1:1000) and β-actin (Cell Signaling Technology, 5125 S, 1:5,000). HRP-conjugated secondary antibodies (Jackson ImmunoResearch, 111-035-003, 1:10,000) used for a 2-h incubation at room temperature. Protein bands were made visible through the use of an ECL kit (Beyotime) and then quantified via ImageJ software (version 1.53t; National Institutes of Health, https://imagej.nih.gov/ij/), taking β-actin as the loading control. Cell migration was assessed using Transwell chambers (Corning, 3422). In short, 5 × 10⁴ cells suspended in serum - free medium were plated into the upper chamber. Following a 24-h incubation period, the cells that had migrated to the lower surface of the membrane were fixed using methanol, stained with 0.1% crystal violet, and then counted under a microscope.
In vivo validation of UPP1
As shown in the previous study38U87 cell suspension was stereotaxically injected into the brain, and the expression of UPP1 was detected by WB method after 3 weeks of modeling.
Statistical analysis
Data processing and visualization were performed using GraphPad Prism (version 8.0; GraphPad Software, San Diego, CA, USA), with outcomes presented as mean values ± SEM. All statistical analyses were performed using R software (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria). The following R packages were used in the analysis: ggplot2 (v3.3.6) for data visualization, pROC (v1.18.0) for ROC curve analysis, clusterProfiler (v4.4.4) for functional enrichment (GO, KEGG, and GSEA), DESeq2 (v1.36.0) for differential gene expression analysis, GSVA (v1.46.0) for immune infiltration analysis via ssGSEA, survival (v3.3.1) and survminer (v 0.4.9) for survival analysis, and STAT (v4.2.1) for correlation testing. The Student’s t-test and Wilcoxon rank-sum test were used to compare normally and non-normally distributed variables between two groups, respectively. A p value < 0.05 was considered statistically significant. The following databases and software tools were used in this study: TCGA database: https://portal.gdc.cancer.gov (accessed May 2025), Human Protein Atlas: https://www.proteinatlas.org (accessed October 7, 2024), STRING database: https://string-db.org (accessed May 2025), Cytoscape software: https://cytoscape.org (version 3.10.2), MethSurv: https://biit.cs.ut.ee/methsurv/ (accessed August 12, 2024), Wanderer: http://maplab.imppc.org/wanderer/ (accessed June 20, 2025).
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
KQ, CM and XL; investigation: KQ, ZJ, XC and TJ; writing and original draft preparation: KQ and XL; software and formal analysis: LG, JY and QC; writing and editing: KQ, YZ, ZD and XL.; funding acquisition: TJ, ZD and XL. All the authors read and approved the final version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No.82474626); the Science and Technology Plan Project jointly established by the Science and Technology Department of the National Administration of Traditional Chinese Medicine and the Zhejiang Provincial Administration of Traditional Chinese Medicine (No. GZY-ZJ-KJ-24021); Zhejiang University of Traditional Chinese Medicine 2024 Student Research Fund Funded Project - A multi-functional multi-site rat fixator.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Ethical considerations
This study involved a retrospective analysis of publicly available, de-identified datasets from The Cancer Genome Atlas (TCGA). All data were collected with informed consent by the original investigators and made accessible under controlled conditions. Therefore, no additional informed consent or ethical approval was required for the use of human data, in accordance with TCGA policies and the guidelines of the Institutional Review Board of Zhejiang Chinese Medical University.
Ethical statement and informed consent
All animal experiments were approved by the Institutional Animal Care and Use Committee of Zhejiang Chinese Medical University (No.20220507-01). All experiments were conducted in accordance with the relevant specified guidelines and regulations, and the author complied with the ARRIVE guidelines.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kecheng Qian, Zhaoxing Jia, Qian Cai and Tianxiang Jiang contributed equally to this work.
Contributor Information
Congcong Ma, Email: macongcong19940307@163.com.
Xianming Lin, Email: linxianming1966@163.com.
References
- 1.Horbinski, C., Berger, T., Packer, R. J. & Wen, P. Y. Clinical implications of the 2021 edition of the WHO classification of central nervous system tumours. Nat. Rev. Neurol.18, 515–529. 10.1038/s41582-022-00679-w (2022). [DOI] [PubMed] [Google Scholar]
- 2.Mei, X. et al. Association between glioblastoma cell-derived vessels and poor prognosis of the patients. Cancer Commun. (London England). 40, 211–221. 10.1002/cac2.12026 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang, R. et al. EGFR/SRC/ERK-stabilized YTHDF2 promotes cholesterol dysregulation and invasive growth of glioblastoma. Nat. Commun.12, 177. 10.1038/s41467-020-20379-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Voort, S. R. et al. Combined molecular subtyping, grading, and segmentation of glioma using multi-task deep learning. Neuro-oncology25, 279–289. 10.1093/neuonc/noac166 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White, K. et al. Identification, validation and biological characterisation of novel glioblastoma tumour microenvironment subtypes: implications for precision immunotherapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol.34, 300–314. 10.1016/j.annonc.2022.11.008 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Kelly, W. J., Giles, A. J. & Gilbert, M. T lymphocyte-targeted immune checkpoint modulation in glioma. J. Immunother. Cancer. 810.1136/jitc-2019-000379 (2020). [DOI] [PMC free article] [PubMed]
- 7.Siddiqui, A. & Ceppi, P. A non-proliferative role of pyrimidine metabolism in cancer. Mol. Metab. 35, 100962. 10.1016/j.molmet.2020.02.005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhasin, N., Alleyne, D., Gray, O. A. & Kupfer, S. S. Vitamin D regulation of the uridine phosphorylase 1 gene and uridine-Induced DNA damage in colon in African Americans and European Americans. Gastroenterology155, 1192–1204e1199. 10.1053/j.gastro.2018.06.049 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu, Z. et al. Identification of crucial genes of pyrimidine metabolism as biomarkers for gastric cancer prognosis. Cancer Cell Int.2110.1186/s12935-021-02385-x (2021). [DOI] [PMC free article] [PubMed]
- 10.Duan, R. et al. LINC01764 promotes colorectal cancer cells proliferation, metastasis, and 5-fluorouracil resistance by regulating glucose and glutamine metabolism via promoting c-MYC translation. MedComm5, e70003. 10.1002/mco2.70003 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okumura, M. et al. Hepatocyte growth factor enhances antineoplastic effect of 5-Fluorouracil by increasing UPP1 expression in HepG2 cells. Int. J. Mol. Sci.2310.3390/ijms23169108 (2022). [DOI] [PMC free article] [PubMed]
- 12.Wang, J. et al. Uridine phosphorylase 1 is a novel immune-related target and predicts worse survival in brain glioma. Cancer Med.9, 5940–5947. 10.1002/cam4.3251 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nwosu, Z. C. et al. Uridine-derived ribose fuels glucose-restricted pancreatic cancer. Nature618, 151–158. 10.1038/s41586-023-06073-w (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du, W. et al. UPP1 enhances bladder cancer progression and gemcitabine resistance through AKT. Int. J. Biol. Sci.20, 1389–1409. 10.7150/ijbs.83774 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darnowski, J. W. & Handschumacher, R. E. Enhancement of fluorouracil therapy by the manipulation of tissue uridine pools. Pharmacol. Ther.41, 381–392. 10.1016/0163-7258(89)90115-0 (1989). [DOI] [PubMed] [Google Scholar]
- 16.Chen, Z. et al. Machine learning-based discovery of UPP1 as a key oncogene in tumorigenesis and immune escape in gliomas. Front. Immunol.1510.3389/fimmu.2024.1475206 (2024). [DOI] [PMC free article] [PubMed]
- 17.Papanicolau-Sengos, A. & Aldape, K. D. N. A. Methylation profiling: an emerging paradigm for cancer diagnosis. Annu. Rev. Pathol.17, 295–321. 10.1146/annurev-pathol-042220-022304 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Turcan, S. et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature483, 479–483. 10.1038/nature10866 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tay, C., Tanaka, A. & Sakaguchi, S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell.41, 450–465. 10.1016/j.ccell.2023.02.014 (2023). [DOI] [PubMed] [Google Scholar]
- 20.Chou, C. et al. Programme of self-reactive innate-like T cell-mediated cancer immunity. Nature605, 139–145. 10.1038/s41586-022-04632-1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, E. J. et al. Bulk and single cell transcriptomic data indicate that a dichotomy between inflammatory pathways in peripheral blood and arthritic joints complicates biomarker discovery. Cytokine127, 154960. 10.1016/j.cyto.2019.154960 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Bonilla, L. et al. Role of microglial and endothelial CD36 in post-ischemic inflammasome activation and interleukin-1β-induced endothelial activation. Brain. Behav. Immun.95, 489–501. 10.1016/j.bbi.2021.04.010 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannasi, C. et al. Unlocking the therapeutic potential of Adipose-Derived stem cell secretome in oral and maxillofacial medicine: A Composition-Based perspective. Biology1310.3390/biology13121016 (2024). [DOI] [PMC free article] [PubMed]
- 24.Carmona-Rodríguez, L., Martínez-Rey, D., Mira, E. & Mañes, S. SOD3 boosts T cell infiltration by normalizing the tumor endothelium and inducing laminin-α4. Oncoimmunology9, 1794163. 10.1080/2162402x.2020.1794163 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, S., Jiang, M., Wang, L. & Yu, S. Combined chemotherapy with cyclooxygenase-2 (COX-2) inhibitors in treating human cancers: recent advancement. Biomed. Pharmacother. (Biomedecine Pharmacotherapie)129, 110389. 10.1016/j.biopha.2020.110389 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Czekay, R. P. et al. SERPINE1: role in cholangiocarcinoma progression and a therapeutic target in the desmoplastic microenvironment. Cells1310.3390/cells13100796 (2024). [DOI] [PMC free article] [PubMed]
- 27.Boudjadi, S. et al. A fusion transcription factor-driven cancer progresses to a fusion-Independent relapse via constitutive activation of a downstream transcriptional target. Cancer Res.81, 2930–2942. 10.1158/0008-5472.Can-20-1613 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji, R. et al. IGF2BP2-meidated m(6)A modification of CSF2 reprograms MSC to promote gastric cancer progression. Cell Death Dis.14, 693. 10.1038/s41419-023-06163-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alzial, G. et al. Wild-type isocitrate dehydrogenase under the spotlight in glioblastoma. Oncogene41, 613–621. 10.1038/s41388-021-02056-1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Draaisma, K. et al. Molecular evolution of IDH wild-type glioblastomas treated with standard of care affects survival and design of precision medicine trials: a report from the EORTC 1542 study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol.38, 81–99. 10.1200/jco.19.00367 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Ceccarelli, M. et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell164, 550–563. 10.1016/j.cell.2015.12.028 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res.44, D457–462. 10.1093/nar/gkv1070 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res.28, 27–30. 10.1093/nar/28.1.27 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu, G., Wang, L. G., Han, Y. & He, Q. Y. ClusterProfiler: an R package for comparing biological themes among gene clusters. Omics J. Integr. Biol.16, 284–287. 10.1089/omi.2011.0118 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Love, M. I., Huber, W. & Anders, S. Moderated Estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol.15, 550. 10.1186/s13059-014-0550-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, J. et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell173, 400–416e411. 10.1016/j.cell.2018.02.052 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia, W. T., Qiu, W. R., Yu, W. K., Xu, Z. C. & Zhang, S. H. Identifying TME signatures for cervical cancer prognosis based on GEO and TCGA databases. Heliyon9, e15096. 10.1016/j.heliyon.2023.e15096 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu, P. et al. PRMT6-mediated transcriptional activation of ythdf2 promotes glioblastoma migration, invasion, and Emt via the wnt-β-catenin pathway. J. Exp. Clin. Cancer Res. CR43, 116. 10.1186/s13046-024-03038-3 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.