Abstract
Dietary calcium’s role in human health and disease prevention is inconclusive. We examined the associations between dietary calcium intake and the risk of overall and specific causes of mortality. A prospective cohort study was performed for 42,146 individuals from 2008 to 2019. Face-to-face interviews used structured semi-quantitative food frequency and demographic lifestyle questionnaires to obtain calcium intake. The cause of 2,494 deaths was determined from medical records. We calculated hazard ratios (HR) and corresponding 95% confidence intervals (95% CI) using a Cox proportional hazards model across eight quantiles of dietary calcium intake. Compared to the reference range 222.5-261.3 mg/day, the lowest dietary calcium intake was associated with an increased mortality risk from all causes, HR (95% CI): 1.22 (1.04, 1.42). In contrast, the highest dietary calcium intake was associated with an increased risk of cancer death, HR (95% CI): 1.43 (1.01, 2.01). By stratified analysis, the lowest dietary calcium intake was associated with an increased risk of mortality from all causes in women, in never smokers, individuals with a BMI < 23 kg/m², never drinkers, no diabetes, no history of hypertension, and no history of cancer. An increased risk of overall mortality and cancer death at the borderline was observed for the lowest and highest calcium intake, respectively. There is a possible U-shaped association between calcium intake and the risk of all causes and cancer. The reference group ranges from 222.5 mg/day to 261.3 mg/day. The findings warrant further research on the association between dietary calcium intake and overall mortality and cancer.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-10484-2.
Keywords: Dietary, Calcium, Overall and specific causes of mortality, Risk factors, Cohort study, Vietnam
Subject terms: Cancer, Medical research, Risk factors
Introduction
Calcium is an essential mineral in humans, accounting for 1.5–2% of total body weight1. The human body needs calcium to build and maintain bones and teeth, and the heart, muscles, and nerves need calcium to function properly. Calcium may also protect against cancer, diabetes, and high blood pressure. The small part of an ionized pool of calcium in the extracellular fluid, circulatory system, and various tissues mediates blood vessel contraction and dilation, blood clotting, muscle function, hormonal secretion, and nerve transmission2.
Many studies suggest that high levels of dietary calcium intake reduce the risk of mortality. Calcium intakes of up to 1348 (± 316) mg/day from food were associated with decreased risks for non-fatal cardiovascular diseases (CVD), stroke, and all-cause mortality3. A dietary calcium intake of 800 mg/day or higher decreases the risk of CVD events in women who have been menopausal for > 10 years4. The meta-analysis results suggest that high levels of calcium intake are associated with a decreased risk of all-cause mortality5. In contrast, some studies have pointed to an increased risk of mortality among people who consume high levels of calcium. For men, supplementary calcium intake of 1000 mg/day or higher may be associated with higher all-cause and CVD mortality6. For women, high intakes of calcium were associated with higher death rates from all causes and cardiovascular disease7. Due to inconsistent results and findings, the role of dietary calcium in human health and disease prevention is inconclusive. For levels of calcium intake related to health outcomes, intake of 800 mg/day or higher has been associated with a decreased risk of CVD mortality3,4. Still, other studies have observed that intake of 800 mg/day or higher has been associated with an increased risk of CVD and all causes of death6,7. A U-shaped association between calcium intake and health events might explain the above phenomenon. Some studies observed a U-shaped association between calcium intake and mortality risk from all causes or specific health events8,9.
We hypothesized that low or high calcium intake increases mortality risk from all causes and possibly specific causes of death, or there is a U-shaped association between dietary calcium intake and mortality risk. In a large population-based prospective cohort study in Northern Vietnam, we examined the associations between dietary calcium and all-cause mortality, including cancer, cardiovascular diseases, respiratory diseases, injury, and other causes.
Method
Study design and population
This Hanoi prospective cohort study (HPCS) involved 52,325 people from the nine general populations in Northern Vietnam in 2008. To select the commune’s general population, the inclusion criteria included a population size of less than 15,000 residents of each commune to reduce the loss of follow-up. The population was stable to minimize migration; daily medical records of inpatients and outpatients who had visited the state commune health station to receive health services were available. There was an active weekly updated mortality registration of deceased persons. At least one physician has been appointed to work full-time in each commune health station. This medical doctor will serve as a family doctor and oversee morbidity cases and the underlying causes of death for each deceased person, the outcome of the present prospective cohort study. At baseline in 2008, participants underwent face-to-face interviews using a specially designed questionnaire to assess dietary food frequency intake, smoking status, demographic details, refrigerator use and alcohol consumption, body weight and height, medical history, and family history of cancer. The outcome from 2008 to 2019 was all causes of death. We excluded 7,005 individuals under ten and those who had migrated (3,174 participants), resulting in a final sample of 42,146 eligible individuals for the present analysis (Supplemental Fig. 1). Detailed characteristics of the study population of HPCS and inclusion and exclusion criteria for recruiting study participants have been published elsewhere10–13.
Registered household and study participants
Over 17,000 registered households lived in these nine selected communes. In 2008, the agreement in written documents of the local government authority of the People Committee was collected. The officers of nine communes verbally invited each family to participate in the study. We planned to complete a baseline survey for all registered households and family members aged ten years and older to avoid selection bias10–13.
Cross-sectional baseline survey
The cross-sectional baseline survey was conducted to collect data from all registered households and study participants. The interviewers were third-year medical students of Hanoi Medical University who had completed four days of training and practice. The trained interviewers worked for seven consecutive days at each commune with the support of local village health volunteers and officers of the commune health stations. A household visit and a face-to-face interview were conducted to collect data on demographic and household facilities and individual study variables using the printed handout of the demographic lifestyle and semi-quantitative food frequency questionnaires10–13.
The development and validation of a semi-quantitative food frequency questionnaire
We derived data from the existing database of the National Nutritional Survey 1999–2010 for the provinces of the selected nine communes of the present prospective cohort study, and a database of 158 households with 741 persons. A direct interview using a validated questionnaire was conducted to obtain information regarding all food intake over the last 24 h of dietary records (24-HDR) for three consecutive days in these 158 households. Contribution analysis using the Nutritive Composition Table of Vietnamese Foods, revision 2000, and stepwise regression analysis was applied to select food items, and the cumulative contribution of 24 primary nutrients, up to 90%, resulted in 63 food items being selected. We also selected 17 other food items related to fermented or salty foods. Eighty food items were included in the semi-quantitative food frequency questionnaire (SQFFQ)14. All selected 80 food items were fresh, natural, local farm food products without processing used by the participants, and we categorized them as whole foods in the present study.
Calcium intake assessment
The pilot survey indicated that calcium supplementation was uncommon among participants because nine participating communes were general populations, and their monthly income was limited to ordering vitamin/mineral supplements. Dietary data were collected using a validated SQFFQ. Study participants were asked how frequently they consumed the food and food group in 6 categories, ranging from “6–11 times/year”, “1–3 times/month”, “1–2 times/week”, “3–4 times/week”, “5–6 times/week”, and “1–3 times/day”, followed by a question on the amount of food consumed from three portion sizes (i.e., small, medium and large). The average daily intake of 95 nutrients and non-nutrient compounds, including calcium, was calculated using the Vietnamese Food Composition Database for each participant14.
The SQFFQ was validated using 24-hour dietary recalls (24-HDRs) for three consecutive days among 298 families (n = 1,327 individuals). The correlation coefficients (R2) between the SQFFQ and 24-HDR ranged from 0.20 (for lipids) to 0.53 (for energy intake). In addition, for calcium and other micronutrients, a reproducibility survey was conducted with 150 individuals using the SQFFQ, with different interviewers administering the study two weeks apart. The Chi-square for calcium intake comparison was 0.65, confirming the SQFFQ’s good reproducibility15. We performed a Kernel density estimate of the residuals of dietary calcium intake. It closely follows a normal distribution (Supplemental Fig. 2). The correlation of dietary calcium intake against total energy intake was very good (R-squared = 0.61). Using 700 mg/day as the boundary, only 94 individuals consumed 700 mg/day or more (6 of whom died). The estimated mean intake of 263.1 mg/day is only 38% of the Ministry of Health’s recommended intake of 700 mg/day (Table 1) and lower than the results of a case-control study in Northern Vietnam (mean intake 340 mg/day)16, and National Nutritional Survey in 2009–2010 (mean intake 506.2 mg/day)17.
Table 1.
Characteristics of the study population by levels of calcium intake.
| Characteristics | Lowest | Level 2 | Level 3 | Level 4 | Level 5 | Level 6 | Level 7 | Highest | Total | P -value |
|---|---|---|---|---|---|---|---|---|---|---|
| Calcium intake, mean (SD), mg/day | 160.4 (19) | 192 (6.3) | 212.7 (5.6) | 241.7 (11.2) | 271.7 (6) | 295.3 (7.9) | 329.8 (12.9) | 422.7 (79.5) | 263.1 (79.8) | |
| Median, mg/day | 165.2 | 192.2 | 212.8 | 241.7 | 271.5 | 294.9 | 328.7 | 397.1 | 251.6 | |
| Min-Max, mg/day | 24-180.9 | 180.9-202.8 | 202.8-222.4 | 222.5-261.3 | 261.3-282.4 | 282.4-309.9 | 310-352.9 | 353-973.3 | 24.0-973.3 | |
| Age, mean (SD) | 41.2 (20.9) | 39.2 (19.8) | 38.9 (19.8) | 38.3 (19.4) | 38 (19.4) | 37.2 (18.8) | 37.6 (19) | 37.5 (18.7) | 38.5 (19.5) | |
| 10–29 | 1,704 | 1,782 | 1,803 | 3,711 | 1,875 | 1,891 | 1,881 | 1,880 | 16,527 | |
| 30–39 | 713 | 744 | 780 | 1,504 | 775 | 866 | 812 | 844 | 7,038 | |
| 40–49 | 680 | 790 | 742 | 1,581 | 815 | 738 | 788 | 751 | 6,885 | |
| 50–59 | 561 | 568 | 561 | 1,110 | 535 | 545 | 549 | 571 | 5,000 | |
| 60–69 | 386 | 337 | 347 | 615 | 266 | 285 | 282 | 296 | 2,814 | |
| 70–79 | 444 | 293 | 286 | 568 | 269 | 241 | 244 | 213 | 2,558 | |
| ≥ 80 | 197 | 168 | 165 | 276 | 156 | 108 | 127 | 127 | 1,324 | < 0.001 |
| History of hypertension | ||||||||||
| Yes | 201 | 178 | 183 | 350 | 175 | 160 | 155 | 152 | 1,554 | |
| No | 4,484 | 4,504 | 4,501 | 9,015 | 4,516 | 4,514 | 4,528 | 4,530 | 40,592 | 0.14 |
| Energy intake (kcal/day), mean (SD) | 1302.5 (227.5) | 1438.6 (140.1) | 1524.4 (172.6) | 1707.8 (287.6) | 1885.4 (359.9) | 1995.8 (380.1) | 2089.4 (401.6) | 2197.3 (436.7) | 1,760.9 (426.5) | |
| Tertile 1 | 4,436 | 3,658 | 2,425 | 2,097 | 601 | 412 | 315 | 140 | 14,084 | |
| Tertile 2 | 244 | 969 | 2,013 | 4,846 | 2,001 | 1,630 | 1,256 | 1,058 | 14,017 | |
| Tertile 3 | 5 | 55 | 246 | 2,422 | 2,089 | 2,632 | 3,112 | 3,484 | 14,045 | < 0.001 |
| Smoking status | ||||||||||
| Never smokers | 3,620 | 3,600 | 3,661 | 7,303 | 3,656 | 3,705 | 3,647 | 3,633 | 32,825 | |
| Past smokers | 243 | 202 | 237 | 474 | 241 | 211 | 246 | 232 | 2,086 | |
| Current smokers | 822 | 880 | 786 | 1,588 | 794 | 758 | 790 | 817 | 7,235 | 0.10 |
| Alcohol consumption | ||||||||||
| Never drinker | 3,854 | 3,820 | 3,841 | 7,710 | 3,821 | 3,819 | 3,775 | 3,809 | 34,449 | |
| Drinker 10–71 ml daily | 417 | 434 | 419 | 831 | 414 | 407 | 451 | 441 | 3,814 | |
| Drinker 72–150 ml daily | 211 | 215 | 234 | 443 | 240 | 232 | 273 | 232 | 2,080 | |
| Drinker 151–750 ml daily | 203 | 213 | 190 | 381 | 216 | 216 | 184 | 200 | 1,803 | 0.33 |
| Highest level of education | ||||||||||
| Primary school or less | 1,170 | 1,010 | 965 | 1,736 | 840 | 753 | 725 | 673 | 7,872 | |
| Secondary school or higher | 3,515 | 3,672 | 3,719 | 7,629 | 3,851 | 3,921 | 3,958 | 4,009 | 34,274 | < 0.001 |
| Family history of cancer | ||||||||||
| Yes | 10 | 7 | 9 | 17 | 11 | 5 | 4 | 5 | 68 | |
| No | 4,675 | 4,675 | 4,675 | 9,348 | 4,680 | 4,669 | 4,679 | 4,677 | 42,078 | 0.51 |
| BMI, kg/m2, mean (SD) a | 19.5 (2.7) | 19.7 (2.8) | 19.7 (2.7) | 19.7 (2.6) | 19.8 (2.9) | 19.7 (2.7) | 19.7 (2.7) | 19.8 (2.8) | 19.8 (7.4) | |
| < 18.5 | 1,243 | 1,116 | 1,132 | 2,270 | 1,152 | 1,083 | 1,120 | 1,051 | 10,167 | |
| 18.5–22.9 | 2,159 | 2,279 | 2,313 | 4,719 | 2,322 | 2,449 | 2,434 | 2,422 | 21,097 | |
| ≥ 23 | 340 | 336 | 345 | 694 | 366 | 364 | 369 | 401 | 3,215 | < 0.001 |
| Diabetes | ||||||||||
| Yes | 28 | 20 | 26 | 52 | 21 | 34 | 29 | 23 | 233 | |
| No | 4,657 | 4,662 | 4,658 | 9,313 | 4,670 | 4,640 | 4,654 | 4,659 | 41,913 | 0.57 |
| Fridge use | ||||||||||
| Yes | 1,792 | 1,942 | 2,122 | 4,523 | 2,317 | 2,529 | 2,645 | 2,775 | 20,645 | |
| No | 2,893 | 2,740 | 2,562 | 4,842 | 2,374 | 2,145 | 2,038 | 1,907 | 21,501 | < 0.001 |
The Vietnam Ministry of Health recommends 700–1000 mg/day. a Based on available data, SD is standard Deviation, and BMI is body mass index (Asian category, kg/m2). Using 700 mg/day as the boundary, only 94 individuals consumed 700 mg/day or more (6 of whom died). The estimated mean intake of 263.1 mg/day is only 38% of the Ministry of Health’s recommended intake of 700 mg/day.
Outcome determination of all causes of death
All causes of death were identified by an active mortality registration of three commune offices: the State Commune Health Station, the Justice Office, and the Office of Maternal Health and Family Planning (Phase one). Validation of the phase one mortality data for the initial mortality registry showed completeness, sensitivity, and specificity of 93.9%, 75.4%, and 98.4%, respectively18. The causes of death obtained from Phase One were determined by linking them with medical records available at the health facilities (Phase Two). All causes of mortality were identified based on medical records available at the health facilities. We used the International Classification of Diseases, Tenth Revision code for cause-specific mortality in our cohort for cancer, cardiovascular diseases, respiratory diseases, injury, and other causes18.
In the current analysis, the last follow-up was on December 31, 2019, when the information on those who died, had events, or moved out of the community was confirmed. Thereafter, from 2020 to date, a follow-up has been undertaken, but the outcome of the mortality data is being validated and is not yet ready for the present analysis. It is in part due to the COVID-19 pandemic, and the follow-up was a temporary interruption. Follow-up time was defined by years from enrolment to the date of death, loss-to-follow-up, or end of follow-up, whichever came first. The study encompassed a total of 457,228 person-years and recorded 2,494 deaths10–12.
Assessment of other covariates
Possible confounding was identified from previous studies on the risk of mortality from all causes16,19,20. In the current study, we included the following covariates: age (continuous), sex, an education level (< six years, ≥ six years), available fridge (yes/no), BMI (kg/m2, continuous), alcohol consumption (never drinker, drinker 10–71 ml daily, drinker 72–150 ml daily, drinker 151–750 ml daily), tobacco smoking (never smoker, past smoker, current smoker), history of diabetes (yes/no), family history of cancer (yes/no), history of hypertension (yes/no), total energy intake (kcal/day, tertile), total protein intake (g/day, tertile), total fat intake (g/day, tertile), total carbohydrate intake (g/day, tertile), and vitamin D intake (µg/day, tertile).
Statistical analysis
Means and standard deviations (SDs) were calculated for continuous variables, whereas counts and proportions were calculated for categorical variables. T-test and χ2 test were used to compare the difference in distributions of continuous and categorical variables, respectively, between cancer death cases and survived participants, and across categories of calcium intake. Person-years for each participant were calculated from the date of the baseline interview to the date of death, migration out of communities, or December 31, 2019, whichever occurred first.
We employed Cox proportional hazards regression analysis to determine the hazard ratios (HR) and 95% confidence intervals (95% CI) for the relationship between different levels of dietary calcium intake and all-cause mortality and cause-specific mortality compared with the reference group. HR and 95% CI were adjusted for age, sex, education level, available fridge, BMI, alcohol consumption, tobacco smoking, history of diabetes, family history of cancer, history of hypertension, total energy intake, total protein intake, total fat intake, total carbohydrate intake, and vitamin D intake.
Nine quantiles of calcium intake were created. Since the fourth and fifth quartiles were closer to each other in the lower range than the mean level, we decided to collapse them into one category. Finally, eight categories of calcium intake were used in our analysis. Since there is no preconceived notification of the recommended or safe calcium intake for Vietnamese, the range 222.5 mg/day − 261.3 mg/day was used as a reference group in the current analysis. The 8-quantile division method was applied in the study of the association between serum calcium and mortality risk21. We estimated p for trend (p_trend) for the quantiles below and those above a reference group.
We further conducted stratified analysis by sex (Men and women), BMI (< 23 kg/m2 vs. ≥23 kg/m2), smoking status (ever vs. never), alcohol drinking status (ever vs. never), hypertension status (ever vs. never), diabetes status (ever vs. never), and no history of cancer. The trend for cancer risk with calcium intake was tested based on the ordinal values of the lower and upper mean intake. The interactions between selected factors, including sex, BMI kg/m2, smoking status, alcohol drinking status, hypertension status, diabetes status, history of cancer, and calcium intake, were determined by including product terms between calcium intake and these factors in the multivariable logistic regression models. Stata software, version 10.0, facilitated these analyses. For all two-sided tests, a p-value of less than 0.05 was considered statistically significant.
Ethics approval and consent to participate
The authors confirm to follow the study protocol that was approved by the Ethics Committee of IRB-Hanoi Medical University, Vietnam, for ethics in biomedical research implementation (Approval number NCS33/HMU-IRB), and the IRB-International University of Health and Welfare, Japan (Approval number 21-Ig-92). All methods were performed and carried out following relevant ethical guidelines and Vietnam’s national regulations. We obtained written informed consent from all study participants.
Results
Our study population’s overall mean (standard deviation, SD) calcium intake was 263.1 (79.7) mg/day. The estimated mean energy intake varied and ranged from 1302.5 (227.5) kcal/day to 1760.9 (426.5) kcal/day between the lowest and highest levels of calcium intake (Table 1). Compared to survival participants, deceased participants were older (66.2 versus 36.7), had lower calcium intake, mean mg/day (254.4 versus 263.7), a higher proportion of a history of hypertension (15.9% versus 2.9%), ever smokers (37.3% versus 21.2%), (Supplemental Table 1). For all causes of death, the survival curve was lowest in the participants with the lowest mean calcium intake, 160.4 mg/day (Supplemental Fig. 3).
Compared to the reference group ranged from 222.5 mg/day to 261.3 mg/day, the results suggested a U-shaped association between calcium intake and risk of mortality by all causes and cancer death. The lowest calcium intake was associated with an increased mortality risk from all causes, HR (95% CI): 1.22 (1.04, 1.42). In contrast, the highest calcium intake was associated with an increased risk of cancer death, HR (95%CI): 1.43 (1.01, 2.01), Table 2.
Table 2.
Calcium intake and risk of mortality by all causes and cancer death.
| Person-year | Calcium intake, mean, mg/day (minimal-maximal) | Death | Rate per 1,000 | Crude HR 95% CI | P for trend | Adjusted HR 95% CI a | P for trend | Death | Rate per 1,000 | Crude HR 95% CI | P for trend | Adjusted HR 95% CI a | P for trend |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All causes of death | Cancer death (ICD-10: C01-C99) | ||||||||||||
| 50,295 | 160.4 (24.0, 180.9) | 374 | 7.4 | 1.46 (1.28, 1.67) | 0.011 | 1.22 (1.04, 1.42) | 0.043 | 70 | 1.4 | 1.26 (0.93, 1.69) | 0.09 | 1.06 (0.76, 1.49) | 0.25 |
| 50,487 | 192.0 (180.9, 202.8) | 290 | 5.7 | 1.13 (0.98, 1.30) | 1.09 (0.93, 1.27) | 69 | 1.4 | 1.23 (0.91, 1.66) | 1.16 (0.84, 1.60) | ||||
| 50,726 | 212.7 (202.8, 222.4) | 287 | 5.7 | 1.11 (0.96, 1.28) | 1.07 (0.92, 1.24) | 64 | 1.3 | 1.14 (0.84, 1.54) | 1.09 (0.79, 1.49) | ||||
| 101,728 | 241.7 (222.5, 261.3) | 518 | 5.1 | 1.00 | 1.00 | 113 | 1.1 | 1.00 | 1.00 | ||||
| 50,779 | 271.7 (261.3, 282.4) | 272 | 5.4 | 1.05 (0.91, 1.22) | 1.02 (0.88, 1.19) | 49 | 1.0 | 0.87 (0.62, 1.22) | 0.89 (0.63, 1.25) | ||||
| 50,783 | 295.3 (282.4, 309.9) | 252 | 5.0 | 0.97 (0.84, 1.13) | 1.06 (0.90, 1.24) | 63 | 1.2 | 1.12 (0.82, 1.52) | 1.24 (0.89, 1.71) | ||||
| 51,077 | 329.8 (310.0, 352.9) | 264 | 5.2 | 1.01 (0.87, 1.18) | 1.07 (0.91, 1.26) | 61 | 1.2 | 1.08 (0.79, 1.47) | 1.22 (0.87, 1.71) | ||||
| 51,353 | 422.7 (353.0, 973.3) | 237 | 4.6 | 0.90 (0.77, 1.05) | 0.25 | 0.99 (0.83, 1.17) | 0.53 | 67 | 1.3 | 1.18 (0.87, 1.59) | 0.20 | 1.43 (1.01, 2.01) | 0.010 |
Abbreviation: HR (95%CI), hazard ratio (95% confidence intervals); BMI is body mass index (Asian category, kg/m2); a Adjusted for age (continuous), sex, an education level (< six years, ≥ six years), available fridge (yes/no), BMI (kg/m2, continuous), alcohol consumption (never drinker, drinker 10–71 ml daily, drinker 72–150 ml daily, drinker 151–750 ml daily), tobacco smoking (never smoker, pass smoker, current smoker), history of diabetes (yes/no), family history of cancer (yes/no), history of hypertension (yes/no), total energy intake (kcal/day, tertile), total protein intake (g/day, tertile), total fat intake (g/day, tertile), total carbohydrate intake (g/day, tertile), and vitamin D intake (µg/day, tertile).
By stratified analysis, compared to the reference group, the lowest intake of calcium was associated with an increased risk of mortality from all causes in women, HR (95% CI): 1.29 (1.01, 1.63), in never smokers, HR (95%CI): 1.22 (1.00, 1.49), body-mass-index kg/m2 < 23, HR (95%CI): 1.24 (1.04, 1.48), never drinkers, HR (95%CI): 1.28 (1.05, 1.55), no diabetes, HR (95%CI): 1.22 (1.04, 1.43), no history of hypertension, and no history of cancer, all ps_heterogeneity >0.05, Table 3.
Table 3.
Calcium intake and risk of mortality by specific subgroups.
| Calcium intake, mean, mg/day (minimal-maximal) | Person-year | Death | Rate per 1,000 | Crude HR 95% CI | P for trend | Adjusted HR 95% CI a | P for trend |
|---|---|---|---|---|---|---|---|
| Men | |||||||
| 160.7 (24.0, 180.9) | 23,269 | 206 | 8.9 | 1.43 (1.20, 1.70) | < 0.001 | 1.19 (0.97, 1.47) | 0.46 |
| 191.9 (180.9, 202.8) | 24,374 | 163 | 6.7 | 1.08 (0.89, 1.30) | 1.00 (0.81, 1.23) | ||
| 212.6 (202.8, 222.4) | 23,703 | 160 | 6.8 | 1.08 (0.89, 1.31) | 0.99 (0.81, 1.21) | ||
| 241.7 (222.5, 261.3) | 48,788 | 304 | 6.2 | 1.00 | 1.00 | ||
| 271.6 (261.3, 282.4) | 24,314 | 149 | 6.1 | 0.98 (0.81, 1.20) | 0.92 (0.75, 1.12) | ||
| 295.3 (282.4, 309.9) | 24,314 | 153 | 6.3 | 1.01 (0.83, 1.23) | 1.02 (0.83, 1.25) | ||
| 330.0 (310.0, 352.9) | 24,446 | 153 | 6.3 | 1.00 (0.82, 1.22) | 1.03 (0.83, 1.27) | ||
| 422.9 (353.0, 973.3) | 24,388 | 128 | 5.2 | 0.84 (0.68, 1.03) | 0.21 | 0.90 (0.71, 1.13) | 0.95 |
| Women | |||||||
| 160.2 (24.6, 180.9) | 27,026 | 168 | 6.2 | 1.54 (1.26, 1.89) | < 0.001 | 1.29 (1.01, 1.63) | 0.028 |
| 192.1 (180.9, 202.8) | 26,113 | 127 | 4.9 | 1.21 (0.97, 1.50) | 1.24 (0.97, 1.58) | ||
| 212.7 (202.8, 222.4) | 27,023 | 127 | 4.7 | 1.16 (0.93, 1.45) | 1.18 (0.94, 1.48) | ||
| 241.7 (222.5, 261.3) | 52,940 | 214 | 4.0 | 1.00 | 1.00 | ||
| 271.7 (261.3, 282.4) | 26,465 | 123 | 4.6 | 1.15 (0.92, 1.44) | 1.19 (0.95, 1.49) | ||
| 295.4 (282.4, 309.9) | 26,469 | 99 | 3.7 | 0.92 (0.73, 1.17) | 1.11 (0.86, 1.43) | ||
| 329.7 (310.0, 352.9) | 26,631 | 111 | 4.2 | 1.03 (0.82, 1.30) | 1.12 (0.87, 1.44) | ||
| 422.6 (353.0, 907.5) | 26,965 | 109 | 4.0 | 1.00 (0.79, 1.26) | 0.81 | 1.12 (0.86, 1.45) | 0.42 |
| P heterogeneity | 0.82 | 0.35 | |||||
| Never smokes | |||||||
| 160.3 (24.0, 180.9) | 39,167 | 229 | 5.8 | 1.54 (1.30, 1.83) | < 0.001 | 1.22 (1.00, 1.49) | 0.026 |
| 192.0 (180.9, 202.8) | 38,969 | 186 | 4.8 | 1.25 (1.05, 1.51) | 1.16 (0.95, 1.41) | ||
| 212.7 (202.8, 222.4) | 39,762 | 190 | 4.8 | 1.26 (1.05, 1.50) | 1.22 (1.01, 1.47) | ||
| 241.8 (222.5, 261.3) | 79,761 | 304 | 3.8 | 1.00 | 1.00 | ||
| 271.7 (261.3, 282.4) | 39,695 | 180 | 4.5 | 1.19 (0.99, 1.43) | 1.15 (0.95, 1.39) | ||
| 295.3 (282.4, 309.9) | 40,427 | 160 | 4.0 | 1.04 (0.86, 1.26) | 1.13 (0.92, 1.39) | ||
| 329.8 (310.0, 352.9) | 39,942 | 163 | 4.1 | 1.07 (0.88, 1.29) | 1.11 (0.90, 1.36) | ||
| 422.8 (353.0, 973.3) | 39,991 | 152 | 3.8 | 0.99 (0.82, 1.21) | 0.90 | 1.05 (0.84, 1.30) | 0.40 |
| Ever smokers | |||||||
| 160.9 (42.3, 180.9) | 11,128 | 145 | 13.0 | 1.34 (1.09, 1.66) | 0.07 | 1.22 (0.96, 1.57) | 0.50 |
| 192.0 (181.0, 202.8) | 11,518 | 104 | 9.0 | 0.93 (0.73, 1.17) | 0.98 (0.76, 1.27) | ||
| 212.7 (202.8, 222.4) | 10,964 | 97 | 8.8 | 0.91 (0.71, 1.15) | 0.86 (0.67, 1.10) | ||
| 241.4 (222.5, 261.3) | 21,967 | 214 | 9.7 | 1.00 | 1.00 | ||
| 271.6 (261.3, 282.4) | 11,084 | 92 | 8.3 | 0.85 (0.67, 1.09) | 0.84 (0.65, 1.08) | ||
| 295.5 (282.4, 309.9) | 10,356 | 92 | 8.9 | 0.91 (0.71, 1.16) | 0.93 (0.72, 1.21) | ||
| 329.8 (310.0, 352.9) | 11,135 | 101 | 9.1 | 0.93 (0.73, 1.17) | 0.99 (0.77, 1.29) | ||
| 422.5 (353.0, 907.5) | 11,362 | 85 | 7.5 | 0.76 (0.59, 0.98) | 0.08 | 0.87 (0.65, 1.15) | 0.68 |
| P heterogeneity | 0.76 | 0.59 | |||||
| BMI kg/m2 < 23 | |||||||
| 160.3 (24.0, 180.9) | 40,263 | 289 | 7.2 | 1.51 (1.30, 1.76) | < 0.001 | 1.24 (1.04, 1.48) | 0.07 |
| 191.9 (180.9, 202.8) | 40,375 | 214 | 5.3 | 1.12 (0.95, 1.32) | 1.09 (0.91, 1.30) | ||
| 212.7 (202.8, 222.4) | 41,133 | 228 | 5.5 | 1.17 (0.99, 1.37) | 1.10 (0.93, 1.30) | ||
| 241.9 (222.5, 261.3) | 83,715 | 398 | 4.8 | 1.00 | 1.00 | ||
| 271.7 (261.3, 282.4) | 41,642 | 219 | 5.3 | 1.11 (0.94, 1.31) | 1.07 (0.90, 1.27) | ||
| 295.3 (282.4, 309.9) | 42,350 | 210 | 5.0 | 1.04 (0.88, 1.23) | 1.12 (0.94, 1.34) | ||
| 329.7 (310.0, 352.9) | 42,884 | 212 | 4.9 | 1.04 (0.88, 1.23) | 1.08 (0.91, 1.30) | ||
| 422.5 (353.0, 973.3) | 42,579 | 199 | 4.7 | 0.98 (0.83, 1.16) | 0.85 | 1.10 (0.91, 1.33) | 0.14 |
| BMI kg/m2 ≥ 23 | |||||||
| 161.2 (42.3, 180.9) | 10,032 | 85 | 8.5 | 1.27 (0.96, 1.68) | 0.10 | 1.23 (0.89, 1.70) | 0.24 |
| 192.1 (180.9, 202.8) | 10,112 | 76 | 7.5 | 1.13 (0.85, 1.51) | 1.17 (0.85, 1.61) | ||
| 212.5 (202.8, 222.4) | 9,593 | 59 | 6.2 | 0.92 (0.68, 1.26) | 0.99 (0.72, 1.36) | ||
| 241.0 (222.5, 261.3) | 18,014 | 120 | 6.7 | 1.00 | 1.00 | ||
| 271.5 (261.3, 282.4) | 9,137 | 53 | 5.8 | 0.87 (0.63, 1.20) | 0.89 (0.64, 1.24) | ||
| 295.4 (282.5, 309.9) | 8,433 | 42 | 5.0 | 0.74 (0.52, 1.05) | 0.81 (0.55, 1.18) | ||
| 330.2 (310.0, 352.9) | 8,192 | 52 | 6.3 | 0.95 (0.68, 1.31) | 1.05 (0.73, 1.52) | ||
| 423.9 (353.8, 907.5) | 8,774 | 38 | 4.3 | 0.64 (0.44, 0.92) | 0.036 | 0.62 (0.41, 0.93) | 0.12 |
| P heterogeneity | 0.14 | 0.44 | |||||
| Never drinkers | |||||||
| 160.2 (24.0, 180.9) | 41,636 | 258 | 6.2 | 1.61 (1.37, 1.89) | < 0.001 | 1.28 (1.05, 1.55) | 0.010 |
| 192.0 (180.9, 202.8) | 41,367 | 196 | 4.7 | 1.23 (1.03, 1.47) | 1.20 (0.99, 1.46) | ||
| 212.6 (202.8, 222.4) | 41,745 | 190 | 4.6 | 1.18 (0.99, 1.41) | 1.18 (0.98, 1.42) | ||
| 241.7 (222.5, 261.3) | 84,179 | 325 | 3.9 | 1.00 | 1.00 | ||
| 271.6 (261.3, 282.4) | 41,503 | 184 | 4.4 | 1.15 (0.96, 1.38) | 1.14 (0.95, 1.37) | ||
| 295.4 (282.4, 309.9) | 41,714 | 163 | 3.9 | 1.01 (0.84, 1.22) | 1.12 (0.91, 1.36) | ||
| 329.8 (310.0, 352.9) | 41,336 | 171 | 4.1 | 1.07 (0.89, 1.29) | 1.13 (0.92, 1.38) | ||
| 421.8 (353.0, 973.3) | 41,978 | 150 | 3.6 | 0.92 (0.76, 1.12) | 0.54 | 0.98 (0.79, 1.21) | 0.63 |
| Ever drinkers | |||||||
| 161.4 (42.3, 180.9) | 8,660 | 116 | 13.4 | 1.22 (0.97, 1.54) | 0.28 | 1.10 (0.84, 1.44) | 0.94 |
| 191.9 (181.0, 202.8) | 9,120 | 94 | 10.3 | 0.94 (0.73, 1.20) | 0.90 (0.69, 1.18) | ||
| 212.9 (202.8, 222.4) | 8,981 | 97 | 10.8 | 0.98 (0.77, 1.25) | 0.90 (0.70, 1.16) | ||
| 242 (222.5, 261.3) | 17,549 | 193 | 11.0 | 1.00 | 1.00 | ||
| 271.7 (261.3, 282.4) | 9,276 | 88 | 9.5 | 0.86 (0.67, 1.11) | 0.85 (0.66, 1.10) | ||
| 295.2 (282.5, 309.9) | 9,069 | 89 | 9.8 | 0.89 (0.69, 1.15) | 0.95 (0.73, 1.24) | ||
| 330.0 (310.0, 352.9) | 9,741 | 93 | 9.5 | 0.86 (0.67, 1.10) | 0.95 (0.73, 1.25) | ||
| 426.9 (353.0, 907.5) | 9,375 | 87 | 9.3 | 0.84 (0.65, 1.08) | 0.14 | 0.98 (0.73, 1.30) | 0.91 |
| P heterogeneity | 0.26 | 0.53 | |||||
| No diabetes | |||||||
| 160.4 (24.0, 180.9) | 50,006 | 368 | 7.4 | 1.48 (1.29, 1.69) | < 0.001 | 1.22 (1.04, 1.43) | 0.041 |
| 192.0 (180.9, 202.8) | 50,307 | 281 | 5.6 | 1.12 (0.97, 1.29) | 1.07 (0.91, 1.25) | ||
| 212.7 (202.8, 222.4) | 50,478 | 279 | 5.5 | 1.11 (0.96, 1.28) | 1.06 (0.92, 1.24) | ||
| 241.7 (222.5, 261.3) | 101,210 | 506 | 5.0 | 1.00 | 1.00 | ||
| 271.7 (261.3, 282.4) | 50,567 | 267 | 5.3 | 1.06 (0.91, 1.23) | 1.03 (0.88, 1.19) | ||
| 295.4 (282.4, 309.9) | 50,422 | 246 | 4.9 | 0.98 (0.84, 1.14) | 1.06 (0.90, 1.25) | ||
| 329.8 (310.0, 352.9) | 50,772 | 256 | 5.0 | 1.01 (0.87, 1.17) | 1.06 (0.90, 1.25) | ||
| 422.8 (353.0, 973.3) | 51,129 | 231 | 4.5 | 0.90 (0.77, 1.05) | 0.23 | 0.98 (0.83, 1.17) | 0.57 |
| Diabetes | |||||||
| 158.4 (84.6, 180.8) | 289 | 6 | 20.8 | 0.89 (0.33, 2.37) | 0.61 | 0.95 (0.29, 3.15) | 0.76 |
| 191.9 (182.1, 202.7) | 180 | 9 | 50.1 | 2.27 (0.95, 5.38) | 2.38 (0.87, 6.47) | ||
| 213.0 (202.9, 221.2) | 248 | 8 | 32.3 | 1.42 (0.58, 3.48) | 1.37 (0.51, 3.69) | ||
| 238.5 (223.1, 259.6) | 519 | 12 | 23.1 | 1.00 | 1.00 | ||
| 271.4 (262.3, 282.0) | 212 | 5 | 23.6 | 1.01 (0.36, 2.88) | 0.92 (0.3, 2.81) | ||
| 294.6 (283.6, 309.4) | 361 | 6 | 16.6 | 0.70 (0.26, 1.88) | 0.58 (0.20, 1.68) | ||
| 328.9 (310.7, 351.0) | 305 | 8 | 26.2 | 1.13 (0.46, 2.76) | 1.44 (0.51, 4.06) | ||
| 418.6 (355.6, 692.5) | 225 | 6 | 26.7 | 1.19 (0.45, 3.17) | 0.76 | 1.46 (0.46, 4.62) | 0.36 |
| P heterogeneity | 0.96 | 0.51 | |||||
| No history of hypertension | |||||||
| 160.5 (24.0, 180.9) | 48,323 | 308 | 6.4 | 1.44 (1.24, 1.67) | < 0.001 | 1.19 (1.01, 1.41) | 0.09 |
| 192.0 (180.9, 202.8) | 48,743 | 244 | 5.0 | 1.13 (0.97, 1.32) | 1.09 (0.92, 1.29) | ||
| 212.7 (202.8, 222.4) | 48,933 | 236 | 4.8 | 1.09 (0.93, 1.27) | 1.05 (0.89, 1.24) | ||
| 241.7 (222.5, 261.3) | 98,217 | 436 | 4.4 | 1.00 | 1.00 | ||
| 271.7 (261.3, 282.4) | 49,048 | 229 | 4.7 | 1.05 (0.90, 1.23) | 1.04 (0.88, 1.22) | ||
| 295.3 (282.4, 309.9) | 49,203 | 209 | 4.2 | 0.96 (0.81, 1.13) | 1.05 (0.88, 1.25) | ||
| 329.8 (310.0, 352.9) | 49,496 | 227 | 4.6 | 1.03 (0.88, 1.21) | 1.08 (0.91, 1.29) | ||
| 422.4 (353.0, 973.3) | 49,776 | 209 | 4.2 | 0.94 (0.8, 1.11) | 0.56 | 1.02 (0.85, 1.22) | 0.40 |
| Hypertension | |||||||
| 158.5 (56.9, 180.8) | 1,973 | 66 | 33.5 | 1.45 (1.05, 2.01) | 0.038 | 1.40 (0.94, 2.07) | 0.19 |
| 191.2 (181.3, 202.7) | 1,744 | 46 | 26.4 | 1.14 (0.8, 1.64) | 1.13 (0.75, 1.69) | ||
| 212.7 (202.9, 222.1) | 1,792 | 51 | 28.5 | 1.23 (0.86, 1.74) | 1.17 (0.81, 1.69) | ||
| 241.2 (222.5, 261.3) | 3,511 | 82 | 23.4 | 1.00 | 1.00 | ||
| 270.4 (261.5, 282.4) | 1,731 | 43 | 24.8 | 1.07 (0.74, 1.54) | 0.99 (0.68, 1.45) | ||
| 295.4 (283.2, 309.9) | 1,580 | 43 | 27.2 | 1.17 (0.81, 1.69) | 1.05 (0.70, 1.56) | ||
| 329.9 (310.5, 352.9) | 1,581 | 37 | 23.4 | 1.00 (0.68, 1.47) | 0.96 (0.63, 1.46) | ||
| 432.9 (355.6, 852.3) | 1,578 | 28 | 17.7 | 0.75 (0.49, 1.15) | 0.35 | 0.84 (0.52, 1.36) | 0.76 |
| P heterogeneity | 0.57 | 0.71 | |||||
| No history of cancer | |||||||
| 160.5 (24.0, 180.9) | 50,213 | 370 | 7.4 | 1.46 (1.28, 1.67) | < 0.001 | 1.21 (1.03, 1.41) | 0.06 |
| 192.0 (180.9, 202.8) | 50,428 | 287 | 5.7 | 1.13 (0.97, 1.30) | 1.09 (0.93, 1.28) | ||
| 212.7 (202.8, 222.4) | 50,642 | 283 | 5.6 | 1.10 (0.96, 1.28) | 1.06 (0.91, 1.23) | ||
| 241.7 (222.5, 261.3) | 101,562 | 514 | 5.1 | 1.00 | 1.00 | ||
| 271.7 (261.3, 282.4) | 50,684 | 269 | 5.3 | 1.05 (0.91, 1.22) | 1.03 (0.89, 1.20) | ||
| 295.4 (282.4, 309.9) | 50,729 | 251 | 4.9 | 0.98 (0.84, 1.14) | 1.06 (0.90, 1.24) | ||
| 329.8 (310.0, 352.9) | 51,038 | 263 | 5.2 | 1.02 (0.88, 1.18) | 1.07 (0.91, 1.26) | ||
| 422.8 (353.0, 973.3) | 51,302 | 236 | 4.6 | 0.90 (0.78, 1.06) | 0.28 | 0.99 (0.84, 1.18) | 0.50 |
| P heterogeneity | 0.32 | 0.19 | |||||
Abbreviation: HR (95%CI), hazard ratio (95% confidence intervals); BMI is body mass index (Asian category, kg/m2); a Adjusted for age (continuous), sex (if applicable), an education level (< 6 years, ≥ six years), available fridge (yes/no), BMI (if applicable, kg/m2, continuous), alcohol consumption (if applicable, never drinker, drinker 10–71 ml daily, drinker 72–150 ml daily, drinker 151–750 ml daily), tobacco smoking (if applicable, never smoker, pass smoker, current smoker), history of diabetes (if applicable, yes/no), family history of cancer (if applicable, yes/no), history of hypertension (if applicable, yes/no), total energy intake (kcal/day, tertile), total protein intake (g/day, tertile), total fat intake (g/day, tertile), total carbohydrate intake (g/day, tertile), and vitamin D intake (µg/day, tertile).
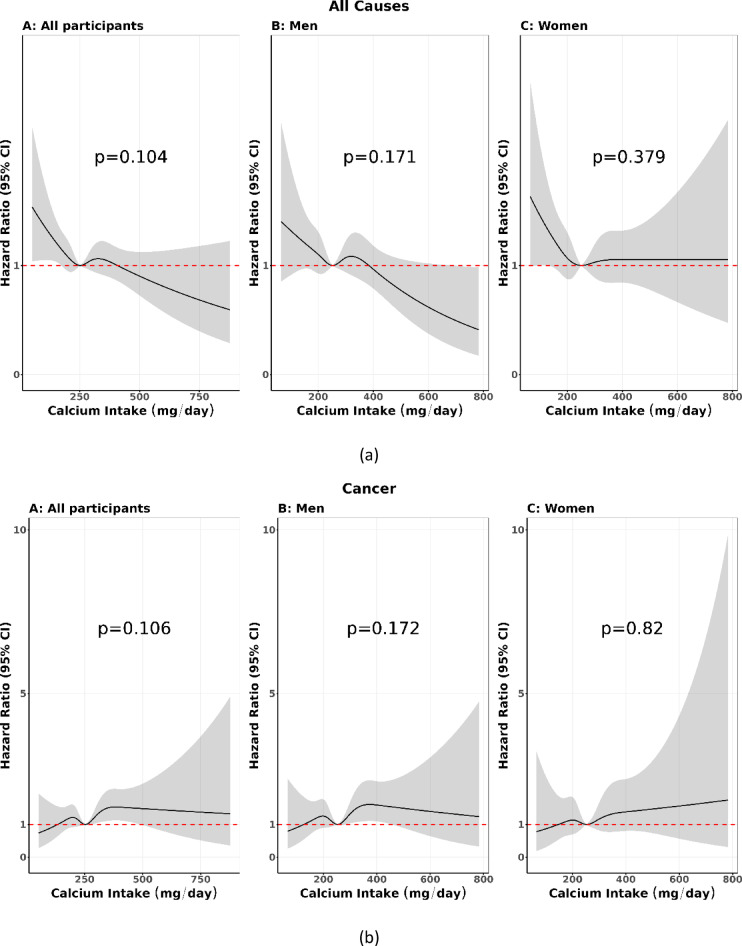
By specific causes of death, a null association between calcium intake and mortality was seen due to cardiovascular diseases, respiratory diseases, and injury. For the other cause due to infectious diseases coded ICD-10: A00-B99, and non-infectious diseases coded D55-G95 (a total of 1,484 deaths), the lowest intake of calcium was associated with an increased risk of mortality, HR (95%CI): 1.41 (1.13, 1.77), Supplemental Table 2. Restricted cubic splines suggest that the risk of all causes among all participants was increased at the borderline when people’s intake of 250 mg/day or lower, but not when it was 250 mg/day or higher (Fig. 1a). In contrast, cancer risk among all participants was slightly increased when people’s intake increased from 250 mg/day to 500 mg/day, then attenuated to a non-significant association (Fig. 1b).
Fig. 1.
(a) Restricted cubic splines calcium intake and all cause, (b) Restricted cubic splines calcium intake and cancer.
Discussion
We observed that the lowest calcium intake was associated at the borderline with an increased mortality risk from all causes among all participants and in the subgroups of women and the subgroups of never smokers, body-mass-index kg/m2 < 23, never drinkers, no diabetes, no history of hypertension, and no history of cancer. The highest calcium intake was also associated at the borderline with an increased cancer risk among all participants. These results suggest that there is a possible U-shaped association between calcium intake and the risk of all causes and cancer. The reference group ranges from 222.5 mg/day to 261.3 mg/day.
The study population’s baseline data in 2008 and also the national nutritional dietary survey in 2009–2010 had a lower dietary calcium intake than the Vietnam Ministry of Health’s recommendation of the mean intake of 700 mg/day17,22. This could be explained that during the 2000s, the Vietnamese social economy situation was developing country and there was a poor diet. The estimated mean dietary calcium intake of the present study is lower than the national nutritional dietary survey in 2009–2010 might be due to differences in the methods of sampling and using SQFFQ and 24-HDR, respectively.
The present findings suggest that the lowest dietary calcium intake is associated with an increased mortality risk, and the highest intake increases cancer risk. These findings are consistent with those of previous prospective cohort studies8,9. A U-shaped association between calcium intake and mortality risk was observed in the Swedish mammography cohort, a population-based cohort established in 1987-907. Supplemental calcium intake of 1000 mg/day or higher may be associated with higher all-cause and CVD-specific mortality in men in the U.S. Cancer Prevention Study II Nutrition Cohort6. In the EPIC-Norfolk study, mortality risk was lowest in the fourth quintile but not in the fifth quintile. This suggests a U-shaped association between calcium intake and mortality risk5. A cross-sectional study in Korea has suggested a U-shaped association between dietary calcium intake and CVD risk among 12,348 women aged 45–70. Medium calcium intake is associated with the lowest risk of CVD4. Recent studies have demonstrated that lower dietary calcium intake and low serum calcium were associated with a higher risk of all-cause mortality. In a large prospective cohort study of Korean adults, lower dietary calcium intake was associated with a higher risk of all-cause mortality23. L-shaped associations of serum calcium with all-cause and CVD mortality were observed in U.S. adults, and hypocalcemia was associated with a higher risk of all-cause and CVD mortality. There is a significantly higher risk of all-cause mortality and CVD mortality in the first quantile of serum calcium24. Low serum calcium is associated with higher long-term mortality in myocardial infarction patients25. The association between low serum calcium and an increased risk of all-cause mortality has been investigated in various populations and hospital settings. Low plasma ionized calcium was associated with an increased all-cause, cancer, and other mortality in the general population21,26. Overall, the study population subgroup of races modified the U-shaped association between calcium and all-cause mortality21. Low serum calcium was an independent predictor of all-cause mortality in patients with acute pulmonary thromboembolism, neonatal sepsis patients, elderly patients with sepsis, and critically ill patients with acute kidney injury27–30.
The study results were consistent with a study in a Swedish population of 61,433 women, with calcium from foods and supplements at the highest level of 1,400 or above mg/day and the lowest level of less than 600 mg/day increasing the risk of death, compared with the reference group of 600–999 mg/day31. A low dietary calcium intake was associated with an increased risk of all-cause mortality in Korean adults32. The association between serum calcium and mortality risk was U-shaped in a historical cohort study in the U.S. Department of Veterans Affairs health care facilities21. A high serum calcium was associated with a high risk of all-cause mortality among type 2 diabetes mellitus patients using the National Health and Nutrition Examination Surveys33. Each 0.1 mmol/L lower plasma ionized calcium below the median of 1.21 mmol/L was associated with an increased risk of all-cause mortality26.
Investigators have a hypothesis that serum calcium is a prospective biomarker of fatal prostate cancer because high levels of calcium in serum may promote the growth of potentially fatal cancers34. Comparing men at the top with men in the bottom tertile of serum calcium, the multivariable-adjusted relative hazard for fatal prostate cancer was 2.68 (95% confidence interval, 1.02–6.99. A similar pattern of serum calcium is associated with an increased risk of deadly prostate cancer, top-to-bottom quintiles comparison using data on the National Health and Nutrition Examination Survey between high levels of total calcium in serum, measured prospectively, and risk of fatal prostate cancer35,36. These findings have supported the present study’s U-shaped association between dietary calcium intake and cancer risk.
Possible explanations and implications
Diets that are lower than the reference ranges of calcium level or very high in calcium can override normal homeostatic control, causing changes in blood levels of calcium or calciotropic hormones. Calcium-enriched meals can reduce calcitriol, the active vitamin D metabolite, by inhibiting 1α-hydroxylase and also increase serum levels of fibroblast growth factor 23. Higher levels of circulating fibroblast growth factor 23 are associated with an increased risk of cardiovascular events and all-cause mortality34,37–41.
Strengths and weaknesses of the study
Our study’s strengths include the population-based prospective design, a large study size, and validated calcium intake measurements. This prospective cohort study included 42,146 participants, both men and women aged ten and older, with a follow-up period of nearly 12 years. This provides a robust sample size for our analysis. Data on calcium intake were derived from many types of whole foods. We adjusted for d for several essential covariates.
This study certainly has limitations. We only evaluated calcium intake from whole foods, but no other sources of supplements, because they are not commonly used among the study population. The accuracy of dietary assessment methods could be concerned with the precision and accuracy of measurements. SQFFQ is used in large studies to assess habitual dietary intake and is a valid method for assessing dietary mineral intake, particularly calcium42. However, these questionnaires may suffer from measurement errors in estimating calcium intake due to inaccurate recall and participant-reported bias. Besides, despite accounting for several confounding factors in the multivariable-adjusted model, residual confounding factors are likely to remain. The outcome was mortality, which might be affected by treatment and palliative care.
In conclusion
The prospective cohort study provides inconclusive results in linking dietary calcium intake to overall and cancer mortality. The results suggest an increased risk of overall mortality and cancer death at the borderline for the lowest and highest calcium intake, respectively. There is a possible U-shaped association between calcium intake and the risk of all causes and cancer. The reference group ranges from 222.5 mg/day to 261.3 mg/day. The findings warrant further research on the association between dietary calcium intake and overall mortality, cancer, and its mechanism.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors appreciate all the participants in the three northern provinces of Vietnam for their time and dedication to this study. The Vietnam Ministry of Science and Technology supported the survey from 2006 to 2011 and from 2017 to 2019. We sincerely thank Jun Tsuda for revising the manuscript.
Abbreviations
- BMI
Body mass index
- SQFFQ
Semi-quantitative food frequency questionnaire
- HR (95%CI)
Hazard ratio (95% confidence interval)
Author contributions
All authors reviewed the manuscript and contributed revisions. N.T.L. T.G.N. N.Y.N.H. L.T.L. and H.L.N. were mainly responsible for drafting, revision, and analysis. N.T.L. T.G.N. and L.T.L. were principally responsible for data collection. N.T.L. extracted data and was mainly responsible for managing and analyzing data. N.T.L. and T.G.N. were major manuscript writing contributors. All authors approved the version for publication.
Funding
The Grant Agreement No.: 18/FIRST/1a/HMU, Under the Project: “Fostering Innovation through Research, Science, and Technology,” during 2017–2019; Viet Nam Ministry of Science and Technology, during 2006–2011.
Data availability
Data is available from the corresponding author on request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ngoan Tran Le and Thinh Gia Nguyen contributed equally as co-first authors.
Contributor Information
Ngoan Tran Le, Email: letngoan@hmu.edu.vn, Email: letranngoan@duytan.edu.vn.
Hieu Lan Nguyen, Email: Nguyenlanhieu.muh@gmail.com.
References
- 1.National Research Council (US). Committee on Diet and Health, Diet and Health: Implications for Reducing Chronic Disease Risk, National Academies Press (US), Washington (DC). https://www.ncbi.nlm.nih.gov/books/NBK218743/ (1989). 10.17226/1222. [PubMed]
- 2.National Institutes of Health. Calcium-Health Professional Fact Sheet, Office of Dietary Supplements, NIH (2024). https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/.
- 3.Khan, B. et al. Higher dietary calcium intakes are associated with reduced risks of fractures, cardiovascular events, and mortality: A prospective cohort study of older men and women. J. Bone Min. Res.30, 1758–1766 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Lee, J. K. et al. S.K. Seo, association between daily dietary calcium intake and the risk of cardiovascular disease (CVD) in postmenopausal Korean women. Nutrients16 (2024). [DOI] [PMC free article] [PubMed]
- 5.Pana, T. A. et al. Calcium intake, calcium supplementation and cardiovascular disease and mortality in the British population: EPIC-norfolk prospective cohort study and meta-analysis. Eur. J. Epidemiol.36, 669–683 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang, B. et al. Calcium intake and mortality from all causes, cancer, and cardiovascular disease: The Cancer prevention study II nutrition cohort. Am. J. Clin. Nutr.103, 886–894 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Michaelsson, K., Melhus, H., Warensjo Lemming, E., Wolk, A. & Byberg, L. Long term calcium intake and rates of all cause and cardiovascular mortality: Community based prospective longitudinal cohort study. BMJ346, f228 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun, X. et al. U-shaped association between dietary calcium density intake during adolescence and hypertension in adulthood: A 20-year longitudinal nationwide study in China. Br. J. Nutr.127, 1723–1730 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Wang, X. et al. Dietary calcium intake and mortality risk from cardiovascular disease and all causes: A meta-analysis of prospective cohort studies. BMC Med.12, 158 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le, N. T. et al. Waterpipe tobacco smoking and risk of Cancer mortality. JAMA Oncol.10, 1237–1244 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le, P. H. et al. Waterpipe tobacco smoking and risk of all-cause mortality: A prospective cohort study. Int. J. Epidemiol.53 (2023). [DOI] [PubMed]
- 12.Kieu, H. D., Van Phan, C., Tran, H. H. & Le, N. T. Novel hazards of waterpipe tobacco and the benefits of stopping smoking in men, a prospective cohort study. Sci. Rep.13, 7346 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le, H. X. et al. A prospective cohort study on the association between waterpipe tobacco smoking and gastric cancer mortality in Northern Vietnam. BMC Cancer. 22, 803 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngoan, L. T. et al. Development of a semi-quantitative food frequency questionnaire for dietary studies, focus on vitamin C intake. Asian Pac. J. Cancer Prev.9, 427–432 (2008). [PubMed] [Google Scholar]
- 15.Le, T. N. et al. Reproducibility of a designed semi-quantitative food frequency questionnaire in general populations in Northern Viet Nam. Southeast-Asian J. Sci.6, 191–200 (2018). http://sajs.ntt.edu.vn/index.php/jst/article/view/127/111 [Google Scholar]
- 16.Nguyen, T. G., Truong, D. T. T., Le, P. H., Kim Vo, T. C. & Ikeda, S. Tran le, calcium intake contributed by whole foods and gastric Cancer in Viet nam: A Case-control study. Nutr. Cancer. 75, 1243–1253 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Health, National Institute of Nutrition. National Nutritional Survey 2009–2010 (Medical Publishing House, 2010).
- 18.Stevenson, M. R. et al. Evaluation of the Vietnamese A6 mortality reporting system: All-Cause mortality, Asia-Pacific. J. Public Health. 27, 733–742 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Yang, J. J. et al. Tobacco smoking and mortality in asia: A pooled meta-analysis. JAMA Netw. Open.2, e191474 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thun, M. J. et al. 50-year trends in smoking-related mortality in the united States. N Engl. J. Med.368, 351–364 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu, J. L. et al. Racial differences in association of serum calcium with mortality and incident cardio- and cerebrovascular events. J. Clin. Endocrinol. Metab.101, 4851–4859 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ministry of Health. Appendix 1: Table of recommended nutrition intakes for Vietnamese. Circular No. 43/2014/TT-BYT providing for the management of functional food, (2014).
- 23.Yoo, J. Y., Cho, H. J. & Lee, J. E. Lower dietary calcium intake is associated with a higher risk of mortality in Korean adults. J. Acad. Nutr. Diet.122, 2072–2086 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Hou, X. et al. L-shaped association of serum calcium with all-cause and CVD mortality in the US adults: A population-based prospective cohort study. Front. Nutr.9, 1097488 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitz, T. et al. Low serum calcium is associated with higher long-term mortality in myocardial infarction patients from a population-based registry. Sci. Rep.11, 2476 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobylecki, C. J., Nordestgaard, B. G. & Afzal, S. Low plasma ionized calcium is associated with increased mortality: A population-based study of 106 768 individuals. J. Clin. Endocrinol. Metab.107, e3039–e3047 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Li, H. et al. Clinical value of serum calcium in elderly patients with sepsis. Am. J. Emerg. Med.52, 208–211 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Wang, X. et al. Association between serum calcium and prognosis in patients with acute pulmonary embolism and the optimization of pulmonary embolism severity index. Respir Res.21, 298 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, Y., Chai, Y., Rong, Z. & Chen, Y. Prognostic value of ionized calcium levels in neonatal Sepsis. Ann. Nutr. Metab.76, 193–200 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Wang, B., Li, D., Gong, Y., Ying, B. & Cheng, B. Association of serum total and ionized calcium with all-cause mortality incritically ill patients with acute kidney injury. Clin. Chim. Acta. 494, 94–99 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Michaëlsson, K., Melhus, H., Lemming, E. W., Wolk, A. & Byberg, L. Long term calcium intake and rates of all cause and cardiovascular mortality: Community based prospective longitudinal cohort study. BMJ346, f228 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoo, J. Y., Cho, H. J. & Lee, J. E. Lower dietary calcium intake is associated with a higher risk of mortality in Korean adults. J. Acad. Nutr. Dietetics. 122, 2072–2086 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Qiu, M. et al. Relationship of serum calcium concentration with chronic kidney disease and mortality in type 2 diabetes mellitus patients: Evidence from the NHANES 1999–2018. Int. Urol. Nephrol.57, 1009–1018 (2025). [DOI] [PubMed] [Google Scholar]
- 34.Schwartz, G. G. Is serum calcium a biomarker of fatal prostate cancer? Future Oncol.5, 577–580 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Skinner, H. G. & Schwartz, G. G. A prospective study of total and ionized serum calcium and fatal prostate cancer. Cancer Epidemiol. Biomark. Prev.18, 575–578 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skinner, H. G. & Schwartz, G. G. Serum calcium and incident and fatal prostate cancer in the National health and nutrition examination survey. Cancer Epidemiol. Biomark. Prev.17, 2302–2305 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vervloet, M. G., van Ittersum, F. J., Buttler, R. M., Heijboer, A. C. & Blankenstein, M. A. Ter wee, effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin. J. Am. Soc. Nephrol.6, 383–389 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kendrick, J. et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J. Am. Soc. Nephrol.22, 1913–1922 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fomina, L. A. [Calcium-phosphorus ratio in relapse of peptic ulcer disease]. Eksp. Klin. Gastroenterol. 22–26. (2011). [PubMed]
- 40.Mirza, M. A., Larsson, A., Melhus, H., Lind, L. & Larsson, T. E. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis207, 546–551 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Bushinsky, D. A., Riera, G. S., Favus, M. J. & Coe, F. L. Evidence that blood ionized calcium can regulate serum 1,25(OH)2D3 independently of parathyroid hormone and phosphorus in the rat. J. Clin. Invest.76, 1599–1604 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serra-Majem, L. et al. Dietary assessment methods for intakes of iron, calcium, selenium, zinc and iodine. Br. J. Nutr.102 (Suppl 1), S38–55 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available from the corresponding author on request.