Abstract
Faecal microbiota transplantation (FMT) has been explored as a potential treatment for obesity, but its long-term effects on metabolic health remain unclear. Here, we report 4-year follow-up findings from a double-blind, randomised, placebo-controlled trial assessing FMT in adolescents with obesity (ACTRN12615001351505, Australian New Zealand Clinical Trials Registry). This unblinded follow-up study evaluated 63% (55/87) of the original participants (27 FMT, 28 placebo). There was no difference in BMI between the two groups, after adjusting for sex, age, diet, and physical activity (−3.6 kg/m2, p = 0.095). However, FMT recipients showed clinical improvements in body composition and metabolic health compared to the placebo group. Specifically, FMT recipients had smaller waist circumference (−10.0 cm, p = 0.026), total body fat (−4.8%, p = 0.024), metabolic syndrome severity score (−0.58, p = 0.003), and systemic inflammation (−68% hs-CRP, p = 0.002) and higher levels of HDL cholesterol (0.16 mmol/L, p = 0.037). No group differences were observed in glucose markers, or other lipid parameters. Shotgun metagenomic sequencing revealed sustained long-term alterations in gut microbiome richness, composition and functional capacity, with persistence of donor-derived bacterial and bacteriophage strains. These findings highlight the potential relevance of FMT as a microbiome-augmenting intervention for obesity management and metabolic health, warranting further investigation.
Subject terms: Obesity, Metabolic syndrome, Microbiology techniques, Colon, Bacterial techniques and applications
In this 4-year unblinded follow-up study, the authors evaluate 63% of the participants of a double-blind randomised control trial that assessed the effects of faecal microbiota transplantation (FMT) on adolescents with obesity.
Introduction
Global obesity rates have nearly tripled over the past 50 years, now affecting 14% of the adult population, and imposing considerable health and financial burdens on society1. Obesity that develops in childhood or adolescence often persists into adulthood2, and is a strong predictor of increased morbidity3 and mortality4 later in life. Thus, there is an urgent need for early interventions to help alter this disease trajectory.
While diet5, physical activity6, and host genetics7 have long been associated with obesity risk, recent attention has begun to examine the role(s) of the gut microbiome, particularly with regards to appetite regulation8 and energy harvest9. Prior association studies have shown that individuals with obesity harbour distinct gut microbiome compositions10–13, which can induce weight gain and metabolic impairments when transplanted into germ-free mice14–18. Motivated by these discoveries, clinical trials have since investigated the effects of transplanting lean donor microbiomes into recipients with obesity and/or metabolic disorders. While several studies have reported short-term improvements in insulin sensitivity19–22, intestinal permeability23, and other markers of glucose metabolism24,25, none have demonstrated the effectiveness of faecal microbiota transplantation (FMT) as a weight loss therapy26–31.
Similarly, in our earlier double-blinded randomised placebo-controlled trial, we found no impact of encapsulated FMT on BMI SDS (the trial’s primary outcome) or total body fat over 6 months among a cohort of 87 adolescents with obesity32. However, we observed an improvement in fat distribution (lower android-to-gynoid-fat ratio, a marker of central adiposity), as well as transient improvements in insulin resistance and glucose metabolism in a subset of participants who had metabolic syndrome at baseline. In parallel, we observed sustained shifts in both the composition and functional potential of recipients’ gut microbiomes33,34, raising the possibility that clinical improvements may manifest over a longer timeframe. To investigate this, participants from the original trial were invited to attend a long-term, unblinded follow-up visit approximately four years after receiving treatment. Assessments reflected the secondary outcomes prespecified in the original trial, including anthropometry, blood pressure, diet and lifestyle questionnaires, and metabolic and microbiome profiling.
Results
Long-term follow-up and participant characteristics
We assessed 63% (55/87) of the original Gut Bugs Trial participants at the 4-year follow-up (4YFU; median 4.4 years, range 3.7–5.5 years) at a median age of 21.8 years (range 18.3–24.5 years). The 4YFU participants were evenly distributed between treatment groups (27 FMT, 28 placebo; Fig. 1). Recapture rates were higher among males and participants of European, Asian, and Indian ethnicity compared to females and those of Pacific and Māori ethnicity (Table 1). Baseline (i.e., initial pre-intervention) age and BMI Z-scores did not differ between returning participants and those lost to follow-up (Table 1). Similarly, there were no differences in demographics or baseline BMI z-scores between FMT and placebo recipients who returned for the 4YFU (Table 1).
Fig. 1. Study flow from treatment randomisation to the 6-month follow-up (end of the original trial period), through to the 4-year follow-up assessment.
Note that some participants who did not complete the initial 6-month follow-up did return for the 4YFU. DXA, whole-body dual-energy X-ray absorptiometry scans; FMT faecal microbiota transplantation.
Table 1.
Baseline characteristics of participants lost to follow-up versus those who returned for the 4-year follow-up, and comparison between treatment groups among returnees
| Lost to follow up | Returned for 4YFU | p | Returned for 4YFU | p | ||
|---|---|---|---|---|---|---|
| FMT | Placebo | |||||
| n | 32 | 55 | 27 | 28 | ||
| Sex | ||||||
| Female | 23 (72%) | 28 (51%) | 0.07 | 14 (52%) | 14 (50%) | >0.99 |
| Male | 9 (28%) | 27 (49%) | 13 (48%) | 14 (50%) | ||
| Ethnicity | ||||||
| European | 10 (31%) | 33 (60%) | 0.021 | 16 (59%) | 17 (61%) | 0.40 |
| Pacific | 11 (34%) | 9 (16%) | 5 (19%) | 4 (14%) | ||
| Māori | 10 (31%) | 8 (15%) | 2 (7%) | 6 (21%) | ||
| Asian | - | 3 (5%) | 2 (7%) | 1 (4%) | ||
| Indian | 1 (3%) | 2 (4%) | 2 (7%) | - | ||
| Age (years) at BL | 17.2 ± 1.4 | 17.2 ± 1.4 | 0.93 | 17.2 ± 1.5 | 17.1 ± 1.4 | 0.78 |
| BMI Z-score at BL | 2.36 ± 0.37 | 2.29 ± 0.35 | 0.38 | 2.30 ± 0.41 | 2.28 ± 0.29 | 0.82 |
Data are n (%) or mean ± standard deviation, as appropriate. p-values were derived from Fisher’s exact tests for categorical variables and one-way ANOVA for continuous variables. Two-sided unadjusted p-values for statistically significant between-group differences at p < 0.05 are shown in bold.
BL baseline, BMI body mass index.
Long-term differences in body composition and metabolic health
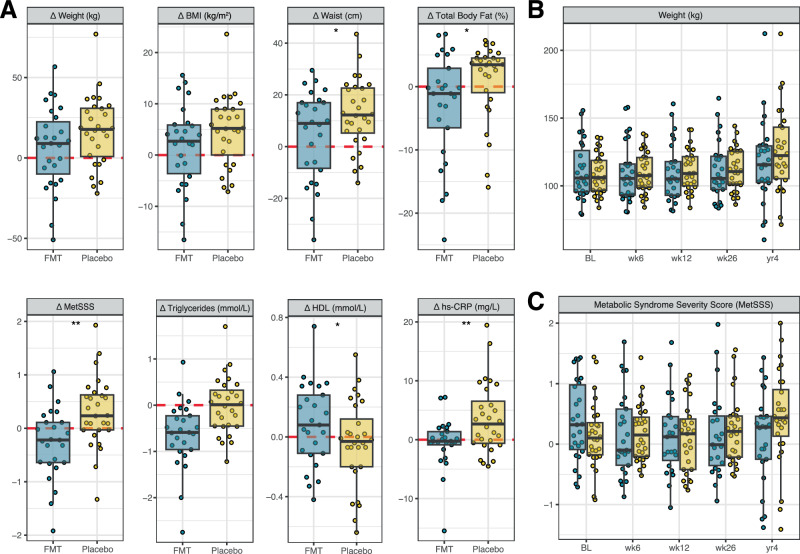
Four years post-treatment, clinical outcomes were highly variable both between and within treatment groups (Fig. 2A). While BMI and body weight trended lower in the FMT group, the differences between treatment groups were not statistically significant (Table 2 and Fig. 2B). However, on average, FMT recipients had a smaller waist circumference (−10.0 cm, p = 0.026) and less total body fat (−4.8%, p = 0.024) compared to the placebo group (Table 2 and Fig. 2A).
Fig. 2. Longitudinal changes in anthropometry and metabolic health among participants treated with FMT or placebo.
A Individual changes (Δ) in anthropometric and metabolic outcomes at 4 years compared to baseline. Points represent individual participants. The boxes represent the interquartile range (IQR) split by the median, with whiskers expanding up to 1.5× the IQR. The dashed red line at 0 represents the reference baseline value. *p < 0.05 and **p < 0.01 for between-group differences, derived from linear models; p-values are nominal (unadjusted for multiple comparisons) and two-sided. Exact sample sizes for each analysis are indicated Fig. 1. BMI body mass index, FMT faecal microbiota transplantation, HDL high-density lipoprotein cholesterol, hs-CRP high-sensitivity C-reactive protein; and metabolic syndrome severity scores (MetSSS). B Weight and C MetSSS over the full study period, including only participants (FMT, blue; Placebo, yellow) who returned for the 4-year follow-up.
Table 2.
Clinical and microbiome outcomes at the 4YFU
| FMT | Placebo | p | ||||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | aMD (95% CI) | ||
| Anthropometry | ||||||
| Weight (kg) | 27 | 117.2 ± 30.3 | 28 | 125.6 ± 30.7 | −12.2 (−24.9, 0.6) | 0.068 |
| BMI (kg/m2) | 26 | 38.5 ± 9.1 | 28 | 41.2 ± 8.8 | −3.6 (−7.6, 0.5) | 0.095 |
| Waist circumference (cm) | 26 | 109.3 ± 19.7 | 28 | 116.2 ± 15.9 | −10.0 (−18.4, −1.5) | 0.026 |
| Hip circumference (cm) | 26 | 124.6 ± 16.6 | 28 | 131.2 ± 17.3 | −6.3 (−12.8, 0.1) | 0.062 |
| Metabolic syndrome | ||||||
| MetSSS | 25 | 0.11 ± 0.76 | 28 | 0.45 ± 0.78 | −0.58 (−0.94, −0.22) | 0.003 |
| Blood pressure | ||||||
| SBP (mmHg) | 26 | 124 ± 9 | 28 | 125 ± 14 | 0 (−6, 6) | 0.90 |
| DBP (mmHg) | 26 | 74 ± 6 | 28 | 76 ± 9 | −2 (−6, 3) | 0.53 |
| MAP (mmHg) | 26 | 91 ± 6 | 28 | 93 ± 10 | −1 (−6, 3) | 0.62 |
| Body composition | ||||||
| Total body fat (%) | 25 | 43.5 ± 10.3 | 28 | 47.9 ± 9.9 | −4.8 (−8.8, −0.8) | 0.024 |
| Android-to-gynoid-fat ratio | 25 | 1.13 ± 0.17 | 28 | 1.16 ± 0.11 | −0.04 (−0.10, 0.02) | 0.20 |
| Glucose metabolism | ||||||
| Fasting insulin (mmol/L)Ln | 25 | 22.4 ± 14.7 | 28 | 28.1 ± 19.3 | −24% (−47%, 8%) | 0.13 |
| Fasting glucose (mmol/L) | 25 | 5.39 ± 0.41 | 28 | 5.43 ± 0.36 | −0.04 (−0.23, 0.15) | 0.68 |
| HOMA-IR Ln | 25 | 5.44 ± 3.73 | 28 | 6.89 ± 4.99 | −25% (−48%, 9%) | 0.14 |
| Lipid profile | ||||||
| Total cholesterol (mmol/L) | 25 | 4.35 ± 1.07 | 28 | 4.14 ± 0.70 | 0.13 (−0.37, 0.64) | 0.61 |
| Triglycerides (mmol/L) Ln | 25 | 1.02 ± 0.50 | 28 | 1.13 ± 0.59 | −23% (−42%, 2%) | 0.070 |
| LDL (mmol/L) | 25 | 2.74 ± 1.01 | 28 | 2.61 ± 0.68 | 0.04 (−0.43, 0.51) | 0.87 |
| HDL (mmol/L) | 25 | 1.28 ± 0.29 | 28 | 1.15 ± 0.34 | 0.16 (0.01, 0.30) | 0.037 |
| Inflammation markers | ||||||
| hs-CRP (mg/L) Ln | 25 | 2.83 ± 3.39 | 28 | 7.03 ± 6.91 | −68% (−84%, −37%) | 0.002 |
| Uric acid (umol/L) | 25 | 388 ± 103 | 28 | 385 ± 62 | −19 (−57, 20) | 0.35 |
| Gut microbiome | ||||||
| Shannon diversity index Ln | 23 | 2.93 ± 0.64 | 26 | 2.74 ± 0.54 | 3% (−10%, 19%) | 0.64 |
| Species richness | 23 | 106 ± 22 | 26 | 96 ± 17 | 13 (5, 21) | 0.003 |
| Gene richness | 23 | 282k ± 73k | 26 | 265k ± 60k | 27k (−3k, 58k) | 0.089 |
| Species composition shift Ln | 23 | 0.63 ± 0.11 | 26 | 0.53 ± 0.15 | 0.11 (0.04, 0.19) | 0.006 |
| Pathway composition shift Ln | 23 | 0.047 ± 0.029 | 26 | 0.027 ± 0.016 | 53% (6%, 121%) | 0.030 |
Between-group differences are reported as the adjusted mean differences (aMD) and 95% confidence intervals (CI) derived from linear models, adjusted for treatment group, sex, age, diet quality score, physical activity (IPAQ total MET) and the baseline value of the outcome. p-values for statistically significant between-group differences at p < 0.05 are shown in bold; p-values are nominal (unadjusted for multiple comparisons) and two-sided. Composition shift represents the Bray-Curtis dissimilarity between baseline and 4YFU microbiome profiles.
LnVariables were log-transformed prior to analysis. The back-transformed aMD and 95% CI are reported as proportional between-group differences (FMT vs. Placebo).
BMI body mass index, DBP diastolic blood pressure, HDL high-density lipoprotein cholesterol, HOMA-IR homoeostatic model assessment of insulin resistance, hs-CRP high-sensitivity C-reactive protein, LDL low-density lipoprotein cholesterol, MAP mean arterial pressure, MetSSS metabolic syndrome severity score, SBP systolic blood pressure.
Differences in metabolic parameters were also observed between FMT and placebo recipients. Notably, metabolic syndrome severity scores (MetSSS), which serve as a proxy for cardiovascular disease risk, differed by over half a standard deviation (p = 0.003, Table 2), continuing to increase over time in the placebo group (Fig. 2C). This was accompanied by a 68% higher level of the systemic inflammatory marker hs-CRP in the placebo group (p = 0.002). FMT recipients also had higher HDL levels (0.16 mmol/L, p = 0.037, Table 2 and Fig. 2A). No differences were observed between groups in other lipid parameters, uric acid, or markers of glucose metabolism (Table 2).
Regarding adverse metabolic phenotypes, there were no differences between treatment groups in the proportion of participants with obesity, high waist circumference, metabolic syndrome, dyslipidaemia, impaired fasting glycose, hypertension, or abnormal liver function (Table 3).
Table 3.
Adverse metabolic phenotypes at baseline and the 4YFU
| FMT | Placebo | pCMH | |||||
|---|---|---|---|---|---|---|---|
| BL | 4YFU | pMcN | BL | 4YFU | pMcN | ||
| Obesitya | 26 (100%) | 23 (88%) | - | 28 (100%) | 24 (86%) | - | >0.99 |
| High waist circumferencea | 26 (100%) | 23 (88%) | - | 28 (100%) | 26 (93%) | - | 0.66 |
| Metabolic syndrome | 9 (35%) | 7 (28%) | 0.72 | 7 (25%) | 12 (43%) | 0.13 | 0.95 |
| Dyslipidaemia | 19 (73%) | 16 (64%) | 0.51 | 21 (75%) | 20 (71%) | >0.99 | 0.75 |
| Impaired fasting glucose | 8 (31%) | 9 (36%) | >0.99 | 9 (32%) | 7 (25%) | 0.68 | 0.75 |
| Hypertension | 7 (27%) | 7 (27%) | >0.99 | 5 (18%) | 10 (36%) | 0.23 | 0.98 |
| Abnormal liver function | 9 (35%) | 5 (20%) | 0.34 | 5 (18%) | 6 (21%) | >0.99 | 0.48 |
Data are n (%) and two-sided p values (unadjusted). Adverse phenotypes are defined in ‘Methods’. Within-group differences between baseline (BL) and the 4-year follow-up (4YFU) were assessed using McNemar’s tests (McN) and between-group differences with Cochran–Mantel–Haenszel (CMH) tests.
aAll participants had obesity and high waist circumference at baseline, so only Fisher’s exact tests were used to compare potential between-group differences at the 4YFU.
Clinical improvements in relation to diet, exercise, and medications
To account for key potential confounders, we investigated whether the observed anthropometric and metabolic improvements were influenced by between-group differences in diet, physical activity, or medication use. A secondary analysis that excluded three participants taking medications known to affect metabolism did not alter the main study findings (Table S1). Analysis of dietary habits revealed no differences in individual food item consumption or overall diet quality scores between FMT and placebo groups at the 4YFU (Tables S2 and S3). Physical activity levels did not differ between the two groups throughout the follow-up period (Table S4).
FMT is associated with long-term changes in gut microbiome composition and functional potential
Shotgun metagenomic sequencing of 430 samples revealed sustained alterations in gut microbiome composition and function four years post-FMT. Previously, we had observed a transient increase in Shannon diversity at 6 weeks post-FMT, which returned to baseline levels at subsequent follow-up visits33. Similarly, there was no difference in Shannon diversity between treatment groups at the 4YFU (Table 2). However, FMT gut microbiomes contained 13 more species, on average, than placebo gut microbiomes (p = 0.003, Table 2 and Fig. 3A).
Fig. 3. Long-term gut microbiome dynamics in FMT and placebo recipients.
A Individual changes (Δ) in species richness from baseline (dashed red line). Points represent individual participants. The boxes represent the interquartile range (IQR) split by the median, with whiskers expanding up to 1.5× the IQR. Total sample size for microbiome analysis (FMT n = 23, Placebo n = 26). Significance based on linear model (*p < 0.05 and **p < 0.01; p-values are nominal (unadjusted for multiple comparisons) and two-sided. B Bray-Curtis dissimilarity in microbiome species or pathway composition at the 4YFU compared to baseline. Boxplot parameters and sample size as described above. C Top 50 differentially abundant species from baseline among FMT recipients, ranked by strength of association (−log(qval) × |coef|) from the Maaslin2 model. ‘+’ and ‘−’ indicate increased and decreased abundance from baseline, respectively. D Proportion of donor-matching bacterial strains in the individual recipient’s gut microbiome. Boxplot parameters and sample size as described above. E Proportion of engrafted bacterial strains detected in FMT recipients at the 4YFU by donor. F Proportion of viral operational taxonomic units (vOTUs) matching the recipient’s baseline strain, matching one or more of their donor strains, or that were newly detected (Other). G Proportion of vOTUs detected in FMT recipients at 4YFU by donor.
FMT gut microbiomes had a higher dissimilarity to their baseline species and pathway composition than placebo gut microbiomes (Table 2 and Fig. 3B). FMT and placebo microbiomes were most similar in composition at baseline but significantly diverged after treatment when adjusted for sex (PERMANOVA, p < 0.05; Table S5), reflecting the sex-matched FMT donor allocation design. However, by the 4YFU, this compositional divergence between the two groups was no longer significant (Table S5).
Differential abundance testing was performed to identify the enrichment or depletion of individual species at the 4YFU (Fig. 3C and Supplementary Data 1). In the FMT group, 48 species were found to be differentially abundant compared to baseline (28 increased and 20 decreased). Conversely, only two species in the placebo group were differentially abundant from baseline, both having decreased (Clostridium sp. CAG-138, Firmicutes bacterium CAG-176; Supplementary Data 1). Four of the 28 species that had increased in abundance within FMT microbiomes (i.e. Alistipes sp. CAG 435, Bacteroides finegoldii, Bacteroides salyersiae, and Desulfovibrionaceae bacterium) were also higher in abundance when compared to placebo microbiomes (Supplementary Data 1).
While many species were enriched in both male and female FMT recipients, we also identified distinct sex-specific enrichments. For example, Desulfovibrio piger was exclusively enriched in female recipients, whereas Megasphaera sp. MJR8396C showed enrichment only in males. Analysis of the donor gut microbiomes linked these sex-specific enrichments to particular donors. However, not all sex-specific enrichments could be solely attributed to differences in donor material. For example, Ruminococcus callidus was enriched exclusively in male FMT recipients, despite being abundant in donors of both sexes. These findings suggest that sex-specific species enrichment/depletion in FMT recipients may be influenced by a combination of donor material, strain fitness, and recipient physiology.
PERMANOVA analysis of metabolic pathways encoded by the microbiome revealed that FMT continued to affect abundance profiles at the 4YFU, accounting for 18% of the variation (Table S5). This effect was more pronounced in males, suggesting greater differences in metabolic capacity between male FMT and placebo recipients (Table S5). In the FMT group, 114 pathways were found to be differentially abundant compared to baseline (6 increased and 108 decreased). While in the placebo group, only 22 pathways were differentially abundant from baseline, all of which decreased (Supplementary Data 1). Despite these temporal changes and overall group differences in pathway profiles, no specific pathways were differentially abundant between FMT and placebo microbiomes at the 4YFU. These findings suggest that the observed differences in pathway profiles between treatment groups were likely driven by small, subtle changes across multiple pathways, rather than by substantial shifts in a few specific pathways.
Engrafted donor strains are stably retained long-term
To assess the long-term persistence of donor-strain engraftment, we compared the genetic similarity of donor and recipient strains before and after treatment. Strains were classified as donor-derived if their genetic profile (SNP haplotype) closely matched donor strains within a conservative genetic distance threshold, distinguishing them from environmentally acquired strains. At the 4YFU, placebo microbiomes showed 1% of strains genetically matching a donor strain, 41% matching the recipient’s baseline strain, and 58% of unknown origin. In contrast, FMT microbiomes contained 23% donor-derived strains, 22% matching the baseline, and 55% of unknown origin. The proportion of donor strains at 4YFU was correlated (Pearson r = 0.55, p = 0.010) and consistent with the proportion present at 6 weeks post-treatment, indicating stable long-term engraftment (Fig. 3D). Donor strain engraftment was, however, highly variable between FMT recipients ranging from no detected donor strains in two recipients to as much as 50% of their microbiome being donor-derived in one recipient (Fig. 3D). Engraftment rates also varied by contributing donors, with one female and one male donor dominating strain engraftment, with lesser contributions from the other donors (Fig. 3E). By tracing recipients’ dominant strain of a given species across the entire time course of the study, we were able to confirm that the majority of donor strains that were present at the 4YFU were stable in their dominance, with only a few instances of inter-donor strain switching (Fig. S1).
Long-term retention and augmentation of the gut phageome
Our microbiome analysis extended beyond bacteria to include bacteriophages, revealing long-term retention of donor-derived phages (Fig. 3F) and sustained alterations in the gut phageome four years after treatment (Fig. S2). Individual donor contributions to engrafted phages were similar to bacterial patterns (Fig. 3G). We observed significant differences in phage community composition between FMT and placebo groups across all post-treatment time points (Fig. S2). Specifically at the 4YFU, FMT recipients exhibited a higher proportion of phages of unknown taxonomy, with lower representation of Crass-like, Podoviridiae, and Siphoviridiae families (Table S6). Regarding phage lifestyle, the microbiomes of FMT recipients contained a higher proportion of temperate, as opposed to virulent, phages compared to placebo microbiomes (Table S6).
Gut microbiome features associated with clinical improvement
We next investigated whether there was a relationship between donor strain engraftment and clinical response. However, there was no correlation between the proportion of donor strains present at 4YFU and changes in body weight (Pearson, r = −0.19, p = 0.40) or MetSSS (Pearson, r = −0.12, p = 0.58). Similarly, we did not identify any particular donor strain/s that were universally detected in all clinical responders. We did, however, detect a relationship between reductions in MetSSS and higher abundances of Bacteroides thetaiotaomicron at 4YFU (Fig. 4B). We also identified three bacterial species (Agathobaculum butyriciproducans, Coprococcus catus, and Dorea formicigenerans) whose abundance at baseline was associated with greater reductions in MetSSS at 4YFU (Fig. 4A). Regarding the phageome, FMT recipients with higher Myoviridae richness and lower relative abundances of Siphoviridae showed greater reductions in weight (Fig. 4B).
Fig. 4. Associations between microbiome components and changes in clinical outcomes at the 4-year follow-up (4YFU) compared to baseline.
A Baseline gut microbiome samples and B 4YFU gut microbiome samples. Bacterial species relative abundances associated with changes in Metabolic Syndrome Severity Score (MetSSS) were identified using Maaslin2 (coefficients (coef) and unadjusted two-sided p-values reported). Spearman rank correlations assessed associations between viral family richness/relative abundance and weight changes (Spearman rho and unadjusted p-values reported). Each point represents an individual participant. The solid line indicates the linear regression fit, with the shaded area showing the 95% confidence interval.
Discussion
Our 4-year follow-up study provides insights into the long-term effects of FMT in adolescents and young adults with obesity. The natural trajectory of obesity from adolescence to adulthood is generally progressive weight gain and metabolic deterioration2, as was observed in the placebo group. By contrast, the FMT group exhibited reductions in total body fat alongside various metabolic improvements. Notably, FMT recipients had a 0.58 standard deviation difference in MetSSS when compared to placebo recipients. This finding is likely to be clinically meaningful, given that a 1.0 standard deviation increase in MetSSS during late childhood is associated with a 7.6-fold increased risk of type 2 diabetes mellitus and a 4.8-fold increased risk of cardiovascular disease in mid-adult life35.
Importantly, we observed long-term retention of donor-derived microbial strains and sustained alterations in the composition and functional capacity of FMT recipient gut microbiomes. These changes were evident in both bacterial and bacteriophage communities, highlighting the effects of FMT are likely sustained across multiple domains within the gut ecosystem. Collectively, our results suggest that modulation of an obesity-associated gut microbiome via the stable engraftment of gut microbiome strains from lean donors may be associated with beneficial metabolic effects that gradually manifest over time.
Due to our relatively small sample size and the complex, heterogeneous nature of the gut microbiome, identifying the precise factors or mechanisms underlying the observed metabolic changes associated with FMT remains challenging. While we identified several taxa associated with clinical improvements, it is important to acknowledge that these are not necessarily causal relationships and could simply be markers of an improved metabolic state. For example, Bacteroides thetaiotaomicron, has previously been shown to be markedly reduced in subjects with obesity, increasing in abundance post-bariatric surgery13. However, experiments in mice treated with B. thetaiotaomicron have yielded conflicting findings with respect to diet-induced body-weight gain and adiposity, suggesting that strain-level variation and experimental context are likely important factors13,36,37. This is consistent with our observations of substantial variability in strain engraftment between competing donor microbiomes and across recipients who received the same set of FMT capsules. Further research into the factors responsible for this variability may help explain why some individuals respond better to FMT than others38.
This study has several limitations. First, treatment allocation was unblinded at the end of the original trial, which might have introduced bias in participant behaviours or reporting during follow-up. Therefore, while we adjusted for diet and physical activity at the 4YFU time point, we lack data on their use of medications, dietary habits, and lifestyle choices in the intervening years, limiting our ability to account for their potential impact. Second, 37% of the original cohort was lost to follow-up, and the extent to which this attrition introduced bias is unknown. These factors should be considered when interpreting our findings. Nonetheless, our study had notable strengths. This is the longest clinical follow-up of adolescents with obesity (or other obesity related comorbidities) treated with FMT. Notably, participants underwent comprehensive anthropometric and metabolic assessments, and we performed extensive characterisation of their gut microbiomes, including taxonomic, functional, and temporal analyses. Our findings suggest that a single FMT treatment is associated with long-term shifts in gut microbiome composition and functional capacity, alongside improvements in metabolic health in adolescents and young adults with obesity. These results underscore the need for larger randomised controlled trials of FMT to firstly establish whether these findings can be replicated across other cohorts, and secondly determine if multiple FMT treatments could potentially enhance or extend these outcomes.
Methods
Ethics
The Northern A Health and Disability Ethics Committee approved the original Gut Bugs Trial (16/NTA/172) and a subsequent amendment for the 4-year follow-up (4YFU) (16/NTA/172/AM08). All participants (and parents of those aged <16 years at the original trial) provided verbal and written informed consent. This study followed all appropriate institutional and international guidelines and regulations for medical research, in line with the Declaration of Helsinki39.
Study design and participant information
The Gut Bugs Trial was a double-blind, randomised, placebo-controlled trial conducted in Auckland (New Zealand) from October 2017 to March 201932,40. Full details of the study protocol, including eligibility criteria, randomisation, and intervention procedures, have previously been published and are open access40. In brief, we recruited 87 post-pubertal adolescents (aged 14–18 years) with obesity BMI ≥ 30 kg/m2. Participants were randomised (stratified by sex) in a 1:1 ratio to receive either FMT capsules containing gut microbiota from four healthy lean sex-matched donors or placebo capsules containing saline. To cleanse their bowels, participants were administered a 70 g Glycoprep-C laxative solution (active ingredient: macrogol 3350; Fresenius Kabi Australia, Mount Kuring-gai, Australia) the day before their first treatment visit. The treatment consisted of 28 acid-resistant, double encapsulated DRcaps capsules (Capsugel, Sydney, Australia) in total (7 from each of the four donors), administered orally over two consecutive mornings in clinic.
Each FMT capsule, prepared as described previously33, contained 0.25 g of microbiota suspended in 0.5 ml cryoprotective saline solution (0.9% NaCl, 15% glycerol). To minimise FMT heterogeneity, the same set of donors was used throughout the trial. Participants underwent clinical assessments at baseline (pre-treatment), and at 6, 12, and 26 weeks post-treatment, after which they were unblinded32,33,40. Throughout the 26-week trial period, participants were instructed to maintain their usual diet and physical activity levels.
4-year follow-up (4YFU) assessment
Approximately 4 years after treatment, we invited participants for a follow-up visit at our clinic. Assessments were performed as previously described32,40 and included:
Medical history and physical examination (including information on any antibiotic, probiotic, and/or other medication use);
Fasting blood samples;
Blood pressure (BP);
Anthropometry (height, weight, and waist and hip circumferences);
Body composition using a whole-body dual-energy X-ray absorptiometry scan;
Self-reported questionnaires on physical activity (IPAQ— International Physical Activity Questionnaire Long Form41) and diet (New Zealand Adolescent Food Frequency Questionnaire42);
Collection of stool samples (at home or in the clinic).
Blood pressure and anthropometric measurements were taken in triplicate, with the median value used for analysis. Fasting blood samples were analysed for markers of metabolic function:
Glucose metabolism—glucose, insulin;
Lipid profile—total cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), and triglycerides;
Liver function—gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate transaminase (APT);
Inflammation—uric acid, and high-sensitivity C-reactive protein (hsCRP).
Adverse metabolic phenotypes
Adverse metabolic phenotypes were defined as previously described32,40.
Obesity: BMI ≥ 30 kg/m2
- High waist circumference:
- ≥77 cm in females or ≥79.9 cm in males (<15 years old)
- ≥78.4 cm in females or ≥81.7 cm males (<16 years old)
- ≥80 cm in females or ≥94 cm in males (≥16 years old)
Hypertension: systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg
Impaired fasting glucose: ≥5.6 mmol/L
- Dyslipidaemia: any of the following:
- High total cholesterol (>5.2 mmol/L)
- High triglycerides (≥1.7 mmol/L)
- High LDL (>2.6 mmol/L)
- Low HDL (<1.29 mmol/L for females or <1.03 mmol/L for males)
- Abnormal liver function:
- Males—ALT > 41 U/L, AST > 40 U/L or GGT ≥ 60 U/L
- Females—ALT > 33 U/L, AST > 32 U/L or GGT ≥ 40 U/L
-
Metabolic syndrome:
1) High waist circumference AND
2) Any two of the following:- Low HDL;
- High triglycerides;
- Impaired fasting glucose;
- Elevated blood pressure (systolic BP ≥ 130 mmHg or diastolic BP ≥ 85 mmHg)
Metabolic syndrome severity score
We derived a metabolic syndrome severity score (MetSSS) using the sex-stratified ‘non-Hispanic White’ formulae43, which have been previously validated in a New Zealand cohort44. The MetSSS is a surrogate marker of metabolic health that incorporates waist circumference, fasting glucose, triglycerides, HDL, and systolic BP measurements.
Dietary analysis
We assessed habitual diet using the New Zealand Adolescent Food Frequency Questionnaire (NZAFFQ)42 for consistency with the original trial33. Foods were grouped into 35 categories (including 34 from Wong et al.42 and an additional alcoholic drinks category) and an overall diet quality score (based on the Healthy Dietary Habits Index) was calculated45 (Table S2).
Physical activity analysis
Physical activity was assessed using the International Physical Activity Questionnaire Long Form (IPAQ)41. Responses were scored per guidelines46, and the parameter of interest was the overall physical activity level expressed as total MET-minutes/week.
Metagenomic sequencing and data processing
Stool sample collection and processing was performed as per the original trial methodology. Stool samples were collected on site and immediately aliquoted and stored at −80 °C. If samples could not be produced during the clinic visit, participants were given a self-collection kit and instructed to store their sample in their home freezer until transfer to the lab could be arranged. No preservation buffer was used. DNA was extracted from a 200 mg aliquot of stool using a modified Qiagen AllPrep DNA/RNA Mini Kit (#80204) protocol33 and included a negative extraction blank and the ZymoBIOMICS Microbial Community Standard (#D6300). DNA extracts were shipped on dry ice to Novogene (Beijing, China) for shotgun metagenomic sequencing on an Illumina NovaSeq6000 platform generating an average sequencing depth of 23 million 150 bp paired-end reads/sample. Metagenomic sequencing data were processed by KneadData47 to trim and remove poor-quality reads and those mapped to the human genome (hg19). The quality-filtered reads were subsequently processed using select tools from the bioBakery3 suite48, namely taxonomic profiling with MetaPhlAn3, dominant strain profiling with StrainPhlAn3, and functional profiling of microbial gene families and pathways with HUMAnN3. Pathway abundance profiles were renormalised from RPK to CPM units.
Donor strain engraftment analysis
Donor strain engraftment was assessed using the multiple sequence alignment output from StrainPhlAn3 as described previously33. This method has been validated in prior studies of strain transmission and provides a robust, conservative framework for detecting engraftment while minimising false positives49–51. The Jukes and Cantor (JC69) model from the phangorn R package was used to calculate pairwise DNA distances between conspecific strains52. To systematically apply the same genetic similarity threshold between species with variable degrees of strain variation, DNA distances were normalised by the median DNA distance of all pairwise comparisons for a given species. Strains with a normalised DNA distance ≤0.2 were considered a strain match. Recipient strains that matched those detected in any of the four contributing donors and did not match their pre-treatment baseline strain, were classified as donor-strain engraftment events. When a recipient’s post-treatment strain had multiple strain matches, the strains ‘source’ was assigned to the sample with the lowest normalised DNA distance. Recipient strains that matched a donor strain at baseline (i.e. pre-FMT) were labelled as ambiguous and not included in FMT engraftment assessments.
Bacteriophage analysis
The bacteriophage population was characterised as performed previously34. In brief, quality-filtered reads were first assembled into contigs with MEGAHIT53. Viral contigs were subsequently isolated with VirSorter2 (version 2.2.3)54, quality assessed with CheckV (version 0.7.0)55, and clustered into vOTUs (viral Operational Taxonomic Units) using FastANI (version 1.33)56 according to MIUVG standards57 (i.e. requiring ≥95% average nucleotide identity (ANI) over ≥85% alignment fraction). The longest contig in the vOTU was selected as the representative sequence. Only vOTUs with a representative contig that was ≥90% complete were included in the analysis. Taxonomy was assigned using vContact2 (version 0.9.19)58 and Hidden Markov Model profile comparison against the ViPhOGs database (29/06/2021)59. Phage lifestyle was predicted using BACPHLIP (version 1.0)60. Relative abundances were determined by the median CPM of the genes within the vOTU genome, calculated with BWA (version 0.17.7)61. Donor phage engraftment was determined by identifying clonal phages (i.e. ANI ≥ 99.4%) in donor and post-FMT recipient samples.
Statistical analyses
For continuous clinical outcomes, linear models were used to assess potential differences between treatment groups, adjusting for sex, age, diet quality score, physical activity (total MET-minutes/week), and the baseline value of the outcome. Between-group differences are reported as the adjusted mean differences (aMD) and respective 95% confidence intervals (CI). Secondary analyses were run, excluding 3 participants taking medications known to affect metabolism. For paired nominal data (i.e. adverse metabolic phenotypes), McNemar’s test assessed changes from baseline within each treatment group, focusing on whether the rate of a condition had increased or decreased by the 4YFU. The Cochran–Mantel–Haenszel (CMH) test examined differences between the Placebo and FMT groups at 4YFU, effectively adjusting for any baseline imbalances. Mann–Whitney U tests assessed potential between-group differences in diet (overall diet quality scores) and physical activity levels (total MET-minutes/week). The McNemar’s, CMH, and Mann–Whitney U tests were carried out in SAS v9.4 (SAS Institute, Cary, NC, USA). The other statistical analyses and figure generation were conducted in R v.4.3.1 (R Foundation for Statistical Computing, Vienna, Austria). All statistical tests for clinical outcomes were 2-sided with significance set at p < 0.05 without adjustment for multiple comparisons. No imputation methods were used for missing data.
For microbiome data, alpha and beta diversity metrics were calculated using the vegan R package62. Alpha diversity metrics included species-, pathway-, and gene-level richness (defined as the number of unique features detected per sample), and the Shannon diversity index. These metrics were compared between treatment groups using linear models as described above. Bray-Curtis dissimilarities were computed for both species-level, pathway-level, and phageome profiles. Shifts in composition were assessed by evaluating the Bray-Curtis dissimilarity between the recipient’s baseline and 4YFU sample. In addition, between-group differences in overall composition (i.e. beta-diversity) were assessed using PERMANOVA, tested separately at each time point. Differentially abundant species and pathways were evaluated with the MaAsLin2 package63, using log-transformed relative abundances, requiring a minimum feature prevalence of 10%, and using a significance threshold of q < 0.2 (False Discovery Rate correction using Benjamini–Hochberg Procedure). Between-group differences were assessed at the 4YFU visit only, while differences from baseline were examined separately for each treatment group with participant ID added as a random effect. MaAsLin2 was also used to assess associations between individual species and changes in MetSSS among FMT recipients (i.e. delta MetSSS). We evaluated species abundances at two time points: (1) at baseline to identify species predictive of MetSSS changes, and (2) at 4YFU to identify species reflective of MetSSS changes.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
We thank all trial participants for their ongoing commitment to this study. We also thank the Rockfield Trust for their generous philanthropic donation that funded this project.
Author contributions
Funding acquisition: J.M.O. and W.S.C. Study design and methodology: B.C.W., M.Z., J.G.B.D., B.B.A., K.S.W.L., K.L.B., T.V., J.M.O. and W.S.C. Clinical assessments: B.B.A., R.Y.T.-C., K.S.W.L. and W.S.C. Confounder analysis: B.C.W., J.G.B.D. and K.L.B. Microbiome analysis: B.C.W., M.Z. and T.V. Statistical analysis: B.C.W., M.Z., J.G.B.D. and K.L.B. Manuscript drafting: B.C.W., J.G.B.D., J.M.O., and W.S.C. Manuscript review: all authors.
Peer review
Peer review information
Nature Communications thanks Kristina Martinez and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
Metagenomic sequencing data have been deposited in NCBI Sequence Read Archive under BioProject PRJNA637785. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Justin M. O’Sullivan, Email: justin.osullivan@auckland.ac.nz
Wayne S. Cutfield, Email: w.cutfield@auckland.ac.nz
On behalf of the Gut Bugs Study Group:
Michele Zuppi, Tommi Vatanen, Benjamin Albert, Kathryn Beck, Valentina Chiavaroli, Cathryn Conlon, Christine Creagh, Wayne Cutfield, Frankie Day, Marysia Depczynski, José Derraik, Taygen Edwards, David Holland, Thilini Jayasinghe, Yannan Jiang, Karen Leong, Justin O’Sullivan, Chris Pook, Theo Portlock, William Schierding, Darren Svirskis, Ry Tweedie-Cullen, Mark Vickers, and Brooke Wilson
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-62752-4.
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet387, 1377–1396 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh, A. S., Mulder, C., Twisk, J. W. R. et al. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes. Rev.9, 474–488 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Must, A., Jacques, P. F., Dallal, G. E. et al. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N. Engl. J. Med.327, 1350–1355 (1992). [DOI] [PubMed] [Google Scholar]
- 4.Twig, G., Yaniv, G., Levine, H. et al. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N. Engl. J. Med.374, 2430–2440 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Popkin, B. M., Adair, L. S. & Ng, S. W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev.70, 3–21 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leskinen, T., Sipilä, S., Alen, M. et al. Leisure-time physical activity and high-risk fat: A longitudinal population-based twin study. Int. J. Obes.33, 1211–1218 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Locke, A. E., Kahali, B., Berndt, S. I. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature518, 197–206 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fetissov, S. O. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol.13, 11–25 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Turnbaugh, P. J., Ley, R. E., Mahowald, M. A. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature444, 1027–131 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Le Chatelier, E., Nielsen, T., Qin, J. et al. Richness of human gut microbiome correlates with metabolic markers. Nature500, 541–546 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: human gut microbes associated with obesity. Nature444, 1022–1023 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Peters, B. A., Shapiro, J. A., Church, T. R. et al. A taxonomic signature of obesity in a large study of American adults. Sci. Rep.8, 9749 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, R., Hong, J., Xu, X. et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med.23, 859–868 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Ridaura, V. K., Faith, J. J., Rey, F. E. et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science341, 1241214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tremaroli, V., Karlsson, F., Werling, M. et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab.22, 228–238 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsson, L. M., Poitou, C., Poitou, C. et al. Gut microbiota of obese subjects with Prader-Willi syndrome is linked to metabolic health. Gut69, 1229–1238 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, T. T., Parajuli, N., Sung, M. M. et al. Fecal transplant from resveratrol-fed donors improves glycaemia and cardiovascular features of the metabolic syndrome in mice. Am. J. Physiol. Endocrinol. Metab.315, E511–E519 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Zhang, L., Bahl, M. I., Roager, H. M. et al. Environmental spread of microbes impacts the development of metabolic phenotypes in mice transplanted with microbial communities from humans. ISME J.11, 676–690 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vrieze, A., Van Nood, E., Holleman, F. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology143, 913–916.e7 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Kootte, R. S., Levin, E., Salojärvi, J. et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab.26, 611–619.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 21.de Groot, P., Scheithauer, T., Bakker, G. J. et al. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut69, 502–512 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mocanu, V., Zhang, Z., Deehan, E. C. et al. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial. Nat. Med.27, 1272–1279 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Craven, L., Rahman, A., Nair Parvathy, S. et al. Allogenic fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: a randomized control trial. Am. J. Gastroenterol.115, 1055–1065 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Yu, E. W., Gao, L., Stastka, P. et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: the FMT-TRIM double-blind placebo-controlled pilot trial. PLOS Med.17, e1003051 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allegretti, J. R., Kassam, Z., Hurtado, J. et al. Impact of fecal microbiota transplantation with capsules on the prevention of metabolic syndrome among patients with obesity. Hormones20, 209–211 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahtinen, P., Juuti, A., Luostarinen, M. et al. Effectiveness of fecal microbiota transplantation for weight loss in patients with obesity undergoing bariatric surgery: a randomized clinical trial. JAMA Netw. Open5, e2247226 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koopen, A. M., Almeida, E. L., Attaye, I. et al. Effect of fecal microbiota transplantation combined with mediterranean diet on insulin sensitivity in subjects with metabolic syndrome. Front. Microbiol.12, 662159 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakker, G. J., Meijnikman, A. S., Scheithauer, T. P. et al. Fecal microbiota transplantation does not alter bacterial translocation and visceral adipose tissue inflammation in individuals with obesity. Obes. Sci. Pract.8, 56–65 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinott, E., Youngster, I., Yaskolka Meir, A. et al. Effects of diet-modulated autologous fecal microbiota transplantation on weight regain. Gastroenterology160, 158–173.e10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng, S. C., Xu, Z., Mak, J. W. Y. et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: a 24-week, double-blind, randomised controlled trial. Gut71, 716–723 (2022). [DOI] [PubMed] [Google Scholar]
- 31.Hartstra, A. V., Schüppel, V., Imangaliyev, S. et al. Infusion of donor feces affects the gut–brain axis in humans with metabolic syndrome. Mol. Metab.42, 101076 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leong, K. S. W., Jayasinghe, T. N., Wilson, B. C. et al. Effects of fecal microbiome transfer in adolescents with obesity: the gut bugs randomized controlled trial. JAMA Netw. Open3, e2030415 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson, B. C., Vatanen, T., Jayasinghe, T. N. et al. Strain engraftment competition and functional augmentation in a multi-donor fecal microbiota transplantation trial for obesity. Microbiome9, 107 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuppi, M., Vatanen, T., Wilson, B. C. et al. Fecal microbiota transplantation alters gut phage communities in a clinical trial for obesity. Microbiome12, 122 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deboer, M. D. & Gurka, M. J. Clinical utility of metabolic syndrome severity scores: considerations for practitioners. Diabetes Metab. Syndr.10, 65–72 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho, S. H., Cho, Y. J. & Park, J. H. The human symbiont Bacteroides thetaiotaomicron promotes diet-induced obesity by regulating host lipid metabolism. J. Microbiol.60, 118–127 (2022). [DOI] [PubMed] [Google Scholar]
- 37.Catlett, J. L., Carr, S., Cashman, M. et al. Metabolic synergy between human symbionts bacteroides and methanobrevibacter. Microbiol. Spectr.10, e0106722 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson, B. C., Vatanen, T., Cutfield, W. S. & O’Sullivan, J. M. The super-donor phenomenon in fecal microbiota transplantation. Front. Cell. Infect. Microbiol.9, 2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Medical Association World Medical Association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA310, 2191–2194 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Leong, K. S. W., Jayasinghe, T. N., Derraik, J. G. B. et al. Protocol for the Gut Bugs Trial: a randomised double-blind placebo-controlled trial of gut microbiome transfer for the treatment of obesity in adolescents. BMJ Open9, e026174 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagströmer, M., Oja, P. & Sjöström, M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr.9, 755–762 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Wong, J. E., Parnell, W. R., Black, K. E. & Skidmore, P. M. L. Reliability and relative validity of a food frequency questionnaire to assess food group intakes in New Zealand adolescents. Nutr. J.11, 65 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gurka, M. J., Lilly, C. L., Oliver, M. N. & Deboer, M. D. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score. Metab. Clin. Exp.63, 218–225 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merry, T. L., Metcalf, P., Scragg, R. et al. Metabolic syndrome severity score (MetSSS) associates with metabolic health status in multi-ethnic Aotearoa New Zealand cohorts. Diabetes Res. Clin. Pract.192, 110088 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Wong, J. E., Skidmore, P. M. L., Williams, S. M. & Parnell, W. R. Healthy dietary habits score as an indicator of diet quality in New Zealand adolescents. J. Nutr.144, 937–942 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Sjostrom, M., Ainsworth, B. & Bauman, A. et al. Guidelines for data processing analysis of the International Physical Activity Questionnaire (IPAQ) - Short and long forms. (2005).
- 47.bioBakery KneadData: Quality control tool on metagenomic and metatranscriptomic sequencing data, especially data from microbiome experiments. https://github.com/biobakery/kneaddata. Accessed 3 Dec 2020.
- 48.Beghini, F., McIver, L. J., Blanco-Míguez, A. et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. eLife10, e65088 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kong, L., Lloyd-Price, J., Vatanen, T. et al. Linking strain engraftment in fecal microbiota transplantation with maintenance of remission in Crohn’s disease. Gastroenterology159, 2193–2202.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valles-Colomer, M., Blanco-Míguez, A., Manghi, P. et al. The person-to-person transmission landscape of the gut and oral microbiomes. Nature614, 125–135 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferretti, P., Pasolli, E., Tett, A. et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe24, 133–145.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schliep, K. P. phangorn: phylogenetic analysis in R. Bioinformatics27, 592–593 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li, D., Liu, C. M., Luo, R. et al. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics31, 1674–1676 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Guo, J., Bolduc, B., Zayed, A. A. et al. VirSorter2: a multi-classifier, expert-guided approach to detect diverse DNA and RNA viruses. Microbiome9, 37 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nayfach, S., Camargo, A. P., Schulz, F. et al. CheckV assesses the quality and completeness of metagenome-assembled viral genomes. Nat. Biotechnol.39, 578–585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jain, C., Rodriguez-R, L. M., Phillippy, A. M. et al. High throughput ANI analysis of 90 K prokaryotic genomes reveals clear species boundaries. Nat. Commun.9, 5114 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roux, S., Adriaenssens, E. M., Dutilh, B. E. et al. Minimum information about an uncultivated virus genome (MIUViG). Nat. Biotechnol.37, 29–37 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bin Jang, H., Bolduc, B., Zablocki, O. et al. Taxonomic assignment of uncultivated prokaryotic virus genomes is enabled by gene-sharing networks. Nat. Biotechnol.37, 632–639 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Moreno-Gallego, J. L. & Reyes, A. Informative regions in viral genomes. Viruses13, 1164 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hockenberry, A. J. & Wilke, C. O. BACPHLIP: predicting bacteriophage lifestyle from conserved protein domains. PeerJ9, e11396 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics26, 589–595 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oksanen, J., Blanchet, F. G., Friendly, M. et al. vegan: community ecology package. R. Package Version2, 5–6 (2019). [Google Scholar]
- 63.Mallick, H., Rahnavard, A., McIver, L. J. et al. Multivariable association discovery in population-scale meta-omics studies. PLOS Comput. Biol.17, e1009442 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Metagenomic sequencing data have been deposited in NCBI Sequence Read Archive under BioProject PRJNA637785. Source data are provided with this paper.