Abstract
The depletion of mineral resources and the escalating environmental pollution caused by industrial waste have underscored the urgent need for efficient metal recovery from these waste streams. This research focuses on the selective extraction of Cu(II), Ni(II), Zn(II), and Cd(II) from industrial lead plant waste, employing a synergistic combination of Dichloromethane (DCM) and Aliquat 336 (A336) and individual solvent extraction using these solvents. The accuracy of the synthesized task-specific ionic liquids (TSILs) (Et3NC2NHC4 and Bu3NC2NHC4) was investigated using FTIR and H-NMR analysis. The structure of the TSIL was optimized by TSIL experiments with a range of anions (Cl−, NO3−, HSO4−, and CH3COO−) and ammonium cations with varying alkyl chain lengths, and [Bu3NC2NHC4][Cl] was chosen as an extractant to extract the mentioned metals. Subsequently, solvent extraction experiments were conducted to optimize key parameters, including pH, TSIL concentration, TSIL reaction time, phase contact time, and the aqueous-to-organic phase ratio (A/O). The stripping stage used sulfuric acid (H2SO4) at varying concentrations. The DCM system demonstrated optimal extraction at a pH of 3, with 150 mg of TSIL, a 5-minute reaction time, a 5-minute contact time, and an A/O ratio of 2:1. The A336 system exhibited similar optimal conditions. Notably, the synergistic combination of DCM and A336 attained the highest extraction efficiency (%E) at a pH of 2, with a 40% DCM fraction in the total organic phase. The concentrations of metal ions in the aqueous phase were specified with atomic absorption spectroscopy. The possibility of metal recovery from the loaded ionic liquid phase using H2SO4 was also investigated, demonstrating the reusability of the ionic liquids.
Keywords: Synergistic, Solvent extraction, Task-Specific ionic liquid, Metal recovery, Industrial waste
Subject terms: Environmental chemistry, Chemical engineering
Introduction
The recent growth in industrial activities has greatly increased the volume and toxicity of heavy metal waste1. For instance, solutions generated from processes like electrowinning, copper refining, electrorefining, leaching, solvent extraction, acid mine drainage, and coal mining effluents often contain elevated levels of metals, including zinc, copper, cadmium, antimony, arsenic, lead, manganese, nickel, aluminum, iron, chromium, mercury, and silver2. These metals must be removed before releasing the solutions into surface water or sewage systems3–5. Due to their high toxicity, heavy metals pose serious concerns to human health and the ecological balance, prompting the establishment of regulations to control their disposal6. As a result, the recovery and extraction of metals from industrial waste have become crucial for reducing waste and mitigating pollution7. Reducing pollution will lead to economic gains in the future as the value of metals will continue to increase 7. Metals can be removed from industrial waste through various methods, like coagulation, membrane separation, solvent extraction, chemical precipitation, ion exchange, electrolysis, and adsorption8–12. Among these, solvent or liquid-liquid extraction (LLE) is a highly effective method for segregating and concentrating metal ions due to its ease of creating operating conditions and the wide range of extractors available from industrial waste13–18. LLE is a technique used to concentrate, purify, and separate metals from aqueous solutions19. In this method, a hydrophobic phase is placed in contact with the aqueous phase or leaching solution together with the extractant so that the extractant separates the metals and is selectively and spontaneously dissolved in the organic phase20. The extraction agents are effectively dissolved in an organic solvent (like toluene or kerosene) that is an optimal diluent. However, a significant disadvantage of this approach is the loss of the organic solvent through volatilization, which poses environmental and health risks21. Recent research efforts have therefore focused on substituting common organic solvents with greener alternatives like ionic liquids (ILs)22–27. Among the various methods discussed, the capabilities of separation and enrichment are key criteria for assessing the practical application potential of a technique. Synergistic extraction has long been explored as an efficient approach to improving extraction efficiency (%E) and selectivity, owing to its theoretical significance and practical utility28. By leveraging the synergistic interaction between two extractants, the distribution ratios of their combined use can be significantly increased compared to the individual performance of each extractant18,29–32.
ILs are eutectic molten salts composed of large, asymmetric organic cations paired with organic or inorganic anions, characterized by melting points below 100° C33. These compounds are gaining recognition as green solvents due to their unique properties, including negligible volatility, thermal stability, and non-flammability34. Their environmentally friendly nature has prompted extensive global research into their use as alternatives to conventional organic solvents in various fields, such as organic synthesis, catalytic processes, electrochemistry, separation techniques, biochemistry, and materials engineering35–40. Task-specific ionic liquids (TSILs) have recently appeared as a significant advancement in the field of ionic liquids. TSILs are designed with functional groups integrated into their cations and/or anions, allowing them to be customized for particular applications6. Unlike traditional ionic liquids, TSILs serve as solvents and functional liquid materials tailored to specific processes41–44. Table 1 summarizes the literature on the use of ionic liquids in the solvent extraction of metals. A review of Table 1 reveals that previous research has predominantly utilized a variety of ionic liquids, such as those based on imidazolium, phosphonium, and ammonium, for separating single metal ions under simplified, laboratory-scale conditions. However, these studies have focused on model systems rather than complex, absolute industrial waste containing multiple metals. Moreover, the synergistic use of ionic liquids in combination with conventional solvents for enhancing extraction efficiency and selectivity remains relatively underexplored.
Table 1.
Summary of the literature reported on the use of ionic liquids in solvent extraction of metals.
| Researcher | Ionic liquids | Metals | Conditions |
|---|---|---|---|
| 46 |
Cyphos IL 101 (Cy IL 101) Cyphos IL 102 (Cy IL 102) |
Gold | pH of 9, A/O ratio of 2, and concentration of IL of 1.25 mmol/L |
| 47 | ionic liquid methyltrioctylammonium chloride, [MTOA+][Cl−] | Zn, Cd, and Fe | Contact time of 5 min and Temperature of 303.15 K |
| 22 | R4PSCN | Cu | Contact time of 5 min, pH of 0.75, Temperature of 25 ⁰C, and phase ratio (A: O) of 1:1 |
| 48 | R4ND and R4NCY | Mo | pH of 2.0, A: O of 5:1, |
| 49 | trialkylmethylammonium di(2-ethylhexyl)orthophosphinate ([N1888][P507]) | Li | O/A ratio of 10:1 |
| 50 | 1-decyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ionic liquid | Li, Co, Ni | low pH (pH < 3) |
| 51 | trihexyltetradecylphosphonium ([PC6C6C6C14], paired with (choloride ([Cl−]), dicyanamide ([-N(CN)2−]), and bis(trifluoromethyl sulfonyl)amide ([-NTf2−]) | As, Cr, Cd, Cu, Zn, Pb, and Hg | pH of 7, quantity of IL (20 mg), contact time (15 min) and stirring speed (100 rpm) |
| 52 | [C8H17NH2][Cyanex 272] | Co2+ | pHeq of 4.5, te of 40 min, CTSILs of 0.29 mol/L, (O/A) of 1:1, and Te of 298 K |
| 53 | trihexyl(tetradecyl)phosphonium thiocyanate or nitrate ([C101][SCN], [C101][NO3]) | Nd(III) and Dy(III) | te of 60 min, T of 25 ⁰C, pH of 2, Shaking speed: 2000 rpm, concentration of CaCl2 in the aqueous phase of 2.5 M, 65.7 g/L Nd(III) and 13.5 g/L Dy(III), Retention time of 15 min, and O:A ratio of 1:1 |
| 54 | carboxymethyl trimethylammonium bis(trifluoromethyl)sulfonimide | Li | pH of 3, A/O of 1:2, 298.15 K, standard atmospheric pressure |
| 13 | Trihexyl (tetradecyl)phosphonium chloride [P66614+][Cl−] and trihexyl(tetradecyl)phosphonium bis(2,4,4- trimethylpentyl)phosphinate [P66614+][BTMPP−] | Ti | room temperature (25 °C), te of 10 min, stirring rate of 450 rpm |
The TSIL synthesized in this study, [Bu3NC2NHC4][Cl], offers several advantages over conventional extractants. Its quaternary ammonium-based cation is functionalized with an amino group, enabling strong interactions and effective chelation with metal ions such as Ni(II), Cu(II), Cd(II), and Zn(II). Additionally, this TSIL exhibits high thermal stability, low volatility, and good compatibility with common diluents such as Dichloromethane (DCM). Moreover, its ability to participate in synergistic extraction systems such as Aliquat 336 (A336)/DCM enhances both selectivity and extraction efficiency in complex multi-metal solutions. These features, along with improved operational safety and reduced environmental impact compared to traditional organic solvents, make [Bu3NC2NHC4][Cl] a promising candidate for sustainable heavy metal recovery from real-world sources such as industrial wastewater and electronic waste.
The objective of this study is to synthesize and characterize a novel task-specific ionic liquid ([Bu3NC2NHC4][Cl]) and to evaluate its synergistic performance with DCM and A336 for the selective extraction and recovery of Ni(II), Cu(II), Cd(II), and Zn(II) from industrial waste solutions. This study analyzed FT-IR and H-NMR spectra to verify the successful TSIL Et3NC2NHC4 and Bu3NC2NHC4 synthesis. These two analyses confirmed the synthesis of the TSILs. Subsequently, the results obtained from experiments related to the structure of the TSIL, namely the cation, and anion, the parameters affecting the solvent extraction process and metal extraction efficiency, and finally, the stripping efficiency for all three hydrophobic systems (DCM, A336, and the synergy of both (DCM/A336)) were investigated. The extractant is a TSIL based on a quaternary ammonium salt cation and a chloride anion. The extractant has an amino group that can chelate various metals with the help of an oxygen atom. Additionally, a hydrogen atom attached to the nitrogen of the amino group protonates the extractant. By examining the hydrophobicity/hydrophilicity of the extractant and the extraction efficiency obtained from the cation determination experiments, butylamine was selected as the functional group forming the cation of the TSIL. Many previous studies have either focused on single-metal systems or overlooked synergistic effects. Consequently, there is a significant gap in exploring environmentally friendly multi-metal extraction systems that provide both efficiency and selectivity. To the best of our knowledge, the synergistic extraction of Ni(II), Cu(II), Cd(II), and Zn(II) ions from industrial waste using [Bu3NC2NHC4][Cl] has not been mentioned in any previous studies. The TSIL with the mentioned structure was used in all experiments, and optimal values for each system were reported. DCM45 and A33617 are widely used in solvent extraction, and the synergistic effect of DCM and A336 may provide promising application potential for enriching and separating Ni(II), Cu(II), Cd(II), and Zn(II) ions, a combination that has not been explored in earlier research. In addition, Sulfuric acid was selected as the stripping agent to optimize the selectivity and efficiency of the stripping stage. Given its ease of implementation, the ability to create a closed-loop system, and enhanced process reproducibility, sulfuric acid was chosen. Additionally, sulfuric acid is regenerated due to the protonation of the TSIL and the hydrogen ions lost during the solvent extraction.
Experimental
Chemicals and reagents
All reagents and chemicals were employed as received without additional purification steps. Sulfuric acid (H2SO4, 98%), ammonium persulfate (APS) ((NH4)2S2O8, 98%), ferrous sulfate (FeSO4.xH2O, 99%), methanol (CH3OH, 99.8%), dichloromethane (CH2Cl2, 99.8%), trimethylamine (N(CH2CH3)3, 99%), tributylamine (N(C3H6CH3)3, 99%), methyl Chloroacetate (C3H5ClO2, 99%), sodium hydrogen sulfite (NaHSO4, 99%), ammonium acetate (NH4CH3COO, 98%), sodium nitrate (NaNO3, 99%), ethylamine (C2H5NH2, 99%), Hexylamine (C6H13NH2, 99%), octylamine (C8H17NH2, 99%), and chloroform (CHCl3, 99%) were purchased from Merck Co. Aliquat 336 (C25H54ClN, 97%), and Butylamine (C4H9NH2, 99%) were obtained from Sigma-Aldrich Co.
Synthesis of TSILs
The synthesis of TSILs involved a three-step process55. Firstly, an amidation reaction introduced an ester group to the desired trialkyl amine. Subsequently, an anion metathesis reaction was performed to exchange the initial anion with the desired anion. Finally, the desired functional group was incorporated into the cation55.
Chloride synthesis of ionic liquids
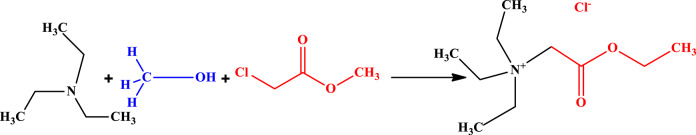
Initially, 60 mmol of trialkyl amine, specifically triethylamine or tributylamine, was added to 15 mL of methanol in a round-bottom flask and stirred for 15 min to ensure homogeneity. Subsequently, 60 mmol of methyl chloroacetate was added dropwise to the solution over 60 min. The round-bottom flask was then capped and stirred at 600 rpm for 24 h. After the reaction, the solution was transferred to a vacuum flask, and the temperature was at 50° C. A vacuum pump was activated to reduce the system’s atmospheric pressure to 0.02 bar, facilitating methanol removal from the mixture. The yellow solid resulted from an ionic liquid, a chloride anion, and a trialkyl amine cation bearing an ester group55. Figure 1 illustrates the first step of the synthesis pathway for the TSILs.
Fig. 1.
The first step of synthesis of TSILs.
Metathesis or anion displacement
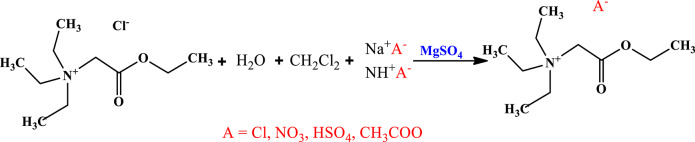
The yellow solid precursor obtained from the previous step (50 mmol) was added to a mixture of 10 mL water, 100 mL DCM, and 50 mmol of the desired sodium or ammonium salt in a round-bottom flask. The solution was vigorously stirred at 600 rpm for 2 h. This study used sodium hydrogen sulfate, ammonium acetate, and sodium nitrate to obtain ionic liquids with hydrogen sulfate, acetate, and nitrate anions. Each salt was dissolved in the water-DCM mixture to facilitate anion exchange between chloride and the desired anion. Subsequently, the organic layer (DCM) was separated, and 1 g of magnesium sulfate was added to remove residual water. Finally, the DCM solution containing the dissolved ionic liquid was filtered through filter paper to remove excess salts. The solution was then placed at 40° C to remove the DCM, leaving behind the desired ionic liquid with the target anion55. Figure 2 illustrates the second step of the synthesis pathway for the TSILs.
Fig. 2.
The second step of synthesis of TSILs.
Amine functionalization with alkylamine
In this step, the compound obtained from the previous step is dissolved in 10 mL of methanol, and 51 mmol of alkylamine (with a chain length of C2-C8) is added. The primary reaction here involves the interaction between the amine group of the alkylamine and the functional groups of the precursor compound, leading to the formation of an ionic liquid (IL). The alkylamine’s amine group (–NH2) attacks the appropriate functional groups on the precursor molecule, forming an ionic bond that constitutes the TSIL structure. The solution is heated under controlled conditions at 60° C and 0.2 bar pressure to remove the excess methanol and obtain the desired ionic liquid. Due to the low vapor pressure of methanol, it evaporates, leaving the ionic liquid product intact55. Figure 3 illustrates the third step of the synthesis pathway for the TSILs.
Fig. 3.
The third step of synthesis of TSILs.
Acid leaching of lead production plant waste
The residue generated from the lead production line at the Delijan plant contains valuable metals like Ni(II), Cu(II), Cd(II), and Zn(II), presenting significant recovery potential. A two-stage acid-leaching process was employed to extract these metals56. Sulfuric acid is commonly used as a leaching agent due to its firm acidity, effectiveness, and cost-efficiency. It extracts metals like Ni(II), Cu(II), Cd(II), and Zn(II) from industrial waste by dissolving metal ions into the aqueous phase, forming metal sulfate salts that aid in extraction57. Sulfuric acid also allows pH adjustment, optimizes leaching, and facilitates silicate removal. Its oxidizing nature helps convert metals into more extractable forms, making it an economical and practical choice for improving metal recovery in industrial applications58. In the initial stage, 10 g of the residue was subjected to leaching in 1.5 L of distilled water at 60–70° C for one hour under continuous stirring. Although no significant chemical reactions take place at neutral pH, this step aids in the dispersion, hydration, and swelling of particles, enhancing reactivity in the following acid-leaching stage. Subsequently, pH was regulated to 1.5 with gradually increasing 5 M sulfuric acid, followed by an additional 2 h of stirring. In acidic conditions, metal oxides and, to some extent, elemental metals are solubilized through proton attack, forming metal sulfate complexes (Eq. 1)59.
 |
1 |
 |
If metals exist in their elemental state, they may react directly with sulfuric acid to form metal sulfates and hydrogen gas, as shown below, except for Cu, which reacts minimally due to its low reactivity (Eq. 2)59.
 |
2 |
 |
After filtration, the resulting solution proceeded to the second stage. In this stage, the solution was stirred at 60–70° C for 2 h to remove silicates by adding ammonium persulfate and iron sulfate60,61 (Eqs. 3 and 4).
 |
3 |
 |
4 |
The pH was then adjusted to 3–4 using sodium hydroxide and sulfuric acid, and the solution was filtered. Thus, the final solution was prepared for subsequent processing steps.
Extraction experiments
This study selected three hydrophobic phase models including DCM, A336, and the synergy of these two solvents, DCM/A336. Also, a TSIL was used as an extractant to extract the mentioned metals, whose structure and operating parameters were optimized by the efficiency of the solvent extraction method. The structure of the extractant and the solvents of the hydrophobic phase are drawn in Fig. 4. The structure of the ionic liquid under study was analyzed, and appropriate anions and cations were chosen for metal extraction experiments. In pure DCM and the DCM/A336 synergistic system, 3 mL of the organic phase was used, while 1 mL was used for the A336 system. The aqueous phase volume was kept at 6 mL. Extraction time was set at 15 min, followed by a 5 min mixing period after adding the organic phase, except for time variation experiments. Stirring speed was maintained at 600 rpm. Initially, ammonium cations were synthesized using alkylamines of varying chain lengths (C2 to C8) for the functionalized moiety in step 3. The extraction efficiency was assessed for ethylamine, butylamine, hexylamine, and octylamine. Subsequently, chloride, acetate, nitrate, and hydrogen sulfate anions were compared for the TSIL.
Fig. 4.
Extractant structure and hydrophobic phase solvents.
The optimal structure for the extractant was selected based on comparing extraction efficiency and solubility in the hydrophobic phase. This structure was then utilized for further experiments to optimize operational parameters such as the amount of extractant, pH, reaction time, contact time with the hydrophobic phase, synergistic ratio of DCM and A336, and phase ratio.
To optimize process parameters, varying amounts of the TSIL (ranging from 1 to 300 mg) were investigated. The TSIL was dissolved in 6 mL of aqueous solution, and after the reaction, the organic phase was brought into contact with the aqueous solution. Upon determining the optimal amount of extractant, 100 mg of the TSIL was added to the aqueous solution in the pH range of 1 to 6.5 to enhance selectivity and stirred for 15 min to investigate the extraction efficiency of metals with varying acidity. The composition of the aqueous phase was analyzed using atomic absorption spectroscopy (AAS). The concentration of metal ions in the IL phase was determined by calculating the difference in metal ion concentrations between the aqueous phase before and after the extraction process. The extraction efficiency was assessed by calculating the percentage (% E) using the following Eq1.: The initial concentrations of metals in the leaching solution, measured by AAS, were as follows: Cu of 58.89 mg/L, Ni of 108.52 mg/L, Zn of 108.03 mg/L, and Cd of 49.12 mg/L.
 |
5 |
Ci (mol/L) and Cf (mol/L) refer to the initial and final aqueous solution concentrations. The percentages of metal extraction (% E) were specified at 25 °C. To guarantee the reliability of the results, experiments were carried out in triplicate, allowing the average extraction yields for each system to be assessed. The distribution ratio (D) is determined by the following formula1:
 |
6 |
Vw is water, while VIL refers to ionic liquid phase volume. The relative uncertainty for D is ± 10%. The results of the experiments, which were conducted in duplicate, show agreement within a margin of 5%. The separation factor (SF) indicates the ratio of the distribution coefficients for two different metals62,63.
 |
7 |
An SF > 1000 indicates efficient separation, while lower values suggest reduced separation efficiency64. After extraction, purification is usually performed before performing a desorption step to remove excess extracted metals in the organic phase. This is generally done using water, a desired metal salt (M) solution, dilute acid, or base. Subsequently, at the optimal pH, the reaction time of the TSIL was studied from 1 to 480 min (1, 5, 15, 30, 60, 120, 240, and 480). At the chosen reaction time, the hydrophobic phase (all three systems) was placed in contact with the aqueous solution and examined for 1 to 60 min, including 1, 5, 15, 30, and 60 min, until equilibrium was obtained. Finally, the organic phase was introduced to the aqueous phase with a hydrophobic phase to aqueous phase ratio (O/A) of 0.125, 0.25, 0.5, 1, and 2 to determine the optimal amount of the hydrophobic phase for DCM, A336, and the synergized solvent. After evaluating the extraction efficiency for the three types of systems, experiments were conducted on the synergized system to investigate selectivity, enhanced solubility of the extractant in the hydrophobic phase, speed of phase separation, and cost reduction by varying the A336 to DCM ratio from 0 to 100% by volume in the synergized system.
Stripping
For stripping, six different concentrations (0.1, 0.25, 0.5, 1, 1.5, and 2.5 M) of sulfuric acid, each in a volume of 6 mL, were added to the hydrophobic phase (of all three systems) and stirred at 600 rpm for 30 min to evaluate the stripping efficiency of sulfuric acid. Figure 5 shows a schematic of the stripping method.
Fig. 5.
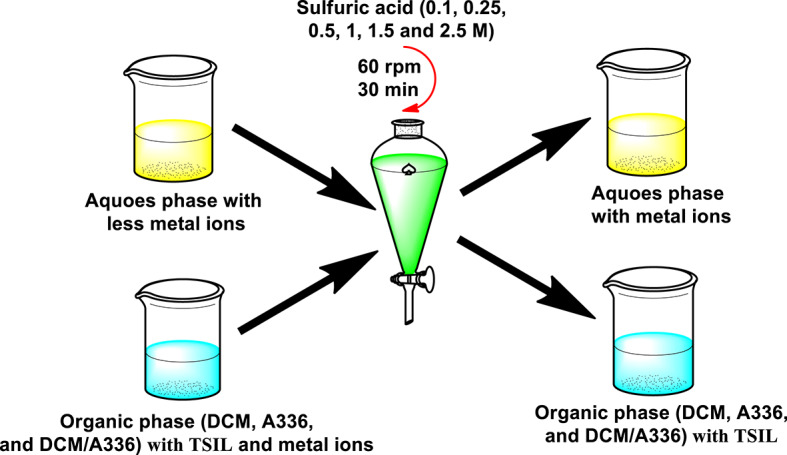
The schematic of the stripping method with the different concentrations of sulfuric acid.
Analytical measurements
Absolute metal ion concentrations in the aqueous phase were quantitatively determined at different stages of the process, including after leaching, extraction, and stripping, using the SHIMADZU 6300 AAS instrument. Fourier transform infrared spectroscopy (FTIR) (Bruker Vertex 70) tests were applied to recognize the chemical composition of two ionic liquids, triethyl with ethyl and tributyl with butyl. The analyses were performed in the wavelength range of 4000–400 cm−1. Proton nuclear magnetic resonance (H-NMR) (BRUKER 400 MHz Ultra Shield) analysis was also performed to detect the intensity and amount of hydrogen in the TSIL structure of two ionic liquids, triethyl with ethyl and tributyl with butyl.
Results and discussion
Characterization of TSILs
FTIR is a widely employed method to identify functional groups in organic compounds, primarily for qualitative analysis. As depicted in Fig. 6, the spectra of TEE and TBE, obtained from the first step of synthesizing the TSILs, Et3NC2NHC4, and Bu3NC2NHC4, as well as the spectra of TEBA and TBBA from the third step of the synthesis process for the same TSILs, are presented. In the region between 3250 cm−1 and 3500 cm−1, a stretching vibration peak corresponding to the OH group is observed, attributed to water molecules adsorbed onto the ILs65. In the region of 2967 cm−1, the stretching vibration peak corresponds to the C-H bond along the carbon chain, while the stretching vibration peak in the area of 1754 cm−1 is associated with the C = O bond of the carbonyl group in the structure of the synthesized ILs64,66. Additionally, the stretching vibration peak in the region of 1310 cm−1 corresponds to the C-O bond, which is observed in TEBA and TBBA owing to the attendance of the ester group67. It is no longer observed in the compounds obtained from the third step because of the substitution of butyl amine. Furthermore, in the region of 1200 cm−1, a stretching vibration peak corresponding to the C-C bond in the carbon chains is observed. In the area of 1100 cm−1, a stretching vibration peak associated with the C-N bond is present. However, during the third synthesis step, butyl amine substitution weakens the bond between C-N, precisely when carbon is also bonded to oxygen in the structure. This shifts the peak toward higher frequencies, causing it to broaden and overlap with adjacent peaks.
Fig. 6.

FTIR spectra of TSILs.
In the H-NMR spectrum of Bu3NC2NHC4 shown in Fig. 7a, three peaks around 0.85 ppm correspond to the hydrogens of the CH3 groups at the ends of the butyl chains. A sextet peak near 1.3 ppm corresponds to the terminal CH2 groups of the butyl chains, while a quintet peak around 1.57 ppm is attributed to the CH2 groups connected to the inner CH2 groups of the butyl chains. A triplet peak at approximately 2.77 ppm corresponds to the CH2 groups attached to the ammonium nitrogen. A singlet peak for the NH group is observed at 5.77 ppm, and the solvent peak appears at 9.5 ppm.
Fig. 7.
H-NMR spectrum of (a) Bu3NC2NHC4, (b) Et3NC2NHC4 ionic liquids.
In Fig. 7b, the H-NMR spectrum of Et3NC2NHC4 is shown. A triplet peak at around 0.83 ppm corresponds to the hydrogen of the terminal CH3 groups in the butyl chains. Another triplet peak at approximately 1.12 ppm represents the CH3 groups at the ends of the butyl chains. A quintet peak near 1.37 ppm is attributed to the CH2 groups connected to both CH3 and CH2 groups. Additionally, a triplet peak at about 3.06 ppm corresponds to the CH2 groups attached to the nitrogen connected to the amide group. A quartet peak at 3.43 ppm represents the CH2 groups bonded to the ammonium nitrogen. Furthermore, a single peak for the NH group appears at 5.77 ppm, and the solvent peak is observed at 9.5 ppm.
The structure of TSIL
The experiments investigating the structure of the TSILs are conducted in two stages: the first focuses on identifying the type of anion, while the second examines the length of the amine chain on the cation. Initial extraction efficiency of anions such as Cl−, HSO4−, NO3−, and CH3COO− is evaluated for Bu3NC2NHC4. Subsequently, with the chloride anion fixed, the lengths of the amine chain are analyzed at 2, 4, 6, and 8 units.
Anionic effect of TSIL
In this study, TSIL was optimized by testing a variety of anions, including Cl−, NO3−, HSO4−, and CH3COO−, to evaluate their effects on the extraction efficiency and overall characteristics of the ionic liquid. The selection of these anions is crucial because they play a significant role in determining the physical and chemical properties of the ionic liquid, such as solubility, viscosity, and extraction performance. Each anion interacts differently with the cationic part of the ionic liquid, which can influence the formation of metal-ligand complexes, the phase separation process, and the transfer of metal ions from the aqueous to the organic phase33. For example, anions like Cl− and NO3− are likely to form more substantial complexes with metal ions, enhancing the extraction efficiency. In contrast, anions like HSO4− or CH3COO− may affect the extraction process differently. These differences in the properties of anions can significantly alter the extraction efficiency for different metals. Figure 8 illustrates the %E of Cu(II), Zn(II), Cd(II), and Ni(II) with variations in the anionic portion of the TSIL. It is evident that in all TSILs, the highest extraction percentage is because of the softer nature of Cd(II) compared to Zn(II), Zn(II) to Cu(II), and Cu(II) to Ni(II), in the order of Cd(II), Zn(II), Cu(II), and Ni(II), respectively. Bu3NC2NHC4-Cl and Bu3NC2NHC4-HSO4 in systems with DCM, A336, and DCM/A336 have significantly extracted more Zn(II) and Cd(II) than Cu(II) and Ni(II). Bu3NC2NHC4-CH3COO has extracted Cd(II) and Zn(II) much more efficiently than the other metals except in the DCM solvent. The Bu3NC2NHC4-NO3 has shown poor performance in DCM but has effectively extracted Cd(II) and Zn(II) in other cases. Due to its simple synthesis and structural properties, Cl was chosen as the best anion for the next steps.
Fig. 8.

Metal extraction efficiency with ionic liquid anion change. The colors represent the metals as follows: orange for Cu, green for Ni, purple for Zn, and yellow for Cd.
Cationic effect of TSIL
The %E is shown in Fig. 9 as a function of the amide moiety’s carbon chain length and the solvent type. As can be seen, the extraction of metals increases with increasing carbon chain length in the DCM solvent for all metals except Cd(II) up to a certain degree in the ionic liquid with a butyl group and then decreases. In solvent, A336 and DCM/A336 synergism, Cu(II) extraction increases with increasing carbon chain length up to the IL with a butyl group. In contrast, in Ni(II), it first increases, then decreases. In contrast, Cd(II) and Zn(II) extraction changes little. The TSIL with a butyl chain was chosen as the extraction agent in this experiment phase because it is hydrophilic, dissolves more quickly in the organic phase, and has better selectivity than other TSIL.
Fig. 9.

Metal extraction efficiency with changing the carbon chain of ionic liquid cation. The colors represent the metals as follows: orange for Cu, green for Ni, purple for Zn, and yellow for Cd.
The effect of pH
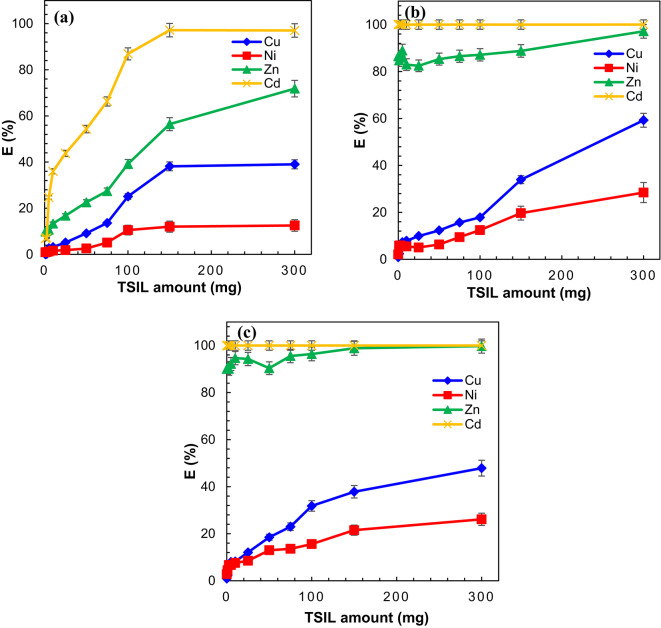
The influence of pH on the extraction performance of Cd(II), Zn(II), Cu(II), and Ni(II) was investigated across three different solvent systems, including DCM, A336, and a synergistic combination of DCM and A336. Figure 10a shows that in the DCM system, the extraction efficiency of Cd(II) increases with increasing pH up to 3, reaching 96%, and then remains constant until a pH of 5 before gradually decreasing. However, due to the low amount of TSIL, the extraction efficiency of other metals remains very low. The extraction of Zn(II) reaches its maximum value of 68% at a pH of 4 and gradually decreases with increasing pH. In the A336 system (Fig. 10b), The data indicate that changes in pH have no significant effect on the extraction percentages of Cd(II) and Zn(II), both exhibiting satisfactory extraction efficiencies. In contrast, the extraction efficiency of Ni(II) shows a slight increase at pH 1.5, reaching 12.18%, followed by a gradual decline at higher pH levels. Furthermore, the extraction efficiency of Cu(II) steadily increases with rising pH, reaching 17.36% at pH 3.5. In the synergistic DCM/A336 system (Fig. 10c), the findings suggest that alterations in pH do not exert a substantial influence on the extraction efficiency of Cd(II) and Zn(II), with both metals exhibiting extraction percentages within acceptable ranges. However, Ni(II) extraction slightly rises to 11.87% at a pH of 3, followed by a gradual decline at higher pH values. Furthermore, the extraction of Cu(II) shows a steady increase of up to 40% as the pH is elevated.
Fig. 10.
Effect of pH on the extraction efficiency of Cd(II), Zn(II), Cu(II), and Ni(II) in three solvent systems, (a) DCM, (b) A336, and (c) DCM/A336 synergistic system.
The distribution coefficient (D) correlates with pH, as shown in Fig. 11a, b, and c. In the DCM system (Fig. 11a), the maximum distribution coefficients for Ni(II), Cu(II), Cd(II), and Zn(II) are obtained at a pH of 3 and are 0.28, 0.14, 1.6, and 15.13, respectively. In the A336 system (Fig. 11b), D for Cd(II) is exceptionally high (~ 10,000) due to complete extraction. Zn(II) shows a maximum D of 16.08 at pH of 3, followed by a decline with increasing pH. Cu(II) and Ni(II) show very low D values (~ 0.1), reflecting poor extraction. Similarly, in the synergistic system (Fig. 11c), Cd(II) maintains a high D (~ 10,000). Zn(II)’s D decreases from 19 to 8 as pH increases from 1 to 5.6. Cu(II)’s D follows the extraction trend and peaks at pH 3, whereas Ni(II)’s D remains negligible.
Fig. 11.
Effect of pH on the distribution coefficient (D) of Cd(II), Zn(II), Cu(II), and Ni(II) in three solvent systems, (a) DCM, (b) A336, and (c) DCM/A336 synergistic system. The colors represent the metals as follows: black for Cu, red for Ni, blue for Zn, and green for Cd.
Figure 12a and b illustrate the selectivity factor (SF), defined as the ratio of the distribution coefficient of one metal to another for Zn(II) and Cd(II) relative to other metals, respectively, as a function of pH in the DCM solvent system. The selectivity factor for Zn(II) relative to Ni(II), Cu(II), and Cd(II) generally increases with increasing pH, reaching a maximum value of 20.5, 24, and 0.7 at pH of 5.6. The increased selectivity with pH is attributed to the higher extraction efficiency of Zn(II) compared to Cu(II) and Cd(II) at higher pH values. In the A336 system (Fig. 12c and d), The selectivity factors of Cu(II) and Ni(II) relative to Zn(II) and Cd(II) are nearly zero due to their low extraction. Zn(II)’s selectivity over Ni(II) and Cu(II) initially decreases and then increases with pH, reflecting their extraction behavior. Cd(II) shows a similar trend, but its selectivity over Zn(II) increases as Zn(II) extraction drops, peaking at 1257.7 at pH of 5. In addition, Fig. 12e and f depict the selectivity factors (SF) for Zn(II) and Cd(II), respectively, relative to other metals as a function of pH within the synergistic solvent system comprising DCM and A336. The selectivity factor for Ni(II) relative to Cu(II), Zn(II), and Cd(II) is negligible. The selectivity factor for Cu(II), while also low relative to Zn(II) and Cd(II), decreases with increasing pH up to pH of 5 and then increases again until pH of 5.6, mirroring the extraction trends of these metals. Due to the extremely high extraction of Cd(II), the selectivity factor for Zn(II) relative to Cd(II) is minimal. However, it exhibits a similar trend relative to Ni(II) and Cu(II), initially decreasing until the pH of 4.5 and then increasing until the pH of 5.6. The maximum distribution coefficient for Zn(II) relative to Cu(II) and Ni(II) occurs at a pH of 1, with values of 113.05 and 584.37, respectively. The selectivity factor for Cd(II) relative to Ni(II) and Cu(II) approaches infinity due to its near-complete extraction. However, when compared to Zn(II), the Cd(II) selectivity factor increases from pH in range of 1 to 5.6, reaching a maximum of 1152.6 at a pH of 6.
Fig. 12.
The variation of metal selectivity factor (SF) as a function of aqueous phase pH for (a) Zn(II) and (b) Cd(II) in a DCM system, (c) Zn(II) and (d) Cd(II) in a A336 system, and (e) Zn(II) and (f) Cd(II) in the DCM/A336 synergistic system.
The effect of TSIL dosage
The influence of TSIL dosage (0–300 mg) on the extraction efficiency of Cd(II), Zn(II), Cu(II), and Ni(II) was investigated in various solvent systems. As illustrated in Fig. 13a, increasing the TSIL content in the DCM system enhances the extraction efficiency for all metals. Cd(II) shows the highest efficiency, reaching a plateau at 150 mg. At the same time, Zn(II) continues to exhibit a gradual increase with higher TSIL amounts, suggesting a strong dependence on TSIL concentration.
Fig. 13.
The variation of extraction efficiency with the amount of ionic liquid in three solvent systems, (a) DCM, (b) A336, and (c) DCM/A336 synergistic system.
In the A336 system (Fig. 13b), the findings revealed that Cd(II) extraction was unaffected by the TSIL concentration. In contrast, the extraction of Zn(II), Cu(II), and Ni(II) increased with higher TSIL concentrations, with Cu(II) and Ni(II) showing a more pronounced increase than Zn(II) beyond 100 mg. As shown in Fig. 13b, A336 alone can extract Cd(II) and Zn(II) from aqueous solutions in a sulfate medium. However, adding of the IL before A336 allows better control over the mechanism of metal extraction, potentially optimizing the process.
In the DCM/A336 synergistic system (Fig. 13c), a consistent rise in extraction efficiency is observed for all metals with increasing TSIL content. Cd(II) demonstrates the highest extraction efficiency across all concentrations, while Zn(II), Cu(II), and Ni(II) follow similar upward trends, reaching efficiencies of 98.99%, 47.87%, and 11.26%, respectively.
The effect of TSIL reaction time
The effect of contact time on the extraction efficiency of Cd(II), Zn(II), Cu(II), and Ni(II) was assessed under optimized conditions using 150 mg of TSIL. As illustrated in Fig. 14a, increasing contact time enhances the extraction of all metals, except for Cd(II), which maintains a consistently high extraction rate. Zn(II), Cu(II), and Ni(II) show time dependent increases in extraction efficiency, with Ni(II) exhibiting the slowest kinetics due to its lower affinity and slower mass transfer rate. This behavior indicates that at shorter contact times, the selectivity for Cd(II) and Zn(II) is higher compared to Cu(II) and Ni(II).
Fig. 14.
The variation of extraction efficiency with the reaction time of the ionic liquid in three solvent systems, (a) DCM, (b) A336, and (c) DCM/A336 synergistic system.
In the A336 system, the trend is similar. As illustrated in Fig. 14b, Cd(II) extraction slightly improves over time, reaching up to 97%, while Zn(II) extraction increases steadily, peaking at 95%. The extraction efficiencies for Cu(II) and Ni(II) also rise gradually with time, reaching 35% and 20%, respectively.
A comparable trend is observed in the synergistic DCM/A336 system shown in Fig. 14c. Cd(II) extraction increases from 95% to nearly 100% as contact time increases. Zn(II) extraction also rises, attaining 98.31%. Meanwhile, Cu(II) and Ni(II) extractions improve significantly with longer contact times, reaching 47% and 14.24%, respectively.
The effect of two phases contact time
The impact of contact time between the two phases on extraction efficiency was systematically investigated across the three solvent systems. As shown in Fig. 15a, increasing the contact time resulted in enhanced extraction efficiencies of Zn(II), Ni(II), and Cu(II) from the aqueous phase, indicating a shift in equilibrium favoring further extraction as metal concentrations in the aqueous phase decreased. In contrast, the extraction of Cd(II) remained unaffected by contact time, suggesting a rapid equilibrium and minimal time requirement for its extraction. In the A336 system (Fig. 15b), extending the contact time led to a gradual increase in Cd(II) extraction efficiency, reaching up to 99.08%. Conversely, Zn(II) extraction efficiency decreased with time, declining to 81%, which may be attributed to Zn(II) stabilization within the A336 phase. Meanwhile, Cu(II) and Ni(II) exhibited improved extraction with prolonged contact time, reaching 56% and 24%, respectively.
Fig. 15.
The variation of extraction efficiency with the time of contact of the hydrophobic phase with the aqueous phase in three solvent systems, (a) DCM, (b) A336, and (c) DCM/A336 synergistic system.
Similar trends were observed when using the synergistic DCM/A336 system (Fig. 15c). Zn(II), Ni(II), and Cu(II) extraction efficiencies generally increased with contact time. However, Cd(II) extraction remained relatively stable, indicating rapid equilibrium. Zn(II) extraction slightly declined from 95% at 5 min to 88% at 60 min, while Ni(II) and Cu(II) increased from 7% and 15–17% and 34%, respectively, over the same period.
The effect of the O/A ratio
The effect of varying the organic-to-aqueous (O/A) phase ratio on the extraction efficiency of Cd(II), Zn(II), Cu(II), and Ni(II) was investigated across three solvent systems: DCM, A336, and the synergistic DCM/A336 system. The corresponding results are shown in Fig. 16a, b, and c. In the DCM system (Fig. 16a), Cd(II) showed a high selectivity over other metals, particularly at an A/O ratio of 2:1. As the organic phase volume increased, the extraction efficiencies of the other metals also improved. However, the optimal extraction ratio was found to be 0.5. Therefore, after evaluating various operational parameters in the DCM system, the optimal conditions for the process were found to be: pH of 3, 150 mg of ionic liquid, 5 min of ionic liquid reaction time, 5 min of phase contact time, and a 2:1 aqueous to organic phase ratio.
Fig. 16.
The variation of extraction efficiency with the ratio of organic phase to aqueous phase three solvent systems, (a) DCM, (b) A336, and (c) DCM/A336 synergistic system.
In the A336 system (Fig. 16b), the extraction efficiency of Cd(II) increased with the organic-to-aqueous (O/A) ratio, reaching a maximum of 98% at a ratio of 0.25. Zn(II) also showed a high extraction efficiency, peaking at 97% at a ratio of 0.5. Observations indicated that Cd(II) was preferentially extracted at lower O/A ratios, while Zn(II) was extracted more effectively at higher ratios. This trend suggests that selective extraction of Cd(II) and Zn(II) can be controlled by adjusting the volume of A336. Additionally, the extraction efficiencies of Cu(II) and Ni(II) showed gradual increases with rising phase ratios, ultimately reaching 71.3% and 14.3%, respectively. As a result, for the ionic liquid solvent A336, the optimal conditions were identified as follows: a pH of 3, 150 mg of the ionic liquid, a reaction time of 5 min for the ionic liquid, a phase contact time of 5 min, and an A/O ratio of 2:1.
A similar trend was observed in the DCM/A336 synergistic system (Fig. 16c). Cd(II) extraction increased with higher phase ratios, achieving 97% at a ratio of 2. Zn(II) also showed high extraction efficiency, peaking at 96% at the same ratio. Meanwhile, Cu(II) and Ni(II) extraction efficiencies improved from 14% to 7% at a ratio of 0.125 to 50% and 17% at a ratio of 2, respectively.
The effect of the ratio of A336 to the hydrophobic phase
The optimal ratio of A336 to DCM was determined by investigating extraction efficiencies at various ratios. As shown in Fig. 17, Zn(II) extraction increased from 85% in pure A336 to 98.84% with increasing DCM content. Conversely, Cu(II) and Ni(II) extraction decreased with increasing A336 proportion. Cd(II) extraction remained relatively unaffected by the changes in the solvent ratio. After evaluating various operational parameters for the synergistic system of DCM and A336, the optimal conditions were determined as follows: a pH of 2, 150 mg of the TSIL, a reaction time of 5 min for the TSIL, a contact time of 5 min, an A/O ratio of 2:1, and a DCM-to-total hydrophobic phase ratio of 40%.
Fig. 17.

The changes of extraction efficiency with varying volume ratios of A336 to the hydrophobic phase in the synergistic DCM/A336 system.
Recovery of metals from the hydrophobic phase of the extractant
The results of metal stripping from the organic phase to the aqueous phase at various H2SO4 concentrations using different solvents were analyzed and are presented in Fig. 18. According to the data, Cd(II) exhibited limited stripping efficiency in DCM. In contrast, no stripping was observed with other solvents. Zn(II), however, showed the highest stripping efficiency in DCM, with its efficiency increasing as the H2SO4 concentration rose. A similar trend was observed for other metals, although their stripping efficiencies were lower than those of DCM. Ni(II), which generally has very low extraction efficiency across all solvents tested, also exhibited low stripping efficiency. However, in solvent A336, Ni(II) showed the highest stripping efficiency, approximately 62%. Unlike other cases, the stripping efficiency for Ni(II) decreased with increasing H2SO4 concentration in solvent A336, whereas it increased with different solvents. Cu(II) displayed the highest stripping efficiency (95%) in the synergistic solvent system. However, as the H2SO4 concentration increased, the stripping efficiency decreased, a trend also observed in solvent A336. In contrast, Cu(II) stripping efficiency in DCM increased with higher H2SO4 concentrations.
Fig. 18.
The stripping efficiency of metals with varying H2SO4 concentrations in three systems: (a) DCM, (b) A336, and (c) DCM/A336 synergistic system.
Metal ion selectivity of TSIL
In this study, the selectivity of TSIL for metal ions follows the sequence of Cd(II) > Zn(II) > Cu(II) > Ni(II). This selectivity order has significant implications for the potential applications of TSIL in metal ion extraction, as it suggests that TSIL could be particularly effective in extracting Cd(II) from a mixture of these metal ions. The selectivity can be attributed to speciation and the extraction mechanism.
Speciation refers to the different forms of metal ions, such as free ions, complexes with ligands, or hydrolyzed species68,69. In the aqueous phase, metal ions are present in various speciation forms, directly impacting their extraction efficiency. For instance, Cd(II) primarily exists as Cd2+ ions, which are small and possess a high charge density, making them highly solvated and readily complexed by TSIL. In contrast, Zn(II) mainly exists as Zn2+ ions but can form weak complexes or undergo minor hydrolysis to create Zn(OH)2. Cu(II) is typically found as Cu2+ ions, but at higher pH, it may hydrolyze to form Cu(OH)2. Ni(II), with its lower charge density and larger ionic radius, is less efficiently extracted due to weaker interactions with the TSIL, and it can also form hydrolyzed species such as Ni(OH)2 at elevated pH. The speciation of these metal ions plays a crucial role in determining their extraction efficiency, with Cd(II) being extracted most efficiently due to its smaller ionic radius and higher charge density70.
The extraction process, a key focus of this study, involves the interaction between the metal ions and the ionic liquid. TSIL is more effective at extracting ions with higher charge densities because it coordinates well with them58. The precision of our research is evident in the fact that the selectivity order is directly related to the charge density of the metal ions. Since Cd(II) has the highest charge density in the series, it is more easily extracted. While Zn(II) and Cu(II) are also extracted, they do so to a lesser extent because of their larger ionic radii and lower charge density. Ni(II), having the lowest charge density, is the least efficiently extracted, which explains its position at the end of the selectivity order.
Although a clear selectivity order of Cd(II) > Zn(II) > Cu(II) > Ni(II) was observed, it is essential to note that the absolute differences in extraction efficiencies among these metal ions under the current experimental conditions were moderate and did not result in complete or highly sharp separations. This limitation indicates that the practical applicability of TSIL for selective metal recovery in multi-metal systems requires further optimization, such as pH adjustment, multiple extraction stages, or the use of more selective stripping agents.
Nevertheless, this study provides valuable insights into the extraction mechanisms of metal ions by TSILs, and the observed selectivity trends offer a foundation for the future development of more efficient and environmentally friendly separation processes. Additionally, the synergistic effect observed from combining TSIL with conventional solvents such as DCM and A336 highlights a promising strategy that has been less explored in prior research. Overall, this work transparently acknowledges the current limitations while positioning its contribution as an essential step toward advancing task-specific ionic liquids for metal extraction, supporting broader efforts to reduce pollution and recover valuable resources.
Conclusion
The study focused on synthesizing and analyzing TSILs, Et3NC2NHC4, and Bu3NC2NHC4, confirming their successful synthesis through FT-IR and H-NMR analyses. The results of experiments related to the structure of the TSILs, the parameters affecting solvent extraction and metal extraction efficiency, and the stripping efficiency for three hydrophobic systems (DCM, A336, and the synergistic combination of both) were thoroughly investigated. Based on a quaternary ammonium salt cation and chloride anion, the extractant demonstrated chelating capabilities for various metals. Butylamine was selected as the functional group forming the cation of the TSIL, showcasing promising results in metal extraction processes. Overall, the TSIL exhibited selectivity in the order of Cd(II) > Zn(II) > Cu(II) > Ni(II) for all three mentioned systems, except in cases of prolonged extraction times where Ni(II) extraction increased while others decreased. This selectivity correlates with the softness order of metals, aligning with the softness of the TSIL’s chelating agents. The TSIL A336 displayed complete removal of Cd(II) and high removal of Zn(II), with lower extraction of Cu(II) and Ni(II), displaying selectivity in the order of Cd(II) > Zn(II) > > Ni(II) > Cu(II). The use of the ionic liquid A336 is advantageous due to the presence of a nitrogen atom in the structure of the ammonium cation, making it particularly effective for extracting softer metals like Cd(II) and Zn(II) than Cu(II) and Ni(II). In the synergistic system with DCM, the extraction sequence prioritizes Cd(II) and Zn(II), followed by Cu(II) and then Ni(II). This trend is consistent across all three systems, with Cu(II) extraction showing variability based on conditions. In contrast, Ni(II) extraction remains low and slow due to its unique kinetics and orbital characteristics. Combining DCM and A336 enhances extraction amounts and selectivity for Cd(II) and Zn(II). DCM’s presence in the hydrophobic phase leads to improved phase separation, decreased viscosity, and lower costs at higher volumes. The similarities in structure between the TSIL and A336 result in their effective separation from the aqueous phase, with A336 increasing the solvent’s boiling point and reducing the hydrophobic phase’s volatility due to stronger intermolecular interactions. With its high charge density, the sulfate anion’s presence in the aqueous phase stabilizes water molecules. It reduces the solubility of the TSIL and the hydrophobic phase in the aqueous phase, impacting extraction mechanisms. The TSIL’s ability to chelate metals and donate hydrogen enables effective metal extraction through metal cation chelation and hydrogen displacement. Significant metal extraction through cation displacement was confirmed by pH measurements, indicating nearly 90% extraction via this mechanism. Extraction by A336 in the hydrophobic phase occurs through ion-pair or ion displacement mechanisms, with sulfate anions contributing to reduced extraction through ion displacement due to their stability and charge density. The TSIL’s dissolution behavior in different solvents follows a specific order, with A336 showing the highest tendency to dissolve, followed by DCM and water. The synergistic combination enhances extraction efficiency, while challenges persist in the disposal stage due to strong interactions between Cd(II) and the hydrophobic phase. The presence of sulfuric acid prompts the re-recovery of the TSIL to ensure its reuse. In conclusion, A336 proves effective for extracting metals like Zn(II) and Cd(II), while the TSIL offers improved selectivity and stability over time. The synergistic combination of DCM and A336 enhances extraction efficiency and selectivity, providing cost savings and reducing waste.
Author contributions
Ahad Ghaemi: Conceptualization, Methodology, Software, Conceived and designed the experiments, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Supervision Visualization, Supervision. Mehran Zallaghi: Conceptualization, Methodology, Conceived and designed the experiments, Validation, Formal analysis, Investigation, Resources, Writing - original draft, Writing - review & editing. Bentolhoda Chenarani: Conceptualization, Methodology, Conceived and designed the experiments, Validation, Formal analysis, Investigation, Resources, Writing - original draft, Writing - review & editing.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Diabate, P. D., Dupont, L., Boudesocque, S. & Mohamadou, A. Novel task specific ionic liquids to remove heavy metals from aqueous effluents. Metals (Basel)8(6), 412 (2018).
- 2.Castillo, J. et al. Cu(II) extraction using quaternary ammonium and quaternary phosphonium based ionic liquid. Hydrometallurgy141, 89–96 (2014). [Google Scholar]
- 3.Navarro, P. & Alguacil, F. J. Adsorption of antimony and arsenic from a copper electrorefining solution onto activated carbon. Hydrometallurgy66 (1–3), 101–105 (2002). [Google Scholar]
- 4.Ahmed Basha, C., Bhadrinarayana, N. S., Anantharaman, N. & Meera Sheriffa Begum, K. M. Heavy metal removal from copper smelting effluent using electrochemical cylindrical flow reactor. J. Hazard. Mater.152 (1), 71–78 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Laus, R., Geremias, R., Vasconcelos, H. L., Laranjeira, M. C. M. & Fávere, V. T. Reduction of acidity and removal of metal ions from coal mining effluents using Chitosan microspheres. J. Hazard. Mater.149 (2), 471–474 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Zhou, Y., Boudesocque, S., Mohamadou, A. & Dupont, L. Extraction of metal ions with task specific ionic liquids: influence of a coordinating anion. Sep. Sci. Technol.50 (1), 38–44 (2015). [Google Scholar]
- 7.Kumbasar, R. A. Extraction and concentration study of cadmium from zinc plant leach solutions by emulsion liquid membrane using trioctylamine as extractant. Hydrometallurgy95 (3–4), 290–296 (2009). [Google Scholar]
- 8.AbiD B, A., BrbootI, M. & Al-ShuwaikI, M. M. Removal of heavy metals using chemicals precipitation. Eng. Technol. J.29 (3), 595–612 (2011). [Google Scholar]
- 9.Deepatana, A. & Valix, M. Recovery of nickel and Cobalt from organic acid complexes: adsorption mechanisms of metal-organic complexes onto aminophosphonate chelating resin. J. Hazard. Mater.137 (2), 925–933 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Fenglian, F. & Qi, W. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manage.92 (3), 407–419 (2011). [DOI] [PubMed] [Google Scholar]
- 11.R, T. S., J. C, C. A. & C, J. L. Use of caustic Magnesia to remove cadmium, nickel, and Cobalt from water in passive treatment systems: column experiments. Environ. Sci. Technol.40 (20), 6438–6443 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Taseidifar, M., Ziaee, M., Pashley, R. M. & Ninham, B. W. Ion flotation removal of a range of contaminant ions from drinking water. J Environ. Chem. Eng7(4), 103263 (2019).
- 13.Martínez-Ponce, M. Á., Ortiz Lara, N., Avila-Rodríguez, M. & Cholico-González, D. Selective extraction of Ti(IV) from acidic sulfate media by phosphonium-based ionic liquids and stripping with sulfuric acid. Hydrometallurgy221, 106136 (2023).
- 14.Niu, Z. et al. Efficient extraction of lithium from alkaline solution using the synergistic extractants ethylhexyl salicylate and trialkylphosphine oxide in kerosene and stripping with acid. Hydrometallurgy228, 106342 (2024).
- 15.Jha, M. K., Kumar, V., Jeong, J. & Lee, J. C. Review on solvent extraction of cadmium from various solutions. Hydrometallurgy111–112 (1), 1–9 (2012). [Google Scholar]
- 16.Komjarova, I. & Blust, R. Comparison of liquid-liquid extraction, solid-phase extraction and co-precipitation preconcentration methods for the determination of cadmium, copper, nickel, lead and zinc in seawater. Anal. Chim. Acta. 576 (2), 221–228 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Nayl, A. A. Extraction and separation of Co(II) and Ni(II) from acidic sulfate solutions using Aliquat 336. J. Hazard. Mater.173 (1–3), 223–230 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Sun, X., Ji, Y., Zhang, L., Chen, J. & Li, D. Separation of Cobalt and nickel using inner synergistic extraction from bifunctional ionic liquid extractant (Bif-ILE). J. Hazard. Mater.182 (1–3), 447–452 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Lee, L. Y., Morad, N., Ismail, N., Talebi, A. & Rafatullah, M. Optimization for liquid-liquid extraction of cd(Ii) over cu(ii) ions from aqueous solutions using ionic liquid Aliquat 336 with tributyl phosphate. Int. J. Mol. Sci.21 (18), 1–16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobekova Foltova, S., Vander Hoogerstraete, T., Banerjee, D. & Binnemans, K. Samarium/cobalt separation by solvent extraction with undiluted quaternary ammonium ionic liquids. Sep. Purif. Technol.210, 209–218 (2019). [Google Scholar]
- 21.Abebe, A., Tilahun, S., Mesfine, M. & Atlabachew, M. Removal of cadmium ions from aqueous solution using very small ionic liquids to water ratio without metal chelator and pH modifications. Ethiop. J. Sci. Technol.10 (1), 51 (2017). [Google Scholar]
- 22.Behera, S. S. & Parhi, P. K. Influence of ionic liquid (R4PSCN) for selective separation and recovery of copper from spent Cu[sbnd]Cr catalyst leach liquor. Hydrometallurgy228, 106352 (2024).
- 23.Freire, M. G. Green Chemistry and Sustainable Technology Ionic-Liquid- Based Aqueous Biphasic Systems Fundamentals and Applications.
- 24.Rodriguez, H. Ionic Liquids for Better Separation Processes. Published online (2015).
- 25.Chen, Ji. Application of Ionic Liquids on Rare Earth Green Separation and Utilization. Springer Berlin, Heidelberg (2016).
- 26.Lertlapwasin, R., Bhawawet, N., Imyim, A. & Fuangswasdi, S. Ionic liquid extraction of heavy metal ions by 2-aminothiophenol in 1-butyl-3-methylimidazolium hexafluorophosphate and their association constants. Sep. Purif. Technol.72 (1), 70–76 (2010). [Google Scholar]
- 27.Huddleston, J. G., Willauer, H. D., Swatloski, R. P., Visser, A. E. & Rogers, R. D. Room temperature ionic liquids as novel media for clean liquid-liquid extraction. Chem. Commun.16, 1765–1766 (1998). [Google Scholar]
- 28.Liu, W. et al. Synthesis of N-(2-ethylhexyl)-pyridine-4-carboxamide and its synergistic behaviors with dinonylnaphthalene sulfonic acid for the selective extraction of nickel and cobalt. Sep Purif. Technol286, 120385 (2022).
- 29.Fouad, E. A. Separation of copper from aqueous sulfate solutions by mixtures of Cyanex 301 and LIX® 984N. J. Hazard. Mater.166 (2–3), 720–727 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Cheng, C. Y. Solvent extraction of nickel and Cobalt with synergistic systems consisting of carboxylic acid and aliphatic hydroxyoxime. Hydrometallurgy84 (1–2), 109–117 (2006). [Google Scholar]
- 31.Darvishi, D., Haghshenas, D. F., Alamdari, E. K., Sadrnezhaad, S. K. & Halali, M. Synergistic effect of Cyanex 272 and Cyanex 302 on separation of Cobalt and nickel by D2EHPA. Hydrometallurgy77 (3–4), 227–238 (2005). [Google Scholar]
- 32.Sun, X., Zhao, J., Meng, S. & Li, D. Synergistic extraction and separation of yttrium from heavy rare earths using mixture of sec-octylphenoxy acetic acid and bis(2,4,4-trimethylpentyl) phosphinic acid. Anal. Chim. Acta. 533 (1), 83–88 (2005). [Google Scholar]
- 33.Sharma, P., Sharma, S. & Kumar, H. Introduction to ionic liquids, applications and micellization behaviour in presence of different additives. J Mol. Liq.393, 123447 (2024).
- 34.Stojanovic, A. & Keppler, B. K. Ionic liquids as extracting agents for heavy metals. Sep. Sci. Technol.47 (2), 189–203 (2012). [Google Scholar]
- 35.P. C, M. I, Z. A, Buriol, L., N. D., & Martins, P. M. A. Pharmaceutical salts: solids to liquids by using ionic liquid design. Ion Liq - New Asp Futur. Published online (2013).
- 36.Li, H. et al. Ionic liquid adsorption and nanotribology at the silica-oil interface: Hundred-fold Dilution in oil lubricates as effectively as the pure ionic liquid. J. Phys. Chem. Lett.5 (23), 4095–4099 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Ionic Liquids: Theory, Properties, New Approaches. Ion Liq Theory, Prop New Approaches. Published online. (2012).
- 38.Chen, F. T., Wang, B. & Ma, H. Z. Novel synthesis of methoxymethyl benzene by electrochemical coupling reaction of toluene with methanol in ionic liquid media. J. Hazard. Mater.165 (1–3), 1253–1257 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Plechkova, N. V. & Seddon, K. R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev.37 (1), 123–150 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Sun, X. Q., Peng, B., Chen, J., Li, D. Q. & Luo, F. An effective method for enhancing metal-ions’ selectivity of ionic liquid-based extraction system: adding water-soluble complexing agent. Talanta74 (4), 1071–1074 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Harjani, J. R., Friščić, T., MacGillivray, L. R. & Singer, R. D. Removal of metal ions from aqueous solutions using chelating task-specific ionic liquids. Dalt Trans.34, 4595–4601 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Visser, A. E. et al. Task-specific ionic liquids for the extraction of metal ions from aqueous solutions. Chem Commun (1), 135–136 (2001).
- 43.Visser, A. E. et al. Task-specific ionic liquids incorporating novel cations for the coordination and extraction of Hg2 + and Cd2+: synthesis, characterization, and extraction studies. Environ. Sci. Technol.36 (11), 2523–2529 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Vladimir, M. et al. Task-specific ionic liquid trioctylmethylammonium salicylate as extraction solvent for transition metal ions. Talanta80 (3), 1177–1182 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Agatonovic-Kustrin, S., Gegechkori, V., Kustrin, E. & Morton, D. W. The effect of lactic acid fermentation on the phytochemical content of Fig leaf extracts compared to single solvent and sequential solvents extraction. South. Afr. J. Bot.166, 218–225 (2024). [Google Scholar]
- 46.Mahandra, H., Faraji, F. & Ghahreman, A. Novel extraction process for gold recovery from thiosulfate solution using phosphonium ionic liquids. ACS Sustain. Chem. Eng.9 (24), 8179–8185 (2021). [Google Scholar]
- 47.De Los Ríos, A. P. et al. Removal of metal ions from aqueous solutions by extraction with ionic liquids. J. Chem. Eng. Data. 55 (2), 605–608 (2010). [Google Scholar]
- 48.Mohapatra, A. S., Behera, S. S., Tripathy, S. K., Parhi, P. K. & Sanjay, K. Extensive investigation on extraction behaviour of organo-phosphrous based bi-functional ionic liquids for separation of molybdenum (Mo) from spent Co-Mo/Al2O3 leach liquor. J Mol. Liq.366, 120087 (2022).
- 49.Bai, R., Wang, J., Wang, D., Zhang, Y. & Cui, J. Selective separation of lithium from the high magnesium Brine by the extraction system containing phosphate-based ionic liquids. Sep Purif. Technol274, 119051 (2021).
- 50.Zante, G., Masmoudi, A., Barillon, R., Trébouet, D. & Boltoeva, M. Separation of lithium, Cobalt and nickel from spent lithium-ion batteries using TBP and imidazolium-based ionic liquids. J. Ind. Eng. Chem.82, 269–277 (2020). [Google Scholar]
- 51.Thasneema, K. K. et al. Removal of toxic heavy metals, phenolic compounds and textile dyes from industrial waste water using phosphonium based ionic liquids. J Mol. Liq323, 114645 (2021).
- 52.Jing, X. et al. Environmentally friendly extraction and recovery of Cobalt from simulated solution of spent ternary lithium batteries using the novel ionic liquids of [C8H17NH2][Cyanex 272]. ACS Sustain. Chem. Eng.9 (6), 2475–2485 (2021). [Google Scholar]
- 53.Riaño, S., Sobekova Foltova, S. & Binnemans, K. Separation of neodymium and dysprosium by solvent extraction using ionic liquids combined with neutral extractants: batch and mixer-settler experiments. RSC Adv.10 (1), 307–316 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng, H. et al. Selective extraction of lithium from spent lithium batteries by functional ionic liquid. ACS Sustain. Chem. Eng.9 (20), 7022–7029 (2021). [Google Scholar]
- 55.Blesic, M. et al. Tunable thermomorphism and applications of ionic liquid analogues of girard’s reagents. Green. Chem.16 (9), 4115–4121 (2014). [Google Scholar]
- 56.Zeng, L. & Cheng, C. Y. A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts. Part I: metallurgical processes. Hydrometallurgy98 (1–2), 1–9 (2009). [Google Scholar]
- 57.Kani, O. S. M., Azizitorghabeh, A. & Rashchi, F. Recovery of Zn(II), Mn(II) and Co(II) from the zinc plant residue using the solvent extraction with CYANEX 302 and D2EHPA/TBP: stoichiometry and structural studies. Miner Eng.169, 106944 (2021).
- 58.Yudaev, P. A. & Chistyakov, E. M. Ionic liquids as components of systems for metal extraction. ChemEngineering6(1), 6 (2022).
- 59.Wellens, S. et al. Dissolution of metal oxides in an acid-saturated ionic liquid solution and investigation of the back-extraction behaviour to the aqueous phase. Hydrometallurgy144–145, 27–33 (2014). [Google Scholar]
- 60.Rao, Y. F., Qu, L., Yang, H. & Chu, W. Degradation of carbamazepine by Fe(II)-activated persulfate process. J. Hazard. Mater.268, 23–32 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Zhu, J. P. et al. Modelling of Iohexol degradation in a Fe(II)-activated persulfate system. Chem. Eng. J.367, 86–93 (2019). [Google Scholar]
- 62.Xie, F., Zhang, T. A., Dreisinger, D. & Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Min. Eng.56, 10–28 (2014). [Google Scholar]
- 63.Gorzin, H., Ghaemi, A., Hemmati, A. & Maleki, A. Equilibrium and kinetics of Praseodymium and neodymium extraction from NdFeB magnet-leaching solutions with [R4N+][NO3–] using single drop column. J Mol. Liq.318, 114376 (2020).
- 64.Gorzin, H., Ghaemi, A., Hemmati, A. & Maleki, A. Studies on effective interaction parameters in extraction of Pr and Nd using Aliquat 336 from NdFeB magnet-leaching solution: multiple response optimizations by desirability function. J Mol. Liq.324, 115123 (2021).
- 65.Helmi, M., Chenarani, B., Ghaemi, A. & Hemmati, A. Multi-functional cao@go catalyst and adsorbent derived from eggshell waste for removal of environmental pollutants. Fuel396, 135308 (2025).
- 66.Chenarani, B., Srivastava, V., Sainio, T. & Lotfollahi, M. N. Mesoporous nanocomposite polydopamine-coated graphene oxide/maghemite for high-efficient adsorption of diclofenac sodium in batch mode: synthesis, characterization, RSM modeling and optimization. J Porous Mater. 32, 965–988 (2025).
- 67.Predoi, D. et al. Properties of Basil and lavender essential oils adsorbed on the surface of hydroxyapatite. Materials (Basel)11(5), 652 (2018). [DOI] [PMC free article] [PubMed]
- 68.Hirose, K. Chemical speciation of trace metals in seawater: A review. Anal. Sci.22 (8), 1055–1063 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Templeton, D. M. et al. Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC recommendations 2000). Pure Appl. Chem.72 (8), 1453–1470 (2000). [Google Scholar]
- 70.Namieśnik, J. & Rabajczyk, A. The speciation and physico-chemical forms of metals in surface waters and sediments. Chem. Speciat. Bioavailab.22 (1), 1–24 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.