Abstract
Pseudomonas syringae pv. tomato, the causal agent of bacterial speck of tomato, and the plant growth-promoting bacterium Azospirillum brasilense were inoculated onto tomato plants, either alone, as a mixed culture, or consecutively. The population dynamics in the rhizosphere and foliage, the development of bacterial speck disease, and their effects on plant growth were monitored. When inoculated onto separate plants, the A. brasilense population in the rhizosphere of tomato plants was 2 orders of magnitude greater than the population of P. syringae pv. tomato (107 versus 105 CFU/g [dry weight] of root). Under mist chamber conditions, the leaf population of P. syringae pv. tomato was 1 order of magnitude greater than that of A. brasilense (107 versus 106 CFU/g [dry weight] of leaf). Inoculation of seeds with a mixed culture of the two bacterial strains resulted in a reduction of the pathogen population in the rhizosphere, an increase in the A. brasilense population, the prevention of bacterial speck disease development, and improved plant growth. Inoculation of leaves with the mixed bacterial culture under mist conditions significantly reduced the P. syringae pv. tomato population and significantly decreased disease severity. Challenge with P. syringae pv. tomato after A. brasilense was established in the leaves further reduced both the population of P. syringae pv. tomato and disease severity and significantly enhanced plant development. Both bacteria maintained a large population in the rhizosphere for 45 days when each was inoculated separately onto tomato seeds (105 to 106 CFU/g [dry weight] of root). However, P. syringae pv. tomato did not survive in the rhizosphere in the presence of A. brasilense. Foliar inoculation of A. brasilense after P. syringae pv. tomato was established on the leaves did not alleviate bacterial speck disease, and A. brasilense did not survive well in the phyllosphere under these conditions, even in a mist chamber. Several applications of a low concentration of buffered malic acid significantly enhanced the leaf population of A. brasilense (>108 CFU/g [dry weight] of leaf), decreased the population of P. syringae pv. tomato to almost undetectable levels, almost eliminated disease development, and improved plant growth to the level of uninoculated healthy control plants. Based on our results, we propose that A. brasilense be used in prevention programs to combat the foliar bacterial speck disease caused by P. syringae pv. tomato.
Tomato plants are hosts to Azospirillum brasilense, a plant growth-promoting bacterium (PGPB) (7, 8), and Pseudomonas syringae pv. tomato, the causal agent of bacterial speck disease(35, 47). This disease is of moderate economic importance to tomato production under greenhouse and field conditions (24). Whereas A. brasilense increases the growth and yield of tomato plants (14), P. syringae pv. tomato decreases production (51). Although A. brasilense is known as a rhizosphere bacterium (7), some strains are ephiphytic (7, 10), and P. syringae pv. tomato is largely an epiphytic foliar bacterium (15, 36). Nevertheless, both bacterial species are capable of colonizing seeds, leaves, and the rhizosphere. Plant foliage is heavily colonized by both bacterial species only under mist chamber conditions (4) that favor bacterial speck infections (12). The presence of low levels of these bacterial species under dry leaf conditions is known as well (4, 36).
Despite progress in our understanding of the molecular mechanisms of P. syringae pv. tomato pathogenicity (20), including the sources of genetic resistance to the pathogen (6, 18, 33, 34, 39, 50; V. F. Lawson and W. L. Summers, abstr., HortScience 17:503) and the genes which confer resistance to the disease (30, 41), this knowledge has not yet translated into an efficient strategy to control this minor, but occasionally devastating, foliar disease. Disease control is still based on traditional chemical and physical methods (3), and despite some significant successes, achieved mainly by the induction of systemic resistance in plants (1, 27, 45, 52) and the displacement of a pathogen by nonvirulent strains of the same pathogen or by ecologically similar antagonistic strains (28, 43, 44, 48, 49), biological control of foliar bacterial pathogens is still largely at the experimental stage.
The aim of this study was to measure the fluctuations in the populations of the two bacterial species, belonging to different genera, on the foliage and in the rhizospheres of tomato plants inoculated with one or both species. The effect of the relative sizes of the bacterial populations on the development of bacterial leaf speck disease in tomato plants and on plant growth was monitored.
MATERIALS AND METHODS
Organisms and growth conditions.
A. brasilense Cd (DSM 1843) and a naturally triple-antibiotic-resistant mutant (oxytetracycline, rifampin, and kanamycin, 200 μg of each per liter) of P. syringae pv. tomato (WT-1-ORK) from our laboratory culture collection were used in this study and grown as described previously (4). P. syringae pv. tomato, strain WT-1 (12), was originally isolated from infected tomato plants growing in a greenhouse during an epidemic in 1975. In preliminary greenhouse and growth chamber tests, there was no difference in virulence between P. syringae pv. tomato WT-1-ORK used in this study and wild-type P. syringae pv. tomato WT-1 (unpublished data).
Tomato plants (Lycopersicon esculentum Mill) of the susceptible fresh-market cultivar Pik Red (Joseph Harris Co., Rochester, N.Y.) (24) were grown in black, 500-ml pots containing a sterile (tyndallized) commercial potting substrate (Sunshine Mix 3, special fine; Fisons Horticulture, Mississauga, Ontario, Canada) in a greenhouse as previously described (16). The commercial greenhouse cultivation of tomato plants in steamed, nonsoil substrates, where plants are subjected to a high relative humidity and the condensation of free water drops on leaf surfaces, is commonplace (12).
Inoculation and detection techniques.
Seeds and leaves were inoculated with A. brasilense Cd and P. syringae pv. tomato, as described previously, after the plants were preconditioned under mist conditions (4, 12). Leaves were inoculated at the three- to five-true-leaf stage with a handheld pneumatic sprayer from a height of 25 to 35 cm above the plant. Plants were sprayed until runoff occurred. Tomato seeds or leaves were inoculated with monocultures of each bacterium separately or with both species together at the intervals mentioned for each experiment. In each case, the total inoculation level was 106 CFU/ml. These concentrations are optimal for plant growth promotion by Azospirillum sp. (14), for avoiding the growth inhibition known to be induced by high cell concentrations (2, 25), and for preventing atypical symptom formation caused by high concentrations of P. syringae pv. tomato (12). Plants were incubated under mist chamber conditions in the greenhouse with a temperature regime of 28°C during the day and 22°C at night and with natural illumination. Mist diffuser jets applied mist every 30 min for 5 s, creating permanently wet leaves with minimal dripping of liquids from the surfaces. To increase the A. brasilense Cd population on the leaves, diluted malic acid was sprayed onto the leaves prior to inoculation; before application, the malic acid was adjusted to pH 6.5 with 0.01 g of NaOH per liter and then dissolved in 0.01 M phosphate-buffered saline, pH 6.5, to achieve a final concentration of 0.02 g/liter.
After the leaves were homogenized as previously described (38), bacteria were specifically detected and enumerated by an enzyme-linked, immunosorbent assay for A. brasilense Cd (11, 26) and by the plate count method for P. syringae pv. tomato on nutrient agar plates supplemented with an antibiotic package (200 μg of each antibiotic [oxytetracycline, rifampin, and kanamycin] per liter of medium). Rhizosphere populations were sampled as previously described (4, 11, 14). The total number of cultured bacteria in the phyllospheres and in the rhizospheres of plants growing under dry ambient temperature conditions were enumerated by the plate count method on nutrient agar (Difco, Detroit, Mich.) supplemented with cycloheximide (250 mg/liter; Sigma) after 72 h of incubation at 28 ± 2°C.
Evaluation of disease severity and bacteriocin activity.
Disease severity was evaluated visually and scored using a disease index with a range of 0 to 3 (0 signifies a healthy-looking plant; 1 signifies 2 to 5 specks together or spread over each leaf; 2 signifies 6 to 10 specks; and 3 signifies more than 10 specks), as previously described (50).
Experimental design and statistical analysis.
The plants subjected to the various treatments were randomly placed in a growth chamber or a mist chamber. Each treatment was replicated five times, and three pots served as a single replicate; each pot contained two plants. Data from the three pots were combined, and the entire experiment was analyzed by analysis of variance (ANOVA) or by Student's t test at a P of ≤0.05. This was done because tomato seedlings are small in the initial stages of growth yet need space to grow. Six individual seedlings supplied enough dry matter to avoid the inaccuracies that occur when dry weight is determined for individual, very small plants. All experiments were repeated two or three times. The detection intervals of both bacterial species varied among the experiments, ranging from the time of inoculation to 15 days postinoculation in short-term experiments and up to 60 days postinoculation in longer experiments. Nevertheless, both A. brasilense Cd and P. syringae pv. tomato organisms were enumerated at the same intervals during each experiment.
RESULTS
Sizes of P. syringae pv. tomato and A. brasilense populations in the rhizospheres and on the foliage of tomato plants when each bacterium was inoculated individually.
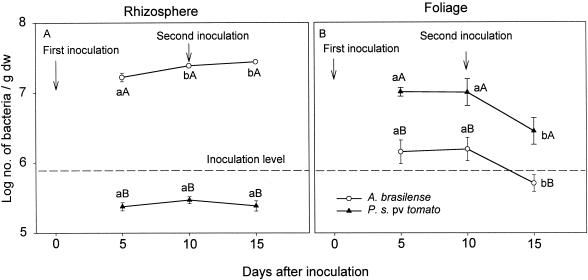
Five or 10 days after seed inoculation, the A. brasilense populations in the rhizospheres of tomato plants (over 107 CFU/g [dry weight] of root) were 2 orders of magnitude greater than the P. syringae pv. tomato populations (over 105 CFU/g [dry weight] of root) in the rhizospheres of separate tomato plants. No further increases in the population sizes were detected later (Fig. 1A). On the same plants, under mist chamber conditions, the leaf populations of P. syringae pv. tomato were 1 order of magnitude greater than the A. brasilense populations 5 days after inoculation. The populations did not increase further with time, and they later decreased (Fig. 1B). All of these changes occurred against the background of a natural microbial population of 100 to 1,000 CFU of culturable phyllosphere bacteria per g (dry weight) of leaf. The total cultured rhizosphere population was constant before and immediately after inoculation, regardless of the preconditioning of the leaves with mist, and it was lower than the population of inoculated bacteria (Table 1). A second inoculation of P. syringae pv. tomato or A. brasilense into the rhizosphere, 10 days after the initial inoculation, did not increase the P. syringae pv. tomato and A. brasilense populations there or in the plant foliage (Fig. 1).
FIG. 1.
Sizes of P. syringae pv. tomato and A. brasilense populations in the rhizospheres and on the foliage of tomato plants when the bacteria were inoculated on separate plants. Points located on the same line and denoted by different lowercase letters differ significantly at a P of ≤0.05 by one-way ANOVA. Points which share the same time position and are denoted by different capital letters differ significantly at a P of ≤0.05 by Student's t test. Bars represent standard errors (SE); missing bars indicate that the SE is smaller than the point.
TABLE 1.
Total cultured bacterial populations on leaves and in the rhizospheres of tomato plants prior to competition experiments
| Location of populations | Mean bacterial population (CFU/g [dry wt]) ± SE
|
||
|---|---|---|---|
| Plants growing under dry ambient conditions | Plants preconditioned with 24-h misting | Plants immediately after inoculationa | |
| Phyllosphere | 2.3 × 102 ± 9 × 101 | 4.4 × 103 ± 1.4 × 103 | 2.1 × 103 ± 6 × 102 |
| Rhizosphere | 1.7 × 105 ± 3 × 104 | 2.1 × 105 ± 4 × 104 | 1.9 × 105 ± 2 × 104 |
Excluding populations of P. syringae pv. tomato and A. brasilense.
Sizes of P. syringae pv. tomato and A. brasilense populations in the rhizospheres of tomato plants and their effects on the dry weights of tomato seedlings when the bacteria were inoculated as a mixed culture on seeds.
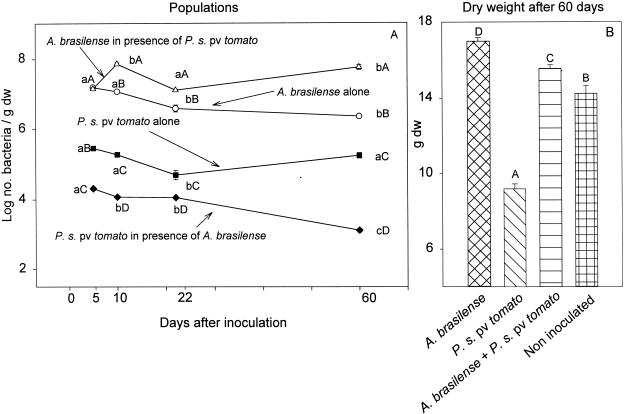
The inoculation of seeds with a mixed culture (A. brasilense and P. syringae pv. tomato) in which the populations of the bacteria were initially similar (106 CFU/ml) resulted in a reduction of the pathogen population in the rhizosphere and a temporary (at 10 days) increase in the A. brasilense population (Fig. 2A). In addition, when plants were grown from seeds inoculated with a mixture of both bacterial species, bacterial speck disease did not develop, even after plants were transferred to mist chamber conditions that favor disease development (data not shown). Compared to inoculation with the pathogen alone, mixed inoculation increased plant biomass, although to a lesser extent than inoculation with A. brasilense alone (Fig. 2B).
FIG. 2.
Sizes of P. syringae pv. tomato and A. brasilense populations in the rhizospheres of tomato plants (A) and their effects on the dry weights (dw) of tomato seedlings (B) when the bacteria were inoculated alone and together onto seeds. (A) Points located on the same line and denoted by different lowercase letters differ significantly at a P of ≤0.05 by one-way ANOVA. Points which share the same time position and are denoted by different capital letters differ significantly at a P of ≤0.05 by one-way ANOVA. (B) Columns denoted by different capital letters differ significantly at a P of ≤0.05 by one-way ANOVA. Bars represent SE; missing bars indicate that the SE is smaller than the point.
Sizes of P. syringae pv. tomato and A. brasilense populations and the development of bacterial leaf speck disease on tomato leaves inoculated with a mixed bacterial culture.
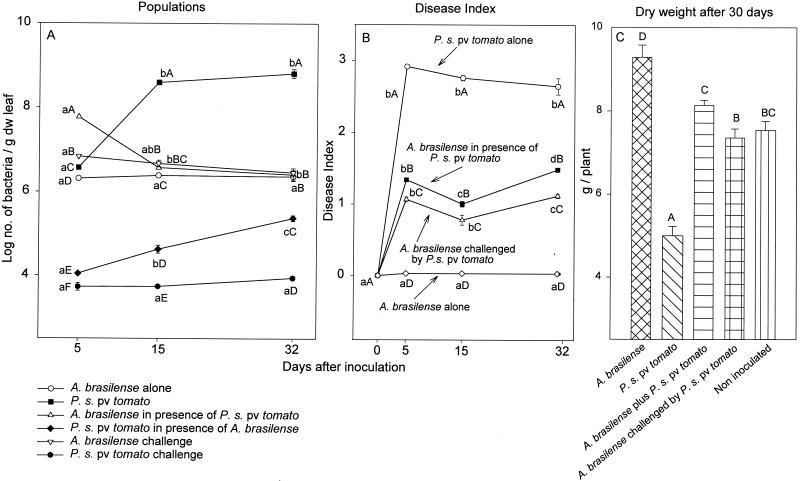
Compared to inoculation with P. syringae pv. tomato alone, leaf inoculation with a mixed bacterial culture under mist conditions significantly reduced the P. syringae pv. tomato population and significantly decreased disease severity (Fig. 3A and B). Challenging plants with P. syringae pv. tomato 4 days after A. brasilense was established on the leaves reduced the population of P. syringae pv. tomato and disease severity even further (Fig. 3A and B). The presence of A. brasilense on the plant, whether from inoculation in a mixed culture with P. syringae pv. tomato or from inoculation alone before challenge with the pathogen, significantly enhanced plant development (Fig. 3C).
FIG. 3.
Sizes of P. syringae pv. tomato and A. brasilense populations (A), the development of bacterial leaf speck disease in tomato leaves (B), and the effect on dry weight of the plants (C) after inoculation of the bacteria onto leaves. (A and B) Points located on the same line and denoted by different lowercase letters differ significantly at a P of ≤0.05 by one-way ANOVA. Points which share the same time position and are denoted by different capital letters differ significantly at a P of ≤0.05 by one-way ANOVA. (C) Columns denoted by different capital letters differ significantly at a P of ≤0.05 by one-way ANOVA. Bars represent SE; missing bars indicate that the SE is smaller than the point.
Both bacteria maintained large populations in the rhizosphere for 45 days when each was inoculated separately onto tomato seeds. However, P. syringae pv. tomato did not survive in the rhizosphere in the presence of A. brasilense (Table 2). Foliar inoculation with A. brasilense after P. syringae pv. tomato was allowed to establish itself on leaves for 2 days did not alleviate bacterial speck disease (Table 3). Under these conditions, A. brasilense did not survive well in the phyllosphere, even under mist conditions.
TABLE 2.
Long-term survival of P. syringae pv. tomato and A. brasilense in the rhizospheres of tomato plants whose seeds were inoculated with one or both species
| Bacterial species inoculated | Mean bacterial population level in roots (CFU/g [dry weight]) ± SE after:
|
|||
|---|---|---|---|---|
| 5 days
|
45 days
|
|||
| A. brasilense | P. syringae pv. tomato | A. brasilense | P. syringae pv. tomato | |
| A. brasilense alone | 2.5 × 107 ± 4 × 106 | 3.4 × 106 ± 7 × 105 | ||
| P. syringae pv. tomato alone | 3.3 × 105 ± 3 × 104 | 1.8 × 105 ± 2 × 104 | ||
| A. brasilense + | 5.5 × 107 ± 8 × 106 | 1.3 × 104 ± 5 × 103 | 4.4 × 106 ± 6 × 105 | Undetectable |
| P. syringae pv. tomato | ||||
TABLE 3.
Development of bacterial leaf speck disease of tomato after leaf inoculation with P. syringae pv. tomato followed by inoculation with A. brasilense 3 days latera
| Bacterial species | Bacterial population level in plants after 10 days (CFU/g [dry wt])
|
Disease severity indexb | Plant dry wt after 15 days (mg) | |
|---|---|---|---|---|
| A. brasilense | P. syringae pv. tomato | |||
| A. brasilense alone | 7.1 × 106 A | 0 | 88 B | |
| P. syringae pv. tomato alone | 7.3 × 108 A | 2.87 A | 63 A | |
| P. syringae pv. tomato + A. brasilense | 4.6 × 104 B | 5.9 × 108 A | 2.79 A | 66 A |
Values followed by a different letter within each column indicate results that differ significantly at a P of ≤0.05 by one-way ANOVA.
Disease severity was scored from 0 to 3 as described in Materials and Methods.
Development of bacterial speck disease on tomato leaves after leaf inoculation with A. brasilense and malic acid.
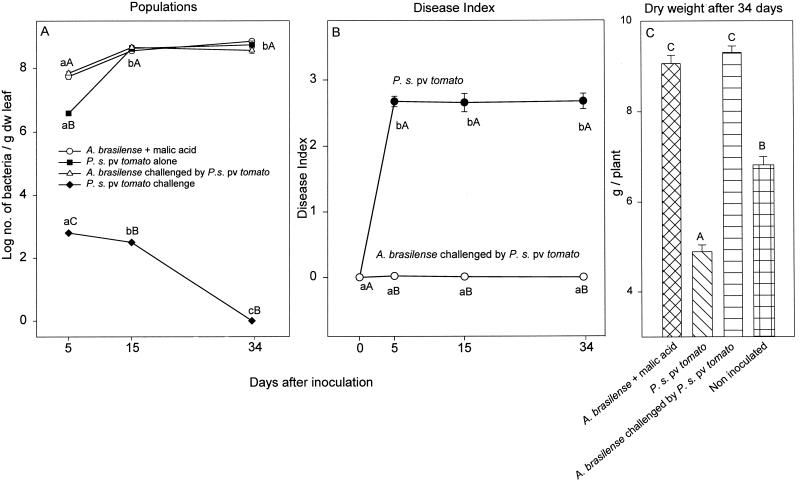
Several applications of a low concentration of buffered malic acid significantly enhanced the size of the A. brasilense leaf population, decreased the population of P. syringae pv. tomato to an almost undetectable level (Fig. 4A), nearly eliminated disease development (Fig. 4B), and improved the growth of the inoculated plants to the level of the uninoculated, healthy control plants (Fig. 4C). Long-term treatment of tomato leaves with malic acid, however, resulted in a pale chlorosis of the leaves after the third and subsequent applications (data not shown).
FIG. 4.
Sizes of P. syringae pv. tomato and A. brasilense populations (A), development of bacterial speck disease in tomato leaves (B), and the effect on dry leaves of the plants (C) after leaf inoculations. (A and B) Points located on the same line and denoted by different lowercase letters differ significantly at a P of ≤0.05 by one-way ANOVA. Points which share the same time position and are denoted by different capital letters differ significantly at a P of ≤0.05 by one-way ANOVA (A) and by Student's t test (B). (C) Columns denoted by different capital letters differ significantly at a P of ≤0.05 by one-way ANOVA. Bars represent SE; missing bars indicate that the SE is smaller than the point. For clarity, values for A. brasilense inoculated alone onto leaves are not presented, as similar information is available in other graphs; values for P. syringae pv. tomato supplemented with malic acid are also not presented, as these were almost identical to those from inoculation with P. syringae pv. tomato alone. For clarity, some letters indicating statistical significance in panel A were also eliminated.
DISCUSSION
Most biological control strategies use biocontrol agents that suppress plant pathogens either by producing inhibitory substances (19) or by displacing (outcompeting) the pathogen (17, 23, 32, 40, 43, 49). Azospirillum spp. are not known as typical biocontrol PGPBs; A. brasilense lacks the capacity to produce significant amounts of antibacterial substances (apart from some bacteriocins and siderophores) (31, 37, 42). This bacterium is also unable to induce systemic resistance in plants (5). However, A. brasilense is considerably rhizo-competent (10) and is able to multiply to form large populations on leaves under wet conditions (4) that are also favorable to many leaf pathogens. These capacities led us to ask whether A. brasilense can displace a leaf pathogen (P. syringae pv. tomato) and in the process reduce disease severity and improve plant growth.
In this study, competition between two different bacterial species, one pathogenic and the other a PGPB hosted by the same plant, was probably a mechanism of biocontrol of this leaf disease. The displacement of P. syringae pv. tomato cells by A. brasilense was demonstrated by the reduced colonization of P. syringae pv. tomato in the rhizosphere and on leaf surfaces in the presence of A. brasilense. A similar phenomenon occurred when A. brasilense was mixed with the mangrove rhizosphere bacterium Staphylococcus sp. (22). The mechanism for the displacement is not known, but it is likely that A. brasilense is better able to obtain nutrients or to colonize plant surfaces. Earlier studies showed that both bacteria are mainly epiphytic on tomato leaves and not endophytic (4, 15).
Alternatively, the protective mechanism of A. brasilense might be indirectly explained by the plant growth promotion effect. That is, due to the positive influence of A. brasilense, a more robust plant might be able to fend off the P. syringae pv. tomato infection more readily. This possibility is consistent with the observation that prior colonization of the plant by the pathogen abolished any protective effects.
Azospirillum prefers malic acid and several other organic acids, like lactate, fumarate, and succinate, as carbon growth substances (29), while the metabolism of organic acids in P. syringae pv. tomato is not efficient. P. syringae pv. tomato prefers sugars, like galactose, glucose, sorbitol, and sucrose (13), which A. brasilense cannot metabolize (21). These affinities were exploited by A. brasilense to preferentially enhance its population on leaves; malic acid has no effect on the proliferation of P. syringae pv. tomato, as it probably cannot metabolize it. Organic acids metabolized by A. brasilense by the Embden-Meyerhof-Parnas hexose phosphate pathway via the enzymes malate dehydrogenase and lactate dehydrogenase (21, 46) are the basis of most Azospirillum culture media (9, 29). The increase in the population of A. brasilense, a consequence of malic acid application, probably helped A. brasilense to displace P. syringae pv. tomato more extensively than it did in the absence of malic acid. Despite the minor yellowing of leaves following malic acid treatment, this approach of enhancing phyllosphere competition by supplying a PGPB with specific nutrients warrants further exploration. Industrial-grade organic acids are available in inexpensive bulk quantities, as they are used regularly by the food industry. Perhaps the yellowish color is a symptom of metabolic inhibition in the host plant caused by the high levels of A. brasilense on the leaves; A. brasilense is known to inhibit the growth of roots at very high concentrations (2, 25). Alternatively, this application perhaps induced a minor chlorosis, a common symptom of nutrient stress in plants.
We propose that our data support the notion that PGPBs, such as Azospirillum sp. and probably other phyllosphere PGPBs (17, 28, 43, 44, 48, 49), can be used in programs to combat foliar bacterial diseases. For the protection of plants, the displacement of a pathogen by a competing species and the competitor's superior adaptation to an ecological niche may prove to be as effective as direct pathogen inhibition or the induction of systemic resistance. This is especially valid when a suitable biocontrol PGPB that inhibits pathogens by secreting antimicrobial compounds has not been identified, as is the case for some foliar pathogens of crops.
Acknowledgments
We thank Martin Romantschuk, Department of Biosciences, University of Helsinki, Finland, for his constructive comments during manuscript preparation; Ira Fogel for English-language editing; and Cheryl Patten for critical reading and English styling.
This study was partially supported by the Bashan Foundation.
Footnotes
This study is dedicated to the memory of the late Avner Bashan and Uzi Bashan from Israel.
REFERENCES
- 1.Alström, S. 1991. Induction of disease resistance in common bean susceptible to halo blight bacterial pathogen after seed bacterization with rhizosphere pseudomonads. J. Gen. Appl. Microbiol. 37:495-501. [Google Scholar]
- 2.Bashan, Y. 1986. Significance of timing and level of inoculation with rhizosphere bacteria on wheat plants. Soil Biol. Biochem. 18:297-301. [Google Scholar]
- 3.Bashan, Y. 1997. Alternative strategies for controlling plant diseases caused by Pseudomonas syringae, p. 575-583. In K. Rudolph, T. J. Burr, J. W. Mansfield, D. Stead, A. Vivian, and J. von Kietzell (ed.), Pseudomonas syringae pathovars and related pathogens, vol. 9. Developments in plant pathology. Kluwer Academic Publishers, Dordrecht, The Netherlands. [Google Scholar]
- 4.Bashan, Y. 1998. Azospirillum plant growth-promoting strains are nonpathogenic on tomato, pepper, cotton, and wheat. Can. J. Microbiol. 44:168-174. [Google Scholar]
- 5.Bashan, Y. 2001. Reduction of bacterial leaf speck disease (Pseudomonas syringae pv. tomato) in tomatoes treated with a combination of Azospirillum brasilense, bactericides and mild heat, p. 296-300. In S. H. De Boer (ed.), Plant pathogenic bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 6.Bashan, Y., E. Fallik, Y. Okon, and N. Kedar. 1981. Lycopersicon pimpinellifolium P.I. 126927: a source of resistance to bacterial speck of tomato. Hassadeh 62:533-534. (In Hebrew.) [Google Scholar]
- 7.Bashan, Y., and G. Holguin. 1997. Azospirillum-plant relationships: environmental and physiological advances (1990-1996). Can. J. Microbiol. 43:103-121. [DOI] [PubMed] [Google Scholar]
- 8.Bashan, Y., and G. Holguin. 1998. Proposal for the division of plant growth-promoting rhizobacteria into two classifications: biocontrol-PGPB (plant growth-promoting bacteria) and PGPB. Soil Biol. Biochem. 30:1225-1228. [Google Scholar]
- 9.Bashan, Y., G. Holguin, and R. Lifshitz. 1993. Isolation and characterization of plant growth-promoting rhizobacteria, p. 331-345. In B. R. Glick, and J. E. Thompson (ed.), Methods in plant molecular biology and biotechnology. CRC Press, Boca Raton, Fla.
- 10.Bashan, Y., and H. Levanony. 1990. Current status of Azospirillum inoculation technology: Azospirillum as a challenge for agriculture. Can. J. Microbiol. 36:591-608. [Google Scholar]
- 11.Bashan, Y., G. Mitiku, O. Ziv-Vecht, and H. Levanony. 1991. Estimation of minimal numbers of Azospirillum brasilense using time-limited liquid enrichment combined with enzyme-linked immunosorbent assay. Soil Biol. Biochem. 23:135-138. [Google Scholar]
- 12.Bashan, Y., Y. Okon, and Y. Henis. 1978. Infection studies of Pseudomonas tomato, causal agent of bacterial speck of tomato. Phytoparasitica 6:135-145. [Google Scholar]
- 13.Bashan, Y., Y. Okon, and Y. Henis. 1982. A note on a new defined medium for "Pseudomonas tomato'. J. Appl. Bacteriol. 52:297-298. [Google Scholar]
- 14.Bashan, Y., Y. Ream, H. Levanony, and A. Sade. 1989. Nonspecific responses in plant growth, yield, and root colonization of noncereal crop plants to inoculation with Azospirillum brasilense Cd. Can. J. Bot. 67:1317-1324. [Google Scholar]
- 15.Bashan, Y., E. Sharon, Y. Okon, and Y. Henis. 1981. Scanning electron and light microscopy of infection and symptom development in tomato leaves infected with Pseudomonas tomato. Physiol. Plant Pathol. 19:139-144. [Google Scholar]
- 16.Carrillo, A., M. E. Puente, and Y. Bashan. 1996. Application of diluted chlorine dioxide to radish and lettuce nurseries insignificantly reduced plant development. Ecotoxicol. Environ. Saf. 35:57-66. [DOI] [PubMed] [Google Scholar]
- 17.Cooksey, D. A. 1988. Reduction of infection by Pseudomonas syringae pv. tomato using a nonpathogenic, copper-resistant stain combined with copper bactericide. Phytopathology 78:601-603. [Google Scholar]
- 18.Fallik, E., Y. Bashan, Y. Okon, A. Cahaner, and N. Kedar. 1983. Inheritance and sources of resistance to bacterial speck of tomato caused by Pseudomonas syringae pv. tomato. Ann. Appl. Biol. 102:365-371. [Google Scholar]
- 19.Glick, B. R., and Y. Bashan. 1997. Genetic manipulation of plant growth-promoting bacteria to enhance biocontrol of phytopathogens. Biotechnol. Adv. 15:353-378. [DOI] [PubMed] [Google Scholar]
- 20.Gu, Y. Q., and G. B. Martin. 1998. Molecular mechanisms involved in bacterial speck disease resistance of tomato. Philos. Trans. R. Soc. Lond. Ser. B 353:1455-1461. [Google Scholar]
- 21.Hartmann, A., and W. Zimmer. 1994. Physiology of Azospirillum, p. 15-39. In Y. Okon (ed.), Azospirillum/plant associations. CRC Press, Boca Raton, Fla.
- 22.Holguin, G., and Y. Bashan. 1996. Nitrogen-fixation by Azospirillum brasilense Cd is promoted when co-cultured with a mangrove rhizosphere bacterium (Staphylococcus sp.). Soil Biol. Biochem. 28:1651-1660. [Google Scholar]
- 23.Janisiewicz, W. J., and A. Marchi. 1992. Control of storage rots on various pear cultivars with a saprophytic strain of Pseudomonas syringae. Plant Dis. 76:555-560. [Google Scholar]
- 24.Jardine, D. J., and C. T. Stephens. 1987. Influence of timing of application and chemical on control of bacterial speck of tomato. Plant Dis. 71:405-408. [Google Scholar]
- 25.Kapulnik, Y., Y. Okon, and Y. Henis. 1985. Changes in root morphology of wheat caused by Azospirillum inoculation. Can. J. Microbiol. 31:881-887. [Google Scholar]
- 26.Levanony, H., Y. Bashan, and Z. E. Kahana. 1987. Enzyme-linked immunosorbent assay for specific identification and enumeration of Azospirillum brasilense Cd in cereal roots. Appl. Environ. Microbiol. 53:358-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, L., J. W. Kloepper, and S. Tuzun. 1995. Induction of systemic resistance in cucumber against bacterial angular leaf spot by plant growth-promoting rhizobacteria. Phytopathology 85:843-847. [Google Scholar]
- 28.May, R., B. Völksch, and J. Kampmann. 1997. Antagonistic activities of epiphytic bacteria from soybean leaves against Pseudomonas syringae pv. glycinea in vitro and in planta. Microb. Ecol. 43:118-124. [DOI] [PubMed] [Google Scholar]
- 29.Okon, Y., S. L. Albrecht, and R. H. Burris. 1977. Methods for growing Spirillum lipoferum and for counting it in pure culture and in association with plants. Appl. Environ. Microbiol. 33:85-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oldroyd, G. E. D., and B. J. Staskawicz. 1998. Genetically engineered broad-spectrum disease resistance in tomato. Proc. Natl. Acad. Sci. USA 95:10300-10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira, R. G. B., and A. Drozdowicz. 1987. Inhibition of bacteriocin producing strains of Azospirillum lipoferum by their own bacteriocin. Zentbl. Mikrobiol. 142:387-391. [Google Scholar]
- 32.O'Sullivan, D. J., and F. O'Gara. 1992. Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol. Rev. 56:662-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilowsky, M., and D. Zutra. 1982. Screening wild tomatoes for resistance to bacterial speck pathogen (Pseudomonas tomato). Plant Dis. 66:46-47. [Google Scholar]
- 34.Pitblado, R. E., and E. A. Kerr. 1980. Resistance to bacterial speck (Pseudomonas tomato) in tomato. Acta Hortic. 100:379-382. [Google Scholar]
- 35.Schneider, R. W., and R. G. Grogan. 1977. Bacterial speck of tomato: sources of inoculum and establishment of a resident population. Phytopathology 67:388-394. [Google Scholar]
- 36.Schneider, R. W., and R. G. Grogan. 1977. Tomato leaf trichomes, a habitat for resident populations of Pseudomonas tomato. Phytopathology 67:898-902. [Google Scholar]
- 37.Shah, S., V. Karkhanis, and A. Desai. 1992. Isolation and characterization of siderophore, with antimicrobial activity, from Azospirillum lipoferum M. Curr. Microbiol. 25:34-35. [Google Scholar]
- 38.Sharon, E., Y. Okon, Y. Bashan, and Y. Henis. 1982. Detached leaf enrichment: a method for detecting small numbers of Pseudomonas syringae pv. tomato and Xanthomonas campestris pv. vesicatoria in seeds and symptomless leaves of tomato and pepper. J. Appl. Bacteriol. 53:371-377. [Google Scholar]
- 39.Sotirova, V., N. Bogatsevska, and L. Stamova. 1994. Sources of resistance to bacterial diseases in tomato wild species. Acta Hortic. 376:353-359. [Google Scholar]
- 40.Stephens, P. M., J. J. Crowley, and C. O'Connell. 1993. Selection of pseudomonad strains inhibiting Pythium ultimum on sugar beet seeds in soil. Soil Biol. Biochem. 25:1283-1288. [Google Scholar]
- 41.Stockinger, E. J., and L. L. Walling. 1994. Pto3 and Pto4: novel genes from Lycopersicon hirsutum var. glabratum that confer resistance to Pseudomonas syringae pv tomato. Theor. Appl. Genet. 89:879-884. [DOI] [PubMed] [Google Scholar]
- 42.Tapia-Hernandez, A., M. A. Mascarua-Esparza, and J. Caballero-Mellado. 1990. Production of bacteriocins and siderophore-like activity in Azospirillum brasilense. Microbios 64:73-83. [PubMed] [Google Scholar]
- 43.Völksch, B., and R. May. 2001. Biological control of Pseudomonas syringae pv. glycinea by epiphytic bacteria under field conditions. Microb. Ecol. 41:132-139. [DOI] [PubMed] [Google Scholar]
- 44.Völksch, B., J. Nüske, and R. May. 1996. Characterization of two epiphytic bacteria from soybean leaves with antagonistic activity against Pseudomonas syringae pv. glycinea. J. Basic Microbiol. 36:99-108. [DOI] [PubMed] [Google Scholar]
- 45.Wei, G., J. W. Kloepper, and S. Tuzun. 1996. Induced systemic resistance to cucumber diseases and increased plant growth by plant growth-promoting rhizobacteria under field conditions. Phytopathology 86:221-224. [Google Scholar]
- 46.Westby, C. A., D. S. Cutshall, and G. V. Vigil. 1983. Metabolism of various carbon sources by Azospirillum brasilense. J. Bacteriol. 156:1369-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilkie, J. P., and D. W. Dye. 1974. Pseudomonas tomato in New Zealand. N. Z. J. Agric. Res. 17:131-135. [Google Scholar]
- 48.Wilson, M., S. S. Hirano, and S. E. Lindow. 1999. Location and survival of leaf-associated bacteria in relation to pathogenicity and potential for growth within the leaf. Appl. Environ. Microbiol. 65:1435-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson, M., and S. E. Lindow. 1994. Ecological similarity and coexistence of epiphytic ice-nucleating (Ice+) Pseudomonas syringae strains and a non-ice-nucleating (Ice−) biological control agent. Appl. Environ. Microbiol. 60:3128-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yunis, H., Y. Bashan, Y. Okon, and Y. Henis. 1980. Two sources of resistance to bacterial speck of tomato caused by Pseudomonas tomato. Plant Dis. 64:851-852. [Google Scholar]
- 51.Yunis, H., Y. Bashan, Y. Okon, and Y. Henis. 1980. Weather dependence, yield losses and control of bacterial speck of tomato caused by Pseudomonas tomato. Plant Dis. 64:937-939. [Google Scholar]
- 52.Zehnder, G. W., C. B. Yao, J. F. Murphy, E. R. Sikora, and J. W. Kloepper. 2000. Induction of resistance in tomato against cucumber mosaic virus by plant growth-promoting rhizobacteria. Biocontrol 45:127-137. [Google Scholar]