Abstract
When cells of Bacillus sp. strain GL1 were grown in a medium containing xanthan as a carbon source, α-mannosidase exhibiting activity toward p-nitrophenyl-α-d-mannopyranoside (pNP-α-d-Man) was produced intracellularly. The 350-kDa α-mannosidase purified from a cell extract of the bacterium was a trimer comprising three identical subunits, each with a molecular mass of 110 kDa. The enzyme hydrolyzed pNP-α-d-Man (Km = 0.49 mM) and d-mannosyl-(α-1,3)-d-glucose most efficiently at pH 7.5 to 9.0, indicating that the enzyme catalyzes the last step of the xanthan depolymerization pathway of Bacillus sp. strain GL1. The gene for α-mannosidase cloned most by using N-terminal amino acid sequence information contained an open reading frame (3,144 bp) capable of coding for a polypeptide with a molecular weight of 119,239. The deduced amino acid sequence showed homology with the amino acid sequences of α-mannosidases belonging to glycoside hydrolase family 38.
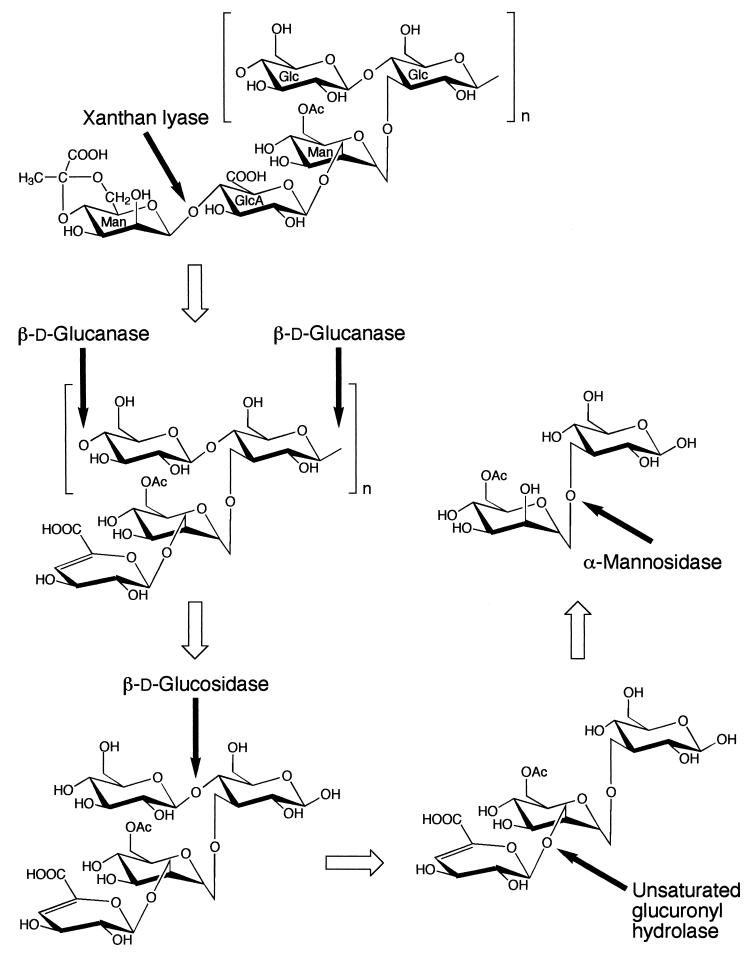
Xanthan is an exopolysaccharide produced by a plant-pathogenic bacterium, Xanthomonas campestris. This polysaccharide has a cellulosic backbone with trisaccharide side chains composed of mannose-(β-1,4)-glucuronic acid-(β-1,2)-mannose attached to alternate glucosyl residues through α-1,3 linkages (15, 24). The mannosyl residues at the reducing and nonreducing termini of the side chains are often acetylated and pyruvylated, respectively, although the extent of modification varies with the culture conditions and bacterial strain (26). We have already analyzed the depolymerization pathway for xanthan (Fig. 1) in a bacterium, Bacillus sp. strain GL1 (20). Xanthan is depolymerized to a tetrasaccharide consisting of unsaturated glucuronyl, acetylated mannosyl, and two glucosyl residues by extracellular xanthan lyase (6) and endo-β-d-glucanase (20). This tetrasaccharide is thought to be degraded to the constituent monosaccharides through the successive actions of intracellular β-d-glucosidase (5), unsaturated glucuronyl hydrolase (4), and α-mannosidase. The functions and molecular structures of the four enzymes other than α-mannosidase involved in the xanthan depolymerization pathway of Bacillus sp. strain GL1 have already been analyzed (4, 5, 6, 20).
FIG. 1.
Xanthan degradation pathway in Bacillus sp. strain GL1. See reference 20. The cleavage sites for xanthan depolymerization enzymes are indicated by solid arrows. The open arrows indicate the degradation pathway for xanthan. Glc, d-glucose; Man, d-mannose; GlcA, d-glucuronic acid; OAc, acetyl group.
The class I α-mannosidase (α-1,2-mannosidase; EC 3.2.1.113) and the class II α-mannosidase (α-1,3-1,6-mannosidase; EC 3.2.1.114) are key enzymes in the maturation of N-linked oligosaccharides in eucaryotic organisms (13). Based on their amino acid sequence similarities, glycoside hydrolases have been classified into 87 families (10, 11, 12; Carbohydrate-Active Enzymes server [http://afmb.cnrs-mrs.fr/∼pedro/CAZY/db.html]). Class I and II α-mannosidases belong to glycoside hydrolase families 47 and 38, respectively.
On the other hand, no extensive studies of bacterial α-mannosidases with properties similar to those of eucaryotic family 38 or family 47 hydrolases have been performed. Maruyama et al. described the purification and characterization of a bacterial α-1,2-mannosidase whose properties resemble those of family 47 enzymes (18). However, it is not clear whether this enzyme is a member of family 47, because its amino acid sequence has not been determined. The nucleotide sequences of the following four kinds of bacterial α-mannosidase genes exhibiting similarity to the sequences of family 38 α-mannosidase genes have been registered:genes of Bacillus halodurans (accession no. AB026114), Mycobacterium tuberculosis (Z92772-28), Synechocystis sp. (D63999-6), and Thermotoga maritima (AE001822-6). There have, however, been no reports of purification of native α-mannosidases. No nucleotide sequences of bacterial genes homologous to family 47 α-mannosidase genes have been registered either.
In order to completely elucidate the xanthan degradation pathway and to gain insight into the nature of α-mannosidases in bacteria, in this study we attempted to purify and characterize the enzyme of Bacillus sp. strain GL1.
MATERIALS AND METHODS
Materials.
Pyruvylated xanthan (average molecular mass, 2 × 106 Da; pyruvylation of terminal mannosyl residues in the side chains, 50%) was a gift from Kohjin Co., Tokyo, Japan. Silica Gel 60/Kieselguhr F254 thin-layer chromatography (TLC) plates were obtained from E. Merck, Darmstadt, Germany. Butyl-Toyopearl 650M was purchased from Tosoh Co., Tokyo, Japan, DEAE-cellulose was obtained from Nacalai Tesque Co., Kyoto, Japan, and Sephacryl S-200HR was obtained from Pharmacia Biotech Co., Uppsala, Sweden. p-Nitrophenyl (pNP) sugars, cellobiose, and α-1,2-, α-1,3-, α-1,4-, and α-1,6-mannobioses were obtained from Sigma Chemical Co., St. Louis, Mo. Restriction endonucleases, DNA-modifying enzymes, vectors pUC118, pUC119, and pET14b, and Escherichia coli BL21(DE3) competent cells were obtained from Takara Shuzo Co., Kyoto, Japan, or Toyobo Co., Tokyo, Japan. A digoxigenin (DIG) high prime DNA labeling and detection starter kit II was obtained from Boehringer GmbH, Mannheim, Germany. The glucose CII test for glucose assays was purchased from Wako Pure Chemicals Co., Osaka, Japan.
Microorganisms and culture conditions.
For purification of α-mannosidase, cells of Bacillus sp. strain GL1 were cultured aerobically at 30°C for 48 h in a liquid xanthan medium (10 liters) consisting of 0.1% (NH4)2SO4, 0.1% KH2PO4, 0.1% Na2HPO4, 0.01% MgSO4·7H2O, 0.01% yeast extract, and 0.5% xanthan (pH 7.2).
Assay for enzyme.
Unless otherwise specified, α-mannosidase activity was assayed at 30°C in a 0.5-ml mixture containing 0.4 mM pNP-α-d-mannopyranoside (pNP-α-d-Man) and 50 mM potassium phosphate buffer (KPB) (pH 7.0). After incubation for 5, 10, 15, 20, or 30 min, the reaction was stopped by the addition of 1.0 ml of 0.25 M Na2CO3. One unit of enzyme activity was defined as the amount of protein required to release 1 μmol of p-nitrophenol per min (λ = 400 nm, ɛ = 18.1 M · cm−1). Protein was determined by the method of Lowry et al. (17) with bovine serum albumin as the standard or by measuring the absorbance at 280 nm, assuming that A280 = 1.0 corresponds to 1 mg/ml. The glucose released from d-mannosyl-(α-1,3)-d-glucose was determined by using glucose oxidase (glucose CII test; Wako).
Purification of α-mannosidase.
Unless otherwise specified, all procedures used for purification of α-mannosidase were performed at 0 to 4°C. Cells (23 g, wet weight) of Bacillus sp. strain GL1 grown on xanthan medium were collected by centrifugation at 13,000 × g for 10 min, washed with 20 mM KPB (pH 7.0), and then resuspended in the same buffer (80 ml). The cells were ultrasonically disrupted (Insonator model 201 M; Kubota, Tokyo, Japan) at 9 kHz for 10 min, and the clear solution obtained after centrifugation at 15,000 × g for 20 min was dialyzed against 20 mM KPB (pH 7.0) overnight. The dialysate was applied to a DEAE-cellulose column (4.7 by 41 cm) equilibrated with 20 mM KPB (pH 7.0). The enzyme was eluted with a linear gradient of NaCl (0 to 1.0 M) in the same buffer (2 liters), and 18-ml portions were collected every 9 min. The active fractions (fractions 66 to 77) were combined (212 ml), and 37 g of ammonium sulfate was dissolved in the solution (30% saturation). The enzyme solution was then applied to a Butyl-Toyopearl 650M column (2.7 by 17 cm) equilibrated with 20 mM KPB (pH 7.0) to which ammonium sulfate (176 g per liter, 30% saturation) had been added. The enzyme was eluted with a linear gradient of ammonium sulfate (30 to 0%) in 500 ml of 20 mM KPB (pH 7.0), and 4-ml portions were collected every 4 min. The active fractions (fractions 67 to 71) were combined (21 ml), concentrated by ultrafiltration with a model 8200 concentrator (Amicon Co., Beverly, Mass.) to a volume of about 3 ml, and then applied to a Sephacryl S-200HR column (2.7 by 64 cm) equilibrated with 20 mM KPB (pH 7.0) containing 0.15 M NaCl. The enzyme was eluted with the same buffer, and 3-ml portions were collected every 6 min. The enzyme eluted in fractions 56 to 63. These fractions were pooled, dialyzed overnight against 20 mM KPB (pH 7.0), and then used as the purified α-mannosidase.
Electrophoresis.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and native gradient PAGE were performed by the methods of Laemmli (16) and Davis (3), respectively.
Isoelectric focusing.
Isoelectric focusing was performed by using an IPGphor system (Amersham Pharmacia Biotech Inc., Piscataway, N.J.) as described in the manufacturer's manual.
Preparation of xanthan and gellan depolymerization products.
Depolymerization products of xanthan [d-mannosyl-(α-1,3)-d-glucose] and gellan [l-rhamnosyl-(α-1,3)-d-glucose] were prepared by the methods described previously (7, 20).
TLC.
Degradation of disaccharides by α-mannosidase was analyzed by TLC with a 1-butanol-acetic acid-water (2:1:1, vol/vol/vol) solvent system. The reaction products were visualized by heating the TLC plates at 110°C for 5 min after they were sprayed with 10% (vol/vol) sulfuric acid in ethanol.
N-terminal amino acid sequence of α-mannosidase.
The N-terminal amino acid sequence of α-mannosidase was determined by Edman degradation by using a Procise 492 protein sequencing system (Applied Biosystems Div., Perkin-Elmer, Foster City, Calif.).
DNA sequence and DNA manipulations.
The DNA sequence of the α-mannosidase gene was determined by the dideoxy chain termination method with an automated DNA sequencer (model 377; Applied Biosystems Div., Perkin-Elmer) (27). Subcloning, transformation, and gel electrophoresis were performed by using the methods described by Sambrook et al. (25).
Molecular cloning of the α-mannosidase gene. (i) First colony hybridization.
A genomic DNA library of Bacillus sp. strain GL1 previously constructed in E. coli (8) was screened by colony hybridization (2) by using a 32P-labeled probe (5′-ATGTTYTGGATHGTNGARAAR-3′) corresponding to the N-terminal amino acid sequence of α-mannosidase. Positive clones obtained by hybridization were cultured in Luria-Bertani medium (19) supplemented with 50 μg of ampicillin per ml. Plasmids were extracted from the clones, and then the nucleotide sequences of the genomic fragments inserted into the plasmids were determined. These sequences were used to search databases with the FASTA program.
(ii) Second colony hybridization.
The Eco47III-NruI fragment (745 bp) in pMan20 (one of the plasmids obtained during the first colony hybridization) was isolated and labeled with digoxigenin (designated probeDA). Genomic DNA of the bacterium was digested with several restriction enzymes. After Southern blotting following electrophoresis on an agarose gel, hybridization with probeDA was performed. Since an approximately 2.5-kb XmaI fragment hybridized with probeDA, genomic DNA of the bacterium was completely digested with XmaI, and 1.4- to 3.5-kbp fragments were obtained by electroelution on an agarose gel. The fragments were ligated into XmaI-digested pUC119. Cells of E. coli DH5α were transformed with the ligated DNAs (designated the XmaI library). The second colony hybridization was carried out by using the XmaI library and a 32P-labeled probe (5′-ATGTTTTGGATCGTGGAGAAGCTTC-3′) encoding the N-terminal amino acids of α-mannosidase, which was determined from the nucleotide sequence of the genomic fragment inserted into pMan20. Plasmids were extracted from positive clones by procedures similar to those described above.
(iii) Third colony hybridization.
The EcoRI-XmaI fragment (467 bp) in pMan86 (one of the plasmids obtained during the second colony hybridization) was isolated and labeled with digoxigenin (designated probeDB). Since an approximately 2.0-kb fragment of HindIII-digested genomic DNA of the bacterium hybridized with probeDB, 1.5- to 3.2-kbp fragments of HindIII-digested genomic DNA were obtained as described above. The fragments were ligated into HindIII-digested pUC118. Cells of E. coli DH5α were transformed with the ligated DNAs (designated the HindIII library). The third colony hybridization was carried out by using the HindIII library and a 32P-labeled probe (5′-CATTACGAGCTCGCGTTCGACGAAG-3′) corresponding to the nucleotide sequence of the 3′ terminus of the genomic fragment inserted into pMan86. Plasmids were extracted from positive clones as described above.
Construction of an expression vector.
The α-mannosidase gene with NcoI and Bpu1102I sites just upstream of the initiation codon and downstream of the stop codon, respectively, was amplified by PCR by using the genomic DNA of Bacillus sp. strain GL1 as a template and synthetic oligonucleotides (5′-GGGCCATGGGCATGTTTTGGATCGTGGAGAAGCTTC-3′ and 5′-TTTGCTGAGCTTAACGCAGCCGAATGAGGAACGTC-3′) as primers. The amplified gene was digested with NcoI and Bpu1102I and then ligated with an expression vector (pET14b), and the resultant plasmid was designated pET14b-Man.
Nucleotide sequence accession number.
The nucleotide sequence of the α-mannosidase gene reported in this paper has been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession no. AB026114.
RESULTS AND DISCUSSION
Purification and characterization of α-mannosidase.
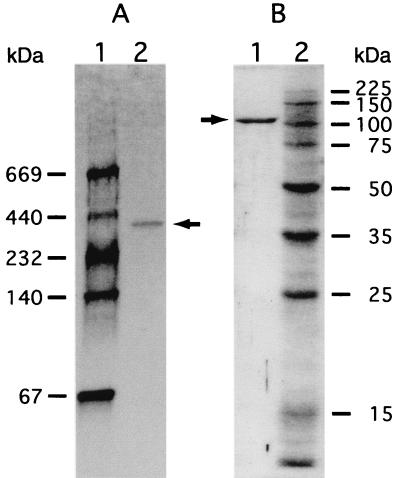
α-Mannosidase was purified approximately 15-fold with an activity yield of 2.6% from a cell extract of Bacillus sp. strain GL1 grown in the xanthan medium (Table 1). The purified enzyme was homogeneous as determined by native gradient PAGE (Fig. 2A), SDS-PAGE (Fig. 2B), and isoelectric focusing (pI 5.5) (data not shown).
TABLE 1.
Purification of α-mannosidase
| Step | Vol (ml) | Total protein (mg) | Total activity (mU) | Sp act (mU/mg) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|---|
| Cell extract | 106 | 850 | 6,500 | 7.65 | 100 | 1.00 |
| DEAE-cellulose | 212 | 93.3 | 1,390 | 14.9 | 21.4 | 1.94 |
| Butyl-Toyopearl 650M | 21.1 | 6.01 | 500 | 83.2 | 7.69 | 10.9 |
| Sephacryl S-200HR | 24.4 | 1.51 | 170 | 112 | 2.62 | 14.6 |
FIG. 2.
Electrophoretic profiles of α-mannosidase. (A) Purified α-mannosidase (0.6 μg) subjected to native gradient PAGE. Lane 1, molecular weight standards, including (from top to bottom) thyroglobulin (molecular weight, 669,000), ferritin (440,000), catalase (232,000), lactate dehydrogenase (140,000), and bovine serum albumin (67,000); lane 2, purified enzyme. (B) Purified α-mannosidase (0.6 μg) subjected to SDS-PAGE. Lane 1, purified enzyme; lane 2, molecular weight standards, including (from top to bottom) synthetic polypeptides with molecular weights of 225,000, 150,000, 100,000, 75,000, 50,000, 35,000, 25,000, and 15,000. The arrows indicate the position of purified α-mannosidase.
(i) Molecular mass.
The molecular mass of the native protein was estimated to be approximately 350 kDa by native PAGE performed with a linear 2 to 15% polyacrylamide gradient (1) (Fig. 2A) and gel filtration with a calibrated column of Sephacryl S-200HR (data not shown). On the other hand, the molecular mass of the denatured protein was determined to be 110 kDa by SDS-PAGE (Fig. 2B), indicating that the 350-kDa enzyme consists of three identical subunits.
(ii) Substrate specificity.
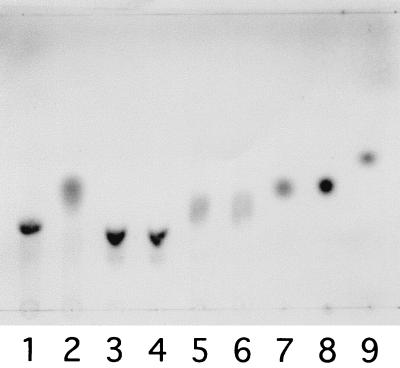
The enzyme specifically hydrolyzed pNP-α-D-Man. In order to determine the Km value of the enzyme for pNP-α-d-Man, the reaction was performed at 30°C in a 0.5-ml mixture containing 0.008, 0.125, 0.2, or 0.4 mM pNP-α-D-Man, 50 mM KPB (pH 7.0), and the purified enzyme (7.4 μg). The Michaelis kinetic parameter (Km) was estimated to be 0.49 mM by using the Lineweaver-Burk representation. Activities for other pNP-sugars (pNP-β-d-mannopyranoside, pNP-α-d-glucopyranoside, pNP-β-d-glucopyranoside, pNP-β-d-glucuronic acid, pNP-cellobioside, pNP-α-l-rhamnoside, pNP-β-d-galactopyranoside, pNP-β-d-xylopyranoside, pNP-α-d-maltopentaoside, and pNP-β-d-fucopyranoside) were not detectable. The enzyme activities with the xanthan depolymerization product [d-mannosyl-(α-1,3)-d-glucose], cellobiose [d-glucosyl-(β-1,4)-d-glucose], and the gellan depolymerization product [l-rhamnosyl-(α-1,3)-d-glucose] were investigated by analyzing the reaction products by TLC (Fig. 3). The enzyme hydrolyzed d-mannosyl-(α-1,3)-d-glucose with the release of equimolar amounts of mannose and glucose, the latter of which was enzymatically determined by using glucose oxidase (data not shown). Cellobiose and l-rhamnosyl-(α-1,3)-d-glucose were inert as substrates for the enzyme. The results indicate that the enzyme catalyzes the last step of the xanthan depolymerization pathway of Bacillus sp. strain GL1 (Fig. 1).
FIG. 3.
Degradation of disaccharides by α-mannosidase. The purified enzyme was incubated with the disaccharides mannosyl-glucose (lanes 1 and 2), cellobiose (lanes 3 and 4), and rhamnosyl-glucose (lanes 5 and 6) (1 mM) at 30°C in KPB (pH 7.0) for 2 h or overnight. The reaction times were as follows: lanes 1, 3, and 5, 0 min; lane 2, 2 h; and lanes 4 and 6, overnight. Authentic d-mannose, d-glucose, and l-rhamnose were electrophoresed in lanes 7, 8, and 9, respectively.
Most of eucaryotic α-mannosidases belonging to families 38 and 47 participate in the biosynthesis of N-glycans having α-1,2-, α-1,3-, and α-1,6-linked mannose residues. Family 38 α-mannosidases hydrolyze α-1,3- and α-1,6-linked mannose residues, while family 47 α-mannosidases hydrolyze α-1,2-linked mannose residues. However, α-mannosidase of Bacillus sp. strain GL1 was specific for α-1,2- and α-1,3-mannobioses and inactive on α-1,4- and α-1,6-mannobioses, indicating that the manner of hydrolysis of the bacterial enzyme is different from that of eucaryotic α-mannosidases.
(iii) pH and temperature.
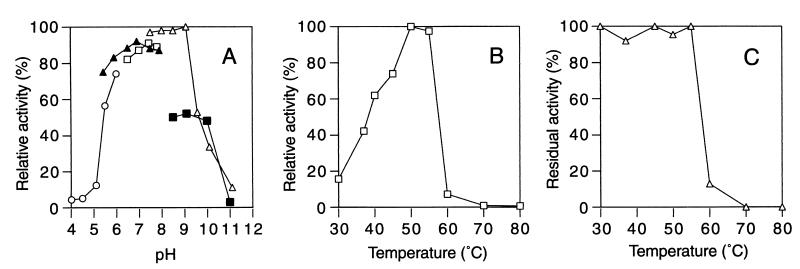
The enzyme was most active at pH 7.5 to 9.0 (Fig. 4A) and 50°C (Fig. 4B) under the conditions employed. About 90% of the activity was lost during incubation at 60°C (Fig. 4C).
FIG. 4.
Effects of pH and temperature on the activity and stability of α-mannosidase. (A) Effect of pH. Reactions were performed in the following buffers (50 mM) at 30°C for 10 min: sodium acetate (○), KPB (▴), sodium HEPES (□), glycine-NaOH (▵), and sodium phosphate (▪). The activity at pH 9.0 in glycine-NaOH buffer was defined as 100%. (B) Effect of temperature. Reactions were performed at various temperatures in KPB (pH 7.0) for 10 min. The activity at 50°C was defined as 100%. (C) Thermal stability. After preincubation of the enzyme for 10 min at various temperatures, the reaction was performed at 30°C in KPB (pH 7.0) for 10 min. The activity of the enzyme before the treatment was defined as 100%.
(iv) Metal ions and other compounds.
Reactions were performed at 30°C for 10 min in the absence or presence of metal ions and other compounds (1 mM). Hg2+ and Cu2+ inhibited the activity of the enzyme by 90 and 70%, respectively. Co2+ and Mn2+ were potent activators of the enzyme, increasing the activity nine- and twofold, respectively. Other metal ions (Mg2+, Al3+, Ca2+, Fe2+, and Zn2+), thiol reagents (dithiothreitol, glutathione [reduced form], and 2-mercaptoethanol), and iodoacetic acid had little effect on the enzyme activity. Recently, the crystal structure of Drosophila melanogaster Golgi α-mannosidase II (a family 38 α-mannosidase) was determined, and this enzyme was found to contain Zn2+ in an active site (29). The α-mannosidase activity of Bacillus sp. strain GL1 decreased 65% in the presence of EDTA (1 mM). However, the presence of Zn2+ (or Co2+) in an active site of the enzyme was not confirmed, since metal replacement experiments were unsuccessful.
(v) N-terminal amino acid sequence.
The N-terminal amino acid sequence of α-mannosidase was determined to be 1MFWIVEKLQK10
Molecular cloning and sequence analysis of the α-mannosidase gene.
The first colony hybridization was carried out as described in Materials and Methods by using a probe encoding the N-terminal amino acid sequence of α-mannosidase. Several positive clones harboring plasmids containing genomic fragments of Bacillus sp. strain GL1 were obtained. The nucleotide sequences of the genomic fragments showed that these clones contained only part of the sequence of the α-mannosidase gene (from the 5′ region upstream of the start codon to the BamHI site 1,258 nucleotides downstream of the start codon). In order to obtain the complete α-mannosidase gene, the second and third colony hybridizations were carried out. The former gave a clone containing a plasmid with the region downstream of the BamHI site in the α-mannosidase gene (2,312 bp; designated pMan86), and the latter gave a clone containing a plasmid with a fragment containing the stop codon of the α-mannosidase gene (ca. 2 kbp; designated pMan87). The nucleotide sequence of the complete α-mannosidase gene was determined by overlapping the sequences of the fragments present in pMan20, pMan86, and pMan87. The gene contained a 3,144-bp open reading frame (ORF) capable of coding for a polypeptide with a molecular weight of 119,239. The predicted amino acid sequence encoded by the ORF was found to contain the N-terminal amino acid sequence (MFWIVEKLQK) (1Met to 10Lys) of α-mannosidase purified from cells of Bacillus sp. strain GL1. A genomic PCR with primers based on the 5′- and 3′-terminal sequences of the α-mannosidase gene was performed, and an amplified fragment with the predicted size was subcloned into pET14b (pET14b-Man). A transformant of E. coli BL21(DE3) containing pET14b-Man exhibited apparent α-mannosidase activity, which is absent in E. coli BL21(DE3) transformed with pET14b. These results indicate that the deduced amino acid sequence represents the primary structure of the enzyme. A probable ribosome-binding site (Shine-Dalgarno sequence; GGAGG) (28) was located just before the start codon of the ORF. No apparent promoter exhibiting homology to the E. coli consensus promoter (9) was found in the 5′ region upstream of the initiation codon of the ORF. A terminator was not observed downstream of the stop codon.
The deduced amino acid sequence of the α-mannosidase of Bacillus sp. strain GL1 was used to search protein databases with the FASTA program (22). The enzyme showed the highest identity score with B. halodurans α-mannosidase (48.2% identity in a 1,050-amino-acid overlap). Fairly high identity scores were also found for human cytosolic α-mannosidase (35.0% identity in a 1,011-amino-acid overlap), rat liver endoplasmic reticulum α-mannosidase (34.1% identity in a 1,012-amino-acid overlap), Emericella nidulans α-mannosidase (33.6% identity in a 977-amino-acid overlap), and D. melanogaster Golgi α-mannosidase II (22.2% identity in a 441-amino-acid overlap). Howard et al. (14) and Numao et al. (21) have reported on the catalytic nucleophile of family 38 α-mannosidases. Family 38 α-mannosidases have a highly conserved consensus sequence, and the Asp in the sequence has been found to function as the catalytic nucleophile. A sequence (355YLWLPDVFGYSWALPQIL372) similar to the consensus sequence was present in the deduced amino acid sequence of the enzyme of Bacillus sp. strain GL1, confirming that the α-mannosidase of Bacillus sp. strain GL1 belongs to family 38. Although the M. tuberculosis α-mannosidase gene exhibiting sequence similarity to the genes encoding rat family 38 α-mannosidases was isolated and expressed in E. coli and the properties of the recombinant enzyme were determined (23), no information on the native α-mannosidases has been presented so far. Therefore, the genetic and enzymatic study of Bacillus sp. strain GL1 α-mannosidase described in this paper is the first complete study of a bacterial α-mannosidase belonging to glycoside hydrolase family 38.
REFERENCES
- 1.Andersson, L.-O., H. Borg, and M. Mikaelsson. 1972. Molecular weight estimations of proteins by electrophoresis in polyacrylamide gels of graded porosity. FEBS Lett. 20:199-202. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. Greene Publishing Associates and Wiley-Interscience, John Wiley and Sons, New York, N.Y.
- 3.Davis, B. J. 1964. Disc electrophoresis. II. Method and application to human serum proteins. Ann. N.Y. Acad. Sci. 121:404-427. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto, W., E. Kobayashi, H. Nankai, N. Sato, S. Kawai, and K. Murata. 1999. Unsaturated glucuronyl hydrolase of Bacillus sp. GL1: novel enzyme prerequisite for metabolism of unsaturated oligosaccharides produced by polysaccharide lyases. Arch. Biochem. Biophys. 368:367-374. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto, W., H. Miki, H. Nankai, N. Sato, S. Kawai, and K. Murata. 1998. Molecular cloning of two genes for β-d-glucosidase in Bacillus sp. GL1 and identification of one as a gellan-degrading enzyme. Arch. Biochem. Biophys. 360:1-9. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto, W., H. Miki, N. Tsuchiya, H. Nankai, and K. Murata. 1998. Xanthan lyase of Bacillus sp. strain GL1 liberates pyruvylated mannose from xanthan side chains. Appl. Environ. Microbiol. 64:3765-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto, W., H. Nankai, N. Sato, S. Kawai, and K. Murata. 1999. Characterization of α-l-rhamnosidase of Bacillus sp. GL1 responsible for the complete depolymerization of gellan. Arch. Biochem. Biophys. 368:56-60. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto, W., N. Sato, S. Kimura, and K. Murata. 1998. Polysaccharide lyase: molecular cloning of gellan lyase gene and formation of the lyase from a huge precursor protein in Bacillus sp. GL1. Arch. Biochem. Biophys. 354:31-39. [DOI] [PubMed] [Google Scholar]
- 9.Hawley, D. K., and W. R. McClure. 1983. Comparison and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 11:2237-2255. [DOI] [PMC free article] [PubMed]
- 10.Henrissat, B. 1991. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 280:309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henrissat, B., and A. Bairoch. 1993. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 293:781-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrissat, B., and A. Bairoch. 1996. Updating the sequence-based classification of glycosyl hydrolases. Biochem. J. 316:695-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herscovics, A. 1999. Importance of glycosidases in mammalian glycoprotein biosynthesis. Biochim. Biophys. Acta 1473:96-107. [DOI] [PubMed] [Google Scholar]
- 14.Howard, S., S. He, and S. G. Withers. 1998. Identification of the active site nucleophile in Jack bean α-mannosidase using 5-fluoro-β-l-gulosyl fluoride. J. Biol. Chem. 273:2067-2072. [DOI] [PubMed] [Google Scholar]
- 15.Jansson, P.-E., L. Kenne, and B. Lindberg. 1975. Structure of the extracellular polysaccharide from Xanthomonas campestris. Carbohydr. Res. 45:275-282. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 18.Maruyama, Y., T. Nakajima, and E. Ichishima. 1994. A 1,2-α-d-mannosidase from a Bacillus sp.: purification, characterization, and mode of action. Carbohydr. Res. 251:89-98. [DOI] [PubMed] [Google Scholar]
- 19.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 20.Nankai, H., W. Hashimoto, H. Miki, S. Kawai, and K. Murata. 1999. Microbial system for polysaccharide depolymerization: enzymatic route for xanthan depolymerization by Bacillus sp. strain GL1. Appl. Environ. Microbiol. 65:2520-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Numao, S., S. He, G. Evjen, S. Howard, O. K. Tollersrud, and S. G. Withers. 2000. Identification of Asp197 as the catalytic nucleophile in the family 38 α-mannosidase from bovine kidney lysosomes. FEBS Lett. 484:175-178. [DOI] [PubMed] [Google Scholar]
- 22.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera-Marrero, C. A., J. D. Ritzenthaler, J. Roman, and K. W. Moremen. 2001. Molecular cloning and expression of an α-mannosidase gene in Mycobacterium tuberculosis. Microb. Pathog. 30:9-18. [DOI] [PubMed] [Google Scholar]
- 24.Rogovin, S. P., R. F. Anderson, and M. C. Cadmus. 1961. Production of polysaccharide with Xanthomonas campestris. J. Biochem. Microbiol. Technol. Eng. 3:51-63. [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Sandford, P. A., J. E. Pittsley, C. A. Knutson, P. R. Watson, M. C. Cadmus, and A. Janes. 1977. Variation in Xanthomonas campestris NRRL B-1459: characterization of xanthan products of differing pyruvic acid content. ACS (Am. Chem. Soc.) Symp. Ser. 45:192-210. [Google Scholar]
- 27.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shine, J., and L. Dalgarno. 1974. The 3′ terminal sequence of E. coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosomal binding sites. Proc. Natl. Acad. Sci. USA 71:1342-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Elsen, J. M., D. A. Kuntz, and D. R. Rose. 2001. Structure of Golgi alpha-mannosidase II: a target for inhibition of growth and metastasis of cancer cells. EMBO J. 20:3008-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]