Abstract
The effect of Mn2+ amendment on peroxidase gene expression was studied during Pleurotus ostreatus growth on cotton stalks. Four peroxidase-encoding genes were expressed differentially and in a manner different from that observed in defined media. Mn2+ affects mnp3 expression even 2 h after its addition to the cultures, suggesting a direct effect of the metal ion on expression.
Pleurotus species belong to the group of white rot fungi that are responsible for the degradation of lignin (16, 20). Pleurotus ostreatus produces three types of oxidative extracellular enzymes: manganese-dependent peroxidases (MnPs) (16a), versatile peroxidases (VPs) (26), and laccases (25). VPs have been found in fungi from the genera Pleurotus and Bjerkandera and are suggested to be hybrids of MnP and lignin peroxidase (8, 21). The importance of Mn2+ ions, which are naturally present in lignocellulosic substrates, for the lignin degradation process by Pleurotus species has been demonstrated (6, 9, 17, 18). In recent years, different peroxidases have been purified from Pleurotus species, and their genes have been sequenced and analyzed. These include mnp1 (2), mnp2 (11), and mnp3 (14) from P. ostreatus as well as vpl (corresponding to the P. ostreatus mnp4 gene analyzed here) and vps1 from Pleurotus eryngii, which show high identity with the P. ostreatus mnp2 gene (7, 24). Ruiz-Dueñas et al. (23) studied the regulation of P. eryngii mnp1 transcript levels in peptone-containing liquid medium and stated that no vpl transcript could be detected when Mn2+ (25 μM) was present in the medium. Previously, we described the regulation of P. ostreatus peroxidase activity and mnp gene expression by Mn2+ as well as the lignin mineralization rate in peptone medium (PM) under solid-state fermentation (SSF) conditions (9). The reduction in VP gene (mnp1, mnp2, and mnp4) transcript levels and the increase in mnp3 (which encodes an MnP) transcript level were colinear with the changes observed in the enzyme activity profiles. Bogan et al. (3) analyzed gene expression during Phanerochaete chrysosporium bioremediation of organopollutants in soil. Janse et al. (15) analyzed the expression of the P. chrysosporium lignin peroxidase-, MnP-, and glyoxal oxidase-encoding genes in wood. In both studies, the observed transcription patterns were different from those found in defined media.
Since the environmental conditions that prevail in defined liquid or solid cultures are different from those in the natural substrate, extrapolation of results obtained during studies performed on cultures growing in defined media to those occurring under natural growth conditions should be performed with caution. As information concerning extracellular peroxidases of white rot fungi accumulates, we believe that it is timely to advance such analyses to conditions which are more similar to the natural lignocellulosic substrate. Such analyses would not only provide information relevant to the natural niche but would also provide a basis for comparing results obtained while analyzing the more amenable liquid culture medium system to the natural growth substrate.
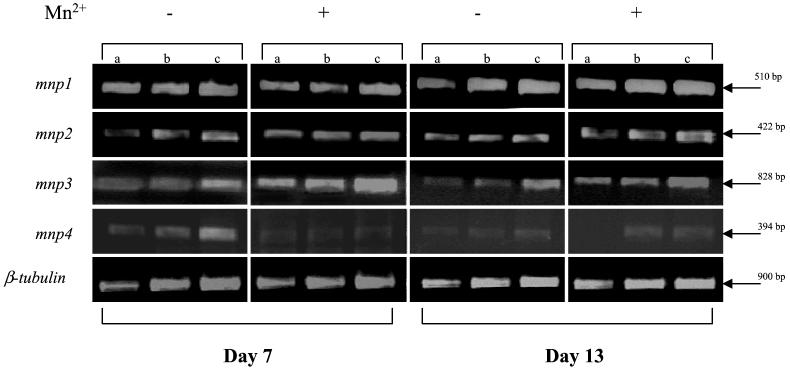
The study of gene expression during SSF in natural substrates is challenging due to the presence of plant polyphenols and polysaccharides, which are known to inhibit PCR and reverse transcription (RT)-PCR (12). In the present study, we report on the comparative abundance of P. ostreatus MnP and VP gene transcripts during fungal SSF on a natural lignocellulosic substrate. P. ostreatus (pregrown on poor medium as described in reference 9) was used to inoculate sterile cotton stalks (3 g in polypropylene cups). In order to determine the expression levels of the mnp genes, we used a relative RT-PCR approach with oligonucleotide primer sets synthesized on the basis of nonconserved sequences of the different peroxidases, as previously described (9). As total RNA could not be used for RT-PCR (due to RT inhibitors present in the natural extract), we used mRNA (isolated with a PolyA-Tract kit; Promega). Quantitative calibration of mRNA was performed using β-tubulin transcript abundance analyses as described previously (9). Briefly, the degrees of abundance of the RT-PCR products obtained from different transcripts and treatments were compared in a reaction that was terminated well within the linear phase of product accumulation. Preliminary results (data not shown) indicate that, under the reaction conditions chosen, 30 cycles of amplification were appropriate. RT-PCR amplifications were performed with RNA samples from different culture conditions in order to compare the relative abundances of mnp1, mnp2, mnp3, mnp4, and β-tubulin gene transcripts. Although the natural substrate contains 5 ppm of manganese, our Mn2+ amendment (73 ppm) to the cotton stalks affected the expression of the different genes (Fig. 1). Transcripts of mnp1, mnp2, mnp3, and mnp4 were detected. The most significant effect of Mn2+ amendment was on mnp3 transcript abundance on day 7. The transcript levels of mnp2 increased on day 13, while mnp1 transcript levels were apparently not affected during the experiment. The mnp4 transcripts were almost undetectable in all the cultures analyzed, especially in the presence of Mn2+, which corresponds to its preferential expression in liquid cultures, and inhibition by Mn2+ as described for P. eryngii (23). These results indicate a trend of expression different from that reported when the fungus was cultured under defined conditions using PM and perlite as solid supports. Under these conditions, Mn2+ amendment increased MnP-encoding gene transcript levels, while the levels of the VP-encoding gene transcripts declined (9). These differences could be explained by additional factors that affect gene expression, such as nutrient nitrogen (22) or natural inducers that are present in the cotton stalks. Such compounds have been reported to increase laccase activity and lignin degradation by P. ostreatus (1) and MnP activity in PM (R. Cohen et al., unpublished results).
FIG. 1.
The effect of Mn2+ amendment (73 ppm) on peroxidase gene expression of P. ostreatus grown under SSF conditions on cotton stalks. Gene expression was determined by relative RT-PCR utilizing specific primer sets for each gene. β-Tubulin gene expression was used as an internal control. Amplicons with increasing amounts (1-, 1.3-, and 1.5-fold amounts of RNA in panels a, b, and c, respectively) of mRNA template per gram of substrate, established on the basis of the linear range of the β-tubulin amplicon produced (see reference 9), are shown.
Mn2+ amendment enhanced lignin degradation during P. ostreatus growth on cotton stalks (18). This effect was demonstrated by measuring the [14C]lignin mineralization rate, as well as lignin content, under the same culture conditions used in the present study. Under these conditions cellulose degradation was not affected. Thus, an increase in preferential lignin degradation was achieved and in vitro digestibility of organic matter was enhanced. It was suggested that this may be the result of an increase in the activity of lignin-degrading enzymes (18). In the present study we aim to support this hypothesis by the analysis of changes in peroxidase gene transcript levels.
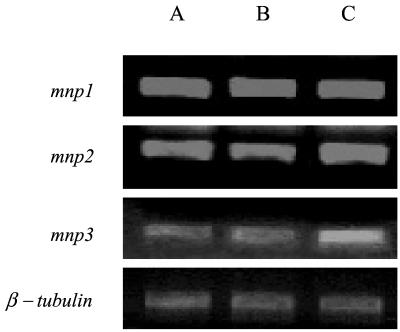
The regulation of mnp and vp genes over short periods in P. chrysosporium and P. eryngii, respectively, has been studied (4, 23). However, induction during SSF on a natural substrate has not been demonstrated in white rot fungi. To determine the kinetics of the alterations in mnp transcript levels following Mn2+ amendment, we conducted a series of short-term (0 to 8 h) Mn2+ induction experiments. Mn2+ (23 ppm) was added to 7-day-old nonamended cultures. The Mn2+ was added in the presence of 1% Tween 20 to enable the uptake of Mn2+ by the fungus, which produces a hydrophobic surface. Samples were collected after 2, 4, and 8 h. Addition of Tween 20 alone did not affect gene expression (Fig. 2B). A significant and positive effect of Mn2+ on mnp3 expression was detected after 2 h, while the expression levels of mnp2 were only slightly affected and mnp1 transcript levels were apparently unchanged. The expression of mnp4 was hardly detected during the course of this experiment (data not shown).
FIG. 2.
P. ostreatus mnp gene expression, 2 h after addition of Mn2+ (23 ppm in 1% Tween 20) to a 7-day-old culture grown on cotton stalks. Gene expression was determined using relative RT-PCR and specific primer sets for each gene as explained in the Fig. 1 legend. Since the same pattern of linear range was observed, only one RNA concentration is presented. β-Tubulin gene expression was used as an internal control. (A) Nonamended control; (B) 1% Tween 20 control; (C) Mn2+ amendment (23 ppm).
The same trend was obtained after 4 and 8 h (data not shown). In P. chrysosporium, detectable mnp mRNA was found within 20 to 40 min of Mn2+ amendment of Mn2+-deficient nitrogen-limited cultures. It was suggested that the Mn2+ may be directly involved in activating gene expression (4).
Different studies have shown that MnP regulation occurs at the level of gene transcription by means of nutrient nitrogen, manganese, heat shock, and other factors (4, 5, 10, 19, 23). These data and those presented here suggest the presence of regulatory elements in the promoter regions of these genes. Since mnp3 was sequenced without its promoter region (14), we isolated this region (1,000 nucleotides [nt]) (using a GenomeWalker kit; Clontech) and analyzed it in comparison with the other mnp genes. Interestingly, this region contained only one metal response element. We therefore continued the sequence analysis further upstream (approximately 2,500 nt sequenced on both strands; GenBank accession number AF435445). Most of the regulatory elements of the mnp3 promoter were located between 1,000 and 2,400 nt upstream of the ATG, whereas in the other genes the regulatory elements were reported in the first 1,000 nt. We identified seven putative metal response elements in mnp3. These findings provide a basis for future functional dissection of the significant and positive effect of Mn2+ on this gene's expression. The presence of nitrogen regulatory elements in the promoter region of mnp3 and in the other genes could explain the differences in gene expression between cotton stalks and medium containing peptone. The advances in use of the P. ostreatus DNA-mediated transformation system (13) should prove useful in future alteration of the regulation of peroxidase genes in this fungus.
Acknowledgments
This study was supported by a grant from the Israeli Ministry of Sciences, Culture, and Sports.
REFERENCES
- 1.Ardon, O., Z. Kerem, and Y. Hadar. 1998. Enhancement of lignin degradation and laccase activity in Pleurotus ostreatus by cotton stalk extract. Can. J. Microbiol. 44:676-680. [Google Scholar]
- 2.Asada, Y., A. Watanabe, T. Irie, T. Nakayama, and M. Kuwahara. 1995. Structures of genomic and complementary DNAs coding for Pleurotus ostreatus manganese (II) peroxidase. Biochim. Biophys. Acta 1251:205-209. [DOI] [PubMed] [Google Scholar]
- 3.Bogan, B. W., B. Schoenike, R. T. Lamar, and D. Cullen. 1996. Manganese peroxidase mRNA and enzyme activity levels during bioremediation of polycyclic aromatic hydrocarbon-contaminated soil with Phanerochaete chrysosporium. Appl. Environ. Microbiol. 62:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, J. A., M. Alic, and M. H. Gold. 1991. Manganese peroxidase gene transcription in Phanerochaete chrysosporium: activation by manganese. J. Bacteriol. 173:4101-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, J. A., D. Li, M. Alic, and M. H. Gold. 1993. Heat shock induction of manganese peroxidase gene transcription in Phanerochaete chrysosporium. Appl. Environ. Microbiol. 59:4295-4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camarero, S., B. Bockle, M. J. Martinez, and A. T. Martinez. 1996. Manganese-mediated lignin degradation by Pleurotus pulmonarius. Appl. Environ. Microbiol. 62:1070-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camarero, S., D. F. G. Ruiz, S. Sarkar, M. J. Martinez, and A. T. Martinez. 2000. The cloning of a new peroxidase found in lignocellulose cultures of Pleurotus eryngii and sequence comparison with other fungal peroxidases. FEMS Microbiol. Lett. 191:37-43. [DOI] [PubMed] [Google Scholar]
- 8.Camarero, S., S. Sarkar, D. F. Ruiz, M. J. Martinez, and A. T. Martinez. 1999. Description of a versatile peroxidase involved in the natural degradation of lignin that has both manganese peroxidase and lignin peroxidase substrate interaction sites. J. Biol. Chem. 274:10324-10330. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, R., Y. Hadar, and O. Yarden. 2001. Transcript and activity levels of different Pleurotus ostreatus peroxidases are differentially affected by Mn2+. Environ. Microbiol. 3:312-322. [DOI] [PubMed] [Google Scholar]
- 10.Gettemy, J. M., B. Ma, R. Alic, and M. H. Gold. 1998. Reverse transcription PCR analysis of the regulation of the manganese peroxidase gene family. Appl. Environ. Microbiol. 64:569-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giardina, P., G. Palmieri, B. Fontanella, V. Rivieccio, and G. Sannia. 2000. Manganese peroxidase isoenzymes produced by Pleurotus ostreatus grown on wood sawdust. Arch. Biochem. Biophys. 376:171-179. [DOI] [PubMed] [Google Scholar]
- 12.Gibb, K., and A. Padovan. 1994. A DNA extraction method that allows reliable PCR amplification of MLO DNA from “difficult” plant host species. PCR Methods Appl. 4:56-58. [DOI] [PubMed] [Google Scholar]
- 13.Honda, Y., T. Matsuyama, T. Irie, T. Watanabe, and M. Kuwahara. 2000. Carboxin resistance transformation of the homobasidiomycete fungus Pleurotus ostreatus. Curr. Genet. 37:209-212. [DOI] [PubMed] [Google Scholar]
- 14.Irie, T., Y. Honda, H. H. T. Watanabe, and M. Kuwahara. 2000. Isolation of cDNA and genomic fragments encoding the major manganese peroxidase isozyme from the white rot basidiomycete Pleurotus ostreatus. J. Wood Sci. 46:230-233. [Google Scholar]
- 15.Janse, B., J. Gaskell, M. Akhtar, and D. Cullen. 1998. Expression of Phanerochaete chrysosporium genes encoding lignin peroxidases, manganese peroxidases, and glyoxal oxidase in wood. Appl. Environ. Microbiol. 64:3536-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerem, Z., D. Friesem, and Y. Hadar. 1992. Lignocellulose degradation during solid-state fermentation: Pleurotus ostreatus versus Phanerochaete chrysosporium. Appl. Environ. Microbiol. 58:1121-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Kerem, Z., and Y. Hadar. 1996. Proceedings of the 6th International Conference on Biotechnology in the Pulp and Paper Industry, Vienna, Austria, p. 369-372.
- 17.Kerem, Z., and Y. Hadar. 1993. Effect of manganese on lignin degradation by Pleurotus ostreatus during solid-state fermentation. Appl. Environ. Microbiol. 59:4115-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerem, Z., and Y. Hadar. 1995. Effect of manganese on preferential lignin degradation by Pleurotus ostreatus during solid-state fermentation. Appl. Environ. Microbiol. 61:3057-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, D., M. Alic, J. A. Brown, and M. H. Gold. 1995. Regulation of manganese peroxidase gene transcription by hydrogen peroxide, chemical stress, and molecular oxygen. Appl. Environ. Microbiol. 61:341-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez, A. T., S. Camarero, F. Guillen, A. Gutierrez, C. Munoz, E. Varela, M. J. Martinez, J. M. Barrasa, K. Ruel, and J. M. Pelayo. 1994. Progress in biopulping of non-woody materials: chemical, enzymatic and ultrastructural aspects of wheat straw delignification with ligninolytic fungi from the genus Pleurotus. FEMS Microbiol. Rev. 13:265-274. [Google Scholar]
- 21.Mester, T., and J. A. Field. 1998. Characterization of a novel manganese peroxidase-lignin peroxidase hybrid isozyme produced by Bjerkandera species strain BOS55 in the absence of manganese. J. Biol. Chem. 273:15412-15417. [DOI] [PubMed] [Google Scholar]
- 22.Pribnow, D., M. B. Mayfield, V. J. Nipper, J. A. Brown, and M. H. Gold. 1989. Characterization of a cDNA encoding a manganese peroxidase, from the lignin-degrading basidiomycete Phanerochaete chrysosporium. J. Biol. Chem. 264:5036-5040. [PubMed] [Google Scholar]
- 23.Ruiz-Dueñas, F. J., F. Guillen, S. Camarero, M. Perez-Boada, M. J. Martinez, and A. T. Martinez. 1999. Regulation of peroxidase transcript levels in liquid cultures of the ligninolytic fungus Pleurotus eryngii. Appl. Environ. Microbiol. 65:4458-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz-Dueñas, F. J., S. Camarero, M. Perez-Boada, M. J. Martinez, and A. T. Martinez. 2001. A new versatile peroxidase from Pleurotus. Biochem. Soc. Trans. 29:116-122. [DOI] [PubMed] [Google Scholar]
- 25.Sannia, G., P. Giardina, M. Luna, M. Rossi, and V. Buonocore. 1986. Laccase from Pleurotus ostreatus. Biotechnol. Lett. 8:797-800. [Google Scholar]
- 26.Sarkar, S., A. T. Martinez, and M. Martinez. 1997. Biochemical and molecular characterization of a manganese peroxidase isoenzyme from Pleurotus ostreatus. Biochim. Biophys. Acta 1339:23-30. [DOI] [PubMed] [Google Scholar]