Abstract
Multicenter research designs are widely recognized for enhancing the generalizability and real-world applicability of findings across diverse healthcare settings. However, conducting experimental studies across international sites presents substantial methodological, logistical, and operational challenges.
Quality CPR was a European multicenter simulation-based experimental study conducted across academic institutions in Portugal, Germany, and Finland. Using a randomized crossover design, the study investigated factors influencing chest compression quality and rescuer fatigue during simulated cardiopulmonary resuscitation.
Rather than serving as a methodological guide, this paper reflects on the practical experience of designing and implementing the Quality CPR study, highlighting twelve key lessons across the research lifecycle—planning, development, execution, and dissemination. These include collaborative protocol design, the development of a practical researcher guide to ensure cross-site harmonization, active support and real-time data monitoring during execution, and integrated dissemination strategies throughout the project lifecycle.
By documenting the enablers of success, this paper provides a practical reference for researchers planning similar international, multicenter studies. The insights offered aim to support the growing community of healthcare researchers engaged in cross-institutional initiatives.
Keywords: Research, Multicenter studies, Experimental design, Simulation, Practical guidance, International collaboration
Background
Multicenter research has become increasingly prominent in the medical and health sciences, offering a rigorous methodological approach to enhance the generalizability, scalability, and clinical applicability of findings. These designs support larger sample sizes, accelerate recruitment, and mitigate site-specific biases by harmonizing protocols across structurally and geographically distinct sites.1, 2, 3 Beyond methodological advantages, such studies foster international collaboration, promote knowledge exchange, and support sustainable research networks that drive long-term innovation.3, 4, 5, 6
Despite these advantages, conducting multicenter experimental studies—particularly at an international level—presents substantial methodological, logistical, and operational challenges. These include standardizing interventions and outcome measures across sites, harmonizing protocols, managing ethical and data protection requirements across jurisdictions, and maintaining data quality and consistency.5, 7 Although several efforts have been made to provide guidance on addressing these complexities,5, 6, 7, 8 our experience suggests that substantial risks for inconsistency remain. These may arise from variations in equipment/materials, protocol deviations, or site-specific constraints.
Simulation-based research is a valuable methodology for investigating healthcare education, clinical performance, and system-level interventions under controlled and replicable conditions.9 Although not suitable for all study designs, when appropriate, simulation can enable rigorous cross-site comparisons while preserving environmental control.7, 10, 11
Quality CPR was a two-year European multicenter experimental study carried out across three academic institutions in Portugal, Germany, and Finland. Conducted between March 2023 and February 2025, following preparatory discussions in November 2022, it used a randomized crossover trial to examine factors influencing chest compression quality and rescuer fatigue during simulated cardiopulmonary resuscitation. The study was designed as a rigorous, cross-national experimental investigation, using simulation as the platform to deliver the intervention. A full description of the Quality CPR study can be found in Nicolau et al. (2024) and Sa-Couto et al. (2025).12, 13
This paper does not intend to serve as a methodological guide for conducting multicenter studies, as the underlying frameworks are already well-documented in scientific literature.4, 7, 10, 14 Rather, it reflects on the authors’ experience of designing and conducting Quality CPR study, focusing on twelve key success factors for navigating common collaborative, methodological, and logistical challenges in multicenter research. These twelve lessons were derived through structured team reflection sessions conducted during and after the project, supplemented by notes from coordination meetings and partner feedback collected throughout the research lifecycle. While not exhaustive, these lessons reflect the consortium collective experience and are intended to support other teams undertaking similar initiatives.
Lessons learnt from Quality CPR project
Drawing on insights gathered throughout the project lifecycle, we identify twelve key lessons that contributed to the success of Quality CPR. To provide a structured overview, these lessons are organized according to common research phases—planning, development, execution, and dissemination,7 Fig. 1.
Fig. 1.
Twelve key lessons drawn from the Quality CPR Project across the project lifecycle.
Planning
Lesson 1. Establishing an interdisciplinary research team
A foundational element of Quality CPR was the early formation of an interdisciplinary team, bringing together complementary expertise from physicians, paramedics, nurses, educators, researchers, biomedical engineers, computer scientists, and biostatisticians. This diversity was instrumental in shaping a research study that was both clinically relevant and technically robust.
Each discipline contributed a unique perspective. Clinicians, educators with experience in healthcare simulation, and researchers ensured that the study design aligned with clinical relevancy and best practices, while engineers and computer scientists advised on equipment selection and supported data acquisition and processing. We strongly recommend involving a biostatistician—or an experienced quantitative researcher—from the outset, to ensure methodological rigor and support the use of advanced inferential statistical methods required to analyze complex, multimodal data.
Lesson 2. Ensuring a clear coordination structure and communication
A dual coordination model was adopted. A central coordinating team provided leadership and oversight across all scientific, operational, and financial dimensions of the study, while local coordinators ensured procedural consistency and accountability.
To support effective collaboration, regular virtual meetings were held throughout the project, complemented by real-time messaging platforms (e.g., WhatsApp). These channels enabled agile decision-making, rapid clarification of protocol-related queries, and the timely resolution of operational issues − both during planning and, crucially, in real-time during data collection (see also lesson 10).
Lesson 3. Building flexibility to accommodate local variations
A structured project timeline was established during the initial planning phase, including critical milestones across all project phases: protocol development, ethics submission, testing, data collection, analysis, and dissemination.
Built-in flexibility accommodated site-specific variations, such as institutional review processes, academic calendars, and infrastructure readiness. Adaptive scheduling and continuous dialogue ensured coordinated progress while respecting local constraints and capabilities.
Lesson 4. Securing funding and leveraging local resources
- Adequate financial support was essential to the feasibility, coordination, and overall success of Quality CPR. A centralized funding application, led by the coordinating institution, secured national and international resources, which were strategically allocated to:
-
•Full-time research fellow, who played a pivotal role in project coordination and day-to-day operations;
-
•Travel and accommodation costs for two in-person consortium meetings, critical for protocol refinement and collaborative data analysis;
-
•The acquisition of three identical biometric sensor sets, ensuring consistent data capture;
-
•Dissemination activities, including participation in international conferences and open-access publications.
-
•
In parallel, substantial in-kind contributions were mobilized from partner institutions, including simulation space, equipment, and protected time for academic and technical staff. This combination of central funding and local resources highlights the importance of multi-level investment and collaborative commitment in enabling large-scale, multicenter research.
Lesson 5. Co-creating a shared and feasible study protocol
A shared protocol was collaboratively developed with contributions from all partners, and included a clear research question, articulated primary and secondary outcomes, and a comprehensive description of the intervention and data collection strategy. A priori sample size calculation was performed to ensure adequate statistical power and guide recruitment (see also lesson 9).
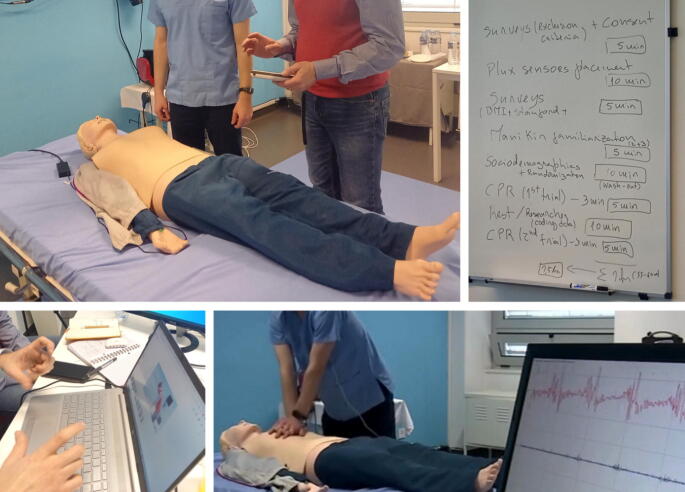
A 2-days in-person consensus meeting was held during the preparatory phase to review, pilot, and refine the protocol. This included refining the intervention phases, real-time scenario testing, evaluation of the technical infrastructure, and clarification of logistical and methodological issues, Fig. 2. This helped close the gap between “work-as-imagined” and “work-as-done,” aligning planned procedures with practical implementation. It was especially valuable for harmonizing interventions across centers and resolving questions related to multi-source data collection.
Fig. 2.
Photographs from the two-day in-person consensus meeting held on March 24–25, 2023, in Porto, Portugal, during the planning phase of the project. Activities included refining the intervention phases, conducting real-time scenario testing, evaluating the technical infrastructure, and addressing logistical and methodological considerations.
Lesson 6. Ensuring ethics and data protection compliance
Streamlining ethical review procedures is particularly important, as regulatory requirements vary between countries—even within the European Union—often causing delays in the initiation of cross-border research. To address this, a standardized template package (including the study protocol, participant information sheets, and informed consent forms) was centrally developed. Local coordinators translated and adapted these documents to meet cultural and linguistic requirements. In accordance with local legal and regulatory frameworks, each participating institution obtained approval from the respective ethics and data protection boards. Queries and requests for clarification from review boards were managed centrally, ensuring consistent and timely responses.
Development
Lesson 7. Creating a comprehensive and practical implementation guide
To promote procedural fidelity, a detailed implementation guide was developed. It was intentionally designed to be both comprehensive and user-friendly, incorporating visual flow diagrams, step-by-step instructions, scripted elements for simulation delivery, and data capture protocols (see supplementary material – Quality CPR Implementation Guide).
This document was reviewed during a dedicated online session with all partner institutions and subsequently disseminated to all involved researchers. It served as a constant reference during local implementation and proved essential for maintaining protocol adherence across geographically distributed teams, due to its clarity and practical employment. This guide supported consistent implementation across sites, standardized data collection and mitigated confounding variables.
Lesson 8. Standardizing data collection tools and procedures
Given the complexity and multimodality of the study data, a comprehensive data collection strategy was implemented, integrating simulator-derived metrics, biomedical sensors, electronic forms, and video recordings. Ensuring inter-site comparability and data integrity was a central priority, achieved through consensus-based standardization of data collection tools and procedures.
- Key strategies included:
-
•Embedding clear, context-sensitive instructions and reminders within the electronic data collection forms to guide researchers during data entry.
-
•Utilizing a centralized platform for uniform data submission and secure storage.
-
•Standardizing hardware and software platforms—such as simulators, sensors, recording systems, and clinical equipment—across all sites to minimize variability. The selection of materials prioritized items with broad commercial availability and accessibility. For example, a commonly available step-stool, purchasable from a Europe-wide retail chain, was used to ensure procedural consistency and reproducibility.
-
•Conducting preliminary testing of all simulation equipment and environmental conditions at each site to verify operational consistency prior to data collection.
-
•
Execution
Lesson 9. Strengthening the recruitment strategy with a built-in safeguard
To further strengthen the study’s validity, the research team adopted a recruitment strategy that deliberately exceeded the minimum required sample size. This approach provided a safeguard against potential participant dropout or data loss due to technical issues – an especially important consideration in studies that rely on multimodal data collection and technology. Moreover, over-recruitment contributed to enhancing the generalizability of the findings, allowing for more balanced representation across study groups and sites.
Lesson 10. Maintaining active support and data monitoring
During the data collection phase, the coordinating team maintained open and direct communication channels with local investigators. Protocol-related questions were addressed promptly via messaging platforms, email, or virtual meetings.
All local investigators and data collectors were briefed on the protocol and instructed to escalate any issues either to their local coordinator or directly to the central team.
Data monitoring was conducted in real-time, with periodic review of incoming data to identify potential errors (e.g., incomplete records, equipment malfunctions), thereby reducing the risk of post hoc data loss.
Lesson 11. Conducting collaborative data analysis and interpretation
Upon completion of data collection and preliminary analysis, a second 2-days in-person meeting was held with all partners. Although the need for in-person meetings should always be weighed against economic implications and sustainability impacts, this format enabled rich, contextual discussion of findings, cross-validation of results, and integrative interpretation of patterns, Fig. 3. This collaborative discussion strengthened the robustness of conclusions and fostered shared ownership of the results.
Fig. 3.
Photograph illustrating the second in-person meeting held on April 22–23, 2024, in Porto, Portugal. The meeting focused on cross-validating results and developing an integrated interpretation of emerging patterns.
Dissemination
Lesson 12. Embedding dissemination across the project lifecycle
Dissemination efforts were integrated throughout the research lifecycle to maximize impact and foster engagement with the broader research community. Early in the project, Quality CPR was presented during an ALERT (Advanced Look Exploratory Research Template) session at SESAM SiReN Online meeting, where it received external mentorship and formative peer feedback during the study design phase.
Preliminary findings were subsequently shared at major international conferences, including SESAM15, 16 and ASPiH,17 through poster and oral presentations. These opportunities not only promoted visibility but also facilitated valuable input from external experts, helping to refine both the methodology and the interpretation of results.
To support sustained knowledge translation, manuscripts were collaboratively developed and published12, 13 in peer-reviewed open-access journals selected for their relevance to the study’s focus, thereby maximizing the visibility and impact of the findings among the intended target audience.
Dissemination efforts included strategic media engagement, initiated during the planning phase and sustained throughout the study. To maximize impact, media activities were aligned with key project milestones and involved all participating institutions. For example, national television coverage was secured during the in-person protocol refinement meeting. In addition, social media amplified visibility via institutional channels and researchers’ professional networks.
Reflections and implications for future research studies
The Quality CPR project provides a practical illustration of how complex multicenter experimental research can be successfully conducted through deliberate planning, cross-site collaboration, and structured implementation.
The planning and development phases proved particularly decisive. Early investment in interdisciplinary team formation, coordination structures, flexible timelines, protocol co-creation, and ethics preparation fostered a shared understanding of objectives and constraints. These foundational efforts enabled each site to operate with a degree of autonomy while preserving consistency in implementation and data integrity. The subsequent development of standardized instruments and a clear, practical implementation guide further reinforced procedural fidelity across institutions.
Our experience also underscores the importance of continuous, open communication and responsive operational support during the execution phase. Real-time troubleshooting and sustained team engagement were critical to addressing technical or procedural challenges as they emerged. Likewise, collaborative data interpretation and embedded dissemination strategies enhanced transparency, promoted shared ownership, and strengthened the translational impact of the findings. Building on the success of the Quality CPR project and the collaborative relationships it fostered, a follow-up multicenter research initiative is currently underway, with a proposal recently submitted for European funding.
While the lessons outlined may not be universally applicable across all multicenter research contexts, they offer a practical reference for future experimental studies. Future studies may build upon these practical lessons to design and implement their processes more effectively, with improved cost-efficiency, sustainability, and risk mitigation across all phases of the research lifecycle. These considerations are particularly relevant in consortia where partners have limited prior experience with multicenter studies, come from different professional or cultural backgrounds, or are unfamiliar with each other’s research practices.
Conclusions
The Quality CPR project illustrates that international multicenter experimental research is both feasible and valuable when supported by structured coordination, methodological rigor, and proactive interdisciplinary engagement. The twelve lessons presented offer practical, experience-based insights to inform the planning, implementation, and management of similar collaborative initiatives. By documenting the strategies that enabled successful execution across diverse institutional and national contexts, this paper contributes to the growing body of applied knowledge essential for advancing high-quality, cross-institutional healthcare research.
Declaration of Generative AI and AI-assisted technologies in the writing process
During the preparation of this work the author(s) used ChatGPT-4o (OpenAI) to assist with writing, including refining language and improving clarity. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Funding sources
This work was supported by national funds of the FCT – Fundação para a Ciência e a Tecnologia, I.P., under the project “QualityCPR, ref. 2022.03731.PTDC”, and by a grant from the Laerdal Foundation (ref. 2022-0083).
CRediT authorship contribution statement
Carla Sa-Couto: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Christoffer Ericsson: Writing – review & editing, Investigation, Conceptualization. Marc Lazarovici: Writing – review & editing, Methodology, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge to all members of the research teams that participated in the project that led to this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2025.101054.
Contributor Information
Carla Sa-Couto, Email: csacouto@med.up.pt.
Christoffer Ericsson, Email: christoffer.ericsson@arcada.fi.
Marc Lazarovici, Email: marc.lazarovici@med.uni-muenchen.de.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Das M.K. Multicenter studies: relevance, design and implementation. Indian Pediatrics. 2022;59:571–579. doi: 10.1007/s13312-022-2561-y. [DOI] [PubMed] [Google Scholar]
- 2.Impellizzeri F.M. Together we are stronger: multicenter studies. Int J Sports Phys Perform. 2017;12(2):141. doi: 10.1123/IJSPP.2016-0818. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz A., Young R., Hicks P.J. Medical education practice-based research networks: facilitating collaborative research. Med Teach. 2016;38(1):64–74. doi: 10.3109/0142159X.2014.970991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huggett K.N., Gusic M.E., Greenberg R., Ketterer J.M. Twelve tips for conducting collaborative research in medical education. Med Teach. 2011;33(9):713–718. doi: 10.3109/0142159X.2010.547956. [DOI] [PubMed] [Google Scholar]
- 5.Johnson J.K., Barach P., Vernooij-Dassen M., HANDOVER Research Collaborative Conducting a multicentre and multinational qualitative study on patient transitions. BMJ Qual Safet. 2012;21(Suppl 1):i22–i28. doi: 10.1136/bmjqs-2012-001197. [DOI] [PubMed] [Google Scholar]
- 6.Fisberg M., Kovalskys I., Salas G.G., et al. Developing a cooperative multicenter study in Latin America: lessons learned from the Latin American study of nutrition and health project. Revista Panamericana De Salud Pública. 2017;41:e111. doi: 10.26633/RPSP.2017.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng A., Kessler D., Mackinnon R., et al. Conducting multicenter research in healthcare simulation: lessons learned from the INSPIRE network. Adv Simul. 2017;2:6. doi: 10.1186/s41077-017-0039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gisbert J.P., Chaparro M. Tips and tricks for successfully conducting a multicenter study. Gastroenterología y Hepatología (English Edition) 2024;47(6):649–660. doi: 10.1016/j.gastre.2024.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Cheng A., Auerbach M., Hunt E.A., et al. Designing and conducting simulation-based research. Pediatrics. 2014;133(6):1091–1101. doi: 10.1542/peds.2013-3267. [DOI] [PubMed] [Google Scholar]
- 10.Whitfill T., Gross I.T., Auerbach M. In: Healthcare simulation research: A practical guide (pp. [specific pages if known]) Nestel D., Hui J., Kunkler K., Scerbo M.W., Calhoun A.W., editors. Springer Cham; 2019. Establishing and maintaining multicenter studies in healthcare simulation research. [DOI] [Google Scholar]
- 11.Lamé G., Dixon-Woods M. Using clinical simulation to study how to improve quality and safety in healthcare. BMJ Simulat Technol Enhan Learn. 2020;6(2):87–94. doi: 10.1136/bmjstel-2018-000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolau A., Bispo I., Lazarovici M., et al. Influence of rescuer position and arm angle on chest compression quality: an international multicentric randomized crossover simulation trial. Resuscit Plus. 2024;20 doi: 10.1016/j.resplu.2024.100815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sa-Couto C., Sa-Couto P., Nicolau A., et al. Impact of rescuer position, arm angle, and anthropometric variables on muscle fatigue during cardiopulmonary resuscitation: an international multicentric randomized crossover simulation study. Resuscit Plus. 2025;100971 doi: 10.1016/j.resplu.2025.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makram A.M., Elsheikh R., Makram O.M., Fahim M., El Sherif D., Khalil H. Tips from an expert panel on the development of a clinical research protocol. BMC Med Res Method. 2024;24:293. doi: 10.1186/s12874-024-02315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolau A., Bispo I., Lazarovici M., et al. Exploring the influence of rescuer position on chest compression quality: an international multicentric pseudo-randomised manikin trial [Conference abstract] Adv Simul. 2024;9(Suppl 1):O13. doi: 10.1186/s41077-024-00287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bispo I., Nicolau A., Lazarovici M., et al. Evaluation of cardiac and muscular parameters during chest compressions: an international multicentric pseudo-randomised manikin trial [Conference abstract] Adv Simul. 2024;9(Suppl 1):O11. doi: 10.1186/s41077-024-00287-2. [DOI] [Google Scholar]
- 17.Bispo I., Nicolau A., Lazarovici M., Ericsson C., Vieira-Marques P., Sá-Couto C. Impact of physical fitness on muscle activity and quality of chest compressions in cardiorespiratory resuscitation: a multicentric manikin study [Conference abstract] J Healthcare Simulat. 2024;4(Suppl 1):A30. doi: 10.54531/EHEV6601. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.