ABSTRACT
Aims
Heart failure (HF) is a leading cause of hospitalizations worldwide. HF can lead to pulmonary hypertension (PH) and co‐occurrence of HF and PH is associated with a poor prognosis. This systematic review and meta‐analysis aim to estimate the prevalence of PH in patients with HF.
Methods
We searched MEDLINE and EMBASE for studies reporting the prevalence of PH amongst HF patients. A meta‐analysis of PH prevalence, including subgroup analyses, was conducted using a random‐effects model. Subgroup analyses and meta‐regressions by comorbidities and patient characteristics were done. Study quality was assessed using the Joanna Briggs Institute Critical Appraisal Tool.
Results
Fifty‐four papers with 259 665 HF patients were included, of which 46 004 also had PH. The overall PH prevalence estimate in individuals with HF is 46.6% (95% CI: 39.6%–53.7%). Prevalence varied by diagnostic method, with studies using right heart catheterization reporting the highest estimates (62.5%; 52.0%–72.0%), hospital recorded data the lowest (18.4%; 14.4%–23.3%), and echocardiography 45.7% (37.1%–54.6%). Prevalence was higher in HF with preserved (47.2%; 34.8%–60.0%) than reduced ejection fraction (35.7%; 22.6%–51.3%). Prospective studies show higher estimates (60.1%; 50.7%–68.8%) than retrospective studies (37.3%; 29.5%–45.9%).
Conclusions
This is the first systematic review and meta‐analysis investigating the prevalence of PH in HF patients and shows that the prevalence of PH in this patient population is strikingly high. There is notable variability in estimates reported by different studies, largely attributed to differences in the diagnostic method of PH. Future studies with robust, standardized methodologies are needed to estimate prevalence more accurately.
Keywords: heart failure, hypertension, meta‐analysis, prevalence, pulmonary, systematic review
This systematic review has shown that the prevalence of pulmonary hypertension among individuals with heart failure is high, and varies by several population characteristics, potentially reflecting patient selection, disease severity, and differences in diagnosis and treatment.

1. Introduction
The prevalence of pulmonary hypertension (PH) is currently reported as 12–53 cases per million people [1]. PH rates are increasing, particularly in resource‐limited areas, and two epidemics have been described in the last 60 years [2]. Thus, the disease is becoming an increasingly serious global health burden. The prognostic outcomes of PH patients are poor, with strikingly high mortality rates; the 1‐year mortality rate in the United States was estimated to be 8%, and 38% in high‐risk patients [3]. Early disease identification and intervention are key in reducing the significant morbidity and mortality associated with PH. This, coupled with new findings from large patient cohorts, has recently led to changes in the definition of PH, with the diagnostic threshold being lowered to a mean pulmonary artery pressure of 20 mmHg, instead of 25 mmHg [3, 4].
Transthoracic echocardiography is an important noninvasive, relatively low‐cost investigation to estimate PH probability. A higher peak tricuspid regurgitant velocity increases the probability of PH, for example, a velocity of > 3.4 m/s confers a high probability of PH in the absence of other features of PH on echocardiography [3]. However, right heart catheterization (RHC), which is a more invasive investigation, is the gold standard diagnostic method for PH. During RHC, pulmonary vascular resistance (PVR) and pulmonary artery wedge pressure (PAWP) can be measured. These measurements are important to differentiate between precapillary PH (PAWP ≤ 15 mmHg and PVR > 2 Wood units), postcapillary PH (PAWP > 15 mmHg and PVR > 2 Wood units), and combined precapillary and postcapillary PH (PAWP > 15 mmHg and PVR ≤ 2 Wood units) [3]. Precapillary PH results from remodeling of the pulmonary vasculature, whilst postcapillary PH results from increased pressure in the pulmonary vasculature due to cardiac disease [3].
PH has many causes which can be broadly split into five categories: idiopathic (Group I), left cardiac disease (Group II), lung disease (Group III), pulmonary artery obstruction (Group IV), and hematological/systemic disorders (Group V). Of these, heart failure (HF) is the most common cause of PH [5]. HF is the inability of the heart to adequately pump blood and meet the metabolic demands of the body. It is classified into three distinct groups, each with differing levels of ejection fraction (preserved, mid‐range, and reduced) [6]. The immense prevalence, mortality, and public health burden has even been described as a “global pandemic,” with reported prevalence rates ranging between 3.9 and 16 per 1000 people [1, 7, 8].
PH is a poor prognostic indicator in HF and is associated with an increased 5‐year mortality rate, independent of HF severity and other comorbidities [9]. These poor outcomes can be largely attributed to the lack of specific treatments available for Group II PH, in comparison to other PH subtypes.
Although the disease mechanisms linking HF and PH are well documented in the literature, little work has been done to establish the epidemiological relationship between the two pathologies in patients. This systematic review and meta‐analysis summarizes the literature investigating the co‐existence of these two pathologies, with a focus on the prevalence of PH in individuals with HF. The aims of this review are to investigate the overall prevalence of PH amongst individuals with HF, and to estimate how this varies across different populations and study characteristics, including the subtype of HF, presence of other comorbidities, and study methodology.
2. Methods
In this systematic review and meta‐analysis, MEDLINE and EMBASE were searched electronically on October 7, 2022, to find any papers related to PH and HF published since inception of the databases (1946). Key terms such as “heart failure”, “pulmonary hypertension”, and “prevalence” were included. The full search strategy of Medline and Embase is included in Supporting Information S1: Table 1. We included prospective, cross‐sectional, or retrospective studies on humans, in which individuals with HF were included and information on PH was mentioned. Studies exclusively on children (< 18 years of age), studies in a language other than English, or review articles were excluded.
Search results were uploaded to Covidence, and duplicates were removed. Two independent reviewers completed title/abstract (J.S., N.D.) and then full‐text screening (J.S., M.K./R.S.), according to the prespecified Prospero protocol CRD42023335272. Conflicts were resolved after discussion with another reviewer (C.K.).
Data extraction was completed in Covidence, using a template predesigned according to the Prospero protocol, by two independent reviewers (J.S., M.K./R.S.). Extracted data were consolidated with any discrepancies resolved by discussion. We extracted data on study characteristics (title, author, year of publication, location, special population, study design [retrospective/prospective], total number of participants, inclusion and exclusion criteria), study population (mean age, proportion female, ethnicity, mean BMI, proportion with pulmonary disease, obstructive sleep apnea, diabetes, anemia, kidney disease, smoking, atrial fibrillation, and ischemic heart disease), HF (number of participants with HF, diagnostic method, definition, subtype of HF [reduced/preserved ejection fraction]), and PH (number of participants with PH, number of participants with HF who also had PH, diagnostic method, definition). Overall and subgroup‐specific prevalence estimates were collected from each paper, if available. Countries were grouped based on their income using the World Bank Income classification.
A meta‐analysis of PH prevalence was done using a logit transformation and a random effects model, and as a sensitivity analysis using an inverse‐variance weighted average. If an overall and subgroup‐specific prevalence was extracted from a study, data from the latter was excluded in the overall analysis and only included in the relevant subgroup analysis. Prevalence estimates and 95% confidence intervals (CI), overall and in subgroups, based on a random effects model, are reported unless stated otherwise. Heterogeneity was assessed using I 2 and Cochran's Q test. Correlations between prevalence of PH and population characteristics (such as demographics and comorbidities, HF features, or etiology) were assessed graphically and using meta‐regression.
The papers included were critically appraised by two independent reviewers (M.K., R.S.) using the Johanna Briggs Institute Critical Appraisal Tool for systematic reviews (Supporting Information S1: Table 2) [10]. Data analysis was done using R version 4.2.2 and package “meta”.
3. Results
3.1. Study Selection
Of the 3390 studies identified by the search strategy, following the removal of duplicates, 1670 full papers were screened. Overall, 54 papers were identified for inclusion in this review (Figure 1).
Figure 1.

A summary of the study selection process.
3.2. Characteristics of the Included Studies
Table 1 shows the characteristics of the studies included. The complete data set extracted from the studies is shown in Supporting Information S1: Table 3. Overall, 259 665 participants with HF were included, of which 46 004 also had a diagnosis of PH. The total number of participants within each study ranges from 10 to 188 991 [11, 12]. Overall, studies from 25 different countries were included, although most studies were conducted in the United States [12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28]. The methodology and patient demographics of the studies vary greatly, and patient data were collected from a variety of sources, including registries [12, 17, 29, 30, 31, 32, 33]. The reported mean age and BMI from the included studies range from 49.5 to 92.33 years and 22.7 to 32.6, respectively [27, 34, 35, 36].
Table 1.
Main characteristics of included studies.
| Paper | Location | Location per income | Study type | Group | HFrEF versus HFpEF | N | PH prevalence | PH diagnosis |
|---|---|---|---|---|---|---|---|---|
| Ahmadi 2021 | North America (Canada) | High | Retrospective | Right ventricular dysfunction | 10 | 60.00% | Echo | |
| Ahmadi 2021 | North America (Canada) | High | Retrospective | Normal right ventricular function | 23 | 13.00% | Echo | |
| Ahmed 2020 | Europe (Sweden) | High | Prospective | Pre‐heart transplant | 26 | 73.10% | RHC | |
| Allison 2016 | Europe (England) | High | Retrospective | Nonagenarians (overall) | 144 | 47.00% | Echo | |
| Allison 2016 | Europe (England) | High | Retrospective | Nonagenarians (HFrEF) | HFrEF | 63 | 50.79% | Echo |
| Allison 2016 | Europe (England) | High | Retrospective | Nonagenarians (HFpEF) | HFpEF | 55 | 32.73% | Echo |
| Alqahtani 2019 | North America (USA) | High | Retrospective | Not for palliative care | 188 991 | 14.90% | Hospital recorded data | |
| Alqahtani 2019 | North America (USA) | High | Retrospective | For palliative care | 2338 | 28.30% | Hospital recorded data | |
| Amadi 2016 | Africa (Nigeria) | Low | Prospective | Overall | 125 | 70.40% | Echo | |
| Arnaert 2021 | Europe (Belgium) | High | Retrospective | Overall | 248 | 41.10% | Echo | |
| Auffret 2020 | Europe (France) | High | Retrospective | Post‐TAVR | 102 | 77.20% | Echo | |
| Benito‐Gonzalez 2020 | Europe | High | Retrospective | HFrEF with fMR | HFrEF | 93 | 14.00% | Echo |
| Bosch 2017 | Asia (Singapore) | High | Prospective | HFrEF | HFrEF | 219 | 52.00% | Echo |
| Bosch 2017 | Asia (Singapore) | High | Prospective | HFpEF | HFpEF | 219 | 39.00% | Echo |
| Butler 1999 | North America (USA) | High | Retrospective | Overall | HFrEF | 320 | 19.00% | RHC |
| Chakraborty 2022 | North America (USA) | High | Retrospective | Hypertrophic cardiomyopathy | 6040 | 30.40% | Hospital recorded data | |
| Choudhary 2014 | North America (USA) | High | Retrospective | Overall | 107 | 74.80% | Echo | |
| Covella 2017 | North America (USA) | High | Retrospective | Hypertrophic obstructive cardiomyopathy | 172 | 50.60% | RHC | |
| Diaconu 2015 | Europe (Romania) | High | Retrospective | Diabetes | 67 | 19.40% | Hospital recorded data | |
| Faggiano 2000 | Europe (Italy) | High | Prospective | Aortic stenosis | 216 | 69.90% | RHC | |
| Gerges 2015 | Europe (Austria) | High | Retrospective | Retrospective: Systolic HF | 664 | 68.80% | RHC | |
| Gerges 2015 | Europe (Austria) | High | Retrospective | Retrospective: Diastolic HF | 399 | 59.40% | RHC | |
| Gerges 2015 | Europe (Austria) | High | Prospective | Prospective: Systolic HF | 172 | 80.80% | RHC | |
| Gerges 2015 | Europe (Austria) | High | Prospective | Prospective: Diastolic HF | 219 | 76.70% | RHC | |
| Guazzi 2017 | Europe (Italy) | High | Prospective | HFpEF TAPSE/PASP ratio < 0.35 | HFpEF | 129 | 91.50% | Echo |
| Guazzi 2017 | Europe (Italy) | High | Prospective | HFpEFTAPSE/PASP ratio 0.35–0.57 | HFpEF | 129 | 85.71% | Echo |
| Guazzi 2017 | Europe (Italy) | High | Prospective | HFpEF TAPSE/PASP ratio > 0.57 | HFpEF | 129 | 81.81% | Echo |
| Haddad 2022 | North America (USA) | High | Retrospective | HFpEF | HFpEF | 96 | 55.00% | Echo |
| Hsieh 2016 | Asia (Taiwan) | High | Retrospective | CKD requiring hemodialysis | 160 | 31.90% | Echo | |
| Huang 2022 | Asia (China) | Upper‐middle | Prospective | HF and coronary artery disease | 182 | 77.50% | RHC | |
| Huang 2022 | Asia (China) | Upper‐middle | Prospective | HFpEF and coronary artery disease | HFpEF | 142 | 82.50% | RHC |
| Huang 2022 | Asia (China) | Upper‐middle | Prospective | HFrEF and coronary artery disease | HFrEF | 40 | 76.10% | RHC |
| Ibe 2016 | Asia (Japan) | High | Retrospective | Overall | 164 | 40.00% | RHC | |
| Jentzer 2022 | North America (USA) | High | Retrospective | Coronary care unit patients | 2651 | 79.10% | Echo | |
| Kanumuri 2019 | Asia (India) | Lower‐middle | Retrospective | Hospitalized patients | 130 | 4.60% | Echo | |
| Karaye 2013 | Africa (Nigeria) | Low | Prospective | Overall | 80 | 66.25% | Echo | |
| Khush 2009 | North America (USA) | High | Retrospective | Overall | HFrEF | 171 | 47.00% | RHC |
| Kushimo 2019 | Africa (Nigeria) | Low | Prospective | Overall | 219 | 38.80% | Echo | |
| Lam 2009 | North America (USA) | High | Prospective | HFpEF | HFpEF | 203 | 83.00% | Echo |
| Lee 2022 | Asia (South Korea) | High | Retrospective | Overall | 1729 | 21.60% | Echo | |
| Leung 2010 | North America (USA) | High | Retrospective | HFpEF | HFpEF | 455 | 68.80% | RHC |
| Lima 2019 | North America (USA) | High | Retrospective | Overall | 5595 | 3.04% | Hospital recorded data | |
| Lin 2022 | Asia (China) | Upper‐middle | Retrospective | Overall | 480 | 44.80% | RHC | |
| Liu 2020 | Asia (China) | Upper‐middle | Prospective | HFpEF | HFpEF | 149 | 39.60% | Echo |
| Lutsey 2022 | North America (USA) | High | Retrospective | Venous thromboembolism patients | 6189 | 22.40% | Hospital recorded data | |
| Mogollon 2008 | Europe (Spain) | High | Retrospective | Pre‐heart transplant | 39 | 48.70% | RHC | |
| Mutlak 2018 | Asia (Israel) | High | Prospective | fTR | 709 | 72.00% | Echo | |
| Nakagawa 2020 | South America (Brazil) | Upper‐middle | Prospective | HFrEF | HFrEF | 70 | 46.00% | Echo |
| Nakamura 2019 | Asia (Japan) | High | Prospective | HFpEF | HFpEF | 198 | 46.50% | RHC |
| Nkoke 2022 | Africa (Cameroon) | Lower‐middle | Prospective | Overall | 66 | 93.90% | Echo | |
| Pandey 2020 | North America (USA) | High | Retrospective | Hospitalized with HFrEF in 2005–2009 | HFrEF | 5440 | 12.20% | Hospital recorded data |
| Pandey 2020 | North America (USA) | High | Retrospective | Hospitalized with HFrEF in 2010–2014 | HFrEF | 7883 | 12.10% | Hospital recorded data |
| Pandey 2020 | North America (USA) | High | Retrospective | Hospitalized with HFpEF in 2005–2009 | HFpEF | 4278 | 13.20% | Hospital recorded data |
| Pandey 2020 | North America (USA) | High | Retrospective | Hospitalized with HFpEF in 2010–2014 | HFpEF | 7335 | 20.70% | Hospital recorded data |
| Pintalhao 2017 | Europe (Portugal) | High | Retrospective | Overall | 117 | 59.80% | Echo | |
| Raina 2015 | North America (USA) | High | Retrospective | Overall | 537 | 59.60% | RHC | |
| Raina 2015 | North America (USA) | High | Prospective | Octogenarians with aortic stenosis | 36 | 80.60% | RHC | |
| Rifaie 2010 | Africa (Egypt) | Lower‐middle | Prospective | Overall | HFpEF | 100 | 20.00% | Echo |
| Santas 2019 | Europe (Spain) | High | Prospective | Overall | HFpEF | 760 | 77.80% | Echo |
| Selvaraj 2017 | North America (USA) | High | Prospective | CKD | 35 | 22.90% | Echo | |
| Shah 2014 | North America (USA) | High | Retrospective | HFpEF | HFpEF | 935 | 36.00% | Echo |
| Sobieszczanska‐Malek 2014 | Europe (Poland) | High | Retrospective | Overall | 559 | 66.70% | RHC | |
| Stein 2012 | Asia (Israel) | High | Retrospective | Overall | 9335 | 29.50% | Echo | |
| Straburzynska‐Migaj 2007 | Europe (Poland) | High | Prospective | Overall | HFrEF | 56 | 41.00% | Echo |
| Torres‐Macho 2012 | Europe (Spain) | High | Retrospective | Overall | 419 | 62.30% | RHC | |
| Vanhercke 2015 | Europe (Belgium) | High | Prospective | Overall | 401 | 69.80% | Echo | |
| Vanhercke 2015 | Europe (Belgium) | High | Prospective | HFrEF | HFrEF | 67.00% | Echo | |
| Vanhercke 2015 | Europe (Belgium) | High | Prospective | HFpEF | HFpEF | 73.00% | Echo | |
| VanWezenbeek 2022 | Europe (Netherlands) | High | Retrospective | HFpEF | HFpEF | 46 | 71.70% | RHC |
| Wang 2014 | Asia (China) | Upper‐middle | Retrospective | HFrEF requiring CRT | HFrEF | 183 | 50.30% | Echo |
| Zotter‐Tufaro 2015 | Europe (Austria) | High | Prospective | HFpEF | HFpEF | 174 | 85.10% | RHC |
Abbreviations: CKD = chronic kidney disease; CRT = cardiac resynchronisation therapy; fMR = functional mitral regurgitation; fTR = functional tricuspid regurgitation; PASP = pulmonary artery systolic pressure; TAPSE = tricuspid annular plane systolic excursion; TAVR = transcatheter aortic valve replacement.
The definitions and diagnostic methods for PH were highly heterogeneous. Thirty‐nine papers formally reported their definition for diagnosing PH [13, 16, 17, 18, 19, 20, 21, 25, 27, 28, 29, 30, 31, 32, 33, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58]. The majority of papers used pulmonary arterial pressure with cut‐offs ranging from > 25 to > 45 mmHg [16, 19, 20, 21, 25, 28, 29, 30, 31, 33, 35, 36, 37, 38, 40, 41, 42, 43, 44, 45, 46, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58]. Echocardiography [11, 15, 17, 18, 20, 26, 27, 29, 31, 32, 34, 35, 37, 39, 42, 45, 47, 48, 50, 51, 52, 53, 54, 56, 57, 59, 60, 61, 62, 63] RHC [13, 16, 19, 21, 25, 28, 30, 33, 36, 38, 40, 41, 43, 44, 46, 49, 55, 58] were the most commonly used methods for diagnosing PH in the included studies.
Generally, HF was more thoroughly assessed and defined, with the New York Heart Association classification or the Framingham criteria being the most used assessment tools. Seventeen papers focused exclusively on patients requiring hospitalization for HF [12, 14, 15, 19, 24, 25, 29, 32, 38, 39, 42, 48, 51, 54, 56, 59, 64]. Some studies calculated prevalence estimates partially and/or exclusively on special HF patient cohorts such as those with valvular disease [28, 49, 54, 59, 60], chronic kidney disease [26, 37], or hypertrophic cardiomyopathy [14, 16].
One study by Lima et al. focused exclusively on post‐partum women; although we did not specify pregnancy/post‐partum as an exclusion criterion in our protocol, we felt that this cohort did not represent the general HF population given its distinct pathophysiology, and thus excluded this study from the main analysis [22].
3.3. Primary Outcome
The overall PH prevalence amongst individuals with HF was 46.6% (95% CI: 39.6%–53.7%) based on the random effects model (Figure 2). The reported prevalence in the included studies ranges from 4.6% [61] to 93.9% [56] (Table 1, Figure 2). The former estimate arises from a retrospective analysis carried out in 130 hospitalized patients in India, whilst the latter is derived from a prospective study in 66 patients hospitalized with acute HF in Cameroon. An overall pooled estimate including Lima et al. is included in Supporting Information S1: Figure 1.
Figure 2.

A forest plot showing the overall prevalence of pulmonary hypertension amongst those in heart failure and the prevalence as per diagnostic method (echocardiography, right heart catheterization, and hospital‐recorded data).
3.4. Secondary Outcomes
There was significant variability in the prevalence ranges which is expected due to the different inclusion criteria and settings of the included studies. We found that the main driver of this observed heterogeneity was attributed to the diagnostic method used. Studies were grouped based on their diagnostic method, and a sensitivity analysis was performed using the common effects model (Figure 2, Supporting Information S1: Figure 2). Thirty studies used echo to report PH prevalence estimates (n = 19 861) [12, 16, 18, 19, 21, 27, 28, 30, 31, 32, 33, 35, 36, 38, 39, 41, 43, 46, 48, 49, 52, 53, 54, 55, 57, 59, 60, 62, 63, 64], 18 used right heart RHC (n = 5648) [14, 17, 20, 22, 26, 29, 34, 40, 42, 44, 45, 47, 50, 51, 56, 58, 61, 65], and 5 used hospital recorded data (n = 228 561) [11, 12, 13, 14, 15, 16] (Table 1, Figure 2). Studies using RHC reported the highest PH prevalence estimates (62.5%; 52.0%–72.0%) amongst those with HF and those using hospital‐recorded data reported the lowest prevalence estimates (18.4%; 14.4%–23.3%). Studies using echo reported prevalence estimates of 45.7% (37.1%–54.6%). Although there was a small overlap between CI for the estimates reported using echo and RHC, the difference observed between these estimates is statistically significant (p = 0.0169).
Subgroup analysis of PH prevalence amongst adults with HF by ejection fraction, continental region, country income classification, and study methodology was performed (Figure 3). Of the investigated factors, only study design (retrospective vs. prospective) showed a significant difference in the reported prevalence estimates. Prospective studies have higher overall PH prevalences (60.1%; 50.7%–68.8%) in comparison to retrospective studies (37.3%; 29.5%–45.9%) (Figure 3, Supporting Information S1: Figure 3). Of the papers included in this review, 30 are retrospective [11, 12, 13, 14, 15, 16, 17, 18, 19, 21, 23, 24, 25, 27, 29, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 59, 60, 61, 64], 21 are prospective [20, 25, 26, 30, 31, 32, 33, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 62, 63], and 1 study had retrospective and prospective subgroups [58] (Table 1, Supporting Information S1: Figure 3).
Figure 3.

A forest plot showing how the prevalence of pulmonary hypertension amongst those with heart failure varies with ejection fraction, location, country income classification, study type, and diagnostic method.
Analysis based on study design and diagnostic method is detailed in Supporting Information S1: Figure 4. Studies using echo and RHC to diagnose PH used a mix of retrospective and prospective methodologies, whilst studies using hospital recorded data were exclusively retrospective in nature (Table 1, Supporting Information S1: Figure 4). Prevalence estimates reported by prospective studies remained higher than estimates reported by retrospective studies, even when split by diagnostic method.
Ten and 15 papers include PH prevalence data on patients with HF with reduced ejection fraction (HFrEF) [13, 19, 24, 30, 34, 45, 48, 60, 62, 63] and/or HF with preserved ejection fraction (HFpEF) [17, 20, 21, 24, 27, 30, 31, 33, 34, 44, 48, 50, 53, 55, 57], respectively (Table 1, Supporting Information S1: Figure 5). PH prevalence was lower in patients with HFrEF (35.7%; 22.6%–51.3%) than HFpEF (47.2%; 34.8%–60.0%) (Figure 3, Supporting Information S1: Figure 5). Analysis based on subtype of HF and diagnostic methods are shown in Supporting Information S1: Figure 6.
When grouping the studies into continental regions, North America [11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 23, 24, 25, 26, 27] and Europe [29, 31, 32, 33, 34, 35, 40, 41, 43, 44, 46, 49, 50, 58, 59, 60, 63, 64] account for most of the papers (17), followed by Asia (12) [30, 36, 37, 38, 39, 42, 45, 48, 53, 54, 55, 61], Africa (5) [47, 51, 52, 56, 57], and South America (1) [62] (Table 1, Supporting Information S1: Figure 7). Studies from North America had the lowest calculated prevalence of PH in HF (33.3%; 23.9%–44.3%) and studies from Africa had the highest (61.0%; 29.5%–85.4%) (Figure 3, Supporting Information S1: Figure 7).
Fourty‐two studies were conducted in high income countries [11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 23, 24, 25, 26, 27, 28, 29, 31, 32, 33, 34, 35, 37, 38, 39, 40, 41, 42, 43, 44, 46, 48, 49, 50, 54, 55, 58, 59, 60, 63, 64], 5 in upper‐middle [30, 45, 53, 62, 65], 3 in lower‐middle [56, 57, 61], and 3 in low income countries [47, 51, 52] (Table 1, Supporting Information S1: Figure 8). The highest estimate of PH amongst those with HF came from low‐income countries (58.6%; 38.3%–76.4%) and the lowest estimates came from lower‐middle income countries (36.1%; 2.0%–94.1%) (Figure 3, Supporting Information S1: Figure 8).
3.5. Other Outcomes
Meta‐regression showed that diabetes is the only investigated factor which shows a significant association with PH prevalence (slope estimate: −3.12; p < 0.003) (Figure 4D). The remaining factors, including smoking and BMI, were not associated with PH prevalence in this meta‐regression analysis (Figure 4, Supporting Information S1: Figure 9).
Figure 4.
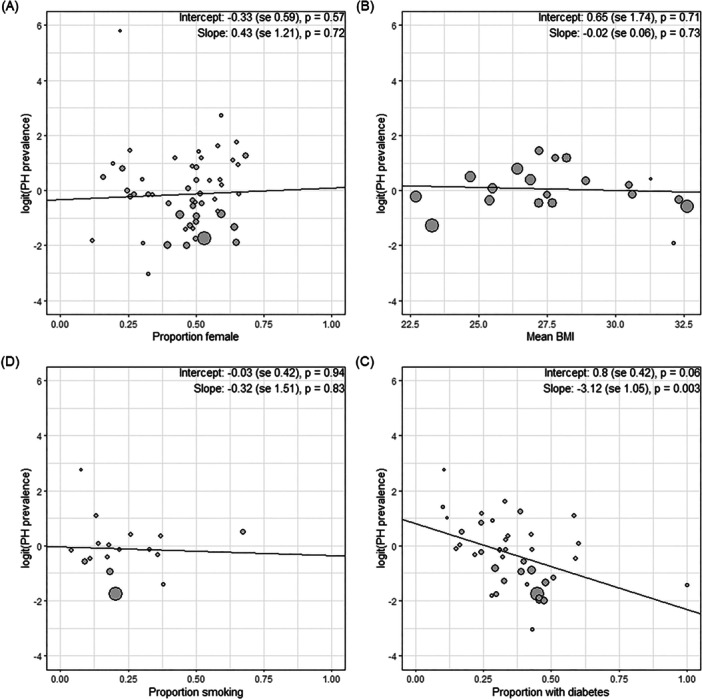
Meta‐regression analysis showing how the prevalence of pulmonary hypertension amongst those with heart failure varies with (A) proportion of female participants, (B) mean BMI, (D) proportion of participants who smoke, and (C) the proportion of participants who have diabetes.
4. Discussion
PH remains a serious complication of HF and is associated with a significant increase in morbidity and mortality. To the best of our knowledge, this is the first systematic review and meta‐analysis investigating the prevalence of PH in individuals with HF. We included 54 papers with 259 665 participants to calculate the global estimate of PH prevalence amongst those with HF. Overall, we show that the prevalence of PH amongst those with HF is strikingly high; approximately of individuals with HF are at risk of developing this complication. Compared to the general population, individuals with HF are nearly 20 times more likely to develop PH [66]. Given that PH can significantly worsen the prognosis in HF, it is of utmost importance that HF patients are carefully monitored to detect early signs of PH to enable prompt interventions.
4.1. Heterogeneity
The included studies were highly heterogeneous in their methodology and patient characteristics. Our subgroup analysis shows that studies which use RHC, are prospective in nature, or have a higher proportion of patients with HFpEF report higher PH prevalence estimates. We show that the PH diagnostic method (RHC, echo, and hospital‐recorded data) is the main contributor to heterogeneity, and this finding is corroborated by our sensitivity analysis performed when studies were split by diagnostic method. Studies using RHC report the highest prevalence estimates, followed by echo and then hospital recorded data, which reports the lowest estimates. In this review, echo was the most commonly used method of diagnosing PH. It should be noted that patients diagnosed by RHC have more severe HF than those diagnosed by echo, presenting a source of inherent bias reflected by the higher PH estimates calculated using RHC.
In this review, six papers used hospital‐recorded data and the majority of these studies relied on ICD‐10 codes to confirm the PH diagnosis. Although hospital‐recorded data tends to be less reliable, these studies used large patient cohorts and may be more representative of a general HF cohort.
Another notable factor affecting prevalence is study methodology. In general, prospective studies are likely to be more reliable as investigators have more control over data collection and standardization of disease diagnosis. By following participants over time, prospective studies can also provide more comprehensive and accurate data of PH prevalence in HF. However, perhaps due to their large time‐ and resource‐intensity, the number of individuals with the outcome of interest in prospective studies is often small.
In this meta‐analysis, we show that the prevalence estimate reported by prospective studies is significantly higher than those reported by retrospective studies. This may be partly due to differences in diagnostic methods used in retrospective and prospective studies (e.g. hospital‐recorded data are exclusively used in retrospective studies). However, this does not appear to fully explain the difference, as prospective studies report higher prevalence estimates even when grouped by diagnostic method. This finding is mirrored by Greges et al., the only paper included in this review which uses retrospective and prospective patient groups, albeit to a lesser extent [58]. Here, the authors used RHC to measure the prevalence of PH in HF and reported that the prevalence was lower in retrospective cohorts (65.3% compared to 78.5%).
4.2. Geographical Location
We show that the continent with the lowest reported PH prevalence rate is North America, which is also the continent contributing to the highest number of papers in the review. Africa shows the highest prevalence, although it has very few data points. These findings may reflect global differences in PH diagnostic practices and HF treatments. Interestingly, studies using RHC to diagnose PH were exclusively carried out in high and/or upper‐middle‐income countries. RHC remains the gold standard for diagnosing PH but may be inaccessible in certain locations due to its invasive nature and cost. Further studies investigating PH prevalence using the gold‐standard diagnostic methods are needed, particularly in low‐income countries.
4.3. Subtype of HF
Our results show that HFpEF is associated with higher PH prevalence compared to HFrEF. This fits in with the underlying pathophysiology of PH, as HFpEF may lead to increased left atrium pressure and subsequent pulmonary venous congestion. Furthermore, there are very limited treatment options for HFpEF in comparison to what is available for HFrEF [66]. This, coupled with the fact that individuals with HFpEF are reported to have higher survival rates, means that such patients may be more likely to develop the long‐term sequelae of HF, which include PH.
4.4. Diabetes
The results of our meta‐regression analysis contradict other studies, which report higher rates of PH in diabetic patients [67, 68, 69]. Our result that diabetes prevalence is negatively associated with PH prevalence is counterintuitive and can likely be explained by the heterogeneity of studies included in our analysis and the ecological biases of study‐level associations. It could also represent a lack of screening for PH in this population.
4.5. Strengths and Limitations
This review has numerous strengths. First, it systematically and comprehensively synthesizes the global prevalence of PH in individuals with HF. Second, our thorough subgroup analysis highlights how different factors contribute to the observed heterogeneity between study prevalence estimates. Our use of the random effects model appropriately accounts for study heterogeneity in our meta‐analysis and enables us to provide an accurate overall estimate of PH prevalence. Third, we have included the common effects as a sensitivity analysis in the Supporting Information S1: Figures.
There are several limitations to this review. The included papers use a variety of mean pulmonary artery pressure thresholds to define PH; 35 mmHg was the most common, but definitions ranged between 25 and 45 mmHg. Also, some participants who may have only had a transient increase in pulmonary pressures due to congestion may have been included in the studies, rather than those with true PH. Similarly, multiple criteria were used to report HF disease diagnosis and severity, including the Framingham criteria and European Society of Cardiology Guidelines [5, 70]. Furthermore, our meta‐regression is limited by study‐level associations and further work is needed to investigate risk factors associated with developing PH in those with HF. Our search yielded one randomized controlled trial which was TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial). Although this could represent a limitation in our search strategy, it more likely reflects the lack of PH assessment in HF trials. Future HF trials should investigate PH development as an outcome, given the increased mortality associated with developing this complication.
5. Conclusion
Overall, the prevalence of PH in individuals with HF reported in the 54 included papers is strikingly high, with an estimate of 45.4% (95% CI: 38.0%–52.9%). There was marked heterogeneity between studies included in this meta‐analysis, and this was largely driven by differences in PH diagnostic methods. Studies using RHC, which is the gold‐standard diagnostic method, report higher PH prevalence estimates (62.5%; 52.0%–72.0%). Studies using hospital‐recorded data report the lowest PH prevalence (15.7%; 10.4%–22.9%) and those using echo report estimates of 45.7% (37.1%–54.6%). Moving forward, future studies should focus on harmonization and standardization of the methodology, diagnostic techniques, and definitions used to improve the accuracy of PH estimates in individuals with HF. In addition to this, further work is needed to investigate the risk factors associated with developing PH amongst those with HF to identify patient subgroups who may benefit from early intervention.
Author Contributions
C.K. conceived and designed the study. J.S. carried out searches. J.S., N.D., M.K., R.S., and C.K. screened search results. J.S., M.K., and R.S. extracted data. C.K. and J.S. analyzed the data. M.K., R.S., J.S., A.P., and C.K. drafted the manuscript. All authors reviewed and edited the final draft. All authors had full access to all the data in the study and the final responsibility for the decision to submit for publication.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Supplementary information.
Supplementary Table 3. Complete data extracted from the included studies.
Acknowledgments
In memory of Eleftheria H. We would like to thank Nia Roberts (Bodelian Libraries, University of Oxford) for help with the search strategy.
Maaedah Khan and Rhea Suribhatla are joint first authors.
Data Availability Statement
All data are available as supplementary information.
References
- 1. Strange G., Playford D., Stewart S., et al., “Pulmonary Hypertension: Prevalence and Mortality in the Armadale Echocardiography Cohort,” Heart 98, no. 24 (December 2012): 1805–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rich S., Haworth S. G., Hassoun P. M., and Yacoub M. H., “Pulmonary Hypertension: The Unaddressed Global Health Burden,” Lancet Respiratory Medicine 6, no. 8 (August 2018): 577–579. [DOI] [PubMed] [Google Scholar]
- 3. “Mortality in Pulmonary Arterial Hypertension in the Modern Era: Early Insights From the Pulmonary Hypertension Association Registry,” PMC [Internet] , November 20, 2023, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9238604/. [DOI] [PMC free article] [PubMed]
- 4. Oldroyd S. H., Manek G., Sankari A., and Bhardwaj A., “Pulmonary Hypertension,” in StatPearls [Internet] (StatPearls Publishing, 2023), http://www.ncbi.nlm.nih.gov/books/NBK482463/. [PubMed] [Google Scholar]
- 5. McDonagh T. A., Metra M., Adamo M., et al., “2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure,” European Heart Journal 42, no. 36 (September 2021): 3599–3726. [DOI] [PubMed] [Google Scholar]
- 6. Hajouli S. and Ludhwani D., “Heart Failure and Ejection Fraction,” in StatPearls [Internet] (StatPearls Publishing, 2023), http://www.ncbi.nlm.nih.gov/books/NBK553115/. [PubMed] [Google Scholar]
- 7. Savarese G. and Lund L. H., “Global Public Health Burden of Heart Failure,” Cardiac Failure Review 03, no. 1 (April 2017): 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McMurray J. J. and Stewart S., “Heart Failure: Epidemiology, Aetiology, and Prognosis of Heart Failure,” Heart 83, no. 5 (May 2000): 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rao S. D., “Pulmonary Hypertension in Heart Failure Patients,” December 13, 2019, https://www.cfrjournal.com/articles/pulmonary-hypertension-heart-failure-patients.
- 10.“JBI Systematic Reviews. JBI Manual for Evidence Synthesis,” JBI Global Wiki [Internet], April 13, 2024, https://jbi-global-wiki.refined.site/space/MANUAL/355598371/1.+JBI+Systematic+Reviews.
- 11. Alqahtani F., Balla S., Almustafa A., Sokos G., and Alkhouli M., “Utilization of Palliative Care in Patients Hospitalized With Heart Failure: A Contemporary National Perspective,” Clinical Cardiology 42, no. 1 (2019): 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chakraborty S., Das S. K., Lorente‐Ros M., et al., “Impact of Pulmonary Hypertension in Patients With Hypertrophic Cardiomyopathy Presented With Cardiogenic Shock/Acute Decompensated Heart Failure,” Current Problems in Cardiology 47 (2022): 101251. [DOI] [PubMed] [Google Scholar]
- 13. Diaconu C. C., “Chronic Heart Failure: Diabetes Fuels a Worse Prognosis,” Archives of the Balkan Medical Union 50, no. 1 (2015): 5–8. [Google Scholar]
- 14. Lima F., Nie L., Yang J., et al., “Postpartum Cardiovascular Outcomes Among Women With Heart Disease From a Nationwide Study,” American Journal of Cardiology 123, no. 12 (2019): 2006–2014. [DOI] [PubMed] [Google Scholar]
- 15. Lutsey P. L., Evensen L. H., Thenappan T., et al., “Incidence and Risk Factors of Pulmonary Hypertension After Venous Thromboembolism: An Analysis of a Large Health Care Database,” Journal of the American Heart Association 11, no. 14 (2022): e024358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pandey A., Vaduganathan M., Arora S., et al., “Temporal Trends in Prevalence and Prognostic Implications of Comorbidities Among Patients With Acute Decompensated Heart Failure: The ARIC Study Community Surveillance,” Circulation 142, no. 3 (2020): 230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmadi A., Renaud J. M., Promislow S., et al., “Increased Myocardial Oxygen Consumption Rates Are Associated With Maladaptive Right Ventricular Remodeling and Decreased Event‐Free Survival in Heart Failure Patients,” Journal of Nuclear Cardiology 28, no. 6 (2021): 2784–2795. [DOI] [PubMed] [Google Scholar]
- 18. Butler J., Chomsky D. B., and Wilson J. R., “Pulmonary Hypertension and Exercise Intolerance in Patients With Heart Failure,” Journal of the American College of Cardiology 34, no. 6 (1999): 1802–1806. [DOI] [PubMed] [Google Scholar]
- 19. Choudhary G., Jankowich M., and Wu W. C., “Elevated Pulmonary Artery Systolic Pressure Predicts Heart Failure Admissions in African Americans: Jackson Heart Study,” Circulation: Heart Failure 7, no. 4 (2014): 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Covella M., Rowin E. J., Hill N. S., et al., “Mechanism of Progressive Heart Failure and Significance of Pulmonary Hypertension in Obstructive Hypertrophic Cardiomyopathy,” Circulation: Heart Failure 10, no. 4 (2017): e003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haddad F., Ataam J. A., Amsallem M., et al., “Insulin Growth Factor Phenotypes in Heart Failure With Preserved Ejection Fraction, an INSPIRE Registry and CATHGEN Study,” Journal of Cardiac Failure 28, no. 6 (2022): 935–946. [DOI] [PubMed] [Google Scholar]
- 22. Jentzer J. C., Wiley B. M., Reddy Y. N. V., Barnett C., Borlaug B. A., and Solomon M. A., “Epidemiology and Outcomes of Pulmonary Hypertension in the Cardiac Intensive Care Unit,” European Heart Journal. Acute Cardiovascular Care 11, no. 3 (2022): 230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khush K. K., Tasissa G., Butler J., McGlothlin D., and De Marco T., “Effect of Pulmonary Hypertension on Clinical Outcomes in Advanced Heart Failure: Analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) Database,” American Heart Journal 157, no. 6 (2009): 1026–1034. [DOI] [PubMed] [Google Scholar]
- 24. Lam C. S. P., Roger V. L., Rodeheffer R. J., Borlaug B. A., Enders F. T., and Redfield M. M., “Pulmonary Hypertension in Heart Failure With Preserved Ejection Fraction,” Journal of the American College of Cardiology 53, no. 13 (2009): 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leung C. C., Moondra V., Catherwood E., and Andrus B. W., “Prevalence and Risk Factors of Pulmonary Hypertension in Patients With Elevated Pulmonary Venous Pressure and Preserved Ejection Fraction,” American Journal of Cardiology 106, no. 2 (2010): 284–286. [DOI] [PubMed] [Google Scholar]
- 26. Raina A., Abraham W. T., Adamson P. B., Bauman J., and Benza R. L., “Limitations of Right Heart Catheterization in the Diagnosis and Risk Stratification of Patients With Pulmonary Hypertension Related to Left Heart Disease: Insights From a Wireless Pulmonary Artery Pressure Monitoring System,” Journal of Heart and Lung Transplantation 34, no. 3 (2015): 438–447. [DOI] [PubMed] [Google Scholar]
- 27. Selvaraj S., Shah S. J., Ommerborn M. J., et al., “Pulmonary Hypertension Is Associated With a Higher Risk of Heart Failure Hospitalization and Mortality in Patients With Chronic Kidney Disease: The Jackson Heart Study,” Circulation: Heart Failure 10, no. 6 (2017): e003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shah A. M., Shah S. J., Anand I. S., et al., “Cardiac Structure and Function in Heart Failure With Preserved Ejection Fraction: Baseline Findings From the Echocardiographic Study of the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial,” Circulation: In Heart failure 7, no. 1 (2014): 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pintalhao M., Castro‐Chaves P., Vasques‐Novoa F., et al., “Relaxin Serum Levels in Acute Heart Failure Are Associated With Pulmonary Hypertension and Right Heart Overload,” European Journal of Heart Failure 19, no. 2 (2017): 218–225. [DOI] [PubMed] [Google Scholar]
- 30. Huang L., Pang L., Gu Q., et al., “Prevalence, Risk Factors, and Survival Associated With Pulmonary Hypertension and Heart Failure Among Patients With Underlying Coronary Artery Disease: A National Prospective, Multicenter Registry Study in China,” Chinese Medical Journal 135, no. 15 (2022): 1837–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santas E., Palau P., Guazzi M., et al., “Usefulness of Right Ventricular to Pulmonary Circulation Coupling as an Indicator of Risk for Recurrent Admissions in Heart Failure With Preserved Ejection Fraction,” American Journal of Cardiology 124, no. 4 (2019): 567–572. [DOI] [PubMed] [Google Scholar]
- 32. Vanhercke D., Pardaens S., Weytjens C., et al., “Prevalence, Determinants, and Prognostic Significance of Pulmonary Hypertension in Elderly Patients Admitted With Acute Decompensated Heart Failure: A Report From the BIO‐HF Registry,” Echocardiography 32, no. 9 (2015): 1333–1338. [DOI] [PubMed] [Google Scholar]
- 33. Zotter‐Tufaro C., Duca F., Kammerlander A. A., et al., “Diastolic Pressure Gradient Predicts Outcome in Patients With Heart Failure and Preserved Ejection Fraction,” Journal of the American College of Cardiology 66, no. 11 (2015): 1308–1310. [DOI] [PubMed] [Google Scholar]
- 34. Raina A., Gertz Z. M., O'Donnell W. T., Herrmann H. C., and Forfia P. R., “Pulmonary Hypertension Is a Manifestation of Congestive Heart Failure and Left Ventricular Diastolic Dysfunction in Octogenarians With Severe Aortic Stenosis,” Pulmonary Circulation 5, no. 3 (2015): 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Allison S. J., Orton C. M., and Al‐Mohammad A., “Nonagenarians Presenting to the Diagnostic Heart Failure Clinic,” European Geriatric Medicine 7, no. 1 (2016): 28–33. [Google Scholar]
- 36. Lin Y., Pang L., Huang S., et al., “The Prevalence and Survival of Pulmonary Hypertension Due to Left Heart Failure: A Retrospective Analysis of a Multicenter Prospective Cohort Study,” Frontiers in Cardiovascular Medicine 9, no. 101653388 (2022): 908215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hsieh C., Lee C., Chen C., Hsu L., Hu H., and Wu J., “Pulmonary Hypertension in Patients on Chronic Hemodialysis and With Heart Failure,” Hemodialysis International 20, no. 2 (2016): 208–217. [DOI] [PubMed] [Google Scholar]
- 38. Ibe T., Wada H., Sakakura K., et al., “Pulmonary Hypertension Due to Left Heart Disease: The Prognostic Implications of Diastolic Pulmonary Vascular Pressure Gradient,” Journal of Cardiology 67, no. 6 (2016): 555–559. [DOI] [PubMed] [Google Scholar]
- 39. Lee J. H., Park J. H., Hwang I. C., Park J. J., and Park J. B., “Decreased Peak Left Atrial Longitudinal Strain Is Associated With Persistent Pulmonary Hypertension Associated With Left Heart Disease,” Journal of Clinical Medicine 11, no. 12 (2022): 3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mogollón M. V., Lage Gallé E., Hinojosa Pérez R., et al., “Prognosis After Heart Transplant in Patients With Pulmonary Hypertension Secondary to Cardiopathy,” Transplantation Proceedings 40, no. 9 (2008): 3031–3033. [DOI] [PubMed] [Google Scholar]
- 41. Sobieszczańska‐Małek M., Zieliński T., Piotrowski W., Korewicki J., and Of Polkard‐Hf O., “Prognostic Value of Pulmonary Hemodynamic Parameters in Cardiac Transplant Candidates,” Cardiology Journal 21, no. 5 (2014): 532–538. [DOI] [PubMed] [Google Scholar]
- 42. Stein G. Y., Kremer A., Shochat T., et al., “The Diversity of Heart Failure in a Hospitalized Population: The Role of Age,” Journal of Cardiac Failure 18, no. 8 (2012): 645–653. [DOI] [PubMed] [Google Scholar]
- 43. Torres‐Macho J., Delgado‐Jiménez J. F., Sanz‐Salvo J., et al., “Predictors of Pulmonary Hypertension in Patients With End‐Stage Heart Failure,” Congestive Heart Failure 18, no. 4 (2012): 212–216. [DOI] [PubMed] [Google Scholar]
- 44. Van Wezenbeek J., Kianzad A., Van De Bovenkamp A., et al., “Right Ventricular and Right Atrial Function Are Less Compromised in Pulmonary Hypertension Secondary to Heart Failure With Preserved Ejection Fraction: A Comparison With Pulmonary Arterial Hypertension With Similar Pressure Overload,” Circulation: Heart Failure 15, no. 2 (2022): E008726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang J., Su Y., Bai J., Wang W., Qin S., and Ge J., “Elevated Pulmonary Artery Pressure Predicts Poor Outcome After Cardiac Resynchronization Therapy,” Journal of Interventional Cardiac Electrophysiology 40, no. 2 (2014): 171–178. [DOI] [PubMed] [Google Scholar]
- 46. Ahmed S., Ahmed A., Bouzina H., Lundgren J., and Rådegran G., “Elevated Plasma Endocan and BOC in Heart Failure Patients Decrease After Heart Transplantation in Association With Improved Hemodynamics,” Heart and Vessels 35, no. 11 (2020): 1614–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Amadi V. N., Ajayi O. E., Akintomide A. O., Abiodun O. O., Bamikole O. J., and Balogun M. O., “Pulmonary Hypertension in Heart Failure Patients Presenting at OAUTHC, Ile‐Ife, Nigeria,” Clinical Medicine Insights Cardiology 10 (2016): 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bosch L., Lam C. S. P., Gong L., et al., “Right Ventricular Dysfunction in Left‐Sided Heart Failure With Preserved Versus Reduced Ejection Fraction,” European Journal of Heart Failure 19, no. 12 (2017): 1664–1671. [DOI] [PubMed] [Google Scholar]
- 49. Faggiano P., Antonini‐Canterin F., Ribichini F., et al., “Pulmonary Artery Hypertension in Adult Patients With Symptomatic Valvular Aortic Stenosis,” American Journal of Cardiology 85, no. 2 (2000): 204–208. [DOI] [PubMed] [Google Scholar]
- 50. Guazzi M., Dixon D., Labate V., et al., “RV Contractile Function and Its Coupling to Pulmonary Circulation in Heart Failure With Preserved Ejection Fraction: Stratification of Clinical Phenotypes and Outcomes,” JACC Cardiovascular Imaging 10, no. 10 (2017): 1211–1221. [DOI] [PubMed] [Google Scholar]
- 51. Karaye K., Saidu H., Bala M., and Yahaya I., “Prevalence, Clinical Characteristics and Outcome of Pulmonary Hypertension Among Admitted Heart Failure Patients,” Annals of African Medicine 12, no. 4 (2013): 197–204. [DOI] [PubMed] [Google Scholar]
- 52. Kushimo O., Mbakwem A., Ajuluchukwu J., and Amadi C., “Clinical and Echocardiographic Correlates of Pulmonary Hypertension Among Heart Failure Patients in Lagos, South‐Western Nigeria,” Cardiovascular Journal of Africa 30, no. 1 (2019): 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu Y.‐X., Li H., Xia Y.‐Y., et al., “Left Atrial Diameter and Atrial Fibrillation, but Not Elevated NT‐proBNP, Predict the Development of Pulmonary Hypertension in Patients With HFpEF,” Journal of Geriatric Cardiology: JGC 17, no. 7 (2020): 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mutlak D., Lessick J., Khalil S., Yalonetsky S., Agmon Y., and Aronson D., “Tricuspid Regurgitation in Acute Heart Failure: Is There Any Incremental Risk?,” European Heart Journal‐Cardiovascular Imaging 19, no. 9 (2018): 993–1001. [DOI] [PubMed] [Google Scholar]
- 55. Nakamura T., Uematsu M., Deyama J., et al., “Pulmonary Vascular Resistance Is Associated With Brachial‐Ankle Pulse‐Wave Velocity and Adverse Clinical Outcomes in Patients With Heart Failure With Preserved Ejection Fraction,” Journal of Cardiac Failure 25, no. 9 (2019): 725–732. [DOI] [PubMed] [Google Scholar]
- 56. Nkoke C., Jingi A. M., Noubiap J. J., and Dzudie A., “Pulmonary Hypertension Among Patients Hospitalized With Acute Heart Failure in Buea‐South West Region of Cameroon,” Pan African Medical Journal 42 (2022): 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rifaie O., El‐Damanhory H., Amr M., and Nammas W., “Prevalence and Predictors of Pulmonary Hypertension in Elderly Patients With Isolated Diastolic Heart Failure,” Kardiologia Polska 68, no. 6 (2010): 655–661. [PubMed] [Google Scholar]
- 58. Gerges M., Gerges C., Pistritto A. M., et al., “Pulmonary Hypertension in Heart Failure. Epidemiology, Right Ventricular Function, and Survival,” American Journal of Respiratory and Critical Care Medicine 192, no. 10 (2015): 1234–1246. [DOI] [PubMed] [Google Scholar]
- 59. Arnaert S., De Meester P., Troost E., et al., “Heart Failure Related to Adult Congenital Heart Disease: Prevalence, Outcome and Risk Factors,” ESC Heart Failure 8, no. 4 (2021): 2940–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Auffret V., Bakhti A., Leurent G., et al., “Determinants and Impact of Heart Failure Readmission Following Transcatheter Aortic Valve Replacement,” Circulation Cardiovascular Interventions 13, no. 7 (2020): e008959. [DOI] [PubMed] [Google Scholar]
- 61. Kanumuri S., Jonnalagadda N., Garre I., and Jyotsna M., “Predictors of In‐Hospital Events for Patients With Nonischemic Acute Heart Failure,” Indian Journal of Cardiovascular Disease in Women WINCARS 04, no. 4 (2019): 195–199. [Google Scholar]
- 62. Nakagawa N. K., Diz M. A., Kawauchi T. S., et al., “Risk Factors for Inspiratory Muscle Weakness in Chronic Heart Failure,” Respiratory Care 65, no. 4 (2020): 507–516. [DOI] [PubMed] [Google Scholar]
- 63. Straburzyńska‐Migaj E., Szyszka A., Trojnarska O., and Cieśliński A., “Restrictive Filling Pattern Predicts Pulmonary Hypertension and Is Associated With Increased Bnp Levels and Impaired Exercise Capacity in Patients With Heart Failure,” Kardiologia Polska 65, no. 9 (2007): 1049–1055. [PubMed] [Google Scholar]
- 64. Benito‐González T., Freixa X., Godino C., et al., “Ventricular Arrhythmias in Patients With Functional Mitral Regurgitation and Implantable Cardiac Devices: Implications of Mitral Valve Repair With Mitraclip,” Annals of Translational Medicine 8, no. 15 (2020): 956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lin D., Li X., Yang H., et al., “Alcohol Intoxication and Sexual Risk Behaviors Among Rural‐to‐Urban Migrants in China,” Drug and Alcohol Dependence 79, no. 1 (2005): 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lam C. S. P., Gamble G. D., Ling L. H., et al., “Mortality Associated With Heart Failure With Preserved vs. Reduced Ejection Fraction in a Prospective International Multi‐Ethnic Cohort Study,” European Heart Journal 39, no. 20 (May 2018): 1770–1780. [DOI] [PubMed] [Google Scholar]
- 67. Takahashi T., Yoshihisa A., Sugimoto K., et al., “Associations Between Diabetes Mellitus and Pulmonary Hypertension in Chronic Respiratory Disease Patients,” PLoS One 13, no. 10 (October 2018): e0205008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Abernethy A. D., Stackhouse K., Hart S., et al., “Impact of Diabetes in Patients With Pulmonary Hypertension,” Pulmonary Circulation 5, no. 1 (March 2015): 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lopez‐Lopez J. G., Moral‐Sanz J., Frazziano G., et al., “Diabetes Induces Pulmonary Artery Endothelial Dysfunction by NADPH Oxidase Induction,” American Journal of Physiology‐Lung Cellular and Molecular Physiology 295, no. 5 (November 2008): L727–L732. [DOI] [PubMed] [Google Scholar]
- 70. Mahmood S. S. and Wang T. J., “The Epidemiology of Congestive Heart Failure: The Framingham Heart Study Perspective,” Global Heart 8, no. 1 (March 2013): 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Supplementary Table 3. Complete data extracted from the included studies.
Data Availability Statement
All data are available as supplementary information.


