Abstract
In Europe the use of the growth promoter avoparcin is considered to have selected for vancomycin-resistant enterococci (VRE). Sweden ceased using avoparcin in 1986, and only occasional cases of VRE from hospitals have been reported since 1995. Within the framework of a European study, samples from urban raw sewage, treated sewage, surface water, and hospital sewage in Sweden (n = 118) were screened for VRE. Surprisingly, VRE were isolated from 21 of 35 untreated sewage samples (60%), from 5 of 14 hospital sewage samples (36%), from 6 of 32 treated sewage samples (19%), and from 1 of 37 surface water samples. Thirty-five isolates from 33 samples were further characterized by geno- and phenotyping, MIC determination, and PCR analysis. Most isolates (30 of 35) carried the vanA gene, and the majority (24 of 35) of the isolates were Enterococcus faecium. Most of the VRE were multiresistant. The typing revealed high diversity of the isolates. However, one major cluster with seven identical or similar isolates was found. These isolates came from three different sewage treatment plants and were collected at different occasions during 1 year. All VRE from hospital sewage originated from one of the two hospitals studied. That hospital also had vancomycin consumption that was 10-fold that of the other. We conclude that VRE were commonly found in sewage samples in Sweden. The origin might be both healthy individuals and individuals in hospitals. Possibly, antimicrobial drugs or chemicals released into the sewage system may sustain VRE in the system.
Enterococci are members of the normal gut flora of animals and humans and are thus released into the environment directly or via sewage outlets, where they can survive for long time periods. Their role in nosocomial infections has increased due to their ability to acquire high-level resistance to antimicrobial agents, which makes them difficult to treat (24). During the last decade the concern has been focused on enterococci that are resistant to the glycopeptide antibiotic vancomycin (vancomycin-resistant enterococci [VRE]). Until now vancomycin has been the drug of last resort against drug-resistant enterococci and methicillin-resistant staphylococci. Two resistance genotypes, vanA and vanB, are considered to be of main importance, since these resistance genes are transferable and since the genes confer high-level resistance to vancomycin (4 to 1,000 μg/ml). The vanA genotype also confers high-level resistance to teicoplanin (3).
There is an epidemiological difference between the occurrences of VRE in the United States and in Europe. In the United States clones of VRE have spread within and between hospitals (9, 30), but VRE among nonhospitalized humans have so far not been reported. Thus, VRE are thought to have evolved and spread due to the heavy antibiotic use in hospitals, a theory that the recent reported recovery of VRE of the vanA genotype from hospital sewage strengthens (14). In contrast, studies from European countries report a high prevalence of VRE, mainly Enterococcus faecium of the vanA genotype, among nonhospitalized individuals, among farmers, among farm animals, in meat products, and in sewage treatment plants (2, 4, 5, 7, 19, 35). Genetic fingerprinting of European nosocomial VRE isolates has often shown polyclonality, indicating the import of various vanA strains from the community (6, 10). The use of the glycopeptide antibiotic avoparcin as a feed additive in animal husbandry is assumed to have created a pool of VRE in the community (1, 5, 18). This assumption led to a ban of the use of avoparcin within the European Union in 1997. In Sweden, the usage of antibiotics as feed additives was banned in 1986. Thus, if the assumptions that usage of avoparcin has selected for VRE among farm animals and that spread to humans occurs mostly via the food chain are true, VRE should not be common in Sweden. Antibiotic use in Swedish hospitals is restrictive, and vancomycin is used only for treatment of serious human infections caused by multiresistant gram-positive bacteria, such as methicillin-resistant staphylococci or multiresistant enterococci, and enterocolitis caused by Clostridium difficile (25).
The first VRE in Sweden was reported in 1995 (23, 28), and in a national investigation of the human fecal flora in 1999, VRE of the vanB genotype were isolated from 9 of 841 patients, all in the same hospital, and from only 1 of 670 healthy individuals (33). Another investigation from 1997 reported the first small hospital outbreak of VRE of the vanA genotype, involving three patients (34). In 2000, 20 VRE infections in humans were reported to the Swedish Institute for Infectious Disease Control (31). In comparison, as many as 1.8% of nonhospitalized individuals and 8.6% of hematology patients carried VRE of the vanA genotype in a cattle-rearing region in France in 1997 (10). In the same year 23% of the tested enterococci in intensive care units in the United States were resistant to vancomycin (27).
The present study formed part of a multinational study in which one of the aims was to compare the prevalences of VRE in different European countries. Screening for VRE in fecal samples is a cumbersome procedure, especially when the prevalence is low. An alternative way to screen for VRE is to search in urban or hospital sewage samples. Sewage contains high numbers of bacteria, mainly of human fecal origin, and has the advantages that samples are easy to obtain and that the samples consist of a mixture of fecal flora of many individuals. One sample could thus be regarded as a pooled sample of many fecal samples. In the present study we used this approach to screen for VRE in the Stockholm and Uppsala areas of Sweden.
MATERIALS AND METHODS
Collection of samples.
Samples were collected eight times during the time period from October 1998 to February 2000 from four different urban sewage treatment plants in the areas of Stockholm and Uppsala. Samples were also collected once in each of three small urban sewage treatment plants in the surroundings of Stockholm. At the larger plants, flow proportional sampling was carried out on incoming raw sewage (n = 32) and on outgoing treated sewage (n = 29). Samples from the smaller plants (n = 6) were collected directly in sterile 1-liter bottles.
Samples from surface water (n = 37) were collected at several locations in the Stockholm archipelago, which is the recipient for treated sewage in Stockholm, and from the river Fyrisån, which is the recipient for treated sewage in Uppsala. The samples were collected at depths of 5 to 10 cm in sterile 1-liter bottles.
Sewage from hospitals (n = 14) was collected seven times from the sewage outlets of the two major hospitals in the city of Stockholm. Samples were collected directly in sterile 1-liter glass bottles.
All samples were kept refrigerated and analyzed within 6 h.
Isolation of vancomycin-resistant enterococci.
Samples from surface water were membrane filtered directly through 0.45-μm-pore-size membrane filters (Millipore Corporation, Bedford, Mass.), while sewage samples were subject to serial 10-fold dilutions with phosphate-buffered saline before filtration.
The membrane filters were put on brain heart infusion agar (Becton Dickinson, Sparks, Md.) plates and preincubated for 2 h at 37°C before they were transferred to m Enterococcus agar (Becton Dickinson) supplemented with 8 mg of vancomycin per liter (SB-8V). Membrane filters were also transferred to m Enterococcus agar without supplement in order to determine the total number of enterococci in each sample. Agar plates were incubated for 48 h at 37°C, whereupon membranes showing distinct colony growth were transferred to prewarmed bile esculin agar (Becton Dickinson) plates and incubated for 2 h at 44°C. Black colonies were tested for catalase activity. The total number of enterococci was recorded, and presumed VRE were defined as the isolates that grew on SB-8V, grew at 44°C, and were esculin positive and catalase negative. Those isolates were further analyzed.
Vancomycin enrichment was done on 10 ml of the undiluted samples mixed with 10 ml of enrichment medium consisting of BBL Enterococcosel broth (Becton Dickinson) supplemented with vancomycin to a final concentration of 8 mg/liter. These enrichment cultures were incubated for 24 h at 37°C, whereupon 10 μl from the enrichment broth was spread on SB-8V and treated as described above.
Phenotyping with the PhenePlate system.
The PhenePlate rapid screening system for enterococci (PhP-RF plates; PhPlate Microplate Techniques AB, Stockholm, Sweden) was used for preliminary typing of isolates (21). The PhP-RF plates contain eight sets of the following chemicals, which were selected for optimal discrimination among enterococci: l-arabinose, lactose, melibiose, d-melezitose, d-raffinose, myo-inositol, d-sorbitol, d-mannitol, d-galactonic acid γ-lactone, d-amygdalin, and d-gluconate. Up to eight colonies from each of both isolation methods for VRE (direct plating and vancomycin enrichment) were picked for PhP typing. Preliminary species identification was done by comparing the PhP patterns of unknown isolates to the patterns in a reference database consisting of known strains of various Enterococcus species (26). Species identification was confirmed by PCR (see below). The similarity between all isolates was calculated and illustrated with a dendrogram by using the PhP software (PhPlate Microplate Techniques AB).
Most samples yielded only one PhP type among isolates that grew in the presence of vancomycin. However, in two samples more than one PhP type was obtained. One enterococcal isolate representing each vancomycin resistance type from each sample was saved in glycerol broth at −70°C for further studies.
Finally, all saved isolates were typed with the PhP-FS plate, which is a more discriminatory phenotyping system using 23 different reagents (20).
Antimicrobial susceptibility testing.
MICs were determined by broth microdilution (VetMic system; National Veterinary Institute, Uppsala, Sweden) for the selected isolates (see above) with the following antimicrobial agents: vancomycin, ampicillin, erythromycin, tetracycline, virginiamycin, and avilamycin. The tests were performed according to the recommendations of the NCCLS, with the inclusion of relevant American Type Culture Collection control strains (29). When the MIC of vancomycin was >16 mg/liter, isolates were further analyzed.
Genetic analysis.
Detection of the resistance genotypes vanA and vanB and identification of E. faecalis and E. faecium to the species level were done by PCR, as described by Dutka-Malen et al. (8).
Genotyping with PFGE.
All VRE were genotyped in order to verify clonal relationships. DNA purification and enzyme digestion for pulsed-field gel electrophoresis (PFGE) analysis of the isolates were performed as previously described (17), using SmaI as a restriction enzyme. Electrophoresis was performed with a CHEF-DR III system (Bio-Rad Laboratories, Hercules, Calif.), using 1.2 or 1.4% agarose (SeaKem) in 0.5× Tris-borate-EDTA at 180 V. Running conditions consisted of two phases used in sequence. Phase 1 was 2 to 8 s with a run time of 20 h. Phase 2 was 8 to 22 s with a run time of 20 h (17). Reconstructed PFGE gel images were prepared using the Gel Compar software, version 4.0 (Applied Maths, Sint-Martens-Latem, Belgium).
RESULTS
Prevalence of VRE.
VRE were isolated from 33 of the 118 sewage and water samples studied. VRE were most commonly isolated from raw sewage (21 of 35 samples), followed by hospital sewage (5 of 14 samples), treated sewage (6 of 32 samples), and surface water (1 of 37 samples) (Table 1). In seven samples VRE were isolated directly from the membrane filter, normally in low numbers (<100 CFU/100 ml), but in most cases VRE were found only after enrichment in vancomycin broth (Fig. 1).
TABLE 1.
Prevalence of VRE in different samples from Sweden
| Sample | No. of samples analyzed | No. of samples with VRE | Prevalence of VRE (%) |
|---|---|---|---|
| Raw sewage | 35 | 21 | 60 |
| Treated sewage | 32 | 6 | 19 |
| Surface water | 37 | 1 | 3 |
| Hospital sewage | 14 | 5 | 36 |
| Total | 118 | 33 |
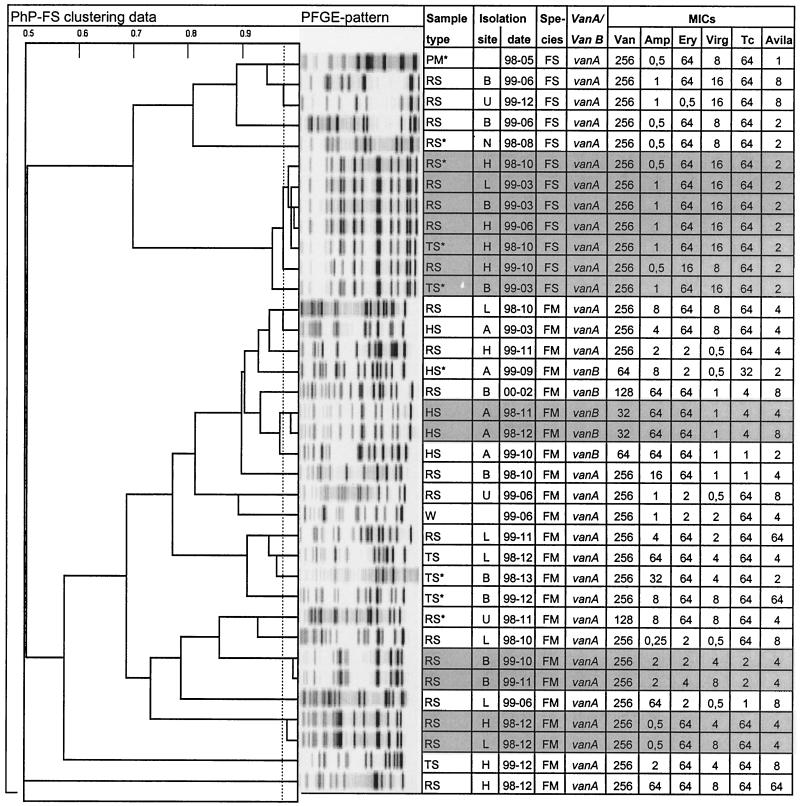
FIG. 1.
Dendrogram showing UPGMA clustering of PhenePlate typing data for Swedish VRE isolated from raw sewage (RS), treated sewage (TS), hospital sewage (HS), and surface water (W) and for the only isolate from animal husbandry, which was found in pig manure (PM) (n = 54) (the PM data are presented only in this figure and are not discussed elsewhere in this publication). An asterisk indicates that the isolate was isolated directly from the membrane filter, in contrast to the other isolates, which were isolated after enrichment in vancomycin broth. The corresponding PFGE patterns of SmaI-digested DNA for each isolate, isolation site and date (year-month), species (FS, E. faecalis; FM, E. faecium), vancomycin resistance genotype (vanA or vanB), and susceptibility to vancomycin (Van), ampicillin (Amp), erythromycin (Ery), virginiamycin (Virg), tetracycline (Tc), and avilamycin (Avila) are also shown. The horizontal axis in the dendrogram shows the similarities between the isolates, and the dotted line indicates that the identity level is set at 0.975. Grey shading indicates identical or highly similar isolates.
VRE were found on several occasions in raw sewage in all four large sewage treatment plants and also in one of the small plants. VRE were recovered from treated sewage in all three large plants in Stockholm but not in that from Uppsala. In one of the two hospitals studied, VRE were isolated on five of seven sampling occasions, compared to none of seven in the other hospital (Fig. 1). The total numbers of enterococci were 103 to 104 per ml in raw sewage and in hospital sewage and 101 to 102 per ml in treated sewage and in surface waters. Samples with and without VRE showed no difference in the total number of enterococci (data not shown).
Characterization of VRE isolates.
According to PhP typing, 2 of the 33 samples contained two different types of VRE, resulting in 35 isolates that were further analyzed. Thirty isolates were of the vanA genotype (86%), and 5 were of the vanB genotype (14%). Twenty-four of the isolates were E. faecium (69%), and 11 were E. faecalis (31%). All five isolates from hospital sewage were E. faecium and four of them were of the vanB genotype.
There was total agreement between the genotyping and the phenotyping results, showing a high diversity for the isolates (diversity index = 0.97), indicating that the isolates belonged to several clonal groups (Fig. 1). However, one major cluster was observed. This cluster contained seven E. faecalis isolates of identical or similar PhP type, PFGE type, and resistance phenotype from three different sewage treatment plants. Four of these seven isolates originated in the same sewage treatment plant (plant H) but were isolated on three different occasions over 1 year. On one occasion this strain was found both in incoming raw sewage and in outgoing treated sewage. Some other pairs of isolates that gave identical typing results and showed similar antimicrobial susceptibility patterns were also found (Fig. 1), i.e., two isolates from plant B isolated 1 month apart, two sewage isolates from plants H and L isolated on the same occasion, and two isolates from the hospital sewage isolated 1 month apart.
The majority of the VRE isolates (27 of 35) were resistant to at least two of the tested antimicrobial agents besides vancomycin (Fig. 1). Of the 24 vancomycin-resistant E. faecium isolates, 67% were resistant to erythromycin (MIC, >4 mg/liter), 38% were resistant to ampicillin (MIC, >8 mg/liter), 29% were resistant to virginiamycin (MIC, >4 mg/liter), and 12% were resistant to avilamycin (MIC, >8 mg/liter). Of the 11 E. faecalis strains, 91% were resistant to erythromycin and 100% were resistant to tetracycline (MIC, >8 mg/liter), whereas all were sensitive to ampicillin and avilamycin.
DISCUSSION
The high prevalence of vancomycin and multiresistant enterococci in Swedish sewage was unexpected in the light of the low prevalence among Enterococcus spp. isolated from production animals and from humans in hospitals and the community (12, 33, 35, 36). The source or origin(s) of these strains is unclear.
One explanation of the high prevalence of VRE in the present study could be the use of an enrichment method, i.e., broth supplemented with vancomycin, for isolation of VRE. This method detects VRE in very low concentrations, which is of importance for the kind of samples that were assayed in this study (water and sewage). Gambarotto et al. used the same procedure and reported that 75% of the 65 recovered VRE from feces would have been missed if the isolation by vancomycin enrichment had been excluded (10).
Our typing of the isolates revealed a high diversity, indicating multiple origins. However, the finding of identical VRE from some different locations implies that a clonal spread of certain strains could have occurred or that certain strains are more prone to acquire resistance. The finding of identical isolates in the same sewage treatment plant but at different sampling occasions indicates that strains are persisting in the sewage system or leaking from a reservoir, e.g., a hospital.
Nine (three from hospital sewage and six from raw and treated sewage) of the 24 vancomycin-resistant E. faecium isolates in the present study (38%) were resistant to ampicillin. In Sweden, the selection pressure for vancomycin- as well as ampicillin-resistant enterococci in animal production is low, since glycopeptides have not been used since 1986 and since the use of ampicillin is sparse (11). Thus, enterococci resistant to ampicillin are rarely isolated from farm animals in Sweden (<1%) (36) and are also quite rare among healthy humans (6%) (33). However, ampicillin resistance is more common among E. faecium isolates from hospitalized patients (22%) (32). A majority of ampicillin-resistant E. faecium isolates from Swedish hospitalized patients have recently been shown to belong to one clonal group, showing a unique PhP pattern that was rare among ampicillin-resistant enterococci isolated outside the hospital environment (E. Torell, I. Kühn, B. Olsson-Liljequist, B. M. Hoffman, S. Haeggman, C. Lindahl, and L. G. Burman, unpublished data). Seven of the nine vancomycin- and ampicillin-resistant isolates in the present study showed PhP patterns identical or similar to that of this clonal group. Since the sewage from hospitals normally is led to community treatment plants and mixed in the pipelines with urban sewage, it is not impossible that we have recovered VRE from hospitals in the community treatment plants.
There was a noticeable difference in vancomycin usage between the two hospitals studied. During the time of the present study, hospital A (1,000 beds) purchased 10 times as much vancomycin as hospital C (500 beds) (2,918 defined daily doses [1 defined daily dose = 2 g] for hospital A and 292 defined daily doses for hospital C [National Corporation of Swedish Pharmacies, personal communications]). The fact that VRE were found only in sewage from hospital A and not in that from hospital C indicates that the amount of vancomycin used within the hospitals influences the prevalence of VRE in the hospital sewage. This could happen through selection of VRE in the intestinal flora of the patients, or, probably less likely, it could be due to excreted drugs in the sewage system. However, it has recently been reported that the predicted concentrations of antibiotics in hospital effluents are in the range of semi-maximum inhibitory concentrations for sensitive bacteria, from which it follows that the development of resistance in biological films cannot be excluded (22).
Most Swedish VRE isolated from hospitalized patients have been E. faecium carrying the vanB gene (13, 33), and ampicillin resistance is common in clinical isolates of E. faecium (74%) (13), which fits with our observation of the isolates from hospital sewage, where four of five isolates carried the vanB gene and four of five isolates were ampicillin resistant. On the other hand, 11 of 29 VRE from urban sewage were E. faecalis and 28 were vanA, which indicates that VRE in urban sewage may be of origins other than hospitals. VRE may, e.g., be harbored by healthy carriers in the community to a greater extent than earlier reported and/or may increase in number in the sewage system. Sewage constitutes a favorable environment, consisting of variable mixtures of bacteria, nutrients, and antimicrobial agents (15) that might promote growth of resistant bacteria or gene transfer between bacteria in the sewage or in the biofilm lining the pipelines.
E. faecalis is more common in nosocomial infections than E. faecium, but E. faecium has a greater ability to acquire drug resistance. This has enabled multiresistant E. faecium to emerge as a severe nosocomial pathogen worldwide, while E. faecalis has remained sensitive to at least one effective antibiotic (16). According to our data, 31% of the Swedish VRE were E. faecalis. This could imply a greater risk for nosocomial infections with VRE. No other investigation has reported such a high prevalence of vancomycin-resistant E. faecalis.
In conclusion, Swedish sewage samples contained VRE more often than was expected, which in turn indicates a higher prevalence of VRE both in hospitals and in the community than earlier reported. Nosocomial infections with VRE are still rare in Sweden, and with the knowledge that VRE exist in the community and in hospitals suitable measures could prevent their further spread into the hospital sphere.
Acknowledgments
Thanks are due to the staff at Stockholm Vatten, Locum in Stockholm, and Kungsängsverket in Uppsala for assistance in the collection of samples, to the Danish Veterinary Laboratory for doing the PFGE typing, and to Maria Finn and Verena Rehbinder at the Swedish National Veterinary Institute for performing PCR and antimicrobial susceptibility testing.
This work was supported by European Commission grant FAIR5-CT97-3709 and Swedish Council for Agricultural and Forestry Research grant 722.1233/97
REFERENCES
- 1.Aarestrup, F. M. 1995. Occurrence of glycopeptide resistance among Enterococcus faecium isolates from conventional and ecological poultry farms. Microb. Drug Resist. Mech. Epidemiol. Dis. 1:255-257. [DOI] [PubMed] [Google Scholar]
- 2.Aarestrup, F. M., P. Ahrens, M. Madsen, L. V. Pallesen, R. L. Poulsen, and H. Westh. 1996. Glycopeptide susceptibility among Danish Enterococcus faecium and Enterococcus faecalis isolates of animal and human origin and PCR identification of genes within the vanA cluster. Antimicrob. Agents Chemother. 40:1938-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur, M., and P. Courvalin. 1993. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob. Agents Chemother. 37:1563-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates, J. 1997. Epidemiology of vancomycin-resistant enterococci in the community and the relevance of farm animals to human infection. J. Hosp. Infect. 37:89-101. [DOI] [PubMed] [Google Scholar]
- 5.Bates, J., Z. Jordens, and J. B. Selkon. 1993. Evidence for an animal origin of vancomycin-resistant enterococci. Lancet 342:490-491. [DOI] [PubMed] [Google Scholar]
- 6.Bonten, M. J., S. Slaughter, M. K. Hayden, C. Nathan, J. van Voorhis, and R. A. Weinstein. 1998. External sources of vancomycin-resistant enterococci for intensive care units. Crit. Care Med. 26:2001-2004. [DOI] [PubMed] [Google Scholar]
- 7.Chadwick, P. R., N. Woodford, E. B. Kaczmarski, S. Gray, R. A. Barrell, and B. A. Oppenheim. 1996. Glycopeptide-resistant enterococci isolated from uncooked meat. J. Antimicrob. Chemother. 38:908-909. [DOI] [PubMed] [Google Scholar]
- 8.Dutka-Malen, S., S. Evers, and P. Courvalin. 1995. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 33:24-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frieden, T. R., S. S. Munsiff, D. E. Low, B. M. Willey, G. Williams, Y. Faur, W. Eisner, S. Warren, and B. Kreiswirth. 1993. Emergence of vancomycin-resistant enterococci in New York City. Lancet 342:76-79. [DOI] [PubMed] [Google Scholar]
- 10.Gambarotto, K., M. C. Ploy, P. Turlure, C. Grelaud, C. Martin, D. Bordessoule, and F. Denis. 2000. Prevalence of vancomycin-resistant enterococci in fecal samples from hospitalized patients and nonhospitalized controls in a cattle-rearing area of France. J. Clin. Microbiol. 38:620-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grave, K., C. Greko, L. Nilsson, K. Odensvik, T. Mork, and M. Ronning. 1999. The usage of veterinary antibacterial drugs for mastitis in cattle in Norway and Sweden during 1990-1997. Prev. Vet. Med. 42:45-55. [DOI] [PubMed] [Google Scholar]
- 12.Greko, C. 1999. Antibiotics as growth promoters. Acta Vet. Scand. Suppl. 92:87-100. [PubMed] [Google Scholar]
- 13.Hallgren, A., H. Abednazari, C. Ekdahl, H. Hanberger, M. Nilsson, A. Samuelsson, E. Svensson, and L. E. Nilsson. 2001. Antimicrobial susceptibility patterns of enterococci in intensive care units in Sweden evaluated by different MIC breakpoint systems. J. Antimicrob. Chemother. 48:53-62. [DOI] [PubMed] [Google Scholar]
- 14.Harwood, V. J., M. Brownell, W. Perusek, and J. E. Whitlock. 2001. Vancomycin-resistant Enterococcus spp. isolated from wastewater and chicken feces in the United States. Appl. Environ. Microbiol. 67:4930-4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch, R., T. Ternes, K. Haberer, and K. L. Kratz. 1999. Occurrence of antibiotics in the aquatic environment. Sci. Total Environ. 225:109-118. [DOI] [PubMed] [Google Scholar]
- 16.Huycke, M. M., D. F. Sahm, and M. S. Gilmore. 1998. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg. Infect. Dis. 4:239-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen, L. B., P. Ahrens, L. Dons, R. N. Jones, A. M. Hammerum, and F. M. Aarestrup. 1998. Molecular analysis of Tn1546 in Enterococcus faecium isolated from animals and humans. J. Clin. Microbiol. 36:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klare, I., H. Heier, H. Claus, G. Bohme, S. Marin, G. Seltmann, R. Hakenbeck, V. Antanassova, and W. Witte. 1995. Enterococcus faecium strains with vanA-mediated high-level glycopeptide resistance isolated from animal foodstuffs and fecal samples of humans in the community. Microb. Drug Resist. Mech. Epidemiol. Dis. 1:265-272. [DOI] [PubMed] [Google Scholar]
- 19.Klare, I., H. Heier, H. Claus, and W. Witte. 1993. Environmental strains of Enterococcus faecium with inducible high-level resistance to glycopeptides. FEMS Microbiol. Lett. 106:23-29. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn, I., L. G. Burman, S. Haeggman, K. Tullus, and B. E. Murray. 1995. Biochemical fingerprinting compared with ribotyping and pulsed-field gel electrophoresis of DNA for epidemiological typing of enterococci. J. Clin. Microbiol. 33:2812-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhn, I., and R. Mollby. 1993. The Php Rs system—a simple microplate method for studying coliform bacterial-populations. J. Microbiol. Methods 17:255-259. [Google Scholar]
- 22.Kummerer, K. 2001. Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—a review. Chemosphere 45:957-969. [DOI] [PubMed] [Google Scholar]
- 23.Larsson, C. 1995. Enterococci as a new nosocomial problem. A vancomycin-resistant strain was isolated in Sweden. Lakartidningen 92:286-289. [PubMed] [Google Scholar]
- 24.Linden, P. K., and C. B. Miller. 1999. Vancomycin-resistant enterococci: the clinical effect of a common nosocomial pathogen. Diagn. Microbiol. Infect. Dis. 33:113-120. [DOI] [PubMed] [Google Scholar]
- 25.LINFO. 2001. FASS Läkemedel i Sverige. Läkemedelsinformation, A. B., Stockholm, Sweden.
- 26.Manero, A., and A. R. Blanch. 1999. Identification of Enterococcus spp. with a biochemical key. Appl. Environ. Microbiol. 65:4425-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martone, W. J. 1998. Spread of vancomycin-resistant enterococci: why did it happen in the United States? Infect. Control Hosp. Epidemiol. 19:539-545. [DOI] [PubMed] [Google Scholar]
- 28.Melhus, A., and I. Tjernberg. 1996. First documented isolation of vancomycin-resistant Enterococcus faecium in Sweden. Scand. J. Infect. Dis. 28:191-193. [DOI] [PubMed] [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 30.Pegues, D. A., C. F. Pegues, P. L. Hibberd, D. S. Ford, and D. C. Hooper. 1997. Emergence and dissemination of a highly vancomycin-resistant vanA strain of Enterococcus faecium at a large teaching hospital. J. Clin. Microbiol. 35:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swedish Institute for Infectious Disease Control. 2001. Smittsamma sjukdomar 2000—epidemiologiska enhetens årsrapport. Swedish Institute for Infectious Disease Control, Solna, Sweden.
- 32.Torell, E., O. Cars, and A. Hambraeus. 2001. Ampicillin-resistant enterococci in a Swedish university hospital: nosocomial spread and risk factors for infection. Scand. J. Infect. Dis. 33:182-187. [DOI] [PubMed] [Google Scholar]
- 33.Torell, E., O. Cars, B. Olsson-Liljequist, B. M. Hoffman, J. Lindback, and L. G. Burman. 1999. Near absence of vancomycin-resistant enterococci but high carriage rates of quinolone-resistant ampicillin-resistant enterococci among hospitalized patients and nonhospitalized individuals in Sweden. J. Clin. Microbiol. 37:3509-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torell, E., H. Fredlund, E. Tornquist, E. B. Myhre, L. Sjoberg, and A. Sundsfjord. 1997. Intrahospital spread of vancomycin-resistant Enterococcus faecium in Sweden. Scand. J. Infect. Dis. 29:259-263. [DOI] [PubMed] [Google Scholar]
- 35.van den Bogaard, A. E., P. Mertens, N. H. London, and E. E. Stobberingh. 1997. High prevalence of colonization with vancomycin- and pristinamycin-resistant enterococci in healthy humans and pigs in The Netherlands: is the addition of antibiotics to animal feeds to blame? J. Antimicrob. Chemother. 40:454-456. [DOI] [PubMed] [Google Scholar]
- 36.van den Bogaard, A. E., and E. E. Stobberingh. 2000. Epidemiology of resistance to antibiotics. Links between animals and humans. Int. J. Antimicrob. Agents 14:327-335. [DOI] [PubMed] [Google Scholar]