Abstract
Bovine manure, with or without added Salmonella enterica serovar Typhimurium (three strains), was incorporated into silty clay loam (SCL) and loamy sand (LS) soil beds (53- by 114-cm surface area, 17.5 cm deep) and maintained in two controlled-environment chambers. The S. enterica serovar Typhimurium inoculum was 4 to 5 log CFU/g in manure-fertilized soil. The conditions in the two environmental chambers, each containing inoculated and uninoculated beds of manure-fertilized soil, simulated daily average Madison, Wis., weather conditions (hourly temperatures, rainfall, daylight, and humidity) for a 1 March or a 1 June manure application and subsequent vegetable growing seasons ending 9 August or 28 September, respectively. Core soil samples were taken biweekly from both inoculated and uninoculated soil beds in each chamber. Radishes, arugula, and carrots were planted in soil beds, thinned, and harvested. Soils, thinned vegetables, and harvested vegetables were analyzed for S. enterica serovar Typhimurium and Escherichia coli (indigenous in manure). After the 1 March manure application, S. enterica serovar Typhimurium was detected at low levels in both soils on 31 May, but not on vegetables planted 1 May and harvested 12 July from either soil. After the 1 June manure application, S. enterica serovar Typhimurium was detected in SCL soil on 7 September and on radishes and arugula planted in SCL soil on 15 August and harvested on 27 September. In LS soil, S. enterica serovar Typhimurium died at a similar rate (P ≥ 0.05) after the 1 June manure application and was less often detected on arugula and radishes harvested from this soil compared to the SCL soil. Pathogen levels on vegetables were decreased by washing. Manure application in cool (daily average maximum temperature of <10°C) spring conditions is recommended to ensure that harvested vegetables are not contaminated with S. enterica serovar Typhimurium. Manure application under warmer (daily average maximum temperature >20°C) summer conditions is not recommended when vegetable planting is done between the time of manure application and late summer. A late fall manure application will not increase the risk of contaminating vegetables planted the next spring, since further experiments showed that repeated freeze-thaw cycles were detrimental to the survival of S. enterica serovar Typhimurium and E. coli in manure-fertilized soil. The number of indigenous E. coli in soil was never significantly lower (P < 0.05) than that of S. enterica serovar Typhimurium, suggesting its usefulness as an indicator organism for evaluating the risk of vegetable contamination with manure-borne S. enterica serovar Typhimurium.
An increasing association between fresh vegetables and foodborne infection outbreaks has led to concern about contamination of vegetables with fecal pathogenic bacteria in the agricultural environment (14). One possible route of contamination is the use of noncomposted bovine manure as fertilizer (11, 14). Both conventional and organic farmers commonly apply bovine manure as a fertilizer to fields used in vegetable crop production. In 2000, produce grown organically represented 2% of United States retail produce sales, and this market share is expected to continue increasing yearly at a 10 to 12% rate (J. Riddle, C. Weakley, P. Riesgraf, C. Winter, M. Doyle, and D. Bowen, Abstr. 88th IAFP Annu. Meet., abstr. S10, 2001). With the increasing popularity of organic produce and the reliance upon manure for fertilization in organic farming, the importance of applying manure such that crops are not contaminated will undoubtedly increase.
To decrease the risk of manure-borne pathogens, such as Salmonella enterica serovar Typhimurium and Escherichia coli O157:H7, contaminating vegetables grown in manure-fertilized soil, it is necessary to establish appropriate time limits between the application of noncomposted manure and vegetable harvest. The U.S. Department of Agriculture (USDA) requires that at least 120 days elapse between noncomposted manure application and harvest of organic crops with edible portions exposed to soil particles (15). A focus of the present study was to determine whether the adequacy of the USDA 120-day limit varies according to seasonal factors such as temperature and frost, as suggested by previous studies (4, 13, 16, 19).
In addition to seasonal factors, soil type is a factor that may affect survival of E. coli and Salmonella in soils and soil-manure mixtures (9, 19). Therefore, the present study compared two Wisconsin vegetable production soils that differ markedly in physical and chemical properties.
Testing directly for the presence of pathogens in manure-fertilized soil would not necessarily indicate whether manure was applied at a sufficient time prior to harvest. For example, detection of Salmonella spp. from recently fertilized soil would be unlikely if the manure happened to be Salmonella free or if it contained very low levels of this pathogen. Therefore, more useful information would be obtained by testing for indicator bacteria that are always present in bovine manure and have survival characteristics comparable to those of fecal enteric pathogens. The present study evaluated E. coli as a potential indicator organism for this testing.
The present study addressed several aspects of S. enterica serovar Typhimurium survival in bovine manure-fertilized soils and on vegetables grown in these soils. The objectives were to determine (i) if following the USDA regulation of ≥120 days between spring or summer manure application and vegetable harvest would ensure the absence of S. enterica serovar Typhimurium on summer- and fall-harvested leaf and root vegetables; (ii) whether differences in soil properties would affect S. enterica serovar Typhimurium survival; (iii) the lethality of a series of soil freeze-thaw cycles, such as occur in fall and winter; and (iv) whether E. coli is a suitable indicator organism for evaluating potential presence of S. enterica serovar Typhimurium.
MATERIALS AND METHODS
Soils.
Silty clay loam (SCL) and loamy sand (LS) soils were obtained from University of Wisconsin—Madison Agricultural Research Stations in Madison and Hancock, Wis., respectively. The University of Wisconsin—Madison Soil and Plant Analysis Laboratory analyzed each soil type for specific chemical and textural properties (Table 1). The soil was not sieved or pulverized prior to use, but large chunks were subdivided into pieces less than 125 cm3 with a shovel.
TABLE 1.
Chemical and textural characteristics of SCL and LS soils
| Soil type | pH | % Texture category |
Total N (%) | Available |
|||
|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | P (ppm) | K (ppm) | |||
| SCL | 6.6 | 24 | 62 | 14 | 0.271 | 70 | 29 |
| LS | 5.8 | 98 | 1 | 1 | 0.0279 | 67 | 64 |
Collection and inoculation of bovine manure.
Fresh manure was collected from lactating Holstein cows on a standard corn- and soy-based diet at the University of Wisconsin—Madison Dairy Research Center. Preliminary testing showed that Salmonella spp. were not present in the manure. Three S. enterica serovar Typhimurium patient isolates, JBL 3266, JBL 3268, and JBL 3270 (John Luchansky, Food Research Institute, University of Wisconsin—Madison) were used to inoculate the bovine manure. Stock cultures were tested for purity by Gram stain, cell morphology, and the API 20E biochemical profiling system (bioMérieux, Inc., Hazelwood, Mo.) and were stored in brain heart infusion medium containing 20% (vol/vol) glycerol at −20°C. Working cultures were obtained by streaking a loopful of stock culture onto nutrient agar (Difco/Becton Dickinson, Mansfield, Mass.) followed by incubation at 35°C for 24 h. A loopful of each working culture was transferred to 9 ml of brain heart infusion (Difco) and incubated for 18 h at 35°C prior to inoculation. To inoculate the manure, 9.0 ml of an 18-h culture of each isolate was added to ca. 8 kg of fresh bovine manure and thoroughly mixed.
Preparation of soil and incorporation of inoculated manure.
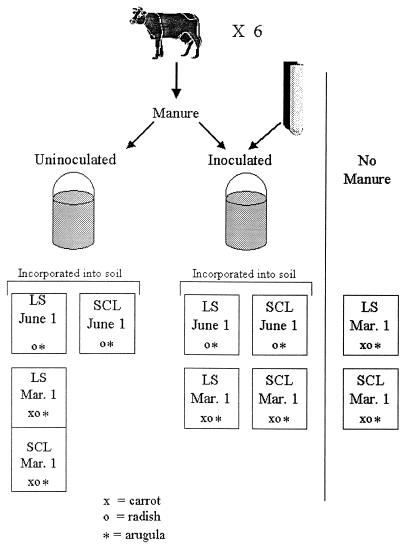
The experimental unit in this study was a bed constructed of a pressure-treated lumber frame with a plywood base and a fine-mesh aluminum screen and plastic to contain soil. The surface area of each bed was 53 by 114 cm, and soil was added to a depth of 17.5 cm. Soil beds were placed into controlled-environment chambers at the University of Wisconsin—Madison Biotron. The environment in one chamber simulated hourly Madison, Wis., conditions from 1 March through 9 August (spring manure application and vegetable planting), and the other chamber simulated conditions for 1 June through 28 September (summer manure application and late-summer vegetable planting). Soil in the beds was thoroughly mixed with either inoculated or uninoculated manure (Fig. 1) at a level of 4 kg per bed by using a small trowel. These manure applications simulated 67.2 metric tons of wet manure per ha distributed in a 15-cm plow depth, a typical application rate in Wisconsin (7). The level of S. enterica serovar Typhimurium in the inoculated manure-fertilized soil ranged from 4.0 to 5.0 log CFU/g. In the chamber simulating the 1 March manure application, there were four beds and two half-beds divided by lumber framing, an aluminum screen, and plastic (Fig. 1): (i) SCL with inoculated manure, full bed; (ii) SCL with uninoculated manure, half-bed (control for manure); (iii) LS with inoculated manure, full bed; (iv) LS with uninoculated manure, half-bed (control for manure); (v) SCL with no manure added, full bed (soil control); and (vi) LS with no manure added, full bed (soil control). Half-beds were used because of space constraints in the controlled-environment chambers.
FIG. 1.
Experimental design. SCL and LS soils were fertilized with inoculated or uninoculated manure and stored under 1 March to 9 August or 1 June to 28 September conditions. Each rectangle indicates a soil bed; two contiguous rectangles denote two half-beds. Vegetables were planted as indicated.
There were four beds in the chamber that simulated a June 1 manure application (Fig. 1): (i) SCL with inoculated manure, (ii) SCL with uninoculated manure (control for manure), (iii) LS with inoculated manure, and (iv) LS with uninoculated manure (control for manure).
Environmental conditions.
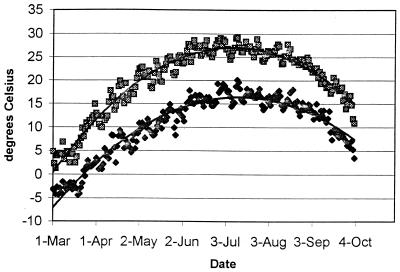
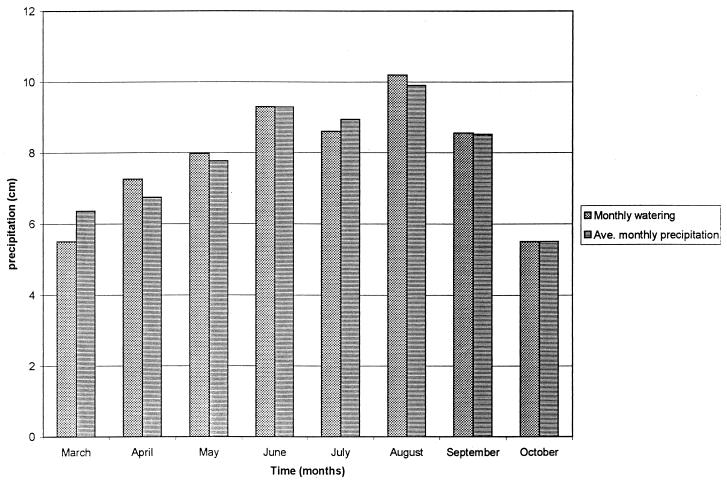
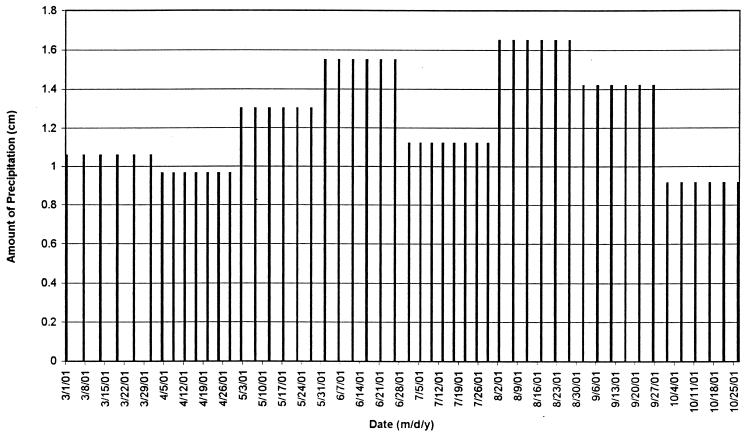
All soil beds were exposed to temperatures corresponding to the average hourly Madison temperatures for each calendar date starting at either 1 March or 1 June, as determined from 1985 to 1994 hourly temperature records obtained from the University of Wisconsin—Madison weather station. The average high and low temperatures for each calendar date beginning 1 March through 3 October are shown in Fig. 2. Soil beds were watered with a calibrated watering can filled with distilled water, with monthly totals based on the average monthly precipitation total for Madison, Wis., as determined by the National Weather Service from 1970 through 1999 (Fig. 3). From the University of Wisconsin—Madison weather station records for 1985 to 1994, the average number of rainfalls of >0.254 cm was determined for each month. For a given month, the number of days in the month was divided by the number of rainfalls of >0.254 cm and rounded to the nearest whole number to give the frequency of watering. The amount of water applied at each watering was then calculated by dividing the total monthly precipitation by the number of waterings (Fig. 4). The relative humidity (RH) in the chambers alternated between 40 and 95% RH, with 40% RH during times of rising temperature (morning to afternoon), and 95% during times of decreasing temperature (afternoon to morning). Times of sunrise and sunset for Madison, Wis., in 2000 (Astronomical Applications Department, U.S. Naval Observatory, Washington, D.C.) dictated the times that beds were illuminated by high intensity fluorescent and incandescent lights on a given date.
FIG. 2.
Average daily high and low temperatures for 1 March to 1 October simulated in the environmental chambers, based on Madison, Wis., averages for 1985 to 1994.
FIG. 3.
Total watering amounts for soil beds compared with average monthly precipitation for Madison, Wis., based on 1970 to 1999 data.
FIG. 4.
Amount and frequency of watering during March to October conditions simulated in controlled-environment chambers. Each bar indicates a watering.
Vegetable production.
Radish (Raphanus sativus) and arugula (Eruca vesicaria) seeds (Cherry Belle radish and Wild Italian Rocket arugula; W. Atlee Burpee & Co., Warminster, Pa.) were planted in manure-fertilized soils on 1 May and 15 August, roughly 9 and 11 weeks after the 1 March and 1 June manure applications, respectively. Vegetables were also planted on 1 May in control soil beds not fertilized with manure. Planting dates were chosen based on average weather conditions being suitable for spring or late-summer planting. Due to the long maturation time, carrot (Daucus carota var. sativus) seeds (Short ’n Sweet; Burpee) could not be planted as a fall crop and were planted only on 1 May. Plastic coverings were placed over each bed after planting to retain moisture until visible germination occurred (i.e., at 3 to 4 days). Liquid iron fertilizer (Fertilome; Voluntary Purchasing Groups, Inc., Bonham, Tex.) was applied to all LS beds shortly after germination to enhance vegetable growth because of a previously observed iron deficiency in the soil (50 ml of fertilizer added to each bed in one regularly scheduled watering). Periodically throughout vegetable growth, radishes and carrots were thinned. At thinning and harvest, radish, arugula, and carrot plants were analyzed for populations of S. enterica serovar Typhimurium (inoculum) and E. coli (indigenous in manure).
Exposure of soils to freezing and thawing cycles after fall harvest.
In an attempt to explain the poorer bacterial survival observed in March and April compared to the June-July conditions, two additional experiments investigated the lethality of repeated freeze-thaw cycling against S. enterica serovar Typhimurium and E. coli in manure-fertilized soils. The first experiment examined the survival of S. enterica serovar Typhimurium and E. coli, applied to soil via manure on 1 June, during various freeze-thaw treatments applied after vegetables were harvested on 28 September (17 weeks later). Individual 19-by-11-cm sample bags (Fisher) were filled with ca. 620 g of LS or SCL manure-fertilized soil and exposed to various freezing-thawing treatments. The second experiment was done to simulate the introduction of S. enterica serovar Typhimurium and E. coli to soil during a fall manure application. On 15 October, soils (620 g per bag) to which uninoculated manure had been added on 1 June were thoroughly mixed with 26 g of fresh bovine manure, inoculated with S. enterica serovar Typhimurium at a rate of 2 ml of each culture added to 620 g of manure, resulting in an inoculum level of 5.5 log CFU/g of manure-fertilized soil. Bags of manure-fertilized soil were assigned specific freezing (−20°C for 16 h)-thawing (4°C for 8 h) treatments with different numbers of freezing-thawing cycles and intervening frozen storage (Table 2). After the final thawing in a given treatment, manure-fertilized soil was analyzed for surviving S. enterica serovar Typhimurium and E. coli as described below.
TABLE 2.
Survival of S. enterica serovar Typhimurium and E. coli in manure-fertilized SCL and LS soils exposed to freezing-thawing treatments
| Treatmenta | Survivalb |
|||
|---|---|---|---|---|
|
S. enterica serovar Typhimuriumc |
E. coli |
|||
| SCL | LS | SCL | LS | |
| Group A | ||||
| −20°C, 4 mo; thaw | NT | NT | + (1) | + (1) |
| 1 freeze-thaw cycled | + (1) | + (1) | + (1) | + (1) |
| 10 freeze-thaw cycles | + (1) | + (1) | + (1) | + (1) |
| 10 freeze-thaw cycles; 4 mo, −20°C; thaw | NT | NT | + (1) | + (1) |
| 10 freeze-thaw cycles; 4 mo, −20°C; 10 freeze-thaw cycles | + (2) | + (1), − (1) | + (2) | + (2) |
| Group B | ||||
| −20°C, 4 mo; thaw | 3.22 (1) | 3.17 (1) | 1.87 (1) | 3.36 (1) |
| 1 freeze-thaw cycle | 4.96 (1) | 4.41 (1) | NT | NT |
| 10 freeze-thaw cycles | 1.57 (1) | <DLe (1) | 3.13 (1) | 2.27 (1) |
| 10 freeze-thaw cycles; 4 mo, −20°C; thaw | 2.31 (2) | 2.55 (2) | 2.80 (2) | 2.41 (2) |
| 10 freeze-thaw cycles; 4 mo, −20°C; 10 freeze-thaw cycles | <DL (2)f | <DL (2) | <DL (2) | <DL (2) |
The history of the soils prior to treatment was as follows. For group A, inoculated manure was applied to soils (in beds), manure-fertilized soils were exposed to 1 June to 28 September conditions, and radish and arugula were grown and harvested. For group B, inoculated manure was applied to soils (in beds), manure-fertilized soils were exposed to 1 June to 28 September conditions, radish and arugula were grown and harvested, and inoculated manure was added to soils in sample bags (initial levels of S. enterica serovar Typhimurium were ca. 5.50 log CFU/g in a soil-manure mixture).
Survival was determined quantitatively (log CFU/g) or qualitatively (+/−). The number of samples is given in parentheses. NT, not tested.
S. enterica serovar Typhimurium was not detected without enrichment in each soil type at the time of fall harvest.
A freeze-thaw cycle consisted of −20°C for 16 h, followed by thawing at 4°C for 8 h.
<DL, less than the detection limit for LS soil (1.64 log CFU/g).
<DL, less than the detection limit for SCL soil (1.23 log CFU/g).
Sampling of manure-fertilized soil.
Three randomly selected core samples of manure-fertilized soil were taken biweekly from each bed, alternating weekly between the 1 March to 9 August and the 1 June to 28 September chambers. At each sampling time, a random location for sampling each bed was chosen based on a 6-by-6 grid pattern for the bed surface, and random numbers were generated by rolling two dice. A sterile spatula was used to obtain a 15- to 25-g core sample. The sterile spatula was inserted at a 45° angle and gradually straightened beneath the surface of the soil. For frozen-thawed bags of manure-fertilized soil, a single core sample was obtained in a similar manner for each bag. Each core sample was mixed with 99 ml of Butterfield's phosphate diluent (BPD; International Bioproducts, Redmond, Wash.) in a stomacher bag (Nasco Whirl-Pak, Atkinson, Wis.). The sample and diluent were manually shaken for 30 s, set still for 30 s, and shaken again for 30 s. This sample homogenization method had previously been found equivalent to using a blender (9). Serial dilutions were made with BPD. To enumerate presumptive S. enterica serovar Typhimurium, one spread plate per dilution was prepared on XLD agar (Oxoid, Inc., Ogdensburg, N.Y.). After a 20- to 24-h incubation at 35°C, black colonies were counted. To enumerate presumptive E. coli, a single Petrifilm E. coli/Coliform Count plate (3M Microbiology Products, St. Paul, Minn.) was inoculated with 1.0 ml of the appropriate dilution. After 48 h of incubation at 35°C, blue colonies with associated gas were counted. This quantitative plating procedure was used for all manure-fertilized soil samples when detectable levels of S. enterica serovar Typhimurium and E. coli were expected. Use of a single plate per dilution is an acceptable practice for food analysis (8), and any decrease in precision resulting from this practice was more than offset by triplicate sampling. The mean log CFU/gram values were calculated, and if no cells were detected by direct plating, an arbitrary value of 1.0 log CFU/g was assigned. The paired t test (version 12.22; Minitab, Inc., State College, Pa.) was used to test for a significant difference between SCL and LS soils in terms of the decrease in microbial numbers at a particular time after the 1 March or 1 June manure application. The paired t test was also used to determine whether a statistically significant (P < 0.05) difference existed between numbers of S. enterica serovar Typhimurium and E. coli in a soil bed at a given sampling time.
Sampling procedure for vegetables at thinning and harvest.
Vegetables were sampled at thinning and harvest. Carrots and radishes were thinned and sampled as follows: a sterile spatula was used to loosen the roots from the soil, and vegetables were then aseptically transferred to a sterile stomacher bag. One bag was filled with either carrots or radishes thinned from a single soil bed. From one such bag, a randomly picked subsample (10 to 25 g of carrot, 30 to 55 g of radish) was aseptically transferred to another sterile stomacher bag, and 99 ml of Peptone-Tween diluent (10 g of Tween 80 [Fisher Scientific, Itasca, Ill.] and 1 g of Bacto Peptone [Difco] per liter of distilled water) was added to each bag and mixed as previously described. This diluent was used to enhance removal of microbes from the vegetable surface. The vegetables and diluent were not homogenized with a stomacher or blender in order to minimize the potential release of antimicrobial phytochemicals into the diluent. The plating procedure used for vegetable analysis is described below.
At harvest from each soil bed, the volume of each type of vegetable from a given bed necessitated the use of two sterile stomacher bags, each of which was filled. Sterile scissors were used to remove the roots (arugula) or leaves (radishes and carrots), and the edible portions were dropped into the stomacher bags. Arugula roots, radish tops, and carrot tops were discarded. From each bag a representative, randomly selected subsample was aseptically transferred to another sterile stomacher bag (50 to 75 g of radishes, 20 to 30 g of arugula, or 20 to 30 g of carrots). Of the two resulting subsamples for a vegetable type, one subsample was not washed and was analyzed within 1 h of harvesting. The second subsample was first washed by adding 99 ml of sterile distilled water to the stomacher bag and shaking it 30 times to simulate consumer washing of the vegetable before use or consumption. The wash water was then removed, diluted in 99 ml of BPD, and plated as described below. For the unwashed samples and the washed samples after water removal, 99 ml of Peptone-Tween diluent was then added to each stomacher bag. The samples were shaken manually for 30 s, allowed to rest for 30 s, and shaken manually for a final 30 s. Subsequent dilutions were made in BPD and either spread onto duplicate XLD agar plates or transferred to duplicate Petrifilm E. coli/Coliform Count plates. The plates were incubated and the colonies were counted as described above for soil analysis. The detection limit for each soil and vegetable type was calculated based on a single colony formed after plating of the initial rinse or dilution of the largest sample tested. Detection limits for soil from beds, radish, arugula, and carrot samples were 1.29, 1.39, 1.60, and 1.76 log CFU/g, respectively.
Enrichment enhances detection of bacteria by allowing repair of injured cells and multiplication of low numbers of target cells to detectable levels. After the levels of S. enterica serovar Typhimurium and E. coli dropped below the detection limits, radish, arugula, and carrot samples were enriched by static incubation in 90 ml of nonselective brain heart infusion for 24 h at 35°C. Samples were then serially diluted in 99 ml of BPD, spread on a single XLD agar plate per dilution, and transferred to a single Petrifilm E. coli/Coliform Count plate for each dilution. The presence or absence of presumptive S. enterica serovar Typhimurium and E. coli was noted after incubation of the plates as previously described. Frozen-thawed manure-fertilized soil samples were also enriched and plated by using this procedure when detectable levels of S. enterica serovar Typhimurium were not expected.
Confirmation of presumptive S. enterica serovar Typhimurium and E. coli.
To confirm S. enterica serovar Typhimurium and E. coli presence in soil and vegetable samples (both enriched and nonenriched), a single colony per sample was randomly selected from a countable plate of each medium type (black colony on XLD agar, blue colony with associated gas on the Petrifilm E. coli/Coliform Count plate) and streaked onto brain heart infusion agar (Difco). The brain heart infusion agar plates were incubated for 24 h at 35°C. Isolates were confirmed based on Gram stain reaction, cell morphology, and biochemical characterization by the API 20E system (bioMérieux). Throughout this study, 91.2% (52 of 57) and 100% (81 of 81) of presumptive S. enterica serovar Typhimurium and E. coli isolates, respectively, were confirmed.
RESULTS
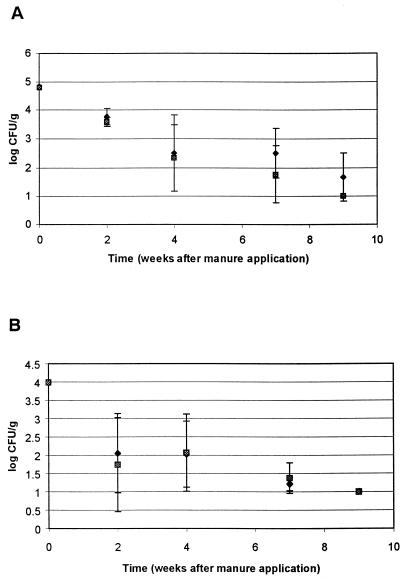
In LS soil fertilized with inoculated manure on 1 March, both S. enterica serovar Typhimurium and E. coli declined from initial levels of ca. 4.8 log CFU/g to 1.68 (S. enterica serovar Typhimurium) and <1.29 (E. coli) log CFU/g (undetectable by direct plating) by the time vegetables were planted nine weeks later (Fig. 5A). A similar decrease in numbers of both organisms occurred in SCL soil after the 1 March manure application (Fig. 5B). However, both organisms were detected after enrichment of both manure-fertilized soils on 26 July, after vegetables were harvested (21 weeks after the 1 March manure application). The presence of low levels of S. enterica serovar Typhimurium and E. coli in manure-fertilized LS soil seldom corresponded with the detection of either organism on vegetables grown in this soil after a 1 March manure application. At thinning and harvest of radish, arugula, and carrot, no S. enterica serovar Typhimurium was detected by direct plating (thinned radish and carrot) or after enrichment (harvested radish, arugula, and carrot) of unwashed or washed vegetables. S. enterica serovar Typhimurium also was not detected in wash water by direct plating. E. coli was only detected after enrichment of harvested radishes. On vegetables grown in SCL soil after 1 March manure application, S. enterica serovar Typhimurium and E. coli were not detected on any enriched samples except for washed harvested arugula (positive for both organisms after enrichment).
FIG. 5.
Survival of S. enterica serovar Typhimurium (⧫) and E. coli (░⃞) in manure-fertilized LS (A) and SCL (B) after a 1 March manure application. Each point represents mean of three samples; error bars indicate ± 1 standard deviation. When cells were not detected by direct plating, a value of 1.0 log CFU/g was assigned for calculation of the mean and standard deviation. Sampling times for which samples were enriched before plating (after 9 weeks) are not shown.
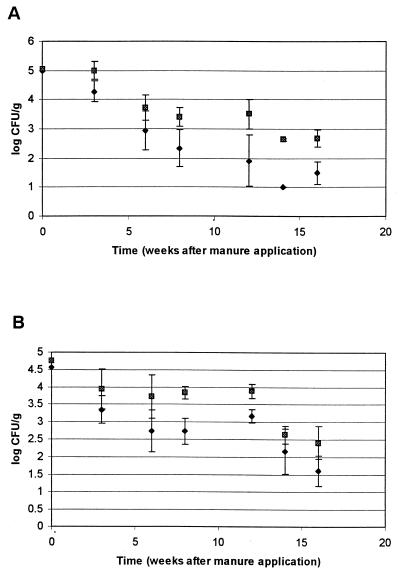
Decreases in levels of S. enterica serovar Typhimurium and E. coli were somewhat less in LS soil after a 1 June manure application (Fig. 6A) than after a 1 March application (Fig. 5A). From initial levels of ca. 5.0 log CFU/g in LS soil, S. enterica serovar Typhimurium and E. coli levels dropped to ca. 2.3 and 3.4 log CFU/g, respectively, by 27 July (8 weeks after the 1 June manure application), and E. coli was still detected by direct plating on 7 September, about 3 weeks after vegetables were planted. As a result, vegetables grown in LS soil after a 1 June manure application were more often contaminated than those grown after March 1 manure application. S. enterica serovar Typhimurium was detected after enrichment of unwashed radish and arugula harvested from LS soil on 28 September (17 weeks after manure application) (Table 3). Washing these vegetables apparently removed S. enterica serovar Typhimurium but not E. coli, despite S. enterica serovar Typhimurium and E. coli originally being present in nearly equal numbers in the manure-fertilized soil (Table 3 and Fig. 6). More than 2.0 log CFU of E. coli/ml was present in a sample of water after the washing of harvested radishes, but neither S. enterica serovar Typhimurium nor E. coli was detected by direct plating of other wash water samples.
FIG. 6.
Survival of S. enterica serovar Typhimurium (⧫) and E. coli (░⃞) in manure-fertilized LS (A) and SCL (B) after the 1 June manure application. Each point represents the mean of three samples; error bars indicate ± 1 standard deviation. When cells were not detected by direct plating, a value of 1.0 log CFU/g was assigned for calculation of the mean and standard deviation.
TABLE 3.
Effect of washing on the presence of S. enterica serovar Typhimurium and E. coli on arugula, radishes, and carrots grown in manure-fertilized SCL and LS soilsa
| Soil type | Vegetable (no. of wk after manure applicationc) | Survival of organism (S. enterica serovar Typhimurium, E. coli)b: |
||
|---|---|---|---|---|
| On unwashed vegetable | On washed vegetable | In wash water | ||
| SCL | Arugula (16) | <DL, <DL | <DL, <DL | <DL, <DL |
| Arugula (17) | +, + | +, + | <DL, <DL | |
| Radish (14) | 1.50, NT | 1.80, NT | 2.79, NT | |
| Radish (16) | 2.61, 2.65 | <DL, 2.53 | <DL, 2.78 | |
| Radish (17) | +, + | +, + | <DL, 1.92 | |
| LS | Arugula (16) | <DL, <DL | <DL, <DL | <DL, <DL |
| Arugula (17) | +, + | −, + | <DL, <DL | |
| Radish (14) | <DL, NT | <DL, NT | <DL, NT | |
| Radish (16) | <DL, 1.93 | <DL, <DL | <DL, <DL | |
| Radish (17) | +, + | −, + | <DL, 2.34 | |
Manure-fertilized soils were exposed to 1 June to 28 September conditions, with planting on 15 August. Values are from quantitative (log CFU/g) or qualitative (+/−) analysis of a single sample.
DL, detection limits were 1.39 and 1.60 log CFU/g for radish and arugula, respectively; NT, not tested.
On 1 June.
With the exception of E. coli counts 8 weeks after the 1 June manure application, there was no significant (P < 0.05) difference between soils in terms of decrease in microbial numbers (Fig. 6). E. coli was detected by direct plating on washed and unwashed radishes from SCL soil harvested on 21 September (16 weeks after the 1 June manure application) (Table 3) and in two radish wash water samples. S. enterica serovar Typhimurium was detected by plating of unwashed radishes harvested from SCL soil on 21 September and after enrichment of washed and unwashed arugula and radish harvested from SCL soil on 28 September (17 weeks after 1 June manure application). S. enterica serovar Typhimurium was detected by plating of one radish wash water sample.
Freeze-thaw treatments did not eliminate the small numbers of S. enterica serovar Typhimurium and E. coli remaining after vegetable harvest 17 weeks after the 1 June manure application. Both organisms were detected in all but one sample following enrichment (Table 2). When freeze-thaw treatments followed the addition of inoculated manure to soil on 15 October, S. enterica serovar Typhimurium decreased by 0.5 to ≥4.3 log CFU/g, depending on the severity of the treatment (Table 2). The most severe treatment reduced numbers of the organisms below the direct plating detection limit. Treatments involving more than one freeze-thaw cycle were clearly more lethal than those involving a single cycle.
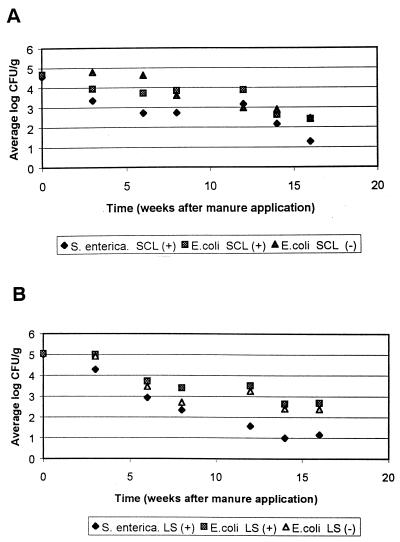
Throughout the study the level of E. coli in a soil bed was never significantly lower than the level of S. enterica serovar Typhimurium (Fig. 5 and 6). The numbers of E. coli were similar in inoculated and uninoculated manure-fertilized soils, showing that the results obtained with the beds of manure-fertilized soil were reproducible and that the presence of added S. enterica serovar Typhimurium did not alter E. coli survival in manure-fertilized soils (Fig. 7).
FIG. 7.
Log CFU of S. enterica serovar Typhimurium and E. coli/gram in SCL (A) and LS (B) soil containing inoculated (+) and uninoculated (−) manure after a simulated 1 June application. Each point represents the mean of three samples; the standard deviations for S. enterica serovar Typhimurium and E. coli in inoculated manure are the same as in Fig. 6. The standard deviations for E. coli in uninoculated manure ranged from 0.09 to 1.17 log CFU/g. A value of 1.0 log CFU/g represents no S. enterica serovar Typhimurium or E. coli detected by direct plating (no enrichment).
DISCUSSION
Spring-summer (1 March to 9 August) conditions were clearly more detrimental to S. enterica serovar Typhimurium and E. coli survival in manure-fertilized soil than the summer-fall (1 June to 28 September) conditions. Frequent fluctuation of ambient temperature, ca. 0°C, in March conditions (Fig. 1) may have caused more rapid bacterial death. Experiments with freeze-thaw cycling to simulate winter conditions caused S. enterica serovar Typhimurium and E. coli levels to decrease by several orders of magnitude (Table 2) and support this hypothesis. Alternatively, S. enterica serovar Typhimurium and E. coli may survive better in manure-fertilized soil at warmer temperatures. However, others (2, 3, 5, 17) have reported more rapid death of Salmonella spp. and pathogenic E. coli in bovine manure (not added to soil) with increasing temperature (4 to 35°C).
Vegetables grown after a 1 March manure application were seldom contaminated with either S. enterica serovar Typhimurium or E. coli, while both organisms were often detected on root and leaf vegetables grown after the 1 June application. Our results strongly recommend the application of bovine manure to soil under cool (daily average maximum temperature of <10°C) early-spring conditions. The USDA-mandated interval between manure application and vegetable harvest should also be followed. The combination of these two practices will minimize the risk of vegetable contamination with manure-borne S. enterica serovar Typhimurium. Our results also clearly point out that application of manure under warmer (daily average maximum temperature of >20°C) summer conditions prior to planting a fall crop cannot be recommended. The USDA 120-day limit was inadequate in our experiment with the 1 June manure application because vegetables harvested 17 weeks (119 days) later still contained detectable levels of S. enterica serovar Typhimurium and E. coli. It is possible, however, that S. enterica serovar Typhimurium would not have been detected if initial cell levels in the manure had been lower. The manure inoculation rate used in our study (ca. log 7 CFU/g) was at the high end of the reported range of 2 to 7 log CFU/g for salmonellae in manure from infected cattle (5, 6), representing a worst-case situation. The present study did not evaluate predation of S. enterica serovar Typhimurium and E. coli by protozoa. Predation has been cited as an important factor affecting bacterial survival in soil (12). It is possible that protozoan activity differing from that in our soil beds alters pathogen survival under field conditions. However, the use of freshly obtained soils in large beds with closely simulated weather conditions is as close an approximation of natural conditions as possible without actual field studies.
Although freeze-thaw cycling did not completely eliminate S. enterica serovar Typhimurium and E. coli in manure-fertilized soil, our results show that it may cause a substantial reduction in cell numbers. A previous study (18) also found that freeze-thaw cycles result in high E. coli mortality and that repeated freeze-thaw cycling is much more lethal to E. coli than a single cycle. A fall manure application would thus be unlikely to result in contamination of vegetables planted the following spring and summer. Applying manure to fields in the fall could have little effect on soil N levels the following spring but would add organic matter to the soil and serve as a low-cost means of manure disposal.
Our results show the potential importance of washing vegetables to reduce or eliminate pathogenic bacteria. Washing vegetables may be critical for safety even if the vegetables are cooked prior to consumption because handling of unwashed contaminated vegetables prior to cooking may initiate person-to-person transmission of pathogenic microorganisms. For example, handling unwashed potatoes grown in manure-fertilized soil was implicated as the initial source in the person-to-person transmission of E. coli O157:H7 in a multifamily illness outbreak (1).
S. enterica serovar Typhimurium and E. coli death rates did not differ significantly between LS and SCL soils. Lau and Ingham (9) found that E. coli and enterococcus populations decreased significantly (P < 0.05) faster in LS soil than in SCL soil, but the latter study utilized a much smaller model system and a markedly different temperature regime. Comparison of results for vegetables grown in LS and SCL soils after the 1 June manure application suggests that contamination of vegetables by manure-borne bacteria is more likely in SCL soil, probably because the higher clay content of SCL soil resulted in visibly greater adherence to vegetables.
The similarity of E. coli and S. enterica serovar Typhimurium survival in manure-fertilized soils and the greater prevalence of E. coli on vegetables grown in these soils clearly established the usefulness of E. coli as an indicator organism for evaluating the risk of S. enterica contamination. The absence of E. coli from manure-fertilized soil would almost certainly indicate that S. enterica serovar Typhimurium was also absent. E. coli has been shown to survive better than another fecal indicator group, Enterococcus spp., in manure-fertilized soils (9) and to be a reliable indicator for potential survival of E. coli O157:H7 in soil (10).
In summary, applying noncomposted bovine manure to soil in early spring or late fall and adhering to the USDA regulation of ≥120 days between manure application and vegetable harvest should ensure that root and leaf vegetable crops will be free of S. enterica serovar Typhimurium. However, S. enterica serovar Typhimurium may be present on vegetables after an early-summer manure application, even if ≥120 days have elapsed between manure application and vegetable harvest. Differences between the two soils studied did not have a significant effect on S. enterica serovar Typhimurium and E. coli survival but may influence contamination of vegetables because of differences in adherence of soil to vegetable surfaces. Repeated freeze-thaw cycles reduce levels of S. enterica serovar Typhimurium and E. coli in soils but may not completely eliminate these organisms. Finally, E. coli can be recommended as a reliable indicator organism for the potential presence of S. enterica serovar Typhimurium in manure-fertilized soil.
Acknowledgments
This research was financially supported by a grant from the USDA Special Food Safety Program.
We gratefully acknowledge the preliminary and supporting laboratory studies of Megan Lang, Maria Lau, and Anthony Arment.
REFERENCES
- 1.Chapman, P. A., C. A. Siddons, J. Manning, and C. Cheetam. 1997. An outbreak of infection due to verocytotoxin-producing Escherichia coli O157 in four families: the influence of laboratory methods on the outcome of the investigation. Epidemiol. Infect. 119:113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forshell, L. P., and I. Ekesbo. 1993. Survival of salmonellas in composted and not composted solid animal manures. J. Vet. Med. B 40:654-658. [DOI] [PubMed] [Google Scholar]
- 3.Fukushima, H., K. Hoshina, and M. Gomyoda. 1999. Long-term survival of Shiga toxin-producing Escherichia coli O26, O111, and O157 in bovine feces. Appl. Environ. Microbiol. 65:5177-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guy, E. M., and J. A. Small. 1977. Survival of streptococci and coliforms of bovine faecal origin in drainage water and soil stored at different temperatures. N. Z. J. Agric. Res. 20:13-18. [Google Scholar]
- 5.Himathongkham, S., S. Bahari, H. Riemann, and D. Cliver. 1999. Survival of Escherichia coli O157:H7 and Salmonella typhimurium in cow manure and cow manure slurry. FEMS Microbiol. Lett. 178:251-257. [DOI] [PubMed] [Google Scholar]
- 6.Jones, P. W. 1976. The effect of temperature, solids content, and pH on the survival of salmonellas in cattle slurry. Br. Vet. J. 132:284-293. [DOI] [PubMed] [Google Scholar]
- 7.Madison, F., K. Kelling, L. Massie, and L. Ward Good. 1995. Guidelines for applying manure to cropland and pasture in Wisconsin. Extension Bulletin R-8-95-2M-E, Madison, Wis.
- 8.Marshall, R. T. (ed.). 1993. Standard methods for the examination of dairy products, 16th ed. American Public Health Association, Washington, D.C.
- 9.Lau, M. M., and S. C. Ingham. 2001. Survival of faecal indicator bacteria in bovine manure incorporated in soil. Lett. Appl. Microbiol. 33:1-6. [DOI] [PubMed] [Google Scholar]
- 10.Ogden, I., D. Fenlon, A. Vinten, and D. Lewis. 2001. The fate of Escherichia coli O157 in soil and its potential to contaminate drinking water. Int. J. Food Microbiol. 66:111-117. [DOI] [PubMed] [Google Scholar]
- 11.Pell, A. N. 1997. Manure and microbes: public and animal health problem? J. Dairy Sci. 80:2673-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Recorbet, G., C. Steinberg, and G. Faurie. 1992. Survival in soil of genetically engineered Escherichia coli as related to inoculum density, predation, and competition. FEMS Microbiol. Lett. 101:251-260. [Google Scholar]
- 13.Tannock, G. W., and J. M. B. Smith. 1972. Studies on the survival of Salmonella typhimurium and Salmonella bovis-morbificans on soil and sheep feces. Res. Vet. Sci. 13:150-153. [PubMed] [Google Scholar]
- 14.Tauxe, R., H. Kruse, C. Hedberg, M. Potter, J. Madden, and K. Wachsmuth. 1997. Microbial hazards and emerging issues associated with produce: a preliminary report to the National Advisory Committee on Microbiological Criteria for Foods. J. Food Prot. 60:1400-1408. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Agriculture, Agricultural Marketing Service. 2000. National organic program. 7 CFR Part 205:203. U.S. Department of Agriculture, Washington, D.C.
- 16.Van Donsel, D. J., E. E. Geldreich, and N. A. Clarke. 1967. Seasonal variations in survival of indicator bacteria in soil and their contribution to storm-water pollution. Appl. Microbiol. 15:1362-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, G., T. Zhao, and M. P. Doyle. 1996. Fate of enterohemorrhagic Escherichia coli in bovine feces. Appl. Environ. Microbiol. 62:2567-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiser, R. S., and C. M. Osterud. 1945. The influence of the freezing temperature, repeated fluctuations of temperature, and the exposure to freezing temperatures on the mortality of Escherichia coli. J. Bacteriol. 50:413-439. [DOI] [PubMed] [Google Scholar]
- 19.Zibilske, L. M., and R. W. Weaver. 1978. Effect of environmental factors on survival of Salmonella typhimurium in soil. J. Environ. Qual. 7:593-597. [Google Scholar]