Abstract
Background
Polycystic Ovary Syndrome (PCOS) is an endocrine disorder affecting women of reproductive age, characterized by hormonal imbalances, reproductive abnormalities, and metabolic disturbances. The diagnosis and management of PCOS is not well documented, particularly in the Ethiopian healthcare setup.
Methods
A cross-sectional study was conducted on 210 healthcare professionals selected from hospitals in Addis Ababa from April 10 to May 24, 2024. Data was collected using a standardized questionnaire and double entered using Microsoft Excel and analyzed by using the Statistical Package for Social Sciences (SPSS) Version 25. Likert scale was used for the attitude analysis and the average score measure of difference was used for knowledge, attitude and practice (KAP) level of measurements. ANOVA was used for testing the association of p-value less than 0.05% and Spearman’s rho was used to test correlation.
Result
Out of 210 respondents, only 43 (20.5%) had “Good Knowledge” about PCOS and its diagnosis. Gynecologists exhibited the highest knowledge (58.3%) and practice (50%) scores compared to other professions. A majority, 187 individuals (89.0%), were classified as having a “Good Attitude” towards PCOS and its diagnosis. Only 42 (22.0%) of participants indicated Good Practice. Among the hormonal markers, Follicle Stimulating Hormone (FSH) on its own was the most frequently used (75 participants, 41.4%), Anti-Müllerian hormone (AMH) and Dehydroepiandrosterone Sulfate (DHEAS) were only utilized by 15.5% (28) and 17.7% (32) of participants respectively.
Conclusion
The study highlighted significant gaps in knowledge, attitude, and practices among healthcare professionals in Ethiopia regarding PCOS diagnosis. Inadequate practices were common, with reliance on ultrasonography and physical symptoms alone. More attention should be given to creating proper diagnosis and referring channels along with teaching and advocacy in academia and the public to promote women’s reproductive health.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12905-025-03948-0.
Keywords: PCOS, KAP, Health care providers, Laboratory diagnosis, Hormonal tests, Ethiopia
Introduction
Polycystic Ovary Syndrome (PCOS) is an endocrine disorder affecting women of reproductive age [1]. It is characterized by excessive ovarian and/or adrenal androgen secretion [2]. Aside from hormonal imbalances and reproductive abnormalities [1], PCOS is often accompanied by psychological impairments, such as depression and other mood disorders, as well as metabolic derangements, including insulin resistance and compensatory hyperinsulinemia. These factors contribute to altered androgen production and metabolism [2, 3]. Additionally, women diagnosed with PCOS exhibiting high depression and anxiety scores showed significant associations with hormonal imbalances (elevated LH: FSH ratios, testosterone levels) and clinical features (irregular menstruation, hirsutism), suggesting PCOS may contribute to mental health risks through endocrine dysregulation [4].
A polycystic ovary contains an increased number of small antral follicles, which are small fluid-filled follicles visible on ultrasonography scanning. These follicles are not “cysts” as they contain potentially healthy oocytes and can be stimulated to grow normally with exogenously administered follicle stimulating hormone(FSH) [2, 5]. A recent study found that while polycystic ovarian morphology (PCOM) is a characteristic feature of PCOS, it is not a definitive indicator of the syndrome, as it is also observed in a significant percentage of normal women (16–25%) [6]. Similar ovarian morphology has been observed in women with congenital adrenal hyperplasia (CAH) and female-to-male transgender individuals [7]. This suggests that PCOM can occur independently of PCOS and does not always lead to its development. These findings collectively emphasize that PCOM in regularly cycling women is not a major risk factor for the development of PCOS, highlighting the complexity of the relationship between PCOM and the development of PCOS [6].
Various diagnostic criteria have been proposed for PCOS over the years. In 1990, the National Institutes of Health (NIH) established criteria that required the simultaneous presence of (a) hyperandrogenism and (b) menstrual dysfunction [8]. This was later updated in 2003 with the Rotterdam criteria which added the (c) presence of PCOM on ultrasonography as a third criterion. The Rotterdam criteria are now widely accepted and require the presence of two out of three of the following: oligo- and/or anovulation, clinical and/or biochemical signs of hyperandrogenism, and polycystic ovaries [9]. Serum Anti-Müllerian hormone (AMH) levels can be used as a diagnostic marker for PCOS when factors such as age, test standardization, PCOS phenotypes, and body mass index are taken into account [10].
Biochemical hyperandrogenism, a consistent feature of PCOS, is an important component considered for diagnosis. AMH levels are a useful marker for PCOS diagnosis [11, 12]. AMH shows higher diagnostic potential than androgens and gonadotropins and levels are typically elevated in PCOS [11, 13]. Several studies have established AMH cutoff values within the range of 4.57–5.20 ng/ml that can be used as an alternative to ultrasonography assessment of follicles or evidence of hyperandrogenism for PCOS diagnosis [11, 14].
Serum concentrations of free testosterone have also been shown to be sensitive biochemical marker for detecting PCOS when measured with reliable methods such as equilibrium dialysis [15]. Other androgens that can be assessed include total testosterone, dehydroepiandrosterone sulfate (DHEAS), and androstenedione [9]. Additionally, a recent study showed a serum protein Sestrin, as an indicator of increased oxidative stress was significantly higher in PCOS cases and can be a potentially supportive biomarker for diagnosis of PCOS [16].
It is estimated 8–13% of women of reproductive age are affected by PCOS worldwide, with up to 70% of cases going undiagnosed [3]. PCOS accounts for 38% of fertility problems in a 2021 meta-analysis in Sudan [17]. Primary healthcare providers play a crucial role in the early diagnosis and management of PCOS. However, limited research exists on the burden of PCOS and healthcare providers’ level of knowledge, attitude and practice on both general and regarding laboratory diagnostic criteria for PCOS in Ethiopia and Africa. Existing studies have, however, highlighted gaps in healthcare providers’ understanding of clinical diagnostic criteria for PCOS around the world including their awareness of the significance of hormonal evaluation through laboratory tests [18–20]. Lack of knowledge of the diagnostic criteria may lead to under diagnosis of PCOS [18]. Given the detrimental mental and physical health consequences of PCOS [1–3], improved and early diagnosis is important to improve the health outcome of women. Therefore, the current study aimed to assess understanding of polycystic ovarian syndrome laboratory diagnosis among health care providers in Addis Ababa, Ethiopia.
Materials and methods
Study area, design and period
The facility-based cross-sectional study was conducted from April 10 to May 24, 2024 at five selected hospitals in Addis Ababa namely, Tikur Anbessa Specialized Hospital, Gandhi Memorial Hospital, Ras Desta Damtew Memorial Hospital, Yekatit 12 Hospital Medical College- Abebech Gobena Maternal-Child Hospital, and Zewditu Memorial Hospital. The mentioned public hospitals were chosen because they are referral hospitals which receive and serve a large number of patients from different parts of the country.
Study population and variables
The study population consists of gynecologists, gynecology residents, interns (final-year medical students in clinical rotations), and midwives (licensed health professional who provides comprehensive reproductive, maternal and neonatal health services) at selected hospitals during the study period that fulfill the eligibility criteria and were willing to participate. Inclusion criteria consisted of general practitioners, gynecologists, gynecology residents, interns and midwives who practice at selected hospitals and had 6 months or more work experience as of the start of data collection and are currently practicing and not on extended leave while the exclusion criteria consisted of health care providers who had less than 6 months of work experience or less than 6 month of professional license of practices (exception for interns as they have not yet received their license but are active in diagnosis) or those on extended leave during data collection.
The paper attempts to assess the KAP of clinicians regarding laboratory diagnosis of PCOS by determining the dependent variables of knowledge, attitude and practice towards PCOS using the independent variable sex, education level, work experience and profession type.
Data collection and analysis
The sample size was calculated using a 50% population proportion assumption yielding a target sample size of 299 participants. A total of 210 participants were enrolled in the survey via a convenient sampling technique. A structured, self-administered, pre tested questionnaire was used to collect the KAP data from different health care providers. The original questionnaire [19] was reviewed and modified to fit the current participants by the principal investigators and subsequently pretested by volunteer health professionals in Hemen Maternal and Children’s Hospital and Legehar General Hospital. Their feedback and responses informed revisions to the questionnaire to enhance its clarity and relevance. The questionnaire was then distributed to target healthcare providers using Google Form and participants who volunteered to participate in the study responded. The number of participants from each hospital and department was determined by the proportion of available health care providers in each hospital and department as per the information provided by their human resources department. Following data collection, a thorough data cleaning process was conducted, which included an evaluation of the questions’ alignment with the study’s scope. Those questions deemed extraneous were excluded from the final analysis. To assess the reliability of the questionnaire, the Spearman-Brown coefficient was calculated for each section. The results indicated a reliability coefficient of 0.795 for the knowledge section (equal parts), 0.659 for the attitude section (unequal parts), and 0.786 for the practice section (unequal parts), demonstrating acceptable internal consistency across the instrument. Data was checked for completeness, entered, and analyzed using SPSS version 25. Descriptive statistics was used to calculate frequencies to give a general overview of the distribution of knowledge, attitude, and practice among healthcare providers. One-way analysis of variance (ANOVA) was performed to analyze the relation of the frequencies of knowledge, attitude and practice scores between different groups of health care providers in relation to the independent variables along with average KAP score differences between different groups and professions. Correlation between knowledge and practice scores was performed by Spearman’s rho. A p value of less than 0.05 was considered statistically significant.
To ensure comprehensive assessment of the questionnaire, the correct answers were not limited to a single diagnostic criterion. Instead, all possible answers derived from various diagnostic criteria were considered valid, as the primary objective of the questionnaire was to evaluate clinicians’ awareness of the full spectrum of diagnostic approaches for PCOS. For the knowledge section, there were a total of eight multiple-choice questions. Each option was assigned a weight of one point, with correct answers increasing the total score by one point and incorrect answers deducting one point. The correctness of each choice was determined based on authoritative sources [21, 22]. The threshold for satisfactory performance was set at 50% of the total maximum possible score, which corresponded to 20 points. The practice section included nine multiple-choice questions, with each option weighted similarly to the knowledge section. The threshold for determining satisfactory performance was set at 50% of the total maximum possible score, which corresponded to eight points. Supplementary File 1 shows this scoring system in detail.
There were a total of 13 attitude questions. Each response was assigned a weighted score ranging from one to five, with one representing ‘strongly disagree’ and five representing ‘strongly agree.’ Based on the criteria outlined in the operational definition and supported by referenced sources, we identified which questions reflected a positive attitude and which indicated a negative attitude. A score above three for each question was considered indicative of a good attitude since in low-resource settings like Ethiopia, where PCOS training is minimal, even neutral responses may indicate latent receptiveness to education. The threshold for a satisfactory overall attitude score was set at 50% of the total maximum possible score, which corresponded to 31 points. Supplementary File 2 shows this in detail.
Results
Characteristics of the study participants
This study enrolled a total of 210 participants from the five selected hospitals. The majority of the participants were midwives (84; 40%), while gynecologists comprised the smallest percentage at 12 participants (5.7%). The study also found that the majority of participants were males (133; 63.3%). In terms of experience the majority of the participants (168, 80%) had less than 8 years of experience. The highest experience level recorded was 28 years. The educational background of the participants was also diverse. The majority held a BSc/MD degree (84; 40%) followed by BSc (69; 32.9%). Socio-demographic data can be found in Supplementary File 3.
Knowledge, attitude and practices of diagnosis methods among the study participants
Knowledge analysis
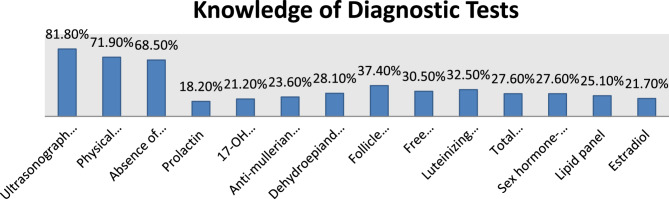
Out of the total 210 respondents, 43 individuals (20.5%) were classified as having “Good Knowledge,” while the majority, 167 participants (79.5%) were categorized as having “Poor Knowledge.” Overall, the result indicated being male, a gynecologist, having a PhD and an experience of 15–20 years is associated with higher knowledge scores. Among the hormonal markers, as presented in Fig. 1, FSH alone (not as part of LH: FSH ratio) had the highest recognition chosen by 79 (37.40%) participants. Other notable hormonal markers included luteinizing hormone (LH), free testosterone, and DHEAS.
Fig. 1.
Knowledge of diagnostic tests (IN THEORY) among health care providers at selected hospitals
The results also explored the knowledge of theoretical criteria among different professions. Our results showed that all of the gynecologists, and gynecology residents revealed they use diagnostic criteria. Of the 37 general practitioners, nine (24.3%) indicated they did not use diagnostic criteria. 36 (42.9%) midwives were not using diagnostic criteria out of the total 84, and eight (17.0%) out of the 47 interns did not use diagnostic criteria either.
The study also examined participants’ knowledge of theoretical diagnosis criteria for PCOS. The results revealed that the Rotterdam criteria (2003) was the most recognized, followed closely by the Androgen Excess Society criteria (2006) (Table 1).
Table 1.
Knowledge of theoretical diagnostic criteria among each category of health care professional at selected hospitals
| Theoretical Diagnosis Criteria | Responses’ Frequency (Percent %) |
|---|---|
| NIH criteria (1990) | 24 (11.1%) |
| Rotterdam criteria (2003) | 72 (33.2%) |
| Androgen Excess Society criteria (2006) | 66 (30.4%) |
| The hospital’s policy | 33 (15.2%) |
| Other* | 22 (10.1%) |
| Total | 217 (100.0%) |
*The ‘Other’ category was included to capture reasons not explicitly listed in the provided options
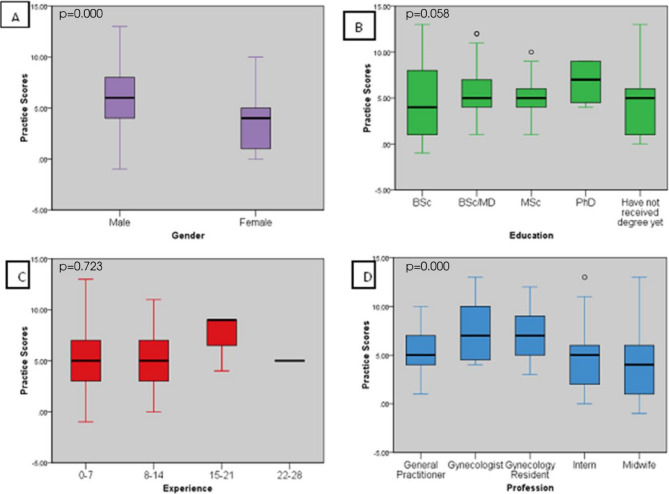
Further analysis by one way analysis of variance revealed a significant effect on knowledge by the independent variables gender (A) (𝐹(1,200) = 12.084,𝑝=0.001), education (B) (𝐹(4,197) = 6.791,𝑝=0.000), and profession (D) (𝐹(4,197) = 16.773,𝑝=0.000), where gynecologists, males and those with PhD having the highest knowledge score while midwives and MSc holders had the lowest scores. On the contrary, experience (F (3,198) = 2.095, p = 0.102) did not show significant impact on knowledge of the health care providers as demonstrated in Fig. 2 although the trend did show higher cores among those with 15–21 years’ experience.
Fig. 2.
Impact of Gender, Education status, Experience, and Profession on knowledge scores of health care providers in selected hospitals. Figure 2A shows impact of gender on knowledge scores of healthcare providers (p = 0.001). Figure 2B shows impact of education on knowledge scores of healthcare providers (p < 0.001). Figure 2C shows impact of Experience on knowledge scores of healthcare providers. Figure 2D shows impact of profession on knowledge scores of healthcare providers (p < 0.001). P-value scores derived from ANOVA
Practice analysis
The results show that out of the 191 valid responses, 22.0% (42 participants) indicated good practice, while 78.0% (149 participants) indicated poor practice. The result, as demonstrated on Fig. 3, shows the criteria used by the clinicians for diagnosing PCOS. The Rotterdam criteria (2003) were the most commonly employed, with 23.3% (49 participants) of the practitioners followed by Androgen Excess Society criteria (2006) and Standardized Protocol at the practice were utilized by 13.3% of participants each (28 per category). The NIH criteria was the least utilized with only 7.6% responses (15 participants).
Fig. 3.

Diagnostic criteria utilized in diagnosing PCOS among health care providers at selected hospitals. ‘Other’ category was included to capture options not explicitly listed in the provided options
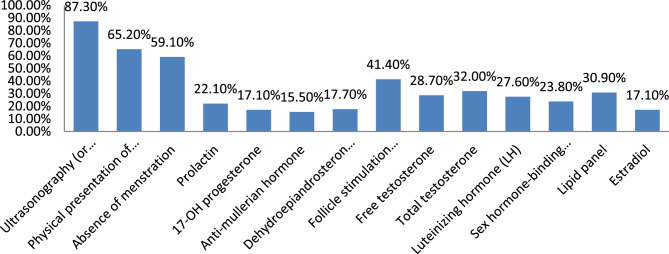
The study further examined the practical diagnosis methods utilized by participants in diagnosing PCOS. The results revealed that ultrasonography (or other imaging of ovaries) was the most commonly used method, with 158 responses (87.3% of cases), followed by physical presentation of hirsutism (118 participants; 65.2% of cases), and absence of menstruation (107 participants; 59.10% of cases). Among the hormonal markers, FSH alone was the most frequently used (75 participants, 41.4% of cases). AMH and DHEAS were only utilized 15.5% (28 participants) and 17.7% (32 participants) respectively. Details can be seen in Fig. 4.
Fig. 4.
Diagnostic tests utilized (IN PRACTICE) in diagnosing PCOS by health care providers at selected hospitals
Moreover, we were interested to see to which type of specialist clinicians refer their patients. The result revealed that, 73.3% of participants (154) referred the patients to gynecologists. Endocrinologists’ referral was the second largest response with 27.9% (65 participants) (Table 2).
Table 2.
Frequency of type of specialists referred to at selected hospitals
| Specialist | Responses’ Frequency (Percent %) |
|---|---|
| Endocrinologist | 65 (27.9%) |
| Gynecologist | 135 (57.9%) |
| Nutrition counselor/dietician | 17 (7.3%) |
| Other* | 16 (6.9%) |
| Total | 233 (100.0%) |
* The ‘Other’ category was included to capture reasons not explicitly listed in the provided options
In association with patient referral, we further tried to explore the reason why 40 participants (19%) did not refer to specialists for the diagnosis and treatment of PCOS. The most significant reason stated was the lack of specialty resources (14 participants; 40% of cases) (Table 3).
Table 3.
Frequency for reasons why respondents don’t refer to specialists
| Reasons | Responses’ Frequency (Percent %) |
|---|---|
| Lack of specialty resources in my community | 14 (40.0%) |
| I feel confident in my practice to diagnose and treat most cases of PCOS | 5 (14.3%) |
| Other* | 18 (51.4%) |
| Total | 37 (105.7%) |
* The ‘Other’ category was included to capture reasons not explicitly listed in the provided options
The ANOVA revealed no significant differences in practice between groups of different experiences and education, with a combined effect of (F(3,187) = 0.442, p = 0.723) and (F(4,186) = 2.328, p = 0.058) respectively. Nevertheless, the general trend showed a peak of practice points for those with 15–21 years of experience, as well as higher practice points for those with PhDs. Moreover, there were significant differences in practice scores for gender (A) (F (1,189) = 26.190, p = 0.000) and professions (D) (F(4,186) = 6.310, p = 0.000.) where males and gynecologists have the highest score respectively (Fig. 5).
Fig. 5.
Impact of Gender, Education status, Experience, and Profession on practice scores of health care providers in selected hospitals. Figure 5A shows impact of gender on practice scores of healthcare providers (p < 0.001). Figure 5B shows impact of education on practice scores of healthcare providers. Figure 5C shows impact of Experience on practice scores of healthcare providers. Figure 5D shows impact of profession on practice scores of healthcare providers (p < 0.001). P-value scores derived from ANOVA
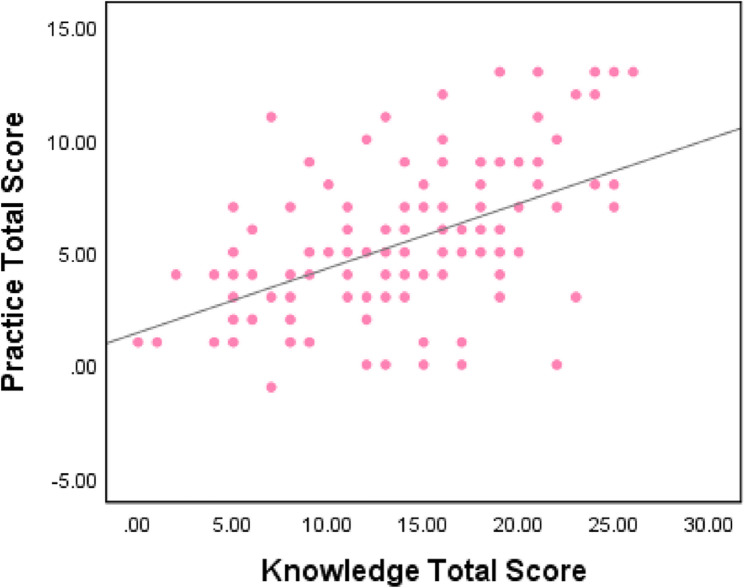
Having observed similar pattern in knowledge and practice score, we did a correlation analysis where a moderate positive correlation between practice and knowledge scores was found as shown by Spearman’s rho correlation coefficient of 0.524 (p < 0.001) (Fig. 6).
Fig. 6.
Correlation between practice and knowledge scores of health care providers at selected hospitals
Attitude analysis
The analysis revealed that the majority of participants, 187 individuals (89.0%), were classified as having a “Good Attitude” towards PCOS and its diagnosis.
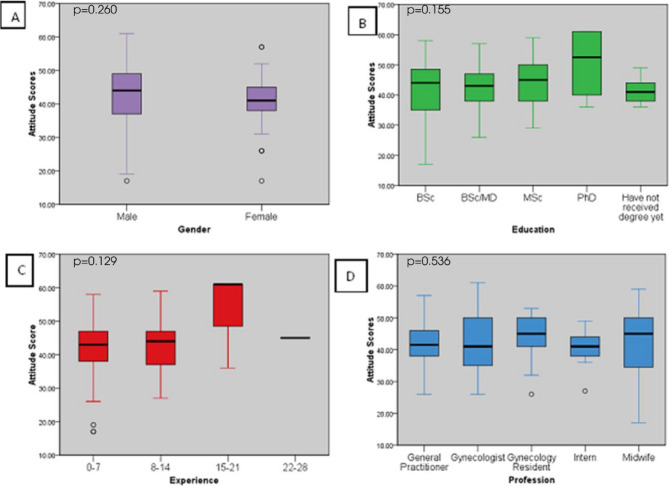
The ANOVA revealed differences in attitude in relation to the variables experience (C) (F(3,199) = 1.910, p = 0.129), gender (A) (F(1,201) = 1.278, p = 0.260), education (B) (F(4,198) = 1.686, p = 0.155), and profession (D) (F(4,198) = 0.786, p = 0.536). Despite no significant difference, there was a trend of higher scores with experience and education where those with 15–21 years of experience and those with a PhD had better attitude scores (Fig. 7).
Fig. 7.
Impact of Gender, Education status, Experience, Profession on attitude scores of health care providers in selected hospitals. Figure 7A shows impact of gender on attitude scores of healthcare providers. Figure 7B shows impact of education on attitude scores of healthcare providers. Figure 7C shows impact of Experience on attitude scores of healthcare providers. Figure 7D shows impact of profession on attitude scores of healthcare providers. P-value scores derived from ANOVA
Discussion
The current study analyzed the level of knowledge, attitude and practice of healthcare professionals across multiple hospitals in Addis Ababa on the diagnosis of PCOS. Diagnosing PCOS based on only ultrasonography, absence of menstruation and physical presentation of hirsutism without biochemical signs of hyperandrogenism is designated as poor knowledge [21]. The majority of participants (79.5%) lacks this understanding of the importance of hormonal tests for PCOS diagnosis; hence fall under “Poor Knowledge” category. The underuse of these tests in our study may reflect a lack of awareness. On the other hand, studies from Nordic Countries and Estonia between 2015 and 2016 recognized high blood androgen levels as a possible marker for PCOS by 93% of participants showcasing deep disparity in using such hormonal markers [24].
Comparatively, ultrasonography (81.8%), physical presentation of hirsutism (71.9%) and absence of menstruation (68.5%) had a significantly high response rate. While ultrasonography can provide valuable information regarding ovarian morphology, it should not be considered a definitive diagnostic tool for PCOS. A study conducted in 2006 [6] on normal women with PCOM over time revealed that at the baseline visit, 57.5% of women exhibited PCOM, but only one subject with PCOM developed irregular menses and presumptive PCOS. This finding suggests that PCOM alone is not a definitive indicator of PCOS development [6]. Therefore, the overreliance of healthcare providers on ultrasonography alone can lead to false diagnosis of PCOS and indicates poor knowledge. Additionally, as introduced by the 2023 edition of the International Evidence-based Guideline for the assessment and management of PCOS, ultrasonography is not deemed necessary for diagnosis of PCOS if both ovulatory dysfunction and hyperandrogenism features are evident, or if one of these features is accompanied by an elevated serum AMH level [21].
This study also investigated the impact of various associated factors on knowledge including experience, gender, level of education, and types of profession. The result highlighted that all gynecologists and gynecology residents displayed the highest knowledge scores, followed by general practitioners and interns while midwives had the lowest scores. Similar to the results of our study, a 2021 study at George Washington University by Conlon evaluating clinicians from different specialties found that specialist clinicians from gynecology knew the importance of following the diagnostic criteria for PCOS diagnosis, while a higher proportion of the general practitioners didn’t respond to this criteria [19]. In another comparable study, physicians in Germany who took a web-based survey in 2023 and 2024 with specialty training in reproductive medicine and gynecologic endocrinology demonstrated a much higher knowledge of correct diagnostic criteria for PCOS (97.9%) compared to those without such training, who had a 51.3% accuracy rate [25].
Furthermore, in the current study, males (31/210) exhibited better knowledge compared to females (12/210), indicating a significant gender-based differences (p = 0.001). This could be due to the greater male representation in specialized professions such as gynecology (10/12 being male). Further research with a larger, and gender balanced, sample size is required to validate this result.
The current study also showed that only 22.0% of respondents exhibited “Good practice” while 78.0% had “Poor practice”. The practice criteria, much like the knowledge criteria, showed various similarities. The results indicated that ultrasonography (or other imaging of ovaries) was the most used method followed by physical presentation of hirsutism. Contrary to our findings, Christ and colleague in 2023 reviewed the current guidelines of PCOS diagnosis and suggests that while acne and female pattern hair loss are common complaints among patients with PCOS, currently, data do not support their use as reliable diagnostic markers for PCOS [26].
PCOS has several recognized phenotypes that present with distinct characteristics and require individualized treatment [19, 23]. This shows that the diagnosis of PCOS based mainly on the physical presentation of symptoms is not good practice, and feeds into poor practice. The Christ, et al. (2023) paper mentioned above on the current guidelines of PCOS recommended the use of the Rotterdam criteria and showed it continues to be the most widely used and accepted criteria for PCOS and were unanimously supported in the 2018 International Evidence-Based Guideline for the Assessment and Management of PCOS [26]. Much like the authors’ suggestion, compared to other criteria, our study shows the Rotterdam criteria is the most identified (33.2%) and used (23.3%) by health care providers.
The practical use of hormonal markers by health care professionals for PCOS diagnosis is low in this study, where FSH alone was the most frequently used, with 41.4% response rate. AMH and DHEAS were utilized only by 15.5% and 17.7% of participants respectively. This aligns with findings from a 2021 United Kingdom study, where despite variations in diagnostic panels, FSH testing on its own was the most frequently requested test by physicians, reported in 86.9% of cases [27]. While FSH remains the most commonly ordered test in our study (41.4%), its utilization rate highlights a notable discrepancy in clinical practice between our setting and the United Kingdom.
Although knowledge regarding the use of hormonal tests for PCOS diagnosis was limited, the actual practice of ordering these tests was even lower. This discrepancy between those who know but do not implement may be largely attributed to the lack of availability and high costs associated with these tests [28, 29]. A 2024 study conducted in Harar [28] emphasized that clinical chemistry tests, including hormonal assays, are among the most frequently interrupted or inaccessible diagnostic tools in Ethiopia [28, 29]. This sentiment is echoed in a 2021 Ugandan study that highlights the incomplete evaluation of the Rotterdam criteria due to lack of laboratory resources [30]. These barriers likely contribute to the underutilization of hormonal tests in clinical practice, despite their importance in the accurate diagnosis of PCOS.
In the current study, 19% of responders indicated they do not refer to other specialties due to various reasons. The most notable reason cited in 40% of cases was the lack of specialty resources. This is unlike the Conlon (2021) mixed method study mentioned earlier, where providers who did not refer indicated that they were confident in their own ability to diagnose and treat most cases of PCOS [19] indicating that lack of accessibility to specialty could be one of the challenges faced only in the context of the low-income country.
An analysis of the effects of type of professions as an independent variable on practice was found to be statistically significant (p < 0.001). The trend analysis showed significant difference observed between professions, where gynecologists and gynecology residents exhibited the highest practice scores, while interns and midwives had the lowest scores in diagnosing PCOS. The relatively poor knowledge and practice score by interns and midwives may partly explain why there were more than 51% of respondents who were in the ‘other’ category for the questions ‘to whom do you refer patients to’, as these group of participants may fail to initially make an assessment and a diagnosis of PCOS and eventually refer them for further investigation to gynecology specialists. More importantly, this underscores the critical need to implement standardized operating procedures for referral criteria and pathways—particularly for interns and midwives, who serve as frontline healthcare providers for patients in most Ethiopian healthcare settings and would benefit significantly from enhanced support and structured guidelines; not just in the referral but also the diagnosis process.
The analysis of participants’ attitudes towards the validity of PCOS information and procedures revealed that the vast majority of participants held a positive and favorable attitude with 89.0% of individuals classified as having a “Good Attitude” towards PCOS and its diagnosis. None of the independent variables had a statistically significant association with participants’ attitude towards PCOS. This suggests that the poor knowledge and practice among healthcare professionals is not due to a dismissal of PCOS as an insignificant condition affecting their patients, but rather stems from insufficient training and education on the subject. The findings indicate that healthcare providers are motivated to address PCOS but lack the necessary knowledge and tools to do so effectively.
Despite its strength of being the first study to investigate the level of knowledge, attitude, and practice of healthcare professionals regarding PCOS in Ethiopia, the study also has some limitations that should be acknowledged. One potential limitation is that the study’s findings may not be fully representative of all healthcare providers due to the relatively small sample size. Additionally, the sample had an imbalance in gender representation, which could influence the interpretation of the results. Future studies should aim for a larger and more gender-balanced sample to enhance the validity and applicability of results. Moreover, in some of the variables, there were responses that were not explicit and grouped as ‘other’ in some categories, which were not prompted and explored further. Hence, future research should incorporate qualitative methods or open-ended questions to explore these ambiguous responses more comprehensively.
Conclusion
The findings of our study highlight significant gaps in knowledge and practices among healthcare professionals regarding the diagnosis of PCOS in a multi-center study in Ethiopia with a significant number relying on insufficient diagnostic methods such as ultrasonography alone. This confirms existing literature regarding the lack of knowledge and, in particular, practice in the use of biochemical methods for the diagnosis of PCOS. Furthermore, this study revealed that certain demographic factors, such as education level and profession, significantly influenced knowledge and practice levels.
Supplementary Information
Supplementary File 1: Scoring System for Knowledge and Practice Questions.
Supplementary File 2: Operational Definition-Categorization of good and poor attitude definition.
Supplementary File 3: Socio-demographic frequencies among respondents at selected hospitals.
Acknowledgements
We are sincerely grateful to the Addis Ababa University College of Health Sciences, Department of Medical Laboratory Sciences, for granting ethical approval for our research. We would also like to express our heartfelt gratitude to the healthcare professionals who voluntarily participated in our study despite their busy schedules, which significantly contributed to the success of this research.
Abbreviations
- AMH
Anti-Müllerian hormone
- ANOVA
Analysis of variance
- BSc
Bachelor of science
- CAH
Congenital adrenal hyperplasia
- DRERC
Departmental research and ethics review committee
- DHEAS
Dehydroepiandrosterone sulfate
- EPHCG
Ethiopian primary healthcare clinical guideline
- FSH
Follicle stimulating hormone
- LH
Luteinizing hormone
- MD
Medical doctor
- MSc
Masters in science
- NIH
National institutes of health
- PCOM
Polycystic ovarian morphology
- PCOS
Polycystic ovary syndrome
- PhD
Doctor of philosophy
- SHBG
Sex hormone-binding globulin
- SPSS
Statistical package for social sciences
Authors’ contributions
The authors confirm contribution to the paper as follows: Study Conception and Design: EWE, MMY, HMM, SFW, EMA, MNData Collection and Processing: EWE, MMY, HMM, SFW, EMAData Analysis and presentation: EWE, MNInterpretation of Results: EWE, MMY, HMM, SFW, EMA, MNDraft Manuscript Preparation: EWE, MMY, HMM, SFW, EMA, MT, AS, MNReviewing and editing thesis and manuscript: EWE, MMY, HMM, SFW, EMA, MT, AS, MNAll authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Ethical clearance was obtained from Addis Ababa University, College of Health Sciences, Department of Medical Laboratory Sciences and Departmental Research and Ethics Review Committee (DRERC) [Ref. No. MLS/198/24]. Letter of permission was obtained from the Department of Medical Laboratory Science to select hospitals. Additionally, ethical clearance was obtained from the Addis Ababa Health Bureau for the hospitals under the Ministry of Health. [Ref, No.A/A/H/16227/227].
Informed oral consent was taken from each participant prior to the questionnaire. Participants were informed that their participation was purely voluntary. Participants were told they were free to not answer any question if unwilling and could withdraw at any time. All data gathered from participants in the study were kept totally confidential. Only principal investigators had access to the data and the Google Forms responses were deleted when they were no longer needed, ensuring data security.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Romitti M, Fabris VC, Ziegelmann PK, Maia AL, Spritzer PM. Association between PCOS and autoimmune thyroid disease: a systematic review and meta-analysis. Endocr Connect. 2018;7(11):1158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan WC. A guide to understanding polycystic ovary syndrome (PCOS). J Fam Plann Reprod Health Care. 2014;40(3):217–25. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Polycystic ovary syndrome 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/polycystic-ovary-syndrome
- 4.Gunkaya O, Tekin A, Bestel A, Arslan O, Şahin F, Taymur B, et al. Is polycystic ovary syndrome a risk factor for depression and anxiety? A cross-sectional study. Rev Assoc Med Bras. 2024;70: e20230918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lujan ME, Chizen DR, Pierson RA. Diagnostic criteria for polycystic ovary syndrome: pitfalls and controversies. J Obstet Gynaecol Can. 2008;30(8):671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy MK, Hall JE, Adams JM, Lee H, Welt CK. Polycystic ovarian morphology in normal women does not predict the development of polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(10):3878–84. [DOI] [PubMed] [Google Scholar]
- 7.Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metab. 2020;106(3):e1071-83. [DOI] [PubMed] [Google Scholar]
- 8.Artini P, Berardino O, Simi G, Papini F, Ruggiero M, Monteleone P, et al. Best methods for identification and treatment of PCOS. Minerva Ginecol. 2010;62:33–48. [PubMed] [Google Scholar]
- 9.Rao P, Bhide P. Controversies in the diagnosis of polycystic ovary syndrome. Therapeutic Adv Reproductive Health. 2020;14:2633494120913032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomes MO, Gomes JO, Ananias LF, Lombardi LA, da Silva FS, Espindula AP. Anti-Mullerian hormone as a diagnostic marker of polycystic ovary syndrome: a systematic review with meta-analysis. Am J Obstet Gynecol. 2025;232(6):506–e237. [DOI] [PubMed] [Google Scholar]
- 11.Casadei L, Fanisio F, Sorge RP, Collamarini M, Piccolo E, Piccione E. The diagnosis of PCOS in young infertile women according to different diagnostic criteria: the role of serum anti-Müllerian hormone. Arch Gynecol Obstet. 2018;298:207–15. [DOI] [PubMed] [Google Scholar]
- 12.Sahmay S, Aydin Y, Oncul M, Senturk LM. Diagnosis of polycystic ovary syndrome: AMH in combination with clinical symptoms. J Assist Reprod Genet. 2014;31:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Köninger A, Koch L, Edimiris P, Enekwe A, Nagarajah J, Kasimir-Bauer S, et al. Anti-mullerian hormone: an indicator for the severity of polycystic ovarian syndrome. Arch Gynecol Obstet. 2014;290(5):1023–30. [DOI] [PubMed] [Google Scholar]
- 14.Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123–9. [DOI] [PubMed] [Google Scholar]
- 15.Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–84. [DOI] [PubMed] [Google Scholar]
- 16.Bestel A, Elmas B. Could sestrin protein in serum be a new marker of oxidative stress in patients with polycystic ovary syndrome? Gynecol Endocrinol. 2022;38(12):1109–13. [DOI] [PubMed] [Google Scholar]
- 17.Abdullah AA, Ahmed M, Oladokun A. Prevalence of infertility in sudan: A systematic review and meta-analysis. Qatar Med J. 2021;2021(3):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muhaidat N, Mansour S, Dardas M, Qiqieh J, Halasa Z, Al-Huneidy L, et al. Current awareness status of and recommendations for polycystic ovarian syndrome: a national cross-sectional investigation of central Jordan. Int J Environ Res Public Health. 2023;20(5): 4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conlon JL. Diagnosis and Management of Adolescents with Polycystic Ovary Syndrome in Primary Care: A Mixed Method Study to Explore Provider Behaviors and Barriers and Facilitators to Practice, PhD Thesis, George Washington University; 2021 Available from: https://hsrc.himmelfarb.gwu.edu/smhs_crl_dissertations/6
- 20.Chemerinski A, Cooney L, Shah D, Butts S, Gibson-Helm M, Dokras A. Knowledge of PCOS in physicians-in-training: identifying gaps and educational opportunities. Gynecol Endocrinol. 2020;36(10):854–9. [DOI] [PubMed] [Google Scholar]
- 21.Teede HJ, Tay CT, Laven JJE, Dokras A, Moran LJ, Piltonen TT, Costello MF, Boivin J, Redman LM, Boyle JA, Norman RJ, Mousa A, Joham AE. International PCOS network. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Eur J Endocrinol. 2023;189(2):G43–64. [DOI] [PubMed] [Google Scholar]
- 22.Teoh WS, Ramu D, Indran IR, Chua MWJ, Thu WPP, Yong EL. Diagnosis and management of polycystic ovary syndrome: perspectives of clinicians in Singapore. Ann Acad Med Singap. 2022;51(4):204–12. [PubMed] [Google Scholar]
- 23.Pfieffer ML. Polycystic ovary syndrome: diagnosis and management. Nurse Pract. 2019;44(3):30–5. [DOI] [PubMed] [Google Scholar]
- 24.Piltonen TT, Ruokojärvi M, Karro H, Kujanpää L, Morin-Papunen L, Tapanainen JS, et al. Awareness of polycystic ovary syndrome among obstetrician-gynecologists and endocrinologists in Northern Europe. PLoS One. 2019;14(12): e0226074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofmann K, Oehler M, Ruckes C, Dionysopoulou A, Stewen K, Schiestl LJ, et al. Gaps in knowledge regarding the diagnostic criteria and management of PCOS in germany: an anonymous web-based survey. Heliyon. 2024;10(22):e40431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christ JP, Cedars MI. Current guidelines for diagnosing PCOS. Diagnostics. 2023;13(6):1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karia AM, Duff CJ, Heald AH, Britton I, Fryer AA, Wu P. Investigation of polycystic ovarian syndrome: variation in practice and impact on the speed of diagnosis. Cardiovasc Endocrinol Metab. 2021;10(2):120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zakir D, Mekonnen GK, Negash B, Marami D. Level of health laboratory service quality, service interruptions, and its predictors in public hospitals in Harar town, Eastern Ethiopia. Front Health Serv. 2024;4:1492766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kedir A, Desale A, Gashu A, Mohammed A, Getahun A, Alemu A et al. ETHIOPIAN ESSENTIAL HEALTH LABORATORY DIAGNOSTICS LIST: Ethiopian Public Health Institute -. First ed January. Addis Ababa, Ethiopia. 2024;1(1):1-120.
- 30.Pebolo FPAA, Alobo G. Polycystic ovarian syndrome: diagnostic challenges in resource-poor settings (Ugandan perspectives). PAMJ Clin Med. 2021;5(41). 10.11604/pamj-cm.2021.5.41.26386.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File 1: Scoring System for Knowledge and Practice Questions.
Supplementary File 2: Operational Definition-Categorization of good and poor attitude definition.
Supplementary File 3: Socio-demographic frequencies among respondents at selected hospitals.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.