Abstract
Wheat (Triticum aestivum) is a staple food crop providing essential nutrition to global population. However, water scarcity and increasing drought stress, because of climate change, threaten its productivity. Oxidative stress increases the production of reactive oxygen species (ROS) due to drought which damages the plant cellular metabolism. Plants counteract this by regulating the transcription of enzymes like catalases, peroxidases, and superoxide dismutase. This research investigated activated biochar’s (AB) role on wheat cultivars under deficit irrigation. Activated biochar significantly reduced lipid peroxidation (27–56%) while increasing antioxidant activities (40–60%) under low irrigation as compared to the control (no biochar), suggesting its potential to improve drought resilience. Peroxidase, for presenting significantly higher antioxidant activity, was selected as a key enzyme for molecular docking. Protein-protein interactions between the DREB1 transcription factor and peroxidase, supported by hydrogen bonding, electrostatic interactions, and hydrophobic forces, highlighted the role of biochar mediated peroxidase in oxidative stress response. This interaction highlighted the role of DREB1 in drought resilience, presenting a range of protein sizes, isoelectric points, and stability indices across TaDREB proteins in the wheat genome. Subcellular localization analysis demonstrated that most TaDREB genes, particularly DREB1, are active in the nucleus. At the same time, some are localized to chloroplasts and mitochondria, suggesting diverse roles in stress response and energy metabolism. Phylogenetic analysis grouped DREB genes from wheat (TaDREB), maize (ZmDREB), and Arabidopsis (AtDREB), indicating conserved evolutionary functions across monocot and dicot species. Motif and domain prediction revealed conserved AP2 domains across TaDREB genes, emphasizing their structural and functional conservation, which likely evolved through gene expansion to enhance stress tolerance. These findings are crucial for understanding biochemical attributes of drought responsive transcription factors and their interactive response with antioxidants, which can further help in gene editing technology.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-025-06938-4.
Keywords: Transcription factors, Antioxidants, Lipid peroxidation, TaDREB, Subcellular localization, Phylogeny
Introduction
Triticum aestivum is the prime food source for many European, African, and Asian countries [1, 2]. It is the major constituent of human diet carrying protein (13%), carbohydrates (71%), fat (1.5%), and water (13%) [3]. By 2023, an exceeded world population is estimated to be 8 billion, and ensuring food security by then will be a major concern for the agricultural sector [4]. Drought severity is growing because of raising temperature and climate change, causing crops to suffer production loss. These production losses compromise food security, economic growth, and crop cultivation. Scientists and breeders have serious concerns about it, particularly considering the concerned forecast predicting that 65% of the world’s population will experience acute water shortages by 2025. It is difficult to understand plant’s resilience against water stress because it depends on several different plant characteristics [5] including physiological functions [6] and patterns of gene activity [7].
During water scarce conditions, plants lower their metabolic activities, especially photosynthesis and respiration, and lower their stomatal conductance among physiological attributes [8]. These factors lead to reduced growth, such as leaf area, stunted plant growth leading to production losses [9]. Under the effect of these harsh conditions, plants produce reactive oxygen species (ROS) from oxygen (such as H2O2 and O2) that are highly reactive and cause oxidative damage to cellular structures and molecules [10], including proteins, lipids, and nucleic acid [11]. Oxidative damage caused by ROS in cells leads to imbalance in free radicals and antioxidants. To counteract the reactive oxygen species, plant cells activate their enzymatic antioxidants such as peroxidase, catalase, superoxide dismutase, and non-enzymatic antioxidants like malondialdehyde, proline, and sugar contents.
Enzymatic antioxidants scavenge free radical superoxide (O2) and hydrogen peroxide (H2O2) and maintain redox balance in cells by mitigating oxidative stress in plants during stress conditions to ensure plant survival [12, 13]. Among the antioxidants, superoxide dismutase converts O2 radicals into H2O2, while catalase and peroxidase further break down H2O2 into harmless water and oxygen, thus mitigating cellular damage caused by ROS accumulation [14]. Compared to other antioxidants, peroxidase catalyses compounds, including amines, p-phenylenediamine, phenols, benzidine, and hydroquinone by hydrogen peroxide. The specificity lies in its high affinity with peroxides that are produced in abiotic stress, especially H2O2. In biochar amended soil, the reduction of H2O2 production can be associated with significantly increased peroxidase activity [6].
Biochar has appeared as an effective soil amendment to improve crop production [15, 16] especially under abiotic stress conditions [17]. It has an alkaline nature with high cation exchange capacity (CEC) that enables it to reclaim degraded soil [18, 19]. Further, the activated form of biochar reduces detrimental effects of abiotic stress by improving soil physiochemical properties, especially water holding capacity [20]. Biochar addition to soils improves potassium (K+) uptake, which ultimately ameliorates plant performance [15–18]. Biochar’s influence on soil properties, especially pH adjustment and increased K+ availability, may trigger calcium- and ROS-mediated signalling, hence promoting hormonal activity [21]. Improved germination and root development were observed due to these hormonal and gene expression changes [22].
Biochar mediated photosynthetic and antioxidant pathways are extended to modulation of gene expression networks enriching photosynthetic and antioxidant pathways [23]. Activation of biochar, using organic sources, has also been acknowledged as an important amendment to enhance crop water use efficiency (WUE) [23]. The use of activated biochar for enhancing carbon assimilation, nutrient uptake, and antioxidant activities leads to enhanced plant growth under limited water conditions [24]. This modulation highlight the need to understand biochar’s interactions with signalling pathways and transcription factors and its potential for enhancing drought resilience in wheat. The early plant response to different abiotic stresses includes cascades of signalling reactions at cellular and molecular levels involving phosphorylation by kinases, membrane permeability, and gene expression regulation by transcription factors [25]. The APETALA2/Ethylene Responsive Factors (AP2/ERF) is a superfamily of transcription factors that play a role in addressing biotic and abiotic stress. This group is the most imperative family of plant transcription factors that share an AP2 conserved DNA-binding domain with about a 60 amino acids long stretch bound to a DNA motif in the gene promoter region such as dehydration-responsive elements [26].
However, the AP2/ERF superfamily is classified into four sub-families based on different conserved domain sequences such as RAV, AP2, ERF (ethylene-responsive factor), and DREB (dehydration-responsive element binding protein). The RAV class has the B3 and AP2 domains, the AP2 has multiple AP2 subdomains, and the ERF and DREB subfamilies comprise one AP2 domain each that diverges in amino acid residues [27]. Researchers reported that increased DREB1 expression led to higher accumulation of mRNA, which contributed to improved dehydration or drought tolerance in plants [28].
Different plant species are reported with a number of AP2/ERF genes including 145 in Arabidopsis [27], 146 in tomato [29], 167 in rice [30], 132 in sesame [31], 119 in kiwifruit [32], and 119 in Chinese jujube [33]. Xie et al. [34] reported that DREB1 regulates genes responsive for abiotic stress in Arabidopsis. But researchers reported that there is a binding preference in DREB1 and DREB2 with a higher affinity for the A/GCCGAC region, which is the dehydration-responsive element (DRE) core motif [27]. Molecular docking of the DREB1 gene in Phaseolus vulgaris revealed its preferential binding to the GCC sequence in the DNA double-helical structure [35]. But there is no previous in-silico study on wheat showing the role of DREB1 in drought stress resilience.
Molecular docking is an effective tool to identify putative inhibitors for protein-protein interactions along with molecular simulation used to identify the binding orientation. To find the most suited binding regions based on the targeted protein active sites of DREB1 protein and biochar-mediated antioxidant enzymes were employed for protein-protein docking. Docking is a time- and cost-effective method for studying the interactive bonding between molecules. Researchers are endeavoring to develop drought-tolerant wheat varieties that should be eco-friendly as well. For this purpose, an insightful understanding of molecular patterns and domain analysis of stress responsive transcription factors is very crucial.
Hence, the current study aimed to find the molecular mechanisms of drought resilience in wheat by investigating the interaction between DREB1 proteins and peroxidase, analyzing the physiochemical properties, subcellular localization, and evolutionary relationships of the TaDREB1 gene family, and predicting conserved motifs and domains. By these findings, the study tends to evaluate whether there is any interaction between the DREB1 and peroxidases under drought stress. Therefore, it was hypothesized that understanding the genome-wide patterns of DREB1 along with its compatibility with the peroxidase enzyme can provide insights into their roles in stress response and gene regulation. Hence, this study can suggest that the enhancement of TaDREB1 gene expression in wheat with specific modifications can improve drought stress tolerance.
Material and method
This study comprised a field experiment conducted at University of the Punjab, Lahore, Pakistan (N 31° 30’ 4.3236”, E 74° 18’ 5.4684). Experiment was designed as split plot with randomized complete block arrangement in three replicates having biochar as main plot divided into cultivars with irrigation levels as subplots. Factors under observation comprised mainly of three cultivars (Dilkash-2020, Akbar-2019, and Faisalabad-2008 as C1, C2, and C3, respectively), activated biochar (0, 5, and 10 tons/ha as AB0, AB1, and AB2, respectively), and irrigation regimes (100%, 70%, and 50% field capacity). Field capacity was measured by soil moisture content throughout the cropping period using a moisture meter (Lutron PMS-714), and irrigation regimes were maintained with regular intervals to maintain the field capacity. Stress was imposed and maintained by applying reduced irrigation, i.e., 70% and 50% field capacity. Activated acacia biochar (AB) was applied manually to the top 15 cm of the soil in plots (8 × 8 ft) and thoroughly mixed. Biochar was produced from pyrolysis of acacia (Acacia nilotica) wood twigs and activated using perlite and vermicompost comprising 1:1:1 ratio of each.
Antioxidant enzyme assay
Lipid peroxidation was observed by the malondialdehyde content quantification using thiobarbituric acid reactive substance (TBARS) [36]. The fresh leaf (1 g) sample was taken, crushed, and homogenized with the trichloroacetic acid (TCA) as 0.1% solution. The supernatant, after centrifugation at 12,000 rpm for 30 min, was incubated with 20% TCA and 0.5% thiobarbituric acid (TBA). By placing it in ice bath for 10 min, the reaction was stopped, and the product was centrifuged at 10,000 rpm for 15 min. Supernatant was taken to check the absorbance at 440 nm, 532 nm, and 600 nm. The malondialdehyde content was then calculated using a standard extinction coefficient (155 mM−1 cm−1) which indicated lipid peroxidation. Beauchamp and Fridovich’s (1971) method was applied to find the superoxide dismutase (SOD) activity [37]. The activity of peroxidase (POD) was analyzed by the method of Gorin and Heidema [38]. Following Iwase et al. (2013), catalase activity in fresh leaf samples was analyzed [39]. Data collected was statistically analyzed utilizing IBM SPSS Statistics 23 and Origin 2024b software.
Protein-protein docking
Based on results observed from antioxidant activity in wheat under deficit irrigation along with activated biochar amendment, an active enzyme was selected. Protein-protein docking was practiced, observing the possible interactions among dehydration-responsive element binding protein (DREB1) with antioxidant peroxidase, as significant variation in peroxidase was observed. The structure of DREB1 (receptor) in PDB format was retrieved from a database named AlphaFood protein structure (https://alphafold.ebi.ac.uk/) and PDB format of the peroxidase (ligand) protein was recovered from the RCSB protein databank (https://www.rcsb.org/). Molecules were prepared for protein-protein docking in PyMol software. Docking was performed using the HDOCK server, and results were retrieved from the server’s webpage (http://hdock.phys.hust.edu.cn/data/66a7dfafcfa39/). The product of docking was then analyzed on the PLIP server, which is an interaction profiler (https://plip-tool.biotec.tu-dresden.de/plip-web/plip/index). Dimensional structures were then observed on the Discovery Studio visualizer.
In-silico analysis of the DREB1 gene family in T. aestivum
The DREB1 protein sequence of Arabidopsis thaliana was obtained from the online database Phytozome v.13 (https://phytozome-next.jgi.doe.gov/). The Sequence obtained from Phytozome was use as a query to identify DREB1 protein sequences in T. aestivum (https://phytozome-next.jgi.doe.gov) using the Protein-basic local alignment search tool (BLAST-P) from Phytozome v13 database. The sequence of amino acids was ratified on the NCBI CDD (Conserved Domain Database) and Motif Finder database using default parameters (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) [40]. The RNA seq data obtained from the NCBI GEO database (https://www.ncbi.nlm.nih.gov/geo/) was analyzed to explore the expression patterns of the DREB1 gene family, which plays a key role in enhancing stress resilience in wheat under drought conditions. The genome map of wheat was sourced from Phytozome.v13 (https://phytozome-next.jgi.doe.gov/info/).
Physiochemical and subcellular localization
Physiochemical characterization of DREB1 genes, such as molecular weight, protein length (amino acid residues), instability index, GRAVY, and isoelectric point (pI), were observed using the program ProtParam (http://web.expasy.org/protparam/) [41]. Subcellular localization of DREB1 was anticipated from the online available program, i.e., WoLF PSORT (https://wolfpsort.hgc.jp/) (Horton et al. 2006). The TBTool-II was operated to construct a heatmap of the localization of DREB1 in different cellular organelles [42].
Phylogenetic analysis of the DREB1 gene
To compare the sequence of amino acids of DREB1 in different plant species for phylogenetic analysis, MEGA-11 software was used. Species under the study included T. aestivum (wheat), O. sativa (rice), Z. mays (maize) and A. thaliana [43]. A phylogenetic tree was then constructed using the full-length sequence of amino acids of DREB1 genes from wheat (Ta), A. thaliana (At), and Z. mays (Zm). The amino acids were initially aligned using MUSCLE (Multiple Sequence Alignment), and a phylogenetic tree was subsequently generated to examine the evolutionary relationship of the DREB1 gene across different species using 1000 bootstrap tests by neighbor-joining (NJ) and paired deletion [44]. The program iTol was utilized for visualization of the phylogenetic tree (https://itol.embl.de/personal_page.cgi).
Motifs and domain prediction
Motif analysis of DREB1 in T. aestivum was performed using Multiple Em for Motif elucidation (https://meme-suite.org/meme/tools/meme). Domain analysis of the DREB1 protein was conducted using the NCBI Conserved Domain Search tool with default parameters (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) [45].
Results
Antioxidant enzyme assay
Statistical analysis of observed antioxidant assays illustrates the complex and variable responses of cultivars towards deficit irrigation and AB treatments. A significant difference in catalase activity of differently treated wheat cultivars was observed under deficit irrigation. For C1 (cultivar Dilkash-2020), maximum catalase activity was observed from AB2 under 50% DI, and the least was observed with no treatment under control. Similar trends were observed for C2 (Akbar 2020) and C3 (Faisalabad 2008) (Fig. 1.A). Results showed that both levels of AB treatment significantly enhanced catalase activity by 44–49% in wheat under deficit irrigation, increasing the stress resilience. Maximum peroxidase activity was observed from C2 treated with AB2 followed by AB1 treatment under 50% DI, and the lowest was in C3 in control with no treatment (Fig. 1.B). Comparatively, peroxidase activity was higher among other antioxidants (Fig. 1). Superoxide dismutase activity was highest in C1 at 50% DI, followed by C2 in 50% DI treated with AB2, and the lowest was in C1 under control with no treatment (AB0) (Fig. 1.C). The AB amendment in deficit irrigation increased the antioxidant activity 44–57% in all cultivars. Whereas AB0 showed low antioxidant activity under deficit irrigation. Lipid peroxidation was significantly reduced by AB under deficit irrigation, whereas DI increased it in all cultivars. Compared to AB0, the treatments AB1 and AB2 decreased lipid peroxidation by 27% and 56%, respectively. Maximum peroxidation was observed from control in C1, followed by C3 with AB0 in control (Fig. 1D). The least was observed from AB2 in control by C1.
Fig. 1.
Effect of activated biochar amendment on activity of catalase (A), peroxidase (B), superoxide dismutase (C), and lipid peroxidation (D) of wheat plants cultivated in normal and deficit irrigation conditions. The error bars show the standard deviation, and alphabets represent the differences among treatments at p ≤ 0.05 level of significance (n = 3)
Peroxidase-DREB1 interaction
A stable protein-protein interaction was observed between peroxidase and dehydration-responsive element binding protein. Protein-protein docking revealed that there is strong hydrogen bonding and electrostatic interactions, indicating a stable interaction (Fig. 2). Key residues involved in hydrogen bonding include ASN 154 and THR 156, both contributing to the binding stability with moderate bond lengths ranging from 2.41 Å to 3.22 Å (Fig. 2C-G). Salt bridges were observed between ASP 157 and ASP 272 with the ligand, providing additional stabilization at longer distances of about 5.36 Å and 5.37 Å, respectively (Table 1). Hydrophobic interactions were also noted, particularly involving THR 156, which further supports the overall binding affinity of the proteins (Table 1). The porphyrin IX (HEM) groups showed several strong hydrogen bonds, especially with ARG 31, GLN 169, and LYS 171, with short bond distances around 2.7 Å to 3 Å, indicating a strong electrostatic bond between the positively charged residues and the ligand (Table 2). The variability in hydrogen bond distance (e.g., 1.91–3.97 Å) and donor angles (e.g., 111.39°–177.2°) reflects structural flexibility in protein interactions, which is perhaps associated with protein stability variation and functional adaptability. The occurrence of salt bridges with different distances (i.e., 5.36 Å, 5.37 Å) reflects differences in charge distribution, which can influence the isoelectric points of proteins. These structural differences in interactions could represent underlying differences in TaDREB protein sizes, stability, and pI, which determine their functions in stress responses. These findings suggest that the binding between DREB1 and the peroxidase enzyme is supported by a combination of hydrogen bonds, hydrophobic interactions, and electrostatic forces, contributing to a stable and specific interaction. These bonding suggest interaction between DREB1 and peroxidase by acting as the primary non-covalent bond between specific amino acid residues on both protein surfaces, allowing them to “dock” together. This docking is attained in a specific orientation due to the complementary distribution of these bonds in each protein molecule. The specific arrangement of polar and nonpolar amino acids is essential on each protein that can facilitate docking interaction with its corresponding complementary residue on the other protein.
Fig. 2.
Structural configuration of protein-protein docking with Ligand protein peroxidase (A) DREB1 protein retrieved from AlphaFood protein structure database (B) GSH-A-1794 Binding sites in Molecule-Protein interaction (C) GSH-A-1794 Binding sites in Molecule-Protein interaction (D) HEM-A-1350 Binding sites in Molecule-Protein interaction (E) HEM-B-1350 Binding sites in Molecule-Protein interaction (F) 2D-structural configuration of Ligand-protein interaction (G-H)
Table 1.
Occurrence of hydrogen bond and salt bridges at GSH-A-1794 and GSH-B-1794 binding sites in protein-protein interaction
| RESNR | RESTYPE | RESCHAIN | DIST_H-A | DIST_D-A | DON_ANGLE |
|---|---|---|---|---|---|
| GSH-A-1794 | |||||
| Hydrogen Bonds | |||||
| 154 | ASN | A | 2.41 | 3 | 117.62 |
| 154 | ASN | A | 3.22 | 3.98 | 139.3 |
| 156 | THR | A | 3.14 | 4.02 | 150.71 |
| Salt Bridges | |||||
| 157 | ASP | A | 13,511,352 | 1794 | 5.36 |
| 272 | ASP | A | 22,232,224 | 1794 | 5.37 |
| GSH-B-1794 | |||||
| Hydrophobic Interactions | |||||
| 156 | THR | B | 3.86 | 4790 | 3652 |
| Hydrogen Bonds | |||||
| 156 | THR | B | 3.09 | 3.93 | 146.07 |
| 156 | THR | B | 3.01 | 3.93 | 150.68 |
Table 2.
Occurrence of hydrogen bond at HEM-1350 (chain A and B) binding sites in protein-protein interaction
| RES NR |
RES TYPE |
DIST_ H-A |
DIST_ D-A |
DON_ ANGLE |
PROT ISDON |
DONOR IDX |
DONOR TYPE |
ACCEPTOR IDX |
ACCEPTOR TYPE |
|---|---|---|---|---|---|---|---|---|---|
| Chain A | |||||||||
| 31 | ARG | 2.7 | 3.44 | 131.99 | TRUE | 431 | Ng+ | 2466 | O3 |
| 31 | ARG | 2.82 | 3.3 | 111.39 | FALSE | 2466 | O3 | 428 | Ng+ |
| 38 | ARG | 2.96 | 3.97 | 170.58 | FALSE | 2446 | N3 | 479 | Ng+ |
| 165 | HIS | 2.16 | 3.18 | 171.86 | FALSE | 2438 | N3 | 1404 | N2 |
| 169 | GLN | 1.91 | 2.89 | 171.96 | TRUE | 1427 | Nam | 2460 | O3 |
| 171 | LYS | 3.13 | 4.09 | 156.63 | TRUE | 1449 | N3+ | 2465 | O3 |
| 171 | LYS | 2.06 | 2.95 | 148.94 | TRUE | 1441 | Nam | 2465 | O3 |
| Chain B | |||||||||
| 28 | SER | 2.68 | 2.76 | 174.45 | TRUE | 389 | O3 | 2437 | N |
| 28 | SER | 2.95 | 3.03 | 168.91 | TRUE | 387 | O1 | 2421 | O |
| 31 | ARG | 2.97 | 3.41 | 150.61 | FALSE | 2467 | N4 | 479 | O |
| 34 | ALA | 2.9 | 3.03 | 151.32 | TRUE | 385 | O1 | 2472 | N |
| 37 | ILE | 2.98 | 3.24 | 173.18 | TRUE | 385 | O2 | 2435 | N |
| 37 | ILE | 2.59 | 2.81 | 161.64 | TRUE | 371 | O2 | 2470 | O |
| 61 | PHE | 2.72 | 3.14 | 177.2 | TRUE | 496 | O3 | 2436 | N |
| 65 | GLU | 2.75 | 2.97 | 170.48 | TRUE | 473 | O2 | 1421 | O |
| 70 | ASP | 2.91 | 2.98 | 174.45 | TRUE | 469 | O2 | 1418 | O |
Physiochemical properties determination
The physiochemical properties of the TaDREB gene family (Supplementary Table 1) provide insights into their structural and functional roles. The length of the TaDREB peptides ranges from 79 amino acids (TaDREB3) to 1256 amino acids (TaDREB15), with molecular weights (MW) ranging from 8,862.08 Da (TaDREB3) to 139,772.88 Da (TaDREB15). Generally, larger proteins, such as TaDREB15, are likely involved in more complex or multi-domain functions, while smaller proteins like TaDREB3 may have more specialized roles. The isoelectric (pI) values of TaDREB proteins range from 4.45 (TaDREB36) to 11.66 (TaDREB3), indicating a wide spectrum of acidic to basic proteins. Proteins with lower pI values (acidic), like TaDREB36 (4.45), are likely to be negatively charged under physiological conditions (pH ~ 7), while those with higher pI values, such as TaDREB3 (11.66), are more likely to be positively charged.
The variation in pI suggests that the TaDREB proteins function in different cellular environments or interact with differently charged molecules. All TaDREB proteins exhibit negative GRAVY values, with TaDREB43 showing the most negative score (−1.010) and TaDREB8 showing the least negative score (−0.145). Negative GRAVY scores indicate that these proteins are hydrophilic and tend to interact with water and polar molecules, suggesting that TaDREB proteins are soluble and may be localized in aqueous environments like the cytoplasm or nucleus. The TaDREB43 with the most negative GRAVY value may have a strong preference for interacting with water, while TaDREB8 may have a slight balance between hydrophobic and hydrophilic residues.
The instability index (II) ranges from 35.10 (TaDREB12) to 73.13 (TaDREB38), with proteins having an instability index above 40 generally considered unstable. Most TaDREB proteins, including TaDREB38, TaDREB13, and TaDREB24, exhibit high instability scores, suggesting they might have short half-lives in vivo or undergo rapid degradation. However, several proteins, such as TaDREB12 and TaDREB8, have low instability indices, indicating they are stable and may function for longer periods without degradation.
The TaDREB genes are spread across various chromosomes (1BL, 2DL, 5BL, etc.), and the strands indicate whether they are found on the forward (F) or reverse (R) DNA strand. This distribution suggests a diverse role across the wheat genome, potentially contributing to stress responses in different tissue types or under various environmental conditions. This analysis underscores the importance of TaDREB proteins in stress response mechanisms, particularly their hydrophilic nature and varying levels of stability, which may influence their functional efficiency under stress conditions. The above-mentioned description of TaDREB proteins describes the binding ability, stability, and distribution of TaDREB proteins. These insights are helpful for researchers to select their gene of interest with detailed specificity to use it for gene editing techniques and develop stress resilient wheat germlines.
Subcellular localization
The heatmap (Fig. 3) shows subcellular localization of TaDREB genes across various cellular organelles such as nucleus (Nucl), mitochondria (Mito), chloroplast (Chlo), cytoplasm (Cyto), endoplasmic reticulum (ER), Golgi apparatus, and more. Most TaDREB genes, especially TaDREB1, TaDREB2, TaDREB13, and TaDREB16, show strong nuclear localization in the nucleus column. This suggests that these genes are highly active within the nucleus, a typical feature of transcription factors like DREBs, which regulate gene expression. Some TaDREB genes, such as TaDREB4, TaDREB5, TaDREB14, and TaDREB37, exhibit significant chloroplast localization. This indicates potential involvement in processes related to photosynthesis or responses to light, which aligns with the fact that some DREB proteins are involved in stress responses triggered by environmental factors. While most genes do not show strong mitochondrial localization, a few (like TaDREB3, TaDREB7, and TaDREB39) do have presence in the mitochondria, hinting at possible roles in regulating stress responses associated with energy metabolism. TaDREB26 is noteworthy for showing localization in both the cytoplasm and nucleus, which may suggest that this gene plays roles in both cytoplasmic processes and nuclear gene regulation.
Fig. 3.
Subcellular localization of DREB1 proteins in Triticum aestivum
Most genes show minimal or no localization in organelles like the Golgi apparatus, vacuole, and endoplasmic reticulum (ER), indicating that these organelles might not be the primary sites for DREB gene activity in wheat. Some genes, specifically TaDREB5, TaDREB28, and TaDREB39, are localized in multiple organelles, indicating a potentially broader functionality (Fig. 3). For example, TaDREB5 is present in the chloroplast, cytoplasm, and plasma membrane, suggesting it may interact with multiple cellular components to mediate stress responses or signalling pathways. A few genes (e.g., TaDREB43) exhibit less common localization patterns, such as in the extracellular matrix (Extr) and Golgi, suggesting unique roles in cellular secretion or transport processes.
Strong nuclear localization is a common trend among TaDREB genes, aligning with their role as transcription factors. Most of the TaDREB genes have specific organelle targets, with very few showing widespread multi-organelle localization. The varied subcellular localization of TaDREB genes indicates a diversity in their functions, potentially allowing them to regulate different aspects of the stress response, energy metabolism, and growth in wheat. Maximum DREB genes were localized in nucleus followed by chloroplast. The TaDREB genes localized in chloroplasts suggest their involvement in photosynthesis-related stress responses. The TaDREB genes localized in the nucleus suggest their role in cell functions (e.g., growth and metabolism). Some were also localized in mitochondria, contributing to energy production for cells especially through respiration and other metabolic pathways mediating stress response and energy metabolism.
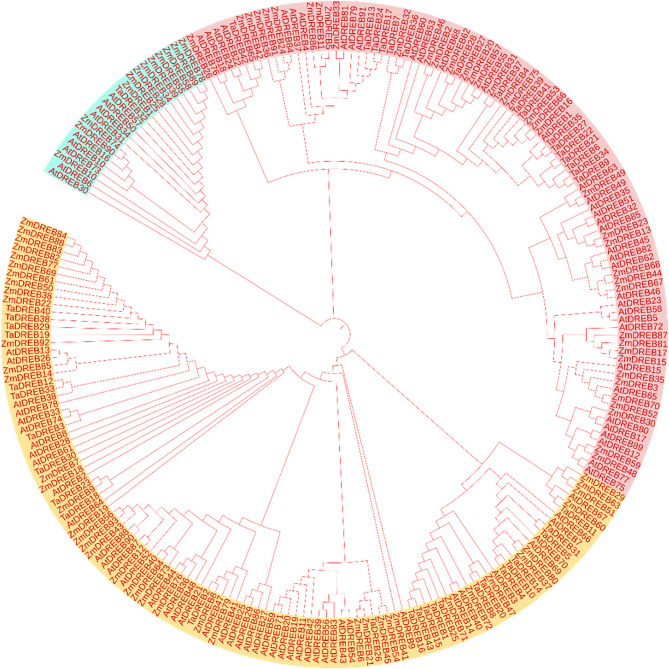
Phylogenetic analysis of the DREB1 gene
To investigate the evolutionary associations, DREB genes (Dehydration Responsive Element Binding proteins) from Triticum aestivum (Ta), Zea mays (Zm), and Arabidopsis thaliana (At) were observed (Fig. 4). There are close groupings between the ZmDREB genes, suggesting that Zea mays possess a high number of closely related DREB genes that likely share common ancestry. For example, genes like ZmDREB84, ZmDREB88, and ZmDREB83 are clustered with nearly zero distance, indicating high sequence similarity or minimal evolutionary divergence. Genes from Triticum aestivum (wheat) are interspersed within groups containing Zea mays genes. TaDREB40 and ZmDREB22 are closely related, showing evolutionary similarity between these species. Similarly, TaDREB12 and TaDREB33 are related to Arabidopsis genes AtDREB38. The larger branch containing genes like ZmDREB14, AtDREB13, and AtDREB26 shows longer branch lengths, reflecting higher divergence between these genes.
Fig. 4.
Phylogenetic tree of DREB genes among three species i.e. Arabidopsis thaliana, Triticum aestivum, and Zea mays
This could indicate functional specialization or adaptation to different environmental stress responses. Based on branch separations and clades, it is likely that the genes are from distinct subfamilies (DREB1, DREB2, etc.). Genes in the same clade, such as those from Arabidopsis (AtDREB25, AtDREB29, etc.), share a common evolutionary origin, forming subfamilies based on sequence conservation. Genes that cluster closely are often expected to have conserved functions. For example, the grouping of ZmDREB and TaDREB genes could indicate shared mechanisms of drought or salinity tolerance in these crop species. This tree highlights evolutionary conservation within the DREB gene family, particularly across monocot species like Zea mays and Triticum aestivum, while also showing divergence with the model dicot Arabidopsis thaliana. Phylogenetic conservation of DREB gene family shows its intricacy and shows that it remained relatively unchanged far back up the phylogenetic tree among different species.
Motif and domain prediction
Investigating conserved domains, AP2, PLN03143 superfamily, and AP2 superfamily emerged as domains within the TaDREB 1 to 43, TaDREB15, and TaDREB14 gene families, respectively (Fig. 5). The MEME tool was used to observe the distribution of 43 motifs within TaDREB, confirming the conservation of these motifs across all proteins (Fig. 6). Motifs (1 & 2) exhibited conservation across all TaDREB genes, indicating that similar motifs were shared by genes existing in the same groups. It shows that a particular role in specific activities is played by these conserved motifs. The existence of similar motifs within different members of the TaDREB gene family indicates that their extension has been a key factor in the evolutionary development of these genes. This conservation was presented through the existence of the AP2 domain in all genes of the TaDREB family (Fig. 5) which remained unchanged. This conservation likely evolved through gene expansion to enhance stress tolerance. Gene expansion refers to the multiple and continuous duplication of a particular gene that enables a cell to respond to environmental stresses by producing corresponding proteins when required. Hence, the AP2 domain of TaDREB being conserved suggests its role in stress response and its mitigation.
Fig. 5.
A color-coded bar graph showing the results of a domain distribution analysis of T. aestivum DREB1 proteins (TaDREB1) using conserved domain database indicating three different domains
Fig. 6.
A color-coded bar graph showing the results of a Motifs distribution analysis of T. aestivum DREB1 proteins (TaDREB1) using MEME version 5.2.2 indicating 43 different Motifs
Discussion
This study demonstrates that the activated biochar (AB) significantly influenced the antioxidants production in wheat cultivars under different irrigation regimes. The results indicated varying responses of antioxidant enzymes to AB and irrigation regimes. Specifically, maximum catalase activity (CAT) was observed in Akbar under 50% irrigation with AB2, while the lowest was found in Dilkash under 100% irrigation with AB0. This suggests cultivar-specific differences in antioxidant levels under drought stress [46]. The increased antioxidant enzyme activity under deficit irrigation with AB amendment is noteworthy. Among antioxidants, peroxidase activity was the highest, followed by superoxide dismutase, and the least was the catalase activity, suggesting an increased role of peroxidase in oxidative stress resilience due to improved soil conditions and water availability [47]. Conversely, the increase in peroxidase activity under low irrigation attributes to enhanced scavenging of reactive oxygen species (ROS) [48]. However, along with an increased antioxidant activity, there are indicators such as lipid peroxidation that also present levels of stress in plants.
Significant reduction in lipid peroxidation with AB application under deficit irrigation reflects decreased cellular damage [49]. In response to abiotic stress, the production of ROS causes damage to lipids in plant cells and tissues, which, if increased, causes severe disturbance in cell structure [50]. The higher activity of antioxidants in AB-mediated stress resilience leads to reduction in ROS production, ultimately reducing lipid peroxidation [51]. The observed increase in lipid peroxidation under deficit irrigation without AB is consistent with the idea that drought stress caused higher lipid peroxidation and cell membrane damage [52]. These findings suggest that lipid peroxidation is inversely associated with antioxidant enzyme activity, which means that the higher the lipid peroxidation, the lower the antioxidant activity. These responses were prominent in wheat when cultivated in biochar amended soil.
Antioxidants scavenge H₂O₂ and O₂⁻ radicals, reducing lipid peroxidation [53]. Researchers revealed that biochar mediated ROS scavenging reduces membrane lipid peroxidation [54]. This process preserves cellular integrity and enhances drought resilience. Biochar plays a role in signalling the expression of drought responsive transcription factors, which bind to dehydration-responsive elements in gene promoters. This triggers the downstream transcription of stress-related genes, improving drought tolerance mechanisms [55]. These transcription factors regulate osmolyte accumulation, antioxidant defense, and stress-protective protein factors further contribute to enhanced membrane stability and metabolic adaptations. Moreover, the interaction of biochar with plant hormones like ABA as well plays role in regulating drought-responsive transcription factors (TFs), including DREB1 [56].
This hormonal synergy promotes stomatal regulation and water-use efficiency. This regulation activates MAPK pathways, amplifying the transcriptional response to stress. Biochar mediated hormonal signal transduction in plants, by activating transcription factors through the MAPK signalling pathway, regulates the expression of a series of functional genes. These regulations reduce abiotic stress, lower cellular ROS, enhance antioxidants, and alleviate the abiotic stress induced growth inhibition [57]. The expression pattern involve regulation of DREB1 and related TFs through these signalling cascades, which further interact with enzymes to play physiological roles [58]. The interaction between the DREB1 protein and peroxidase enzyme, as indicated by our docking studies, shows significant hydrogen bonding and electrostatic interactions. These bonds suggest that DREB1 and peroxidase interact with each other forming a stable complex, which can promote the function of peroxidase. These interactions ultimately present the role of DREB1 in promoting drought stress resilience in wheat [59]. This is due to strong binding affinity and stability of the DREB1-peroxidase complex suggesting its functional role in abiotic stress tolerance, potentially through regulation of antioxidant response mechanism [60].
Exploration of the TaDREB gene family revealed significant variability in peptide length, molecular weight, isoelectric point, and instability index. The diversity in these properties indicates a range of functional roles and stability under stress [56]. The existence of both hydrophilic and hydrophobic regions, as indicated by GRAVY scores, supports the notion that these proteins interact with various cellular components, developing their roles in stress adaptation [61]. The distribution of TaDREB genes across different chromosomes and their varying subcellular localization further emphasizes their complex roles in stress response and cellular regulation. Nucleus localized DREB1 suggests their role in cell functions, chloroplast-localized DREB1 control photosynthesis-related stress responses under deficit water conditions, alleviating oxidative stress, whereas mitochondrial-localized DREB1 genes facilitate respiration and ATP synthesis, maintaining energy homeostasis under stress. Their localization in these organelles underscores their function in stress adaptation and metabolic control.
The phylogenetic analysis revealed close clustering of DREB genes from Triticum aestivum and Zea mays, suggesting common evolutionary origins and potentially similar stress response mechanisms [62]. The divergence observed with Arabidopsis thaliana genes revealed the functional specialization and adaptation to specific environmental stresses [27]. This evolutionary perspective supports the idea of conserved and diversified roles of DREB proteins in stress tolerance across different plant species. The MEME analysis identified conserved motifs and domains within the TaDREB gene family, including AP2 and PLN03143 superfamilies. The conservation of these motifs across different TaDREB genes highlights their importance in regulatory functions as a response to stress [63]. The occurrence of similar motifs in related gene families suggests evolutionary expansion and functional conservation within the DREB family. The evolutionary expansion and functional conservation present the stability and survival of genes over a period of evolution and its ability to stand severe environmental stresses.
When a particular sequence is maintained throughout evolution, possibly due to its essential role in regulating gene expression under challenging conditions, essentially across diverse organisms, it is likely to serve a vital function in adapting or resisting stress [64]. Therefore, a study finds TaDREB with the AP2 domain playing its role in stress resistance in wheat plants. This study allows researchers to comprehensively analyze the function, structure, and potential interaction of the DREB1 transcription factor with stress responsive antioxidant peroxidase. This study leads to insights into the potential role of DREB1 in drought stress response and evolutionary processes, providing a starting point for further experimental validation without the need for extensive physical evaluation of the gene. This study is helpful for gene editing technology by allowing researchers to computationally analyze the entire genome-wide traits of TaDREB1 to identify the best target sites. These target sites can be further used for editing, predicting potential off-target effects, and augmenting guide RNA design. The insights observed by the study can significantly improve the efficiency and specificity of TaDREB1 gene editing procedures before conducting laboratory experiments, particularly with the CRISPR-Cas9 technique. Future studies need to involve functional confirmation of TaDREB1 genes using gene mutations in addition to field trials to determine actual drought tolerance. Investigation of the regulation networks and interactions between other stress-responsive genes will further streamline breeding processes for creating more durable wheat varieties with enhanced yield stability during stress.
Conclusion
The current study evaluated the response of antioxidants along with lipid peroxidation levels in wheat cultivars with activated biochar amendment to combat deficit irrigation. The study concludes that 10 tons per hectare of activated biochar amendment in soil under deficit irrigation can enhance wheat crop stress resilience particularly for Dilkash cultivar as can be witnessed by enhanced antioxidant activities. Antioxidant enzymatic responses might be achieved through signalling transcriptional patterns. The in-silico analysis was performed particularly with peroxidase enzyme that provided an understanding of molecular mechanisms underlying drought resilience in wheat. Strong affinity of peroxidase with DREB1 transcription factor was evident by strong hydrogen bonding and hydrophobic interaction in protein-protein docking. Further observations of DREB1 through phylogenetic, subcellular, motif and domain analysis provided insights into its conservative role in drought stress resilience. These findings can be helpful in gene editing technology for improving wheat crop resistance to drought stress for breeding more resilient cultivars.
Supplementary Information
Acknowledgements
The authors extend their gratitude towards Higher Education Commission (HEC) of Pakistan for providing funds for NRPU research project (Grant Number 20-16716). Authors are sincerely thankful to the Ongoing Research Funding program, (ORF-2025-347), King Saud University, Riyadh, Saudi Arabia.
Authors' contributions
L.k. Wrote the original draft, Investigated, and Conceptualized the study; A.K. Wrote, reviewed & edited the final manuscript and Supervised the study; S.J. administered the project and performed data curation; W.A. Reviewed and visualized the study; M. H. S. and S.A. Validated, Conceptualized, analyzed data, Wrote-reviewed & edited the manuscript; M.S. Reviewed the manuscript.
Funding
This study was funded by Higher Education Commission (HEC) of Pakistan for providing funds for NRPU research project (Grant Number 20-16716) and the Ongoing Research Funding program, (ORF-2025-347), King Saud University, Riyadh, Saudi Arabia.
Data availability
All data used for the study is provided in manuscript along with one supplementary data file.
Declarations
Ethics approval and consent to participate
All authors adhere to the plagiarism policy and ethical considerations of the journal.
Consent for publication
All authors agree to submit this paper for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kamran S, Shahid I, Baig DN, Rizwan M, Malik KA, Mehnaz S. Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Front Microbiol. 2017;8:2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shewry PR, Wheat. J Exp Bot. 2009;60:1537–53. [DOI] [PubMed] [Google Scholar]
- 3.Abid M, Tian Z, Ata-Ul-Karim ST, Cui Y, Liu Y, Zahoor R, et al. Nitrogen nutrition improves the potential of wheat (Triticum aestivum L.) to alleviate the effects of drought stress during vegetative growth periods. Front Plant Sci. 2016;7:981. [DOI] [PMC free article] [PubMed]
- 4.Lal R. Food security in a changing climate. Ecohydrol Hydrobiol. 2013;13:8–21. [Google Scholar]
- 5.Hashmi QA, Ahmad M, Ahmad E, Tariq M, Rafeh A, Awais M. Exploring drought tolerance in wheat: insights from biochemical, morphological, and physiological responses. AJRCS. 2023;8:344–60. [Google Scholar]
- 6.Komal L, Jahan S, Kamran A, Hashem A, Avila-Quezada GD, Abd_Allah EF. Optimizing soil health through activated acacia Biochar under varying irrigation regimes and cultivars for sustainable wheat cultivation. PeerJ. 2025;13:e18748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheoran S, Thakur V, Narwal S, Turan R, Mamrutha HM, Singh V, et al. Differential activity and expression profile of antioxidant enzymes and physiological changes in wheat (Triticum aestivum L.) under drought. Appl Biochem Biotechnol. 2015;177:1282–98. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Wang J, Hui W, Zhao F, Wang P, Su C, et al. Physiology of plant responses to water stress and related genes: A review. Forests. 2022;13:324. [Google Scholar]
- 9.Ahluwalia O, Singh PC, Bhatia R. A review on drought stress in plants: implications, mitigation and the role of plant growth promoting rhizobacteria. Resour Environ Sustain. 2021;5:100032. [Google Scholar]
- 10.Vijayaraghavareddy P, Lekshmy SV, Struik PC, Makarla U, Yin X, Sreeman S. Production and scavenging of reactive oxygen species confer to differential sensitivity of rice and wheat to drought stress. Crop Environ. 2022;1:15–23. [Google Scholar]
- 11.Wang Y, Zhang X, Huang G, Feng F, Liu X, Guo R, et al. iTRAQ-based quantitative analysis of responsive proteins under PEG-induced drought stress in wheat leaves. Int J Mol Sci. 2019;20:2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul GK, Mahmud S, Dutta AK, Sarkar S, Laboni AA, Hossain MS, et al. Volatile compounds of Bacillus pseudomycoides induce growth and drought tolerance in wheat (Triticum aestivum L). Sci Rep. 2022;12:19137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simova-Stoilova L, Vassileva V, Feller U. Selection and breeding of suitable crop genotypes for drought and heat periods in a changing climate: which morphological and physiological properties should be considered? Agriculture. 2016;6:26. [Google Scholar]
- 14.Jena AB, Samal RR, Bhol NK, Duttaroy AK. Cellular Red-Ox system in health and disease: the latest update. Biomed Pharmacother. 2023;162:114606. [DOI] [PubMed] [Google Scholar]
- 15.Moragues-Saitua L, Arias-González A, Blanco F, Benito-Carnero G, Gartzia-Bengoetxea N. Effects of Biochar and wood Ash amendments in the soil-water-plant environment of two temperate forest plantations. Front Forests Global Change. 2023;5:878217. [Google Scholar]
- 16.Palviainen M, Aaltonen H, Laurén A, Köster K, Berninger F, Ojala A, et al. Biochar amendment increases tree growth in nutrient-poor, young Scots pine stands in Finland. For Ecol Manag. 2020;474:118362. [Google Scholar]
- 17.Tang J, Ahmadi A, Alizadeh A, ad, Abedinzadeh R, Abed AM, Smaisim GF, et al. Investigation of the mechanical properties of different amorphous composites using the molecular dynamics simulation. J Mater Res Technol. 2023;24:1390–400. [Google Scholar]
- 18.Lashari MS, Liu Y, Li L, Pan W, Fu J, Pan G, et al. Effects of amendment of biochar-manure compost in conjunction with pyroligneous solution on soil quality and wheat yield of a salt-stressed cropland from central China great plain. Field Crops Res. 2013;144:113–8. [Google Scholar]
- 19.Lehmann J, Joseph S. Biochar for environmental management: science, technology and implementation. Routledge; 2015.
- 20.Jahan S, Ahmad F, Rasul F, Amir R, Shahzad S. Physicochemical analysis of Vermicompost-Perlite based activated Biochar and its influence on wheat (Triticum aestivum L.) growth under water stress. J Soil Sci Plant Nutr. 2023;23:3034–50. [Google Scholar]
- 21.Wu Y, Wang X, Zhang L, Zheng Y, Liu X, Zhang Y. The critical role of Biochar to mitigate the adverse impacts of drought and salinity stress in plants. Front Plant Sci. 2023;14:1163451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Racioppi M, Tartaglia M, De la Rosa JM, Marra M, Lopez-Capel E, Rocco M. Response of ancient and modern wheat varieties to Biochar application: effect on hormone and gene expression involved in germination and growth. Agronomy. 2019;10:5. [Google Scholar]
- 23.Mushtaq T, Bano A, Ullah A. Effects of rhizospheric microbes, growth regulators, and Biochar in modulating antioxidant machinery of plants under stress. J Plant Growth Regul. 2024;44:1–22. [Google Scholar]
- 24.Usman M, Liedl R, Shahid MA. Managing irrigation water by yield and water productivity assessment of a Rice-Wheat system using remote sensing. J Irrig Drain Eng. 2014;140:04014022. [Google Scholar]
- 25.Mohanta TK, Bashir T, Hashem A, Abd Allah EF, Khan AL, Al-Harrasi AS. Early events in plant abiotic stress signaling: interplay between calcium, reactive oxygen species and phytohormones. J Plant Growth Regul. 2018;37:1033–49. [Google Scholar]
- 26.Zhang Y, Yue S, Liu M, Wang X, Xu S, Zhang X, et al. Combined transcriptome and proteome analysis reveal the key physiological processes in seed germination stimulated by decreased salinity in the seagrass Zostera marina L. BMC Plant Biol. 2023;23:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis drebs, transcription factors involved in dehydration-and cold-inducible gene expression. Biochem Biophys Res Commun. 2002;290:998–1009. [DOI] [PubMed] [Google Scholar]
- 28.Li T, Huang Y, Khadr A, Wang Y-H, Xu Z-S, Xiong A-S. DcDREB1A, a DREB-binding transcription factor from Daucus carota, enhances drought tolerance in Transgenic Arabidopsis thaliana and modulates lignin levels by regulating lignin-biosynthesis-related genes. Environ Exp Bot. 2020;169:103896. [Google Scholar]
- 29.Pirrello J, Prasad BN, Zhang W, Chen K, Mila I, Zouine M, et al. Functional analysis and binding affinity of tomato ethylene response factors provide insight on the molecular bases of plant differential responses to ethylene. BMC Plant Biol. 2012;12:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharoni AM, Nuruzzaman M, Satoh K, Shimizu T, Kondoh H, Sasaya T, et al. Gene structures, classification and expression models of the AP2/EREBP transcription factor family in rice. Plant Cell Physiol. 2011;52:344–60. [DOI] [PubMed] [Google Scholar]
- 31.Dossa K, Wei X, Li D, Fonceka D, Zhang Y, Wang L, et al. Insight into the AP2/ERF transcription factor superfamily in Sesame and expression profiling of DREB subfamily under drought stress. BMC Plant Biol. 2016;16:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Q, Wang Z, Hu G, Yao X. Genome-wide identification and characterization of AP2/ERF gene superfamily during flower development in Actinidia Eriantha. BMC Genomics. 2022;23:650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Li X. Genome-wide identification of AP2/ERF superfamily genes and their expression during fruit ripening of Chinese jujube. Sci Rep. 2018;8:15612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie X, Yin X, Chen K. Roles of APETALA2/Ethylene-Response Factors in Regulation of Fruit Quality. Crit Rev Plant Sci. 2016;35:120–30. 10.1080/07352689.2016.1213119. [Google Scholar]
- 35.Maqsood H, Munir F, Amir R, Gul A. Genome-wide identification, comprehensive characterization of transcription factors, cis-regulatory elements, protein homology, and protein interaction network of DREB gene family in Solanum lycopersicum. Front Plant Sci. 2022;13:1031679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du Zhanyuan, Bramlage WJ. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem. 1992;40:1566–70. [Google Scholar]
- 37.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87. [DOI] [PubMed] [Google Scholar]
- 38.Gorin N, Heidema FT. Peroxidase activity in golden delicious apples as a possible parameter of ripening and senescence. J Agric Food Chem. 1976;24:200–1. [DOI] [PubMed] [Google Scholar]
- 39.Iwase T, Tajima A, Sugimoto S, Okuda K, Hironaka I, Kamata Y, et al. A simple assay for measuring catalase activity: A visual approach. Sci Rep. 2013;3:3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, et al. Pfam: the protein families database. Nucleic Acids Res. 2014;42:D222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horton MW, Hancock AM, Huang YS, Toomajian C, Atwell S, Auton A, et al. Genome-wide patterns of genetic variation in worldwide Arabidopsis thaliana accessions from the regmap panel. Nat Genet. 2012;44:212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keklik G. Understanding evolutionary relationships and analysis methods through mega software. Int J New Horizons Sci. 2023:83–90.
- 43.Wen CP, Wai JPM, Tsai MK, Yang YC, Cheng TYD, Lee M-C, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–53. [DOI] [PubMed] [Google Scholar]
- 44.Carocha VJMT. Critical Players and Gene Expression Regulation in Eucalyptus Xylogenesis. PhD Thesis. Universidade NOVA de Lisboa (Portugal); 2016.
- 45.Islam S, Yu Z, She M, Zhao Y, Ma W. Wheat gluten protein and its impacts on wheat processing quality. Front Agricultural Sci Eng. 2019;6:279–87. [Google Scholar]
- 46.Iqbal B. Microplastics and invasive alien plants: A change in soil ecology deliberately impacts the aboveground productivity of the crops. JSPAE. 2024;3:1–7. [Google Scholar]
- 47.Baillo K, Zhang X. Transcription factors associated with abiotic and biotic stress tolerance and their potential for crops improvement. Genes. 2019;10:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nair AS, Abraham TK, Jaya DS. Studies on the changes in lipid peroxidation and antioxidants in drought stress induced Cowpea (Vigna unguiculata L.) varieties. J Environ Biol. 2008;29:689–91. [PubMed] [Google Scholar]
- 49.Yekti R, Bukhari A, Jafar N, Thaha AR. Measurement of malondialdehyde (MDA) as a good indicator of lipid peroxidation. Int J Allied Med Sci Clin Res (IJAMSCR). 2018;6:1–3. [Google Scholar]
- 50.Rao A, Ahmad SD, Sabir SM, Awan S, Shah AH, Khan MF, et al. Antioxidant activity and lipid peroxidation of selected wheat cultivars under salt stress. J Med Plants Res. 2013;7:155–64. [Google Scholar]
- 51.Lehmann J, Gaunt J, Rondon M. Bio-char sequestration in terrestrial Ecosystems– A review. Mitig Adapt Strat Glob Change. 2006;11:403–27. [Google Scholar]
- 52.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–10. [DOI] [PubMed] [Google Scholar]
- 53.Urbanavičiūtė I, Bonfiglioli L, Pagnotta MA. One hundred candidate genes and their roles in drought and salt tolerance in wheat. IJMS. 2021;22:6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bashir SS, Hussain A, Hussain SJ, Wani OA, Zahid Nabi S, Dar NA, et al. Plant drought stress tolerance: Understanding its physiological, biochemical and molecular mechanisms. Biotechnol Biotechnol Equip. 2021;35:1912–25. [Google Scholar]
- 55.Haghpanah M, Hashemipetroudi S, Arzani A, Araniti F. Drought tolerance in plants: physiological and molecular responses. Plants. 2024;13:2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yadav NR, Taunk J, Rani A, Aneja B, Yadav RC. Role of Transcription Factors in Abiotic Stress Tolerance in Crop Plants. In: Tuteja N, Gill SS, editors. Climate Change and Plant Abiotic Stress Tolerance. 1st edition. Wiley. 2013. pp. 605–40.
- 57.Xu N, Zhang N, Yi P, Chen L, Dai H, Zhang J, et al. Integrated physio-biochemistry and RNA-seq revealed the mechanism underlying biochar-mediated alleviation of compound heavy metals (Cd, pb, As) toxicity in cotton. Ecotoxicol Environ Saf. 2024;284:116974. [DOI] [PubMed] [Google Scholar]
- 58.Gao Y, Wang L, Liu R, Tian J, Cai K. Physiological response and proteomic profiling of biochar-induced tomato resistance to bacterial wilt. Sci Hort. 2023;317:112055. [Google Scholar]
- 59.Huang H, Ullah F, Zhou D-X, Yi M, Zhao Y. Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci. 2019;10:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhuang J, Chen J-M, Yao Q-H, Xiong F, Sun C-C, Zhou X-R, et al. Discovery and expression profile analysis of AP2/ERF family genes from Triticum aestivum. Mol Biol Rep. 2011;38:745–53. [DOI] [PubMed] [Google Scholar]
- 61.Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, et al. Protein identification and analysis tools on the expasy server. In: Walker JM, editor. The proteomics protocols handbook. Totowa, NJ: Humana; 2005. pp. 571–607.
- 62.Han J, Xie X, Zhang Y, Yu X, He G, Li Y, et al. Evolution of the DEHYDRATION-RESPONSIVE ELEMENT-BINDING PROTEIN subfamily in green plants. Plant Physiol. 2022;190:421–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riechmann JL, Heard J, Martin G, Reuber L, Jiang C-Z, Keddie J, et al. Arabidopsis transcription factors: Genome-Wide comparative analysis among eukaryotes. Science. 2000;290:2105–10. [DOI] [PubMed] [Google Scholar]
- 64.Gregorio J, Hernández-Bernal AF, Cordoba E, León P. Characterization of evolutionarily conserved motifs involved in activity and regulation of the ABA-INSENSITIVE (ABI) 4 transcription factor. Mol Plant. 2014;7:422–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used for the study is provided in manuscript along with one supplementary data file.