Abstract
We present the case of a female dog that was evaluated following an episode of heart failure and was subsequently diagnosed with anaplasmosis. Cardiac assessment revealed evidence of myocardial injury, systolic dysfunction, and conduction system abnormalities. This case highlights the importance of considering Anaplasma phagocytophilum infection as a potential cause of myocarditis, especially in instances of unexplained heart failure and elevated troponin levels in the absence of other underlying conditions.
Keywords: Myocarditis, Echocardiography, Myocardial injury, Anaplasmosis
Background
The incidence of tick-borne illnesses is rising in Europe, with reported human cases showing more than a threefold increase over several years of surveillance. Concurrently, the geographic range of Ixodes ricinus—the vector responsible for transmitting Anaplasma phagocytophilum—is expanding. As a result, and due to the increasingly close contact between humans and dogs, the latter have become valuable sentinels for these diseases and are themselves increasingly affected [1].
Anaplasma phagocytophilum is an intracellular rickettsial bacterium transmitted by Ixodes spp. ticks and is the causative agent of granulocytic anaplasmosis in dogs, horses, and humans [2, 3]. If clinical signs are observed, they most often occur during the acute phase of infection, typically 1 to 2 weeks after transmission. These signs may be nonspecific and include fever, lethargy, anorexia, lameness (polyarthritis), polyuria, jaundice, lymphadenomegaly, splenomegaly, and thrombocytopenia—which can lead to petechiae or epistaxis—in affected dogs [2–4]. Less common signs include vomiting, diarrhea, coughing, and dyspnea. The extent to which A. phagocytophilum can persist in tissues and contribute to subclinical or chronic disease manifestations in dogs remains a subject of debate [2]. In veterinary medicine, other associated syndromes, such as peritoneal effusion, have also been diagnosed in a young dog and in two horses, according to the literature [5, 6]. Interestingly, A. phagocytophilum has also been reported to cause cardiovascular disease, such as pericarditis, in both dogs and humans [7–9], and may be a rare cause of myocarditis in a few human cases [7, 10, 11].
Myocarditis and myocardial injury (MI) are not synonymous. In both humans and dogs, the term MI refers to patients with elevated cardiac biomarkers, particularly cardiac troponin I (cTnI). MI may be classified as acute if cTnI concentrations display a dynamic pattern—an initial rise followed by a subsequent fall—or chronic if cTnI levels remain persistently elevated [12]. In contrast, myocarditis is defined as an inflammatory infiltration of non-ischemic origin that is associated with cardiomyocyte damage. The incidence of myocarditis in the canine population is unknown; however, a histopathologic diagnosis was reported in 1.5% of dogs in one study [13]. A wide range of infectious and non-infectious conditions can lead to MI, often through either an imbalance between myocardial oxygen supply and demand or direct cardiomyocyte damage [12]. Examples include pharmacologic agents or toxins (e.g., snake envenomation, dicoumarol poisoning, doxorubicin), immune-mediated diseases, trauma, heat stroke, metabolic disorders (e.g., renal disease), anaphylactic shock, neoplasia (such as hemangiosarcoma, lymphoma, and pheochromocytoma), nutritional factors (including non-traditional diets), sepsis, systemic inflammatory response syndrome, and idiopathic myocarditis [12, 13]. Some infectious diseases in dogs that can lead to MI include viruses (e.g., canine parvovirus, canine coronavirus type 2, West Nile virus [13], and COVID-19), bacteria (Borrelia burgdorferi, Bartonella henselae, Leptospira spp. or Streptococcus spp. as infective endocarditis), fungi (cryptococcosis, coccidioidomycosis or aspergillosis), rickettsiae, algae, and various parasites (e.g., Toxoplasma gondii, Dirofilaria immitis, Trypanosoma cruzi, Sarcosporidia spp., Leishmania infantum, or Neospora caninum) [3, 11].
In addition to measuring cTnI concentrations, transthoracic echocardiography and surface electrocardiography (ECG) are commonly used first-line, non-invasive diagnostic tools for evaluating suspected MI or myocarditis in both human and veterinary medicine [12]. However, in up to one-third of dogs diagnosed with MI, the underlying cause remains unidentified despite extensive diagnostic evaluation [14]. The aim of this case report is to describe the clinical, ECG, echocardiographic, and laboratory findings in a dog positive for A. phagocytophilum, presenting with MI and reversible cardiac dysfunction.
Case summary
A 9-year-old neutered female mixed-breed dog was presented to the Small Animal Referral Centre Sibra in Bratislava, Slovak Republic, with an acute onset of tachypnea, fatigue, and a single episode of collapse over the preceding two days. The referring veterinarian had initiated treatment with furosemide and pimobendan; however, the dog’s condition progressively worsened. According to the owner, the dog exhibited increased weakness and dizziness after receiving pimobendan. The past medical history was unremarkable. The dog was currently being fed a commercial grain-free dry dog food. She resided in a western suburb of Bratislava near the Austrian border, in a wooded area endemic for I. ricinus (castor bean tick) (Fig. 1), and was regularly walked outdoors.
Fig. 1.

Geographic distribution of I. ricinus ticks in the Slovak Republic is shown in gray color. The patient’s place of residence is indicated by a red star (highlighted in the enlarged inset on the left), located within a tick-endemic area. Modified from: Petko B, Majláth I, Majláthová V, Víchová B. Map of the potential occurrence of ticks in Slovakia. 1:500,000. First edition. Košice: Institute of Parasitology, Slovak Academy of Sciences.
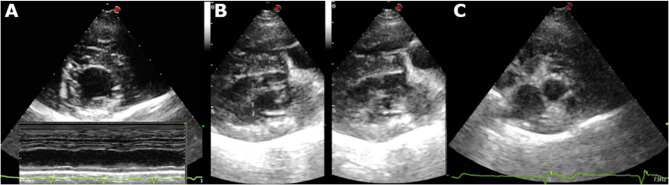
Thoracic radiographs, ECG, and echocardiography were also done at the referring veterinarian demonstrating signs of cardiomegaly, reduced myocardial systolic function, and possible congestive heart failure (CHF). At presentation, general physical examination was otherwise unremarkable with the exception of mild lethargy and weak femoral pulses. A follow-up echocardiogram performed at our hospital demonstrated reduced systolic function (Figs. 2A, B) in both B-mode and M-mode, consistent with a dilated cardiomyopathy (DCM) phenotype. Additionally, there was mild hyperechoic thickening of the left ventricular walls (Fig. 3A), mild left ventricular impaired relaxation (mitral inflow: E/A waves = 0.30/0.34 m/s), and borderline left atrial size (Fig. 3C).
Fig. 2.
Thoracic radiographs in (A) Left laterolateral and (B) dorosoventral views. The heart though mildly round has normal size (vertebral heart size 10v, reference range 9.7–12.2). There is also decreased filling of pulmonary vessels. Also there is mild bronchial pattern in the caudal lung lobes. C Six-lead ECG. Sinus bradycardia. Heart rate 42–70 bpm and large tall T waves. Main electrical axis 60o. Paper speed 50 mm/s, amplitude 2 cm = 1mV
Fig. 3.
A Right parasternal short axis view at the level of papillary muscles: notice the mild epicardial irregularities and generalized hyperechogenicity observed as linear/patch-like hyperechoic areas (also seen in 3B). Lower part. M-mode on the same window displaying hypokinesis of the interventricular septum and the left ventricular wall. B Right parasternal long axis 4-chamber view: marked left ventricular systolic dysfunction (left diastole, right systole) and myocardial mild hyperechoic texture. C right parasternal short axis view at the level of the left atrium and aorta: left atrial enlargement LA/Ao ratio 1.61
Laboratory tests initially included a complete blood count (CBC), biochemical analysis, thyroid profile, cTnI, and urinalysis. Clinical-pathological abnormalities identified leukopenia (4.1 × 10⁹/L, Reference Interval [RI] 5.32–16.92), neutropenia (2.83 × 10⁹/L, RI 3.05–12.1), thrombocytopenia (91.1 × 10⁹/L, RI 140–520), systemic inflammation (C-reactive protein: 28 mg/L, RI < 8.9), and myocardial injury (cTnI: 0.17 µg/L, RI < 0.08) (Table 1). Furosemide (2 mg/kg PO, BID) was continued to prevent further episodes of CHF, while pimobendan was discontinued. The dog’s diet was switched to a commercial cardiac diet (Royal Canin Cardiac VD).
Table 1.
Overview of blood tests performed in the patient
| Parameter | Result | RI | Parameter | Result | RI |
|---|---|---|---|---|---|
| Leukocytes | 4.10 109/L | 5.32–16.92 | Total protein | 64 g/l | 55–95 |
| Basophils | 0.01 109/L | 0.00–0.13 | Albumin | 36 g/l | 23–34 |
| Neutrophils | 2.83 109/L | 3.05–12.10 | ALP | 0.40 ukat/l | 0.10–4.00 |
| Eosinophils | 0.14 109/L | 0.04–1.28 | Glucose | 5.6 mmol/l | 3.1–6.7 |
| Lymphocytes | 0.87 109/L | 0.70–4.95 | Total bilirubin | 3 umol/l | 0–7 |
| Monocytes | 0.25 109/L | 0.20–1.38 | Phosphorus | 1.37 mmol/l | 1.00–2.10 |
| Erythrocytes | 7.38 1012/L | 5.20–8.69 | Cholesterol | 5.04 mmol/l | 3.50–7.80 |
| Hemoglobin | 17.2 g/dL | 11.5–20.1 | GGT | 0.18 ukat/l | 0.00–0.16 |
| MCV | 64.3 fL | 60.0–77.5 | ALT | 0.86 ukat/l | 0.10–1.00 |
| MCH | 23.3 pg | 20.0–27.0 | Calcium | 2.75 mmol/l | 2.30–3.00 |
| MCHC | 362 g/L | 300–380 | Creatinine | 45 umol/l | 35–110 |
| Hematocrit | 47.5% | 35.0–60.0 | Blood urea | 5.11 mmol/l | 3.30–8.30 |
| Platelets | 91 109/L | 140–520 | Sodium | 146 mmol/l | 140–155 |
| Reticulocytes | 12.5 109/L | 9.0–115.0 | Potassium | 4.3 mmol/l | 4.0–5.5 |
| Troponin I | 0.13 ng/ml | < 0.08 | Chloride | 112 mmol/l | 100–115 |
| NT-proBNP | 195.55 pmol/l | < 500 | Globulins | 28 g/l | 16–37 |
| Total thyroxin-T4 | 1.91 ug/dL | 0.8-5.0 | |||
| cTSH | 0.64 ng/mL | 0.0–0.5 |
MCV Mean corpuscular volume, MCH Mean corpuscular hemoglobin, MCHC Mean corpuscular hemoglobin concentration, NT-proBNP n-terminal-pro b-type natriuretic peptide, cTSH Canine thyroid stimulating hormone, ALP Alkaline phosphatase, GGT Gamma-glutamyl transferase, ALT Alanine transaminase
The initial differential diagnosis included diet-associated DCM, myocardial infiltration, and myocarditis with concurrent CHF. Abdominal ultrasound was performed to rule out internal bleeding and potential sources of infection, but no abnormalities were found. A 24-hour ambulatory Holter ECG monitoring revealed generalized bradycardia with pauses longer than 1.5 s during both sleep and daytime, but no other arrhythmias were recorded. Next, blood samples were taken to investigate possible infections that could induce MI. Antibody screening tests for B. burgdorferi, N. caninum, B. canis, D. immitis, T. gondii, B. henselae, and E. canis were all negative. However, the titer for A. phagocytophilum was positive at 25.53 TE (RI < 8). Based on these results, doxycycline (10 mg/kg PO, SID) was added to the treatment regimen for 3 weeks.
Approximately one month later, the owner reported clinical improvement, and a follow-up clinical examination revealed no abnormalities. Echocardiography showed normal left atrial size, improved systolic function with reduced irregularity and hyperechogenicity of the left ventricle, normalization of the mitral inflow, and a decrease in both cTnI and A. phagocytophilum titers (0.12 µg/L and 12.99 TE, respectively). However, mild enlargement of the heart during both systole and diastole was still noted (Fig. 4). Based on these findings, doxycycline was continued for an additional 2 weeks. Two months after the initial diagnosis, the owner reported that the dog had returned to normal health. Echocardiography continued to show mild systolic dysfunction. CBC, biochemistry, and A. phagocytophilum titers were within normal limits (6.86 TE), although a mild elevation in cTnI (0.11 µg/L) persisted. All medications were discontinued, and six months after the initial diagnosis, the owner reported that the dog remained in excellent health. Echocardiography showed a slight reduction in left ventricular size during both systole and diastole, but mild left ventricular systolic dysfunction and volume overload were still evident (Fig. 4).
Fig. 4.
Trend graph showing selected echocardiographic parameters at the time of diagnosis, 1 month and after 2 months. A. Ventricular volumes showed initially increase but 1 month after showed decrease while systolic function improved. EF: % ejection fraction, FS: % fraction shortening, LVIDd: left ventricular internal diameter in diastole, LVIDs: left ventricular internal diameter in systole, ESV: end systolic volume, EDV: end diastolic volume
Discussion
There is evidence suggesting that our patient likely experienced MI and myocarditis related to anaplasmosis (AM), as demonstrated by peripheral blood changes, elevated inflammatory and cardiac markers, systolic dysfunction, A. phagocytophilum seroconversion, and recovery of cardiac function following prompt treatment. Additionally, the patient probably suffered from acute cardiac decompensation, characterized by CHF and weakness, as a consequence of the infection.
Anaplasmosis is a zoonotic disease transmitted to both humans and dogs by I. ricinus ticks [1]. It has been documented worldwide, with northern and central Europe being the regions where it has been most commonly reported [3, 8]. In Slovakia, where our patient resides, the incidence of anaplasmosis can be as high as 11.7% in certain urban and non-urban dog populations [15]. Furthermore, A. phagocytophilum has been detected in questing ticks in Slovakia, with prevalence rates ranging from 1 to 10% [16]. Given the zoonotic nature of the disease, screening for human anaplasmosis should be considered in areas with a high incidence of animal anaplasmosis [1, 3].
No specific gender or breed predisposition has been confirmed for A. phagocytophilum infections. While retriever breeds have been reported as overrepresented, this may reflect the popularity of these dogs accompanying their owners on outdoor activities [3]. Older dogs are more frequently seropositive compared to younger dogs, likely due to increased exposure over time [2, 4]. However, this observation may also be influenced by the seasonal nature of the infection, with most cases occurring during the spring and summer months [8].
The few reported cases of AM in humans demonstrate considerable variability in clinical presentation, ranging from subclinical findings to life-threatening complications such as arrhythmias, CHF, and even death. This is an uncommon but serious condition, with fewer than six published cases in humans to date, most of which are associated with immunocompromised individuals [7, 8, 10, 11, 17–19]. In contrast, our patient—though older—had no evidence of immunosuppression and presented with nonspecific clinical signs, including lethargy, collapse, and bradycardia. These symptoms are relatively uncommon in dogs with MI, in whom fever or heart murmurs are more frequently observed [13]. While CHF could not be definitively confirmed at presentation, it is strongly suspected based on the referring veterinarian’s findings, the owner’s observations, and the presumed resolution of pulmonary edema on thoracic radiographs. To our knowledge, this is the first report of a myocardial syndrome associated with anaplasmosis in a dog, aside from a previously documented case involving pericardial effusion [9]. It remains unclear whether such cases are directly attributable to A. phagocytophilum infection alone or whether other predisposing factors or comorbidities contribute to the cardiac involvement. Notably, a small study reported elevated cardiac markers in a significant number of people positive for anaplasmosis, and these elevations were associated with a more complicated clinical course [20].
Infectious agents can induce acute or chronic myocardial changes through several mechanisms: (i) direct infiltration of inflammatory cells into the myocardium, (ii) the action of microbial toxins, or (iii) a secondary inflammatory response following myocardial damage [14]. The exact pathogenesis of cardiac involvement in anaplasmosis remains incompletely understood. Immunohistochemical staining has demonstrated A. phagocytophilum within the cytoplasm of inflammatory cells in perivascular myocardial tissue. However, it remains unclear whether the pathogen directly causes MI or whether it leads to transient immunosuppression and associated inflammatory cell dysfunction, which in turn damages cardiomyocytes [8]. In some cases, pericardial effusion may result from localized vasculitis triggered by proinflammatory cytokines or the formation of antigen–antibody complexes [9, 10].
Definitive diagnosis of myocarditis is based on histopathological confirmation of myocardial inflammation. Although myocardial biopsy in dogs is considered relatively safe, it is typically performed only in specialized facilities due to the complexity of the procedure and the need for general anesthesia. In our case, myocardial biopsy was not authorized; therefore, a presumptive antemortem diagnosis of canine myocarditis was made using the criteria proposed by Lakhdhir et al. This diagnostic framework includes a combination of major and minor criteria. In our patient, we identified two major criteria—elevated troponin I (cTnI) and positive A. phagocytophilum serology—and four minor criteria: thrombocytopenia, bradycardia, reduced systolic function, and heterogeneous echogenicity of the left ventricular myocardium. However, this approach remains presumptive and is subject to the clinician’s interpretation of the overall clinical and clinicopathological picture [13].
The clinical presentation of myocarditis is highly variable and may include both rhythm disturbances and structural changes that resemble DCM [14]. In both humans and dogs, MI is often associated with echocardiographic abnormalities, including transient or permanent alterations in ventricular function such as global or segmental hypokinesia, akinesia, or dyskinesia—particularly in breeds not genetically predisposed to primary DCM [12, 19, 21]. One study reported that 7 out of 11 dogs with confirmed myocarditis exhibited echocardiographic features suggestive of DCM [14]. In our case, we observed global akinesia from the initial presentation, which persisted at the six-month follow-up. Additional long-term evaluations will be necessary to assess the extent of myocardial recovery or lasting impairment, as well as the potential influence of dietary changes. Structural changes such as diffuse or segmental wall thickening and heterogeneous myocardial echogenicity may also be present in myocarditis. It is important to distinguish true left ventricular (LV) hypertrophy from other conditions that can mimic it, such as systemic arterial hypertension or pseudohypertrophy due to hypovolemia and reduced LV preload [12, 21]. Interestingly, our case exhibited some transient findings, including atrial enlargement and heterogeneous thickening and echogenicity of the myocardium. These alterations may have contributed to the development of CHF, potentially in combination with the observed bradycardia.
In contrast to humans, the list of ECG changes associated with MI in dogs differs considerably. In human medicine, repolarization abnormalities are a central feature of MI diagnosis, whereas in veterinary medicine, they are less emphasized [12]. Although myocardial ischemia can affect all aspects of the ECG—from rhythm disturbances to QT interval alterations—the most prominent and diagnostically reliable changes in humans occur during the repolarization phase, particularly in the ST segment and T-wave. In dogs, ventricular and supraventricular arrhythmias, as well as atrioventricular blocks, are relatively common ECG findings in cases of MI, especially in breeds not predisposed to arrhythmogenic cardiac diseases, such as Boxers [14, 21]. Despite this general trend, our patient demonstrated the development of large, prominent T-waves—an uncommon ECG pattern in dogs. This rare finding has been previously reported in canine MI and is characterized by the acute appearance of prominent T-waves during MI, followed by normalization upon recovery [12, 22]. In human medicine, persistent repolarization abnormalities often suggest irreversible myocardial damage, whereas their resolution may indicate myocardial healing, which holds significant clinical implications. While conduction and rhythm abnormalities are also commonly associated with myocarditis in both humans and dogs, they have not been previously documented in cases of human anaplasmosis [7, 20]. Nonetheless, such abnormalities may emerge during clinical deterioration. In our case, it remains unclear whether the observed sinus bradycardia was directly related to anaplasmosis, a consequence of CHF, or due to another, unidentified mechanism.
Serology remains a commonly used method for detecting A.phagocytophilum infections. However, it has limited diagnostic sensitivity in the early stages of infection, as it typically takes 1 to 2 weeks post-infection for detectable antibody levels to develop. By four weeks, serologic detection exceeds 90% sensitivity. In contrast, polymerase chain reaction (PCR) testing is the most sensitive diagnostic tool for acute infections, although it is not readily available in all veterinary institutions [18, 23]. In chronic cases, PCR testing of tissue biopsies—such as spleen, lymph node, or bone marrow—may offer greater sensitivity than peripheral blood. Nevertheless, PCR testing of whole blood collected during the acute illness phase generally provides an acceptable level of sensitivity [8]. PCR results may take several days to return, and similar to blood smear evaluation, a negative result does not rule out infection, particularly in chronic infections with low bacteremia.
Neutrophils containing A. phagocytophilum morulae are more likely to be observed during the acute phase in clinically ill dogs. In these cases, blood smear evaluation can yield moderate to high sensitivity (ranging from 20 to 75%) [8]. However, the absence of morulae on a blood smear does not exclude infection, especially in chronic phases where bacteremia tends to be lower. Thrombocytopenia, as observed in our case, is the most common hematological abnormality associated with clinical anaplasmosis and is present in over 80% of acutely infected dogs [3]. Due to these diagnostic limitations, a multimodal approach—incorporating serology, PCR, blood smears, and clinical signs—is recommended. Ideally, samples should be collected at the onset of clinical illness to maximize diagnostic accuracy.
In human and veterinary medicine, cTnI has generally been the chosen marker for MI. Cardiac troponin T in dogs is less sensitive than cTnI, being released only with more severe cardiac injury. As dogs and cats rarely develop AMI, and primary cardiac disease is often associated with low-grade myocardial injury, cTnI became the obvious choice in the initial workup (24). Troponins can be used also in cardiac inflammation, trauma, infiltration also with high sensitivity and specificity. They offer quantitative values of myocardial injury which can be reliably measured in dogs and cats and which provide prognostic information, seemingly irrespective of clinical presentation (acute or chronic), suspected type of myocardial injury (reversible or irreversible), and underlying disease (cardiac or noncardiac). Clinically, the greatest strength of troponins can be summed up in their exceptional negative predictive value in both cardiac and noncardiac disease with low troponin concentrations generally associated with improved chances of survival. Increased concentrations, on the other hand, identify individuals at increased risk of death (24).
It is important to note that antibodies can persist in a dog’s system for years. Therefore, a positive serologic result does not necessarily indicate an active infection, as these antibodies may also be present in clinically healthy dogs. For this reason, it is essential to interpret serological findings in conjunction with clinical signs and other diagnostic results suggestive of active disease. Additionally, a low potential for cross-reactivity between E. canis and A. phagocytophilum has been reported, particularly in cases involving prolonged infection or very high antibody titers to one of the pathogens 10. Co-infections with other tick-borne pathogens are also possible, potentially enhancing each other’s pathogenicity and complicating the clinical picture 10. Consequently, a broad infectious disease screening approach is warranted in suspected cases. In our case, the dog initially stabilized with diuretic therapy alone after an adverse reaction to pimobendan, manifesting as weakness. It remains unclear whether this reaction was directly drug-induced, a result of underlying systolic dysfunction, or related to the systemic effects of the infection. Notably, the symptoms resolved following discontinuation of pimobendan. Although A. phagocytophilum is generally susceptible to all tetracyclines, doxycycline is the treatment of choice in both humans and dogs. A dosage of 5 mg/kg twice daily or 10 mg/kg once daily for four weeks is typically effective. When treatment is initiated during the acute phase, dogs usually improve rapidly—often within 24 to 48 h—and the overall prognosis is favorable if the full course of therapy is completed [2]. The optimal duration of treatment, however, has not been definitively established. While most patients recover within 1–2 weeks, doxycycline’s therapeutic effects develop over several days to weeks and do not directly impact antibody production or seroconversion. This explains the persistence of elevated antibody titers and the rationale for extending the treatment duration in some cases. Several treatment parameters remain undefined, including the most effective dose, the ideal duration of doxycycline therapy, and the potential benefit of combination antibiotic regimens [2]. While corticosteroids have been used in some cases, we chose not to administer them due to the risk of exacerbating infection or worsening clinical signs [3]. Other antibiotics were not deemed necessary in this case due to the clear clinical and echocardiographic improvement observed. However, alternative treatments have shown efficacy against A. phagocytophilum in dogs, including orbifloxacin (5 mg/kg SC on day 1, then PO once daily for two weeks), enrofloxacin (5 mg/kg SC, then PO once daily from days 2–8) [3], and other agents like rifampicin and levofloxacin, which have demonstrated in vitro activity [2].
Chloramphenicol has been cited as an alternative treatment option for puppies with A. phagocytophilum infection. In dogs with moderate to severe clinical disease or those who fail to respond rapidly to doxycycline therapy, co-infection with other tick-borne pathogens should be strongly considered and tested for [2]. In our case, this option was explored, yet the delayed clinical and serological recovery may be more reasonably attributed to the underlying electrical and mechanical impairment of the heart rather than antimicrobial failure alone. It remains unclear whether natural infection confers long-term immunity against clinical anaplasmosis in dogs. While re-infection following successful treatment has not been documented in dogs, a single case has been reported in human medicine. Interestingly, horses appear to develop resistance to re-infection following recovery from A. phagocytophilum infection. Given this uncertainty, we advised the client to maintain strict tick prevention protocols to minimize the risk of recurrence [2].
There is growing evidence linking certain diets—especially non-traditional formulations rich in pulses, potatoes, or legumes—to a potentially reversible form of DCM. The exact cause of diet-associated DCM remains complex and multifactorial, but it is now generally advised to avoid grain-free diets that heavily rely on these ingredients. This may have been a contributing factor in our patient’s cardiac changes. Fortunately, awareness of diet-related DCM has increased within the veterinary community, leading to a significant reduction—over 80%—in reported cases in recent years due to diet modifications. In our case, due to the DCM-like echocardiographic phenotype, the patient’s diet was switched to a commercial cardiac formulation. However, six months post-intervention, substantial improvement in cardiac function was not yet observed and this can suggest that cardiac changes occurred after the myocarditis. Recent studies suggest that recovery in some individuals may take up to a year or longer, supporting the need for ongoing dietary management and regular cardiac monitoring [24].
Conclusions
To our knowledge, this is one of the first reported cases of A. phagocytophilum infection causing concurrent electrical conduction abnormalities, MI, and hemodynamic instability in a dog with no evidence of co-infection. This case underscores the importance of including anaplasmosis in the differential diagnosis for clinical syndromes suggestive of myocarditis, particularly when bradycardia or signs of heart failure are present—especially in endemic regions during late spring through early fall. Although A. phagocytophilum is an uncommon cause of MI, it is a treatable condition, and early recognition with prompt doxycycline therapy may reduce disease severity, limit myocardial damage, and improve overall outcomes.
Acknowledgements
Not applicable.
Abbreviations
- AM
Anaplasma myocarditis
- CBC
Complete blood count
- CHF
Congestive heart failure
- cTnI
Cardiac troponin I
- DCM
Dilated cardiomyopathy
- ECG
Electrocardiography
- MI
Myocardial injury
- PCR
Polymerase chain reaction
Authors’ contributions
S.H. design, interpretation of data and critical review.C.F.A. clinical follow-ups and manuscript draft.B.L. ECG and radiography interpretation, concept and critical review. A.B. serology interpretation and critical review. All authors read and approved the final manuscript version.
Funding
This study was supported by grant VEGA 1/0381/25 funded by the Scientific Grant Agency of Ministry of Education of the Slovakian Republic and Slovak Academy of Sciences.
Data availability
On request (images, results) at authors’ archive.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mesquita CAM, Guimarães AM, Guedes E, Silveira JAG, Rocha GC, Nogueira CI, Varaschin MS, de Souza Santos MA, da Rocha CMBM. Dogs (Canis familiaris) as sentinels for determining the risk of occurrence of rickettsia spp. and Anaplasma phagocytophilum in previously undiagnosed areas. Vet Parasitol Reg Stud Reports. 2023;46: 100930. 10.1016/j.vprsr.2023.100930. [DOI] [PubMed] [Google Scholar]
- 2.Sainz Á, Roura X, Miró G, et al. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit Vectors. 2015;8:75. 10.1186/s13071-015-0649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atif FA, Mehnaz S, Qamar MF, Roheen T, Sajid MS, Ehtisham-Ul-Haque S, Kashif M, Ben Said M. Epidemiology. Diagnosis, and control of canine infectious cyclic thrombocytopenia and granulocytic anaplasmosis: emerging diseases of veterinary and public health significance. Vet Sci. 2021;8: 312. 10.3390/vetsci8120312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohn B, Silaghi C, Galke D, Arndt G, Pfister K. Infections with Anaplasma phagocytophilum in dogs in Germany. Res Vet Sci. 2011;91:71–6. [DOI] [PubMed] [Google Scholar]
- 5.Restifo MM, Bedenice D, Thane KE, Mazan MR. Cavitary effusion associated with Anaplasma phagocytophilum infection in 2 equids. J Vet Intern Med. 2015;29:732–5. 10.1111/jvim.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kane A, Block G, Heeb LA. An unusual presentation of granulocytic anaplasmosis in a young dog. J Am Anim Hosp Assoc. 2011;47:276–9. 10.5326/JAAHA-MS-5652. [DOI] [PubMed] [Google Scholar]
- 7.Levy AM, Martin LM, Krakower DS, Grandin EW. Case report: human granulocytic anaplasmosis causes acute myopericarditis with atrial fibrillation. Eur Heart J. 2023. 10.1093/ehjcr/ytad026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik A, Jameel MN, Ali SS, Mir S. Human granulocytic anaplasmosis affecting the myocardium. J Gen Intern Med. 2005;10:C8–10. 10.1111/j.1525-1497.2005.0218_4.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murdock BK, Bach JF, Qurollo BA, Lashnits EW, Friedrichs KR. Detection of Anaplasma phagocytophilum in an inflammatory pericardial effusion of a dog. J Vet Intern Med. 2024;38:2339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misra S, Frampton J, Friedman S. The tick that infected the ticker: a case of Anaplasma myopericarditis in a Vermont veteran. J Am Coll Cardiol. 2020;75:11. 10.1016/S0735-1097(20)33157-0. Suppl A:2530. [Google Scholar]
- 11.Rzechorzek W, Bandyopadhyay D, Pitaktong A, Ranjan P, Fuisz A, El-Khoury MY, Aronow WS, Pan S. (2023). Acute Myopericarditis Due to Human Granulocytic Anaplasmosis. Futur Cardiol 2023;19:197–202. 10.2217/fca-2023-0028 [DOI] [PubMed]
- 12.Romito G, Cipone M. Transient deep and giant negative T waves in dogs with myocardial injury. J Vet Cardiol. 2021;36:131–40. 10.1016/j.jvc.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Lakhdhir S, et al. Clinical presentation, cardiovascular findings, etiology, and outcome of myocarditis in dogs: 64 cases with presumptive antemortem diagnosis (26 confirmed postmortem) and 137 cases with postmortem diagnosis only (2004–2017). J Vet Cardiol. 2020;30:44–56. 10.1016/j.jvc.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janus I, Noszczyk-Nowak A, Nowak M, Cepiel A, Ciaputa R, Pasławska U, Dzięgiel P, Jabłońska K. Myocarditis in dogs: etiology, clinical and histopathological features (11 cases: 2007–2013). Ir Vet J. 2014;67:28. 10.1186/s13620-014-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Čabanová V, Pantchev N, Hurníková Z, Miterpáková M. Recent study on canine vector-borne zoonoses in Southern Slovakia - serologic survey. Acta Parasitol. 2015;60:749–58. 10.1515/ap-2015-0107. [DOI] [PubMed] [Google Scholar]
- 16.Derdáková M, Václav R, Pangrácova-Blaňárová L, Selyemová D, Koči J, Walder G, Špitalská E. CandidatusNeoehrlichia mikurensis and its co-circulation with Anaplasma phagocytophilum in Ixodes ricinus ticks across ecologically different habitats of central Europe. Parasit Vectors. 2014;7:160. 10.1186/1756-3305-7-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Temtanakitpaisan Y, Methachittiphan N, Barbosa FB, Treadwell TL, Love DG, editors. Cardiac involvement in human granulocytic anaplasmosis. 17th world Congress on heart Disease / Annual scientific sessions of the International-Academy-of-Cardiology. Canada: Toronto; 2012 Jul. pp. 27–30.
- 18.Fisher M, Patel P, Slenker A, Langstengel J, Smith S. A fatal case of cardiomyopathy secondary to human granulocytic anaplasmosis in an immunocompromised patient. Chest. 2020;158: A235. [Google Scholar]
- 19.Garofalo V, Walgamage M, Cuello L, Wester K, Robinson S, Gemayel CY. Anaplasmosis: from multi-system Mayheim to ventricular tachycardia in a heartbeat. J Am Coll Cardiol. 2024;83. 10.1016/S0735-1097(24)06496-9. :13. Suppl A:4506.
- 20.Dumic I, Jevtic D, Veselinovic M, Nordstrom CW, Jovanovic M, Mogulla V, Veselinovic EM, Hudson A, Simeunovic G, Petcu E, Ramanan P. Human granulocytic anaplasmosis-a systematic review of published cases. Microorganisms. 2022;10:1433. 10.3390/microorganisms10071433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romito G, Palatini L, Sabetti MC, Cipone M. Myocardial injury in dogs: a retrospective analysis on etiological, echocardiographic, electrocardiographic, therapeutic, and outcome findings in 102 cases. J Vet Cardiol. 2024;53:36–51. 10.1016/j.jvc.2024.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Bosimini E, Giannuzzi P, Temporelli PL, Gentile F, Lucci D, Maggioni AP, Tavazzi L, Badano L, Stoian I, Piazza R, Heyman I, Levantesi G, Cervesato E, Geraci E, Nicolosi GL. Electrocardiographic evolutionary changes and left ventricular remodeling after acute myocardial infarction: results of the GISSI-3 echo substudy. J Am Coll Cardiol. 2000;35:127–35. 10.1016/s0735-1097(99)00487-8. [DOI] [PubMed] [Google Scholar]
- 23.Langhorn R, Willesen JL. Cardiac troponins in dogs and cats. J Vet Intern Med. 2016;30(1):36–50. 10.1111/jvim.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haimovitz D, Vereb M, Freeman L, Goldberg R, Lessard D, Rush J, Adin D. Effect of diet change in healthy dogs with subclinical cardiac biomarker or echocardiographic abnormalities. J Vet Intern Med. 2022;36:1057–65. 10.1111/jvim.16416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
On request (images, results) at authors’ archive.