Abstract
Background
Brain-computer interface (BCI) has been shown to be beneficial in improving lower limb motility in stroke, but their effectiveness on balance and attention is unclear. In addition, current BCIs are mostly in single-task mode. The BCI system used in this study was based on a dual-task model of motor imagery (MI) and virtual reality (VR). Previous studies have demonstrated that dual-task seems to be beneficial for balance and attention. The purpose of this study was to validate the effects of MI-VR-based dual-task BCI on balance and attention in participants with stroke.
Methods
This pilot, single-blind, randomized controlled trial involved 38 stroke participants, randomized to the BCI (BCI pedaling training) or control group (conventional pedaling). Both groups trained 20 min daily, 5 days a week for 4 weeks, alongside conventional rehabilitation. Thirty participants completed the program (mean age: 56.56 years, mean disease duration: 4.48 months). Assessments were made before and after 4 weeks. The primary outcome was the Berg Balance Scale (BBS), and secondary outcomes included the Timed Up and Go Test (TUGT), Fugl-Meyer Lower Extremity Assessment (FMA-LE), Symbol Digit Modalities Test (SDMT), and average attention index.
Results
30 participants completed the study (14 in the BCI and 16 in the control group). The retention rates were 73.68% and 84.21% respectively. No adverse events were reported in this study and participants did not report any discomfort. The changes in BBS, TUGT and SDMT values in the BCI group were significantly better than those in the control group (P < 0.05). Average attention index of the BCI group's participants grew with the number of training sessions, and there was a significant difference comparing pre- to post-treatment (p < 0.05). The value of BBS change is linearly correlated with the value of SDMT change (F = 8.778, y = 0.59x + 1.90, P < 0.001).
Conclusions
This study initially showed positive effects of dual-task mode of BCI pedalling training on balance and attention in stroke participants. However, given the preliminary nature of this study and its limitations, the results need to be treated with caution.
Trial registration Chinese Clinical Trial Registry Identifier: ChiCTR2300071522. Registered on 2023/05/17.
Keywords: Stroke, Brain computer interface, Motor imagery, Virtual reality, Pedaling training, Dual task, Balance, Attention
Introduction
The incidence of stroke is rising due to an aging population and a high prevalence of cardiovascular comorbidities [1]. Balance and cognitive decline are common post-stroke complications, with up to 70% of patients experiencing balance dysfunction in the first year after stroke and 16–30% experiencing impaired attention [2, 3]. Studies have indicated that decreased balance function and attention deficits are significant factors in increasing the incidence of falls among chronic stroke patients [4]. Non-invasive brain-computer interfaces (BCI) are emerging as novel therapeutic tools for stroke rehabilitation [5]. BCI can establish direct communication or control channels with external devices by converting brain signals into computer commands. Studies have shown that BCI can promote neuroplastic changes in stroke patients, leading to clinically meaningful improvements in motor function [6]. Many studies have reported the positive effects of BCI on the rehabilitation of upper limb and hand function [7, 8]. However, there is insufficient research evidence supporting its benefits for lower limb function (particularly balance) and non-motor effects in stroke patients. The use of a multimodal BCIpedaling system has been reported to improve lower limb motor and cognitive function in stroke, but it has mainly been studied in early subacute stroke patients [9]. Another study demonstrated that training with a low-cost BCI pedaling system could significantly improve lower limb motor ability in stroke patients; however, the sample size of this trial was only two cases [10]. A small-sample clinical study reported that BCI-based functional electrical stimulation was more beneficial in improving balance and gait function than functional electrical stimulation alone in stroke patients [11]. However, there is a lack of studies related to the effects of BCI on balance and attention in subacute or chronic stroke.
In addition, current BCI designs tend to use a single training modality, such as upper-limb, lower-limb, or cognitive training. Multimodal-based BCI design is also becoming a trend in research. The BCI system used in this study integrates BCI pedaling training based on motor imagery (MI) with upper extremity virtual reality (VR) games, thus providing users with a novel dual-task BCI training model that combines synchronized upper and lower extremity movement, limb and cognitive function training. Dual task refers to the successful performance of two tasks, such as motor-motor tasks and motor-cognitive tasks [12]. Balance dysfunction in stroke patients is often associated with decreased ability to allocate attentional resources. Dual-task training is to add secondary tasks to the primary tasks, to improve the ability of attention allocation through continuous training, and then to improve the motor and cognitive functions. Studies have shown that dual-task training is more compatible with patients' daily life than single-task training, and can effectively strengthen the functional network connections between motor and cognitive areas of the brain, which is conducive to the improvement of balance and attention levels [13].
This study was the first to use a dual-task modality BCI system for rehabilitation of patients with stroke. We aimed to clarify the effects of a dual-task modal BCI system based on MI and VR on balance and attention in stroke.
Methods
Study design, randomization and blinding
This was a single-blind, randomized controlled pilot study comparing BCI pedaling training with the conventional pedaling training. All reporting of this trial is in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement [14].
The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. Written informed consent was obtained from all participants. The study was registered in the Chinese Clinical Trial Registry (ChiCTR2300071522).
Participants were randomly assigned to receive either BCI pedaling training or conventional pedaling training in a 1:1 allocation ratio. The computer-generated random allocation scheme was concealed from the researcher's view in sequentially numbered, sealed, opaque envelopes. Upon formal enrollment of participants for the intervention protocol, the therapist in charge opened the randomization envelope to determine the intervention modality for the patient. Participants were not blinded because they needed to adhere to the protocol. We used standardized instructions in our study and limited unnecessary investigator-participant interactions to reduce potential bias from the Hawthorne effect. The assessor and other study team members were blinded to the allocation.
Participants
We recruited stroke patients from a rehabilitation specialty hospital. Participants met the following inclusion criteria: (1) age between 18 and 75 years; (2) Within 1 to 12 months of stroke onset (ischemic infarct or intracerebral hemorrhage); (3) Lower-extremity Brunnstrom stage ≤ IV (4) Montreal Cognitive Assessment (MoCA) attention dimension scores of 2–5 points[15]; (5) a certain level of MI ability, indicated by a score of ≥ 55 points on the Kinesthetic and Visual Imagery Questionnaire [16]; (6) capability to walk continuously for at least 10 m with or without assistance[17]; (7) good comprehension and verbal communication skills to participate and cooperate effectively in the study (verbal or physical yes/no indication). The exclusion criteria were: (1) Moderate and severe cognitive impairment (MoCA < 18) [18]; (2) Hemineglect (meet left lateralized neglect diagnostic criteria by the Chinese Test of Behavioral Neglect-Hong Kong version of the scale) [19]; (3) Aphasia (meet the aphasia diagnostic criteria by the China rehabilitation research center aphasia examination (CRRCAE) [20]; (4) intense pain of the lower-extremity or spasticity that hinders pedaling training (Participants complained of lower limb pain during pedalling or the Modified Ashworth Scale (MAS) > 2) [21]; (5) the presence of other conditions leading to cognitive impairment or attention deficits, such as Alzheimer’s disease, Parkinson’s disease, or similar disorders; (6) skull defects affecting electrode–skin fitting.
Interventions
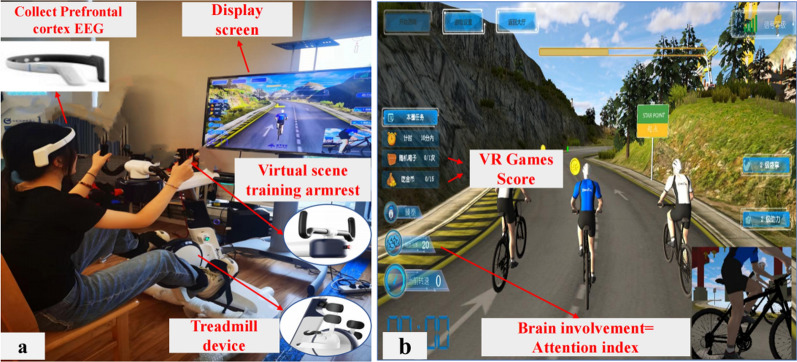
This study used a MI-VR-based dual-task modal BCI system (Fig. 1). It consists of a lower limb BCI-controled pedaling module and an upper limb VR activity module. The BCI-controlled pedaling module includes a lower limb pedaling robot (ZhenTec R1 pedaling training robot) and an EEG acquisition system (ZhenTec NH1 EEG collection system). Studies have demonstrated that human attention is associated with the Alpha (7–13 Hz) and Beta (14–30 Hz) bands [22, 23]. Attention intensity characteristics can be calculated from the energy ratio of Alpha band and Beta band signals. In this study, the alpha and beta band signals from the prefrontal cortex of the participants were captured using an EEG system, and the energy ratio of the beta band and alpha band signals was used to respond to the degree of concentration of the participants during the training, which was defined as the Attention Index (atn). The energy ratio is normalized to an integer value between 0 and 100. The attention index can be defined as follows [9]:
 |
1 |
 |
2 |
Fig. 1.
The dual-task modal BCI system based on MI and VR. a.Participants performed BCI-controlled pedaling training while performing upper limb VR game tasks; b. Upper limbs VR game tasks include riding direction adjustments for different road conditions, eating gold coins, and opening treasure chests.The screen displays real-time information on participants' brain engagement (average attention index), VR game task scores, etc.
In the formula, R is the beta/alpha ratio, Eα is the total power in the Alpha band, Eβ is the total power in the Beta band, and atn is the attention index. MAX = 100 and MIN = 0, which are the upper and lower bounds of the normalised range. max and min are empirical constants obtained by calculating the data from the initial learning phase. Attention index is the key index to control the rotational speed of the lower limb pedalling system, the higher the attention index, the higher the rotational speed of the lower limb pedalling, and the relationship between the two is the following equation: n = k × atn (k is the constant, atn is the participant's attention index). When the attention index rises above a predetermined threshold, the lower limb pedalling will be activated and the participant can perform pedalling exercises. The trigger pedal threshold consists of four levels: 50%, 75% and 100%. The upper limb VR activity module presents a task game for cycling. Users are encouraged to actively participate in the training by completing gaming tasks such as directional control, avoiding obstacles, and earning gold coins using the upper limb handle. The gaming interface displays the participants' prefrontal cortex EEG data, transformed into an identifiable attention index in brain-controlled mode.
The BCI group and the control group used BCI pedaling training and conventional pedaling training respectively, once a day for 20 min, 5 days a week for 4 weeks. Additionally, both groups received the same frequency of daily rehabilitation activities provided by professional physical therapists and occupational therapists, including active physical activity, balance training, walking training, and activities of daily living training.
Participants in the BCI group were required to undergo an additional brief BCI study of about 5 min before each start of the formal pedalling training session, which was designed to initially monitor the fluctuation of the participants' EEG characteristics, determine the range of fluctuation of the R (Eq. 1) value, and ensure that the participants were able to better control the BCI through the attention index. The formal training programme begins immediately after the short BCI learning period. Participants were asked to imagine that their lower limbs were performing a pedalling movement. During this process, the EEG collection system records the participant's prefrontal cortex EEG signals as they imagine the pedaling movement. After computer processing, these EEG signals are converted into a real-time attention index, displayed on the screen to visualize brain engagement levels. The rotation speed of the lower limb training robot is regulated by this index. The higher the attention index, the faster the rotation speed of the lower limb training robot. At the same time, participants were required to synchronize their upper limb VR gameplay tasks. The multisensory feedback provided through VR gaming tasks, including visual and kinesthetic stimuli, supports the motor imagery process by enhancing patient engagement and motivation. Whilst motor imagery is still required to control the lower limb pedal system, VR gaming reduces the system's sole reliance on motor imagery by integrating sensory cues from the virtual environment, which helps participants to remain focused and engaged in the task. This multisensory approach aims to create a more interactive and motivating experience, which in turn fosters greater patient compliance and sustained attention during training. The BCI system in this study was operated by specially trained physical therapists, who adjusted the training difficulty based on the participants' performance during the experiment.
In order to ensure the consistency of training intensity between the two groups, participants in the control group will also receive about 5 min of systematic learning before each formal training session. Control group completed only non-BCI-controlled pedaling training and were not required to perform synchronized upper extremity play tasks. A physiotherapists set the difficulty of the training according to the patient's own situation.
Outcome measures
Assessments were conducted pre- and post-intervention.The assessors were physical therapists who were not involved in the study, had a clinical experience of > 5 years, and were well-trained in the assessments used in the study. They are not clear about the allocation of participants.
Primary outcome
The primary outcome measure in this trial was the Berg Balance Scale (BBS). The BBS assesses balance function in patients with cerebrovascular and brain injuries, predicting fall risk [24]. It includes 14 tasks assessing balance function, such as maintaining static uprightness, standing and sitting, weight transfer, turning, standing on one leg, and shifting weight between both feet. Each task is scored on a scale of 0–4, where 0 denotes inability and 4 indicates meeting a standardized criterion, yielding a maximum score of 56. Higher scores indicate better balance function, whereas scores below 40 indicate increased fall risk [25]. Prior studies have established high test–retest reliability of the BBS in assessing balance among stroke patients, with inter-rater reliability up to 0.98 [26]. Minimum clinically important difference (MCID) for BBS in subacute stroke patients requiring assisted walking and those not requiring assisted walking were 5 and 4 points, respectively [27].
Secondary outcomes
Secondary outcomes included the Timed Up and Go Test (TUGT), Fugl-Meyer Lower Extremity Assessment (FMA-LE), Symbol Digit Modalities Test (SDMT) and the average attention index (β- and α signal band power ratio).
The Timed Up and Go Test (TUGT) assesses the dynamic balance and mobility function of stroke patients by timing a series of movements: rising from a chair, walking three meters forward, turning, walking back to the chair, and sitting down. The test records the time taken from rising to sitting back down. The test showed excellent reliability in stroke patients with intraclass correlation coefficient (ICC) > 0.95 [28]. For subacute stroke patients, the minimal detectable change (MDC) of TUGT is 2.9 s [29].
The Fugl-Meyer Lower Extremity Assessment (FMA-LE) is a 17-item scale used primarily to assess lower extremity motor function in stroke patients. It yields a total score of 34, with higher scores indicating better motor function. FMA-LE is highly recommended in clinical studies assessing motor dysfunction changes post-stroke [30]. The MCID for this indicator in patients with subacute stroke was 3.3 points [31].
The Symbol Digit Modalities Test (SDMT) is widely utilized for assessing cognitive function, specifically information processing speed [32]. Participants were instructed to match each number with its corresponding symbol within a given digital-symbol key, recording the number of correct matches completed in 90 s. Many cognitive tasks necessitate adequate information processing speed within the allotted time. Slow processing often underlies attention deficits, making SDMT useful for assessing attention levels [33]. A lower SDMT score indicates slower information processing speed and greater attention impairment. SDMT demonstrates high predictive validity and applicability in assessing attention in stroke patients.
The attention index is calculated based on the ratio of beta and alpha band signals in prefrontal EEG. Researchers often use the beta and alpha band energy ratio as a quantitative measure of attention level [22, 34]. Durinsg training, the BCI system in this study recorded the real-time EEG signals' β- and α-band energy ratio from the prefrontal cortex of participants, using it as their attention index. When the value exceeds a preset threshold, the lower limb pedaling device is activated, allowing the patient to engage in lower limb pedaling exercises. Thus, this indicator reflects the degree of participants engagement in the training, with higher thresholds indicating greater attention levels. Moreover, the speed of lower limb pedaling is determined by the attention index, with higher values resulting in faster rotation speeds. The attention index was updated every second, and the arithmetic mean of all values in a single training session was used as the average attention index for this training. Considering potential fluctuations in participants' training states, we compared changes in average attention scores between the first and last three training sessions.
It should be noted that in the original trial registration (Chinese Clinical Trial Registry Identifier: ChiCTR2300071522), the FMA-LE and MoCA were listed as the primary outcomes, with all other measures categorized as secondary outcomes. However, after the initial phases of recruitment and early feasibility assessment, we recognized that our intervention had a stronger theoretical and clinical rationale for targeting balance, rather than lower extremity motor recovery. Specifically, the design of the dual-task BCI cycling system emphasizes the simultaneous performance of motor and cognitive tasks through virtual reality and motor imagery, as well as upper and lower limb coordination tasks. Based on this, and given the exploratory nature of the study as a pilot trial, we shifted our focus to BBS as the more appropriate primary outcomes to reflect these domains. This decision was made before data analysis and reflects the more directly targeted domains of the intervention. The FMA-LE and MoCA are still reported as important secondary outcomes. This change is disclosed here in accordance with the CONSORT 2010 guidelines, which recommend reporting and justifying any changes to trial outcomes made after the start of the trial.
Sample size calculation
This study employed PASS 2021 software to determine the minimum sample size. Based on previous studies [35] and clinical experience, it was assumed that the value of change in BBS in the BCI group reached the minimum clinically important difference value of 4 points, and it was assumed that the value of change in BBS scores in the conventional training group was 1 points with a standard deviation of 2.3. A total of 28 patients were required for a 2-tailed α = 0.05 with 90% power. Assuming a potential dropout rate of 25%, we planned to enroll a total of 38 patients (19 in each group).
Statistical analysis
Continuous variables are reported as means (SDs) or medians (IQRs), based on the distribution determined by the Shapiro–Wilk test. Categorical variables are presented as frequencies and percentages. Comparisons before and after intervention were conducted using paired t-tests or Wilcoxon signed rank-test, while between-group comparisons utilized independent sample t-tests, Welch t-test or Mann–Whitney U tests as appropriate. Given that baseline scores of BBS, TUGT, MoCA, SDMT, DST-f, and DST-b might influence post-intervention outcomes, we focused on comparing changes in each index after training in both groups to assess intervention effectiveness. Additionally, we analyzed the change trend in the attention index of the BCI group during the intervention and compared the difference in average attention index between the three training sessions before and after to assess the impact of BCI training on attention changes. Pearson's correlation coefficient and linear regression analysis were used to explore the relationship between the value of change in balance indicators and the value of change in attention indicators. Given the exploratory and pilot nature of this study, we opted for a complete case analysis to provide a clearer estimate of the treatment effect for participants who adhered to the protocol. No formal correction for multiplicity was applied, as this study was designed to be exploratory and hypothesis-generating rather than confirmatory. As such, any statistically significant findings should be interpreted as preliminary. Statistical analyses were conducted using SPSS software version 26.0 (IBM, Chicago, IL, USA) and Prism 9 (Graphpad Software, Inc., USA). Tests were two-sided, with P values < 0.05 considered statistically significant for all analyses.
Results
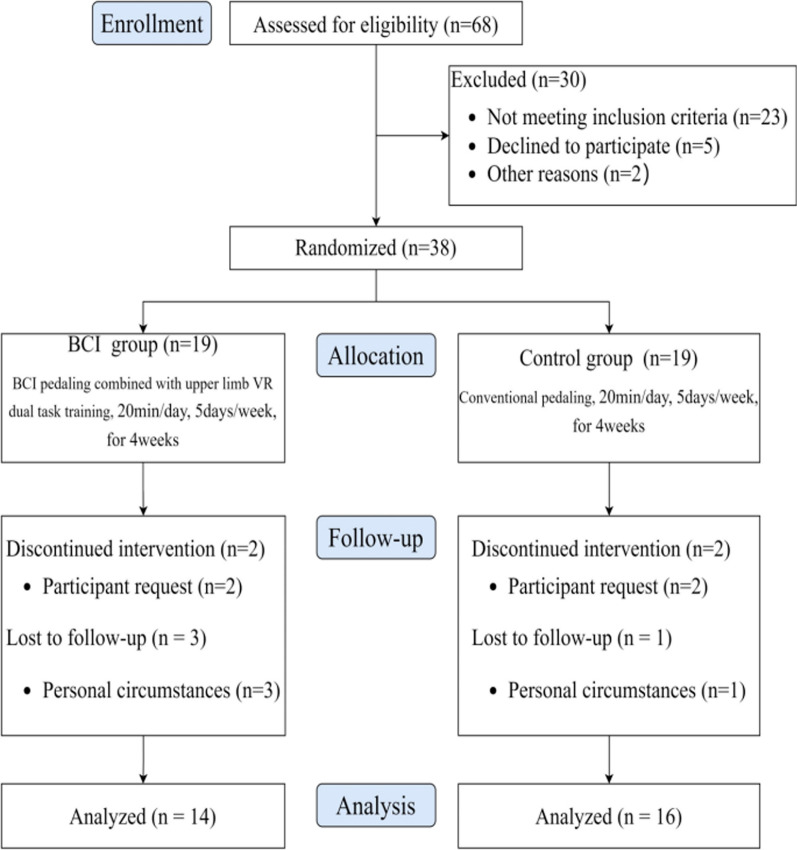
The Consolidated Standards of Reporting Trials (CONSORT) diagram (Fig. 2) illustrates the flow of participants from screening to post-intervention. Since the end of December 2022, a total of 68 stroke participants were screened, out of which 45 were assessed as eligible, and 7 declined or were unable to attend. Thirty-eight participants were randomized into either the BCI pedaling training group (n = 19) or the conventional pedaling training group (n = 19). Thirty participants completed the full training program, received a post-intervention assessment, and were included in the analysis. The retention rates in the BCI and control groups were 73.68% and 84.21%, respectively. It is clear that the BCI group had a relatively higher dropout rate. However, the reasons for dropout were mainly related to random factors such as participants' personal feelings, physical condition and temporary discharge from hospital, and not so much to the intervention itself (One participant withdrew after two sessions, feeling that the BCI pedalling training would not benefit his functional improvement; another found the training more difficult than expected and chose to withdraw; one patient suspended training due to a change in condition, which was deemed unrelated to the intervention by the supervising physician; and two participants were discharged early for personal reasons.). No adverse events were reported in this study and participants did not report any discomfort. Participants' characteristics at baseline are detailed in Table 1.
Fig. 2.
Flow diagram of participants
Table 1.
Characteristics of Patients at Baseline
| Characteristics | BCI group (N = 14) | Control group (N = 16) |
 /z /z |
P |
|---|---|---|---|---|
| Sexa | 1.205 | 0.272 | ||
| Male | 10 (71.43) | 14 (87.50) | ||
| Female | 4 (28.57) | 2 (12.50) | ||
| Age (years)b | 57.07 ± 11.65 | 56.25 ± 15.19 | 0.164 | 0.871 |
| Duration of disease (months)c | 4 (1.38–6.00) | 2.5 (1.00–7.00) | -0.420 | 0.675 |
| Stroke Typea | 2.010 | 0.156 | ||
| Cerebral infarction | 7 (50) | 12 (75.00) | ||
| Cerebral hemorrhage | 7 (50) | 4 (25.00) | ||
| Injury sitea | 3.884 | 0.143 | ||
| Cerebral cortex | 6 (42.86) | 2 (12.50) | ||
| Subcortical | 6 (42.86) | 12 (75.00) | ||
| Mixed | 2 (14.29) | 2 (12.50) | ||
| BBSb | 31.43 ± 12.79 | 37.13 ± 9.82 | -1.378 | 0.179 |
| TUGT (s)b | 52.29 ± 19.42 | 40.06 ± 27.74 | 1.380 | 0.179 |
| FMA-LEb | 17.29 ± 5.25 | 20.50 ± 6.63 | -1.456 | 0.157 |
| SDMTb | 18.29 ± 9.97 | 22.00 ± 11.40 | -0.944 | 0.353 |
BCI, brain-computer interface; FMA-LE, fugl-meyer assessment for the lower extremity; TUGT, timed up and go test; BBS, berg balance scale; MoCA, montreal cognitive assessment; SDMT, symbol digit modalities test; DST-f, digital span test-forward; DST-b: digital span test-backward
aReported as frequency (percentage), analyzed by Chi-square test
bReported as mean ± standard deviation, analyzed by independent sample t-tests or Welch t-test
cReported as median (interquartile range), analyzed by Mann–Whitney U test
The primary outcomes in this revised analysis were balance, assessed by the BBS This outcome was selected based on a post-registration adjustment to better align with the core objectives of the dual-task BCI intervention. Original registered primary outcomes-FMA-LE and MoCA-are reported as secondary outcomes. Results for all outcomes, both primary and secondary, are presented below.
Primary outcome
Table 2 displays the results of the BBS scores. Paired t-tests (within-group analysis) indicated significant improvement in the BCI group, t = 6.623, P < 0.001, with a large effect size (d = 5.500, 95% CI [3.706–7.294]). Similarly, the conventional group also demonstrated significant improvement (t = 5.783, P < 0.001, d = 3.375, 95% CI [2.131–4.619]). An independent sample t-test was used to compare the differences in BBS scores and change values between the two groups after treatment. There was no significant difference in the BBS scores between the two groups after treatment (t = -0.936, P = 0.357, 95% CI [-11.384, 4.241]). However, the change in BBS score in the experimental group was significantly higher than that in the control group after intervention (t = 2.134, P = 0.042, 95% CI [0.086–4.164]). The BCI group showed a mean improvement in BBS of 5.5 points, exceeding the minimum clinically important difference value of 5.0 points.
Table 2.
Primary Outcome and Secondary Outcome Assessments
| Outcome | Group | Pre | Post | Change values | P Value | |
|---|---|---|---|---|---|---|
| Pre VS Post | Change values | |||||
| Primary outcome | ||||||
| BBS a | BCI | 31.43 ± 12.79 | 36.93 ± 12.09 | 5.50 ± 3.11 | < 0.01 | 0.042* |
| Control | 37.13 ± 9.82 | 40.50 ± 8.73 | 3.38 ± 2.34 | < 0.01 | ||
| Secondary outcome | ||||||
| TUGT a, s | BCI | 52.29 ± 19.41 | 40.27 ± 16.10 | -12.03 ± 9.93 | 0.001 | 0.038* |
| Control | 40.06 ± 27.74 | 34.15 ± 21.87 | -4.65 ± 8.63 | 0.027 | ||
| FMA-LE a | BCI | 17.29 ± 5.25 | 21.64 ± 4.50 | 4.36 ± 2.68 | < 0.01 | 0.476 |
| Control | 20.50 ± 6.63 | 24.00 ± 5.15 | 3.63 ± 2.85 | < 0.01 | ||
| SDMT a | BCI | 18.29 ± 9.97 | 23.57 ± 9.26 | 5.29 ± 2.30 | < 0.01 | 0.013* |
| Control | 22.00 ± 11.40 | 25.19 ± 11.36 | 3.19 ± 2.04 | < 0.01 | ||
BCI, brain-computer interface; BBS, berg balance scale; TUGT, timed up and go test; FMA-LE, fugl-meyer assessment for the lower extremity; MoCA, montreal cognitive assessment; SDMT, symbol digit modalities test; DST-f, digital span test-forward; DST-b, digital span test-backward
a Reported as mean ± standard deviation, analyzed by independent sample t test, Welch t-test or paired t test;
*P < 0.05
Secondary outcomes
Table 2 also presents the results of several secondary outcome analyses. The secondary outcomes include evaluation of walking stability, lower extremity movement, and Attention-related outcomes before and after intervention.
Both TUGT and FMA-LE showed significant improvement in both groups after the intervention, with no significant difference between the groups (t = 0.862, P = 0.396, 95% CI [-8.424, 20.667]; t = -1.325, P = 0.196, 95% CI [-6.000, 1.286]). When comparing the changes in TUGT and FMA-LE values between the two groups, the results indicated that the TUGT time was significantly shorter in the BCI group compared to the control group (t = -2.178, P = 0.038, 95% CI [-14.319, -0.440]). There was no significant difference in FMA-LE improvement values (t = 0.722, P = 0.476, 95% CI [-1.345, 2.809]).
SDMT showed significant improvement in both groups after the intervention, with no significant differences between the groups (t = -0.042, P = 0.676, 95% CI [-9.442, 6.210]). When comparing the change values between the two groups, the results indicated a significant difference in the change values of SDMT (t = 2.648, P = 0.013, 95% CI [0.475, 3.722].
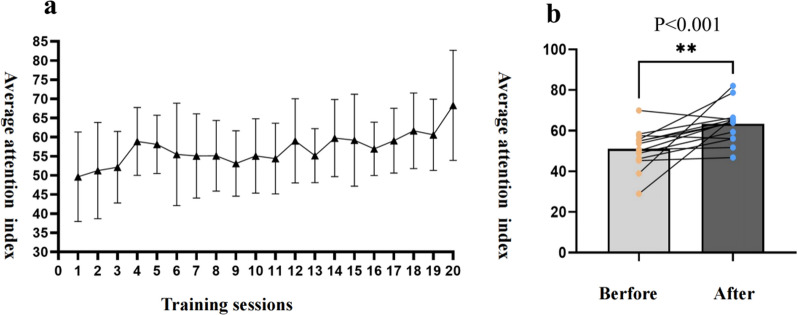
Since the EEG collection device for participants in the conventional training group was inactive during cycling sessions, we only recorded prefrontal EEG data from the BCI group. During each training session, the BCI's EEG acquisition system automatically computes the beta-alpha-band energy ratio as the attention index, at a frequency of once per second. We computed the average attention index of participants during each training session and observed its change trend over 20 sessions. Figure 3 (a) illustrates that as training sessions increased, the average attention index of BCI group participants showed a fluctuating upward trend. Additionally, we observed fluctuations in the attention index of participants during each training session. To assess changes in the attention index before and after the intervention, we averaged the attention scores from the first 3 and last 3 training sessions. Figure 3 (b) demonstrates that after 20 training sessions, the average attention index in the last 3 sessions significantly improved compared to the first 3 sessions.
Fig. 3.
Average attention index trend and comparison before and after treatment (a: The error bars represent the standard deviation, SD; b: **represent P < 0.001). Abbreviations: BCI, brain-computer interface; VR, virtual reality
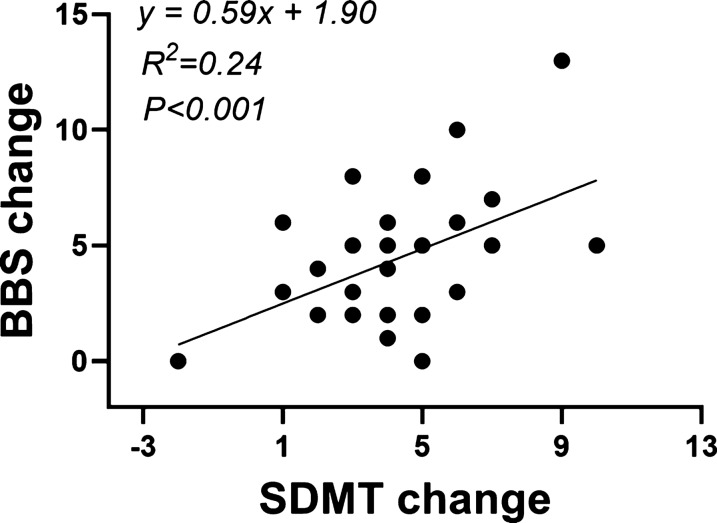
Pearson correlation analysis showed a moderate positive correlation between BBS changes and SDMT changes (r = 0.489, P = 0.006, 95% CI [0.156–0.722]). No significant correlations were observed among the other variables. Figure 4 showed a linear correlation between the BBS change values and SDMT change values (F = 8.778, P = 0.006, 95%CI [0.183, 1.001]).
Fig. 4.
Linear regression analysis of BBS change values and SDMT change values. Abbreviations: BBS, berg balance scale; SDMT: symbol digit modalities test
Discussion
This study compared the effects of MI-VR-based dual-task BCI pedaling training and conventional pedaling on balance and attention in participants with stroke. Compared to conventional pedaling training,dual-task BCI pedaling training based on MI and VR seems to be more beneficial in improving balance and attention levels in stroke participants. In addition, participants' improved balance seemed to correlate with improved attention levels.
The BCI group demonstrated a mean within-group improvement of 5.5 points in the BBS score from baseline to post-intervention, exceeding the minimum clinically important difference (MCID) of 5 points. The mean change in TUGT was 12.03 s, surpassing the minimal detectable change (MDC) of 2.9 s. Both improvements were significantly greater than those observed in the control group. while the mean TUGT change was 12.03 s, which exceeded the minimal detectable change-MDC (2.9 s). This was similar to previous findings [36]. Balance dysfunction in stroke participants is associated with reduced postural control during multitasking in addition to reduced lower limb muscle strength. In particular, when performing cognitive-motor dual-tasking, additional cognitive tasks cause distraction and affect functional activity control. Studies have shown that dual-task training has a positive effect on balance and walking function improvement, and the mechanisms involved in the central resource sharing theory and the bottleneck theory [37, 38].
It has been shown that MI-BCI-controlled pedaling training promotes a significant event-related desynchronization pattern (ERD) in the corresponding cortical areas of the foot [39]. Yuan et al. [40]. also demonstrated that BCI significantly enhanced functional connectivity between area M1, supplementary motor area (SMA), premotor cortex, and parietal regions, thereby promoting improved corticospinal tract integrity and thus improved motor performance. The activation of mirror neurons is another possible neurobiological mechanism for improving balance function through motor imagery and virtual reality BCI [41]. Both patient-initiated motor imagery and motor observation with the aid of video or mirroring can effectively activate mirror neurons to promote motor function recovery [42, 43]. The improvement of balance function by BCI may also be related to upper limb movement. Participants in this study needed to synchronize virtual reality upper limb task training with BCI pedaling, and participants had to turn the handlebars as required by the road surface and game task settings, necessitating a certain degree of proximal upper limb control ability. Studies have shown that repeated motor control training in both upper limbs of stroke patients, whether in the same or opposite directions, is beneficial for improving proximal motor function and control, promoting rapid and symmetrical activation of trunk muscles, enhancing trunk stability and symmetry, and thus improving trunk control and balance function [44, 45].
No significant difference was observed in FMA-LE scores. This aligns with previous research. Sebastián et al. [46]. performed 25 sessions of BCI-based functional electrical stimulation training in 25 stroke patients with walking impairment. The results showed a significant improvement in walking stability and speed in the participants, while there was no significant difference in FMA-LE scores. The reasons for this may be related to the characteristics of the disease course and the degree of dysfunction of the enrolled population. Participants in this study were stroke patients with some ability to walk, and they tended to have high baseline FMA-LE scores. The potential "ceiling effect" may limit the participants' further improvement in this score.
In attention-related indicators, participants in the BCI group had significantly better SDMT change values than the control group, suggesting that the dual-task mode of BCI pedaling training seems to be more conducive to improving the level of attention in stroke participants, which is in line with previous studies [47]. A clinical study of BCI based on motor imagery found that this intervention increased the attention subscale [48]. Virtual reality-based BCI technology provides participants with immersive environments, resulting in a greater sense of immersion, reduced difficulty in performing motor imagery tasks, and improved patient focus during BCI training [49]. Our findings suggest that dual-task BCI pedaling training with MI-VR significantly improves attention in stroke participants. In the secondary outcome, the prefrontal β-and α-band energy ratio (average attention index) of patients in the BCI group tended to increase with the number of training sessions. The difference between pre- and post-intervention was statistically significant. This further confirms the positive effect of BCI on attention enhancement.
The results of the correlation analysis between balance and attention indicators showed that improvement in BBS score was moderately positively correlated with improvement in SDMT that there was a linear relationship between the two. Decreased balance function and walking stability in stroke participants may be associated with inadequate overall attentional resources or imbalanced distribution of attention [50]. Limited attentional resources cannot be allocated to multiple tasks at the same time, leading to decreased postural control in stroke participants, which in turn manifests as balance and walking dysfunction. Attention is fundamental to cognitive processing and crucial, for example, for the (re)learning of motor skills. Individuals with attention disorders often struggle to focus or shift goals, exhibit reduced responsiveness to sustained stimuli, and may have difficulty attending to multiple tasks simultaneously or selecting target stimuli from among multiple tasks. Deficits in attentional focus and breadth have been reported to frequently result in reduced balance and an increased risk of falls [51]. In the present study, participants in BCI group performed brain-controlled lower limb pedaling movements along with upper limb virtual reality gaming tasks. The latter involved cognitive gaming tasks such as judging the road information in the virtual environment, steering at any time to get gold coins or tapping on treasure chests to get higher game scores, in addition to upper limb motor control training. The study found that dual-task motor and cognitive training significantly improved attention and balance function in stroke patients [52], in turn, improved concentration facilitates improved balance and walking stability [53], this is consistent with our findings.
This study explored the possible reasons of MI-VR based dual-task BCI pedaling training for improving balance and attention in participants with stroke from multiple perspectives. Moreover, improving these non-motor domains through BCI appears to positively impact motor function as well. Of course, due to the large span of disease duration of the stroke participants included in this study, participants with shorter disease duration (especially 1–3 months) may have overestimated functional improvement when comparing within groups due to natural neurological recovery. However, given that the main aim of this study was to see whether BCI pedalling efficacy was superior to conventional pedalling regimens, and randomising the groups allowed the potential impact of natural recovery from disease to be evenly distributed between the two groups. This reduces the likelihood that differences between the two groups are due to differences in natural recovery. It was also noted that the mean duration of disease in the BCI group was 4 months compared to 2.5 months in the control group, clearly the control group was superior in terms of natural course recovery compared to the BCI group. However, the results showed that the BCI group generally had a better outcome than the control group. This partly reflected the fact that the improved effect of BCI group was not entirely attributable to spontaneous recovery.
In summary, this study used a novel MI-VR dual-task brain-computer interface system for balance and attention rehabilitation in stroke patients, and preliminary results showed positive results.
Limitations
This study also has some limitations. The BCI system used in this study captured EEG signals from only one brain region in the prefrontal cortex and did not adequately isolate the EEG signals from the motor imagery task, which can result in the BCI pedals being driven by other cognitive tasks that are not motor imagery. The future should be based on more sophisticated signal separation techniques to more directly separate motion imagery signals and reduce the potential influence of non-motion processes on motion interpretation. Another limitation of this study is that the brain-computer interface group had a higher dropout rate (26.3%), which may affect the balance between the groups and the validity of the statistical results. However the reasons for dropping out were largely unrelated to the intervention itself, including technical difficulties and participant discomfort. The study met statistical power requirements but, due to its small sample size and multiple outcomes, may have an increased family-wise error rate. No correction for multiplicity was applied because it was an exploratory pilot trial aimed at generating preliminary data for a larger validation study, and strict corrections could obscure meaningful effects. As such, the findings should be interpreted with caution, and future studies with larger samples are recommended for confirmation. Another limitation of this study is the change in primary outcome measures after trial registration. The original trial registration listed the FMA-LE and MoCA as primary outcomes. However, based on the nature of the dual-task BCI intervention-targeting balance and attention through virtual reality and motor imagery-we shifted our primary focus to the BBS, as these better reflect the domains most directly impacted by the intervention. While this adjustment aligns with the study's clinical objectives, it introduces the potential for outcome reporting bias. We have clearly stated and justified this change in the manuscript in accordance with CONSORT guidelines. Nonetheless, readers should interpret the findings with caution, particularly given the favorable result on the new primary outcome. Finally, this study was that the patients’ task participation and the accuracy of the BCI system could not be objectively verified during the intervention sessions. While participants were instructed to engage in motor imagery and attention tasks, we were not able to implement real-time or post-hoc validation of neural engagement, such as EEG-based performance metrics or classification accuracy. This limits our ability to confirm whether participants were actively and consistently modulating their brain activity as intended. Future studies should incorporate objective measures of BCI performance, such as alpha/beta ratio modulation during relaxation versus attention trials, to assess user engagement and improve system reliability.
Conclusions
The results of this preliminary randomized clinical trial suggest that 4 weeks of MI-VR based dual-task BCI system was beneficial for improving balance and attention in participants with stroke. However, due to the exploratory nature of our study, the lack of multiple corrections for results, and the lack of data support for functional brain connectivity, the findings still need to be viewed with caution. More rigorous clinical randomized controlled trials should be designed in the future. The positive effects of dual-task BCI on balance and attention in stroke should be further clarified by larger sample sizes, better statistical analysis methods, and the addition of objective evaluation indexes that can reflect changes in brain function.
Acknowledgements
We thank all those involved in this work who have contributed to running this study.
Abbreviations
- BCI
Brain Computer Interface
- MI
Motor Imagery
- VR
Virtual Reality
- FMA-LE
Fugl-Meyer Lower Extremity
- BBS
Berg Balance Scale
- TUGT
Timed Up and Go Test
- MoCA
Montreal Cognitive Assessment
- SDMT
Symbol Digit Modalities Test
Author contributions
CLW: Writing-original draft preparation and substantively revision. Qiyuan Zhang: Writing-review & editing and revision. Yu Qiu: Interpretation of data. WTZ: Investigation. YN: Investigation. SYZ: Investigation. JW: Interpretation of data. XWS: Methodology. CY: Interpretation of data. XXW: Methodology and conception. YTZ: Writing-Review&Editing. YQL: Design of the work and conception. All authors revised and agreed to the final version of this article. CLW, QYZ and YQ contributed equally to this work.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was supported by Jiangsu Provincial People's Hospital, Clinical Diagnosis and Treatment Technology Innovation 'Open bidding for selecting the best candidates' Project (No. JBGS202414), Major sports research projects of Jiangsu Sports Bureau (No. ST242102), Ministry of Industry and Information Technology and National Health Commission High-end Equipment Promotion and Application Project (2024TGYY51), Project supported by the National Natural Science Foundation of China (No.82572957).
Availability of data and materials
The datasets associated with the study are not publicly available due patient data protection policy but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (No.2023-SR-049). and registered at Chinese ClinicalTrials.gov (ChiCTR2300071522). All participants and their parents provided informed consent before participating in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xixi Wu, Email: 1010464860@qq.com.
Yuting Zhang, Email: Zhangwish@126.com.
Yongqiang Li, Email: liyongqiang_1980@163.com.
References
- 1.GBD Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weerdesteyn V, de Niet M, van Duijnhoven HJ, Geurts AC. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45(8):1195–213. [PubMed] [Google Scholar]
- 3.Hua J, Dong J, Chen GC, Shen Y. Trends in cognitive function before and after stroke in China. BMC Med. 2023;21(1):204. 10.1186/s12916-023-02908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu T, Clemson L, O’Loughlin K, Lannin NA, Dean C, Koh G. Risk factors for falls in community stroke survivors: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2018;99(3):563-573.e5. [DOI] [PubMed] [Google Scholar]
- 5.Marín-Medina DS, Arenas-Vargas PA, Arias-Botero JC, Gómez-Vásquez M, Jaramillo-López MF, Gaspar-Toro JM. New approaches to recovery after stroke. Neurol Sci. 2024;45(1):55–63. 10.1007/s10072-023-07012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cervera MA, Soekadar SR, Ushiba J, Millán JDR, Liu M, Birbaumer N, Garipelli G. Brain-computer interfaces for post-stroke motor rehabilitation: a meta-analysis. Ann Clin Transl Neurol. 2018;5(5):651–63. 10.1002/acn3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Zhang W, Li W, Zhang S, Lv P, Yin Y. Effects of motor imagery based brain-computer interface on upper limb function and attention in stroke patients with hemiplegia: a randomized controlled trial. BMC Neurol. 2023;23(1):136. 10.1186/s12883-023-03150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang Y, Yin J, Zhao B, Zhang Y, Peng T, Zhuang W, Wang S, Huang S, Zhong M, Zhang Y, Tang G, Shen B, Ou H, Zheng Y, Lin Q. Motor Imagery Brain-Computer Interface in Rehabilitation of Upper Limb Motor Dysfunction After Stroke. J Vis Exp. 2023;(199).10.3791/65405. [DOI] [PubMed]
- 9.Yuan Z, Peng Y, Wang L, Song S, Chen S, Yang L, Liu H, Wang H, Shi G, Han C, Cammon JA, Zhang Y, Qiao J, Wang G. Effect of BCI-controlled pedaling training system with multiple modalities of feedback on motor and cognitive function rehabilitation of early subacute stroke patients. IEEE Trans Neural Syst Rehabil Eng. 2021;29:2569–77. 10.1109/TNSRE.2021.3132944. [DOI] [PubMed] [Google Scholar]
- 10.Romero-Laiseca MA, Delisle-Rodriguez D, Cardoso V, Gurve D, Loterio F, Posses Nascimento JH, Krishnan S, Frizera-Neto A, Bastos-Filho T. A low-cost lower-limb brain-machine interface triggered by pedaling motor imagery for post-stroke patients rehabilitation. IEEE Trans Neural Syst Rehabil Eng. 2020;28(4):988–96. 10.1109/TNSRE.2020.2974056. [DOI] [PubMed] [Google Scholar]
- 11.Chung E, Park SI, Jang YY, Lee BH. Effects of brain-computer interface-based functional electrical stimulation on balance and gait function in patients with stroke: preliminary results. J Phys Ther Sci. 2015;27(2):513–6. 10.1589/jpts.27.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akin H, Senel A, Taskiran H, Kaya Mutlu E. Do motor-cognitive and motor-motor dual task training effect differently balance performance in older adults? Eur Geriatr Med. 2021;12(2):371. 10.1007/s41999-020-00434-8. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q, Yang H, Zhou Q, Pan H. Effects of cognitive motor dual-task training on stroke patients:A RCT-based meta-analysis. J Clin Neurosci. 2021;92:175–85. 10.1016/j.jocn.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, CruzRivera S, Moher D, Calvert MJ, Denniston AK, The SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat Med. 2020;26(9):1364–1374.10.1136/bmj.m3164. [DOI] [PMC free article] [PubMed]
- 15.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. 10.1111/j.1532-5415.2005.53221. [DOI] [PubMed] [Google Scholar]
- 16.Sakai K, Hosoi Y. Relationship between the vividness of motor imagery and physical function in patients with subacute hemiplegic stroke: a cross-sectional preliminary study. Brain Inj. 2022;36(1):121–6. [DOI] [PubMed] [Google Scholar]
- 17.Cai H, Lin T, Chen L, Weng H, Zhu R, Chen Y, Cai G. Evaluating the effect of immersive virtual reality technology on gait rehabilitation in stroke patients: a study protocol for a randomized controlled trial. Trials. 2021;22(1):91. 10.1186/s13063-021-05031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. 10.1111/j.1532-5415.2005.53221.x.Erratum.In:JAmGeriatrSoc.2019Sep;67(9):1991. [DOI] [PubMed] [Google Scholar]
- 19.Kenneth N.K. Fong a, Marko K.L. Chan b, Bill Y.B. Chan b, Peggie P.K. Ng b, Mei Ling Fung b, May H.M. Tsang b, Kathy K.Y. Chow b. Reliability and Validity of the Chinese Behavioural Inattention Test–Hong Kong Version (CBIT-HK) for Patients with Stroke and Unilateral Neglect. Hong Kong Journal of Occupational Therapy. 2007;17(1):23–33.10.1016/S1569-1861(07)70004-9.
- 20.Zhang X, Zhang Q. Correlation between post-stroke upper limb motor dysfunction and aphasia. Chin J Rehabil. 2024;39(09):521–4. [Google Scholar]
- 21.Meseguer-Henarejos AB, Sánchez-Meca J, López-Pina JA, Carles-Hernández R. Inter- and intra-rater reliability of the modified Ashworth scale: a systematic review and meta-analysis. Eur J Phys Rehabil Med. 2018;54(4):576–90. 10.23736/S1973-9087.17.04796-7. [DOI] [PubMed] [Google Scholar]
- 22.Liu NH, Chiang CY, Chu HC. Recognizing the degree of human attention using EEG signals from mobile sensors. Sensors. 2013;13(8):10273–86. 10.3390/s130810273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pope AT, Bogart EH, Bartolome DS. Biocybernetic system evaluates indices of operator engagement in automated task. Biol Psychol. 1995;40(1–2):187–95. 10.1016/0301-0511(95)05116-3. [DOI] [PubMed] [Google Scholar]
- 24.Sapmaz M, Mujdeci B. The effect of fear of falling on balance and dual task performance in the elderly. Exp Gerontol. 2021;147: 111250. 10.1016/j.exger.2021.111250. [DOI] [PubMed] [Google Scholar]
- 25.Lopes JB, Lameira de Melo GE, Lazzari RD, Santos CA, Franco de Moura RC, Dumont AJ, Braun LA, Duarte NA, Pareira RB, Miziara IM, Oliveira CS. Measures used for the evaluation of balance in individuals with Parkinson’s disease: a systematic review. J Phys Ther Sci. 2016;28:1936–42. 10.1589/jpts.28.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther. 2008;88(5):559–66. 10.2522/ptj.20070205. [DOI] [PubMed] [Google Scholar]
- 27.Tamura S, Miyata K, Kobayashi S, Takeda R, Iwamoto H. The minimal clinically important difference in Berg Balance Scale scores among patients with early subacute stroke: a multicenter, retrospective, observational study. Top Stroke Rehabil. 2022;29(6):423–9. 10.1080/10749357.2021.1943800. [DOI] [PubMed] [Google Scholar]
- 28.Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil. 2005;86(8):1641–7. 10.1016/j.apmr.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Gupta A, Prakash NB, Sannyasi G, Mohamad F, Honavar P, Jotheeswaran S, Khanna M, Ramakrishnan S. Effect of overground gait training with “mobility assisted robotic system-MARS” on gait parameters in patients with stroke: a pre-post study. BMC Neurol. 2023;23(1):296. 10.1186/s12883-023-03357-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16(3):232–40. [DOI] [PubMed] [Google Scholar]
- 31.Chen RQ, Wu JX, Shen XS. A research on the minimal clinically important differences of Chinese version of the Fugl-Meyer motor scale. Acta UniversitatisMedicinalis Anhui. 2015;50(04):519–22. 10.19405/j.cnki.issn1000-1492.2015.04.025. [Google Scholar]
- 32.Chiaravalloti ND, Stojanovic-Radic J, DeLuca J. The role of speed versus working memory in predicting learning new information in multiple sclerosis. J Clin Exp Neuropsychol. 2013;35(2):180–91. [DOI] [PubMed] [Google Scholar]
- 33.Li MG, He JF, Liu XY, Wang ZF, Lou X, Ma L. Structural and functional thalamic changes in Parkinson’s disease with mild cognitive impairment. J Magn Reson Imaging. 2020;52(4):1207–15. 10.1002/jmri.27195. [DOI] [PubMed] [Google Scholar]
- 34.Rogers JM, Johnstone SJ, Aminov A, Donnelly J, Donnelly J, Wilson PH. Test-retest reliability of a single-channel, wireless EEG system. Int J Psychophysiol. 2016;106:87–96. 10.1016/j.ijpsycho.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Doğğan A, MengüllüoĞĞlu M, Özgirgin N. Evaluation of the effect of ankle-foot orthosis use on balance and mobility in hemiparetic stroke patients. Disabil Rehabil. 2011;33:1433–9. 10.3109/09638288.2010.533243. [DOI] [PubMed] [Google Scholar]
- 36.Tang H, Su B, Che P, Liangcheng P, Wang T. Effects of a brain-computer interface combined with an end-driven lower limb robot on the balance and walking function in stroke patients. Chin J Rehabil Med. 2024;39(06):791–7. [Google Scholar]
- 37.Kim H, Fraser S. Neural correlates of dual-task walking in people with central neurological disorders: A systematic review. J Neurol. 2022;269(5):2378–402. 10.1007/s00415-021-10944-5. [DOI] [PubMed] [Google Scholar]
- 38.Bayot M, Dujardin K, Tard C, Defebvre L, Bonnet CT, Allart E, Delval A. The interaction between cognition and motor control: a theoretical framework for dual-task interference effects on posture,gait initiation,gait and turning. Neurophysiol Clin. 2018;48(6):361–75. [DOI] [PubMed] [Google Scholar]
- 39.Cardoso VF, Delisle-Rodriguez D, Romero-Laiseca MA, Loterio FA, Gurve D, Floriano A, Valadão C, Silva L, Krishnan S, Frizera-Neto A, Freire Bastos-Filho T. Effect of a brain-computer interface based on pedaling motor imagery on cortical excitability and connectivity. Sensors. 2021;21(6): 2020. 10.3390/s21062020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan K, Wang X, Chen C, Lau CC, Chu WC, Tong RK. Interhemispheric functional reorganization and its structural base after BCI-guided upper-limb training in chronic stroke. IEEE Trans Neural Syst Rehabil Eng. 2020;28(11):2525–36. 10.1109/TNSRE.2020.3027955. [DOI] [PubMed] [Google Scholar]
- 41.Bilika P, Karampatsou N, Stavrakakis G, Paliouras A, Theodorakis Y, Strimpakos N, Kapreli E. Virtual reality-based exercise therapy for patients with chronic musculoskeletal pain: a scoping review. Healthcare. 2023;11(17): 2412. 10.3390/healthcare11172412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cook R, Bird G, Catmur C, Press C, Heyes C. Mirror neurons: from origin to function. Behav Brain Sci. 2014;37(2):177–92. 10.1017/S0140525X13000903. [DOI] [PubMed] [Google Scholar]
- 43.Mao H, Li Y, Tang L, Chen Y, Ni J, Liu L, Shan C. Effects of mirror neuron system-based training on rehabilitation of stroke patients. Brain Behav. 2020;10(8): e01729. 10.1002/brb3.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JH, Choi JD. The effects of upper extremity task training with symmetric abdominal muscle contraction on trunk stability and balance in chronic stroke patients. J Phys Ther Sci. 2017;29(3):495–7. 10.1589/jpts.29.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee DH, Park SH, Han JW. Effect of bilateral upper extremity exercise on trunk performance in patients with stroke. J Phys Ther Sci. 2017;29(4):625–8. 10.1589/jpts.29.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sebastián-Romagosa M, Cho W, Ortner R, Sieghartsleitner S, Von Oertzen TJ, Kamada K, Laureys S, Allison BZ, Guger C. Brain-computer interface treatment for gait rehabilitation in stroke patients. Front Neurosci. 2023;17:1256077. 10.3389/fnins.2023.1256077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mroczkowska D, Białkowska J, Rakowska A. Neurofeedback as supportive therapy after stroke. Case report Postępy Psychiatrii i Neurologii. 2014;23:190–201. [Google Scholar]
- 48.Fateeva VV, Kushnir AB, Grechko AV, Mayorova LA. Rehabilitation of patients with post-stroke cognitive impairment using the P300-based brain-computer interface: results of a randomized controlled trial. Zh Nevrol Psikhiatr Im S S Korsakova. 2023;123:68–74. 10.17116/jnevro202312312268. [DOI] [PubMed] [Google Scholar]
- 49.Coogan CG, He B. Brain-computer interface control in a virtual reality environment and applications for the internet of things. IEEE Access. 2018;6:10840–9. 10.1109/ACCESS.2018.2809453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang L, Lam F, Huang M. Dual-task mobility among individuals with chronic stroke:changes in cognitive-motor interference patterns and relationship to difficulty level of mobility and cognitive tasks. Eur J Phys Rehabil Med. 2017;54(4):526–35. [DOI] [PubMed] [Google Scholar]
- 51.Hyndman D, Ashburn A. People with stroke living in the community: attention deficits, balance. ADL ability and falls Disabil Rehabil. 2003;25:817–22. 10.1080/0963828031000122221. [DOI] [PubMed] [Google Scholar]
- 52.Shu Y, Bi MM, Zhou TT, Liu L, Zhang C. Effect of dual-task training on gait and balance in stroke patients: an updated meta-analysis. Am J Phys Med Rehabil. 2022;101(12):1148–55. 10.1097/PHM.0000000000002016. [DOI] [PubMed] [Google Scholar]
- 53.Peters S, Handy TC, Lakhani B, Boyd LA, Garland SJ. Motor and visuospatial attention and motor planning after stroke: considerations for the rehabilitation of standing balance and gait. Phys Ther. 2015;95(10):1423–32. 10.2522/ptj.20140492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets associated with the study are not publicly available due patient data protection policy but are available from the corresponding author on reasonable request.