Abstract
Background
Large carnivores in human-dominated landscapes face significant risks from increased anthropogenic pressure, making it crucial to understand their movement behaviour for conservation strategies.
Methods
We used conventional and generalised hidden Markov models (HMMs) to analyse GPS telemetry data collected from 2016 to 2022 on 15 subadult tigers to classify behavioural states across three life stages (pre-dispersal, dispersal, post-dispersal) in the Eastern Vidarbha Landscape, India. We further examined how intrinsic and extrinsic factors influenced transitions between these behavioural states.
Results
Three distinct behavioural states were identified: resting (stationary movement with very short step lengths), area-restricted movement (tortuous movement with short to intermediate step lengths), and travelling (highly directional movement with long step lengths). During the pre-dispersal phase, tigers displayed exploratory movement within their natal range, with significant emphasis on area-restricted movement (42.10%), followed by travelling (30.47%), and resting (27.42%). Travelling peaked at dusk and showed the highest probability of occurrence throughout the night until dawn and exhibited faster movement in areas with high human density. Area-restricted movement was most frequent during the day and peaked between 09:00–11:00 h, while resting showed the highest probability between 22:00–23:00 h. Dispersing tigers allocated their activity budget equally among resting (32.09%), area-restricted movement (35.77%), and travelling (32.14%), as they navigated fragmented landscapes comprising of forests, wildlife corridors, agricultural fields, and human settlements. They exhibited faster, directed movements in low-cover areas and increased step lengths in fragmented, non-forest habitats, with a greater likelihood of travelling at dusk and night. Tigers in the post-dispersal phase had stable home ranges and maintained well-defined territorial boundaries. During area-restricted movement, they exhibited longer step lengths in forest habitats and faster travel speeds in a human‒agricultural matrix. Moreover, they tended to rest at high temperatures and travelled more when the temperatures were between 20 and 30 °C.
Conclusions
Our study provides crucial insights on tiger movements in human-dominated landscapes across different life stages and habitats. Understanding their behavioural patterns and implementing effective conservation efforts can ensure the long-term survival of tigers and their coexistence with humans.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40462-025-00594-x.
Keywords: Behaviour, Large carnivore, Hidden markov model, Human-dominated landscape, Movement, Panthera Tigris, Telemetry
Background
Anthropogenic activities contribute to fragmentation, degradation, and loss of habitats worldwide, affecting animal movements [1–3]. High-quality habitats are typically separated into small patches surrounded by unsuitable habitats and anthropogenic features [4]. Consequently, organisms with relatively extensive spatial requirements, such as large carnivores, are forced to inhabit multiple landscapes and move through heterogeneous and human-dominated environments [5, 6]. As a result, large carnivores regularly come into conflict with humans and face significant challenges, including persecution [6]. For example, tigers (Panthera tigris) are sometimes killed in retaliation for preying on livestock or in response to perceived danger to people. Additionally, anthropogenic disturbances like roads also negatively impact wildlife populations [7], e.g., increased wildlife mortality from roadkill [8, 9], reduced ecological connectivity by inhibiting movement [10, 11] or altered species activity and individual behaviour [12, 13]. For example, large carnivores have been shown to modify their movement patterns in response to high human pressure, like increasing movement speed or altering activity patterns to avoid human conflict [14, 15]. This is important because the efficiency of carnivore movement and behaviour determines their ability to survive and persist in human-dominated landscapes [16].
Understanding movement behaviour is crucial in determining how and when individuals traverse landscapes to access resources, mates, avoid competition, and evade predators at various spatial and temporal scales [2, 17, 18]. For example, dispersal is a key ecological process that links movement behaviour to landscape structure, facilitating gene flow, and is important for maintaining functional connectivity between populations [19, 20]. However, increasing landscape fragmentation and the expansion of linear infrastructures, like roads, settlements and fencing, can influence animal dispersal and movement by creating physical barriers and high mortality risks, and force animals to navigate through unsuitable habitats [3, 21, 22]. In response, many species have shown behavioural strategies to avoid spatial or temporal interactions with humans while efficiently using resources, by adjusting their movement patterns or habitat use [14, 23, 24]. For example, large carnivores like lions (Panthera leo), cougars (Puma concolor), and leopards (Panthera pardus) have been observed altering their activity to more nocturnal patterns or increasing movement speeds in human-modified areas to reduce encounter risk and energy expenditure [25–27].
Quantifying and interpreting movement adaptations is essential for promoting long-term human-wildlife coexistence and deriving appropriate conservation management strategies [2, 28], and advances in telemetry and analytical tools have made it increasingly feasible to derive fine-scale insights [29]. For example, hidden Markov models (HMMs) applied to GPS telemetry data can be used to classify movement into distinct behavioural states like resting, foraging or travelling [30], based on movement parameters like step lengths and turn angles [31–33] and investigate how intrinsic (e.g., age, sex) and extrinsic (e.g., habitat type, human disturbance) covariates influence the probability of animals to switch between these different movement states [34, 35]. HMMs thus provide a powerful framework for understanding how large carnivores adapt their movement to navigate complex and fragmented landscapes [30].
Large carnivores, such as tigers in India, are primarily found in protected areas (PAs), although approximately 35% of the tiger population is estimated to occur outside these PAs [14]. Areas outside PAs are typically fragmented landscape, comprising forest patches interspersed with agricultural fields, human settlements and other developed lands. In such environments, tigers may have to move further or faster to mitigate conflicts with humans [14, 36]. Consequently, the long-term persistence of tiger populations depends on the successful dispersal of individuals through human-dominated landscapes, enabling access to suitable habitats and facilitating gene flow and population connectivity. Recent research has shown that tigers adjust their movement patterns in response to both environmental variation and anthropogenic pressures [37, 38]. However, no previous study has systematically investigated how tigers modulate their movement behaviour across distinct life stages (pre-dispersal, dispersal, and post-dispersal) in response to environmental and anthropogenic conditions. In particular, our understanding remains limited regarding how tigers modify their behaviour while residing within their natal range (pre-dispersal), navigating through complex, multi-use landscapes during dispersal, and converging to stable home ranges during the post-dispersal phase. We hypothesised that tiger movement behaviour would differ across these life stages, reflecting environmental and anthropogenic conditions. For example, dispersing individuals are expected to encounter novel and often high-risk areas, requiring greater behavioural flexibility [19]. In contrast, tigers in the post-dispersal phase are likely to exhibit more consistent and structured movement patterns, reflecting habituation to familiar environmental features and risk exposure. We further predicted that movement metrics (step length and turn angle) would vary across habitat types (e.g., forest vs. non-forest), given that increasing human modification of landscapes has been shown to constrain movement behaviour in terrestrial mammals [3].
Here, we studied the movement behaviour of tigers during three life stages in the Eastern Vidarbha Landscape of India. Specifically, we used HMMs to examine how tiger behavioural states are associated with ecological, environmental, and anthropogenic factors, including temperature, diel period (time of day), habitat type (forest and non-forest), proximity to roads, and human population density. Our study had two main objectives: (i) to identify and characterise the behavioural states exhibited by tigers across life stages and (ii) to assess how environmental variables (ambient temperature, time of day, and habitat type) and anthropogenic variables (proximity to roads and human population density) influence behavioural states.
Methods
Study area
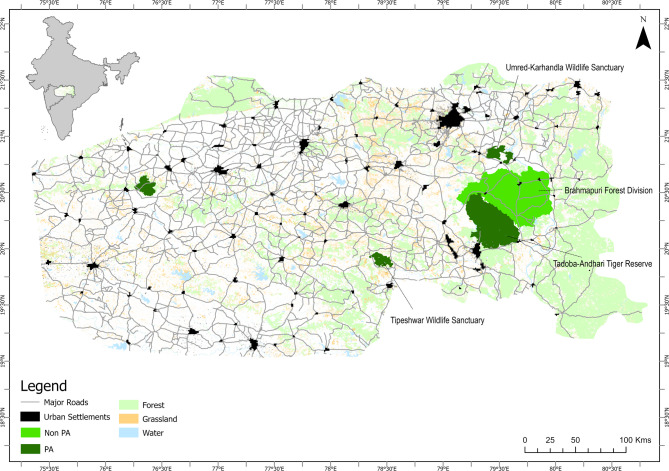
This study was conducted in the Eastern Vidarbha Landscape (EVL) of Maharashtra, a part of the Central Indian Tiger Landscape (Fig. 1), covering approximately 97,320 km² with 27.5% forest cover (Habib et al., 2021). The tiger-bearing PAs in the study include Tadoba-Andhari Tiger Reserve (TATR), Umred-Karhandla Wildlife Sanctuary (UKWLS), and Tipeshwar Wildlife Sanctuary (TWLS). The area outside the PAs comprises the Brahmapuri Forest Division, which is interspersed with urban, semi-urban and rural settlements, and encompasses 8540 villages [39].
Fig. 1.
Study area of the Eastern Vidarbha Landscape showing protected and non-protected areas, land use types (forest, grassland, water bodies), major roads, and human settlements (urban, semi-urban, rural), where 15 tigers were captured and fitted with GPS collars during 2016–2022.
Across the EVL, human settlements vary in density, with urban areas reaching over 3870 person/km², while rural areas maintain lower densities, averaging around 305 person/km². The region is traversed by an extensive road network, comprising highways, primary, and minor roads, with a density of 0.20 km/km². The vegetation is primarily dry deciduous, dominated by teak (Tectona grandis) and bamboo (Dendrocalamus strictus). Faunal species include the tiger, cooccurring with other predators like leopard, dhole (Cuon alpinus), and sloth bear (Melursus ursinus). The ungulate prey base includes species like chital (Axis axis), sambar (Rusa unicolor), nilgai (Boselaphus tragocamelus), and wild pig (Sus scrofa), which are distributed within and outside PAs [40]. The study area experiences a tropical monsoon climate with dry and hot summers, rainy monsoon seasons, and mild winters [41]. Annual precipitation in the EVL varies widely, ranging from 400 to 2000 mm.
Telemetry data collection
We fitted 15 subadult tigers from the EVL of Maharashtra, India, with GPS collars between 2016 and 2022. Of those tigers, we captured ten tigers from PAs (eight males and two females) and five from outside PAs (one male and four females) of different ages (Additional file 1: Supplementary Table S1). Within PAs, which included wildlife sanctuaries and tiger reserves, tigers were captured from various locations. In tiger reserves such as TATR, tigers were captured from the core and buffer areas. Core areas are critical habitats for tigers without human activity, whereas buffer areas lie on the periphery of core zones and include a combination of wild- and human-dominated areas.
Tigers were captured and immobilised using a combination of medetomidine hydrochloride, ketamine hydrochloride, and xylazine (dosages based on body weight, age, and sex). The collars fitted were equipped with GPS and VHF telemetry (GPS Plus; Vectronic Aerospace, Berlin, Germany), allowing for remote data acquisition through satellite and real-time ground-based tracking in the field using VHF. All captured tigers were older than one year at the time of collaring, and collars were padded with foam to accommodate neck growth into adulthood. GPS locations were received at 1–5 h intervals, depending on the life stage of the individual (see next section) and were downloaded remotely via satellite using the GPS Plus X software (Vectronic Aerospace). We used VHF telemetry to track individual tiger movements and observe their behaviour until they established territories. Once individuals established a stable and defined home range, collars were removed using a remote drop-off mechanism upon completion of monitoring.
Dispersal movements
Dispersal is a three-stage ecological process encompassing emigration (departure from the natal range), transience (exploratory movement through unfamiliar areas), and settlement (establishment of a new, stable home range) [19, 42]. In this study, we focused on natal dispersal, which refers to the movement of individuals from their birth site to the location where they eventually establish a breeding territory [19]. To initially distinguish between dispersing and non-dispersing individuals, we visually assessed semivariogram plots, which represent the spatial autocorrelation of movement paths over time and are commonly used to evaluate range residency [43, 44].
Following the identification of dispersing individuals, we aligned the conceptual dispersal framework with movement-based phases derived from net squared displacement (NSD) to classify them into distinct life stages i.e., pre-dispersal, dispersal, and post-dispersal [45]. We distinguished between the ecological stages of dispersal (emigration, transience, settlement) and the movement phases used in our analysis (pre-dispersal, dispersal, post-dispersal), which, though conceptually aligned, reflect behavioural and empirical perspectives, respectively. While ecological stages represent conceptual definitions from dispersal theory, our movement phases are empirically defined based on observed movement patterns extracted from NSD analyses. The pre-dispersal phase is characterised by the movement of individuals within their natal range prior to permanent departure. The dispersal phase involves a permanent, one-way movement from a natal range to another habitat in search of new territory and potential mates [46, 47]. The post-dispersal phase involves movement in an area with a relatively stable and well-defined home range that persists over time. Furthermore, the individual’s post-dispersal range does not overlap with the pre-dispersal or dispersal range.
Processing of movement data
HMMs typically require animal locations that are temporally regular and recorded with high positional accuracy [48]. In our dataset, GPS locations for tigers were recorded at irregular intervals ranging from 1 to 5 h, depending on the life stages. As HMMs are sensitive to temporal resolution (i.e., scale-dependent), inconsistent time steps can bias behavioural inference. Therefore, we filtered the data to include only 2-h and 3-h intervals (the most frequent in our dataset) and regularised the movement paths to consistent 2-h time steps using continuous-time correlated random walk (CTCRW) models [43, 49]. This approach allowed us to predict temporally regular tracks at 2-h intervals suitable for HMM analysis [50, 51].
Environmental and anthropogenic covariates
The time of day (hours), temperature (°C), habitat (forest or non-forest), human population density (people/km²), and distance to the nearest road (km) were determined for each GPS fix and included as predictor variables in the HMM (see below) to test their influence on state transition probabilities (i.e., the probability of switching between behavioural states) and state-dependent probability distribution parameters (distribution of step lengths and turn angles). The time of day was included as a cosine function to account for cyclical behaviour (24 h). The Euclidean distance to the nearest road was calculated using OpenStreetMap geospatial data (OpenStreetMap 2020) in Google Earth Engine [52]. The temperature data were retrieved from the GPS collar sensor. Human population density data were obtained from SEDAC, NASA, at a spatial resolution of 1 × 1 km (https://sedac.ciesin.columbia.edu/). We obtained land use data from Bhuvan (NRSA, 2016; http://bhuvan.nrsc.gov.in/), with a spatial resolution of 60 m. We classified the landscape into two broad habitat types: forest habitat (forest, grassland, and water bodies) and non-forest habitat (comprising built-up areas and agriculture).
Hidden Markov models
We used conventional and generalised HMMs to examine how tiger behavioural states are influenced by environmental and anthropogenic covariates [48]. The conventional HMM allows covariates to affect the state transition probabilities (i.e., the probabilities of switching between behavioural states such as resting, foraging, or travelling, including remaining in the same state i.e., state-persistence). Generalised HMMs extend this framework by allowing covariates to also influence the state-dependent probability distribution parameters of the movement characteristics [53]. This added flexibility is valuable for investigating how covariates influence both the likelihood of switching between behavioural states and the characteristics of movement within states. For example, generalised HMMs allow us to assess if the speed and directionality of tiger movement when travelling varies according to the habitat they are moving through (e.g., roads or human-dominated landscapes), offering greater ecological inference about avoidance or risk-sensitive behaviours.
Statistical analysis using HMMs
The HMMs were fitted by modelling the step lengths with a gamma distribution and turning angles using a von Mises distribution, which is a circular analogue of the normal distribution [54]. We fitted the models using three distinct behavioural states because 3-state HMMs are statistically well supported and biologically meaningful for terrestrial mammalian movement [e.g., 29, 55]. These behavioural states were defined as resting (stationary movement with very short step lengths), area-restricted movement (tortuous movement with short to intermediate step lengths), and travelling (highly directional movement with longer step lengths). We fitted 25 HMMs using different sets of randomly selected starting values for step length and turning angle parameters to ensure optimal maximum likelihood estimates. These starting values were chosen within plausible ranges determined by inspecting histograms of step lengths and turning angles. The model outputs were robust to different starting values, reflecting the convergence value of the maximum likelihood. The model with the lowest AIC value was considered the best model for starting values and used to fit a priori models (N = 19), consisting of ecological and anthropogenic covariates across the life stages, which were fitted using conventional and generalised HMM frameworks (Additional file 1: Supplementary Table S2). The most likely sequence of behavioural states for these models was decoded using the Viterbi algorithm [55]. Model assumptions were verified by visually inspecting the pseudo-residual plots [54, 55]. We used the package crawl to fit CTCRW models [49] and momentuHMM to implement HMMs [48] in R version 4.3.2 [56].
Results
We identified three distinct behavioural states exhibited by tigers during the pre-dispersal, dispersal, and post-dispersal phases. The ‘resting state’ refers to stationary behaviour, whereas tigers in the ‘area-restricted movement’ state indicate foraging or movement within a small area. The ‘travelling state’ corresponds to fast and directed movement over a long distance. The best-supported models of tiger behaviour influenced by environmental and anthropogenic variables across life stages are listed in Table 1. Furthermore, the HMMs revealed different predictor variables that affected the stationary and transition probabilities, and state-dependent movement characteristics. Model validation confirmed that the assumptions of the HMMs were met, based on the visual inspection of pseudo-residual plots for each life-stage-specific model, with no substantial deviations from normality or homoscedasticity, supporting the reliability of model inferences (Additional file 1: Supplementary Figure S3).
Table 1.
The top two hidden Markov models (HMMs), ranked by ΔAIC, for each tiger life stage (pre-dispersal, dispersal, post-dispersal), describing 3-state movement behaviours (resting, area-restricted movement, and travelling) at 2-hour intervals. All other candidate models and parameters are in table S4 (Additional file 1). Habitat type (forest, non-forest) influenced state-dependent distribution parameters (step length, turning angle) across all life stages. Regression coefficients and state transition probabilities for the top three models are presented in tables S5 and S6 (Additional file 1)
| Life stage | Covariate effects on stationary and transition probabilities | AIC | ΔAIC |
|---|---|---|---|
| Pre-dispersal | Ecological (time of day) + Anthropogenic (human population density) | 19740.91 | 0 |
| Ecological (time of day and habitat) + Environmental (temperature) | 19760.79 | 19.87 | |
| Dispersal | Ecological (time of day and habitat) + Environmental (temperature) | 15468.86 | 0 |
| Ecological (time of day) + Environmental (temperature) | 15476.48 | 7.62 | |
| Post-dispersal | Ecological (time of day + habitat) + Environmental (temperature) | 38201.44 | 0 |
| Ecological (time of day + habitat) | 38211.65 | 10.2 |
Pre-dispersal
We analysed data from 10 individuals (6 males and 4 females) ranging from 15 to 154 days per individual, with 9420 GPS locations of tigers from the Eastern Vidarbha Landscape. State 1 (resting) corresponded to very short step lengths (mean = 14.21 m) and variable turning angles (mean = 3.14 radians; concentration = 0.08), indicating stationary and localized movement. State 2 (area-restricted movement) had short step lengths (mean = 99.81 m) and a wide distribution of turning angles (mean = 0.10 radians; concentration = 0.34), indicating tortuous movement within a small area. State 3 (travelling) had longer step lengths (mean = 751.04 m) and a high turning angle concentration parameter (mean = -0.02 radians; concentration = 1.07), indicating faster movement with high directionality (Additional file 2: Figure S1). On average, the tigers spent 30.47% of their time travelling, 42.10% in area-restricted movement, and 27.42% resting. The best model describing the pre-dispersal phase in tigers included the effects of time of day and human population density on the transition probabilities. The movement characteristics (step length and turn angle) within the behavioural states (i.e., state-dependent parameter distributions) were associated with the habitat type (forest or non-forest).
State-dependent distribution parameters (step length and turn angle)
In the resting and area-restricted movement states, the mean step lengths between forest and non-forest habitats were relatively similar (~ 13 m in the resting state and ~ 30 m in area-restricted movement; Additional file 2: Figure S2). However, during the travelling state, the mean step length was greater in non-forest habitats (~ 900 m) compared to forest habitats (~ 750 m). There was no consistent directionality of individuals in the resting state in either habitat (Additional file 2: Figure S3). During area-restricted movements, tigers had higher directionality in forest habitats (concentration = ~ 0.35) compared to non-forest habitats (concentration = ~ 0.1). During the travelling state, tigers exhibited similarly high directional persistence in non-forest (angle concentration = 1.08) and forest habitats (1.04).
Stationary-state probabilities
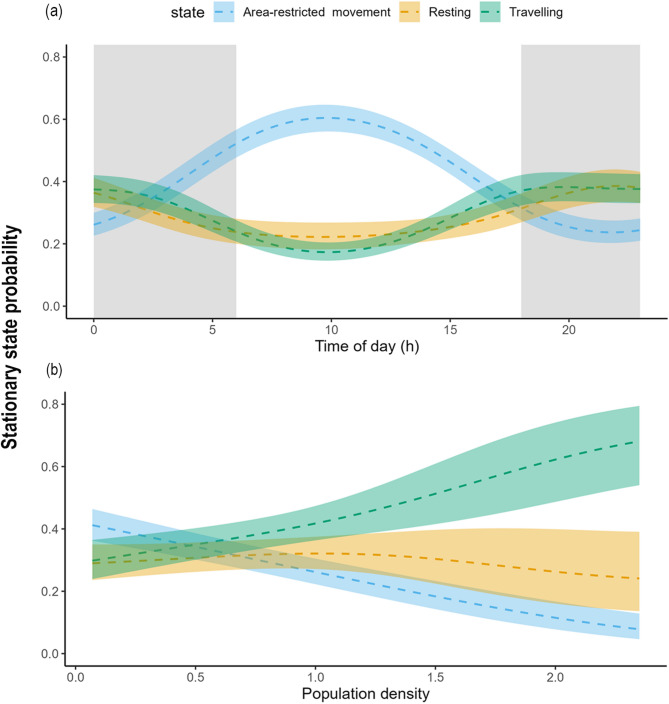
Tigers modulated their behaviour in response to time of day (Fig. 2a). The travelling state was most likely to occur during dawn and dusk, whereas area-restricted movement was prominent between 06:00–13:00 h, peaking at 10:00 h. In contrast, tigers primarily rested at night, with the highest probability of resting at midnight. Furthermore, the stationary probability exhibited distinct behavioural fluctuations corresponding to human population density. Tigers moving through areas with relatively high population density showed the highest probability of travelling. In contrast, the probability of tigers exhibiting area-restricted movement was highest in areas with low human population density and declined with increasing human presence. The likelihood of the resting state remained relatively consistent across human population densities, with a slight decrease observed in areas of highest human presence (Fig. 2b).
Fig. 2.
Effects of (a) time of day (hour), grey shaded area represents night, while the white areas denote day and (b) human population density (person/km²) on stationary-state probabilities during the pre-dispersal phase of tigers in the Eastern Vidarbha Landscape of Maharashtra, India. Bold dashed lines represent mean estimates for each behavioural state, and shaded areas indicate 95% confidence intervals
Transition probabilities
During the pre-dispersal phase, ecological parameters, such as time of day, influenced the transition probability of the tigers switching between movement states (Additional file 2: Figure S4). When resting, the probability of switching from resting to area-restricted movement peaked at noon, and the probability of switching to travelling peaked during the early morning. The probability of transitioning from area-restricted movement to rest was typically low, and travel increased in the early morning and in the evening between 15:00–20:00 h. Once in a travelling state, the probability of transitioning to area-restricted movement peaked at noon, with a low probability of switching to resting. Moreover, human population density also affected transition probabilities (Additional file 2: Figure S5). As human population density increased, the probability of transitioning from a resting state to a travelling state increased. However, tigers remained in the same state when in areas with low human population density (e.g., remained resting when already in a resting state, or remained travelling when already in a travelling state).
Dispersal
We analysed data from six dispersing tigers (five males and one female), tagged for 19–162 days for a total of 5400 GPS locations. State 1 (resting) corresponded to short step lengths (mean = 17.71 m) and variable turning angles (mean = 3.14 radians; concentration = 0.12), indicating very slow and localized movement. State 2 (area-restricted movement) had a moderate step length (mean = 220.88 m) and wide turning angle distribution (mean = -0.10 radians; concentration = 0.28). State 3 (travelling) had longer step lengths (mean = 1387.19 m) and a more concentrated turning angle distribution (mean = 0.03 radians; concentration = 1.79), indicating faster movement with high directional persistence (Additional file 3: Figure S6). Of the total activity budget during dispersal, approximately 32.09% of the tigers’ time was classified as resting, 35.77% as area-restricted movement, and 32.14% as travelling. The best-fitting HMM included the effects of time of day, habitat, and temperature on the state transition probabilities. Furthermore, the movement characteristics (step length and turn angle) within behavioural states were associated with habitat type.
State-dependent distribution parameters (step length and turn angle)
In the resting state, the mean step lengths were similar between the forest and non-forest habitats (Additional file 3: Figure S7). The mean step length in area-restricted movement in non-forest habitats (~ 500 m) was more than twice that in forest habitats (~ 200 m). Similarly, the mean step length in the travelling state for dispersing tigers was longer in non-forest habitats (~ 2500 m) than in forests (~ 1400 m). The difference in directionality between the two habitats was negligible for resting and area-restricted movements. However, in the travelling state, tigers had greater directional persistence in non-forest habitats (angle concentration = 3.5) than in forest habitats (angle concentration = 1.8) (Additional file 3: Figure S8).
Stationary-state probabilities
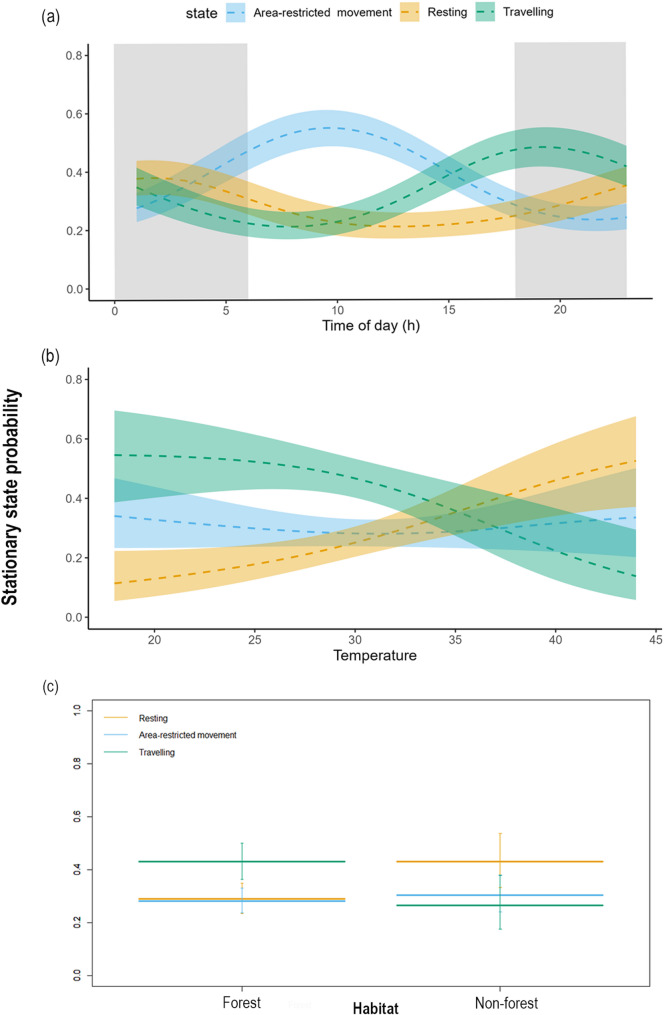
The stationary probability of dispersing tigers varied with the time of day (Fig. 3a). Dispersing tigers were more likely to travel at dusk and night while resting at midnight until dawn. These behavioural states were followed by area-restricted movements in the morning and afternoon, which peaked at 10:00 h. The results also indicated that ambient temperature influenced the stationary probability of the tigers during the dispersal phase (Fig. 3b). Specifically, individuals were more likely to travel when the temperature was between 20 and 30 °C. However, with a further increase in temperature, individuals were more likely to rest. The probability of being in an area-restricted movement state remained steady in response to increased temperature. Tigers had the highest probability of travelling in forest habitats and the lowest probability of travelling in non-forest habitats, coupled with a high probability of resting in non-forest habitats (Fig. 3c). The probability of being in an area-restricted movement state was lowest in forest habitats. Whereas, in non-forest habitats, this state occurred more frequently than travelling but less than resting.
Fig. 3.
Effects of (a) time of day (hour), grey shaded area represents night, while the white areas denote day, (b) temperature (°C), and (c) habitat: forest and non-forest on stationary-state probabilities during the dispersal phase of tigers in the Eastern Vidarbha Landscape of Maharashtra, India. For figure (a) and (b), bold dashed lines represent mean estimates for each behavioural state, with shaded areas indicating 95% confidence intervals. In figure (c), bold horizontal bars indicate mean estimates, and vertical error bars represent 95% confidence intervals
Transition probabilities
During dispersal, the top model predicted that time of day influenced the probability of tigers switching behavioural states (Additional file 3: Figure S9). When resting, tigers were most likely to remain in the same state (i.e., continue resting), with a low probability of switching to area-restricted movement or travelling. Similarly, individuals in the area-restricted movement state were more likely to remain in that state, with the peak probability occurring at 09:00 h, and transition to travelling in the evening (18:00 h). When travelling, the most likely transition for dispersing tigers was to remain in the same state, with the maximum probability occurring at night. The transition from travelling to area-restricted movement peaked at 10:00 h. The response of the transition probabilities to temperature also varied across the states (Additional file 3: Figure S10). With an increase in temperature, the probability of transitioning from resting to area-restricted movement or travelling remained low. However, individuals in the area-restricted movement state showed an increased probability of transitioning either to a resting state or remaining in the same state with increasing temperature. Furthermore, increased temperatures decreased the likelihood of transitioning to the travelling state and increased the likelihood of individuals shifting from travelling to resting or area-restricted movement. Habitat also influenced state transitions, with individuals in non-forest habitats having a slightly higher probability of switching from resting to area-restricted movement compared to those in forest habitats (Additional file 3: Figure S11). Similarly, in area-restricted movement, the likelihood of transitioning to resting was high, whereas the probability of remaining in the same state was lower in non-forest habitat. In the travelling state, tigers showed a slight increase in transitions from travelling to resting in non-forest habitats, whereas they were more likely to remain in the travelling state when in forest habitats.
Post-dispersal
The results for the post-dispersal movements were derived from seven individuals (five males and two females), tagged for 84–305 days, totalling 8980 GPS locations across all individuals. State 1 (resting) had short step lengths (mean = 25.26 m) and variable turning angles (mean = 3.14 radians, concentration = 0.08). State 2 (area-restricted movement) had long step lengths (mean = 724.80 m) and high turning angles (mean = 3.12 radians; concentration = 0), suggesting long distances were travelled with directional persistence. State 3 (travelling) had a higher mean step length (867.48 m) and high directional persistence (mean = -0.01 radians; concentration = 34.07; Additional file 4: Figure S12). Approximately 36.28% of the total activity budget was classified as resting, 38.81% as area-restricted movement, and 24.91% as travelling. Time of day, temperature, and habitat type affected the stationary and transition probabilities. Moreover, habitat type influenced state-dependent parameters (step length and angle).
State-dependent distribution parameters (step length and turn angle)
In the resting state, there was minimal variation in the mean step length between forest and non-forest habitats (Additional file 4: Figure S13). In contrast, during area-restricted movement, the mean step length in forest habitats (~ 720 m) was greater than that in non-forest habitats (~ 440 m). In the travelling state, the mean step length (~ 2000 m) in the non-forest habitat was twice that in the forest habitat (~ 900 m). No consistent directional pattern was observed across habitats for resting or area-restricted movement. However, tiger movement was more directional while travelling in forest habitats (Additional file 4: Figure S14).
Stationary-state probabilities
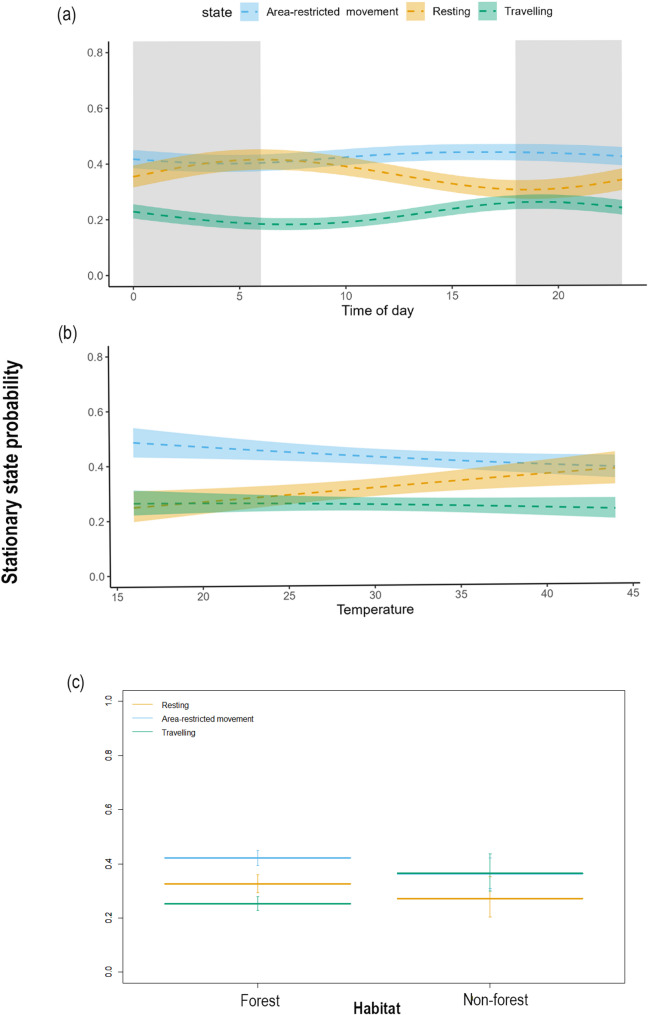
The probability of different behavioural states in tigers during post-dispersal varied according to the time of day (Fig. 4a). Area-restricted movement was the most likely state overall, although the probability of a resting state peaked in the early morning hours (05:00 and 07:00 h). In the evening, there was an increased probability of tigers remaining in a travelling state, reaching a peak between 17:00–18:00 h, although this remained a lower probability than the other two states. The ambient temperature also influenced stationary behaviour; tigers were more likely to rest and travel less with increasing temperature (Fig. 4b). Moreover, it showed the highest probability of area-restricted movement with a decrease in temperature. Tigers in forested habitats showed the highest probability of remaining in the area-restricted movement state and the lowest probability of travelling. In contrast, tigers in non-forest habitats had high probabilities of travelling and area-restricted movement, with resting being the least likely state (Fig. 4c).
Fig. 4.
Effects of (a) time of day (hour), grey shaded area represents night, while the white areas denote day (b) temperature (°C), and (c) habitat: forest and non-forest on stationary-state probabilities during the post-dispersal phase of tigers in the Eastern Vidarbha Landscape of Maharashtra, India. For figures (a) and (b), bold dashed lines represent mean estimates for each behavioural state, with shaded areas indicating 95% confidence intervals. In figure (c), bold horizontal bars indicate mean estimates, and vertical error bars represent 95% confidence intervals
Transition probabilities
The transition probabilities showed less variation with time of day during post-dispersal than in the other two life stages (Additional file 4: Figure S15). During the resting state, the highest probability was to remain in the same state, which peaked at midnight. The probability of switching from resting to area-restricted movements peaked at noon. However, no clear trend was observed for switching from area-restricted movement or travelling to other states. The model also predicted variation in the state-switching behaviour in response to temperature (Additional file 4: Figure S16). With increasing temperature, the probability of transitioning from resting to area-restricted movement increased. However, individuals in the area-restricted movement state showed a decreased probability of remaining in the same state with increasing temperature. Furthermore, higher temperatures increased the likelihood of individuals shifting from the travelling to the resting state. Habitat also played a role in state transitions and tigers in the area-restricted movement had a slightly higher probability of remaining in the same state in non-forest habitats, whereas they were more likely to transition to travelling in forest habitats. In contrast, tigers in the travelling state had a greater chance of switching to area-restricted movement in forest habitats, whereas in non-forest habitats, they were more likely to continue travelling (Additional file 4: Figure S17).
Discussion
This study advances our understanding of tiger movement ecology by characterising three distinct behavioural states into resting, area-restricted movement, and travelling using HMMs applied across life stages (pre-dispersal, dispersal, and post-dispersal) in a human-dominated landscape. While tigers responded consistently to key environmental and anthropogenic drivers, including diel period, temperature, habitat structure, and human disturbance, these behavioural responses were expressed differently according to the life stage. Such behavioural plasticity underscores the adaptive strategies necessary for large carnivores persisting in a fragmented, human-dominated landscape [3, 57]. Similar segmentation of movement behaviours across wide-ranging carnivores suggests a general behavioural framework supporting adaptive decision-making in response to spatial complexity and anthropogenic risks [15, 38, 58].
Across all life stages, tiger movement exhibited strong diel patterns. Travelling was predominantly crepuscular and nocturnal, aligning with their peak prey activity [59] while minimising human encounters [60]. Resting generally dominated during daylight, especially in dispersing individuals. Nocturnal movement during dispersal, combined with increased travelling bouts, likely served to reduce conflict risk and facilitate navigation through unfamiliar, human-modified landscapes in search of suitable habitats. This adaptation has been previously observed, where tigers shift to faster movement at night to navigate fragmented landscapes and maximise their chances of reaching suitable habitats during dispersal [61]. Similar nocturnal activity has been observed across a range of carnivore species, which tend to increase mobility under the cover of darkness to avoid detection and disturbance in human-modified landscapes [62, 63]. In contrast, post-dispersal individuals exhibited peak area-restricted movement during evening and remained high through the night, suggesting a shift towards localised territorial activity once settled. Similar behavioural adaptations have been observed in Florida panthers, which rest during the day and shift from intermediate to exploratory states when traversing open or fragmented landscapes [64].
Similarly, ambient temperature consistently influenced tiger behaviour, where active movements were most frequent at moderate temperatures (~ 20–30 °C), while resting predominated at higher temperatures. These patterns reflect thermoregulatory adaptations aimed at minimising energy expenditure and heat stress; a behavioural strategy common among large carnivores [65]. Specifically, in the post-dispersal phase, higher temperatures reduced the probability of area-restricted movement and increased the likelihood of resting. Elevated temperatures also prompted a shift from travelling to resting, reflecting temperature-sensitive energy optimisation, consistent with thermoregulatory strategies in large carnivores [66].
Tigers also consistently adjusted their movement behaviour in response to habitat type and human presence. Non-forest areas, particularly those with high human density, prompted faster, more directional movements, likely reflecting a risk-avoidance strategy in response to perceived threats [14, 61]. In contrast, forested habitats facilitated localised area-restricted movements associated with foraging and territory maintenance. This capacity for flexible risk navigation is consistent with findings in other wide-ranging carnivores like cougars [67] and leopards [68], where non-forested or human-influenced areas act as semi-permeable barriers, triggering increased movement speed and reduced site fidelity. Furthermore, dispersing tigers in non-forest habitats frequently switch between resting and area-restricted movement in response to frequent human disturbances, like agricultural activities and human movement, which may force individuals to shift resting sites or engage in localised movement within agricultural fields. Such frequent switching may represent a behavioural strategy to minimise exposure to human activity in landscapes outside PAs. However, post-dispersal tigers limited their behavioural switching and were more likely to persist in either area-restricted movement or travelling states. This behavioural consistency or plasticity likely reflects adaptive responses to navigating fragmented, high-risk landscapes characterised by frequent human disturbance [38, 60]. Within forest habitats, tigers were more likely to remain travelling, suggesting higher landscape permeability, high resource access, and reduced perceived risk.
The relative importance of the different movement phases varied across life stages. During the pre-dispersal phase, tigers primarily exhibited area-restricted movement interspersed with resting and short-distance travel, indicating limited-range activity centred on a familiar landscape and close to maternal resources. The predominance of localised movements with low directional persistence suggests foraging or spatial learning behaviour within known areas. Risk avoidance was evident even at this early stage, as individuals displayed increased directional travelling through human-impacted areas, suggesting early development of behavioural strategies to mitigate anthropogenic risk [69]. Nocturnal activity and short-distance movements near dawn and dusk further support adaptive temporal adjustments aimed at minimising exposure during vulnerable periods.
In the dispersal phase, movement patterns shifted towards greater mobility. Dispersing tigers exhibited longer, more frequent travelling bouts, reflecting the need to navigate unfamiliar and fragmented landscapes in search of suitable territories. Movement through non-forest habitats became faster and more directed, likely reflecting an adaptive strategy to minimise time spent in high-risk zones [70]. Increased nocturnal travelling likely mitigated conflict potential during dispersal, a behaviour consistent with reports of increased nocturnality among large carnivores in human-dominated landscapes [14]. Behavioural flexibility was particularly evident during dispersal, as tigers traversed agricultural fields and human settlements, often moving long distances between remnant forest patches. Such mobility and responsiveness to landscape structure are critical for successful dispersal and gene flow in fragmented systems [6].
In contrast, tigers in the post-dispersal phase exhibited increased behavioural stability, reflecting a transition from exploration to territory establishment, consistent with patterns observed in other territorial carnivores [71, 72]. Movements became more localised within forest patches, dominated by area-restricted movement and resting. Reduced behavioural transition reflects stabilisation of home ranges and familiarity with local risks. However, when moving through non-forest areas within established territories, tigers maintained fast, directional movement, underscoring the persistent influence of anthropogenic risk even among established individuals [73]. The dominance of area-restricted movement in forested habitats likely reflects activities related to territory defence, foraging, and reproductive investment, all central to long-term survival and reproductive success [74].
These findings highlight that while tigers rely on a consistent suite of behavioural responses to environmental and anthropogenic variables, the ecological context of each life stage modulates the expression of those responses. These life-stage-specific strategies reveal the importance of maintaining landscape connectivity not just for dispersing individuals but also for territorial adults navigating human-dominated spaces.
Strengths and limitations
Our ability to interpret these complex behavioural responses is strengthened by the methodological framework adopted in this study. A major strength lies in its life-stage-specific approach, providing a rare temporal perspective on how movement behaviour changes as individuals transition from dependence to dispersal and finally territory establishment. Additionally, this study is one of the few to compare movement within and outside PAs, capturing how tigers adapt to variable levels of anthropogenic disturbance across the landscape. The application of generalised hidden Markov models (HMMs) enabled covariate-informed state modelling, offering ecologically meaningful interpretations of how external factors shape behavioural states. Conventional HMMs may oversimplify behavioural responses by assuming movement parameter distributions are constant within states [30, 53]. However, the study is limited by a small, male-biased dispersal sample, which may have skewed inferences toward male-specific movement strategies. Male bias is a common limitation in large carnivore telemetry due to sex differences in movement and capture likelihood [75]. As dispersal behaviour and habitat sensitivity may differ between sexes, caution is warranted in generalising these findings to female tigers. Future research should prioritise balanced sampling to explore sex-specific variation in movement behaviour. Furthermore, while generalised HMMs offer richer modelling potential, they carry a higher risk of overfitting when applied to small datasets [31, 76], emphasising the need for cautious interpretation.
Conclusions
This study offers detailed, life-stage-specific insights into tiger movement ecology, demonstrating how behavioural flexibility enables tigers to navigate human-modified landscapes. Using HMMs, we show that tiger movement patterns are shaped by temporal, environmental, and human factors, reflecting trade-offs between resource acquisition and risk avoidance. In India, where rapid land-use change, infrastructure development, and human-tiger conflict are escalating, particularly in regions like Vidarbha, these findings offer actionable insights for conservation planning. The observed behavioural flexibility underscores the importance of maintaining functional connectivity, via forest corridors, buffer zones, and areas outside PAs, to facilitate dispersal, reduce conflict, support long-term gene flow and allow the survival of large carnivores in human-dominated areas [18]. Integrating movement ecology into landscape and infrastructure planning is therefore vital to mitigate fragmentation impacts, regulate development near protected areas, and promote coexistence strategies that support tiger persistence in shared landscapes.
Furthermore, behavioural flexibility may become even more critical under future climate change, which is projected to intensify environmental variability and resource unpredictability. Evidence from other large carnivores, such as lions, shows that drought conditions can drive range expansion as individuals seek increasingly scarce resources [77]. Similarly, climate change may shift species and prey distributions, indirectly influencing the movement patterns and habitat use of individuals [78]. Rising temperatures may further affect muscle mechanics, altering power and speed, which can influence animal movement and behaviour [79]. These climatic pressures, compounded by human-driven habitat fragmentation, are likely to exacerbate dispersal risks by reducing connectivity, increasing energetic demands, and heightening exposure to anthropogenic threats [80–82]. Consequently, range shifts may be constrained, subpopulations isolated, and gene flow disrupted, with implications for metapopulation stability and long-term viability [83, 84].
In summary, the persistence of wide-ranging species like tigers will depend not solely on protected areas but on managing multifunctional, human-dominated landscapes that preserve connectivity and support behavioural resilience. Conservation strategies that incorporate movement ecology principles will be essential for sustaining tiger populations in an increasingly fragmented and climate-affected future.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Ministry of Environment, Forest and Climate Change (MOEFCC) and the Maharashtra Forest Department (MFD) for granting permission to collar animals and conduct this research. We acknowledge MFD for the financial support provided for this project. We also thank the Telangana Forest Department for their logistical support during tiger tracking in their jurisdiction. Our sincere thanks go to the Field Directors, DFOs, RFOs, forest guards, and watchers for their unwavering support, advice, and input during fieldwork in the study area. We are also grateful to our dedicated field assistants: Irfan, Mangesh, Noor, Rama, Roshan, and Akshay for their continuous assistance throughout the study. We thank Dr. Shaheer Khan and Dr. Hussain Reshamwala for their constant guidance and insightful discussions that helped shape this manuscript. We also thank the Director, Dean, and Research Coordinator of the Wildlife Institute of India, and the Chief Wildlife Warden, Government of Maharashtra, for their institutional support.
Author contributions
Bilal Habib conceived the initial idea and designed the methodology for the study, and subsequently discussed and finalized this with Zehidul Hussain and Luca Börger; Zehidul Hussain and Pallavi Ghaskadbi collected the data; Zehidul Hussain and William Kay analysed the data; Zehidul Hussain led the writing of the manuscript; Bilal Habib acquired the funds and necessary permits; Bilal Habib and Parag Nigam supervised the project; William Kay, Luca Börger, and Bilal Habib edited the manuscript. All authors gave final approval for publication.
Funding
Maharashtra Forest Department, Government of Maharashtra.
Data availability
The data will be provided upon request by the corresponding author. The data location belongs to some critical locations outside protected areas and is prone to poaching and conflict. Therefore, location data will be made available upon request which is owned by the the corresponding author and the Wildlife Insitute of India, Governemnt of India.
Declarations
Ethics approval and consent to participate
Animals were captured following standard and approved protocols after obtaining permission from the Ministry of Environment, Forests and Climate Change, Government of India, and the Maharashtra Forest Department. Individual tigers were collared under the specific permit number MOEF&CC-F. No. 1–36/2014-WL I/05.09.2014.
Consent for publication
All authors provided permission for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anadón JD, Wiegand T, Giménez A. Individual-based movement models reveal sex-biased effects of landscape fragmentation on animal movement. Ecosphere. 2012;3(7):art64. 10.1890/es11-00237.1. [Google Scholar]
- 2.Gomez S, English HM, Bejarano Alegre V, Blackwell PG, Bracken AM, Bray E, et al. Understanding and predicting animal movements and distributions in the anthropocene. J Anim Ecol. 2025;00:1–19. 10.1111/1365-2656.70040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tucker MA, Böhning-Gaese K, Fagan WF, Fryxell JM, Van Moorter B, Alberts SC, et al. Moving in the anthropocene: global reductions in terrestrial mammalian movements. Science. 2018;359(6374):466–9. 10.1126/science.aam9712. [DOI] [PubMed] [Google Scholar]
- 4.van der Ree R, Smith DJ, Grilo C. The ecological effects of linear infrastructure and traffic. Handbook of road ecology. Wiley-Blackwell; 2015. pp. 1–9. 10.1002/9781118568170.ch1.
- 5.Crooks KR, Burdett CL, Theobald DM, Rondinini C, Boitani L. Global patterns of fragmentation and connectivity of mammalian carnivore habitat. Philos Trans R Soc Lond B Biol Sci. 2011;366(1578):2642–51. 10.1098/rstb.2011.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ripple WJ, Estes JA, Beschta RL, Wilmers CC, Ritchie EG, Hebblewhite M, et al. Status and ecological effects of the world’s largest carnivores. Science. 2014;343(6167):1241484. 10.1126/science.1241484. [DOI] [PubMed] [Google Scholar]
- 7.Barrientos R, Ascensão F, D’Amico M, Grilo C, Pereira HM. The lost road: do transportation networks imperil wildlife population persistence? Perspect Ecol Conserv. 2021;19(4):411–6. 10.1016/j.pecon.2021.07.004. [Google Scholar]
- 8.Ascensão F, Lucas PS, Costa A, Bager A. The effect of roads on edge permeability and movement patterns for small mammals: a case study with montane Akodont. Landsc Ecol. 2017;32(4):781–90. 10.1007/s10980-017-0485-z. [Google Scholar]
- 9.Grilo C, Coimbra MR, Cerqueira RC, Barbosa P, Dornas RAP, Gonçalves LO, et al. Brazil road-kill: a data set of wildlife terrestrial vertebrate road-kills. Ecology. 2018;99(11):2625. 10.1002/ecy.2464. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho F, Carvalho R, Mira A, Beja P. Assessing functional landscape connectivity in a forest carnivore using path selection functions. Landsc Ecol. 2016;31(5):1021–36. 10.1007/s10980-015-0326-x. [Google Scholar]
- 11.Chen HL, Koprowski JL. Barrier effects of roads on an endangered forest obligate: influences of traffic, road edges, and gaps. Biol Conserv. 2016;199:33–40. 10.1016/j.biocon.2016.03.017. [Google Scholar]
- 12.Prokopenko CM, Boyce MS, Avgar T. Characterizing wildlife behavioural responses to roads using integrated step selection analysis. J Appl Ecol. 2017;54(2):470–9. 10.1111/1365-2664.12768. [Google Scholar]
- 13.Dickie M, Serrouya R, McNay RS, Boutin S. Faster and farther: Wolf movement on linear features and implications for hunting behaviour. J Appl Ecol. 2017;54(1):253–63. 10.1111/1365-2664.12732. [Google Scholar]
- 14.Habib B, Ghaskadbi P, Khan S, Hussain Z, Nigam P. Not a cakewalk: insights into movement of large carnivores in human-dominated landscapes in India. Ecol Evol. 2021;11(3):1485–96. 10.1002/ece3.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naha D, Dash SK, Kupferman C, Dey S, Ghosh S, Roy M, et al. Movement behavior of a solitary large carnivore within a hotspot of human-wildlife conflicts in India. Sci Rep. 2021;11:3862. 10.1038/s41598-021-83262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter NH, Linnell JD. Co-Adaptation is key to coexisting with large carnivores. Trends Ecol Evol. 2016;31(8):575–8. 10.1016/j.tree.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, et al. A movement ecology paradigm for unifying organismal movement research. Proc Natl Acad Sci U S A. 2008;105(49):19052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittemyer G, Northrup JM, Bastille-Rousseau G. Behavioural valuation of landscapes using movement data. Philos Trans R Soc Lond B Biol Sci. 2019;374(1781):20180046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clobert J, Baguette M, Benton TG, Bullock JM. Dispersal ecology and evolution. Oxford: Oxford University Press; 2012. 10.1093/acprof:oso/9780199608898.001.0001. [Google Scholar]
- 20.Morales-González A, Ruiz-Villar H, Ordiz A, Penteriani V. Large carnivores living alongside humans: brown bears in human-modified landscapes. Glob Ecol Conserv. 2020;22:e00937. 10.1016/j.gecco.2020.e00937. [Google Scholar]
- 21.Naha D, Périquet S, Kilian JW, Kupferman CA, Hoth-Hanssen T, Beasley JC. Fencing affects movement patterns of two large carnivores in Southern Africa. Front Ecol Evol. 2023;11:1031321. 10.3389/fevo.2023.1031321. [Google Scholar]
- 22.Rytwinski T, Fahrig L. Why are some animal populations unaffected or positively affected by roads? Oecologia. 2013;173(4):1143–55. [DOI] [PubMed] [Google Scholar]
- 23.Doherty TS, Hays GC, Driscoll DA. Human disturbance causes widespread disruption of animal movement. Nat Ecol Evol. 2021;5(4):513–9. [DOI] [PubMed] [Google Scholar]
- 24.Ordiz A, Sæbø S, Kindberg J, Swenson JE, Støen OG. Seasonality and human disturbance alter brown bear activity patterns: implications for circumpolar carnivore conservation? Anim Conserv. 2017;20(1):51–60. [Google Scholar]
- 25.Schuette P, Creel S, Christianson D. Coexistence of African lions, livestock, and people in a landscape with variable human land use and seasonal movements. Biol Conserv. 2013;157:148–54. [Google Scholar]
- 26.Fattebert J, Robinson HS, Balme G, Slotow R, Hunter LTB. Structural habitat predicts functional dispersal habitat of a large carnivore: how leopards change spots. Ecol Appl. 2015;25(7):1911–21. 10.1890/14-1631.1. [DOI] [PubMed] [Google Scholar]
- 27.Suraci JP, Clinchy M, Zanette LY, Wilmers CC. Fear of humans as apex predators has landscape-scale impacts from mountain lions to mice. Ecol Lett. 2019;22(10):1578–86. 10.1111/ele.13344. [DOI] [PubMed] [Google Scholar]
- 28.Zeller KA, McGarigal K, Whiteley AR. Estimating landscape resistance to movement: a review. Landsc Ecol. 2012;27(6):777–97. 10.1007/s10980-012-9737-0. [Google Scholar]
- 29.Gardiner R, Hamer R, Leos-Barajas V, Peñaherrera‐Palma C, Jones ME, Johnson C. State‐space modelling reveals habitat perception of a small terrestrial mammal in a fragmented landscape. Ecol Evol. 2019;9(17):9804–14. 10.1002/ece3.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClintock BT, Langrock R, Gimenez O, Cam E, Borchers DL, Glennie R, et al. Uncovering ecological state dynamics with hidden Markov models. Ecol Lett. 2020;23(12):1878–903. 10.1111/ele.13610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glennie R, Langrock R, King R. Hidden Markov models: pitfalls and opportunities in ecology. Methods Ecol Evol. 2022;14(5):1002–14. 10.1111/2041-210X.13801. [Google Scholar]
- 32.McClintock BT. Worth the effort? A practical examination of random effects in hidden Markov models for animal telemetry data. Methods Ecol Evol. 2021;12(8):1475–97. 10.1111/2041-210x.13619. [Google Scholar]
- 33.Patterson TA, Parton A, Langrock R, Blackwell PG, Thomas L, King R. Statistical modelling of individual animal movement: an overview of key methods and a discussion of practical challenges. AStA Adv Stat Anal. 2017;101(4):399–438. 10.1007/s10182-017-0302-7. [Google Scholar]
- 34.Morales JM, Haydon DT, Frair J, Holsinger KE, Fryxell JM. Extracting more out of relocation data: Building movement models as mixtures of random walks. Ecology. 2004;85(9):2436–45. 10.1890/03-0269. [Google Scholar]
- 35.Patterson TA, Basson M, Bravington MV, Gunn JS. Classifying movement behaviour in relation to environmental conditions using hidden Markov models. J Anim Ecol. 2009;78(6):1113–23. [DOI] [PubMed] [Google Scholar]
- 36.Thatte P, Joshi A, Vaidyanathan S, Landguth E, Ramakrishnan U. Maintaining tiger connectivity and minimizing extinction into the next century: insights from landscape genetics and spatially-explicit simulations. Biol Conserv. 2018;218:181–91. [Google Scholar]
- 37.Carter NH, Zuckerwise A, Pradhan NMB, Subedi N, Lamichhane BR, Hengaju KD, et al. Rapid behavioral responses of endangered Tigers to major roads during COVID-19 lockdown. Glob Ecol Conserv. 2023;42:e02388. 10.1016/j.gecco.2023.e02388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dutta S, Krishnamurthy R. Multiphasic movement and step-selection patterns of dispersed Tigers in the central Indian landscape. PLoS ONE. 2024;19(10):e0309517. 10.1371/journal.pone.0309517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Habib B, Nigam P, Mondal I, Ghaskadbi P, Hussain Z. Ensuring safety in the killer fields: identifying potential villages for measures to reduce electrocution of Tigers and associated species in Eastern Vidarbha landscape. Maharashtra, India. Dehradun: Wildlife Institute of India; 2017. [Google Scholar]
- 40.Habib B, Nigam P, Sepat R, Malhotra D, Reza S, Ahuja R. Status of tigers, co-predators and prey in Brahmapuri forest division. Maharashtra, India: Dehradun: Wildlife Institute of India and Maharashtra Forest Department; 2025. pp. 2023–4. [Google Scholar]
- 41.Nandankar PK, Dewangan PL, Surpam RV. Climate of Nagpur. Nagpur: India Meteorological Department, Regional Meteorological Centre; 2011. [Google Scholar]
- 42.Van Dyck H, Baguette M. Dispersal behaviour in fragmented landscapes: routine or special movements? Basic Appl Ecol. 2005;6(6):535–45. [Google Scholar]
- 43.Calabrese JM, Fleming CH, Gurarie E. Ctmm: an R package for analyzing animal relocation data as a continuous-time stochastic process. Methods Ecol Evol. 2016;7(9):1124–34. [Google Scholar]
- 44.Fleming CH, Calabrese JM, Mueller T, Olson KA, Leimgruber P, Fagan WF. From fine-scale foraging to home ranges: a semivariance approach to identifying movement modes across Spatiotemporal scales. Am Nat. 2014;183(5):E154–67. 10.1086/675504. [DOI] [PubMed] [Google Scholar]
- 45.Börger L, Fryxell J. Quantifying individual differences in dispersal using net squared displacement. In: Clobert J, Baguette M, Benton TG, Bullock JM, editors. Dispersal ecology and evolution. Oxford: Oxford University Press; 2012. pp. 222–30. [Google Scholar]
- 46.Howard WE. Innate and environmental dispersal of individual vertebrates. Am Midl Nat. 1960;63(1):152–61. [Google Scholar]
- 47.Waser PM, Jones WT. Natal philopatry among solitary mammals. Q Rev Biol. 1983;58(3):355–90. 10.1086/413385. [Google Scholar]
- 48.McClintock BT, Michelot T, momentuHMM. R package for generalised hidden Markov models of animal movement. Methods Ecol Evol. 2018;9(6):1518–30. 10.1111/2041-210X.12995. [Google Scholar]
- 49.Johnson DS, London JM, Lea MA, Durban JW. Continuous-time correlated random walk model for animal telemetry data. Ecology. 2008;89(5):1208–15. [DOI] [PubMed] [Google Scholar]
- 50.Hooten MB, Johnson DS, McClintock BT, Morales JM. Animal movement: statistical models for telemetry data. Boca Raton: CRC; 2017. [Google Scholar]
- 51.McClintock BT. Incorporating telemetry error into hidden Markov models of animal movement using multiple imputation. J Agric Biol Environ Stat. 2017;22(3):249–69. 10.1007/s13253-017-0285-6. [Google Scholar]
- 52.Gorelick N, Hancher M, Dixon M, Ilyushchenko S, Thau D, Moore R. Google Earth engine: planetary-scale Geospatial analysis for everyone. Remote Sens Environ. 2017;202:18–27. 10.1016/j.rse.2017.06.031. [Google Scholar]
- 53.Carter MID, McClintock BT, Embling CB, Bennett KA, Thompson D, Russell DJF. From pup to predator: generalised hidden Markov models reveal rapid development of movement strategies in a Naïve long-lived vertebrate. Oikos. 2020;129(5):630–42. 10.1111/oik.06853. [Google Scholar]
- 54.Michelot T, Langrock R, Patterson TA. MoveHMM: an R package for the statistical modelling of animal movement data using hidden Markov models. Methods Ecol Evol. 2016;7(11):1308–15. 10.1111/2041-210X.12578. [Google Scholar]
- 55.Zucchini W, MacDonald IL, Langrock R. Hidden Markov models for time series: an introduction using R. 2nd ed. Boca Raton: Chapman and Hall/CRC; 2017. [Google Scholar]
- 56.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2024. Available from: https://www.R-project.org/
- 57.Gaynor KM, Hojnowski CE, Carter NH, Brashares JS. The influence of human disturbance on wildlife nocturnality. Science. 2018;360(6394):1232–5. 10.1126/science.aar7121. [DOI] [PubMed] [Google Scholar]
- 58.Farhadinia MS, Michelot T, Johnson PJ, Hunter LTB, Macdonald DW. Understanding decision making in a food-caching predator using hidden Markov models. Mov Ecol. 2020;8(1):1–11. 10.1186/s40462-020-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karanth KU, Sunquist ME. Behavioural correlates of predation by tiger (Panthera tigris), Leopard (Panthera pardus) and Dhole (Cuon alpinus) in nagarahole, India. J Zool. 2000;250(2):255–65. 10.1111/j.1469-7998.2000.tb01076.x. [Google Scholar]
- 60.Carter NH, Shrestha BK, Karki JB, Pradhan NMB, Liu J. Coexistence between wildlife and humans at fine Spatial scales. Proc Natl Acad Sci U S A. 2012;109(38):15360–5. 10.1073/pnas.1210490109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hussain Z, Ghaskadbi P, Panchbhai P, Govekar R, Nigam P, Habib B. Long-distance dispersal by a male subadult tiger in a human-dominated landscape. Ecol Evol. 2022;12(5):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gehr B, Hofer EJ, Muff S, Ryser A, Vimercati E, Vogt K, et al. A landscape of coexistence for a large predator in a human dominated landscape. Oikos. 2017;126(10):1389–99. 10.1111/oik.04182. [Google Scholar]
- 63.Moreira-Arce D, Vergara PM, Boutin S. Diurnal human activity and introduced species affect occurrence of carnivores in a human-dominated landscape. PLoS ONE. 2015;10(9):e0137854. 10.1371/journal.pone.0137854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van de Kerk M, Onorato DP, Criffield MA, Bolker BM, Augustine BC, McKinley SA, et al. Hidden semi-Markov models reveal multiphasic movement of the endangered Florida Panther. J Anim Ecol. 2015;84(2):576–85. 10.1111/1365-2656.12290. [DOI] [PubMed] [Google Scholar]
- 65.Bothma JDP, Le Riche EAN. The relationship between minimum air temperature and daily distances moved by Kalahari leopards. S Afr J Wildl Res. 1994;24(1):18–20. [Google Scholar]
- 66.Muñoz AR, Márquez AL, Real R. An approach to consider behavioral plasticity as a source of uncertainty when forecasting species’ response to climate change. Ecol Evol. 2015;5(12):2359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benson JF, Sikich JA, Riley SPD. Survival and competing mortality risks of mountain lions in a major metropolitan area. Biol Conserv. 2020;241:108294. 10.1016/j.biocon.2019.108294. [Google Scholar]
- 68.Fattebert J, Balme G, Dickerson T, Slotow R, Hunter L. Density-dependent Natal dispersal patterns in a Leopard population recovering from over-harvest. PLoS ONE. 2015;10(4):e0122355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carter NH, Levin S, Barlow A, Grimm V. Modeling tiger population and territory dynamics using an agent-based approach. Ecol Model. 2015;312:347–62. [Google Scholar]
- 70.Miller JR, Jhala YV, Jena J, Schmitz OJ. Landscape-scale accessibility of livestock to tigers: implications of Spatial grain for modeling predation risk to mitigate human–carnivore conflict. Ecol Evol. 2015;5(6):1354–67. 10.1002/ece3.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whittington J, Hebblewhite M, Baron RW, Ford AT, Paczkowski J. Towns and trails drive carnivore movement behaviour, resource selection, and connectivity. Mov Ecol. 2022;10:17. 10.1186/s40462-022-00318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Behr DM, McNutt JW, Ozgul A, Cozzi G. When to stay and when to leave? Proximate causes of dispersal in an endangered social carnivore. J Anim Ecol. 2020;89(7):1613–24. 10.1111/1365-2656.13300. [DOI] [PubMed] [Google Scholar]
- 73.Singh R, Pandey P, Qureshi Q, Sankar K, Krausman PR, Goyal SP. Philopatric and Natal dispersal of Tigers in a semiarid habitat, Western India. J Arid Environ. 2021;184:104320. 10.1016/j.jaridenv.2020.104320. [Google Scholar]
- 74.Karanth KU, Nichols JD, Kumar NS, Link WA, Hines JE. Tigers and their prey: predicting carnivore densities from prey abundance. Proc Natl Acad Sci U S A. 2004;101(14):4854–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Janečka JE, Blankenship TL, Hirth DH, Kilpatrick CW, Tewes ME, Honeycutt RL. Evidence for male-biased dispersal in bobcats Lynx rufus using relatedness analysis. Wildl Biol. 2007;13(1):38–47. 10.2981/0909-6396(2007)13[38:EFMDIB]2.0.CO;2.
- 76.Langrock R, King R, Matthiopoulos J, Thomas L, Fortin D, Morales JM. Flexible and practical modeling of animal telemetry data: hidden Markov models and extensions. Ecology. 2012;93(11):2336–42. 10.1890/11-2241.1. [DOI] [PubMed] [Google Scholar]
- 77.Ferreira SM, Viljoen P. African large carnivore population changes in response to drought. Afr J Wildl Res. 2022;52(1):1–7. 10.3957/056.052.0001. [Google Scholar]
- 78.Salmanpour F, Shakoori Z, Rahbarizadeh A, et al. Climate change impacts on altitudinal movements of society large mammals in the Alborz. Sci Rep. 2025;15:12735. 10.1038/s41598-025-96738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.James RS, Tallis J. The likely effects of thermal climate change on vertebrate skeletal muscle mechanics with possible consequences for animal movement and behaviour. Conserv Physiol. 2019;7(1):coz066. 10.1093/conphys/coz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caplat P, Edelaar P, Dudaniec RY, Green AJ, Okamura B, Cote J, et al. Looking beyond the mountain: dispersal barriers in a changing world. Front Ecol Environ. 2016;14(5):261–8. 10.1002/fee.1280. [Google Scholar]
- 81.Seebacher F, Post E. Climate change impacts on animal migration. Clim Chang Responses. 2015;2(1):1–11. 10.1186/s40665-015-0013-9. [Google Scholar]
- 82.Bonte D, Van Dyck H, Bullock JM, Coulon A, Delgado M, Gibbs M, et al. Costs of dispersal. Biol Rev Camb Philos Soc. 2012;87(2):290–312. [DOI] [PubMed] [Google Scholar]
- 83.Robillard CM, Coristine LE, Soares RN, Kerr JT. Facilitating climate-change-induced range shifts across continental land-use barriers. Conserv Biol. 2015;29(6):1586–95. 10.1111/cobi.12571. [DOI] [PubMed] [Google Scholar]
- 84.Hagen SB, Kopatz A, Aspi J, Kojola I, Eiken HG. Evidence of rapid change in genetic structure and diversity during range expansion in a recovering large terrestrial carnivore. Proc Biol Sci. 2015;282(1810):20150092. 10.1098/rspb.2015.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be provided upon request by the corresponding author. The data location belongs to some critical locations outside protected areas and is prone to poaching and conflict. Therefore, location data will be made available upon request which is owned by the the corresponding author and the Wildlife Insitute of India, Governemnt of India.