Abstract
l-Homoserine is a valuable intermediate with broad applications in the food, pharmaceutical, and chemical industries. Although Corynebacterium glutamicum has been engineered for the efficient biosynthesis of l-homoserine, both production efficiency and glucose conversion remain suboptimal. In this study, an engineered C. glutamicum strain capable of high-yield l-homoserine production from glucose was successfully developed. First, an engineered C. glutamicum strain capable of biosynthesizing l-homoserine using glucose as the sole carbon source was constructed with a yield of 0.38 g/g. To further enhance conversion efficiency, the expression of key genes in the tricarboxylic acid (TCA) cycle was repressed. Among the strategies evaluated, deletion of the aceE gene proved most effective in decoupling glycolysis from the TCA cycle, and acetate supplementation successfully restored cell growth in the decoupled strain. Subsequent metabolic rewiring, including modulation of acetylation efficiency, enhancement of the glyoxylate cycle, and promotion of fumarate-to-l-aspartate conversion, led to substantial l-homoserine accumulation. The engineered strain ultimately achieved an l-homoserine titer of 17.35 g/L with a yield of 0.56 g/g glucose, representing a 48 % increase. Finally, fed-batch fermentation was performed in a 5-L bioreactor using glucose and acetate as mixed carbon sources. The optimized strain, ACg23-6, produced 70.54 g/L l-homoserine within 96 h, with a yield of 0.58 g/g glucose and a productivity of 0.73 g/L/h, while consuming 80 g/L acetate. This decoupling strategy provided valuable insights for improving glucose conversion efficiency and acetate utilization in the microbial production of l-aspartate-derived compounds.
Keywords: Corynebacterium glutamicum, l-homoserine, TCA, EMP, Acetate
1. Introduction
l-Homoserine is a non-proteinogenic amino acid that serves as a crucial precursor for the biosynthesis of several high-value compounds, including amino acids such as l-methionine and l-threonine, as well as the potent herbicide l-glufosinate. Among these, the global demand for l-glufosinate is projected to reach 60,000 tons by 2026 [1], thereby driving the rising market demand for l-homoserine. Currently, industrial production of l-homoserine primarily relies on chemical synthesis. However, this approach results in the formation of a significant amount of d-homoserine, compromising enantiomeric purity and increasing downstream purification costs. In recent years, microbial biosynthesis of l-homoserine has attracted increasing attention as a sustainable alternative. Escherichia coli and Corynebacterium glutamicum are the most commonly used microbial chassis, both showing promising performance in l-homoserine production [[2], [3], [4], [5], [6], [7]]. l-Aspartate is the precursor of l-homoserine, and its biosynthesis mainly proceeds via two pathways: (1) carboxylation of pyruvate to oxaloacetate, followed by transamination to l-aspartate; and (2) decarboxylation of pyruvate to acetyl-CoA, which enters the tricarboxylic acid (TCA) cycle, yielding fumarate that is subsequently converted to l-aspartate. Therefore, rational redistribution of pyruvate flux, favoring its conversion to oxaloacetate over acetyl-CoA, is critical for enhancing the carbon efficiency of l-homoserine biosynthesis in C. glutamicum, as acetyl-CoA formation results in carbon loss via CO2 release.
Acetyl-CoA, generated via pyruvate decarboxylation, serves not only as the entry point for the TCA cycle but also as a key precursor in the biosynthesis of essential cellular components, including isoprenoids, terpenoids, fatty acids, and fatty acid-derived compounds [8,9]. Moreover, it plays pivotal roles in diverse physiological and metabolic processes [10,11]. As such, insufficient acetyl-CoA synthesis may impair cellular growth and metabolic activity. Conversely, unregulated entry of acetyl-CoA into the TCA cycle could lead to excessive CO2 emission, resulting in substantial carbon loss and reduced glucose conversion efficiency. Many previous studies had focused on enhancing acetyl-CoA supply for the biosynthesis of acetyl-CoA-derived compounds by redirecting glucose metabolism [12]. For pyruvate-derived products, strategies such as oxygen limitation or attenuation of key TCA cycle enzymes had been explored [13], although most acetyl-CoA in these cases was still generated through pyruvate decarboxylation.
In our previous work, Saccharomyces cerevisiae was engineered to enhance acetyl-CoA biosynthesis via caveolin-mediated lipid uptake and β-oxidation pathways, thereby improving the production of acetyl-CoA-derived metabolites [14,15]. More recently, a novel biosynthetic route was identified in which l-threonine was converted to acetyl-CoA and l-glycine [9]. Besides, two primary pathways exist for converting acetate to acetyl-CoA: (1) via acetyl-CoA synthetase (EC: 6.2.1.1), which utilizes two molecules of ATP to drive the condensation of acetate and CoA, and (2) via the ackA-pta pathway, where acetate is first converted to acetyl phosphate by acetate kinase (ackA), followed by conversion to acetyl-CoA by phosphate acetyltransferase (pta) [12]. Our earlier study also demonstrated that acetate supplementation could increase acetyl-CoA availability and promote the accumulation of O-acetyl-l-homoserine [16]. Currently, acetate is emerging as a promising feedstock for the microbial production of value-added chemicals [17]. It can be sourced from a variety of processes, including cellulosic biomass hydrolysis, anaerobic digestion, syngas fermentation, microbial electrosynthesis, and chemical synthesis from waste materials and CO2 [18]. Integration of electrocatalysis with biosynthesis enables CO2 to be concentrated into acetate at high titers, which can then be converted by engineered microbes into high-value products [19]. This innovative platform offered a sustainable route for artificial or semi-artificial production of “food”.
In this study, to enhance the carbon flux from pyruvate to l-homoserine, the expression of key genes in the TCA cycle was attenuated to minimize excessive acetyl-CoA consumption. Several strategies were then designed and validated to decouple glycolysis from the TCA cycle, with successful pathway disconnection achieved via deletion of aceE gene (encoding the E1 component of the pyruvate dehydrogenase complex). Following this, the acetate tolerance of C. glutamicum was evaluated and an optimal supplementation strategy was determined. Intermittent feeding of acetate, coupled with upregulation of acetylation and glyoxylate cycle genes, improved both cell growth and l-homoserine accumulation. Finally, fed-batch fermentation using glucose and acetate as mixed carbon sources achieved a high glucose-to-l-homoserine conversion efficiency. Overall, these findings provided a promising strategy for the biosynthesis of l-aspartate-derived compounds in C. glutamicum using glucose and acetate as carbon sources.
2. Materials and methods
2.1. Strains and plasmids
E. coli JM109 was used for plasmid construction. Engineered C. glutamicum strains were employed for l-homoserine production. The plasmid pEC-XK99E was used for the free expression of genes in C. glutamicum, while pK18mosacB was used for gene knockout and chromosomal integration. The Engineered C. glutamicum strains used in this study are listed in Table 1.
Table 1.
Strains used in this study.
| Strain | Description |
|---|---|
| E. coli JM109 | Plasmid amplification |
| C. glutamicum ATCC 13032 | Wild-type |
| ACg1 | C. glutamicum ATCC 13032 ΔmcbR ΔmetD ΔthrB ΔNCgl2360::Psod-thrAS345FΔNCgl2688 ΔmetY dapAM1VicdM1VΔlysA Δmdh Δpck::Psod-aspC Psod-pycP458SPsod-lysCT311IPsod-asd Psod-homV59APsod-brnFE ΔCgl1066::Ptuf-gapN |
| ACg1-1 | ACg1 harboring pEC-thrAS345F_Ec |
| ACg2-1 | ACg1 acn3-7 harboring pEC-thrAS345F_Ec |
| ACg3-1 | ACg1 odhAM1V harboring pEC-thrAS345F_Ec |
| ACg4-1 | ACg1 sucCM1VsucDV1L harboring pEC-thrAS345F_Ec |
| ACg5-1 | ACg1 acn3-7odhAM1V harboring pEC-thrAS345F_Ec |
| ACg6-1 | ACg1 odhAM1VsucCM1VsucDV1L harboring pEC-thrAS345F_Ec |
| ACg7 | ACg1 acn3-7odhAM1VsucCM1VsucDV1L |
| ACg7-1 | ACg1 acn3-7odhAM1VsucCM1VsucDV1L harboring pEC-thrAS345F_Ec |
| ACg8 | ACg7 ΔCgl0551 ΔCgl0552 |
| ACg9 | ACg7 ΔCgl2089 |
| ACg10 | ACg7 ΔCgl2089 ΔCgl2910 |
| ACg11 | ACg7 ΔCgl0551 ΔCgl0552 ΔCgl2089 ΔCgl2910 |
| ACg12 | ACg7 ΔgltA |
| ACg13 | ACg7 ΔaceE |
| ACg14 | ACg7 ΔgltA ΔaspA::Ptuf-aspB_Pa |
| ACg15 | ACg7 ΔaceE ΔaspA::Ptuf-aspB_Pa |
| ACg15-1 | ACg7 ΔaceE ΔaspA::Ptuf-aspB_Pa harboring pEC-thrAS345F_Ec |
| ACg15-2 | ACg7 ΔaceE ΔaspA::Ptuf-aspB_Pa harboring pEC-thrAS345F_Ec-Ptrc-acsL641P_Se |
| ACg15-3 | ACg7 ΔaceE ΔaspA::Ptuf-aspB_Pa harboring pEC-thrAS345F_Ec-Ptrc-pta-ackA_Cgl |
| ACg16 | ACg7 ΔaspA::Ptuf-aspB_Pa |
| ACg16-1 | ACg7 ΔaspA::Ptuf-aspB_Pa harboring pEC-thrAS345F_Ec |
| ACg17 | ACg7 ΔaceE ΔaspA::Ptuf-aspB_Pa ΔgapN |
| ACg17-1 | ACg7 ΔaceE ΔaspA::Ptuf-aspB_Pa ΔgapN harboring pEC-thrAS345F_Ec |
| ACg17-2 | ACg7 ΔaceE ΔaspA::Ptuf-aspB_Pa ΔgapN harboring pEC-thrAS345F_Ec-Ptrc-acsL641P_Se |
| ACg17-3 | ACg7 ΔaceE ΔaspA::Ptuf-aspB_Pa ΔgapN harboring pEC-thrAS345F_Ec-Ptrc-pta-ackA_Cgl |
| ACg18 | ACg7 ΔaceE::Ptuf-acsL641P ΔaspA::Ptuf-aspB_Pa ΔgapN |
| ACg18-1 | ACg7 ΔaceE::Ptuf-acsL641P ΔaspA::Ptuf-aspB_Pa ΔgapN harboring pEC-thrAS345F_Ec |
| ACg19 | ACg7 ΔaceE::Ptuf-aspA_Cgl ΔaspA::Ptuf-aspB_Pa ΔgapN |
| ACg19-3 | ACg7 ΔaceE::Ptuf-aspA_Cgl ΔaspA::Ptuf-aspB_Pa ΔgapN harboring pEC-thrAS345F_Ec-Ptrc-pta-ackA_Cgl |
| ACg20 | ACg7 ΔaceE::Ptuf-aspA_Ec ΔaspA::Ptuf-aspB_Pa ΔgapN |
| ACg20-3 | ACg7 ΔaceE::Ptuf-aspA_Ec ΔaspA::Ptuf-aspB_Pa ΔgapN harboring pEC-thrAS345F_Ec-Ptrc-pta-ackA_Cgl |
| ACg21-3 | ACg7 ΔaceE::Ptuf-aspA_Cgl ΔaspA::Ptuf-aspB_Pa ΔgapN Ptuf-icl harboring pEC-thrAS345F_Ec-Ptrc-pta-ackA_Cgl |
| ACg22-3 | ACg7 ΔaceE::Ptuf-aspA_Ec ΔaspA::Ptuf-aspB_Pa ΔgapN Ptuf-icl harboring pEC-thrAS345F_Ec-Ptrc-pta-ackA_Cgl |
| ACg23-1 | ACg7 ΔaceE::Ptuf-aspA_Ec ΔaspA::Ptuf-aspB_Pa ΔgapN Ptuf-icl Ptrc-pta-Ptrc-ackA_Cgl harboring pEC-thrAS345F_Ec |
| ACg23-4 | ACg7 ΔaceE::Ptuf-aspA_Ec ΔaspA::Ptuf-aspB_Pa ΔgapN Ptuf-icl Ptrc-pta-Ptrc-ackA_Cgl harboring pEC-thrAS345F_Ec-Ptrc-icl |
| ACg23-5 | ACg7 ΔaceE::Ptuf-aspA_Ec ΔaspA::Ptuf-aspB_Pa ΔgapN Ptuf-icl Ptrc-pta-Ptrc-ackA_Cgl harboring pEC-thrAS345F_Ec-Ptrc-aspA_Ec |
| ACg23-6 | ACg7 ΔaceE::Ptuf-aspA_Ec ΔaspA::Ptuf-aspB_Pa ΔgapN Ptuf-icl Ptrc-pta-Ptrc-ackA_Cgl harboring pEC-thrAS345F_Ec-Ptrc-aspA_Ec-Ptrc-icl |
2.2. Strains culture
E. coli strains were cultured at 37 °C in Luria-Bertani (LB) medium composed of 10 g/L peptone, 5 g/L yeast extract, and 10 g/L NaCl. C. glutamicum strains were grown at 30 °C in LBHIS medium, a modified LB medium, which was supplemented with brain heart infusion (BHI) and D-sorbitol to enhance nutrient availability and osmoregulatory capacity. The acronym “LBHIS” reflects the initial letters of these key components (LB + BHI + Sorbitol). LBHIS medium contained 18.5 g/L brain heart infusion, 10 g/L peptone, 5 g/L yeast extract, 10 g/L NaCl, and 91 g/L d-sorbitol [20]. Epo medium, used for preparing competent cells, consisted of 18.5 g/L brain heart infusion, 10 g/L peptone, 5 g/L yeast extract, 10 g/L NaCl, 30 g/L glycine, and 1 g/L Tween 80. All strains were cultivated in shaking flasks at 220 rpm. For both shake flask and bioreactor fermentation, the seed culture and fermentation media were prepared as previously described [3]. For fed-batch fermentation, the feeding medium contained 500 g/L glucose, 10.0 g/L peptone, 10.0 g/L yeast extract, 5.0 g/L KH2PO4, 2.5 g/L MgSO4, 0.05 g/L MnSO4·H2O, 0.05 g/L FeSO4·7H2O, 5 mg/L vitamin B1, 30 mg/L vitamin B6, 20 mg/L vitamin B12, and 0.125 mg/L biotin. Glucose concentration in the bioreactor was maintained 10–20 g/L through fed-batch feeding. Dissolved oxygen (DO) was maintained at 25 % by adjusting the agitation and aeration rates, and pH was controlled at 6.50 using 50 % (v/v) ammonium hydroxide. Two acetate feeding strategies were evaluated. Strategy 1: 20 % (v/v) acetate was added at 0 h to a final concentration of 5 g/L, followed by subsequent feeding every 12 h. Strategy 2: 20 % (v/v) acetate was added at 0 h to a final concentration of 5 g/L, followed by subsequent feeding every 6 h.
2.3. Plasmids construction
The expression plasmid pEC-thrAS345F_K12, carrrying a mutant E. coli thrA gene (thrAS345F), was obtained from our previous study [3]. Plasmid linearization for subsequent gene insertion was performed by restriction endonuclease digestion. The first gene was inserted using BamHI and XbaI, while the second gene was inserted following XbaI and SalI digestion. The DNA fragment containing the Ptrc promoter, derived from pEC-XK99E and including the Shine-Dalgarno sequence 5′-AGAAGGAGACTAGTA-3′, was fused with the target gene fragment and subsequently assembled into the appropriate linearized vector via Gibson Assembly. For gene knockout or integration, plasmid pK18mosacB was linearized using BamHI and HindIII, and the upstream and downstream homologous arms were assembled into it using Gibson Assembly. Primers were designed with Primer Premier 5.0 software, and sequences as well as plasmid construction details are provided in the Supplementary Materials. DNA amplification was performed using 2 × Phanta Max Master Mix (Vazyme, Nanjing, China).
2.4. Analytical methods
Calcium carbonate in the fermentation broth was appropriately diluted with dilute hydrochloric acid before measuring cell density at OD600 using a spectrophotometer. Glucose and amino acid concentrations were determined using an Agilent 1100 high-performance liquid chromatography (HPLC) system [20]. For glucose analysis, an Aminex HPX-87H column (Bio-Rad, Richmond, CA, USA) and refractive index detector were used, with 5 mM H2SO4 as the mobile phase at a flow rate of 0.5 mL/min. For amino acid analysis, pre-column derivatization with O-phthalaldehyde (OPA) was performed. Detection was carried out using a Hypersil ODS-2 column (250 × 4.6 mm, 5 μm; Thermo Fisher Scientific) with UV detection at 338 nm.
3. Results and discussion
3.1. Construction of l-homoserine-producing strains utilizing glucose as the carbon source
The Wild-type C. glutamicum harbors a complete l-homoserine biosynthetic pathway but fails to accumulate detectable levels of l-homoserine. Based on our previous study [3] (Fig. 1), genes involved in downstream consumption of l-homoserine, including thrB (encoding l-homoserine kinase), metB (NCgl2360, NCgl2688, encoding cystathionine beta-lyases/cystathionine gamma-synthases), and metY (encoding O-acetylhomoserine sulfhydrylase), were disrupted. In addition, the key genes in competing pathways were attenuated, including dapA (encoding aspartate ammonia-lyase) and lysA (encoding diaminopimelate decarboxylase) in the l-lysine biosynthetic route; icd, encoding NADP+-dependent isocitrate dehydrogenase in the TCA cycle; and mdh, encoding malate dehydrogenase. Moreover, mcbR (encoding negative regulatory protein), metD (encoding amino acid import protein), and pck (encoding phosphoenolpyruvate carboxykinase) were deleted.
Fig. 1.
Construction of an l-homoserine producing strain utilizing glucose as a carbon source via metabolic engineering.
The enzymes encoded by the genes are as follows, asd: aspartate-semialdehyde dehydrogenase, EC: 1.2.1.11; aspA: aspartate ammonia-lyase, EC: 4.3.1.1; aspC: PLP-dependent aspartate aminotransferase, EC: 2.6.1.1; brnFE: a two-component export system; dapA: dihydrodipicolinate synthase, EC: 4.2.1.41; gapN: NADP+-dependent glyceraldehyde-3-phosphate dehydrogenase, EC: 1.2.1.9; hom: homoserine dehydrogenase, EC: 1.1.1.3; icd: NADP+-dependent monomeric isocitrate dehydrogenase, EC: 1.1.1.42; lysA: diaminopimelate decarboxylase, EC: 4.1.1.20; lysC: aspartokinase, EC: 2.7.2.4; mdh: malate/lactate dehydrogenases, EC: 1.1.1.37; metB: cystathionine beta-lyases/cystathionine gamma-synthases, EC: 2.5.1.48; metD: amino acid import protein; metY: O-acetylhomoserine sulfhydrylase, EC: 2.5.1.49; pck: phosphoenolpyruvate carboxykinase (GTP), EC: 4.1.1.32; pyc: pyruvate carboxylase, EC: 6.4.1.1; thrA: bifunctional aspartokinase/homoserine dehydrogenase 1, EC: 1.1.1.3, 2.7.2.4; thrB: l-homoserine kinase, EC: 2.7.1.39.
To enhance l-homoserine biosynthesis, several key endogenous and heterologous genes, including mutant variants, were overexpressed at the genomic level. As described in our previous study [3], these included: a mutant endogenous pyruvate carboxylase gene (pycP458S) [21], a mutant endogenous l-aspartate kinase gene (lysCT311I) [21], the l-aspartate-4-semialdehyde dehydrogenase gene (asd), a mutant endogenous l-homoserine dehydrogenase gene (homV59A) [22], the endogenous two-component protein gene (brnFE), the E. coli-derived l-aspartate aminotransferase gene (aspC) and a mutant thrA gene (thrAS345F) encoding l-homoserine dehydrogenase [23,24]. To improve cofactor balance, the NADP+-dependent glyceraldehyde-3-phosphate dehydrogenase gene (gapN) was also integrated into the genome, establishing a dual-channel glycolysis and generating strain ACg1. Further overexpression of the E. coli-derived thrAS345F via plasmid pEC-XK99E resulted in strain ACg1-1. This strain accumulated 17.12 g/L of l-homoserine after 60 h of shake-flask cultivation, consuming 45.61 g/L of glucose, with a yield of 0.38 g/g and an OD600 of 16.91.
In this strain, pyruvate was carboxylated to form oxaloacetate, which was then transaminated to l-aspartate, the precursor of l-homoserine. However, a portion of pyruvate was decarboxylated to form acetyl-CoA and enters the TCA cycle to support energy production and intermediate biosynthesis. This dual fate of pyruvate not only caused substrate competition but also resulted in carbon loss through acetyl-CoA formation. Although the expression level of icd was attenuated, this modification alone did not significantly restrict TCA cycle activity [20]. Under aerobic conditions, the TCA cycle consumed a substantial portion of the carbon source, producing excess energy and reducing power, which reduced the efficiency of glucose conversion to l-homoserine [3]. Therefore, further reduction of the TCA cycle flux was necessary to minimize carbon loss and reduce the surplus generation of energy and reducing equivalent.
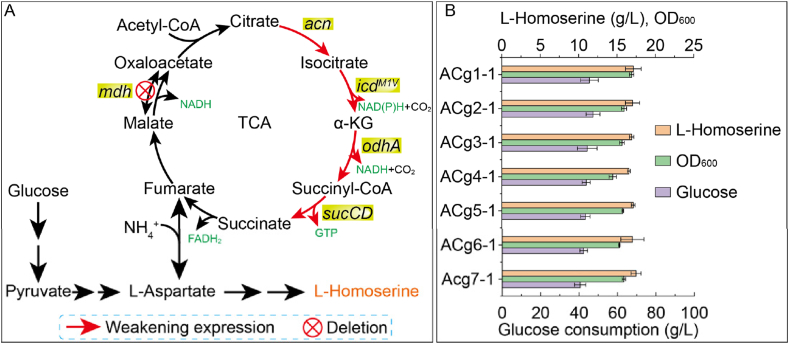
3.2. Effects of attenuating the TCA cycle on l-homoserine biosynthesis
Referring to previous study, key TCA cycle genes in strain ACg1-1 were attenuated via start or rare codon substitution to reduce TCA cycle flux [25,26]. As shown in Fig. 2, the acn gene (Cgl1540) of strain ACg1-1, encoding aconitase (EC: 4.2.1.3), was weakened through rare codon substitution at codons 3 to 7, generating strain ACg2-1. The odhA gene (Cgl1129) of strain ACg1-1, encoding the E1 component of the α-ketoglutarate dehydrogenase complex (EC: 1.2.4.2), was weakened through start codon substitution, generating strain ACg3-1. Similarly, the sucCD operon (Cgl2565 and Cgl2566) of strain ACg1-1, encoding succinyl-CoA synthetase (EC: 6.2.1.5), was also attenuated through start codon substitution, generating strain ACg4-1. The results showed that the l-homoserine titers of strains ACg2-1, ACg3-1, and ACg4-1 were 17.02 g/L, 16.91 g/L, and 16.54 g/L, with yields of 0.36 g/g, 0.38 g/g, and 0.38 g/g, respectively. In addition, cell growth was not adversely affected. These results suggested that individual attenuation of acn, odhA, or sucCD did not compromise either production performance or cellular growth.
Fig. 2.
Attenuation of the TCA cycle in l-homoserine producing strains.
(A) Strategies of the weakening TCA cycle. The enzymes encoded by the genes are as follows, acn: aconitase, EC: 4.2.1.3; icd: NADP+-dependent monomeric isocitrate dehydrogenase, EC: 1.1.1.42; mdh: malate/lactate dehydrogenases, EC: 1.1.1.37; odhA: α-ketoglutarate dehydrogenase complex, EC: 1.2.4.2; sucCD: succinyl-CoA synthetase, EC: 6.2.1.5. (B) Fermentation results of the engineered strains.
To further explore potential synergistic effects, the gene acn and sucCD of strain ACg3-1 were individually attenuated, generating strains ACg5-1 and ACg6-1, respectively. These strains exhibited l-homoserine titers of 17.11 g/L and 16.92 g/L, with yields of 0.39 g/g and 0.40 g/g, and final OD600 values of 15.75 and 15.24, respectively. Compared to the control strain (ACg1-1), there were no significant changes in l-homoserine production, glucose conversion efficiency, or biomass accumulation. Finally, the genes acn, odhA, and sucCD of strain ACg1-1 were simultaneously attenuated to generate strain ACg7-1. This strain produced 17.40 g/L of l-homoserine with the yield was 0.43 g/g and OD600 of 15.81. Although the l-homoserine titer remained comparable to that of previous strains, the yield increased by 14.41 %, indicating enhanced carbon conversion efficiency. These results collectively suggested that suppressing TCA cycle flux in C. glutamicum could improve glucose-to-l-homoserine conversion efficiency without severely affecting growth.
Aconitase, which catalyzed the isomerization of citrate to isocitrate, was a major regulatory point in the TCA cycle [[27], [28], [29]]. Complete deletion of acn was found to severely impair cell growth, hence rare codon substitution was adopted as a strategy to fine-tune its expression [25,26]. Similarly, α-ketoglutarate dehydrogenase complex and succinyl-CoA synthetase were critical nodes in TCA cycle [30,31]. In E. coli, succinyl-CoA was essential for biomass formation and peptidoglycan synthesis [32]. However, in C. glutamicum, deletion of sucCD did not significantly affect cellular growth, likely due to activation of the glyoxylate shunt. This bacterium harbored both isocitrate lyase (aceA/icl, EC: 4.1.3.1) and malate synthase (aceB/mas, EC: 2.3.3.9), enabling bypass of the TCA cycle and supporting biomass formation [27]. Nevertheless, excessive attenuation of multiple TCA cycle genes could lead to an undesirable reduction in metabolic flux, potentially impairing growth and hindering high-level production of target metabolites. Therefore, a rate-controlled TCA cycle would be essential for achieving high glucose conversion efficiency in l-homoserine-producing C. glutamicum strains.
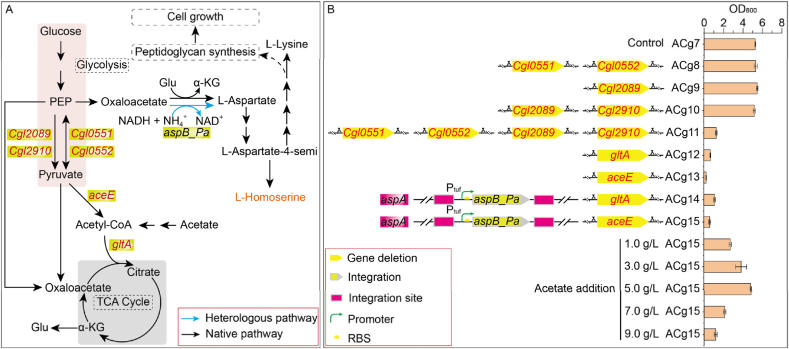
3.3. Disrupting the link between glycolysis and the TCA cycle
Under aerobic conditions, pyruvate generated via glycolysis is predominantly decarboxylated to acetyl-CoA, which enters TCA cycle. This results in reduced oxaloacetate availability for l-homoserine biosynthesisand and leads to carbon loss via CO2 release, thereby reducing glucose-to-product conversion efficiency. Hence, disrupting the connection between glycolysis and the TCA cycle presents a promising strategy to enhance product yield. Although four enzymes were reportedly inactivated to achieve glycolysis-TCA cycle decoupling in previous studies, the specific genes critical for this process remained ambiguous [33]. To gain clearer mechanistic insights, three targeted strategies were employed based on Kyoto Encyclopedia of Genes and Genomes (KEGG) (Fig. 3A): (1) Blocking phosphoenolpyruvate to pyruvate conversion. The ppsA genes (Cgl0551 and Cgl0552), encoding phosphoenolpyruvate synthase (EC: 2.7.9.2), were deleted in strain ACg7 to generate ACg8. Additionally, one of pyk genes (Cgl2089), encoding pyruvate kinase (EC: 2.7.1.40), was deleted in strain ACg7 to generate strain ACg9. Iterative deletion of two pyk genes (Cgl2089 and Cgl2910) generated strain ACg10. Disruption of both ppsA and pyk genes generated to strain ACg11. (2) Disrupting the pyruvate-to-acetyl-CoA pathway. The gltA gene (Cgl0829), encoding citrate synthase (EC: 2.3.3.1) [34], was deleted in strain ACg7 to generate strain ACg12. (3) Blocking citrate formation. The aceE gene (Cgl2248), encoding the E1 component of the pyruvate dehydrogenase complex (EC: 1.2.4.1), was deleted in strain ACg7 to generate strain ACg13.
Fig. 3.
Decoupling strategies of the TCA cycle and glycolytic pathway and its effects on strain growth.
(A) Decoupling strategies of the TCA cycle and glycolytic pathway. The enzymes encoded by the genes are as follows, aceE: the E1 component of the pyruvate dehydrogenase complex, EC: 1.2.4.1; aspB_Pa: NADH-dependent l-aspartate dehydrogenase from P. aeruginosa; Cgl0551: phosphoenolpyruvate synthase, EC: 2.7.9.2; Cgl0552: phosphoenolpyruvate synthase, EC: 2.7.9.2; Cgl2089: pyruvate kinase, EC: 2.7.1.40; Cgl2910: pyruvate kinase, EC: 2.7.1.40; gltA: citrate synthase, EC: 2.3.3.1. (B) Fermentation results of the engineered strains.
For strategy 1, single deletions of ppsA or pyk did not affect colony growth on LBHIS agar plates. However, the double-deletion strain (ACg11) exhibited significantly reduced growth. For strategy 2, deletion of gltA (ACg12) led to severe growth impairment. For strategy 3, deletion of aceE (ACg13) also resulted in slow-growing colonies. Adding 5.0 g/L acetate (for acetyl-CoA biosynthesis) or citrate to the plate could restore growth in aceE-deleted strains, facilitating rapid screening. The growth trends in LBHIS liquid medium (Fig. 3B) mirrored those observed on plates. The findings demonstrated that deletion of aceE or gltA gene alone could effectively decouple glycolysis from the TCA cycle without requiring extensive genetic modifications, offering a more streamlined strategy than previous approaches [33].
The impaired growth observed in strains with disrupted gltA or aceE were likely due to reduced α-ketoglutarate availability, which was essential for l-glutamate biosynthesis. This deficiency hampered the transamination process from oxaloacetate to l-aspartate, leading to reduced biosynthesis of intermediates essential for cell wall formation, thereby affecting cell division. Notably, meso-2,6-diaminoheptanedioate, a key precursor in peptidoglycan synthesis, was derived from l-lysine biosynthesis pathway, which was compromised under these conditions [35]. To address this issue, the aspB_Pa gene from Pseudomonas aeruginosa, encoding NADH-dependent l-aspartate dehydrogenase [36], was integrated into the aspA locus of strains ACg12 and ACg13, generating strains ACg14 and ACg15. This modification restored the transamination capacity independently of l-glutamate and significantly improved growth. Supplementation with acetate further enhanced recovery, likely due to improved acetyl-CoA availability (Fig. 3B). Considering the cost-effectiveness of acetate (∼$510/t) compared to citrate (∼$690/t) [37,38], acetate was selected for further studies, and strain ACg15 was used in subsequent investigations.
3.4. Effects of acetate on the growth of C. glutamicum
Previous studies on acetate metabolism and its regulatory mechanisms in C. glutamicum had revealed that this bacterium possesses the capacity to simultaneously utilize multiple carbon sources [39,40]. Elkasaby et al. reported that itaconic acid-producing C. glutamicum strains could tolerate acetate concentrations exceeding 50 g/L [41]. However, this finding was inconsistent with our previous results, which demonstrated growth inhibition at high acetate concentrations [16,42]. To evaluate the effects of acetate on cell growth, strain ACg15 was cultured in LBHIS medium supplemented with acetate at concentrations of 0.0, 1.0, 3.0, 5.0, 7.0, and 9.0 g/L. As shown in Fig. 3B, OD600 increased from 0.61 (no acetate) to 2.72, 3.84, 4.83, 2.12, and 1.19, representing increases of 345.90 %, 529.51 %, 691.80 %, 247.54 %, and 95.08 %, respectively. The optimal concentration was 5.0 g/L, at which the cell density was comparable to that of the control strain (ACg7). These results indicated that low concentrations of acetate promoted growth of engineered C. glutamicum, whereas high concentrations inhibited growth. Therefore, 5.0 g/L acetate was chosen for subsequent experiments.
In the presence of multiple carbon sources, bacteria typically prioritize the utilization of the most favorable source while repressing pathways for others [43]. Similar inhibitory effects of high acetate concentrations had been observed in E. coli [44,45]. Despite similarities in acetate assimilation pathways across species, C. glutamicum exhibited unique genetic and regulatory traits. Previous transcriptomic analyses had identified acetate-responsive genes in C. glutamicum, confirming transcriptional regulation of acetate-activating enzymes [39,41]. The results of this study further demonstrated that excessive acetate inhibited the growth of engineered strains, highlighting the importance of improving acetate tolerance to enable efficient biosynthesis of high-value chemicals from acetate-based feedstocks.
3.5. Energy supply and l-homoserine accumulation in module-decoupled strains
After decoupling the pyruvate-to-acetyl-CoA pathway, growth restoration was achieved via acetate-derived acetyl-CoA. However, its ability to support efficient l-homoserine biosynthesis required evaluation. To investigate this, the thrAS345F gene was overexpressed in the module-decoupled strain ACg15, as described in our previous study [20], generating strain ACg15-1. Acetate supplementation was performed at 12-h intervals (0, 12, 24, 36, and 48 h) during fermentation, establishing six groups with cumulative acetate additions of 0.0–25.0 g/L. As shown in Fig. 4, increasing acetate supplementation improved the growth recovery of strain ACg15-1 and l-homoserine production. However, the final l-homoserine titer (4.12 g/L) was significantly lower than that of strain without the aceE deletion (ACg16-1, 13.61 g/L). It was hypothesized that energy limitations, rather than cofactor imbalance, were the primary constraint. The expression of the gapN gene in strain ACg15–1 may have imposed a metabolic burden and disturbed cofactor homeostasis. To test this hypothesis, the gapN gene was deleted in strain ACg15-1 to generate strain ACg17-1, which achieved a titer of 7.11 g/L with 25 g/L acetate, supporting the hypothesis that energy and precursor supply, rather than redox imbalance, were limiting factors.
Fig. 4.
Acetate-activated energy supply module enhanced cell growth and l-homoserine biosynthesis.
The acetate utilization pathway was constructed, forming two systems: carbon supply system and energy supply system. The metabolic connection between the two systems was decoupled through deletion of aceE gene (encoding encoding the E1 component of the pyruvate dehydrogenase complex). Glucose was used as the substrate of the carbon supply system to supply the carbon skeleton of the target product; Acetate, as the substrate of energy supply, entered the TCA cycle to supply energy and reducing power.
To further enhance the conversion of acetate to acetyl-CoA, the mutant acetyl-CoA synthetase gene (acsL641P) from Salmonella enterica was introduced into strains ACg15-1 and ACg17-1 [46], generating strains ACg15-2 and ACg17-2, respectively. As shown in Fig. 5, the introduction of acsL641P gene enhanced l-homoserine accumulation in both strains (5.92 g/L and 7.63 g/L), indicating the significance of energy supply following aceE inactivation. However, cell growth did not improve proportionally, speculating a metabolic burden from plasmid-based acsL641P expression. Therefore, the acsL641P gene was integrated into the ΔaceE locus of strain ACg17-1 to generate strain ACg18-1. However, strain ACg18-1 exhibited only modest improvement in growth or l-homoserine titer (7.95 g/L). Considering the irreversibility of the Acs pathway [12], the endogenous AckA-Pta pathway based on KEGG was enhanced via plasmid expression in strain ACg17-1, generating strain ACg17-3. This strain reached a titer of 8.15 g/L, indicating that the AckA-Pta pathway was more favorable under the tested condition.
Fig. 5.
Effects of enhanced acetate acetylation on l-homoserine biosynthesis.
To improve the conversion of acetate into acetyl-CoA, two pathways—the acetyl-CoA synthetase (ACS) pathway and the AckA-Pta pathway—were compared. Strains ACg15-2, ACg17-2, ACg17-3, and ACg18-1 were constructed for this purpose. Strain ACg15-1 served as the control for ACg15-2, while ACg17-1 was used as the control for ACg17-2, ACg17-3, and ACg18-1.
Previous studies had shown that relying solely on the Acs pathway for acetate assimilation was insufficient in C. glutamicum [18]. High Acs expression may cause excessive acetyl-CoA accumulation, leading to enzyme acetylation and reduced pathway activity. In contrast, the AckA-Pta pathway enabled dynamic regulation of intracellular acetyl-CoA and free CoA pools. Notably, the Acs pathway consumed two ATP equivalents per acetyl-CoA molecule (forming AMP), whereas the AckA-Pta pathway required only one ATP, offering an energetic advantage. Thus, optimizing both acetyl-CoA formation and utilization was critical for efficient acetate assimilation. While the AckA-Pta pathway offered metabolic flexibility, it also risked forming a futile cycle, wasting ATP. Therefore, directing acetyl-CoA flux toward biosynthetic pathways remained an important area for further study.
3.6. Driving acetyl-CoA utilization to enhance l-aspartate supply
l-Aspartate ammonia-lyase (EC: 4.3.1.1) catalyzes the reversible conversion of fumarate to l-aspartate, a precursor for l-homoserine biosynthesis. In biocatalysis, this enzyme was widely used for l-aspartate synthesis, and substrate concentration typically dictates the reaction direction [47,48]. To prevent the unpredictable negative effects from excess acetyl-CoA, the TCA cycle was leveraged to direct carbon flux toward l-aspartate synthesis. In detail, the aspA gene from C. glutamicum (aspA_Cgl) and from E. coli K-12 MG1655 (aspA_Ec) were separately introduced into the ΔaceE locus of strain ACg17-3, generating strains ACg19-3 and ACg20-3. As shown in Fig. 6, these strains produced 7.73 g/L and 8.32 g/L l-homoserine, with yields of 0.27 g/g and 0.29 g/g, respectively. The slight decrease was attributed to the bifunctional activity of l-aspartate ammonia-lyase, which may have driven the reverse reaction due to insufficient fumarate.
Fig. 6.
Optimization of the glyoxylate cycle and l-aspartate ammonia-lyase expression enhanced l-aspartate biosynthesis.
To address this, the glyoxylate cycle was activated to boost fumarate availability and minimize carbon loss [49]. The native promoter of the icl gene (encoding isocitrate lyase, EC: 4.1.3.1) was replaced with the strong promoter Ptuf in strains ACg19-3 and ACg20-3, generating strains ACg21-3 and ACg22-3, respectively. As shown in Fig. 6, strains ACg21-3 and ACg22-3 produced 13.32 g/L and 14.22 g/L of l-homoserine, with yields of 0.48 g/g and 0.49 g/g, and OD600 values of 16.96 and 17.82, respectively. To further optimize the metabolic flux from acetate to l-aspartate, modifications were made to the ackA-pta, icl, and aspA_Ec genes in strain ACg22-3 via a combination of genome integration and plasmid expression, generating strains ACg23-1, ACg23-4, ACg23-5, and ACg23-6, respectively. The l-homoserine titers of these strains were 14.14 g/L, 15.12 g/L, 14.87 g/L, and 17.35 g/L, with yields of 0.46 g/g, 0.54 g/g, 0.51 g/g, 0.56 g/g, respectively. These results suggested that the synergistic interaction of l-aspartate ammonia-lyase and the glyoxylate cycle effectively redirected transient excess acetyl-CoA derived from acetate toward l-homoserine biosynthesis, preventing its futile recycling and improving energy efficiency. Rapidly channeling acetate into productive pathways was thus key to developing efficient acetate-based microbial factories.
3.7. Fed-batch fermentation in a 5-L bioreactor promotes l-homoserine production
To evaluate the performance of strain ACg23-6 and ensure continuous acetate supply, fed-batch fermentations were performed in a 5-L bioreactor with two acetate feeding strategies. As shown in Fig. 7, for acetate feeding strategy 1, the accumulated l-homoserine reached 58.63 g/L, with 109.20 g/L glucose and 40 g/L acetate consumption, achieving a yield of 0.53 g/g glucose, 0.39 g/g carbon source and productivity of 0.61 g/L/h. For acetate feeding strategy 2, the accumulated l-homoserine reached 70.54 g/L, with 121.39 g/L glucose and 80 g/L acetate consumption, achieving a yield of 0.58 g/g glucose, 0.35 g/g carbon source, and productivity of 0.73 g/L/h. In the decoupled strains, the expressed l-aspartate ammonia-lyase catalyzed a reversible reaction. To drive the reaction toward l-aspartate formation, a sustained and sufficient supply of fumarate was required. This necessitated a continuous supply of acetate, the concentration of which must be carefully regulated to avoid inhibitory effects on cell growth. In Strategy 2, the acetate feeding profile was more continuous, which supported a more consistent fumarate supply and thus enabled higher l-homoserine production. These findings indicated that continuous acetate supply, combined with rational acetyl-CoA utilization, enabled decoupled C. glutamicum strains to efficiently produce high levels of l-homoserine. Although this was the highest reported glucose conversion efficiency to l-homoserine to date, the absolute titer still required improvement to compete with engineered E. coli strains used glucose as substrates [50], and engineered C. glutamicum strain used the mixed sugars as substrates [51], even no inducer should be added [7].
Fig. 7.
Fed-Batch fermentation in a 5-L bioreactor enhances l-homoserine biosynthesis.
4. Conclusion
Glucose conversion efficiency was a critical parameter in l-homoserine biosynthesis. Reducing pyruvate decarboxylation and downregulating TCA cycle flux were effective strategies to minimize carbon loss. In this study, attenuation of key TCA cycle genes via rare codon substitution successfully decreased CO2 release and improved glucose-to-product conversion efficiency. Decoupling the TCA cycle from glycolysis, coupled with acetate supplementation to replenish acetyl-CoA, enabled the recovery of cell growth. Further optimization of acetylation levels, enhancement of glyoxylate cycle flux, and redirection of acetate-derived carbon through fumarate to l-aspartate biosynthesis effectively mitigated acetyl-CoA accumulation and its inhibitory effects on cellular metabolism. In summary, the high conversion rate of l-homoserine biosynthesis from glucose by C. glutamicum was achieved through a combined strategy of TCA cycle-glycolysis decoupling and the optimized co-utilization of glucose and acetate as dual carbon sources.
CRediT authorship contribution statement
Daobin Wang: Writing – review & editing, Writing – original draft, Investigation, Data curation. Lu Xu: Writing – original draft, Software, Investigation, Formal analysis. Junwen Yuan: Writing – review & editing, Software. Ruisi Wu: Methodology, Data curation. Xiyao Cheng: Writing – review & editing, Conceptualization. Jidong Liu: Writing – review & editing. Ning Li: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Funding
This work was supported by the National Natural Science Foundation of China (22108098), the Natural Science Foundation of Guangxi Province (2023JJA130304).
Declaration of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under the responsibility of Editorial Board of Synthetic and Systems Biotechnology.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.synbio.2025.07.012.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.NEWSIJIE.COM L-homoserine downstream market demand. http://newsijie.com/chanye/huagong/jujiao/2023/1109/11336835.html
- 2.Liu Z., Cai M., Zhou S., You J., Zhao Z., Liu Z., Xu M., Rao Z. High-efficient production of L-homoserine in Escherichia coli through engineering synthetic pathway combined with regulating cell division. Bioresour Technol. 2023;389 doi: 10.1016/j.biortech.2023.129828. [DOI] [PubMed] [Google Scholar]
- 3.Li N., Li L., Yu S., Zhou J. Dual-channel glycolysis balances cofactor supply for L-homoserine biosynthesis in Corynebacterium glutamicum. Bioresour Technol. 2023;369 doi: 10.1016/j.biortech.2022.128473. [DOI] [PubMed] [Google Scholar]
- 4.Cai M., Zhao Z., Li X., Xu Y., Xu M., Rao Z. Development of a nonauxotrophic L-homoserine hyperproducer in Escherichia coli by systems metabolic engineering. Metab Eng. 2022;73:270–279. doi: 10.1016/j.ymben.2022.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Mu Q., Zhang S., Mao X., Tao Y., Yu B. Highly efficient production of L-homoserine in Escherichia coli by engineering a redox balance route. Metab Eng. 2021;67:321–329. doi: 10.1016/j.ymben.2021.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Zhong Z.Y., Ma Z.P., Cao Y.Z., Zhang H.Y., Qi Y.T., Wei L., Jiang J.Q., Xu N., Liu J. Metabolic engineering of for efficient L-homoserine production from lignocellulose-derived sugars. ACS Sustainable Chem Eng. 2025;13(9):3441–3451. doi: 10.1021/acssuschemeng.4c07838. [DOI] [Google Scholar]
- 7.Zhang Y., Wei M., Zhao G., Zhang W., Li Y., Lin B., Li Y., Xu Q., Chen N., Zhang C. High-level production of L-homoserine using a non-induced, non-auxotrophic Escherichia coli chassis through metabolic engineering. Bioresour Technol. 2021;327 doi: 10.1016/j.biortech.2021.124814. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q., Zeng W., Xu S., Zhou J. Metabolism and strategies for enhanced supply of acetyl-CoA in Saccharomyces cerevisiae. Bioresour Technol. 2021;342 doi: 10.1016/j.biortech.2021.125978. [DOI] [PubMed] [Google Scholar]
- 9.Nie M.Z., Wang J.Y., Zhang K.C. Engineering a novel acetyl-CoA pathway for efficient biosynthesis of acetyl-CoA-derived compounds. ACS Synth Biol. 2024;13(3):973. doi: 10.1021/acssynbio.4c00072. 973. [DOI] [PubMed] [Google Scholar]
- 10.Liu M., Huo M.T., Liu C.S., Guo L.K., Ding Y.M., Ma Q.J., Qi Q.S., Xian M., Zhao G. Lysine acetylation of Escherichia coli lactate dehydrogenase regulates enzyme activity and lactate synthesis. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.966062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu M., Huo M., Guo L., Fu Y., Xian M., Qi Q., Liu W., Zhao G. Lysine acetylation decreases enzyme activity and protein level of Escherichia coli lactate dehydrogenase. Eng Microbiol. 2022;2(4) doi: 10.1016/j.engmic.2022.100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu L., Zhang J., Yang J., Jiang Y., Yang S. Strategies for optimizing acetyl-CoA formation from glucose in bacteria. Trends Biotechnol. 2022;40(2):149–165. doi: 10.1016/j.tibtech.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Yu T., Liu Q., Wang X., Liu X., Chen Y., Nielsen J. Metabolic reconfiguration enables synthetic reductive metabolism in yeast. Nat Metab. 2022;4(11):1551–1559. doi: 10.1038/s42255-022-00654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q., Yu S., Lyu Y., Zeng W., Zhou J. Systematically engineered fatty acid catabolite pathway for the production of (2S)-naringenin in Saccharomyces cerevisiae. ACS Synth Biol. 2021;10(5):1166–1175. doi: 10.1021/acssynbio.1c00002. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q., Li N., Lyv Y., Yu S., Zhou J. Engineering caveolin-mediated endocytosis in Saccharomyces cerevisiae. Synth Syst Biotechnol. 2022;7(4):1056–1063. doi: 10.1016/j.synbio.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N., Zeng W., Zhou J., Xu S. O-Acetyl-L-homoserine production enhanced by pathway strengthening and acetate supplementation in Corynebacterium glutamicum. Biotechnol Biofuels Bioprod. 2022;15(1):27. doi: 10.1186/s13068-022-02114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y., Lama S., Agrawal D., Kumar V., Park S. Acetate as a potential feedstock for the production of value-added chemicals: metabolism and applications. Biotechnol Adv. 2021;49 doi: 10.1016/j.biotechadv.2021.107736. [DOI] [PubMed] [Google Scholar]
- 18.Vo T.M., Park J.Y., Kim D., Park S. Use of acetate as substrate for sustainable production of homoserine and threonine by Escherichia coli W3110: a modular metabolic engineering approach. Metab Eng. 2024;84:13–22. doi: 10.1016/j.ymben.2024.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Zheng T., Zhang M., Wu L., Guo S., Liu X., Zhao J., Xue W., Li J., Liu C., Li X., Jiang Q., Bao J., Zeng J., Yu T., Xia C. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering. Nat Catal. 2022;5(5):388–396. doi: 10.1038/s41929-022-00775-6. [DOI] [Google Scholar]
- 20.Li N., Xu S., Du G.C., Chen J., Zhou J.W. Efficient production of L-homoserine in Corynebacterium glutamicum ATCC 13032 by redistribution of metabolic flux. Biochem Eng J. 2020;161 doi: 10.1016/j.bej.2020.107665. [DOI] [Google Scholar]
- 21.Ohnishi J., Mitsuhashi S., Hayashi M., Ando S., Yokoi H., Ochiai K., Ikeda M. A novel methodology employing Corynebacterium glutamicum genome information to generate a new L-lysine-producing mutant. Appl Microbiol Biotechnol. 2002;58(2):217–223. doi: 10.1007/s00253-001-0883-6. [DOI] [PubMed] [Google Scholar]
- 22.Park S.D., Lee J.Y., Sim S.Y., Kim Y., Lee H.S. Characteristics of methionine production by an engineered Corynebacterium glutamicum strain. Metab Eng. 2007;9(4):327–336. doi: 10.1016/j.ymben.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Wei L., Wang Q., Xu N., Cheng J., Zhou W., Han G., Jiang H., Liu J., Ma Y. Combining protein and metabolic engineering strategies for high-level production of O-acetylhomoserine in Escherichia coli. ACS Synth Biol. 2019;8(5):1153–1167. doi: 10.1021/acssynbio.9b00042. [DOI] [PubMed] [Google Scholar]
- 24.Angeles T.S., Viola R.E. The kinetic mechanisms of the bifunctional enzyme aspartokinase-homoserine dehydrogenase I from Escherichia coli. Arch Biochem Biophys. 1990;283(1):96–101. doi: 10.1016/0003-9861(90)90617-8. [DOI] [PubMed] [Google Scholar]
- 25.Long M., Xu M., Ma Z., Pan X., You J., Hu M., Shao Y., Yang T., Zhang X., Rao Z. Significantly enhancing production of trans-4-hydroxy-l-proline by integrated system engineering in Escherichia coli. Sci Adv. 2020;6(21) doi: 10.1126/sciadv.aba2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng B., Ma X., Wang N., Ding T., Guo L., Zhang X., Yang Y., Li C., Huo Y. Utilization of rare codon-rich markers for screening amino acid overproducers. Nat Commun. 2018;9(1):3616. doi: 10.1038/s41467-018-05830-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Zhao J., Wang X., Tang Y., Liu S., Wen T. Model-guided metabolic rewiring for gamma-aminobutyric acid and butyrolactam biosynthesis in Corynebacterium glutamicum ATCC13032. Biology (Basel) 2022;11(6):846. doi: 10.3390/biology11060846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han S.O., Inui M., Yukawa H. Effect of carbon source availability and growth phase on expression of Corynebacterium glutamicum genes involved in the tricarboxylic acid cycle and glyoxylate bypass. Microbiology. 2008;154:3073–3083. doi: 10.1099/mic.0.2008/019828-0. [DOI] [PubMed] [Google Scholar]
- 29.Bott M. Offering surprises: TCA cycle regulation in Corynebacterium glutamicum. Trends Microbiol. 2007;15(9):417–425. doi: 10.1016/j.tim.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Rifqi Ghiffary M., Surya Prabowo C.P., Adidjaja J.J., Sang Yup L., Hyun Uk K. Systems metabolic engineering of Corynebacterium glutamicum for the efficient production of beta-alanine. Metab Eng. 2022;74:121–129. doi: 10.1016/j.ymben.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Kind S., Becker J., Wittmann C. Increased lysine production by flux coupling of the tricarboxylic acid cycle and the lysine biosynthetic pathway-Metabolic engineering of the availability of succinyl-CoA in Corynebacterium glutamicum. Metab Eng. 2013;15:184–195. doi: 10.1016/j.ymben.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Theodosiou E., Breisch M., Julsing M.K., Falcioni F., Bühler B., Schmid A. An artificial TCA cycle selects for efficient α-ketoglutarate dependent hydroxylase catalysis in engineered Escherichia coli. Biotechnol Bioeng. 2017;114(7):1511–1520. doi: 10.1002/bit.26281. [DOI] [PubMed] [Google Scholar]
- 33.Li K., Li C., Liu C., Zhao X., Ou R.W., Swofford C.A., Bai F., Stephanopoulos G., Sinskey A.J. Engineering carbon source division of labor for efficient α-carotene production in Corynebacterium glutamicum. Metab Eng. 2024;84:117–127. doi: 10.1016/j.ymben.2024.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Li N., Shan X., Zhou J., Yu S. Identification of key genes through the constructed CRISPR-dcas9 to facilitate the efficient production of O-acetylhomoserine in Corynebacterium glutamicum. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.978686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sgobba E., Stumpf A.K., Vortmann M., Jagmann N., Krehenbrink M., Dirks-Hofmeister M.E., Moerschbacher B., Philipp B., Wendisch V.F. Synthetic Escherichia coli-Corynebacterium glutamicum consortia for L-lysine production from starch and sucrose. Bioresour Technol. 2018;260:302–310. doi: 10.1016/j.biortech.2018.03.113. [DOI] [PubMed] [Google Scholar]
- 36.Wu W., Zhang Y., Liu D., Chen Z. Efficient mining of natural NADH-utilizing dehydrogenases enables systematic cofactor engineering of lysine synthesis pathway of Corynebacterium glutamicum. Metab Eng. 2019;52:77–86. doi: 10.1016/j.ymben.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 37.outhmoney.com Market price quotation of acetic acid. http://www.southmoney.com/shuju/hysj/202410/54859642.html
- 38.Chemicalbook Citric acid price. https://www.chemicalbook.com/priceindex_cb9854361.htm#changjiabaojia
- 39.Gerstmeir R., Wendisch V.F., Schnicke S., Ruan H., Farwick M., Reinscheid D., Eikmanns B.J. Acetate metabolism and its regulation in Corynebacterium glutamicum. J Biotechnol. 2003;104(1–3):99–122. doi: 10.1016/s0168-1656(03)00167-6. [DOI] [PubMed] [Google Scholar]
- 40.Muffler A., Bettermann S., Haushalter M., Horlein A., Neveling U., Schramm M., Sorgenfrei O. Genome-wide transcription profiling of Corynebacterium glutamicum after heat shock and during growth on acetate and glucose. J Biotechnol. 2002;98(2–3):255–268. doi: 10.1016/s0168-1656(02)00136-0. [DOI] [PubMed] [Google Scholar]
- 41.Elkasaby T., Hanh D.D., Kawaguchi H., Toyoshima M., Kondo A., Ogino C. Co-utilization of maltose and sodium acetate via engineered Corynebacterium glutamicum for improved itaconic acid production. Biotechnol Bioprocess Eng. 2023;28(5):790–803. doi: 10.1007/s12257-023-0091-7. [DOI] [Google Scholar]
- 42.Mutyala S., Kim J.R. Recent advances and challenges in the bioconversion of acetate to value-added chemicals. Bioresour Technol. 2022;364 doi: 10.1016/j.biortech.2022.128064. [DOI] [PubMed] [Google Scholar]
- 43.Görke B., Stülke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol. 2008;6(8):613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 44.Lama S., Kim Y., Nguyen D.T., Im C.H., Sankaranarayanan M., Park S. Production of 3-hydroxypropionic acid from acetate using metabolically-engineered and glucose-grown Escherichia coli. Bioresour Technol. 2021;320(Pt A) doi: 10.1016/j.biortech.2020.124362. [DOI] [PubMed] [Google Scholar]
- 45.Nam S.H., Ye D.Y., Hwang H.G., Jung G.Y. Convergent synthesis of two heterogeneous fluxes from glucose and acetate for high-yield citramalate production. J Agric Food Chem. 2024;72(11):5797–5804. doi: 10.1021/acs.jafc.3c09466. [DOI] [PubMed] [Google Scholar]
- 46.Starai V.J. Residue Leu-641 of Acetyl-CoA synthetase is critical for the acetylation of residue Lys-609 by the protein acetyltransferase enzyme of Salmonella enterica. J Biol Chem. 2005;280(28):26200–26205. doi: 10.1074/jbc.M504863200. [DOI] [PubMed] [Google Scholar]
- 47.Liu H., Zong X., Wang Y., Yin X., Liu M., Liu S., Zhu G., Fang S. One-pot biosynthesis of L-aspartate from maleic anhydride via a thermostable dual-enzyme system under high temperature. J Agric Food Chem. 2022;70(44):14247–14254. doi: 10.1021/acs.jafc.2c05662. [DOI] [PubMed] [Google Scholar]
- 48.Liu Z., Yu L., Zhou L., Zhou Z. One-pot biosynthesis of L-aspartate from maleate via an engineered strain containing a dual-enzyme system. Appl Environ Microbiol. 2019;85(19) doi: 10.1128/aem.01327-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwentner A., Feith A., Munch E., Busche T., Ruckert C., Kalinowski J., Takors R., Blombach B. Metabolic engineering to guide evolution-Creating a novel mode for L-valine production with Corynebacterium glutamicum. Metab Eng. 2018;47:31–41. doi: 10.1016/j.ymben.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 50.Niu K., Zheng R., Zhang M., Chen M.Q., Kong Y.M., Liu Z.Q., Zheng Y.G. Adjustment of the main biosynthesis modules to enhance the production of L-homoserine in Escherichia coli W3110. Biotechnol Bioeng. 2025;122(1):223–232. doi: 10.1002/bit.28861. [DOI] [PubMed] [Google Scholar]
- 51.Zhong Z., Ma Z., Cao Y., Zhang H., Qi Y., Wei L., Jiang J., Xu N., Liu J. Metabolic engineering of Corynebacterium glutamicum for efficient L-homoserine production from lignocellulose-derived sugars. ACS Sustainable Chem Eng. 2025;13:3441–3451. doi: 10.1021/acssuschemeng.4c07838. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.