Abstract
Background
Recent studies have suggested that the effectiveness of environmental intervention is crucial in reducing levels of the mouse allergen Mus musculus (Mus m 1) in inner-city homes. However, the impact of mouse control alone on mouse allergen reduction has not been studied.
Objective
Our aim was to evaluate the effectiveness of 3 house mouse control programs on mouse allergen reduction.
Methods
A total of 18 buildings in 3 cities in New Jersey were randomly divided and assigned to 1 of 3 treatment groups: (1) trapping and baiting (T&B); (2) trapping, baiting, and rodent exclusion (T&B+E); and (3) existing pest control service with no or limited use of bait or glue boards (control). Dust samples from kitchen floors, bedroom floors, and beds were collected and analyzed for Mus m 1 levels at baseline, at 6 months, and at 12 or 24 months after the intervention. The T&B and T&B+E groups were combined for Mus m 1 analyses because there were no differences in mouse infestation rates after intervention between these groups.
Results
Compared with the control, T&B and T&B+E caused greater reduction in Mus m 1 allergen levels in the kitchens in all 3 cities (P < .05). After T&B and T&B+E, kitchen levels of Mus m 1 in New Brunswick, Trenton, and Paterson were reduced by 97.4%, 85.8%, and 34.9%, respectively. In contrast, the kitchen levels of Mus m 1 in the control were reduced by 61.4%, –671.4%, and –289.6%, respectively. However, no significant reduction was observed in the bedroom in the intervention group versus in the control group in any of the 3 cities at the end of the study period.
Conclusion
Effective mouse control alone greatly reduced mouse allergen levels compared with conventional pest control.
Key words: Pest control, Mus m 1, low-income housing
In low-income communities in the United States, mice (Mus musculus domesticus Schwarz and Schwarz) are the second most common indoor pest after cockroaches, with 20% of surveyed apartments having active mouse infestations.1 They cause significant property damage, produce aeroallergens, and carry ectoparasites.2,3 Mouse allergens, particularly Mus m 1, are significant contributors to allergic diseases, including asthma.4 Sensitization to mouse allergens has been linked to increased asthma severity and decreased lung function.5 Studies have also shown that individuals sensitized to mouse allergens experience more frequent emergency department visits owing to asthma exacerbations.4 Multiple studies have shown that there is a widespread presence of mouse allergens in settled reservoir dust samples in certain low-income urban communities, specifically, in the northeastern and midwestern regions of the United States.6 Moreover, approximately 75% to 80% of homes in suburban areas have detectable levels of mouse allergen in settled dust.6 However, levels of mouse allergen in accumulated dust are 100 to 1000 times greater in certain low-income urban regions than in suburban regions.7, 8, 9, 10, 11, 12, 13 Mouse allergens may exist even without documented mouse sightings inside the household, especially in those areas in which the mouse infestation is widespread.6
The quantity of mouse allergen is correlated with the presence of rodent infestation.13 In one study, when patients reported the presence of mice, 90% of the homes had levels of the mouse allergen Mus musculus (Mus m 1) higher than 0.5 μg/g in settled dust.14 In spite of the high mouse infestation rates, many low-income housing communities continue to use ineffective pest control practices that rely solely on rodenticides and/or glue boards for control of mice.15,16 Treatment visits are commonly based on complaints or reports from residents rather than on proactive identification of apartments with unreported mouse activity. Because residents are often unaware of the mouse activity or fail to report mouse infestations to the management office, a high proportion of infested homes remain untreated.1 Follow-up treatments are often not provided, allowing mice to continue to infest and spread among apartments within multiunit dwellings.
On the basis of the knowledge that house mouse allergen contributes to asthma severity, there is a critical need to reduce the amount of this allergen in homes. Several studies have evaluated the effectiveness of mouse allergen reduction techniques alone or in combination with pest control.17, 18, 19 Integrated pest management (IPM) is a pest management strategy that involves education of residents, monitoring of pest populations, reduction of available food sources, exclusion practices, use of nonchemical control measures, and if needed, the application of rodenticides.6,20 A preliminary study of implementation of IPM for house mice plus house cleaning (vacuuming, cleaning surfaces with detergents) by professionals showed that in the intervention group, kitchen and bedroom levels of mouse allergen were lowered by more than 77%, whereas the control group (no intervention received) had an increase of at least 319%.17 Another study9 reported that after 2 years, use of the combination of IPM and allergen reduction techniques (using a vacuum cleaner with an air filter and high-efficiency particle air [HEPA] filter) reduced the amount of mouse allergens on bedroom floors by 27%, whereas the amount of mouse allergens on bedroom floors in control homes increased by 28% during the same period. DiMango et al21 showed that over a 40-week treatment period, allergen reduction methods, including mattress coverings, cleaning, and provision of vacuums, mops, and HEPA air purifiers (without any rodent control) significantly reduced mouse allergen levels in the kitchen versus in the control.
Although environmental intervention trials that included multiple methods (cleaning, vacuuming, mouse control, and resident education) are effective in reducing mouse allergens, no studies have been conducted to evaluate the impact of pest control alone on mouse allergen levels. Furthermore, the multifaceted allergen reduction strategy reported in past studies is not sustainable owing to its high cost. It is especially impractical to implement in low-income communities, in which financial resources are scarce. Past studies have also failed to accurately document the level of mouse infestation before and after interventions or the association between house mouse reduction and reduction of mouse allergen level. A cost-effective and sustainable mouse allergen reduction program requires elimination of the mouse allergen sources (ie, mouse infestations). Therefore, we designed a study to determine whether effective mouse control alone will result in high mouse allergen reduction. We hypothesized that (1) effective mouse control alone would achieve a high level of mouse allergen reduction after 12 or 24 months and (2) a researcher-led mouse control program would result in a greater reduction in the mouse allergen levels than achieved by conventional pest control services.
Methods
Experimental sites and selection of apartments
This research was conducted in Public Housing Authority–managed low-income apartments in New Brunswick, Trenton, and Paterson in New Jersey. In all, 9 buildings in New Brunswick, 6 in Trenton, and 3 in Paterson were randomly allocated to 1 of 3 treatment groups: trapping and baiting (T&B); trapping, baiting, and rodent exclusion (T&B+E); or existing pest control service with no or limited use of bait or glue boards (control). A total of 10 infested apartments from each treatment group and each location were examined for mouse allergens. More details can be found in the Experimental Sites and Selection of Apartments section of the Supplementary Methods (available in the Online Repository at www.jaci-global.org).
Mouse control
The T&B+E intervention included snap traps, rodenticide baits, and exterior and interior mouse proofing. The T&B intervention was similar to T&B+E except without mouse proofing. Residents in T&B+E and T&B groups received an educational flyer at the onset of the study. In the control group, the contractor either did not do any treatment or used glue boards or rodenticides only when residents complained about mice. A detailed description of the T&B intervention, the T&B+E intervention, and the control group can be found in the Mouse Control section in the Supplementary Methods.
Evaluation of mouse control work
Building-wide inspections were conducted at 6, 12, and 24 months after initiation of the treatment programs in New Brunswick and Trenton, whereas the Paterson site had inspections only at 6 and at 12 months. The infested apartments in the T&B and T&B+E groups were visited monthly, with retreatment performed until monitoring of traps and bait stations indicated no new mouse activity.
Dust sample collection and analysis
Baseline dust samples were collected from the kitchens and bedrooms within 1 week after the apartments had been selected. Dust samples were collected using an Atrix backpack vacuum (model VACBP1, Atrix International, Burnsville, Minn) with a HEPA filter. A dust collector (Indoor Biotechnologies, Charlottesville, Va) was fitted into the vacuum hose inlet. In the kitchens, the perimeter of the kitchen floor was vacuumed. In 1 bedroom per apartment, a combined dust sample was collected from the bed, the bedding, and the floor adjacent to the bed. After vacuuming to collect each of the individual dust samples, the filter was removed from the vacuum machine attachment. The tip of the vacuum machine attachment was cleaned and dried. Samples from the kitchen and 1 bedroom of each apartment were labeled and stored separately. They were placed in a cooler with ice packs before being transported to the laboratory. After the samples were transported to the laboratory, they were stored at –20°C until they were sent to the Dermatology, Allergy, and Clinical Immunology Reference Laboratory at Johns Hopkins University. Dust samples were collected from the same apartments at 6 and 12 or 24 months after the intervention. When there was more than 1 bedroom per unit, the same bedroom was sampled across sampling periods. Mus m 1 levels were determined by using mAb-based ELISA within 3 months after sample collection.22, 23, 24 The numbers of samples at each sampling period for each room type at each site are summarized in Table E1 (available in the Online Repository at www.jaci-global.org).
Statistical analysis
The treatment groups (T&B and T&B+E) were combined (into a single intervention group) for the Mus m 1 analyses because there were no differences in mouse infestation levels or Mus m 1 levels between the 2 groups (see Table E2 in the Online Repository at www.jaci-global.org). To analyze the percentage of apartments with Mus m 1 levels higher than 1 μg/g, all of the Mus m 1 data were used. However, when analyzing the geometric mean and reduction of mouse allergen levels, we excluded those records in which Mus m 1 levels were below the clinically relevant threshold level (1 μg/g) at all sampling periods.7
The logarithmically transformed Mus m 1 and percentage of reduction of Mus m 1 levels from 0 to 6 months, 0 to 12 months, and 0 to 24 months between the intervention and control groups were compared by using t tests (PROC TTEST in SAS).25
To analyze the Mus m 1 levels between the kitchens and the bedrooms at month 0, the data from all groups were combined. The logarithmically transformed Mus m 1 levels between the kitchens and bedrooms in each group were analyzed by using a paired t test. The signed rank test was used to analyze seasonal changes (winter vs summer) in Mus m 1 levels in homes without mouse activity. The month 6 data were from the winter season, and the month 12 data were from the summer season.
Results
Comparison of mouse allergen levels
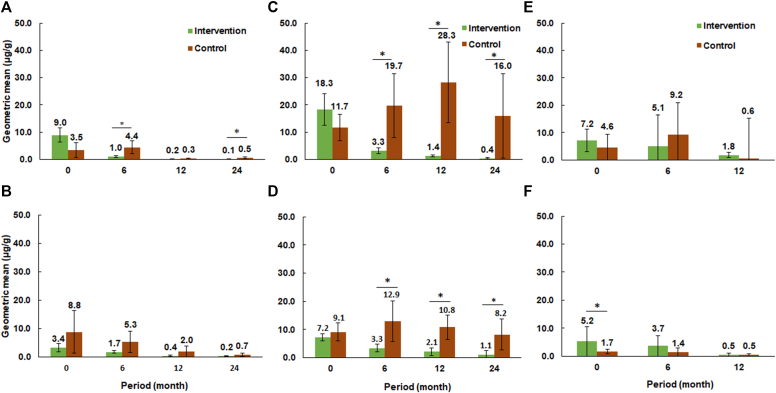
The geometric mean values of Mus m 1 levels in kitchens and bedrooms at the 3 study sites are shown in Fig 1. In New Brunswick, the Mus m 1 level in kitchens was lower in the intervention group than in the control group at month 6 (t = 2.11; df = 25; P = .045) and month 24 (t = 2.94; df = 25; P = .007) (Fig 1, A). No differences were found in the Mus m 1 levels in bedrooms between the intervention and control groups at any of the observation periods (Fig 1, B). From 0 to 24 months, the Mus m 1 levels in kitchens decreased by 98.9% and 85.7% in the intervention and control groups, respectively. The geometric mean levels of Mus m 1 in bedrooms decreased by 93.1% and 91.6% in the intervention and control groups, respectively.
Fig 1.
Changes in the geometric mean of Mus m 1 levels in New Brunswick sampled from kitchens (A) and bedrooms (B); in Trenton sampled from kitchens (C) and bedrooms (D); and in Paterson sampled from kitchens (E) and bedrooms (F). Bars with asterisks indicate statistical differences in logarithmically transformed Mus m 1 levels between the treatments in each period (t test; P < .05).
In Trenton, the Mus m 1 levels in kitchens in the intervention group were lower than in the control group at 6 months (t = 2.99; df = 28; P = .006), 12 months (t = 5.73; df = 28; P < .001), and 24 months (t = 3.97; df = 21; P < .001) (Fig 1, C). The Mus m 1 level in bedrooms in the intervention group were lower than in the controls only at 12 months (t = 2.11; df = 24; P = .046) and 24 months (t = 2.64; df = 20; P = .016) (Fig 1, D). From 0 to 24 months, the mean Mus m 1 levels in kitchens decreased by 97.1% in the intervention group but increased by 36.8% in the control group; the mean Mus m 1 level in bedrooms decreased by 86.1% in the intervention group and 9.9% in the control group.
No differences in Mus m 1 levels between the intervention and control groups were observed in kitchens in Paterson over time (Fig 1, E). Mus m 1 levels in the bedroom were higher in the intervention group than that in the control group at month 0 (t = 2.25; df = 16; P = .039) (Fig 1, F). However, no statistical differences in Mus m 1 levels in the bedroom were found between the intervention and control groups at 6 and 12 months.
In New Brunswick, 90% of apartments had Mus m 1 levels in the kitchen greater than 1 μg/g in the intervention group at month 0. This number decreased to 5% after 24 months (see Table E3 in the Online Repository at www.jaci-global.org). In Trenton, 95% of apartments had kitchen levels of Mus m 1 higher than 1 μg/g in the intervention group at month 0. After 24 months, that number decreased to 38% (see Table E3). In Paterson, 95% of apartments had kitchen levels of Mus m 1 higher than 1 μg/g in the intervention group at month 0. After 12 months, 69% of apartments had Mus m 1 higher than the threshold levels.
Reduction of Mus m 1 levels
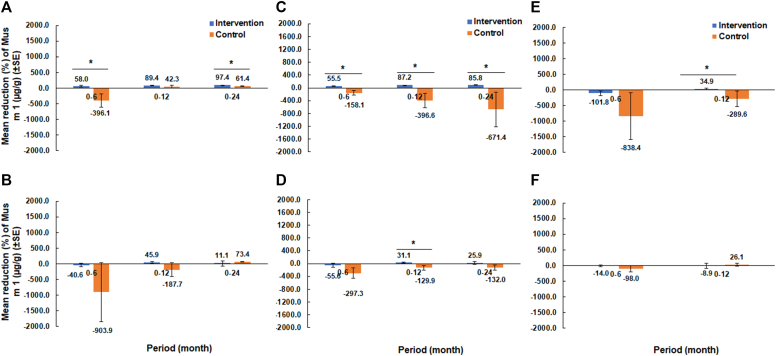
After 12 or 24 months of intervention, greater reduction of Mus m 1 levels in kitchens was observed in the intervention groups than in the control at all 3 cities (Fig 2). However, greater reduction of bedroom levels of Mus m 1 in the intervention group was observed only in Trenton.
Fig 2.
Changes in the percentage of Mus m 1 reduction in New Brunswick sampled from kitchens (A) and bedrooms (B); in Trenton sampled from kitchens (C) and bedrooms (D); and in Paterson sampled from kitchens (E) and bedrooms (F). Asterisks above bars indicate statistical differences between the treatments within each period (t test; P < .05).
In New Brunswick, kitchen levels of Mus m 1 were reduced by 58.0% from 0 to 6 months in the intervention group compared with an increase of 396.1% in the control group (t = 2.95; df = 25; P = .007). Also, a greater reduction in kitchen levels of Mus m 1 was observed from 0 to 24 months, at which time the Mus m 1 level was reduced by 97.4% in the intervention group versus by 61.4% in the control group (t = 4.11; df = 24; P < .001) (Fig 2, A).
In Trenton, the intervention group had an 85.8% reduction in kitchen levels of Mus m 1 after 24 months. Meanwhile, Mus m 1 levels in the control group increased by 671.4%. The changes in Mus m 1 levels between the 2 groups were significantly different at each observation period (P < .001) (Fig 2, C). In bedrooms, the changes in Mus m 1 levels were significantly different between the 2 groups only at month 12 (t = 2.45; df = 23; P = .022) (Fig 2, D).
In Paterson, kitchen levels of Mus m 1 were reduced by 34.9% from 0 to 12 months in the intervention group whereas they increased by 289.6% in the control group (t = 2.14; df = 16; P = .048) (Fig 2, E). However, no significant difference was found in percentage of reduction of bedroom levels of Mus m 1 between the 2 groups (Fig 2, F).
Comparison of Mus m 1 concentrations between the kitchen and the bedroom
The Mus m 1 levels at baseline were higher in the kitchen than in the bedroom in New Brunswick (t = 2.27; df = 15; P = .038) and Trenton (t = 3.31; df = 24; P = .005). However, in Paterson, the Mus m 1 level was similar between the kitchen and the bedroom (t = 1.34; df = 16; P = .200) (Fig 3).
Fig 3.
Comparison of geometric means of Mus m 1 levels between kitchens and bedrooms at 3 study sites before the intervention. Bars with an asterisk indicate significant differences in logarithmically transformed Mus m 1 levels (paired t test; P < .05).
Seasonal changes in mouse allergen levels
Seasonal variation in mouse allergen levels (Mus m 1) was evident at 2 study sites, with notable reductions in allergen concentrations from the 6- to 12-month time points (Fig 4). At the New Brunswick Housing Authority, the mouse allergen level decreased significantly (from 1.69 μg/g at 6 months to 0.41 μg/g at 12 months [S = –121.5; P = .006]). Similarly, in Trenton, the allergen levels dropped from 4.12 μg/g at 6 months to 2.71 μg/g at 12 months (S = –93.5; P = .041). However, in Paterson, despite a decrease in allergen levels from 3.28 μg/g at 6 months to 0.84 μg/g at 12 months, this reduction was not statistically significant (S = –42.5; P = .173).
Fig 4.
Seasonal variation in Mus m 1 levels (μg/g) in units without mouse infestations at the 3 study sites (New Brunswick, Trenton, and Paterson) between the 6-month (December-January) and 12-month (May-June) time points. Bars with an asterisk indicate significant differences in logarithmically transformed Mus m 1 levels.
Discussion
This is the first study comparing the impact of researcher-led mouse control with conventional pest management services on Mus m 1 levels over a 12- or 24-month period. We showed that researcher-led mouse control using simple yet highly effective protocols was much more effective in reducing mouse allergen levels in kitchens than were the conventional pest control services adopted by low-income communities. The difference was most distinct in Trenton, which had the highest mouse activity based on the number of mice caught. The much greater reduction of kitchen levels of Mus m 1 in the treatment group than in the control group was presumably due to a greater level of control in New Brunswick and Trenton (see Table E2). The Paterson site had similar rates of mouse infestation between the treatment groups from 6 months. But still, the intervention group might have been characterized by faster elimination within the first few months than the control as a result of elimination of mice through snap traps and baits. During the intervention period, the numbers of mice caught in the intervention groups in New Brunswick, Trenton, and Paterson were 42, 143, and 19, respectively. The conventional pest control service was calendar based, was reactive (ie, sites were treated only when complaints were received), used ineffective materials (such as glue boards), or relied on only 1 method (ie, a rodenticide only), with little or no follow-up monitoring to confirm pest elimination.15,16 Glue traps are not recognized as an effective tool for controlling mice.26,27 Relying solely on rodenticides is also ineffective in controlling house mouse infestations owing to factors such as bait shyness, genetic resistance, and need for integrated pest management strategies.28,29 Studies have shown that residents often fail to notify property management of pest issues because of their lack of awareness about the problem or their unwillingness to disclose the presence of pests.1
The effect of mouse control on Mus m 1 level reduction is more easily detected in kitchens. The researcher-led mouse control resulted in higher reduction of Mus m 1 levels in bedrooms at only 1 of the 3 sites (Trenton). As mentioned earlier, this site had the highest numbers of mice per apartment. Thus, the effect of mouse control on allergen reduction is more easily detected when the initial mouse numbers are high, which is also true for kitchen allergens. Mice usually deposit urine and feces in kitchens or living rooms in which the food sources are located. Mice usually have a short activity range (on average, 3.6 meters).30
Phipatanakul et al17 found a 78.8% reduction in mouse allergen levels in kitchens during a 5-month period when using an IPM intervention plus multiple allergen reduction procedures (vacuuming, cleaning). In our study, we found 58.0% and 55.5% reductions in Mus m 1 levels in kitchens at the New Brunswick and Trenton sites, respectively, after 6 months. The percentages of reduction increased to 97.4 and 85.8% at the end of the study in New Brunswick and Trenton after 24 months, respectively. The benefit of pest control alone for Mus m 1 level reduction in Paterson appears low, with only a 35% reduction in kitchens after 12 months. However, there was a 289.6% increase in Mus m 1 levels in kitchens in the control group. Overall, the impact of pest control alone on Mus m 1 level reduction was most profound in Trenton, where rodent activity had been eliminated in the intervention group; in contrast, 67% of the apartments still had mouse activity in the control group at the end of the study. There are many differences between our study and previous studies. Phipatanakul et al17 conducted home visits at 0, 1, 3, and 5 months. At each home visit, they vacuumed with HEPA filters and cleaned surfaces with mild detergents. A study by Matsui et al19 included targeted cleaning to remove reservoirs, installation of allergen-proof mattress and pillow encasements, and use of 2 portable air purifiers, resulting in a 67% reduction in level of Mus m 1 from the bedroom floor with a 12-month intervention. These enhanced allergen reduction techniques contributed to the faster reduction in Mus m 1 level than that in our study. However, including multiple cleaning procedures at each visit by researchers involves a much higher cost than using pest control alone, and it is not sustainable. Another study by Kass et al18 found that a 1-time intervention including pest control and cleaning did not reduce mouse populations or mouse allergen levels at 3 months and 6 months after treatment in either the kitchen or the bedroom, likely owing to the fact that the study did not effectively reduce the presence of mice.
Ahluwalia et al5 stated that the clinically relevant threshold level for Mus m 1 is 1 μg/g. Another study by Phipatanakul et al4 found that mouse allergen levels higher than 1.6 μg/g have a higher risk of mouse sensitization than levels less than 1.6 μg/g do. Because kitchen allergen levels are higher than bedroom levels and are more sensitive to mouse interventions, it is intuitive to monitor the kitchen allergen levels over time. In New Brunswick, the percentage of apartments with Mus m 1 levels in their kitchens that were higher than 1 μg/g was reduced from 90% to 5%, whereas in the control group, the percentage decreased from 60% to 30% over 24 months (see Table E3). In Trenton, the percentage of apartments with Mus m 1 levels in the kitchen higher than 1 μg/g was reduced from 95% to 38%, whereas in the control group, the percentage was reduced from 90% to 71% over 24 months. Thus, intervention alone could have significant clinical benefits. But a 24-month period or longer may be necessary to reduce mouse allergen levels below this threshold in most of the apartments. As shown in Table E3, the percentage of apartments with Mus m 1 levels in the kitchen higher than 1 μg/g continues to decrease beyond 12 months in the intervention group. Had the 24-month data been collected in Paterson, we expect that the same trend as in New Brunswick and Trenton would have been observed. These variances in reduction of Mus m 1 level among the different time periods suggest that significant amounts of Mus m 1 can remain for more than 12 months from the beginning of the intervention. A longer observation period (>12 months) may thus be needed to determine the speed of natural Mus m 1 level reduction. Phipatanakul et al17 demonstrated that mouse allergen levels may take time to be reduced effectively. Our study supports their conclusion that when our protocols are used, it takes a long time (many months) to reduce the allergen levels below the threshold. Incorporating resident education on the importance of house cleaning to reduce indoor allergens could help shorten the period required to reduce levels of Mus m 1 allergen below the threshold. From a clinical standpoint, prolonged allergen persistence highlights the importance of sustained and comprehensive interventions to achieve reductions in exposure below sensitization thresholds. This is especially relevant given that cumulative exposure over time is a critical factor in allergic sensitization and asthma severity.31,32 In addition to direct pest control, resident education on allergen mitigation strategies, such as performing routine cleaning with HEPA filters, storing food properly, and sealing potential allergen reservoirs, could further accelerate allergen reductions and amplify the clinical benefits of interventions. Evidence from previous studies suggests that incorporating such environmental modifications alongside pest management can yield more durable and widespread reductions in allergen levels.33,34
We found significant reductions in mouse allergen levels from the winter (December-January) to the summer months (May-June) at the New Brunswick and Trenton sites. These findings suggest that without the presence of house mouse infestations, mouse allergen levels may reduce naturally owing to degradation and resident housekeeping activities, but this change could be very slow and is not significant within a short period, as shown in Paterson. This variation may be due to local environmental factors, building conditions, or differences in mouse infestation levels before the initial sampling. Studies have shown that allergen levels can fluctuate seasonally due to changes in rodent behavior, environmental conditions, and indoor housing factors, which may influence the persistence and distribution of allergens in homes.8 The research of Matsui et al35 indicates that settled dust allergen levels are higher in the spring than in the other seasons. Matsui et al35 suggest that the higher dust allergen levels in the spring may be a result of increased mouse infestation in the fall and winter and subsequent accumulation of allergens after a few months. However, this study did not document the presence of mouse infestation in the sampled homes when dust samples were collected. As the current and previous studies have indicated, indoor levels of dust mouse allergen are associated with rodent activities.13,14 Our results confirm that without mouse activity, mouse allergen levels will likely decrease over a period of a few months. This reduction is most likely due to natural degradation and resident cleaning activity rather than to a seasonal effect. A longer period of dust sampling in the same homes without house mouse activity would be ideal to accurately predict the effect of season and time on mouse allergen levels in the home environment.
Given the relatively small number of allergen samples analyzed at the 12-month follow-up, especially in Paterson and in the control groups, the potential for type II error should be considered when interpreting city-specific trends. Although reductions in allergen levels were observed in some locations, the large variance in baseline Mus m 1 levels, which is consistent with the findings of earlier large-scale surveys, suggests that such differences may partly reflect the natural fluctuation rather than the intervention effects alone. This study serves as a valuable foundation for future research and highlights the need for larger-scale investigations to confirm these patterns. Furthermore, the variation in outcomes may also be related to differences in pest control contractor performance. Unlike studies with centralized, highly monitored pest control efforts, this project worked with multiple vendors, which may have introduced inconsistencies in service delivery. To improve future public health programs, we recommend that pest control contracts include clear performance expectations, incentives for high compliance, and active oversight mechanisms to ensure consistent implementation across sites.
We conclude that an effective mouse control program alone can significantly reduce levels of Mus m 1, outperforming conventional pest control in low-income communities. Its effectiveness is comparable to that of IPM combined with allergen reduction procedures, but it requires a longer time frame. Similarly, a 12-month study found that effective cockroach control alone resulted in mean reductions of the levels of Blattella germanica 1 and Blattella germanica in settled dust of 96% and 90%, respectively.36 Adopting an effective pest control protocol is more economical and sustainable for long-term management of environmental risks associated with mice and other pests (such as cockroaches). Therefore, in future indoor pest allergen reduction studies, greater emphasis must be placed on effective pest control. Incorporating other allergen reduction methods will be necessary to reduce the allergen levels below the clinically relevant levels faster. Documenting the cost-effectiveness of allergen reduction and the clinical benefit of such strategies will also be important to advance our knowledge of sustainable indoor allergen reduction strategies in low-income communities.
This article is New Jersey Experiment Station publication no. D-08-08127-01-24.
Disclosure statement
Supported by the US Department of Housing and Urban Development (grant NJHHU005-20) and the National Institute of Food and Agriculture, US Department of Agriculture (Hatch accession no. 1019198), through the New Jersey Agricultural Experiment Station.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Acknowledgments
We would like to thank the New Brunswick Housing Authority, Trenton Housing Authority, and Paterson Housing Authority for their support and staff assistance in conducting the experiment.
Supplementary data
References
- 1.Abbar S., Cooper R., Ranabhat S., Pan X., Sked S., Wang C. Prevalence of cockroaches, bed bugs, and house mice in low-income housing and evaluation of baits for monitoring house mouse infestations. J Med Entomol. 2022;59:940–948. doi: 10.1093/jme/tjac035. [DOI] [PubMed] [Google Scholar]
- 2.Sked S., Abbar S., Cooper R., Corrigan R., Pan X., Ranabhat S., et al. Monitoring and controlling house mouse, Mus musculus domesticus, infestations in low-income multi-family dwellings. Animals. 2021;11:648. doi: 10.3390/ani11030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Rahman E.H., Abdelgadir M., AlRashidi M. Ectoparasites burden of house mouse (Mus musculus linnaeus, 1758) from Hai’l region, Kingdom of Saudi Arabia. Saudi J Biol Sci. 2020;27:2238–2244. doi: 10.1016/j.sjbs.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phipatanakul W., Eggleston P.A., Wright E.C., Wood R.A. Mouse allergen. II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J Allergy Clin Immunol. 2000;106:1075–1080. doi: 10.1067/mai.2000.110795. [DOI] [PubMed] [Google Scholar]
- 5.Matsui E.C., Simons E., Rand C., et al. Airborne mouse allergen in the homes of inner-city children with asthma. J Allergy Clin Immunol. 2005;115:358–363. doi: 10.1016/j.jaci.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Ahluwalia S.K., Matsui E.C. Indoor environmental interventions for furry pet allergens, pest allergens, and mold: looking to the future. J Allergy Clin Immunol Pract. 2018;6:9–19. doi: 10.1016/j.jaip.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahluwalia S.K., Peng R.D., Breysse P.N., Diette G.B., Curtin-Brosnan J., Aloe C., et al. Mouse allergen is the major allergen of public health relevance in Baltimore City. J Allergy Clin Immunol. 2013;132:830–835. doi: 10.1016/j.jaci.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phipatanakul W., Eggleston P.A., Wright E.C., Wood R.A., Mouse allergen I. The prevalence of mouse allergen in inner-city homes. The National Cooperative Inner-City Asthma Study. J Allergy Clin Immunol. 2000;106:1070–1074. doi: 10.1067/mai.2000.110796. [DOI] [PubMed] [Google Scholar]
- 9.Pongracic J.A., Visness C.M., Gruchalla R.S., Evans I.I.I.R., Mitchell H.E. Effect of mouse allergen and rodent environmental intervention on asthma in inner-city children. Ann Allergy Asthma Immunol. 2008;101:35–41. doi: 10.1016/S1081-1206(10)60832-0. [DOI] [PubMed] [Google Scholar]
- 10.Torjusen E.N., Diette G.B., Breysse P.N., Curtin-Brosnan J., Aloe C., Matsui E.C. Dose-response relationships between mouse allergen exposure and asthma morbidity among urban children and adolescents. Indoor Air. 2013;23:268–274. doi: 10.1111/ina.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsui E.C., Eggleston P.A., Buckley T.J., Krishnan J.A., Breysse P.N., Rand C.S., et al. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann Allergy Asthma Immunol. 2006;97:514–520. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- 12.Matsui E.C., Wood R.A., Rand C., Kanchanaraksa S., Swartz L., Eggleston P.A. Mouse allergen exposure and mouse skin test sensitivity in suburban, middle-class children with asthma. J Allergy Clin Immunol. 2004;113:910–915. doi: 10.1016/j.jaci.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 13.Cohn R.D., Arbes S.J., Jr., Yin M., Jaramillo R., Zeldin D.C. National prevalence and exposure risk for mouse allergen in US households. J Allergy Clin Immunol. 2004;113:1167–1171. doi: 10.1016/j.jaci.2003.12.592. [DOI] [PubMed] [Google Scholar]
- 14.Curtin-Brosnan J., Matsui E.C., Breysse P., McCormack M.C., Hansel N.N., Tonorezos E.S., et al. Parent report of pests and pets and indoor allergen levels in inner-city homes. Ann Allergy Asthma Immunol. 2008;101:517–523. doi: 10.1016/S1081-1206(10)60291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C., Abou El-Nour M.M., Bennett G.W. Survey of pest infestation, asthma, and allergy in low-income housing. J Community Health. 2008;33:31–39. doi: 10.1007/s10900-007-9064-6. [DOI] [PubMed] [Google Scholar]
- 16.Corrigan R.M., Rats and Mice, Mallis A. 10th ed. Mallis Handbook LLC; Cleveland, OH: 2011. Handbook of pest control; pp. 11–149. [Google Scholar]
- 17.Phipatanakul W., Cronin B., Wood R.A., Eggleston P.A., Shih M.C., Song L., et al. Effect of environmental intervention on mouse allergen levels in homes of inner-city Boston children with asthma. Ann Allergy Asthma Immunol. 2004;92:420–425. doi: 10.1016/S1081-1206(10)61777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kass D., McKelvey W., Carlton E., Hernandez M., Chew G., Nagle S., et al. Effectiveness of an integrated pest management intervention in controlling cockroaches, mice, and allergens in New York City public housing. Environ Health Perspect. 2009;117:1219–1225. doi: 10.1289/ehp.0800149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsui E.C., Perzanowski M., Peng R.D., Wise R.A., Balcer-Whaley S., Newman M., et al. Effect of an integrated pest management intervention on asthma symptoms among mouse-sensitized children and adolescents with asthma: a randomized clinical trial. JAMA. 2017;317:1027–1036. doi: 10.1001/jama.2016.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner B.L., Markowitz S., Rivera M., Romero H., Weeks M., Sanchez E., et al. Integrated pest management in an urban community: a successful partnership for prevention. Environ Health Perspect. 2003;111:1649. doi: 10.1289/ehp.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiMango E., Serebrisky D., Narula S., Shim C., Keating C., Sheares B., et al. Individualized household allergen intervention lowers allergen level but not asthma medication use: a randomized controlled trial. J Allergy Clin Immunol Pract. 2016;4:671–679.e4. doi: 10.1016/j.jaip.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton R.G., Chapman M., Platts-Mills T.A., Adkinson N.F. House dust aeroallergen measurements in clinical practice: a guide to allergen-free home and work environments. Immunol Allergy Pract. 1992;14:96–112. [Google Scholar]
- 23.Huss K., Adkinson N.F., Jr., Eggleston P.A., Dawson C., Van Natta M.L., Hamilton R.G. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol. 2001;107:48–54. doi: 10.1067/mai.2001.111146. [DOI] [PubMed] [Google Scholar]
- 24.Pate A.D., Hamilton R.G., Ashley P.J., Zeldin D.C., Halsey J.F. Proficiency testing of allergen measurements in residential dust. J Allergy Clin Immunol. 2005;116:844–850. doi: 10.1016/j.jaci.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 25.SAS Institute . SAS Institute; Cary, NC: 2013. SAS/STAT 14.3 user’s guide. [Google Scholar]
- 26.Frantz S.C., Padula C.M. In: Vertebrate pest control and management materials: fourth symposium, ASTM STP 817. American Society for Testing and Materials. Kaukeinen D.E., editor. ASTM International; Philadelphia, PA: 1983. A laboratory test method for evaluating the efficacy of glueboards for trapping house mice; pp. 209–225. [Google Scholar]
- 27.Corrigan R.M. 1998. The efficacy of glue traps against wild populations of house mice, mus domesticus rutty. Proceedings of the vertebrate pest conference. [Google Scholar]
- 28.Buckle A.P., Smith R.H. 2nd ed. CAB International; Wallingford, UK: 2015. Rodent pests and their control. [Google Scholar]
- 29.Meehan A.P. Rentokil Ltd; UK: 1984. Rats and mice: their biology and control. East Grinstead. [Google Scholar]
- 30.Young H., Strecker R.L., Emlen J.T. Localization of activity in two indoor populations of house mice, Mus musculus. J Mammal. 1950;31:403–410. [Google Scholar]
- 31.Arbes S.J., Cohn R.D., Yin M., Muilenberg M.L., Burge H.A., Friedman W., et al. House dust mite allergen in U.S. beds: results from the first national survey of lead and allergens in housing. J Allergy Clin Immunol. 2003;111:408–414. doi: 10.1067/mai.2003.16. [DOI] [PubMed] [Google Scholar]
- 32.Rosenstreich D.L., Eggleston P., Kattan M., Baker D., Slavin R.G., Gergen P., et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 33.Eggleston P.A., Breysse P.N., Randolf C.E., Kattan M., Mitchell H.E., Buckley T.J., et al. The effects of cockroach and dust mite allergens on inner-city children with asthma. J Allergy Clin Immunol. 1999;104:1058–1063. [Google Scholar]
- 34.Wood R.A., Randolf C.E., Kattan M., Eggleston P.A., Fuhlbrigge A., Gergen P.J., et al. The relationship between cockroach allergen and asthma morbidity in inner-city children. J Allergy Clin Immunol. 2008;122:557–563. [Google Scholar]
- 35.Matsui E.C., Eggleston P.A., Breysse P., Diette G.B. Mouse allergen levels vary over time in inner-city homes. J Allergy Clin Immunol. 2007;120:956–959. doi: 10.1016/j.jaci.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 36.Wang C., Eiden A., Cooper R., Zha C., Wang D., Hamilton R.G. Abatement of cockroach allergens by effective cockroach management in apartments. J Allergy Clin Immunol Pract. 2020;8:3608–3609. doi: 10.1016/j.jaip.2020.06.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.