Abstract
Aims
Metabolic syndrome (MetS) increases the risk of atrial arrhythmias (AA). Hypokalaemia, often secondary to diuretics or diet, frequently coexists with MetS and influences calcium handling. This study investigates the role of apamin-sensitive small-conductance calcium-activated potassium (SK) channels in atrial arrhythmogenesis under hypokalaemic MetS conditions.
Methods and results
High-fat diets (HFD) were fed to mice at 8 weeks old for 16 weeks to mimic MetS. SK current was measured via voltage clamp, and Kcnn1–Kcnn3/CACNA1S, CACNA1C, and CACNA1D expression was assessed by qRT–PCR. We used optical mapping of intact mice hearts to assess the atrial action potential duration (APD), calcium transient duration (CaTD), conduction velocity (CV), conduction vector standard deviation, and AA inducibility in mice during hypokalaemia. HFD mice showed increased SK current and Kcnn3 expression. Apamin prolonged APD significantly in HFD mice. The APD heterogeneity of HFD was higher than that of the control mice. APD alternans threshold was significantly increased after apamin administration in HFD than that in control mice. Heterogeneity of conduction vectors in HFD is greater than that in control mice after apamin application. After apamin injection, AA inducibility is higher in HFD than that in control mice. AA inducibility was higher after apamin application in HFD, where AA was successfully induced.
Conclusion
Our study demonstrated that apamin-sensitive SK channels may exert an antiarrhythmic effect in a hypokalaemic heart of the MetS model. These findings underscore the importance of evaluating the potential risks associated with the use of SK channel blockers in patients with hypokalaemic MetS.
Keywords: Metabolic syndrome, Hypokalaemia, SK channel, Atrial arrhythmias, Optical mapping
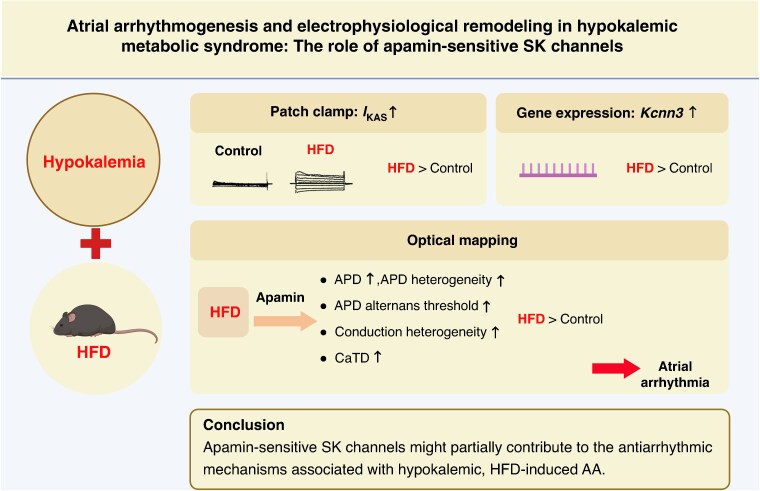
Graphical Abstract
Graphical Abstract.
What’s new?
Atrial apamin-sensitive SK channels are upregulated in hypokalaemic hearts of high-fat diet (HFD)-fed mice.
Action potential duration heterogeneity, alternans threshold, and conduction vector heterogeneity are significantly increased after SK channel blockade with apamin in hypokalaemic hearts of HFD mice.
After apamin application, atrial arrhythmia induction is significantly higher in hypokalaemic hearts of HFD mice, suggesting that the SK channel may play an antiarrhythmic role in a hypokalaemic metabolic syndrome model.
The potential clinical consequence is the induction of atrial arrhythmia in patients with metabolic syndrome treated with SK channel blockers when concurrent hypokalaemia is noticed.
When patients with metabolic syndrome need the SK channel blocker treatment, plasma potassium levels should be carefully monitored, and hypokalaemia should be avoided.
Introduction
The rising prevalence of metabolic syndrome (MetS) in modern society might be related to cardiac arrhythmias.1 Hypokalaemia and MetS are a common comorbidity, as many patients with MetS are treated with diuretics, leading to hypokalaemia,2 which can increase the risk of arrhythmias.3 Furthermore, patients with MetS have been shown to exhibit significant kaliuresis within the first 2 weeks of ingesting a low-carbohydrate diet.4 However, previous studies have focused only on the association between hypokalaemia or MetS alone and arrhythmias,5,6 rather than discussing the arrhythmogenic mechanism related to the interaction between MetS and hypokalaemia. Therefore, we anticipated investigating the underlying mechanism of the atrial arrhythmogenesis via the interaction between MetS and hypokalaemia.
The small-conductance Ca2+-activated K+ (SK) channel is a calcium-sensitive potassium channel primarily expressed in atria. There are three different types of SK channels, SK1–SK3, of which SK2 and SK3 are sensitive to apamin, a selective SK channel blocker, with SK2 demonstrating the highest affinity.7 SK channels are believed to modulate action potential duration and intracellular calcium homeostasis, thereby contributing to the initiation of atrial fibrillation (AF).8 Other studies have shown that hypokalaemia can affect intracellular calcium dynamics,9,10 which we hypothesize may alter SK channel activity and trigger AF.11 However, the synergy between MetS, hypokalaemia, and the SK channel remains unclear. Therefore, we conducted this study to investigate how the interaction between hypokalaemia and MetS modulates SK channel activity and promotes AF.
Methods
The study protocol has been approved by the Institutional Animal Care and Use Committee of Kaohsiung Medical University and complied with the Guide for the Care and Use of Laboratory Animals.
Animal model, cell preparation, and cellular electrophysiological study
Male C57BL/6 mice, aged 8–10 weeks, were used in this study. The mice were randomly assigned to two groups fed the following diets: (i) a regular diet (76% carbohydrate, 10% fat, and 14% protein) and (ii) a high-fat diet (26% carbohydrate, 60% fat, and 14% protein), for 4 months before sacrifice. High-fat diet (HFD) mice were used to mimic MetS in humans.
The atrial myocyte isolation protocol followed our previous study.12 The isolated atrial myocytes were then prepared for patch-clamp studies. We used the whole-cell patch-clamp technique to assess the SK current in hypokalaemic HFD atrial myocytes.13 Please refer to the online supplement for more details.
Optical mapping and induction of atrial arrhythmia
We used optical mapping to assess intact mice hearts’ membrane potential (Vm) and calcium transient (Cai). After establishing Langendorff perfusion, the heart was stained with 20 μL of the voltage-sensitive dye RH237 (Invitrogen) (1.25 mg/mL) for Vm mapping and RHOD-2 AM (Invitrogen) (1 mg/mL) for Cai mapping. Optical signals were processed with spatial (3 × 3 pixel Gaussian filter) and temporal (three-frame moving average) filtering.
We recorded the optical mapping signal after 100 beats of stable pacing at each pacing cycle length (PCL). The heart was paced from PCL 150 to 50 ms until loss of capture. After resting for 5 min, the heart was stimulated by nine pacing bursts from PCL 20 to 40 ms to evaluate the atrial arrhythmias (AA) inducibility. AA was defined as atrial tachycardia or AF lasting >1 s. Then, apamin was added and washed for 10 min. We repeated the pacing protocol above to evaluate the effect of SK channel blockade on action potential duration (APD) and calcium transient duration (CaTD) of right atria (RA). APD80 was measured as 80% repolarization of APD. The CaTD at 80% (CaTD80) compared with baseline was also analysed. APD alternans was defined as the difference in APD measured at APD80 of two consecutive beats of ≥4 ms. Cai alternans was defined as the beat-to-beat change in Cai amplitude ≥5%.14 The isochrone map at PCL 150 ms was used to calculate the conduction velocity (CV) and the standard deviation (SD) of the conduction angles (STDangle).15
mRNA collection and qRT–PCR analysis
The hearts were collected following Langendorff perfusion for subsequent mRNA analysis. The online Supplementary material provides detailed information.
Statistical analysis
Numerical variables were summarized as mean ± SD. Variables were compared with the Wilcoxon signed-rank test or the Mann–Whitney test for the data within and between groups. Categorical parameters are presented as percentages and compared with the McNemar or Fisher exact tests for the data within and between groups. Data were analysed using a repeated-measures mixed-effects model, as the same set of analysis units was exposed to multiple interventions in the patch-clamp study. A two-tailed P value of ≤0.05 was considered statistically significant.
Results
I KAS upregulation in hypokalaemic hearts of high-fat diet mice
The apamin-sensitive small-conductance calcium-activated potassium current (IKAS) was assessed utilizing whole-cell patch clamp on atrial myocytes isolated from CTL and HFD mice. The current–voltage (I–V) relationship was investigated using voltage steps. Representative IKAS traces, obtained by applying voltage steps ranging from −120 to +80 mV with a holding potential of −60 mV, are depicted in Figure 1A. The current density (I[pA/pF]) vs. voltage plot (Figure 1B) demonstrates a significantly higher IKAS current density in the HFD group (n = 6 vs. 7, P = 0.001), indicating an upregulation of IKAS within the atrial myocytes of HFD mice under hypokalaemic condition.
Figure 1.
Increased apamin-sensitive current and expression of Kcnn gene in hypokalaemic hearts of HFD mice. (A) The representative voltage-clamp tracings of atrial myocytes from CTL and HFD mice under hypokalaemic condition. The free Ca2+ 500 nmol/L was used in the patch-clamp study. (B) The current density–voltage relationship of the apamin-sensitive current of atrial myocyte from CTL and HFD mice under hypokalaemic condition. The apamin-sensitive current is significantly prominent in the HFD group (n = 6 vs. 7, P = 0.001). (C) The results indicated that Kcnn3 expression was significantly upregulated in the HFD group, while Kcnn1 and Kcnn2 exhibited upward trends. (D) There is no significant difference in gene regulation in CACNA1S, CACNA1C, and CACNA1D after feeding HFD.
Gene expression of Kcnn1– Kcnn3 and CACNA1S, CACNA1C, and CACNA1D
The gene expression of Kcnn1–Kcnn3 and CACNA1S, CACNA1C, and CACNA1D was conducted. The results indicated that Kcnn1 and Kcnn2 exhibited upward trends, while Kcnn3 expression was significantly upregulated in the HFD group (P = 0.184, 0.141, and 0.041, respectively; n = 3 vs. 3) (Figure 1C). However, there is no significant difference in gene regulation in CACNA1S, CACNA1C, and CACNA1D after feeding HFD (Figure 1D), suggesting the role of SK channels in arrhythmia modulation.
Atrial optical mapping protocol
The experimental protocol for optical mapping is depicted in Figure 2. Figure 2A shows the time frame of the Langendorff perfused heart from the normokalaemic to the hypokalaemic Tyrode’s solution. Figure 2B illustrates a representative heart during optical mapping, with the bipolar pacing lead placed on the RA and the unipolar electrode placed on the left atrium (LA) for LA electrogram (LAE) recording.
Figure 2.
The illustration of the optical mapping protocol. (A) Overview of the optical mapping protocol. Experiments were initially conducted under normokalaemic conditions. Once the mouse heart stabilized, hypokalaemia was induced. Optical mapping measurements were first obtained under baseline hypokalaemic conditions, followed by recordings after apamin administration. (B) Representative image of a heart used in the optical mapping study, showing the pacing lead positioned on the RA and the LAE electrode placed on the LA.
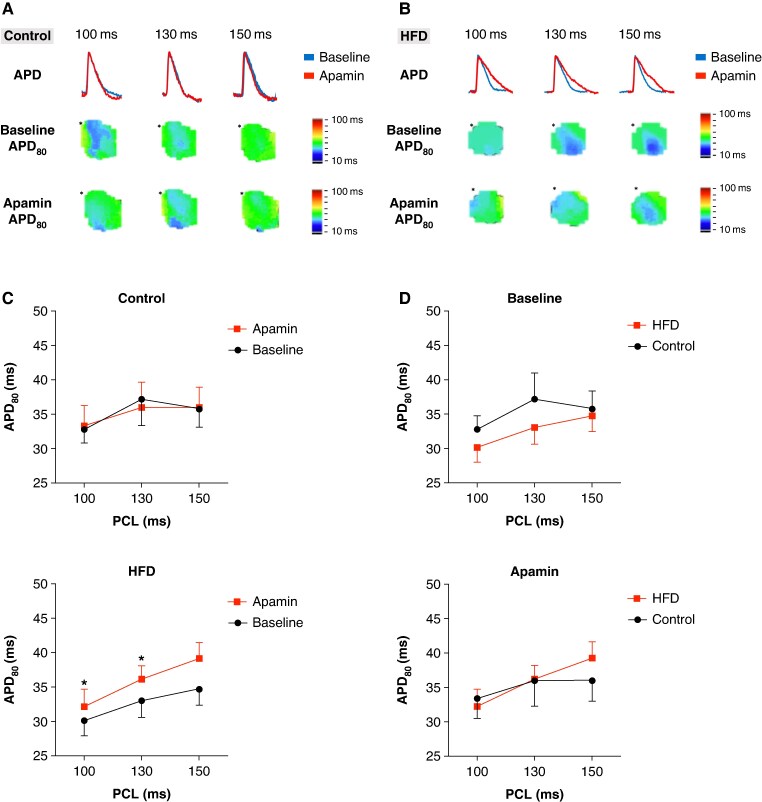
Action potential duration changes and SK upregulation
Figures 3A and B present representative examples of action potential tracing (top panel) and Vm map before (middle panel) and after (bottom panel) apamin application in control and HFD mice, respectively. The asterisks indicate the pacing sites. APD prolongation was observed in HFD mice following apamin application, as shown in Figure 3B. Figure 3C demonstrates that the apamin application significantly increased the APD80 of HFD mice at PCL 100 and 130 ms (30.2 ± 7.4 vs. 32.2 ± 8.0 and 33.1 ± 7.7 vs. 36.2 ± 6.6 ms, P = 0.047 and 0.028, n = 9 vs. 11, respectively), but not in control mice, indicating that SK channel upregulation occurred in hypokalaemic hearts of HFD. Figure 3D shows no significant difference between the APD80 of HFD and the control mice before and after apamin application.
Figure 3.
The APD prolonged after apamin application in HFD mice. (A) Representative example of action potential tracings (top panel) and Vm map changes before (middle panel) and after (bottom panel) apamin application in the control group. The asterisk indicates the pacing site. (B) Representative example of action potential tracings (top panel) and Vm map changes before (middle panel) and after (bottom panel) apamin application in the HFD group. The asterisk indicates the pacing site. (C) Hypokalaemic hearts of HFD mice have significantly prolonged APD80 after apamin application at PCL 100 and 130 ms. (D) There is no significant difference in APD between HFD and control mice at baseline and after apamin conditions.
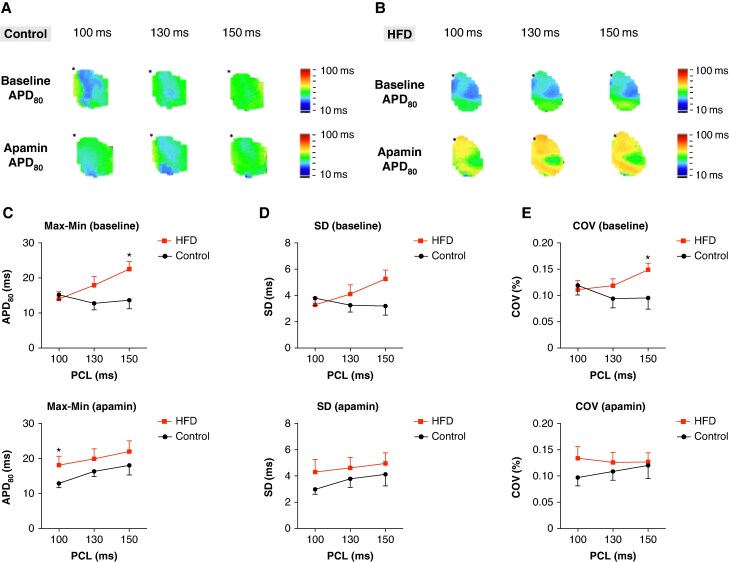
Action potential duration heterogeneity
Figures 4A and B display representative Vm maps illustrating APD heterogeneity in control and HFD mice before and after apamin application. Figures 4C, D, and E show the maximum minus minimum (Max–Min) value, SD, and coefficient of variation (COV) of APD80 within the analysed atrial region at PCL 150, 130, and 100 ms, both before (top panels) and after (bottom panels) apamin application. The Max–Min was significantly higher in HFD than that in control mice at PCL 150 ms (22.5 ± 7.4 vs. 15.0 ± 8.0, n = 11 vs. 9, P = 0.016). After apamin application, Max–Min was significantly higher in HFD than that in control mice at PCL 100 ms (18.10 ± 8.1 vs. 13.29 ± 3.9, n = 11 vs. 9, P = 0.035) (Figure 4C). Figure 4D shows a non-significant trend towards increased SD of APD in HFD mice. Notably, HFD mice had a significantly higher COV than that in controls at PCL 150 ms prior to apamin application (0.148 ± 0.04 vs. 0.095 ± 0.06, n = 11 vs. 9, P = 0.046). The results indicate that APD heterogeneity is greater in HFD compared with that in controls.
Figure 4.
Increased APD heterogeneity in HFD mice. (A, B) The representative Vm map of APD heterogeneity before (top panel) and after (bottom panel) apamin application in control and HFD mice, respectively. (C) Maximum minus minimum value of APD of RA in HFD and control mice. The result shows greater APD heterogeneity in HFD mice at PCL 150 ms at baseline and PCL 100 ms after apamin application. (D) APD SD of RA in HFD and control mice. The result shows no significant difference between the two groups of mice. (E) COV of APD of RA in HFD and control mice. The result shows greater APD heterogeneity of HFD at PCL 150 ms at baseline (*P < 0.05).
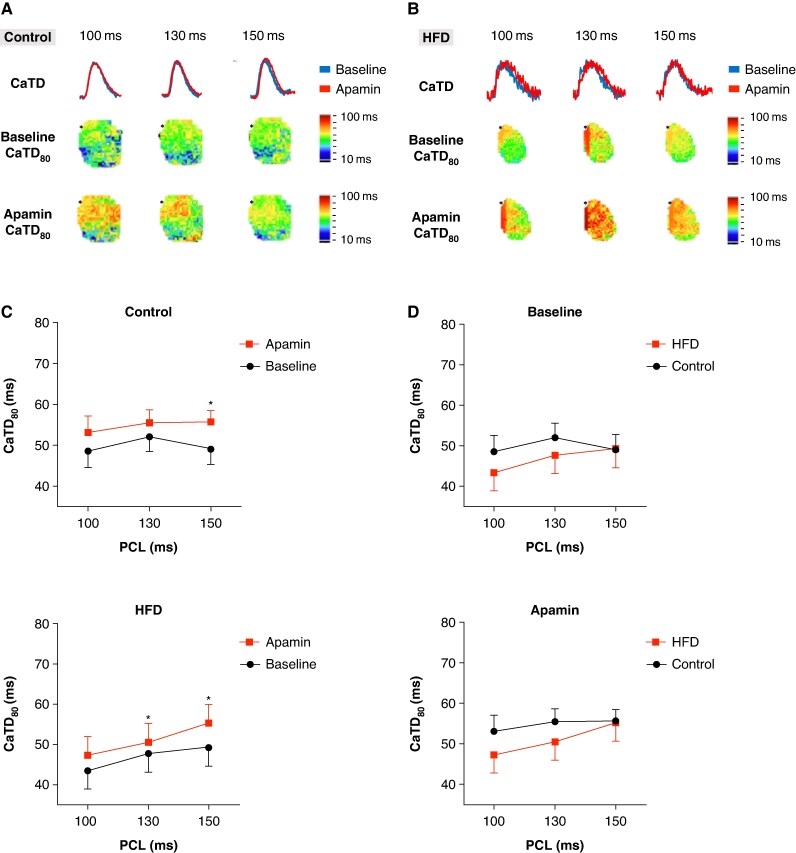
Effect of apamin on calcium transient duration
Figures 5A and B present representative examples of CaTD tracings (top panels) and typical calcium (Cai) maps before (middle panels) and after (bottom panels) apamin application in the right atrium (RA) of control and HFD mice, respectively. Figure 6C shows that HFD mice exhibited a significant prolongation of CaTD80 following apamin administration at pacing cycle lengths (PCL) of 150 and 130 ms (49.3 ± 15.4 vs. 55.30 ± 15.4 and 47.70 ± 14.4 vs. 50.54 ± 15.5 ms, n = 9 vs. 11, P = 0.041 and 0.037, respectively). Control mice also showed a significant prolongation of CaTD80 after apamin application at PCL 150 ms (49.1 ± 11.0 vs. 55.7 ± 8.3, n = 9 vs. 9, P = 0.025). Consistent with our previous findings in normokalaemic HFD mice,12 these results suggest that SK channels also affect Cai in hypokalaemic hearts of HFD mice. No significant differences in CaTD were observed between HFD and control mice before or after apamin treatment (Figure 5D). Collectively, these findings indicate that SK channel inhibition may modulate Cai and contribute to altered calcium homeostasis.
Figure 5.
Increased CaTD after apamin administration in HFD mice. (A, B) A representative example of the Cai map at the RA of control and HFD mice, respectively. The asterisk indicates the pacing site. (C) CaTD is significantly prolonged after apamin application in control mice at PCL 150 ms. We also find that HFD mice have a significant prolongation of CaTD after apamin application at PCL 150 and 130 ms. (D) No significant difference in CaTD between HFD and control mice before or after apamin status.
Figure 6.
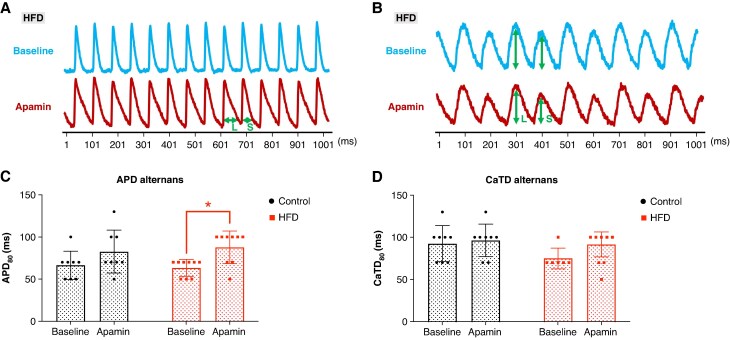
Increased APD alternans threshold after apamin application in HFD mice. (A) Representative example of APD alternans observed after apamin administration but not at baseline in an HFD mouse. The double-headed arrows indicate the long (L) and short (S) APD, demonstrating APD alternans. (B) A representative example of CaTD alternans is seen at baseline and after apamin. The double-headed arrows indicate the large (L) and small (S) CaTD, demonstrating CaTD alternans. (C) The APD alternans threshold significantly increases after apamin application only in HFD mice. (D) The CaTD alternans threshold is not different after apamin application in both groups.
Effect of apamin on action potential duration alternans threshold
Figures 6A and B show representative examples of APD and CaTD alternans before (upper panel) and after (lower panel) apamin administration in HFD mice. APD alternans became more pronounced in the HFD mouse after apamin application. As shown in Figure 6C, the APD alternans threshold significantly increased in HFD mice following apamin administration (63.3 ± 10.0 and 87.8 ± 19.2 ms, n = 9 vs. 9, P = 0.038) but not in control mice (75.6 ± 32.0 and 82.5 ± 25.5, n = 9 vs. 8, P = 0.066) (Figure 6C). Although the CaTD alternans threshold did not reach statistical significance in either group, a non-significant increase was observed in HFD mice (75.0 ± 12.2 and 91.4 ± 14.6 ms, n = 6 vs. 7, P = 0.083) (Figure 6D). These results may indicate the proarrhythmic effect of SK channel blockade in HFD under hypokalaemia.
Effect of apamin on the conduction velocity heterogeneity
Figure 7A presents isochrone maps (right panels) obtained via optical mapping, alongside corresponding CV and SD of conduction angles (STDangle) analyses (left panels).15 No significant differences in CV were found between HFD and control mice, either before or after apamin administration (Figure 7B). However, the STDangle was significantly higher in HFD than that in control mice after apamin administration (1.03 ± 0.55 vs. 0.52 ± 0.07, n = 9 vs. 9, P = 0.038), indicating that the heterogeneity of CV was more pronounced in HFD after apamin administration (Figure 7C).
Figure 7.
Increased conduction heterogeneity after apamin in HFD mice. (A) The combination of the isochrone and CV angle map of a control and an HFD mouse. The arrows in the CV angle map indicate the direction of impulse propagation. The HFD mouse has a more heterogeneous CV angle than the control mouse. (B) There is no significant difference in CV in HFD and control mice before or after apamin administration. (C) STDangle was greater in HFD than that in control mice after the apamin administration.
Effect of apamin on atrial arrhythmia inducibility
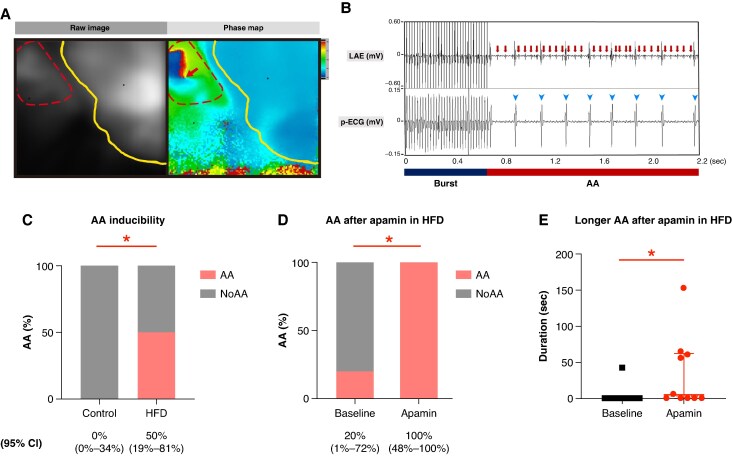
The left panel of Figure 8A displays a representative raw optical mapping image where red and yellow dotted lines demarcate the RA and ventricle, respectively. The right panel shows the corresponding phase map of an induced AA, with a red arrow indicating a phase singularity point. Figure 8B illustrates the LAE (upper panel) and corresponding pseudo-ECG (p-ECG) (lower panel), demonstrating induced AA with variable ventricular conduction (arrowheads) following RA burst pacing. The arrows highlight high-rate atrial signals recorded in LAE. HFD mice exhibited greater AA inducibility than that in control mice at baseline and post-apamin phases, with no AA induced in controls at either phase (5/10 vs. 0/9, P = 0.032) (Figure 8C). We also analysed whether the AA was induced before or after apamin application in the AA-induced HFD mice. Interestingly, the inducibility of AA was significantly increased after apamin administration (20 vs. 100%, n = 5, P = 0.015) in AA-induced HFD mice (Figure 8D). Figure 8E shows that the duration of induced AA was longer after apamin application in AA-induced HFD mice (4.2 ± 13.3 vs. 34.1 ± 50.4 s, n = 5, P = 0.043).
Figure 8.
Higher AA inducibility in HFD mice, especially after apamin administration. (A) The left panel shows an example of the raw image in an optical mapping heart. The (left) dotted line and (right) solid lines indicate the RA and ventricle, respectively. The right panel shows the phase map in an HFD mouse during AF. The arrow indicates the phase ingularity point during AF. (B) Upper and lower panels represent the tracings of LAE and p-ECG in an HFD mouse when AF was induced by burst pacing. The burst bar (left) indicates the period of burst pacing (PCL 20 ms), and the AA bar (right) indicates the period when AF was induced after burst pacing. (C) Higher AA inducibility in HFD than that in control mice at baseline and apamin phase. (D) Higher AA inducibility after apamin application in AA-induced HFD mice. (E) Duration of induced AA is longer after apamin application in AA-induced HFD mice.
Discussion
Our main findings demonstrate that the SK channel is antiarrhythmic in hypokalaemic hearts of HFD-associated AA and partially responsible for the mechanism of HFD-related atrial arrhythmogenesis. The SK channel also influences calcium homeostasis in hypokalaemic hearts of HFD mice, as evidenced by the prolongation of CaTD upon SK channel blockade. SK channel inhibition further elevates the threshold for APD alternans, increases conduction heterogeneity, and enhances AA inducibility.
Our study observed a significant upregulation of the IKAS in atrial myocytes of HFD mice under hypokalaemic condition, along with increased Kcnn3 gene expression in the HFD model. In addition, apamin significantly prolonged atrial APD, suggesting increased activity of SK channels in the atria of hypokalaemic hearts of HFD mice. In conjunction with previous studies,12 our findings illustrate a dual role of the SK channel: although upregulated in HFD mice and contributing to AF, it exhibits antiarrhythmic properties under hypokalaemia. Apamin application notably prolonged CaTD in both control and HFD mice, indicating the influence of SK channels on calcium transients. Prior studies have shown that a reduction in inward potassium currents may prolong APD and CaTD.16 The activation of the L-type calcium channel may lead to Ca2+-induced Ca2+ release from the sarcoplasmic reticulum. Apamin raised the APD alternans threshold in HFD mice, which may partially explain the proarrhythmic effect of SK blockade under hypokalaemic HFD conditions. Cardiac alternans, a periodic beat-to-beat oscillation in electrical activity, is associated with arrhythmias.17 We measured cardiac alternans at the cellular level, assessing both APD and CaTD. SK channel activation might avert APD and APD alternans in ventricular myocytes.18 However, hypokalaemia activated SK channels in rabbit ventricles, whereas apamin, the specific SK channel blocker, increased APD alternans threshold and heterogeneity,19 potentially triggering ventricular fibrillation. Spatial APD heterogeneity has also been implicated in AF.20,21 Employing three methods, we observed greater APD heterogeneity in HFD mice than that in controls, suggesting that HFD may increase APD variability. After apamin application, atrial conduction angle heterogeneity was notably higher in HFD mice than that in controls, indicating that SK channels contribute to the conduction heterogeneity. Gatta et al. similarly reported altered conduction direction following SK channel inhibition and rapid pacing.22 In contrast to controls, approximately half of the HFD mice developed AA post-apamin, linking SK channel inhibition to atrial arrhythmogenesis in hypokalaemic hearts of HFD. Our study highlights that hypokalaemia and HFD together markedly influence cardiac electrophysiology, while SK channels may play an antiarrhythmic role.
Metabolic syndrome and atrial arrhythmias
Obesity and MetS affected the cardiovascular system by promoting hypertension, left ventricular hypertrophy, and the production of inflammatory substances.23–25 MetS has been shown to disrupt energy metabolism in HFD-fed mice, leading to fat accumulation and electrophysiological remodelling.26 Myocardial adipocyte accumulation impairs CV and facilitates reentrant circuit formation via fibrosis, further increasing arrhythmia susceptibility.27 Upregulation of delayed rectifier K⁺ currents shortens APD and heightens AF inducibility.28 A study reveals the increase in L-type calcium channels in MetS.29 Our findings reveal enhanced SK channel expression in HFD mice at the molecular level. APD heterogeneity was greater in HFD, potentially due to SK channel remodelling. Furthermore, conduction angle heterogeneity significantly increased after SK channel blockade in HFD mice. Together with MetS-induced changes, the SK channel indeed plays an important role in AA induction in a hypokalaemic state. Mariosa et al. observed a correlation between abdominal obesity and potassium depletion, suggesting that obese individuals may have lower potassium and higher glucose levels following diuretic therapy.30 These observations support the notion that hypokalaemia and MetS exacerbate one another, jointly predisposing individuals to arrhythmias. Therefore, it is important to elucidate the underlying mechanism of hypokalaemic HFD-related AA.
Hypokalaemia and arrhythmias
Prior researches have documented the association between hypokalaemia and the incidence of AF. Mechanistically, hypokalaemia promotes arrhythmogenesis by (i) reducing potassium channel current, (ii) inhibiting Na+–K+ ATPase, and (iii) activating Ca2+/calmodulin-stimulated protein kinase II (CaMKII) via increased intracellular calcium.9,10 Reduced potassium currents may lead to APD prolongation, thereby enabling the recovery of L-type Ca²⁺ channels from inactivation and enhancing calcium influx.10 Together with previously reported mechanisms in hypokalaemia-induced arrhythmias, we demonstrate that the SK channel plays an important antiarrhythmic role in hypokalaemic hearts of HFD mice.
Hypokalaemia, SK channel activation, and atrial arrhythmias
SK3 overexpression has been shown to shorten APD and increase AF susceptibility.31 In hypokalaemia, Na+/Ca2+ exchanger inhibition might increase the intracellular calcium concentration and further activate the SK channel.19 Li et al. reported that SK2 knockout mice can prolong APD, trigger early afterdepolarization, and increase AF inducibility.13 While SK channel expression is reduced in diabetic atrial myocytes,32 our study demonstrates a significant upregulation of IKAS in hypokalaemic hearts of HFD mice, along with an increased APD alternans threshold following apamin administration. Combined with other research, this supports APD heterogeneity in HFD conditions due to SK channel alternans and spatial heterogeneity under hypokalaemia. Previous research has demonstrated that SK channels are regulated by protein kinases, including casein kinase II (CKII) and CaMKII, as well as protein phosphatase 2A (PP2A).33 Under specific pathophysiological conditions, such as cardiac hypertrophy and hypokalaemia, CaMKII may become activated through autophosphorylation. This activation enables CaMKII to interact with SK channels via calmodulin, increasing calcium ion sensitivity.34 Furthermore, Heijman et al. reported that PP2A and CKII modulate SK channel activity. The phosphorylation of calmodulin may alter calcium ion sensitivity and, consequently, IKAS. Additionally, the number of SK channels targeted to the plasma membrane via cytoskeletal proteins may influence the amplitude of IKAS.35 We hypothesize that hypokalaemia and HFD induce differential activation of CKII, CaMKII, or PP2A, altering calcium sensitivity and SK channel distribution. This could underlie the APD and conduction heterogeneity observed in our model. Further investigations are needed to confirm these regulatory mechanisms.
Arrhythmic events were mostly observed after apamin application, likely resulting from prolonged APD and CaTD, as well as elevated APD alternans thresholds. Increased conduction angle heterogeneity after SK channel blockade may also contribute to arrhythmogenesis in hypokalaemic hearts of HFD mice. The spatial variance between the SK channel and atrial fibrosis may cause conduction deviations and arrhythmias.36 The CTL group showed no evidence of increased AA induction after apamin application, including APD, alternans threshold, and conduction heterogeneity. Although acute hypokalaemia did not affect the transcriptional regulation of SK channels in our study, it did interact with HFD status and induce arrhythmogenic changes. Compared with our previous study,12 current study showed that under acute hypokalaemia, a common scenario in patients with MetS, the SK channel may exhibit an antiarrhythmic role. This novel finding underscores the context-dependent function of SK channels in atrial electrophysiology in HFD status, raising the clinical impact of inducing atrial arrhythmia in MetS patients treated with SK channel blockers when hypokalaemia is present.
Study limitations
First, data from the LA may be unreliable, as the LA was cannulated for left ventricular decompression; therefore, our analysis focused exclusively on right atrial (RA) changes. Secondly, the burst pacing employed to induce AA in this study may not apply to all arrhythmia mechanisms. Thirdly, the very short cycle length and APD of the mouse heartbeat mean that mice are not as ideal as larger animals for demonstrating changes in APD alternans. A stepwise pacing approach may have reduced sensitivity for detecting subtle alternans thresholds or dynamic transitions between experimental groups. Fourthly, due to the small size of the murine atrium, the resolution of the voltage mapping images is inherently limited. Fifthly, although we assessed the statistical power to detect differences in APD between HFD and control groups, some datasets lacked sufficient power. Therefore, further studies with larger sample sizes are warranted for more robust findings. Sixthly, dropout cases in this study were primarily due to perfusate leakage or prolonged ischaemia during optical mapping, resulting in the exclusion of hearts with inadequate perfusion quality.
Conclusions
Hypokalaemic hearts of HFD showed upregulation of atrial apamin-sensitive SK channels. Blockade of apamin-sensitive SK channels contributed to APD and conduction heterogeneity, increased APD alternans threshold, and increased intracellular calcium, leading to AA. Electrophysiological changes modulated by apamin-sensitive SK channels might be partially protective against AA in hypokalaemic hearts of HFD mice. This observation underscores the need for further research to clarify the potential role of SK channel modulation in AA related to hypokalaemic hearts of HFD.
Supplementary Material
Acknowledgements
The authors thank Yi-Lin Shiou and Wun-Jyun Jhuang for their assistance in the experimental preparation and the Center for Laboratory Animals in Kaohsiung Medical University for the animal care.
Contributor Information
Chia-Hao Kuo, Department of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan; Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, No. 100 Ziyou 1st Rd, Kaohsiung 80756, Taiwan.
Jo-Yun Shih, Department of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Yi-Hsiung Lin, Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, No. 100 Ziyou 1st Rd, Kaohsiung 80756, Taiwan; Center for Lipid Biosciences, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan.
Chien-Wei Chang, Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, No. 100 Ziyou 1st Rd, Kaohsiung 80756, Taiwan.
Shih-Jie Jhuo, Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, No. 100 Ziyou 1st Rd, Kaohsiung 80756, Taiwan.
Tzu-Chieh Lin, Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, No. 100 Ziyou 1st Rd, Kaohsiung 80756, Taiwan.
Tien-Chi Huang, Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, No. 100 Ziyou 1st Rd, Kaohsiung 80756, Taiwan.
I Hsin Liu, Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, No. 100 Ziyou 1st Rd, Kaohsiung 80756, Taiwan.
Pei-Heng Kao, Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, No. 100 Ziyou 1st Rd, Kaohsiung 80756, Taiwan.
En-Ying Lin, Department of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Pin-Chieh Huang, Department of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Xin-Hui Chen, Department of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Chao-Yi Chen, Department of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Yu-Chen Feng, Department of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Ying-Xuan Zheng, Department of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Min-Huei Lin, Department of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Guan-Lin Chen, Department of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Po-Chao Hsu, Department of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan; Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, No. 100 Ziyou 1st Rd, Kaohsiung 80756, Taiwan.
Chien-Hung Lee, Department of Public Health, College of Health Science, Kaohsiung Medical University, Kaohsiung, Taiwan.
Chun-Chieh Wu, Department of Pathology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
Hsiang-Chun Lee, Department of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan; Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, No. 100 Ziyou 1st Rd, Kaohsiung 80756, Taiwan.
Bin-Nan Wu, Department of Pharmacology, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Shien-Fong Lin, Institute of Biomedical Engineering, National Yang Ming Chiao Tung University, Hsin-Chu, Taiwan.
Wen-Ter Lai, Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, No. 100 Ziyou 1st Rd, Kaohsiung 80756, Taiwan.
Wei-Chung Tsai, Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, No. 100 Ziyou 1st Rd, Kaohsiung 80756, Taiwan; Faculty of Medicine, College of Medicine, Kaohsiung Medical University, No. 100, Shiquan 1st Rd, Sanmin Dist., Kaohsiung 807378, Taiwan; Drug Development and Value Creation Research Center, Kaohsiung Medical University, No. 100, Shiquan 1st Rd, Sanmin Dist., Kaohsiung 807378, Taiwan.
Supplementary material
Supplementary material is available at Europace online.
Funding
This study was supported in part by grants from the National Science and Technology Council, Taiwan (grant number MOST 110-2314-B-037-111, NSTC 113-2314-B-037-054-MY3), Kaohsiung Medical University Hospital (grant number KMUH111-1T10, SI11001, SI11101, KMUH-DK(B) 111003-4).
Data availability
The datasets for this study can be inquired by contacting the corresponding author, W.C.T.
References
- 1. Aromolaran AS, Boutjdir M. Cardiac ion channel regulation in obesity and the metabolic syndrome: relevance to long QT syndrome and atrial fibrillation. Front Physiol 2017;8:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weiss JN, Qu Z, Shivkumar K. Electrophysiology of hypokalemia and hyperkalemia. Circ Arrhythm Electrophysiol 2017;10:e004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daniell HW. Arrhythmia in hypokalemia. N Engl J Med 1971;284:1385. [DOI] [PubMed] [Google Scholar]
- 4. Rabast U, Vornberger KH, Ehl M. Loss of weight, sodium and water in obese persons consuming a high- or low-carbohydrate diet. Ann Nutr Metab 1981;25:341–9. [DOI] [PubMed] [Google Scholar]
- 5. Middeldorp ME, Ariyaratnam J, Lau D, Sanders P. Lifestyle modifications for treatment of atrial fibrillation. Heart 2020;106:325–32. [DOI] [PubMed] [Google Scholar]
- 6. Georgakopoulos C, Vlachopoulos C, Lazaros G, Tousoulis D. Biomarkers of atrial fibrillation in metabolic syndrome. Curr Med Chem 2019;26:898–908. [DOI] [PubMed] [Google Scholar]
- 7. Voos P, Yazar M, Lautenschläger R, Rauh O, Moroni A, Thiel G. The small neurotoxin apamin blocks not only small conductance Ca(2+) activated K(+) channels (SK type) but also the voltage dependent Kv1.3 channel. Eur Biophys J 2017;46:517–23. [DOI] [PubMed] [Google Scholar]
- 8. Diness JG, Bentzen BH, Sørensen US, Grunnet M. Role of calcium-activated potassium channels in atrial fibrillation pathophysiology and therapy. J Cardiovasc Pharmacol 2015;66:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skogestad J, Aronsen JM. Hypokalemia-induced arrhythmias and heart failure: new insights and implications for therapy. Front Physiol 2018;9:1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tazmini K, Frisk M, Lewalle A, Laasmaa M, Morotti S, Lipsett DB et al. Hypokalemia promotes arrhythmia by distinct mechanisms in atrial and ventricular myocytes. Circ Res 2020;126:889–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faggioni M, Knollmann BC. Arrhythmia protection in hypokalemia: a novel role of Ca2+-activated K+ currents in the ventricle. Circulation 2015;132:1371–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsai WC, Lin YH, Kuo CH, Jhuo SJ, Shih RY, Wu CC et al. Up-regulated small-conductance calcium-activated potassium currents contribute to atrial arrhythmogenesis in high-fat feeding mice. Europace 2023;26:euae004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li N, Timofeyev V, Tuteja D, Xu D, Lu L, Zhang Q et al. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol 2009;587:1087–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang L, Olivas A, Francis Stuart SD, Tapa S, Blake MR, Woodward WR et al. Cardiac sympathetic nerve transdifferentiation reduces action potential heterogeneity after myocardial infarction. Am J Physiol Heart Circ Physiol 2020;318:H558–h565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sung YL, Lin TT, Syu JY, Hsu HJ, Lin KY, Liu YB et al. Reverse electromechanical modelling of diastolic dysfunction in spontaneous hypertensive rat after sacubitril/valsartan therapy. ESC Heart Fail 2020;7:4040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spencer CI, Baba S, Nakamura K, Hua EA, Sears MA, Fu CC et al. Calcium transients closely reflect prolonged action potentials in iPSC models of inherited cardiac arrhythmia. Stem Cell Rep 2014;3:269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards JN, Blatter LA. Cardiac alternans and intracellular calcium cycling. Clin Exp Pharmacol Physiol 2014;41:524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanaporis G, Blatter LA. Activation of small conductance Ca(2+) -activated K(+) channels suppresses Ca(2+) transient and action potential alternans in ventricular myocytes. J Physiol 2023;601:51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan YH, Tsai WC, Ko JS, Yin D, Chang PC, Rubart M et al. Small-conductance calcium-activated potassium current is activated during hypokalemia and masks short-term cardiac memory induced by ventricular pacing. Circulation 2015;132:1377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsieh YC, Chang PC, Hsueh CH, Lee YS, Shen C, Weiss JN et al. Apamin-sensitive potassium current modulates action potential duration restitution and arrhythmogenesis of failing rabbit ventricles. Circ Arrhythm Electrophysiol 2013;6:410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bohne LJ, Jansen HJ, Daniel I, Dorey TW, Moghtadaei M, Belke DD et al. Electrical and structural remodeling contribute to atrial fibrillation in type 2 diabetic db/db mice. Heart Rhythm 2021;18:118–29. [DOI] [PubMed] [Google Scholar]
- 22. Gatta G, Sobota V, Citerni C, Diness JG, Sørensen US, Jespersen T et al. Effective termination of atrial fibrillation by SK channel inhibition is associated with a sudden organization of fibrillatory conduction. Europace 2021;23:1847–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm 2010;2010:535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alpert MA, Lavie CJ, Agrawal H, Aggarwal KB, Kumar SA. Obesity and heart failure: epidemiology, pathophysiology, clinical manifestations, and management. Transl Res 2014;164:345–56. [DOI] [PubMed] [Google Scholar]
- 25. Alpert MA, Omran J, Bostick BP. Effects of obesity on cardiovascular hemodynamics, cardiac morphology, and ventricular function. Curr Obes Rep 2016;5:424–34. [DOI] [PubMed] [Google Scholar]
- 26. Suffee N, Baptista E, Piquereau J, Ponnaiah M, Doisne N, Ichou F et al. Impacts of a high fat diet on the metabolic profile and the phenotype of atrial myocardium in mice. Cardiovasc Res 2022;118:3126–39. [DOI] [PubMed] [Google Scholar]
- 27. Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol 2017;70:2022–35. [DOI] [PubMed] [Google Scholar]
- 28. Christophersen IE, Olesen MS, Liang B, Andersen MN, Larsen AP, Nielsen JB et al. Genetic variation in KCNA5: impact on the atrial-specific potassium current IKur in patients with lone atrial fibrillation. Eur Heart J 2013;34:1517–25. [DOI] [PubMed] [Google Scholar]
- 29. Lima-Leopoldo AP, Leopoldo AS, Silva DC, Nascimento AF, Campos DH, Luvizotto Rde A et al. Influence of long-term obesity on myocardial gene expression. Arq Bras Cardiol 2013;100:229–37. [DOI] [PubMed] [Google Scholar]
- 30. Mariosa LS, Ribeiro-Filho FF, Batista MC, Hirota AH, Borges RL, Ribeiro AB et al. Abdominal obesity is associated with potassium depletion and changes in glucose homeostasis during diuretic therapy. J Clin Hypertens (Greenwich) 2008;10:443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang XD, Timofeyev V, Li N, Myers RE, Zhang DM, Singapuri A et al. Critical roles of a small conductance Ca²⁺-activated K⁺ channel (SK3) in the repolarization process of atrial myocytes. Cardiovasc Res 2014;101:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yi F, Ling TY, Lu T, Wang XL, Li J, Claycomb WC et al. Down-regulation of the small conductance calcium-activated potassium channels in diabetic mouse atria. J Biol Chem 2015;290:7016–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang XD, Thai PN, Lieu DK, Chiamvimonvat N. Cardiac small-conductance calcium-activated potassium channels in health and disease. Pflugers Arch 2021;473:477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tenma T, Mitsuyama H, Watanabe M, Kakutani N, Otsuka Y, Mizukami K et al. Small-conductance Ca(2+)-activated K(+) channel activation deteriorates hypoxic ventricular arrhythmias via CaMKII in cardiac hypertrophy. Am J Physiol Heart Circ Physiol 2018;315:H262–h272. [DOI] [PubMed] [Google Scholar]
- 35. Heijman J, Zhou X, Morotti S, Molina CE, Abu-Taha IH, Tekook M et al. Enhanced Ca(2+)-dependent SK-channel gating and membrane trafficking in human atrial fibrillation. Circ Res 2023;132:e116–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y, Guzadhur L, Jeevaratnam K, Salvage SC, Matthews GD, Lammers WJ et al. Arrhythmic substrate, slowed propagation and increased dispersion in conduction direction in the right ventricular outflow tract of murine Scn5a+/- hearts. Acta Physiol (Oxf) 2014;211:559–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets for this study can be inquired by contacting the corresponding author, W.C.T.