Abstract
The soil fungus Cladophialophora sp. strain T1 (= ATCC MYA-2335) was capable of growth on a model water-soluble fraction of gasoline that contained all six BTEX components (benzene, toluene, ethylbenzene, and the xylene isomers). Benzene was not metabolized, but the alkylated benzenes (toluene, ethylbenzene, and xylenes) were degraded by a combination of assimilation and cometabolism. Toluene and ethylbenzene were used as sources of carbon and energy, whereas the xylenes were cometabolized to different extents. o-Xylene and m-xylene were converted to phthalates as end metabolites; p-xylene was not degraded in complex BTEX mixtures but, in combination with toluene, appeared to be mineralized. The metabolic profiles and the inhibitory nature of the substrate interactions indicated that toluene, ethylbenzene, and xylene were degraded at the side chain by the same monooxygenase enzyme. Our findings suggest that soil fungi could contribute significantly to bioremediation of BTEX pollution.
A considerable amount of gasoline enters the environment as result of leakage from underground storage tanks, accidental spills, or improper waste disposal practices (4). When gasoline is in contact with water, benzene, toluene, ethylbenzene, and the xylene isomers (BTEX) account for as much as 90% of the gasoline components that are found in the water-soluble fraction (19). Consequently, these chemicals are some of the most common contaminants found in drinking water. BTEX are toxic to humans, and their removal from polluted environments is of special interest (15).
It has been assumed that soil bioremediation of BTEX pollution relies upon indigenous bacterial populations; the significance of fungi has been overlooked (4). Fungi generally withstand harsher environmental conditions than bacteria and could play an important role in the degradation of petroleum hydrocarbons in the soil (3). Nevertheless, fungal degradation of BTEX mixtures has been studied only to a limited extent with white-rot fungi (5, 22). BTEX were mineralized but did not support fungal growth when they were supplied as the sole source of carbon and energy. The extracellular lignin-degrading enzymes are capable of oxidizing a wide range of aromatic hydrocarbons, but they appear not to be involved in BTEX degradation. The low degradation rates and the requirement of an additional carbon source limit the use of white-rot fungi in bioremediation. Interestingly, new non-white-rot fungal strains have been isolated and used successfully for biofiltration of air contaminated with volatile aromatic hydrocarbons (i.e., toluene and styrene), which were metabolized as sole carbon and energy sources (7, 8, 17). When hydrocarbon-degrading microbes are used for bioremediation of gasoline pollution, it is very unlikely that they encounter a sole substrate. Some papers dealing with substrate interactions during degradation of BTEX mixtures by bacteria have been published (1, 6, 9, 10, 16), but analogous data for fungi are still very scarce.
The objective of the present study was to investigate the pattern of degradation of BTEX mixtures by a fungus capable of growth on aromatic hydrocarbons. The deuteromycete Cladophialophora sp. strain T1 (= ATCC MYA-2335), which grows on toluene, was selected as a model fungus. This strain was isolated previously from a BTEX-polluted soil and showed the best degradative capacity in terms of substrate specificity among the fungal isolates examined (17). Special attention was paid to the kinetics of utilization of multiple substrates and to the extent of degradation of every BTEX component. This information is important for devising fungus-based techniques for treatment of BTEX pollution, especially under solid state-like environmental conditions, such as those in air biofilters or acidic soils, which usually favor fungal growth over bacterial growth (3, 17).
MATERIALS AND METHODS
Chemicals.
BTEX hydrocarbons and reference compounds for intermediate identification were obtained from Acros Organics (Geel, Belgium), Sigma-Aldrich Chemicals (Steinheim, Germany), Jansen Chimica (Geel, Belgium), Lab-Scan Ltd. (Dublin, Ireland), and Merck KGaA (Darmstadt, Germany). All chemicals were analytical grade. Deuterium oxide (>99.9% deuterium) was supplied by M. G. Chemicals (Toronto, Canada). Deuterated chloroform (99.8% deuterium) was obtained from Isotec Inc. (Miamisburg, Ohio).
Fungal strain.
Cladophialophora sp. strain T1 (= ATCC MYA-2335) was isolated as previously described (17). During the present investigation this organism was routinely maintained at 4°C on mineral medium (11) agar slants supplemented with 2% glucose.
Growth experiments.
Tests were performed in 250-ml Boston flasks containing 25 ml of buffered (35 mM K2HPO4, NaH2PO4·2H2O; pH 7) mineral salts medium (11) and sealed with Teflon-coated valves (Mininert; Phase Separations, Waddinxveen, The Netherlands). BTEX compounds were added as the sole carbon and energy source. After water-air substrate equilibration, flasks were inoculated with a fungal spore suspension containing approximately 104 viable spores. Incubation was performed at 25°C under static conditions. Growth was monitored by gas phase measurement of substrate consumption and CO2 production compared with substrate consumption and CO2 production in noninoculated controls. The concentration of BTEX in the water phase was calculated by using previously reported water-air partition coefficients (2, 14). Cometabolic degradation of the BTEX compounds that individually did not support growth was assayed by using combinations of these compounds with toluene. The carbon recovered as CO2 (C-CO2) and the carbon recovered as biomass (C-biomass) were determined after substrate exhaustion, and the values were corrected by using control flasks inoculated and incubated with no carbon source. The pH of the medium was checked at the end of incubation for adjustment of the water-air partition of CO2, but no significant variations were observed. The biomass was collected by filtration, and the dry matter content was determined. C-biomass was calculated by assuming that 26 g (dry weight) contains approximately 1 mol of carbon (21). The culture filtrate was stored at −20°C for identification of metabolites. Carbon mass balance experiments were performed for both cultures and controls in triplicate.
Identification of intermediates.
The filtrate from the cultures described above was thawed and divided in two 5-ml portions. The first fraction was freeze-dried under a vacuum, and the residue was redissolved in 1 ml of deuterium oxide; the second fraction was extracted with deuterated chloroform (1 ml). Both extracts were analyzed by proton nuclear magnetic resonance (1H NMR). Products of BTEX conversion were identified by comparing their 1H NMR chemical shift values with those of authentic reference compounds, when they were commercially available, and by performing two-dimensional NMR experiments with nuclear Overhauser spectroscopy (12).
Degradation kinetics.
A toluene-grown liquid culture of fungal strain T1 was prepared and harvested as described elsewhere (17). Cells were resuspended in 25 ml of phosphate buffer (50 mM, pH 7) and incubated in 250-ml Boston flasks sealed with Teflon valves (120 rpm, 25°C). The apparent half-saturation constant (Km) for degradation of BTEX was determined from the substrate depletion curves. From these data points, the specific substrate consumption rates were calculated at different concentrations and fitted to a Lineweaver-Burk plot. The maximum biodegradation rate (Vmax) was calculated by linear regression of the data points for which the substrate concentration was more than five times higher than the Km value. The amount of biomass was set for every substrate (between 1 and 6 g [dry weight] liter−1) in order to obtain a biodegradation activity below the rates of mass transfer between the gas and aqueous phases for BTEX, as reported previously for a similar batch system (6). BTEX adsorption onto the biomass was determined in additional batches containing heat-inactivated (25 min, 120°C) cells.
Analytical methods.
BTEX and CO2 contents were determined by injecting 100-μl headspace samples into an HP 6890 Series gas chromatograph (Hewlett-Packard). For BTEX, the stationary phase used was a 10% SE-30 Chromosorb WMP column (Chrompack B.V., Middelburg, The Netherlands). The carrier gas was nitrogen at a flow rate of 1.9 ml min−1. The temperatures of the column and the flame ionization detector were 110 and 300°C, respectively. A CP-Wax 52CB column (Chrompack B.V.) at a temperature of 50°C was used to resolve mixtures of ethylbenzene and the three xylene isomers. For CO2 a CP-Poraplot Q column (Chrompack B.V.) and a thermal conductivity detector were used. Helium at a flow rate of 3.0 ml min−1 was the carrier gas. The column and detector temperatures were 70 and 250°C, respectively. 1H NMR measurements were obtained with a Bruker AMX 500-MHz NMR spectrometer. The sample volume was 0.5 ml, and the temperature used for measurement was 23°C. Biomass dry weight was determined after cell suspensions were filtered and dried (24 h, 105°C) with glass fiber paper with >1-μm retentivity (Schleicher & Schuell, Dassel, Germany). The filters were previously rinsed with demineralized water, dried, and weighed.
RESULTS
Growth experiments.
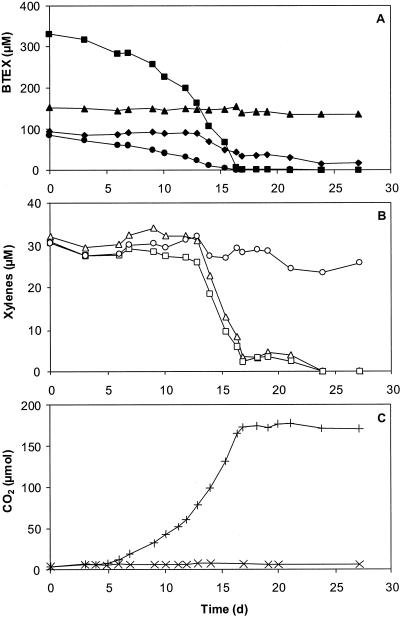
The time course concentration of every component in a mixture of all BTEX compounds, provided as the sole source of carbon and energy, was monitored during growth of Cladophialophora sp. strain T1 in a batch system. The benzene concentration remained constant throughout the experiment, but toluene and ethylbenzene were exhausted within 17 days of inoculation (Fig. 1A). The degradation patterns for the xylenes also differed, and only the ortho and meta isomers were depleted (Fig. 1B). Uptake of these xylenes started after toluene and ethylbenzene had been partially removed. During growth, CO2 was produced at an exponential rate (0.28 day−1; r2 ≥ 0.98) equivalent to a doubling time of 2.5 days (Fig. 1C).
FIG. 1.
(A) Degradation of a mixture of benzene (▴), toluene (▪), ethylbenzene (•), and xylenes (⧫) by a batch culture of the fungus Cladophialophora sp. strain T1 incubated at 25°C. (B) Degradation curves for the xylene isomers o-xylene (▵), m-xylene (□), and p-xylene (○). (C) Production of CO2 (+) during growth compared with production of CO2 in inoculated controls incubated without BTEX (×).
The type of metabolism for every individual BTEX compound (namely, assimilation or cometabolism) was determined in additional growth experiments with single substrates and binary combinations of substrates (Table 1). Carbon balances were determined by measuring the amounts of consumed C substrate that were recovered as C-CO2 and C-biomass. Toluene and ethylbenzene were both used for growth, and carbon recovery values of about 90% were obtained when these compounds were added either individually or together. Neither benzene nor the xylenes supported growth as single substrates, but the latter were successfully cometabolized in the presence of toluene. The carbon recovery values in batches containing toluene plus o- or m-xylene were lower than in those obtained with an identical amount of toluene alone. In contrast to the lack of p-xylene degradation observed with BTEX mixtures, about 60% of the p-xylene was depleted when this compound was combined with toluene. Whereas o- and m-xylenes were consumed simultaneously with toluene, degradation of p-xylene occurred only after the toluene was exhausted. The carbon recovery value with mixtures of p-xylene and toluene was as high as the values measured for toluene and ethylbenzene (Table 1).
TABLE 1.
Carbon mass balance after growth of Cladophialophora sp. strain T1 on single compounds and binary combinationsa
| Substrate(s) | Depletion of second substrate
|
C-CO2 (μmol) | C-biomass (μmol) | % C recoveryc | |
|---|---|---|---|---|---|
| % | Patternb | ||||
| Ethylbenzene (197 μmol) | 132 ± 03 | 42 ± 04 | 88 ± 03 | ||
| Toluene (196 μmol) | 131 ± 07 | 41 ± 04 | 89 ± 04 | ||
| Toluene (196 μmol) + benzene (68 μmol) | <5d | 135 ± 10 | 38 ± 05 | 88 ± 08 | |
| Toluene (190 μmol) + ethylbenzene (66 μmol) | 100 ± 00 | S | 160 ± 23 | 64 ± 04 | 86 ± 07 |
| Toluene (196 μmol) + o-xylene (67 μmol) | 100 ± 00 | S | 134 ± 15 | 45 ± 02 | 68 ± 07 |
| Toluene (196 μmol) + m-xylene (65 μmol) | 100 ± 00 | S | 138 ± 02 | 45 ± 06 | 70 ± 02 |
| Toluene (196 μmol) + p-xylene (65 μmol) | 58 ± 12 | D | 163 ± 12 | 58 ± 07 | 93 ± 04 |
The values are averages ± standard deviations based on three different experiments.
S, simultaneous; D, diauxie.
Percentage of carbon recovered as CO2 and biomass.
Considered not degraded.
Identification of intermediates.
Extracts from the cultures described above, grown on single substrates and binary BTEX mixtures, were analyzed by 1H NMR, and the resonances from aromatic intermediates were assigned (Table 2). No aromatic metabolites were detected in cultures incubated with toluene, ethylbenzene, p-xylene, or benzene or binary combinations of those with toluene. A similar pattern of 1H signals in the aliphatic chemical shift region (0 to 6 ppm) was observed for all cultures (data not shown), indicating excretion of downstream metabolites. To address the possibility that volatile intermediates, such as aromatic aldehydes, alcohols, phenols, and catechols, were lost during the vacuum extraction, we extracted some cultures with deuterated chloroform. No evidence of these metabolites was found in deuterated chloroform extracts.
TABLE 2.
1H NMR chemical shift values and coupling constants of metabolites formed from xylene degradationa
| Substrates | Intermediate |
1H NMR chemical shifts
|
||||||
|---|---|---|---|---|---|---|---|---|
| −CH3 | −CH2OH | H2 | H3 | H4 | H5 | H6 | ||
| o-Xylene + toluene | 4-Hydroxy-2-methylbenzoic acidb | 2.26 | 7.21 | 6.64 (3JH5-H6 = 8.3) | 6.61 | |||
| 2-Methylbenzoic acid | 2.25 | 7.18 (3JH3-H4 = 7.0) | 7.12 (3JH4-H5 = 7.0) | 7.14 (3JH5-H6 = 7.0) | 7.20 | |||
| o-Phthalic acid | 7.36 (3JH3-H4 = 7.0) | 7.29 (3JH4-H5 = 7.0) | 7.29 (3JH5-H6 = 7.0) | 7.36 | ||||
| m-Xylene + toluene | 3-Methylbenzoic acid | 2.30 | 7.63 | 7.30 (3JH4-H5 = 7.0) | 7.30 (3JH5-H6 = 7.0) | 7.60 | ||
| 3-Hydroxymethylbenzoic acidb | 4.65 | 7.73 | 7.42 (3JH4-H5 = 7.7) | 7.38 (3JH5-H6 = 7.6) | 7.71 | |||
| m-Phthalic acid | 8.17 | 7.87 (3JH4-H5 = 7.6, 4JH4-H6 = 1.3) | 7.41 (3JH5-H6 = 7.6) | 7.87 | ||||
Chemical shifts were determined relative to the HDO signal (4.7 ppm) at 23°C. Resonances which showed strong coupling were simulated and iteratively fitted onto the measured data.
Identified by two-dimensional NMR experiments with nuclear Overhauser spectroscopy.
Degradation kinetics.
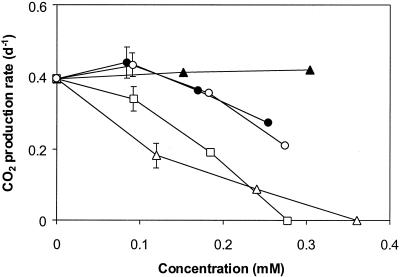
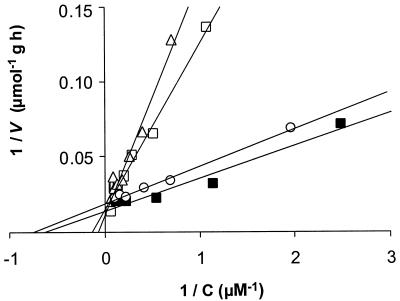
The effect of a second substrate, present at different concentrations, on growth on toluene was studied (Fig. 2). Both o-xylene and m-xylene negatively affected the growth rate, causing complete inhibition when they were present at a concentration similar to that of toluene. Under equivalent conditions, ethylbenzene and p-xylene inhibited growth moderately, whereas benzene did not have any significant effect. The nature of BTEX inhibitory interactions was characterized by performing degradation activity tests using toluene-grown harvested mycelia and different substrate combinations. Besides toluene, ethylbenzene and the xylenes were degraded without a lag phase, and the Michaelis-Menten kinetic parameters for these substrates were determined (Table 3). Additionally, Lineweaver-Burk plots were obtained for depletion of toluene in the presence of a second substrate (added at concentrations up to 0.1 mM), as illustrated in Fig. 3 for the xylenes. The presence of p-xylene did not affect the toluene degradation kinetics. However, the Km values for toluene in batches that were supplemented with o-xylene and m-xylene were 13.3 and 7.8 μM, respectively, as determined from Fig. 3. These values are significantly higher than 2.7 ± 1.0 μM, the value measured for toluene when it was the single substrate. It was not possible to generate similar Lineweaver-Burk plots with binary combinations of ethylbenzene and toluene. Ethylbenzene at a concentration of 0.1 mM hindered toluene exhaustion, and if ethylbenzene was added at a lower concentration, toluene and ethylbenzene were consumed simultaneously. Controls containing heat-inactivated mycelia showed that adsorption was insignificant in these experiments.
FIG. 2.
Effects of benzene (▴), ethylbenzene (•), o-xylene (▵), m-xylene (□), and p-xylene (○) added at different concentrations in combination with toluene (0.3 mM) on the growth of Cladophialophora sp. strain T1. Growth was characterized by determining the rate coefficient of CO2 production at 25°C (n ≥ 5, r2 ≥ 0.98). When present, error bars indicate the standard deviation based on three independent experiments.
TABLE 3.
Michaelis-Menten kinetic parameters for the degradation of toluene, ethylbenzene, and xylenes by toluene-grown cells of Cladophialophora sp. strain T1, as measured in batch incubation preparations (120 rpm, 25°C)
| Substrate | Vmax (μmol g−1 h−1)a |
Kmb
|
Vmax/Km (liters g−1 h−1) | |
|---|---|---|---|---|
| Water (μM) | Air equivalent (mg m−3) | |||
| Toluene | 75 ± 5c | 2.7 ± 1.0c | 66 | 30.7 |
| Ethylbenzene | 45 | 5.8 | 195 | 7.8 |
| o-Xylene | 21 | 4.2 | 88 | 4.4 |
| m-Xylene | 19 | 3.8 | 112 | 5.0 |
| p-Xylene | 2 | 1.1 | 33 | 1.8 |
Measured rates (n ≥ 6, r2 ≥ 0.99).
Data from Lineweaver-Burk plot linearization (n ≥ 8, r2 ≥ 0.97).
Average ± standard deviation based on three independent experiments.
FIG. 3.
Lineweaver-Burk plot from toluene depletion curves for cells of Cladophialophora sp. strain T1 incubated with toluene as the only substrate (▪) and together with 0.1 mM o-xylene (▵), m-xylene (□), or p-xylene (○) (25°C, r2 ≥ 0.98). The kinetic parameters (Vmax and Km) are given in the text.
DISCUSSION
Spores of the soil fungus Cladophialophora sp. strain T1 germinated and grew on a complex mixture of BTEX hydrocarbons as the sole carbon and energy source. This BTEX solution was comparable to a real gasoline water-soluble fraction (19). The ability of fungi to grow on the water-soluble fraction of petroleum fuels is well documented, but it was generally believed that only the aliphatic hydrocarbons supported fungal growth (3, 13). Interestingly, this is to our knowledge the first report of a fungus growing on aromatic hydrocarbons from a model gasoline water-soluble fraction, which included all six BTEX components. Most of the previous research in this field has focused on aerobic bacteria growing on simpler BTEX mixtures containing only two or three components (1, 6, 9, 10, 16). Although the substrate specificity and the extent of degradation were found to be highly strain specific, cometabolism and competitive inhibition were the most common substrate interactions. Like bacteria, Cladophialophora sp. strain T1 degraded BTEX components by a combination of assimilation and cometabolism, but only the alkylated benzenes were metabolized. Toluene and ethylbenzene served as carbon and energy sources, whereas the xylenes were cometabolized. Carbon balance experiments and 1H NMR metabolic profiles revealed that the o- and m-xylene isomers were partially oxidized to dead-end products. For p-xylene, a higher carbon recovery value together with the lack of accumulation of intermediates points to mineralization. However, the low rate of degradation of p-xylene seems to be insufficient to sustain growth if this compound is supplied as the single substrate. In BTEX mixtures, p-xylene appears to be outcompeted by the other substrates.
The Michaelis-Menten model provided a good description of the degradation kinetics in batch experiments in which whole cells were used. The specific affinity (Vmax/Km) obtained for every individual BTEX component was consistent with the degradation pattern seen in a complex BTEX mixture, where toluene and ethylbenzene were consumed preferentially, followed by o- and m-xylenes and finally p-xylene, which was hardly degraded. Comparisons of the Km values for degradation of toluene, alone and in combination with a second substrate, suggest that competitive inhibition is the main substrate interaction. Competition for substrates that, like o- and m-xylenes, led to neither carbon assimilation nor energy-yielding reactions consequently resulted in growth inhibition (Fig. 2). Strong competition might induce a sequential pattern for the utilization of the different substrates, a phenomenon usually referred as diauxie, which makes treatment of mixtures in a continuous system difficult. Diauxie during BTEX degradation has been described, for example, for a Rhodococcus strain (9), where the presence of ethylbenzene blocked degradation of any additional substrate. In our study, sequential degradation of p-xylene (in combination with toluene) was observed, but for all other toluene, ethlybenzene, and xylene components a certain degree of simultaneous uptake occurred (Fig. 1).
As reported in our previous study on the assimilation of toluene by Cladophialophora sp. strain T1, toluene is first hydroxylated to benzyl alcohol and subsequently converted to benzoic acid, prior to hydroxylation and cleavage of the aromatic ring (18). Here we have shown that o- and m-xylenes were also oxidized at the side chain to form dibenzoic acids, which were not metabolized further. Considering in addition the likely competitive nature of utilization of multiple substrates, we propose that fungal strain T1 oxidizes the alkylated compounds (toluene, ethylbenzene, and xylenes) at the alkyl side chain via the same monooxygenase enzyme. This conclusion explains the immediate degradation of ethylbenzene and the xylenes observed with toluene-grown mycelia. Conversely, the toluene degradation capacity needs to be induced in glucose-grown mycelia (data not shown). According to this view, the metabolism of benzene requires a very different enzymatic mechanism, apparently absent in our fungus, which is capable of performing the initial oxidation at the aromatic ring.
The results described in this report suggest that fungi with the ability to grow on aromatic hydrocarbons might contribute significantly to bioremediation of BTEX pollution. At present, a great deal of attention in the field of fungal bioremediation is being paid to the development of fungal biofilters for the treatment of polluted air (7). In relation to this, the low Km of Cladophialophora sp. strain T1 for degradation of toluene, ethylbenzene, and xylene makes this organism very suitable for treatment of toluene, ethylbenzene, and xylene vapors. The Km values reported here (Table 3) are equivalent to air concentrations below the recommended threshold limit values for exposure to toluene, ethylbenzene, and xylene (2). Considering the similarities between air biofiltration and soil bioventing, fungi could also be used to enhance bioremediation of polluted soil. The lack of benzene degradation appears to be the main drawback for application of our strain. Nevertheless, taking into account the high microbial diversity for metabolism of BTEX and the environmental variability of field conditions, effective bioremediation is most likely to rely on a consortium of organisms rather than on the action of a single microorganism. In support of this hypothesis, an earlier study showed that there is synergism between fungi and bacteria for mineralization of aromatic hydrocarbons in an acidic soil (20). In this study we have shown that soil fungi possess a metabolic capacity for degradation of BTEX similar in many aspects to that of bacteria. Fungi thus should not be ignored in the development of more efficient bioremediation strategies.
Acknowledgments
This work was supported by grant BIO4 CT 972295 from the European Commission.
We acknowledge Nadia Nikolova (Bourgas University, Bourgas, Bulgaria) for performing some of the preliminary experiments.
REFERENCES
- 1.Alvarez, P. J. J., and T. M. Vogel. 1991. Substrate interactions of benzene, toluene, and p-xylene during microbial degradation by pure cultures and mixed culture aquifer slurries. Appl. Environ. Microbiol. 57:2981-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amoore, J. E., and E. Hautala. 1983. Odor as an aid to chemical safety: odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 3:272-290. [DOI] [PubMed] [Google Scholar]
- 3.Bossert, I., and R. Bartha. 1984. The fate of petroleum in soil ecosystems, p. 435-473. In R. M. Atlas (ed.), Petroleum microbiology. Macmillan Publishing Co., New York, N.Y.
- 4.Bowlen, G. F., and D. S. Kosson. 1995. In situ processes for bioremediation of BTEX and petroleum fuel products, p. 515-542. In L. Y. Young and C. E. Cerniglia (ed.), Microbial transformations and degradation of toxic organic chemicals. Wiley-Liss, Inc., New York, N.Y.
- 5.Braun-Lüllemann, A., C. Johannes, A. Majcherzyk, and A. Hüttermann. 1995. The use of white-rot fungi as active biofilters, p. 235-241. In R. E. Hinchee, G. D. Sayles, and R. S. Skeen (ed.), Biological unit processes for hazardous waste treatment. Battelle Press, Columbus, Ohio.
- 6.Chang, M. K., T. C. Voice, and C. S. Criddle. 1993. Kinetics of competitive inhibition and cometabolism in the biodegradation of benzene, toluene, and p-xylene by two Pseudomonas isolates. Biotechnol. Bioeng. 41:1057-1065. [DOI] [PubMed] [Google Scholar]
- 7.Cox, H. H. J., and H. J. Doddema. May1996. Biological filter for removing volatile hydrophobic compounds from gas emissions. EP-0,710,147.
- 8.Cox, H. H. J., R. E. Moerman, S. Van Baalen, W. N. M. Van Heiningen, H. J. Doddema, and W. Harder. 1997. Performance of a styrene-degrading biofilter containing the yeast Exophiala jeanselmei. Biotechnol. Bioeng. 53:259-266. [DOI] [PubMed] [Google Scholar]
- 9.Deeb, R. A., and L. Alvarez-Cohen. 1999. Temperature effects and substrate interactions during the aerobic biotransformation of BTEX mixtures by toluene-enriched consortia and Rhodococcus rhodochrous. Biotechnol. Bioeng. 62:526-536. [PubMed] [Google Scholar]
- 10.Duetz, W. A., B. Wind, J. G. van Andel, M. R. Barnes, P. A. Williams, and M. Rutgers. 1998. Biodegradation kinetics of toluene, m-xylene, p-xylene and their intermediates through the upper TOL pathway in Pseudomonas putida (pWWO). Microbiology 143:2331-2338. [DOI] [PubMed] [Google Scholar]
- 11.Hartmans, S., and J. Tramper. 1991. Dichloromethane removal from waste gases with a trickle-bed bioreactor. Bioprocess Eng. 6:83-92. [Google Scholar]
- 12.Jeener, J., B. H. Meier, P. Bachmann, and R. R. Ernst. 1979. Investigation of exchange processes by two-dimensional NMR spectroscopy. J. Chem. Phys. 71:4546-4553. [Google Scholar]
- 13.Lindley, N. D. 1992. Hydrocarbon-degrading yeast and filamentous fungi of biotechnological importance, p. 905-929. In D. K. Arora, R. P. Elander, and K. G. Mukerji (ed.), Handbook of applied mycology, vol. 4. Marcel Dekker, Inc., New York, N.Y. [Google Scholar]
- 14.Mackay, D., and W. Y. Shiu. 1981. A critical review of Henry's law constants for chemicals of environmental interest. J. Phys. Chem. Ref. Data 10:1175-1199. [Google Scholar]
- 15.Mehlman, M. A. 1992. Dangerous and cancer-causing properties of products and chemicals in the oil refining and petrochemical industry. VIII. Health effects of motor fuels: carcinogenicity of gasoline—scientific update. Environ. Res. 59:238-249. [DOI] [PubMed] [Google Scholar]
- 16.Oh, Y. S., Z. Shareefdeen, B. C. Baltzis, and R. Bartha. 1994. Interactions between benzene, toluene, and p-xylene (BTX) during their biodegradation. Biotechnol. Bioeng. 44:533-538. [DOI] [PubMed] [Google Scholar]
- 17.Prenafeta-Boldú, F. X., A. Kuhn, D. Luykx, H. Anke, J. W. van Groenestijn, and J. A. M. de Bont. 2001. Isolation and characterisation of fungi growing on volatile aromatic hydrocarbons as their sole carbon and energy source. Mycol. Res. 105:477-484. [Google Scholar]
- 18.Prenafeta-Boldú, F. X., D. M. A. Luykx, J. Vervoort, and J. A. M. de Bont. 2001. Fungal metabolism of toluene: monitoring of fluorinated analogs by 19F nuclear magnetic resonance spectroscopy. Appl. Environ. Microbiol. 67:1030-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saeed, T., and M. Al Mutairi. 1999. Chemical composition of the water-soluble fraction of the leaded gasolines in seawater. Environ. Int. 25:117-129. [Google Scholar]
- 20.Stapleton, R. D., D. C. Savage, G. S. Sayler, and G. Stacey. 1998. Biodegradation of aromatic hydrocarbons in an extremely acidic environment. Appl. Environ. Microbiol. 64:4180-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tijhuis, L., M. C. M. van Loosdrecht, and J. J. Heijnen. 1993. A thermodynamically based correlation for maintenance Gibbs energy requirements in aerobic and anaerobic chemotrophic growth. Biotechnol. Bioeng. 42:509-519. [DOI] [PubMed] [Google Scholar]
- 22.Yadav, J. S., and C. A. Reddy. 1993. Degradation of benzene, toluene, ethylbenzene, and xylenes (BTEX) by the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl. Environ. Microbiol. 59:756-762. [DOI] [PMC free article] [PubMed] [Google Scholar]