Abstract
The hydrovoltaic effect, based on interactions at the solid-liquid interface, offers a promising route for ion sensing. However, it is hampered by long response times, typically several minutes, due to slow ion diffusion equilibrium in nanochannels. Here, we demonstrate a rapid, flexible hydrovoltaic ion sensing strategy enabled by fast ion transport. Apart from the drag resistance reduction resulting from the ordered nanochannels and gravity elimination along the nanochannel direction, the liquid-driven effect concurrent with low-resistance shear flow at the liquid-liquid transport zone in semi-dry nanochannels are proposed to achieve an open-circuit voltage exceeding 4.0 V within 0.17 s, being two orders of magnitude faster than previous works with infiltration channels. Moreover, the obtained flexible hydrovoltaic device exhibits a wide ion sensing range of 10−7 to 100 M, a maximum sensitivity up to −1.69 V dec-1 for NaCl, and distinctive multi-dimensional signals, enabling its application in selective ion sensing and sweat electrolyte monitoring.
Subject terms: Electronic devices, Nanopores, Sensors, Polymers
The hydrovoltaic effect offers a promising route for ion sensing but is limited by a long response time. Here, the authors leverage rapid ion transport within nanochannels to achieve a high voltage output of 4.0 V with a response time of just 0.17 s.
Introduction
Hydrovoltaic technology1–7 based on the direct interaction between solid-liquid interface blazes a new trail for ion sensing8,9 in addition to energy harvesting from water10. However, restricted to flow resistance and gravity, it often takes several or even tens of minutes to achieve the diffusion equilibrium of water and ions under the driving of capillary infiltration and evaporation-induced flow in the functionalized nanochannels11,12, which is an unacceptable long process for ion sensing. Looking back at the field, massive efforts have been devoted to developing hydrovoltaic devices by materials and structural design, but mostly for the enhancement of the output voltage or current up to now13–16. A rapid response time is an urgent bottleneck issue when applying the hydrovoltaic effect to active ion sensing in fields from environmental monitoring to human electrolyte perspiration monitoring17–19.
Currently, the directional migration of ions with opposite charges to the nanochannels to form a gradient distribution is the essence of generating voltage signals for various hydrovoltaic devices20–23. The implication here is that a strong driving force from water flow can shorten the equilibrium time of ion migration to achieve a fast response. At the micro-nanoscale, increasing the channel size is a highly effective method to reduce the influence of viscous resistance at the solid-liquid interface and accelerate the water flow velocity24,25. But for hydrovoltaic devices that rely on the electric double layer (EDL) within narrow distances on the solid-liquid interface26–28, it’s a highly challenging trade-off between accrescent size induced high waterflow velocity and weakened interfacial interaction. In addition, the orientations of the nanochannels, surface wettability, and gravity are important factors as well to be cautiously considered for improving water transport velocity29–34. Exploring efficient and fast ion driving mechanisms in nanochannels for fast, sensitive hydrovoltaic ion sensing can bring broad opportunities for the development of hydrovoltaic sensing devices, but that is fraught with difficulties.
In this work, we demonstrate rapid hydrovoltaic ion sensing enabled by a fast ion transport and accumulation mechanism in nanochannels. By constructing a flexible horizontal hydrovoltaic device containing flexible oriented functionalized nylon-66 nanofilms (NNFs), the flow resistance introduced by structural dislocation and gravity in the solution transport direction is effectively reduced. Furthermore, the mechanism of the low-resistance shear flow at the liquid-liquid transport zone within semi-dry nanochannels is employed to realize a maximum water transport velocity of 2.86 cm s−1 to drive the migration of ions. Moreover, the rapid transport of new droplets also gives rise to a liquid-driven effect, promoting the synchronous forward migration and accumulation of the previous residual liquid containing ions. The synergistic advantages of the ion transport–accumulation mechanism enable rapid voltage generation, producing an open-circuit voltage (Voc) signal exceeding 4.0 V within 0.17 s using only a 3 µL water droplet, which is approximately two orders of magnitude faster than previously reported works. This hydrovoltaic device shows a wide ion sensing range of 10−7 to 100 M and a maximum sensitivity up to −1.69 V dec−1 for NaCl. More than expected, due to the tail drop in hydrodynamically-governed ion transport within nanochannels, which provides the fabricated hydrovoltaic device with more dimensional signal signatures for selective ion sensing, including electrolyte ions monitoring in sweat. Our hydrovoltaic ion-sensitive device, based on the rapid ion transport-accumulation, breaks through the limit of slow infiltration of solution in the nanochannels, bridging the hydrovoltaic effect and ion sensing.
Results
Ordered nanochannels and gravity elimination along the nanochannel direction resulted in drag resistance reduction
Figure 1a schematically shows the design of the hydrovoltaic device. Overall, the stable structural binding of nanochannels and the surface polarity enhancement are achieved by dip-coating carboxylated polystyrene (CPS) onto ordered NNFs fabricated by high-speed electrospinning. The fabrication process is presented in the “Methods” section. The CPS/DMF-coated NNF films undergo obvious shrinkage during solvent (DMF) evaporation, while the capillary force generated at the liquid-air interface induces compressive stress across the film, which compresses the NNF network vertically. Due to the presence of carboxyl groups (-COOH), CPS can form hydrogen bonds with carbonyl (C = O) and amino (-NH-) on NNFs to achieve interfacial binding and a compression ratio of approximately 3.2 times the initial thickness, shown in Fig. 1b–d and Supplementary Fig. 1a, b. The enhancement of the interface binding force of the CPS-coated NNF (CPS@NNF) film is demonstrated by a 10-fold increase from 19.84 to 199.97 MPa in elastic modulus of the CPS@NNF film perpendicular to the axis (Supplementary Fig. 2a, b). Apart from modifying CPS with a high carboxyl density of 368 mmol kg−1 to improve the surface polarity of the hydrovoltaic film (Supplementary Fig. 3), oxygen plasma treatment is also performed on the obtained oxidized CPS@NNFs (OCPS@NNFs) film to further increase the oxygen-containing functional groups on the surface, resulting an enhanced surface zeta potential of −50.9 mV (Fig. 1e). This result is further supported by the hydroxyl (C-OH) and C = O peaks located at 286 and 287.5 eV in X-ray photoelectron spectroscopy and the enhanced vibration peaks at 3301 cm−1 in Fourier transform infrared spectroscopy, shown in (Fig. 1f, Supplementary Fig. 4). Besides, the oxygen plasma treatment introduces oxygen groups (-COOH/-OH) on CPS@NNF, stabilizing local pH via acid-base equilibria and enhancing surface charge, which strengthen the EDL, promoting ion adsorption and transport efficiency35,36.
Fig. 1. Structural and surface property regulation effect of the Ordered O-CPS@NNF films.
a Schematic diagram of the composition, structure, and preparation of the ordered OCPS@NNF hydrovoltaic device. b Schematic diagram of the molecular composition of the OCPS@NNFs. c SEM images of Ordered NNF. Scale bar, 2 μm. d SEM images of Ordered OCPS@NNF. Scale bar, 2 μm. e Zeta potential of the NNF (Nylon-66 nanofilms), CPS@NNF (CPS-coated NNF), and OCPS@NNF (Oxidized CPS@NNF). f X-ray photoelectron spectroscopy (XPS) of NNF, CPS@NNF, and OCPS@NNF. g Optical photograph of the diffusion paths of D-OCPS@NNF (Dis-ordered OCPS@NNF) and O-OCPS@NNF (Ordered OCPS@NNF) with 3 μL deionized water droplets. Scale bar, 1 cm. h Optical images of the spreading process of D-OCPS@NNF and O-OCPS@NNF with a 3 μL deionized water droplet. Scale bars, 1 mm. i Pore size distribution of NNF, D-OCPS@NNF, and O-OCPS@NNF measured by mercury intrusion method. j Diffusion height of horizontally placed O-OCPS@NNF, D-OCPS@NNF, and vertically placed O-OCPS@NNF (right figure is an enlarged view within the time range of 0–1 s). k Infrared thermal images of the final diffusion paths of D-OCPS@NNF, and O-OCPS@NNF (left: horizontally placed; right: vertically placed). Scale bars, 1 cm. The error bars in (e) represent the standard deviations from 4 parallel measurements (n = 4). Source data are provided as a Source Data file.
The orientation of the fibers plays an important role in the flow resistance in the hydrovoltaic OCPS@NNF film. To visualize the effect of fiber orientation on water transport, two drops of 3 μL deionized water were dropped on ordered and disordered OCPS@NNF films (abbreviated O-OCPS@NNF and D-OCPS@NNF, respectively). When the fiber diameter is constant, the elliptic diffusion path formed by water droplets on the O-OCPS@NNF film is larger than that of water droplets on the D-OCPS@NNF film (Fig. 1g, Supplementary Fig. 5, and Supplementary Movie 1). The spread of droplet into the film was recorded in detail by a high-speed CCD camera with a side view: 3 μL droplets showed a smaller contact Angle (~12°) in contact with the ordered film and fully spread out in 9.23 s in about half the time of that on D-OCPS@NNF film, which proves the lower flow resistance of O-OCPS@NNF film (Fig. 1h, Supplementary Fig. 6, and Supplementary Movie 2). Quantitatively, when the sizes of the nanochannels formed by the adjacent OCPS@NNFs is about 350 nm (Fig. 1i), the maximum water transport velocity in the horizontal dry O-OCPS@NNF hydrovoltaic film reaches 2.39 cm s−1 higher than that of 2.06 cm s−1 in the dry D-OCPS@NNF film, shown in Fig. 1j, k. Moreover, with the increase of water transport distance on the films, the deceleration of water transport on the D-OCPS@NNF film caused by the flow resistance is more severe than that of the O-OCPS@NNF film. As shown in the Supplementary Fig. 7a, the O-OCPS@NNF film in the horizontal orientation achieved a peak voltage of 4.0 V, while the D-OCPS@NNF film (horizontal) exhibited a significantly lower peak voltage of 3.1 V. The above results strongly demonstrate the flow resistance reduction in the O-OCPS@NNF film. The effect of gravity is also taken into account, and the O-OCPS@NNF film placed horizontally in the dry state shows a faster water transport velocity of about 2.39 cm s−1 and a longer transport distance of 3.6 cm in 1800 s, greater than the vertically placed O-OCPS@NNF film of 1.51 cm s−1and 2.6 cm correspondingly. Furthermore, the vertically placed hydrovoltaic device generated a reduced peak voltage of 2.5 V (Supplementary Fig. 7b), compared to the horizontal one (4.0 V). The optical image in Supplementary Fig. 7c illustrates the vertical testing setup, where gravity counteracts capillary-driven water transport, slowing flow velocity and weakening ion accumulation. This can be explained by the Lucas-Washburn37,38 equation describing liquid climbing in capillaries:
| 1 |
Where is the capillary rise velocity, is the unbalanced atmospheric pressure, is the liquid surface tension, is the capillary radius, is the contact angle, is the liquid density, g is the gravity acceleration, is the capillary rise height, and is the liquid viscosity. When the film is placed horizontally, the influence of gravity in the vertical direction is nullified (that is, the term in the equation), enabling a greater driving force and higher water transport velocity.
Liquid-liquid transport zone design and fast ion transport triggered a rapid response
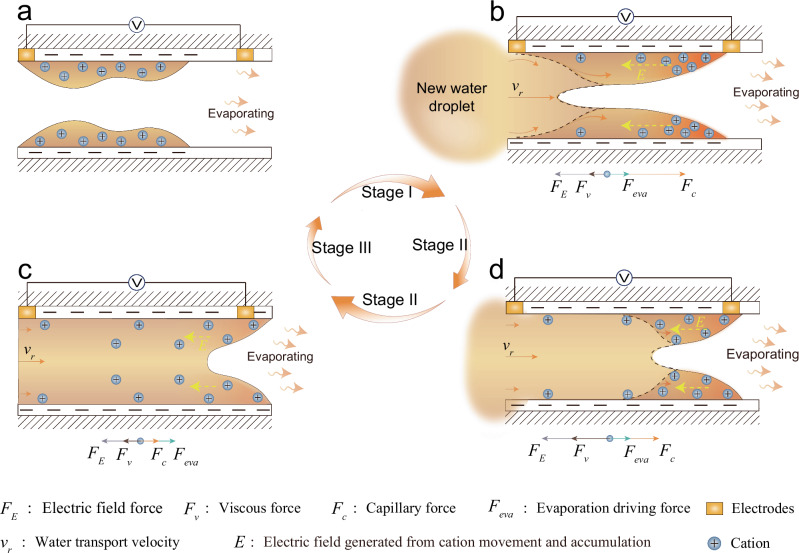
Naturally, the fabricated O-OCPS@NNF film was assembled with a flexible PET frame printed with patterned carbon electrodes to build a flexible hydrovoltaic device (Fig. 2a). Here, the carbon electrodes are inert and do not serve any role beyond acting as electrodes for hydrovoltaic devices. (Supplementary Figs. 8–10). When only a 3 μL small DI water droplet is applied to the bottom of a horizontally placed obtained device, as shown in the optical photograph Fig. 2b, an eye-catching voltage signal can be quickly generated. As shown in Fig. 2c and Supplementary Movie 3, the complete real-time Voc signal of the hydrovoltaic device from the dry state to the steady state is further investigated in detail. After the accumulation of the first three drops, the maximum Voc signal exceeding 4.0 V can be repeatedly realized. Staring at the Voc signal in Fig. 2d, a strongest voltage signal above 4.0 V is generated within 0.17 s after dropping the droplet (here we called Stage I), and then rapidly decays to a plateau period of slow decay at about 1.5 V with the advance of time (Stage II), and finally goes through a rapid decay to 0 V (Stage III).
Fig. 2. Device design and fast response performance of the hydrovoltaic device.
a Schematic diagram of the construction and sensing process of a fabricated hydrovoltaic device. O-OCPS@NNF refers to Ordered oxidized CPS-coated Nylon-66 nanofilm. b Optical photograph of the testing process of the device. c Real-time Voc signal of the hydrovoltaic device from the dry state to the steady state by regularly adding 3 μL of deionized water droplets. d Enlarged view of a single Voc signal. e Optical images of the diffusion process in a semi-dry channel of hydrovoltaic films (upper row: applying a 3 μL of deionized water droplet; bottom row: applying a 3 μL of 1 wt% sodium fluorescein aqueous solution and observed under ultraviolet irradiation). Scale bars, 2 mm. f Temperature distribution of the hydrovoltaic film at 0.17 s when applying a 3 μL hot water droplet. Scale bar, 1 cm. g Water content variation of the film at 0.1, 0.3, and 0.5 cm from the bottom electrode over a complete drop cycle. Testing conditions: 25 ± 1 °C, 30 ± 2% RH. The error bars in (g) represent the standard deviations from measurements of 3 independent samples (n = 3). Source data are provided as a Source Data file.
For our fast-response hydrovoltaic device, the essence of voltage generation lies in the unique strategy of fast and directional ion migration and accumulation driven by water flow. Therefore, the distinctive transmission state of water droplets in the channels on the O-OCPS@NNF film is then investigated. The transport process of a drop of 3 μL 1 wt% sodium fluorescein aqueous solution on the film after the end of the previous stable droplet cycle was first demonstrated by fluorescence rendering technology and optical images in Fig. 2e. Here the stable droplet cycle is defined as the signal generated by the droplet consisting of stable Stage I-III. After the droplets contact the film, the transport of water is carried out at a maximum linear and area velocities of 0.56 cm s−1 and 0.27 cm2 s−1 within 0.17 s, which corresponds to the rapid voltage peaks of Stage I on the timeline (Supplementary Fig. 11a–d). It must be highlighted that the rapid transport of droplets on the semi-dry film after the previous stable droplet cycle is actually no longer a liquid transport between solid and liquid interfaces, but is transformed into a composite system involving liquid transport at the solid-liquid and liquid-liquid transport zones. These remaining water from the previous cycle in the slit nanochannels can conspicuously diminish the contact area of the solid-liquid interface between the newly instilled droplets and the nanochannels, as well as decrease the interfacial friction and the local resistance arising from the geometrical shape variations of the solid nanochannel surface. Driven by the nanochannels-induced capillary force, the aqueous solution will form a sliding transport on the surface of the water film liquid with minute internal frictional forces, resulting in a visually faster water transport velocity than that on the completely dry O-OCPS@NNF film. As evidence, when the water source is bulk water, the maximum transport velocity on the semi-dry film reached 2.86 cm s−1, which was nearly 0.5 cm s−1 faster than that on the dry film (Supplementary Fig. 11a, b). At Stage II (t = 40 s to t = 60 s), the linear and area velocities gradually decay to the more stable 0.0058 cm s−1 and 0.0043 cm2 s−1 (Supplementary Fig. 11e, f), which is a typical case where the flow resistance increases sharply with the elongating of the transmission distance. According to the Washburn Equation for horizontal capillaries, we can infer that the capillary rise velocity is inversely proportional to the capillary rise height :
| 2 |
Throughout the whole process, the Laplacian pressure drives the diffusion of DI water towards the front dry area or regions with low water content of the O-OCPS@NNF film. Ultimately, as a result of the continuous consumption by evaporation, the water content on the O-OCPS@NNF film gradually reduces, leading to a decrease in the area covered by water on the film.
In addition to dramatic drag reduction and shear-driven transport mechanisms, the small amount of water in the nanochannels at the end of a stable droplet cycle is highly important to achieve rapid ion accumulation. Comparing the above optically and fluorescent synchronous images, an interesting phenomenon can be found that the upper edge of the new water droplet is lower than the edge covered by the remaining water from the previous cycle on the film, shown in Fig. 2e, t = 10 s. And with the water droplets transmitted on the film, the upper edge of the water from the last cycle arrives at the upper electrode before the new water droplets edge (Fig. 2e). Apart from optical and fluorescent images, infrared thermal images also demonstrated that new droplet could be transported about 0.25 cm within 0.17 s less than the electrodes spacing of 0.6 cm (Fig. 2f, Supplementary Figs. 12, 13), although the water transport velocity is quickish.
To further quantify the water transport model on the O-OCPS@NNF film, the water content of the film at 0.1, 0.3, and 0.5 cm from the bottom electrode over a complete drop cycle was measured. As shown in Fig. 2g, before the start of a new droplet cycle (t = 0 s), the water content on the film decreases from 1.17 to 0.19 mg cm−2, corresponding to 0.1 to 0.5 cm, which proves that the hydrovoltaic device is semi-dry at the end of the previous droplet cycle. Surprisingly, when a 3 μL droplet contact with the semi-dry O-OCPS@NNF film, the water content increased to 0.46 mg cm−2 in a very short time of 0.17 s as far as 0.5 cm away from the bottom electrode, which can be understood as the liquid-driven effect, meaning that new water droplets push the water in the semi-dry nanochannels forward while moving forward driven by capillary force, thus achieving an instantaneous increase in water content at the top of the nanochannels. Because this synchronous pushing effect is faster than the capillary-driven water transport, the maximum water content at 0.3 cm and 0.5 cm comes at about 10 s and 40 s, respectively. Finally, the water content on the whole film decreased gradually after 40 s due to the evaporation of water.
Mechanism analysis
At this point, a working mechanism consistent with our hydrovoltaic device can be delineated. The polar -COOH groups on the surface of the semi-dry film, covered with a small amount of water, release freely moving hydronium ions (H3O+). When the hydrovoltaic device is in its initial semi-dry state, water within the O-OCPS@NNF film remains confined beneath the top electrode without establishing contact (Fig. 3a). After the new drops of water enter, hydronium ions are pushed to the top of the nanochannel instantaneously with the water from the previous cycle, forming a high-density cation layer that generates an electric field (Stage I) under the combined effects of low-resistance shear flow and synchronous push force of newly added water droplets (Fig. 3b and Supplementary Figs. 14, 15). The residual liquid film enables the detection of the electric field generated by the rapid accumulation of cations (e.g., H3O+) at the top electrode during Stage I. As time progresses, reduced water transport velocity in the nanochannels leads to insufficient thrust to counteract the Coulomb repulsion from the electric field formed by high-density cations. This causes the reflux of hydronium ions at the top and a corresponding decline in the voltage signal (Stage II, Fig. 3c, d). Here, due to the relatively slow water transport, the influence of water evaporation on flow velocity in the nanochannels becomes significant and will be discussed in detail below. Finally, with the continuous consumption of water by evaporation, the area covered by water on the film fails to cover the top electrode, resulting in a rapid decay of the voltage signal to zero during Stage III (Fig. 3d and Fig. 3a).
Fig. 3. Mechanism of rapid hydrovoltaic ion sensing.
a Schematic diagram of the semi-dry hydrovoltaic nanochannel at the end of a Voc signal cycle. b Schematic diagram showing dynamics of water and ions in the semi-dry nanochannels when applying a new water droplet (Stage I). An unbalanced force exists: . c Schematic diagram of the nanochannel when the Voc signal starts to decrease (Stage II, t = 0.17 s to t = 40 s) because of the existence of unbalanced force that . d Schematic diagram of the nanochannel when the Voc signal is relatively stable (Stage II, t = 40 s to t = 60 s) due to the existence of force balance .
The effect of device geometry is first investigated. As shown in Fig. 4a, b, when the electrode spacing (i.e., film length) increases from 2 mm to 10 mm at a fixed film width of 8 mm, the peak open-circuit voltage (Voc) rises from 2.6 V to 4.0 V and then slightly declines to 3.8 V. The maximum output is achieved at an electrode spacing of 6 mm. This trend is attributed to the increase in freely moving hydronium ions (H₃O⁺) provided by the carboxylated nanochannel walls with longer channel lengths. However, longer nanochannels also introduce greater flow resistance. An optimal balance between ion supply and flow resistance is achieved at 6 mm. Similarly, with the electrode spacing fixed at 6 mm, films with smaller widths contain fewer hydronium ions, while excessively wide films cause lateral droplet diffusion, which reduces ion transport efficiency along the nanochannel direction and decreases electrical output (Supplementary Fig. 16a, b). Therefore, a hydrovoltaic device with an electrode spacing of 6 mm and a width of 8 mm was selected for this study.
Fig. 4. Influencing factors of the hydrovoltaic device performance.
a Schematic diagram showing the definition of electrode distance and film width. b Voc signal of the hydrovoltaic device with different electrode distances at a fixed film width of 8 mm. c Voc signal of the hydrovoltaic device when applying water droplets of different volumes. d Voc signal of the hydrovoltaic device when applying a 3 μL water droplet at different Stages. e Voc signal of the hydrovoltaic device exposed to air flow with speeds of 0, 1.0, and 1.5 m s−1. f Voc signal of the hydrovoltaic devices fabricated with NNF of 13 wt%, 17 wt%, and 21 wt% (right figures show the amplified rise curves of their corresponding Voc signal). g Voltages and response times of hydrovoltaic devices working with deionized water via capillary infiltration. The detailed parameters are listed in Supplementary Table 1. h Dynamics of water and charge species in the semi-dry hydrovoltaic nanochannels with small (upper) and large (bottom) pore sizes when applying new water droplets. Testing conditions: 25 ± 1 °C, 30 ± 2% RH. Source data are provided as a Source Data file.
Water, as the carrier of energy, is central to the electricity generation of the hydrovoltaic device. Therefore, the effect of droplet volume was subsequently investigated. As depicted in Fig. 4c, when the droplet volume increases from 1 μL to 9 μL, the peak voltage signal remains approximately 4.1 V, except for a slight decrease to 3.9 V with the 9 μL droplet. This is primarily because the droplet volume does not alter the water transport behavior during Stage I. However, the 9 μL droplet is excessively large, and the water extends slightly beyond the bottom electrode, covering parts of the O-OCPS@NNF film that do not contribute effectively to charge transport. This leads to relatively slower ion migration in that region and a slight reduction in voltage (Supplementary Fig. 17). It is interesting to note that as the droplet volume increases, the duration of Stage II gradually becomes longer, and a voltage plateau of approximately 1.3 V appears when the droplet volume exceeds 5 μL. This plateau can be attributed to a dynamic equilibrium between the water flow driven by evaporation and capillarity, and the Coulombic repulsion resulting from the electric field generated by ion accumulation.
Particularly, if new droplets are introduced midway through the preceding droplet cycle, no Voc peak signal is observed. As shown in Fig. 4d, when droplets are added at the end of Stage II, the hydrovoltaic signal exhibits a stable plateau at approximately 1.3 V instead of a peak. This plateau can be sustained by subsequent droplets. This phenomenon is attributed to the incompressibility of water. Once the nanochannels are filled with a high water content, they cannot accommodate a large volume of new water. As a result, rapid forward transport is suppressed. The newly added droplets can only move slowly by replenishing the evaporated water at the front end (Supplementary Fig. 18). Furthermore, if the droplet is placed near the upper electrode instead of the inlet, the hydrovoltaic voltage signal is inverted, confirming the directionality of ion migration and accumulation along the water flow path (Supplementary Fig. 19).
Since our hydrovoltaic devices operate in an open environment, the influence of water evaporation is further investigated. As shown in Fig. 4e, when exposed to air flow with speed of 0, 1.0 and 1.5 m s−1, the Voc peak signal of the hydrovoltaic device in Stage I exhibits almost no discernible change, remaining at about 4.1 V. Nevertheless, the Voc signal value in Stage II exhibits a consistent increase with rising air flow velocity, aligning with the mechanism previously discussed. In Stage I, liquid-liquid transport zone slippage and synchronous propulsion primarily dominate the rapid ion transport and accumulation, and at this point, the influence of evaporation on the water velocity is insignificant. In Stage II, the increase in flow resistance and the attenuation of the synchronous propulsion effect dramatically manifest the influence of water evaporation on the water velocity, and a high wind speed has a positive facilitating effect on the Voc signal. Accompanied by the enhancement of a high evaporation rate in Stage II, the water droplet cycle will also be conspicuously shortened from 56 s to approximately 40 s (Fig. 4e). Ambient relative humidity (RH) also plays an important role in influencing hydrovoltaic signal, as shown in Supplementary Fig. 20. When RH increases from 20 to 40%, the peak voltage (~4.0 V) remains consistent, indicating that the initial ion transport and accumulation processes are unaffected within this humidity range. However, the post-peak voltage plateau decreases from 1.7 V (lasting ~20 s) to 1.1 V (extending to ~80 s), demonstrating that higher humidity levels prolong water retention within the semi-dry nanochannels, thereby sustaining ion transport and inflecting the plateau duration and amplitude.
At the nanoscale, the influence of the channel diameter on the flow resistance dominated by viscous forces is highly significant. According to Supplementary Equation (13), the rate of work performed in response to the frictional force on the capillary wall can be written as , where is the one-order derivative of the capillary height . Combing the Washburn Equation for horizontal capillary, , and , we have:
| 3 |
From which we can tell that is proportional to , and viscous forces sharply increase with the increase of the radius of the capillary. Subsequently, O-OCPS@NNF films with channel diameters ranging from 678 nm to 151 nm were constructed through regulating the concentration of the electrospinning solution from 21 wt% to 13 wt% correspondingly to investigate the influence pattern of channel scale (Supplementary Fig. 21). As anticipated, the hydrovoltaic device with a channel diameter of 151 nm shows a response time of 34.85 s, a maximum peak Voc of approximately 2.0 V and a water droplet cycle time of 121.87 s due to the considerable channel flow resistance-induced slow water transport, which is conspicuously inferior to the 0.17 s response time and 4.1 V peak Voc of the device with a channel diameter of 350 nm (Fig. 4f and Supplementary Fig. 22a). To the best of our knowledge, 0.17 s represents the fastest response time for the current hydrovoltaic devices, which is approximately two orders of magnitude faster compared to the previously reported works (Fig. 4g and Supplementary Table S1). Nevertheless, although the hydrovoltaic device with channel diameter of 676 nm exhibits a marginally faster response time than the device with 350 nm channel, the maximum peak Voc signal is merely approximately 2.51 V (Fig. 4f and Supplementary Fig. 22b). Explaining this anomalous phenomenon of voltage reduction with the increase of flow velocity requires a return to the ion distribution at the solid-liquid interface. As illustrated in Fig. 4h, the interface between fibers possessing -COOH groups and water engenders an electrical double layer (EDL) resulting from the dissociation of these carboxyl groups. The EDL consists of a compact region of ions that remains immobile relative to the solution, along with a diffusive layer with ions that can flow with the solution, within which, the ion concentration decays exponentially from the compact layer to a finite range in the water body. In this work, the essence of electricity generation resides in the utilization of high-velocity water flow to drive the directional migration and accumulation of ions in the diffusion layer. The velocity distribution at the cross-section of the hydrovoltaic nanochannel satisfies:
| 4 |
Where is the capillary radius, is the pressure drop, is distance from the center of the channel. From the standpoint of fluid dynamics, while larger channels exhibit reduced overall flow resistance, the transmission of water flow is predominantly concentrated in the central region of channels characterized by low ion concentration, resulting in a diminished migration of ions to the upper sections of the channels along with the water flow and a declined Voc signal.
Highly sensitive and selective ion sensing
In general, the Debye length () denotes the distance within which charges or electric fields can propagate in a plasma, as well as farthest distance that the EDL is capable of covering. The corresponding Debye-Hückel equation39,40 is expressed as follows:
| 5 |
where , , , and are the permittivity of water, the permittivity of a vacuum, the concentration of the solution, and the valence number, respectively. F, T, and R represent Faraday’s constant, absolute temperature, and the universal gas constant, respectively. Consequently, the EDL is theoretically suppressed sharply with the increase of ion concentration in the solution. In order to validate the aforementioned speculation, we investigated the response performance of our rapid-responsive hydrovoltaic device to NaCl solutions. As shown in Fig. 5a, b, Supplementary Fig. 23a, b and Supplementary Fig. 24a–f, when the ion concentration increases from 10-7 to 100 M, the peak Voc signal of the device drops from 3.6 to 0.03 V, demonstrating the wide ion sensing range of the device. Particularly in the interval from 10−4 to 10−2 M, the device possesses a sensitivity as high as −1.69 V dec−1. Here, we define the sensitivity (S) as
| 6 |
where V and c are the Voc and ion concentrations, respectively.
Fig. 5. Ion sensing performance of the hydrovoltaic device.
a Voc response of the hydrovoltaic device to NaCl solutions. The shaded regions from left to right represent the sensing signals in response to NaCl solutions ranging from 10−7 to 100 M. b Peak voltages (Vpeak, T = 0.17 s) of the Voc signal responses to NaCl solutions from 10−7 M to 100 M. c Schematic diagram showing the dynamics of the Ion species in the semi-dry hydrovoltaic nanochannels when applying a drop of salt solution. d Real-time Voc signal of the hydrovoltaic device when applying droplets of salt solution in the order of 10−6 M, 10−4 M and 10−3 M. e Voltages at different time points of the Voc signal responses to NaCl solutions from 10−7 M to 100 M. f Three-dimensional (3D) sensing surface of the hydrovoltaic device to NaCl solutions from 10−7 M to 100 M. g Vpeak signals of the hydrovoltaic device in NaCl, KCl and CaCl2 solutions from 10−6 M to 10-1 M. h 3D sensing surfaces of the hydrovoltaic device to NaCl, KCl and CaCl2 solutions from 10−6 M to 10−1 M. i Amplified figure of the 3D sensing surfaces at 10−4 M. Testing conditions: 25 ± 1 °C, 30 ± 2% RH. The error bars in (b, e, g) represent the standard deviations from 3 parallel measurements (n = 3). Source data are provided as a Source Data file.
Systematic analysis of the ionic sensing performance and the EDL model of the hydrovoltaic devices can rationally explain the ionic sensing mechanism of our hydrovoltaic devices. As illustrated in Fig. 5c, within the nanochannels of our hydrovoltaic device, the EDL at the nanochannel-solution interface is suppressed sharply with the increase of ion concentration in the solution, which leads to a corresponding reduction in the net charge that can be accumulated forward with the water transport as ion concentration rises, ultimately resulting in a lower Voc signal. In line with the aforementioned trend is that while the overlapping EDL within 350 nm diameter nanopores facilitates ion selectivity, this selectivity is diminished as the presence of ion pairs suppresses the double layer. Additionally, when solutions of 10−6 M, 10−4 M, and 10−3 M are sequentially and repeatedly dropped onto the same obtained device, the Voc sensing signal of the hydrovoltaic device for ions exhibits reliable repeatability (Fig. 5d), which can still be maintained when the dropping sequence is 100 μM–1 μM–100 μM (Supplementary Fig. 25).
Due to the fact that the Voc signal of the hydrovoltaic device within the water droplet cycle encompasses a highly distinctive variation process, which offers the possibility for our hydrovoltaic device to be employed in selective ion sensing. We extracted the Voc signals at 0.17, 1, 3, 5, 10, 20, 30, 40, and 50 s within a droplet cycle to reconstitute the NaCl concentration-Voc relationship curves. As depicted in Fig. 5e, the signal clusters formed by Voc measurements at multiple time points enhance the richness and differentiation of the sensing signals for various concentrations of NaCl solutions. Moreover, when we present the data of time, ion concentration, and Voc signal in a three-dimensional fashion in Fig. 5f, the response curves of the device at different concentrations become readily distinguishable to the naked eye, which suggests that our hydrovoltaic device possesses the potential for ion-selective recognition capability.
To verify the selective recognition ability of our designed hydrovoltaic device for ions, the response performance of the device to the solutions of NaCl, KCl, and CaCl2 within the concentration range from 10−6 to 10−1 M was investigated. As shown in Supplementary Fig. 26 and Fig. 5g, under the identical concentration condition, the peak Voc signals of the device in response to the three distinct solutions exhibit marked discrepancies and decrease in the order of NaCl, KCl, and CaCl2, which can be ascribed to the flow resistance effect of large-sized cations and the more severe compression effect of high-valence ions on the EDL under the premise of the same anion type. Nevertheless, such a simple peak signal poses challenges for discriminating solutions of different concentrations and types. In marked contrast, upon the introduction of the time scale, the aforementioned minute differences are significantly magnified and can be distinctly separated (Fig. 5h, i). For instance, the sensing signals for 10 μM CaCl2 and 10 μM KCl at 0.17 s, 30 s, and 60 s respectively correspond to 2.25, 1.09, 0.86 and 2.08, 0.78, 0.65 V. Compared with the single peak voltage signals under the aforementioned ionic conditions, the abundant variances offer a reliable foundation for highly selective identification of ion types and concentrations.
Application
The fast and highly ion-sensitive response properties of our hydrovoltaic devices motivate their application in the rapid monitoring of electrolytes in sweat. Moreover, the inherent flexibility and scalable preparation characteristics of the flexible hydrovoltaic devices render this application more feasible (Fig. 6a). Electrolyte ions such as Na+, K+, Ca2+, and Cl- are essential for maintaining the body’s water balance and electrolyte balance41,42. Long-term intense exercise can cause dehydration, electrolyte loss, fluid disorders, and cramps43. The utilization of our rapid-response hydrovoltaic device to monitor sweat is therefore expected to provide a basis for the timely replenishment of electrolyte water to prevent excessive water loss. Among the diverse electrolyte constituents present in sweat, NaCl exhibits the most pronounced variation in concentration during physical exertion, whereas other components demonstrate only marginal changes44,45. Nevertheless, in the interest of maintaining rigor, before the body test, simulated sweat containing 5 mM KCl, 5 mM CaCl2 and a concentration gradient-varying NaCl from 10 mM to 80 mM was subjected. As shown in Fig. 6b, c, the voltage signal of the device decreases regularly in correspondence with the increase in the concentration of NaCl, demonstrating the device’s reliable sensing ability for the variation of a single component in complex ion circumstances. Subsequently, our hydrovoltaic device was used on a subject cycling at a constant power of 100 W. As shown in Fig. 6d, from the beginning of sweating in 10 min, the subject’s sweat was tested every 20 min until the completion of the 90 min exercise without replenishing water. Voc signal exhibited a reduction as the exercise duration prolonged. Typically, the peak voltage signal decreased from 98 mV to 71 mV, which corresponds to a Na+ concentration of 20.1 mM to 68.2 mM, when we approximately adopt the curves in Fig. 6b, c as the standard curve. In contrast, during exercise, continuously replenishing water for 90 min at a rate of 100 mL per 10 min, the voltage signal can stabilize at 88 mV after 50 min, indicating that the Na+ in sweat is stable at about 38.0 mM (Fig. 6e). In actuality, the subtleties of the influence of water replenishment on the body’s electrolytes are conspicuously displayed in three-dimensional data (Fig. 6g), which illustrate that effective water replenishment contributes to the stability of electrolyte ion concentrations in sweat, suggesting that our hydrovoltaic device may serve as a promising tool for sweat health monitoring. Furthermore, the Voc signals of the hydrovoltaic sensor for pure DI water and triglyceride-containing DI water (5 wt%) exhibited nearly identical profiles (Supplementary Fig. 27), with peak voltages of 4.0 V, which validates the robustness of the hydrovoltaic sensor for sweat electrolyte monitoring, even in the presence of oily secretions.
Fig. 6. Application of the hydrovoltaic device.
a Optical photograph of (left) laser cut electrode array and (right) hydrovoltaic device bent at 120°. b Voc responses of the hydrovoltaic device to the simulated sweat with different NaCl concentrations from 10 mM to 80 mM. c 3D sensing surface of the hydrovoltaic device to the simulated sweat. d Real-time Voc signal responses of the hydrovoltaic device to the sweat at different time points during cycling without water replenishment. e Real-time Voc signal responses to the sweat at different time points with multiple water replenishment. f Vpeak signal responses of the hydrovoltaic device to the sweat at different time points during cycling with and without water replenishment. g 3D sensing surface to the conditions of water intake and no water intake. Testing conditions: 25 ± 1 °C, 30 ± 2% RH. The error bars in (f) represent the standard deviations from 3 parallel measurements (n = 3). Source data are provided as a Source Data file.
Discussion
In summary, we developed a mechanism that facilitates rapid directional transport and accumulation of ions in nanochannels, achieving a Voc signal exceeding 4.0 V within 0.17 s, bringing the hydrovoltaic effect one step closer to application in reliable ion sensing. To achieve a breakthrough in the response time from several or even tens of minutes, the orientation of nanochannel structures and the elimination of gravity were initially demonstrated to be conducive to accelerating water transport. Nevertheless, the most core element lies in the liquid-driven effect of new droplets on the residual solution of the previous droplet cycle, which occurs concurrently with the low-resistance shear flow at the liquid-liquid transport zone within the semi-dry nanochannels. Furthermore, the developed flexible hydrovoltaic device exhibited remarkable ion sensing capabilities with a NaCl sensing range of 10−7 to 100 M and a maximum sensitivity up to −1.69 V dec−1. Well beyond expectations, in the water droplet circulation influenced by flow resistance, the Voc signal of the hydrovoltaic device encompasses highly distinctive variation processes, enabling the construction of a multi-dimensional signal of time-ion concentration-voltage system for highly selective ion sensing and electrolyte ion monitoring in sweat. Prospectively, hydrovoltaic ion sensors could achieve higher sensitivity and faster response times by minimizing device dimensions and increasing the density of polar functional groups (-COOH/-OH). These strategies would enhance ion selectivity and transport efficiency, enabling applications in real-time health monitoring and environmental sensing.
Methods
Fabrication of electrospinning nylon-66 nanofiber film (NNF)
Nylon-66 polymer solution with a mass fraction of 17 wt% was prepared by dissolving Nylon-66 (N303420, Aladdin, China) into formic acid (99%, Aladdin, China) under continuous stirring at room temperature for 3 h. The prepared solution was then used in electrospinning. The polymer solution was loaded into a 5 mL plastic syringe that was attached to a stainless-steel needle with an inner diameter of 0.5 mm. The effluent velocity, the low voltage, the high voltage, the translation distance of needle, the receiving distance, the operating temperature, and the relative humidity of the electrospinning process were fixed at 0.5 mL h−1, −2 kV, 28 kV, ±5 cm, 6 cm, 25 °C, and 70%, respectively. The Nylon-66 fibers were layer by layer collected on a target rotating collector for 230 min. The rotation speed of the rotating collector was set at 3500 rpm to obtain the ordered nanofibers film and 100 rpm to obtain the disordered nanofibers film. Then, the obtained free-standing Nylon-66 electrospinning fiber membranes were dried at 80 °C for 12 h to remove residual solvents for further use.
Fabrication of carboxyl-functionalized polystyrene (CPS)
Carboxyl-functionalized polystyrene (CPS) was successfully obtained by the soap-free emulsion method. The corresponding chemical reaction scheme is shown in Supplementary Fig. 30.
0.9 g of acrylic acid (AA, 98%, Adamas, China), 6 g of styrene (St, 99%, Aladdin, China), and 96 mL of DI water were added into a three-neck round-bottom flask. Then the mixture was stirred vigorously under a nitrogen atmosphere using a magnetic stirrer. When the mixture was heated to 70 °C, 4 mL of ammonium persulfate (APS, 98%, Greagent, China) aqueous solution (mAPS = 0.2 g) was added into the reactor drop by drop to initiate polymerization. The reaction continued for 8 h at 70 °C under uniform stirring of 350 rpm. The obtained suspension was centrifuged at approximately 21,600 × g for 20 min and then washed with ethanol and DI water 3 times, respectively. Ultrasonic dispersion is used in the middle of each washing. The obtained CPS was poured into a glass dish and dried at 60 °C for 12 h to remove residual moisture. Finally, the obtained white powder was dissolved in N,N-dimethylformamide (DMF, 99.8%, Adamas, China) with a mass fraction of 1 wt% for the dip coating procedure.
Fabrication of OCPS@NNF hydrovoltaic ion sensors
The dried nylon-66 nanofiber film was cut into strips of 0.8 × 1 cm2. The cut films were dipped in the 1 wt% CPS/DMF solution for a few seconds to obtain CPS coating, and then were placed on a hot plate at 100 °C for complete drying. The obtained film was then treated with Oplasma cleaner (PDC-MG, Mingheng, China) for 1 min, the current used is 180 mA. Poly(ethylene terephthalate) (PET) films (≈150 μm) were cut into specific shapes by laser direct writing to serve as the electrode substrate and hollow film cap. The distance between the top and bottom electrodes was 0.6 cm. The obtained PET films were then cleaned in ethanol with ultrasonication and then dried in an 80 °C oven. Chemically inert conductive carbon paste was printed on the substrate by screen printing with two L-shaped structures as electrodes. The hydrovoltaic ion sensors were finally obtained by assembling the OCPS@NNF film and the PET films.
Characterization
A digital camera (Canon EOS 72D) was used to take the photographs in this study. The FOTRIC 850-Ex infrared thermal imager was used to detect the temperature and infrared thermal images. The morphology of the samples was examined by scanning electron microscopy (SEM, JSM-7001 F). The Nylon-66 Electrospinning fiber membranes were fabricated by an Electrospinning machine (YFSP-T) manufactured by Yunfan (Tianjin) Instrument Co., LTD. A centrifuge (Heraeus Multifuge X3R, Thermo Fisher Scientific, USA) is used for centrifugation of the SF aqueous solution. The voltage and current signals were recorded in real time using a Keithley 6514 electrometer. NS-EM Electrometer Control Software v1.0.8 was used for the data collection. The ambient temperature and humidity were controlled by a temperature and humidity cabinet manufactured by Guangzhou Wusuo Environmental Equipment Co., Ltd. Origin version 2024 (OriginLab, USA) was used to analyze and visualize the data.
Ethics
The performance validation of the sweat sensor was conducted with a single human subject. The participant (a 25-year-old male) is one of the authors of this article and therefore participated in the study without compensation. Sex was self-reported. Due to the single-subject design, no sex- or gender-based analyses were performed, and disaggregated sex and gender data are not applicable. Written informed consent was obtained from the participant prior to the study. The study was conducted in accordance with ethical regulations and was approved by the Ethics Committee of the Suzhou Institute of Nano-Tech and Nano-Bionics (Approval ID: SINANO/EC/2022-075).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary File
Source data
Acknowledgements
The authors acknowledge the funding support from the National Natural Science Foundation of China (22109173, Lianhui Li, 62471465, Lianhui Li, 62071463, Ting Zhang, 62271479, Shuqi Wang, 62401561, Mingxu Wang), the National Natural Science Foundation for Distinguished Young Scholars of China (62125112, Ting Zhang), China Postdoctoral Science Foundation (2023M742562, Lianhui Li, 2024M762320, Mingxu Wang), the Strategic Priority Research Program of the Chinese Academy of Science (Grant No. XDB0520301, Ting Zhang), and the Natural Science Foundation of Jiangsu Province (BK20243004, Ting Zhang). The authors are grateful for the technical support from the Nano-X Vacuum Interconnected Workstation & Key Laboratory of Multifunctional Nanomaterials and Smart Systems of Suzhou Institute of Nano-Tech and Nano-Bionics (SINANO), Chinese Academy of Sciences (CAS).
Author contributions
T.Z. and L.L. conceived the idea and designed the experiments. C.G., L.L., M.W., and Y.Z. performed the experiments. L.L., C.G., Y.W., F.Z., C.Z., J.M., F.W., S.W., M.L., S.W., Y.L., and H.S. analyzed the data, discussed the results. L.L. and C.G. wrote the original manuscript. F.S. and T.Z. helped revise it.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data supporting the findings of this study are available within the article and its supplementary files. Any additional requests for information can be directed to, and will be fulfilled by, the corresponding authors. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lianhui Li, Email: lhli2015@sinano.ac.cn.
Ting Zhang, Email: tzhang2009@sinano.ac.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-63549-1.
References
- 1.Xue, G. et al. Water-evaporation-induced electricity with nanostructured carbon materials. Nat. Nanotechnol.12, 317–321 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Zhang, Z. et al. Emerging hydrovoltaic technology. Nat. Nanotechnol.13, 1109–1119 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Li, L. et al. Enhancing hydrovoltaic power generation through heat conduction effects. Nat. Commun.13, 1043 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim, H. et al. Hydrovoltaic electricity generator with hygroscopic materials: a review and new perspective. Adv. Mater.36, 2301080 (2024). [DOI] [PubMed] [Google Scholar]
- 5.Wang, X. F. et al. Hydrovoltaic technology: from mechanism to applications. Chem. Soc. Rev.51, 4902–4927 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Tan, J. et al. Harvesting energy from atmospheric water: grand challenges in continuous electricity generation. Adv. Mater.36, 2211165 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Yin, J., Zhou, J. X., Fang, S. M. & Guo, W. L. 11 Hydrovoltaic energy on the way. Joule4, 1852–1855 (2020). [Google Scholar]
- 8.Li, L. et al. A flexible tough hydrovoltaic coating for wearable sensing electronics. Adv. Mater.35, 2304099 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Ge, C. et al. Silk fibroin-regulated nanochannels for flexible hydrovoltaic ion sensing. Adv. Mater.36, 2310260 (2024). [DOI] [PubMed] [Google Scholar]
- 10.Li, L. et al. A hydrovoltaic power generation system based on solar thermal conversion. Nano Energy99, 107356 (2022). [Google Scholar]
- 11.Yun, T. G., Bae, J., Rothschild, A. & Kim, I. D. Transpiration driven electrokinetic power generator. ACS Nano13, 12703–12709 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Xia, H. et al. Electricity generated by upstream proton diffusion in two-dimensional nanochannels. Nat. Nanotechnol.19, 1316–1322 (2024). [DOI] [PubMed] [Google Scholar]
- 13.Li, L. et al. Sustainable and flexible hydrovoltaic power generator for wearable sensing electronics. Nano Energy72, 104663 (2020). [Google Scholar]
- 14.Hu, Q. C., Ma, Y. J., Ren, G. P., Zhang, B. T. & Zhou, S. G. Water evaporation-induced electricity with Geobacter sulfurreducens biofilms. Sci. Adv.8, eabm8047 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang, C. et al. Transfer learning enhanced water-enabled electricity generation in highly oriented graphene oxide nanochannels. Nat. Commun.13, 6819 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren, G. et al. Growth of electroautotrophic microorganisms using hydrovoltaic energy through natural water evaporation. Nat. Commun.15, 4992 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu, X. H. et al. Ionic-liquid doping enables high transconductance, fast response time, and high ion sensitivity in organic electrochemical transistors. Adv. Mater.31, 1805544 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Zheng, M. et al. Fast response and high sensitivity europium metal organic framework fluorescent probe with chelating terpyridine sites for Fe3+. ACS Appl. Mater. Interfaces5, 1078–1083 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature529, 509–514 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin, Y. S. et al. Constant electricity generation in nanostructured silicon by evaporation-driven water flow. Angew. Chem., Inter. Ed.59, 10619–10625 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Li, L. et al. A novel, flexible dual-mode power generator adapted for wide dynamic range of the aqueous salinity. Nano Energy85, 105970 (2021). [Google Scholar]
- 22.Hu, Q. et al. Hydrovoltaic electricity generation induced by living leaf transpiration. Nat. Water2, 988–998 (2024). [Google Scholar]
- 23.Liu, X. M. et al. 40 Power generation from ambient humidity using protein nanowires. Nature578, 550–554 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Chen, H. et al. Continuous directional water transport on the peristome surface of Nepenthes alata. Nature532, 85–89 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Lv, C. et al. Substrate curvature gradient drives rapid droplet motion. Phys. Rev. Lett.113, 026101 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Liu, X. et al. Microbial biofilms for electricity generation from water evaporation and power to wearables. Nat. Commun.13, 4369 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He, N. et al. Ion engines in hydrogels boosting hydrovoltaic electricity generation. Energ. Environ. Sci.16, 2494–2504 (2023). [Google Scholar]
- 28.Garemark, J. et al. Advancing hydrovoltaic energy harvesting from wood through cell wall nanoengineering. Adv. Funct. Mater.33, 2208933 (2023). [Google Scholar]
- 29.Chen, H. et al. Ultrafast water harvesting and transport in hierarchical microchannels. Nat. Mater.17, 935–942 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Li, J. et al. Directional transport of high-temperature Janus droplets mediated by structural topography. Nat. Phys.12, 606–612 (2016). [Google Scholar]
- 31.Feng, S. et al. Three-dimensional capillary ratchet-induced liquid directional steering. Science373, 1344–1348 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Li, C. et al. Bioinspired inner microstructured tube controlled capillary rise. Proc. Natl. Acad. Sci.116, 12704–12709 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang, J. et al. Directional pumping of water and oil microdroplets on slippery surface. Proc. Natl. Acad. Sci. USA116, 2482–2487 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun, Q. et al. Surface charge printing for programmed droplet transport. Nat. Mater.18, 936–941 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Ma, Y., Yeh, L. H., Lin, C. Y., Mei, L. & Qian, S. pH-regulated ionic conductance in a nanochannel with overlapped electric double layers. Anal. Chem.87, 4508–4514 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Mei, L., Yeh, L.-H. & Qian, S. Buffer anions can enormously enhance the electrokinetic energy conversion in nanofluidics with highly overlapped double layers. Nano Energy32, 374–381 (2017). [Google Scholar]
- 37.Washburn, E. W. The Dynamics of Capillary Flow. Phys. Rev.17, 273–283 (1921). [Google Scholar]
- 38.Szekely, J., Neumann, A. W. & Chuang, Y. K. The rate of capillary penetration and the applicability of The Washburn equation. J. Colloid Inferface Sci.35, 273–278 (1971). [Google Scholar]
- 39.Kortschot, R. J., Philipse, A. P. & Erne, B. H. Debye length dependence of the anomalous dynamics of ionic double layers in a parallel plate capacitor. J. Phys. Chem. C.118, 11584–11592 (2014). [Google Scholar]
- 40.Schoch, R. B., Han, J. Y. & Renaud, P. Transport phenomena in nanofluidics. Rev. Mod. Phys.80, 839–883 (2008). [Google Scholar]
- 41.Patterson, M. J., Galloway, S. D. R. & Nimmo, M. A. Variations in regional sweat composition in normal human males. Exp. Physiol.85, 869–875 (2004). [DOI] [PubMed] [Google Scholar]
- 42.van Doremaele, E. R. W., Ji, X., Rivnay, J. & van de Burgt, Y. A retrainable neuromorphic biosensor for on-chip learning and classification. Nat. Electron.6, 765–770 (2023). [Google Scholar]
- 43.Wang, S. et al. Wearable sweatband sensor platform based on gold nanodendrite array as efficient solid contact of ion-selective electrode. Anal. Chem.89, 10224–10231 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Nyein, H. Y. Y. et al. A wearable microfluidic sensing patch for dynamic sweat secretion analysis. ACS Sens.3, 944–952 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Min, J. et al. Skin-interfaced wearable sweat sensors for precision medicine. Chem. Rev.123, 5049–5138 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary File
Data Availability Statement
All data supporting the findings of this study are available within the article and its supplementary files. Any additional requests for information can be directed to, and will be fulfilled by, the corresponding authors. Source data are provided with this paper.