Abstract
The Chinese Expert Consensus on central precocious puberty (CPP) defines girls’ rapid sexual development before age 8 as CPP; while after age 8 as early normal puberty (ENP). And the use of recombinant human growth hormone (rhGH) for CPP and ENP is off-label and lacks reliable evidence for clinical practice. This study only included girls due to the low prevalence among boys. We aimed to compare the long-term efficacy and safety of gonadotrophin releasing hormone analogue (GnRHa) in combination with or without rhGH for the treatment of CPP and ENP, and to explore the differences in the efficacy of different age of intervention. The medical information of girls with CPP or ENP at a women’s and children’s hospital from January 2013 to December 2018 was retrospectively collected. The primary outcome of efficacy was final adult height (FAH), and the secondary outcome included height gain, genetic height gain, standard deviation score of final adult height (FAHSDS), and standard deviation score of height (HSDS) gain. The safety outcomes were the rate of at least one adverse event of any type and the rate of each adverse event. We included factors with P < 0.05 in baseline analysis, and factors from systematic review and clinical experience as covariates in multivariable linear regression to adjust the clinical treatment choice, and subgroup analysis was taken to explore the efficacy of interventions at different ages. A total of 182 girls with CPP or ENP were finally included in this study. The adjusted results of multivariable linear regression showed that the mean(SD) of FAH in the combination therapy group (CG) (162.58 [0.46] cm) was higher than the monotherapy group (MG) (160.25 [0.35] cm) and the no treatment group (NG) (158.39 [0.47] cm) (P < 0.001), and the height gain(CG: 4.00 [0.46] cm, MG: 1.68 [0.36] cm, NG: − 0.18 [0.47] cm, P < 0.001), genetic height gain(CG: 6.18 [0.46] cm, MG: 3.85 [0.35] cm, NG: 1.99 [0.47] cm, P < 0.001), FAHSDS (CG: 0.66 [0.08], MG: 0.24 [0.06], NG: − 0.13 [0.08], P < 0.001), and HSDS gain(CG: 0.26 [0.08], MG: − 0.17 [0.06], NG: − 0.54 [0.08]) in CG were all higher than MG and NG. Besides, the incidence of at least one adverse event of any type in the CG was higher than MG and NG (CG: 83.30%, MG: 15.00%, NG: 16.70%, P < 0.001), among which the incidence of fasting insulin elevation (CG: 55.60%, MG: 1.25%, NG: 2.08%, P < 0.001) and hypothyroidism(CG: 44.4%, MG: 0.00%, NG: 0.00%, P < 0.001) was higher than the other two groups. Subgroup analysis indicated that, compared with the NG, the MG showed no differences in FAH in girls who entered puberty after the age of 8 years (1.46 [− 0.01, 2.93], P = 0.051) and those treated with GnRHa for less than 1 year (0.30 [− 1.34, 1.94], P = 0.718). Compared with the NG, there were no differences in FAH between the CG and MG (1.41 [− 0.76, 3.58], P = 0.204, 1.70 [− 0.77, 4.16], P = 0.178) in girls who initiated pharmacotherapy at the age of 10–12 years. Compared with the MG, the CG showed no differences in FAH in girls treated with rhGH in combination for less than 1 year (1.48 [− 0.09, 3.05], P = 0.064). The combination of GnRHa and rhGH can improve the FAH of girls with CPP and ENP to a certain extent, especially for those who began pharmacotherapy before 10 years of age and continued treatment for more than 1 year, but meanwhile increased the incidence of adverse events. The benefits, risks, and affordability of medication should still be comprehensively considered before decisions on pharmacotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-02740-2.
Keywords: Gonadotropin-releasing hormone agonist, Recombinant human growth hormone, Central precocious puberty, Early normal puberty, Retrospective cohort study
Subject terms: Diseases, Endocrinology, Health care, Medical research
Introduction
Puberty refers to the period from the appearance of secondary sexual characteristics to the maturation of the gonads1. The average age of onset of puberty is approximately 10.96 (1.88) years for normal girls and 11.81 (1.04) years for normal boys; the timing of puberty depends on genetic and environmental factors2–4. Precocity should be considered when the onset age of puberty is 2 to 2.5 standard deviations earlier than the average age of the normal population5. Among these, the early sexual development triggered by the premature activation of the hypothalamic–pituitary–gonadal axis (HPGA) before the age of 8 years for girls and 9 years for boys is deemed to be central precocious puberty (CPP), with an approximately tenfold higher incidence in girls than in boys6–8. Besides, those who experience the early stage of puberty but with rapid progression in the Tanner scale within 6 months are defined as early normal puberty (ENP), generally between 8–10 years of age in girls and 9–11 years of age in boys9,10. CPP and ENP have similar pathogenesis and clinical manifestations. The difference is whether rapid sexual development occurs before or after the defined time of puberty. The Chinese expert consensus of CPP holds that children are defined as fast-progressing CPP if the onset of rapid sexual development occurs before the age of 8 for girls or 9 for boys, or defined as ENP if the onset of rapid sexual development occurs later11. And both can be potentially harmful to children, including accelerated linear growth, advanced bone age (BA), earlier closure of epiphyses, shorter final adult height (FAH), and related psychological or social problems due to sexual maturity 12–14.
According to the Chinese expert consensus of CPP, GnRHa is recommended for the treatment of both CPP and ENP due to its similar therapeutic mechanisms in these two diseases15. Commonly used GnRHa, for example leuprolide and triptorelin, work by occupying the gonadotropin-releasing hormone (GnRH) receptors. This prevents the physiologic activation from the hypothalamus. It also continuously stimulates the pituitary gonadotrophs, causing the desensitization of gonadotroph cells. This leads to the suppression of gonadotropins and a reduction in sex steroids. As a result, it helps delay pubertal development and the advancement of bone age16. However, further observation and study of GnRHa revealed some issues. After treatment, the growth rate decreased to varying degrees among different patients. Some children even showed obvious growth deceleration. Their growth velocity (GV) was lower than that of normal prepuberty. This resulted in impaired height and failure to achieve the target height (TH). However, the exact mechanism of GnRHa-induced growth deceleration is still unclear17. In addition, some parents have high expectations for the FAH of their children and do not prefer the efficacy of GnRHa, hoping to achieve the growth catch-up with peers through further treatment.
It was proposed that recombinant human growth hormone (rhGH), as an adjunctive therapy, combined with GnRHa might compensate for the loss of height18,19, which takes effect by stimulating the hepatic insulin-like growth factor 1 (IGF-1) production to synergistically promote cellular proliferation and differentiation20. Treatment with rhGH is most commonly used for growth hormone deficiency, idiopathic short stature, and small for gestational age, etc. However, the utilization in CPP and ENP represents off-label applications. The recommendations of GnRHa combined with rhGH in relevant guidelines have not reached an agreement and more evidence is needed to evaluate the benefits and risks of combination therapy15,21,22.
Previous studies reported contradictory results on the FAH and height gain between the GnRHa combined with rhGH regimen and the GnRHa alone regimen. Kim and his colleagues23 indicated that the FAH of girls with CPP who received GnRHa in combination with rhGH (159.62 [3.22] cm) was lower than that of those who received GnRHa alone (162.03 [4.19] cm) (P = 0.02), and the height gain was similar between the two groups. However, a cohort study published by Fu et al.24 found no difference presented in FAH among the combination therapy group (160.0 [0.51] cm), the monotherapy group (159.7 [0.28] cm), and the no treatment group (158.0 [0.55] cm) (P = 0.174), but the height gain of the combination therapy group (9.51 [0.53] cm) was higher than the monotherapy group (8.07 [0.37] cm) (P = 0.048). The opposite conclusions among the studies may be attributed to the baseline imbalance between the groups. The impacts on outcomes from the uncontrolled potential confounding factors may affect the authenticity and reliability of the results, which cannot effectively support clinical decision-making. Besides, unclear adverse effects are potential risks of off-label use of rhGH. Some studies have suggested that the incidence of adverse effects of rhGH (for example, dislocation capital femoral epiphysis and scoliosis, benign intracranial hypertension, decreased insulin sensitivity) may be relevant to the underlying diseases of the treated children, which is the lowest in idiopathic growth hormone deficiency and idiopathic short stature (78 per 100 000 per year) and highest in Prader-Willi syndrome and craniopharyngioma (184 per 100 000 per year), respectively20,25. However, there is still a lack of long-term observation in CPP or ENP, and the safety of combination therapy is not yet clear.
Considering the off-label nature of rhGH in the treatment of CPP or ENP, this study aimed to evaluate the efficacy and safety of GnRHa in combination with or without rhGH for girls with CPP or ENP through a retrospective cohort study via multivariable linear analysis to control for the confounding factors, and explore the differences in the efficacy of interventions at different ages to provide evidence-based support for guiding rational medication in clinical practice.
Materials and methods
Study design and data source
We conducted a retrospective cohort study at West China Second University Hospital, Sichuan University, the largest women’s and children’s hospital located in western China. Considering that rhGH was introduced in the hospital since 2013, and the treatment duration required for final adult height (FAH), we recruited girls who were first diagnosed with CPP and ENP from January 1, 2013, to December 31, 2018. The data were extracted from the electronic medical databases, including the hospital information system, picture archiving and communication system, and laboratory information system.
Participants
The eligible patients were consecutively selected based on the following inclusion criteria15:
-
(i)
Girls with CPP or ENP;
-
(ii)
CPP: the appearance of thelarche before the age of 8 years or menarche before the age of 10 years; ENP: the development of above secondary sexual characteristics between the age of 8–10 years but each of Tanner stage progression less than 6 months;
-
(iii)
The basal serum luteinizing hormone (LH) ≥ 0.83 IU/L, or the GnRH stimulation test with positive based on immunochemiluminescence (i.e., the peak value of LH ≥ 5.0 IU/L, and the ratio of the peak value of LH and the peak value of follicle stimulating hormone (FSH) ≥ 0.6);
-
(iv)
The pelvic ultrasound showed the ovarian volume ≥ 1 mL with multiple follicles ≥ 4 mm in diameter;
-
(v)
BA exceeded the chronological age (CA);
The patients were excluded if those who:
-
(i)
were diagnosed as secondary CPP with clear etiology, including central nervous system abnormalities such as occupational lesions, tumors, infections, acquired injuries, etc., or other diseases such as congenital hypothyroidism, congenital adrenocortical hyperplasia, McCune-Albright syndrome, etc.;
-
(ii)
or combined with other gonad-related disorders, such as ovarian tumors, Turner syndrome, etc.;
-
(iii)
or combined with other diseases that may affect height, such as growth hormone deficiency, small for gestational age, etc.;
-
(iv)
or missed the key data, such as height or BA at the initiation or end of intervention, and FAH (defined as the height at BA > 15 years, or annual GV < 1 cm/year, or > 2 years after menarche);
-
(v)
or received treatment outside the hospital and had no consecutive follow-up data in electronic medical databases.
Exposures and outcomes
According to the clinician’s prescriptions, patients who received GnRHa combined with rhGH consisted of the combination therapy group (CG) (exposure group I), those who received GnRHa alone consisted of the monotherapy group (MG) (exposure group II), and those who refused or did not receive pharmacotherapy consisted of the no treatment group (NG) (control group). Among them, GnRHa was adopted with Leuprorelin Acetate Microspheres for Injection (BEIYI®, 3.75 mg, Shanghai Livzon Pharmaceutical Group Inc.; or ENANTONE®, 3.75 mg, Takeda Pharmaceutical Co., Ltd.), or Triptorelin Acetate for Injection (Diphereline®, 3.75 mg, Ipsen Pharma Biotech.), with a therapeutic dose of 3.75 mg intramuscularly injected every 4 weeks; and rhGH was adopted with Recombinant Human Growth Hormone Injection (Jintropin®, 30IU/10mg, Changchun Genescience Pharmaceutical Co., Ltd.), or Recombinant Human Growth Hormone for Injection (Ansomone®, 4IU/1.33mg, Anhui Anke Biotechnology (Group) Co., Ltd.), with a therapeutic dose 0.05–0.07mg/kg subcutaneously injected once a day before sleep.
The basic information of eligible patients was collected including age of puberty onset, age of treatment initiation, disease type, height, weight, body mass index (BMI), standard deviation score of height (HSDS, = [height − average height at the same age]/[standard deviation of height at the same age])26, target height (TH, = [height of father + height of mother]/2 − 6.5), Tanner stage, BA, BA advancement (BA-CA), predicted adult height (PAH)27, age at menarche, treatment duration of GnRHa, treatment duration of rhGH, the number of PAH below the normal range (PAH < average height of peers at the age of achieving final adult height [AHPA]-2SD, the number of PAH below the parental target range (PAH < TH-2SD), the number of PAH below the both (PAH < Both [AHPA-2SD and TH-2SD]), the number of FAH below the normal range, the number of FAH below the parental target range, the number of FAH below the both.
The primary outcome was FAH, defined as the height at BA > 15 years or annual GV < 1 cm/year or > 2 years after menarche. Secondary outcomes included: (i) height gain, defined as the difference between FAH and initial PAH (FAH-PAH0); (ii) genetic height gain, defined as the difference between FAH and TH (FAH-TH); (iii) FAHSDS, defined as the standard deviation score of FAH, which can eliminate the effects of differing standard deviations in different age, gender, and ethnicity; and (iv) HSDS gain, defined as the difference between FAHSDS and initial HSDS (FAHSDS-H0SDS). Besides, the safety outcomes included: (i) the rate of at least one adverse event of any type (including injection site local reactions, post-injection vaginal bleeding, elevated fasting blood glucose, impaired glucose tolerance, hypothyroidism, elevated uric acid, elevated aminotransferases, skin rashes, menstrual disorders, and overweight/obesity), and (ii) the rate of each adverse events.
Statistical analysis
Continuous variables of normally distributed data (including age of puberty onset, age of treatment initiation, height, weight, BMI, HSDS, TH, BA, BA advancement and PAH) were described as mean (standard deviation, SD) and median (P25, P75) for non-normally distributed data (including treatment duration of GnRHa, treatment duration of rhGH dose of rhGH and age of menarche), while categorical variables (including disease type, Tanner stage, PAH < AHPA-2SD, PAH < TH-2SD, PAH < Both, FAH < AHPA-2SD, FAH < TH-SD and FAH < Both) were described as frequency (percentage). For missing data, median for continuous variables (including age of puberty onset, age of treatment initiation, height, weight, BMI, HSDS, TH, BA, BA advancement, PAH, treatment duration of GnRHa, treatment duration of rhGH dose of rhGH and age of menarche) and mode for categorical variables (including disease type, Tanner stage, PAH < AHPA-2SD, PAH < TH-2SD, PAH < Both, FAH < AHPA-2SD, FAH < TH-SD and FAH < Both) were used for imputation. To identify differences of baseline characteristics among treatment groups, we compared baseline characteristics using various statistical methods, continuous variables of normally distributed were compared by ANOVA and continuous variables of non-normally distributed were compared by Kruskal–Wallis test, while categorical variables were compared by chi-square test. Multivariable linear regression was employed to compare the differences in FAH, height gain, genetic height gain, FAH standard deviation score (FAHSDS), and HSDS gain across treatment groups. We included factors with P < 0.05 (including age of treatment initiation, TH, HSDS before treatment initiation, BA before treatment initiation and PAH before treatment initiation) in baseline analysis, and age of puberty onset from systematic review and clinical experience as covariates in multivariable linear regression to adjust the clinical treatment choice. In terms of sample size, we conducted a post-hoc power analysis through the final effect (power > 0.8) and sample size (184 cases) by PASS (Power Analysis and Sample Size 2021, NCSS, USA), and the power is > 0.8, which means the sample size is adequate. Subgroup analysis was further used to explored FAH differences stratified by disease type (CPP ≤ 8 years vs. ENP > 8 years), age at treatment initiation (6–8, 8–10, 10–12 years), GnRHa treatment duration (≤ 1, 1–2, > 2 years), and rhGH (≤ 1, 1–2, > 2 years). In the sensitivity analysis, we 1) excluded individuals with missing data on baseline variables; and 2) compared the baseline characteristics of those who followed and those who were lost-to-follow-up populations within groups to test the robustness. All statistical analysis were performed using IBM SPSS (IBM Statistical Product and Service Solutions, Chicago, IL, US) software (version 26.0).
Results
Characteristics of participants
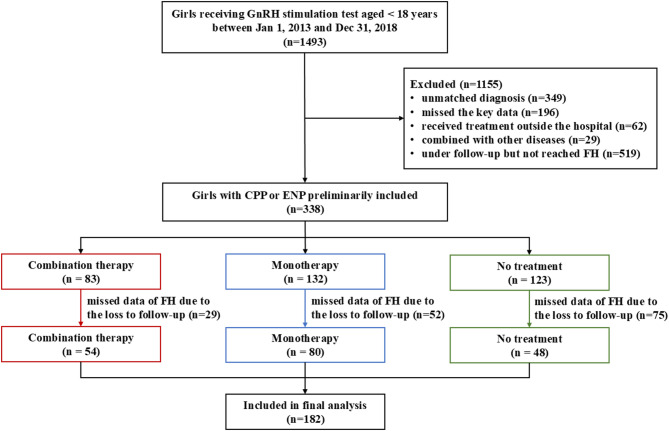
In total, the information of 1493 potentially eligible girls was extracted from the electronic medical databases. According to the inclusion and exclusion criteria, 338 girls with CPP or ENP were preliminarily included, among which 156 girls with missing data of FAH were excluded. A total of 182 patients were finally enrolled in this study, including 54 girls in the CG, 80 girls in the MG, and 48 girls in the NG (Fig. 1).
Fig. 1.
Flow diagram of participants with CPP and ENP. Notes: GnRH: gonadotropin-releasing hormone; CPP: central precocious puberty; ENP: early normal puberty; FAH: final adult height.
Before the initiation of treatment, there were no differences in the age of puberty onset, disease type, height, weight, BMI, Tanner stage, BA advancement, PAH below the normal range, PAH below the parental target range and PAH below both among the three groups (P > 0.05). The age of treatment initiation of CG (9.61 [0.86]) was larger than MG (9.25 [0.73]) and NG (9.12 [0.63]) (P = 0.003). The HSDS of NG (0.78 [1.00]) was larger than CG (0.02 [0.82]) and MG (0.45 [0.91]) (P < 0.001). The TH of NG (157.39 [4.24]) was larger than CG (155.04 [3.50]) and MG (156.73 [3.97]) (P = 0.007). The BA of CG (11.08 [0.60]) was larger than MG (10.68 [0.60]) and NG (10.58 [0.68]) (P < 0.001). And the PAH of NG (160.86 [5.92]) was larger than CG (156.23 [4.76]) and MG (158.78 [5.56]) (P = 0.007). In addition, the treatment duration of GnRHa was similar between the CG and the MG. The characteristics of the included girls are shown in Table 1.
Table 1.
Characteristics of the included participants in this study.
| Variables | Combination therapy (n = 54) | Monotherapy (n = 80) | No treatment (n = 48) | F/χ2 | P |
|---|---|---|---|---|---|
| Baseline | |||||
| Age of puberty onset (years) | 8.43 (1.19) | 8.29(1.03) | 8.14(0.74) | 1.000 | 0.370 |
| Age of treatment initiation (years) | 9.61 (0.86) | 9.25 (0.73) | 9.12(0.63) | 6.075 | 0.003 |
| Disease type | 2.282 | 0.319 | |||
| CPP | 16 (29.6%) | 31 (38.8%) | 21 (43.8%) | ||
| ENP | 38 (79.4%) | 49 (61.2%) | 27 (56.2%) | ||
| H0 (cm) | 138.14 (5.58) | 138.43 (5.50) | 139.66(6.10) | 1.024 | 0.361 |
| W0 (kg) | 33.29 (4.92) | 32.77(4.64) | 34.28 (5.80) | 1.349 | 0.262 |
| BMI0 (kg/cm2) | 17.39 (1.93) | 17.06(1.94) | 17.48(1.92) | 0.850 | 0.429 |
| H0SDS | 0.02 (0.82) | 0.45(0.91) | 0.78(1.00) | 9.010 | < 0.001 |
| TH (cm) | 155.04 (3.50) | 156.73(3.97) | 157.39(4.24) | 5.053 | 0.007 |
| Tanner stage | 0.848 | ||||
| B2 | 24 (44.4%) | 38 (47.5%) | 25 (52.1%) | ||
| B3 | 18 (33.3%) | 27 (33.8%) | 16 (33.3%) | ||
| B4 | 9 (16.7%) | 13 (16.2%) | 7 (14.6%) | ||
| B5 | 3 (5.56%) | 2 (2.50%) | 0 (0.00%) | ||
| BA0 (years) | 11.08 (0.60) | 10.68 (0.60) | 10.58 (0.68) | 9.731 | < 0.001 |
| PAH0 (cm) | 156.23 (4.76) | 158.78 (5.56) | 160.86 (5.92) | 9.317 | < 0.001 |
| BA0-CA0 (years) | 1.47 (0.70) | 1.43 (0.60) | 1.47 (0.58) | 0.100 | 0.905 |
| Treatment duration of GnRHa (years) | 1.50 (1.17–2.15) | 1.33 (1.06–1.83) | / | 0.051 | |
| Treatment duration of rhGH (years) | 1.25 (0.85–1.73) | / | / | / | |
| Dose of rhGH (IU) | 6.50 (5.80–7.38) | / | / | / | |
| PAH < AHPA-2SD* | 3/54 (5.55%) | 1/80 (1.25%) | 0/48 (0.00%) | 4.245 | 0.120 |
| PAH < TH-2SD* | 3/54 (5.55%) | 2/80 (2.50%) | 0/48 (0.00%) | 2.968 | 0.227 |
| PAH < Both* | 3/54 (5.55%) | 1/80 (1.25%) | 0/48 (0.00%) | 4.245 | 0.120 |
| Treatment end | |||||
| age of treatment end (years) | 12.08 (0.95) | 10.58 (0.73) | – | 107.033 | < 0.001 |
| H1 (cm) | 153.95 (4.72) | 146.10 (6.29) | – | 60.829 | < 0.001 |
| W1 (kg) | 46.06 (6.32) | 39.05 (6.32) | – | 39.702 | < 0.001 |
| BMI1 | 19.41 (2.25) | 18.23 (2.30) | – | 8.690 | 0.004 |
| H1SDS | 0.26 (0.81) | 0.30 (0.87) | – | 0.082 | 0.775 |
| BA1 (years) | 12.44 (0.85) | 11.45 (0.57) | – | 65.254 | < 0.001 |
| PAH1 (cm) | 164.85 (5.44) | 161.60 (5.85) | – | 10.564 | 0.001 |
| ΔPAH (cm) | 8.62 (4.66) | 2.82 (3.13) | – | 74.273 | < 0.001 |
| BA1-CA1 (years) | 0.51 (0.73) | 0.93 (0.64) | – | 12.393 | 0.001 |
| age of menarche (years) | 12.0 (11.5–12.8) | 12.0 (11.6–12.4) | 11.0 (10.3–11.9) | – | < 0.001 |
| FAH < AHPA-2SD* | 0–54 (0.00%) | 0–80 (0.00%) | 0–48 (0.00%) | – | – |
| FAH < TH-2SD* | 0–54(0.00%) | 0–80 (0.00%) | 0–48 (0.00%) | – | – |
| FAH < Both* | 0–54(0.00%) | 0–80 (0.00%) | 0–48 (0.00%) | – | – |
Numbers 0 and 1 refer to the phase at the initiation and end of intervention, respectively.
H, height; W, weight; BMI, body mass index; TH, target height; HSDS, standard deviation score of height; BA, bone age; PAH, predicted final height; BA-CA, BA advancement, refers to the difference between bone age and chronological age; ΔPAH, the difference between predicted final height before and predicted final height after treatment; GnRH, gonadotropin-releasing hormone; rhGH, recombinant human growth hormone. FAH, final adult height; AHPA, average height of peers at the age of achieving final adult height.
*PAH < AHPA-2SD = PAH below the normal range; PAH < TH-2SD = PAH below the parental target range; PAH < Both = PAH below the normal range and the parental target range; FAH < AHPA-2SD = FAH below the normal range; FAH < TH-2SD = FAH below the parental target range;FAH < Both = FAH below the normal range and the parental target range.
Significant values are in [bold].
Final adult height
The unadjusted analysis showed that the mean (SD) of FAH of the CG, MG, and NG was 160.31(5.11) cm, 160.61(5.03) cm, and 160.35(5.06) cm, respectively, and no difference was observed among the three groups (P = 0.935). The FAHSDS was also similar among the CG (0.24[0.87]), MG (0.31[0.93]), and NG (0.24[0.92]) (P = 0.858). Besides, the height gain (CG: (4.08 [4.22])cm, MG: (1.83 [3.61])cm, NG(− 0.51 [3.81]), P1[CG VS MG] = 0.001, P2[CG VS NG] < 0.001, P3[MG VS NG] = 0.001;P < 0.001), genetic height gain (CG: 5.27 [4.51] , MG: 3.88[4.37], NG: 2.97[4.98] , P1 = 0.086, P2 = 0.012, P3 = 0.278, P = 0.039), and HSDS gain (CG: 0.21[0.72], MG: − 0.14[0.62], NG: − 0.54[0.78], P1 = 0.004, P2 < 0.001, P3 = 0.002, P < 0.001) were all different among the three groups.
There are six potential covariates included in the multivariable linear regression analysis model, of which age of treatment initiation, TH, H0SDS, BA0, and PAH0 are selected based on P < 0.05 in the univariate analysis of baseline characteristics, and age of puberty onset is from clinical experience and expert consultation. After adjustment, the results of multivariable linear regression revealed that all of the height outcomes were different among the three groups (P < 0.001). The adjusted mean FAH of the CG, MG, and NG were 162.58 (0.46) cm, 160.25 (0.35) cm, and 158.39 (0.47) cm, respectively. The height gain of the three groups was 4.00 (0.46) cm, 1.68 (0.36) cm, and − 0.18 (0.47) cm, and the genetic height gain of the three groups was 6.18 (0.46) cm, 3.85 (0.35) cm, and 1.99 (0.47) cm. The FAHSDS of the CG, MG, and NG were 0.66 (0.08), 0.24 (0.06), and − 0.13 (0.08), and the HSDS gain of the three groups was 0.26 (0.08), − 0.17 (0.06), and − 0.54 (0.08), respectively (Table 2).
Table 2.
The final height of the included participants.
| Variables | Combination therapy (n = 54) | Monotherapy (n = 80) | No treatment (n = 48) | F | P | P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|---|
| Before adjustment | ||||||||
| FAH | 160.31 (5.11) | 160.61 (5.03) | 160.35 (5.06) | 0.068 | 0.935 | 0.739 | 0.964 | 0.785 |
| FAH-PAH0 | 4.08 (4.22) | 1.83 (3.61) | − 0.51 (3.81) | 17.997 | < 0.001 | 0.001 | < 0.001 | 0.001 |
| FAH-TH | 5.27 (4.51) | 3.88 (4.37) | 2.97 (4.98) | 3.308 | 0.039 | 0.086 | 0.012 | 0.278 |
| FAHSDS | 0.24 (0.87) | 0.31 (0.93) | 0.24 (0.92) | 0.153 | 0.858 | 0.636 | 0.994 | 0.655 |
| FAHSDS-H0SDS | 0.21 (0.72) | − 0.14 (0.62) | − 0.54 (0.78) | 15.051 | < 0.001 | 0.004 | < 0.001 | 0.002 |
| After adjustment | ||||||||
| FAH | 162.58 (0.46) | 160.25 (0.35) | 158.39 (0.47) | 17.682 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| FH-PAH0 | 4.00 (0.46) | 1.68 (0.36) | − 0.18 (0.47) | 17.682 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| FH-TH | 6.18 (0.46) | 3.85 (0.35) | 1.99 (0.47) | 17.682 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| FHSDS | 0.66 (0.08) | 0.24 (0.06) | − 0.13 (0.08) | 21.250 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| FHSDS-H0SDS | 0.26 (0.08) | − 0.17 (0.06) | − 0.54 (0.08) | 21.303 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
P1: combination therapy VS monotherapy; P2 combination therapy VS no treatment; P3: monotherapy VS no treatment; FAH: final adult height; FAH-PAH0: height gain, refers to the difference between final adult height and predicted adult height before treatment; FAH-TH: genetic height gain, refers to the difference between final adult height and target height; FAHSDS: the standard deviation score of final adult height; FAHSDS- H0SDS: HSDS gain, refers to the difference between the standard deviation score of final adult height and that of height before treatment.
*The age of puberty onset, age of treatment initiation, the standard deviation score of height before treatment, target height, bone age before treatment, and predicted final height before treatment were included as covariates in the general linear model to adjust for the impacts of confounding factors.
Significant values are in [bold].
Adverse events
A total of 45 girls (83.30%) had at least one adverse event of any type in the CG, 12 girls (15.00%) in the MG, and 8 girls (16.70%) in the NG, which were different among the three groups (P < 0.001). There were 11 kinds of adverse events involved in this study. Among them, the rate of elevated fasting insulin (CG: 55.6% [30/54], MG: 1.25% [1/80], NG: 2.08% [1/48], P < 0.001) and hypothyroidism (CG: 44.4% [24/80], MG: 0.00% [0/80], NG: 0.00% [0/48], P < 0.001) in the CG were higher than in the MG and the NG. The rates of other adverse effects are listed in Table 3.
Table 3.
The adverse events of the included participants.
| Variables | Combination therapy (n = 54) | Monotherapy (n = 80) | No treatment (n = 48) | P |
|---|---|---|---|---|
| At least one adverse event of any type | 45 (83.30%) | 12 (15.00%) | 8 (16.70%) | < 0.001 |
| Local reactions at the injection site | 0 (0.00%) | 2 (2.50%) | 0 (0.00%) | 0.505 |
| Vaginal bleeding after injection | 2 (3.70%) | 1 (1.25%) | 0 (0.00%) | 0.464 |
| Elevated fasting blood glucose | 2 (3.70%) | 1 (1.25%) | 0 (0.00%) | 0.464 |
| Impaired glucose tolerance | 2 (3.70%) | 1 (1.25%) | 0 (0.00%) | 0.464 |
| Elevated fasting insulin | 30 (55.60%) | 1 (1.25%) | 1 (2.08%) | < 0.001 |
| Hypothyroidism | 24 (44.4%) | 0 (0.00%) | 0 (0.00%) | < 0.001 |
| Elevated uric acid | 3 (5.56%) | 0 (0.00%) | 1 (2.08%) | 0.060 |
| Elevated transaminase | 0 (0.00%) | 1 (1.25%) | 0 (0.00%) | 1.000 |
| Rash | 1 (1.85%) | 0 (0.00%) | 0 (0.00%) | 0.560 |
| Menstrual disorders | 4 (7.41%) | 4 (5.00%) | 2 (4.17%) | 0.772 |
| Overweight/obesity | 4 (7.41%) | 3 (3.75%) | 4 (8.33%) | 0.487 |
Significant values are in [bold].
The reported adverse events were all mild and no serious adverse event had happened in this study. In addition to 6 patients with elevated fasting insulin and 3 patients with hypothyroidism who failed to recover to normal levels after the discontinuation of medication in the CG, the remaining adverse events that occurred during medication all got back to normal levels after withdrawal.
Subgroup analysis
Among the girls whose puberty onset before 8 years of age, who were also defined as fast progressive CPP, the FAH of the CG and MG was higher than that of NG (5.76 [3.65, 7.88], P < 0.001, 2.30[0.59, 4.02], P = 0.009). When puberty was onset after 8 years of age, who were also defined as ENP, the FAH of the CG was higher than that of NG (3.19 [1.55, 4.83], P < 0.001), and difference was presented between the MG and the NG (1.46 [− 0.01, 2.93], P = 0.051), suggesting that the monotherapy may not improve the FAH in girls who began the puberty after the age of 8 years (Fig. 2).
Fig. 2.
Subgroup analysis of final height among girls with CPP and ENP. Notes: FAH: final adult height; MD: mean difference; CI: confidence interval; GnRH: gonadotropin-releasing hormone; rhGH: recombinant human growth hormone.
For the girls who initiated the treatment at 6–8 years of age, the difference in FAH was observed between the MG and the NG (2.16 [− 1.61, 5.03], P = 0.262), while the FAH of the CG was higher than NG (6.67 [2.77, 10.56], P = 0.001). When the treatment initiation was at 8–10 years of age, the FAH of the CG and MG was higher than NG (4.81 [3.40, 6.23], P < 0.001, 1.80 [0.66, 2.93], P = 0.002). However, the pharmacotherapy may not improve the FAH in girls who initiated the treatment at 10–12 years of age, neither combination therapy nor monotherapy (1.41 [− 0.76, 3.58], P = 0.204, 1.70 [− 0.77, 4.16], P = 0.178) (Fig. 2).
There was a difference in FAH presented between the MG and the NG when the treatment duration of GnRHa was more than 1 year (1–2 years: 2.38 [1.13, 3.62], P < 0.001; > 2 years: 2.19 [0.31, 4.08], P = 0.023), and a difference was not observed between the two groups when the treatment duration of GnRHa was less than 1 year (0.30 [− 1.34, 1.94], P = 0.718). Besides, a difference was also presented between the CG and the MG when the treatment duration of rhGH was more than 1 year (1–2 years: 2.21 [0.74, 3.68], P = 0.003; > 2 years: 4.29 [2.20, 6.38], P < 0.001), but similar efficacy was observed between the two groups when the treatment duration of rhGH was less than 1 year (1.48 [− 0.09, 3.05], P = 0.064) (Fig. 2).
Sensitivity analysis
After excluding the girls with missing data of baseline variables, the results were consistent with those of the main analysis, suggesting that the results of multivariable linear regression were robust (Appendix S1). The baseline characteristics of enrolled patients with available data of FAH in each group were compared with those who were excluded due to the lack of FAH on account of the loss to follow-up, and the results showed that there was no difference in the baseline variables between the follow-up population and the loss to follow-up population in each group, indicating that the possibility of selection bias caused by the loss to follow-up was relatively low and had little effect on the results (Appendix S2).
Discussion
There is no consensus on the classification of CPP. The Chinese expert consensus of CPP holds that CPP can be classified into slow-progressing and rapidly-progressing types according to the speed of sexual development in children. In children with slow-progressing type, sexual development and bone age progress relatively slowly, and linear growth remains in the corresponding percentile, so some of the children should be followed up and timely intervention should be made when abnormalities are found. In children with rapidly-progressing that children are classified as rapidly progressing CPP and ENP based on whether the time of rapid sexual development occurs before or after 8 years of age (girls) or 9 years of age (boys)15, the clinical presentation is characterized by accelerated progression through sexual development stages (with intervals from one stage to the next < 6 months), accompanied by a more marked increase in growth rate, bone maturation, and bone age than actual age in a short period. Besides, the epiphyses close prematurely, resulting in the impairment of FAH, so children with rapidly-progressing should be treated by GnRHa.
This cohort study retrospectively analyzed the real-world data from 182 girls with fast-progressing CPP and ENP, evaluated the long-term efficacy and safety of GnRHa combined with or without rhGH in the treatment of CPP and ENP, and further explored the differences in the efficacy of pharmacotherapy under interventions of different ages. Our results found that the group of GnRHa combined with rhGH was associated with increases in FAH of girls with CPP and ENP, especially for those who initiated the treatment before 10 years of age and those who were treated more than 1 year, but the increasing risk of adverse events such as elevated fasting insulin and hypothyroidism in combination therapy also should not be ignored despite it was tolerated.
The analysis of baseline characteristics of enrolled patients showed that those who received combination therapy were mainly presented with a late age of treatment initiation, lower initial HSDS, lower TH, older BA, and lower PAH compared with the monotherapy group and no treatment group, which might produce selection bias. Before controlling for the potential confounders, the mean and SD of FAH were similar among the three groups, but differences were present in height gain, genetic height gain, and HSDS gain, indicating that the patients receiving pharmacotherapy seemed to obtain more benefits than no treatment. However, it was arguable whether the gain was the result of medication or the effect of confounding factors due to the inconsistent baseline. For more reliable results, multivariable linear regression was adopted to control the impacts on outcomes from confounders that were determined mainly based on previous studies23,28–30, results of baseline analysis, and clinical experience. The adjusted results revealed that the FAH, height gain, genetic height gain, FAHSDS and HSDS gain of girls receiving GnRHa combined with rhGH were better than those receiving GnRHa alone and no treatment, which meant that the combination therapy could improve the FAH of girls with CPP or ENP to some extent, narrow the gap in height with their normal peers, and provide benefits compared with PAH, TH and HSDS before intervention.
In this study, only a minority of children in the CG and the MG had a predicted short stature based on their PAH, while the majority had a normal PAH. This raises questions about treatment selection: Should combination therapy be given to all children with a normal PAH? Future research should explore selective treatment strategies, reserving therapy for children predicted to be short, thereby avoiding overtreatment.
Nevertheless, due to the lack of recognized evaluation criteria of efficacy in CPP and ENP, it was still not possible to determine whether the differences were clinically meaningful for patients. The minimum clinically important difference (MCID) for quantitatively evaluating the short-term and long-term effectiveness of CPP and ENP pharmacotherapy could be codetermined based on group decision-making and expert consensus, so as to further assist the judgment of evidence quality and guide clinical decision-making.
In addition, subgroup analysis found that monotherapy appeared to have little effect on FAH among the girls whose puberty onset was after the age of 8 years, indicating that GnRHa alone may not improve the FAH in those with ENP. These results were in agreement with the previous observations of Bouvattier et al.31. GnRHa alone may not improve the FAH in those with ENP may be related to a compensation mechanism. Specifically, even without additional treatment, children who enter puberty after the age of 8 retain residual genetic growth potential. This is because their growth period is longer than that of children who developed prematurely, which is sufficient to allow them to naturally attain similar FAH to the normal population (those with puberty onset at 12 years of age). These findings suggest that GnRHa therapy has limited impact on the height outcomes of children with ENP32.
The enrolled girls who received pharmacotherapy before the age of 10 years appeared to have an improvement in FAH, and those adopting combination therapy at the age of 6–8 years had a greater benefit. However, the differences between the MG and the NG were not significant at 6–8 years of age and differed from the results of Park et al.9, which might be related to the small sample size in this subgroup and needed to be further confirmed based on larger studies. While the girls initiating treatment after the age of 10 years seemed to have no benefit from pharmacotherapy, neither combination therapy nor monotherapy. A possible explanation for this might be the higher bone age and growth space reduction at the time of treatment in this group. Some studies have pointed out that GnRHa has a limited inhibitory effect on girls with a bone age of more than 12 years and a closed epiphyseal state, and recommended that GnRHa could be discontinued when the bone age reached 12.0–12.5 years33,34. Due to the low awareness of precocity in China35, some patients miss the best opportunity for intervention, leading to the limited growth potential and unsatisfactory efficacy of GnRHa and rhGH. Thus, early intervention plays an important role in improving the long-term prognosis of CPP and ENP.
The efficacy was also closely related to the duration of pharmacotherapy. The relevant expert consensus has recommended that GnRHa treatment should be continued for more than 2 years for the target of FAH improvement15. Subgroup analysis showed that the FAH of girls treated with GnRHa for more than 1 year was improved, suggesting that the height could still benefit before the bone age was limited, so long as the GnRHa treatment was for at least 1 year. Moreover, those treated with GnRHa combined with rhGH for more than 1 year had an improvement than those treated with GnRHa alone, but no difference was found in patients who combined with rhGH for less than 1 year, which was consistent with the findings reported by Liu et al.36, suggesting that the application of rhGH in the short term has little effect on height improvement.
A large cohort study based on the registration database of 83,803 children receiving rhGH stated that the overall incidence of adverse events of rhGH treatment was 14.4%, of which the incidence of serious adverse events was 3.7%37. However, the registered population in this database was mainly children with growth hormone deficiency, and the proportion of precocious puberty and other non-indications was less than 5.0%. In this study, the overall incidence of more than one adverse event of any type in the CG was 83.3%, which was much higher than the results of previous investigations37. Among them, the risk of elevated fasting insulin and hypothyroidism increased, but all adverse events were mild, and no serious adverse events occurred. The negative effect of rhGH on insulin and thyroid might be associated with the direct or indirect regulatory function of growth hormone on endosomatic hormones38–40. Considering the potential effect of rhGH on the endocrine system of children, it is still necessary to closely monitor and regularly evaluate the glucose metabolism and thyroid function before and during rhGH treatment, and adjust the medication in time when abnormal conditions occur.
The median age at menarche in the girls of two groups treated with GnRHa was 12 years of age and similar to the normal girls in China41, while that in those without treatment was 11 years of age, suggesting that GnRHa can inhibit gonadal development and delay menarche in girls with CPP and ENP. After the onset of menstruation, 94.5% of girls had regular menstrual cycles, and 5.5% of girls had menstrual disorders such as amenorrhea and prolonged menstruation. However, the incidence of menstrual disorders was similar among the three groups, which may be attributed to immature gonads, environmental stress, and irregular sleep42. Besides, some parents reported that their children became overweight or obese after using GnRHa. There is still controversy about the effect of GnRHa on BMI in children with CPP and ENP. Lee and his colleagues 43 have found that CPP girls with normal weight had an increase in BMI after GnRHa treatment compared with pre-treatment, while Sinthuprasith et al.44 and Vuralli et al.45 indicated that although the BMI increased during treatment with GnRHa, it could return to baseline by 2 years after discontinuation of the medication. Our results showed that the proportion of girls with normal weight before treatment who progressed to overweight or obese at the stage of FAH was similar among the three groups, implying that pharmacotherapy appeared to have little effect on weight. Previous studies proposed that precocious puberty might be one of the risk factors for overweight and obesity in children46, thus, the incidence of overweight or obesity in this study was possibly related to the disease itself. It is recommended that children with CPP and ENP strengthen weight management throughout the growth and development stage.
This study also has the following limitations. First, this study was a single-center retrospective study, and the applicability of the results may be insufficient. Second, most patients missing FAH data may result in selection bias, but sensitivity analysis showed that the baseline characteristics of the excluded population were similar to those of the enrolled population in each group, so the impact of selection bias on results could be considered relatively small. Third, the FAH of children is also affected by nutrition, exercise, sleep quality, family economic conditions, and social and psychological factors. Since the detailed information mentioned above was not recorded in the hospital information system during follow-up before and corresponding measurement tools were lacking, the above factors were not taken into account in the analysis model of confounder control in this study, which may have a certain impact on the results. Fourth, due to the lower incidence in boys, the efficacy and safety of different regimens in boys with CPP and ENP have not been evaluated in this study.
Conclusions
With the increasing pressure of social competition in recent years, contemporary parents are deeply immersed in “parenting anxiety” and gradually extend it to the height of their children, resulting in higher and higher expectations for children’s height. To achieve further growth in height, the off-label use of rhGH proposed by some parents of children with CPP and ENP should be given more attention in clinical practice. Although GnRHa combined with rhGH could make a somewhat positive contribution to the FAH of girls with CPP and ENP, especially those who initiate the pharmacotherapy before the age of 10 years and continue treatment for more than 1 year, it also increases the incidence of adverse events. Despite the adverse events were generally tolerated based on the current observation, it is still necessary to strengthen medication monitoring and follow-up management during treatment. The benefits, risks, and affordability of rhGH should be taken into consideration comprehensively before decision-making, and the abuse of rhGH due to the blind pursuit of height growth should be avoided. Multicenter and larger prospective studies are still needed to conduct and consider the nutrition, exercise, sleep quality, family economic condition, social psychology, and other factors of children with CPP or ENP, to provide more high-quality evidence to guide rational drug use and formulate a scientific management mode.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Abbreviations
- HPGA
Hypothalamic–pituitary–gonadal axis
- CPP
Central precocious puberty
- ENP
Early normal puberty
- BA
Bone age
- GnRHa
Gonadotropin-releasing hormone agonist
- GnRH
Gonadotropin-releasing hormone
- GV
Growth velocity
- TH
Target height
- rhGH
Recombinant human growth hormone
- IGF-1
Insulin-like growth factor 1
- LH
Luteinizing hormone
- FSH
Follicle-stimulating hormone
- FAH
Final adult height
- CA
Chronological age
- BMI
Body mass index
- HSDS
Standard deviation score of height
- PAH
Predicted adult height
- FAHSDS
Standard deviation score of final adult height
- SD
Standard deviation
- LSD
Least significant difference
- MCID
Minimum clinically important difference
- AHPA
Average height of peers at the age of achieving final adult height
- CG
Combination therapy group
- MG
Monotherapy group
- NG
No treatment group
Author contributions
CSY XH and ZL designed the study. CSY, ZL, and JW collected the data. CSY and ZL verified the data, analyzed the study, and wrote the draft. LLZ and JW supervised the study and reviewed the final manuscript. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Funding
This study was supported by the Science and Technology Project of the Health Commission of Sichuan Province (No.23LCYJ030), the China International Medical Foundation (Z-2017-26-1902-5), and the Cross-Straits Medicine Exchange Association.
Data availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The ethics committee of West China Second University Hospital, Sichuan University approved this study (No. 2023[012]), and exempted the requirement for written informed consent due to the retrospective nature of the study. All methods were performed in accordance with the Declaration of Helsinki.
Consent to participate
The ethics committee of West China Second University Hospital, Sichuan University exempted the requirement for written informed consent due to the retrospective nature of the study.
Consent for publication
The ethics committee of West China Second University Hospital, Sichuan University consented to publish this paper after removing the private information of included patients. The ethics committee of West China Second University Hospital, Sichuan University exempted the requirement for written informed consent due to the retrospective nature of the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chunsong Yang, Email: yangchunsong_123@126.com.
Jin Wu, Email: wangdo620@163.com.
Lingli Zhang, Email: zhanglingli@scu.edu.cn.
References
- 1.Abreu, A. P. & Kaiser, U. B. Pubertal development and regulation. Lancet Diabetes Endocrinol.4, 254–264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi, J. H. & Yoo, H. W. Control of puberty: Genetics, endocrinology, and environment. Curr. Opin. Endocrinol. Diabetes Obes.20, 62–68 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Sun, S. S. et al. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics110, 911–919 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Juul, A. et al. Pubertal development in Danish children: comparison of recent European and US data. Int. J. Androl.29, 247–255 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Harrington, J., Palmert, M. R. Definition, etiology, and evaluation of precocious puberty. In J H, MR P (eds). UpToDate (2022).
- 6.Cheuiche, A. V., da Silveira, L. G., de Paula, L. C. P., Lucena, I. R. S. & Silveiro, S. P. Diagnosis and management of precocious sexual maturation: An updated review. Eur. J. Pediatr.180, 3073–3087 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Bräuner, E. V. et al. Trends in the incidence of central precocious puberty and normal variant puberty among children in Denmark, 1998 to 2017. JAMA Netw. Open3, e2015665 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckert-Lind, C. et al. Worldwide secular trends in age at pubertal onset assessed by breast development among girls: A systematic review and Meta-analysis. JAMA Pediatr.174, e195881 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park, H. K., Choo, M. S. & Shim, Y. S. Adult height after gonadotropin-releasing hormone agonist treatment in girls with early puberty: A meta-analysis. Clin. Endocrinol. (Oxf)93, 135–145 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Carel, J. C., Lahlou, N., Roger, M. & Chaussain, J. L. Precocious puberty and statural growth. Hum. Reprod. Update10, 135–147 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Branch PEaGMGotCMAsP. Consensus on the diagnosis and treatment of central precocious puberty. Chin. J. Pediatr. 53 (6), 412–418. (2015). [PubMed]
- 12.Bradley, S. H., Lawrence, N., Steele, C. & Mohamed, Z. Precocious puberty. BMJ368, l6597 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Mul, D., Oostdijk, W. & Drop, S. L. Early puberty in girls. Best Pract. Res. Clin. Endocrinol. Metab.16, 153–163 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Bourguignon, J. P. Variations in duration of pubertal growth: A mechanism compensating for differences in timing of puberty and minimizing their effects on final height. Belgian Study Group for Paediatric Endocrinology. Acta Paediatr. Scand. Suppl.347, 16–24 (1988). [PubMed] [Google Scholar]
- 15.The Society of Pediatrics CMA. Expert consensus on the diagnosis and treatment of central precocious puberty (2022). Chin. J. Pediatr.61, 16–22 (2023) (Chinese). [DOI] [PubMed] [Google Scholar]
- 16.Bangalore Krishna, K. et al. Use of gonadotropin-releasing hormone analogs in children: Update by an International Consortium. Horm. Res. Paediatr.91, 357–372 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Pasquino, A. M. et al. Combined treatment with gonadotropin-releasing hormone analog and growth hormone in central precocious puberty. J. Clin. Endocrinol. Metab.81, 948–951 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Pasquino, A. M., Pucarelli, I., Segni, M., Matrunola, M. & Cerrone, F. Adult height in girls with central precocious puberty treated with gonadotropin-releasing hormone analogues and growth hormone. J. Clin. Endocrinol. Metab.84, 449–452 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Tuvemo, T. et al. Final height after combined growth hormone and GnRH analogue treatment in adopted girls with early puberty. Acta Paediatr.93, 1456–1462 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Ranke, M. B. & Wit, J. M. Growth hormone - past, present and future. Nat. Rev. Endocrinol.14, 285–300 (2018). [DOI] [PubMed] [Google Scholar]
- 21.The Society of Pediatrics CMA. Recommendations for the clinical use of recombinant human growth hormone in children. Chin. J. Pediatr.51, 426–432 (2013) (Chinese). [PubMed] [Google Scholar]
- 22.Kim, S. J. et al. 2022 Clinical practice guidelines for central precocious puberty of Korean children and adolescents. Ann. Pediatr. Endocrinol. Metab.28, 168–177 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, M. S., Koh, H. J., Lee, G. Y., Kang, D. H. & Kim, S. Y. Comparing adult height gain and menarcheal age between girls with central precocious puberty treated with gonadotropin-releasing hormone agonist alone and those treated with combined growth hormone therapy. Ann. Pediatr. Endocrinol. Metab.24, 116–123 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu, J. et al. Long-term outcomes of treatments for central precocious puberty or early and fast puberty in Chinese girls. J. Clin. Endocrinol. Metab.105, 027 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Bell, J. et al. Long-term safety of recombinant human growth hormone in children. J. Clin. Endocrinol. Metab.95, 167–177 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Hui, L., Cheng-ye, J., Xin-nan, Z. & Ya-qin, Z. Body mass index growth curves for Chinese children and adolescents aged 0 to 18 years. Chin. J. Pediatr.47(07), 493–498 (2009). [PubMed] [Google Scholar]
- 27.Bayley, N. & Pinneau, S. R. Tables for predicting adult height from skeletal age: Revised for use with the Greulich-Pyle hand standards. J. Pediatr.40, 423–441 (1952). [DOI] [PubMed] [Google Scholar]
- 28.Wu, W. et al. Development and validation of a model for predicting the adult height of girls with idiopathic central precocious puberty. Eur. J. Pediatr.182, 1627–1635 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Swaiss, H. H., Khawaja, N. M., Farahid, O. H., Batieha, A. M. & Ajlouni, K. M. Effect of gonadotropin-releasing hormone analogue on final adult height among Jordanian children with precocious puberty. Saudi Med. J.38, 1101–1107 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gyon, Y., Yun, Y. J., Kim, Y. D. & Han, H. S. Age at menarche and near final height after treatment with gonadotropin-releasing hormone agonist alone or combined with growth hormone in Korean girls with central precocious puberty. Clin. Pediatr. Endocrinol.24, 175–183 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouvattier, C. et al. Lack of effect of GnRH agonists on final height in girls with advanced puberty: A randomized long-term pilot study. J. Clin. Endocrinol. Metab.84, 3575–3578 (1999). [DOI] [PubMed] [Google Scholar]
- 32.Llop-Viñolas, D. et al. Onset of puberty at eight years of age in girls determines a specific tempo of puberty but does not affect adult height. Acta Paediatr/93, 874–879 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Arrigo, T. et al. Analysis of the factors affecting auxological response to GnRH agonist treatment and final height outcome in girls with idiopathic central precocious puberty. Eur. J. Endocrinol.141, 140–144 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Bereket, A. A critical appraisal of the effect of gonadotropin-releasing hormon analog treatment on adult height of girls with central precocious puberty. J. Clin. Res. Pediatr. Endocrinol.9, 33–48 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.(2017) Care and growth—the public health education project of precocious puberty in children helps to standardize the treatment of the disease. In: Limited TPC (ed)
- 36.Liu, S., Liu, Q., Cheng, X., Luo, Y. & Wen, Y. Effects and safety of combination therapy with gonadotropin-releasing hormone analogue and growth hormone in girls with idiopathic central precocious puberty: A meta-analysis. J. Endocrinol. Invest.39, 1167–1178 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Maghnie, M. et al. Safety and efficacy of pediatric growth hormone therapy: Results from the full KIGS cohort. J. Clin. Endocrinol. Metab.107, 3287–3301 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okawa, M. C. et al. Insulin signaling through the insulin receptor increases linear growth through effects on bone and the GH-IGF-1 Axis. J. Clin. Endocrinol. Metab.109, e96–e106 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glynn, N. et al. Alterations in thyroid hormone levels following growth hormone replacement exert complex biological effects. Endocr. Pract.24, 342–350 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Jørgensen, J. O. et al. Growth hormone administration stimulates energy expenditure and extrathyroidal conversion of thyroxine to triiodothyronine in a dose-dependent manner and suppresses circadian thyrotrophin levels: studies in GH-deficient adults. Clin. Endocrinol. (Oxf)41, 609–614 (1994). [DOI] [PubMed] [Google Scholar]
- 41.Ma, N. et al. Trend of age of menarche among Chinese Han girls aged 9 to 18 years from 2010 to 2019. Chin. J. Prevent. Med.57, 486–491 (2023) (Chinese). [DOI] [PubMed] [Google Scholar]
- 42.Chung, P. W., Chan, S. S., Yiu, K. W., Lao, T. T. & Chung, T. K. Menstrual disorders in a paediatric and adolescent gynaecology clinic: Patient presentations and longitudinal outcomes. Hong Kong Med. J.17, 391–397 (2011). [PubMed] [Google Scholar]
- 43.Lee, H. S., Yoon, J. S., Roh, J. K. & Hwang, J. S. Changes in body mass index during gonadotropin-releasing hormone agonist treatment for central precocious puberty and early puberty. Endocrine54, 497–503 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Sinthuprasith, P., Dejkhamron, P., Wejaphikul, K. & Unachak, K. Near final adult height, and body mass index in overweight/obese and normal-weight children with idiopathic central precocious puberty and treated with gonadotropin-releasing hormone analogs. J. Pediatr. Endocrinol. Metab.32, 1369–1375 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Vuralli, D., Ozon, Z. A., Gonc, E. N., Alikasifoglu, A. & Kandemir, N. Long-term effects of GnRH agonist treatment on body mass index in girls with idiopathic central precocious puberty. J. Pediatr. Endocrinol. Metab.33, 99–105 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Song, Y., Kong, Y., Xie, X., Wang, Y. & Wang, N. Association between precocious puberty and obesity risk in children: A systematic review and meta-analysis. Front. Pediatr.11, 1226933 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.