Abstract
Marine bacterioplankton were isolated and grown in batch cultures until their growth became limited by organic carbon (C), nitrogen (N), or phosphorus (P). Samples were taken from the cultures at both the exponential and stationary phases. The elemental composition of individual bacterial cells was analyzed by X-ray microanalysis with an electron microscope. The cell size was also measured. The elemental content was highest in exponentially growing cells (149 ± 8 fg of C cell−1, 35 ± 2 fg of N cell−1, and 12 ± 1 fg of P cell−1; average of all isolates ± standard error). The lowest C content was found in C-limited cells (39 ± 3 fg of C cell−1), the lowest N content in C- and P-limited cells (12 ± 1 and 12 ± 2 fg of N cell−1, respectively), and the lowest P content in P-limited cells (2.3 ± 0.6 fg of P cell−1). The atomic C:N ratios varied among treatments between 3.8 ± 0.1 and 9.5 ± 1.0 (average ± standard error), the C:P ratios between 35 ± 2 and 178 ± 28, and the N:P ratios between 6.7 ± 0.3 and 18 ± 3. The carbon-volume ratios showed large variation among isolates due to different types of nutrient limitation (from 51± 4 to 241 ± 38 fg of C μm−1; average of individual isolates and treatments ± standard error). The results show that different growth conditions and differences in the bacterial community may explain some of the variability of previously reported elemental and carbon-volume ratios.
To quantify the flow of energy and nutrients among organisms in an ecosystem, one must be able to determine their elemental content. For microorganisms, this is usually done by the use of conversion factors, e.g., carbon-cell, carbon-volume, or elemental ratios. Cell content of carbon (C), nitrogen (N), and phosphorus (P) as well as conversion factors reported in literature vary substantially (reviewed in references 7 and 8). The large variability of these factors can be due to several reasons. The elemental content can vary among taxa (27), and the growth conditions can also affect the elemental composition (7, 10, 20, 30). Furthermore, there may be methodological problems, such as large errors in cell size estimations and systematic differences between different methods (19).
Most conversion factors have been derived from bulk experiments in which the elemental content is determined from cells collected on a filter, followed by a chemical analysis. The conversion factor is then calculated from these values, and cell number or cell volume is determined with a microscope (1, 19, 27). With these methods, there may be problems with interference from detritus, especially in natural systems. Furthermore, these analyses give an average value of the elemental content per cell and no measurement of individual cells. Therefore, little is known about how the elemental content of individual cells varies.
It has been shown for both marine and freshwater bacteria that the cell size decreases when the bacterial cells become C limited (12, 16, 27). Growth limitation by inorganic nutrients has been reported to both increase and decrease bacterial cell size (12, 16). The C content per bacterial cell generally decreases when the cell growth rate becomes growth limited, while the carbon-volume (C:V) ratios have been reported to both increase and decrease (7, 27). Most of the studies of the C content of bacteria have been conducted with native cells, cells that are enriched or C limited, or cells with unknown growth conditions. However, studies of the effect of limitation by inorganic nutrients on the elemental content of bacteria are more rare. P and N limitation of bacterioplankton growth seems to be more common in both marine and limnetic systems than previously thought (6, 29). It is therefore important to increase the knowledge of how the growth conditions affect the elemental content and the elemental content-volume ratios of cells.
The macromolecule composition and thereby the stoichiometry of a bacterial cell is affected by both growth rate and nutrient availability. For example, the RNA content is correlated with growth rate (25). RNA is usually the largest P pool of the cell, and the growth rate will therefore affect the P content of the cell. However, P can also be stored as polyphosphate during growth limitation and thereby affect the P content of the cell (32). Other compounds, such as carbohydrates, can also be stored during growth limitation (31) and therefore affect the stoichiometry of the cell.
In the present study, it was investigated how C, N, and P limitation affect the elemental content (C, N, and P) and cell volume in pure cultures of bacteria isolated from the marine water column. The analyses of the C, N, and P content of individual bacterial cells were conducted by X-ray microanalysis with a transmission electron microscope.
MATERIALS AND METHODS
Isolation of bacterioplankton.
Seawater was sampled from Raunefjorden, 15 km south of Bergen, Norway (60°16′N, 5°14′E), in October 1997. Four Erlenmeyer flasks received 250 ml of autoclaved seawater. No nutrients were added to one of the Erlenmeyer flasks. One flask was enriched with a full medium containing P (20 μM KH2PO4, final concentration), N (160 μM KNO3, final concentration), organic C (350 μM glucose, final concentration), and trace metals (f/2 medium [11]). One flask received full medium except that the amount of P was reduced to 1/10, and one flask received full medium except that the concentration of organic C was reduced to 1/10 of that in full medium.
To each flask, 10 ml of seawater filtered through 1.0-μm filter was added. The cultures were allowed to grow for 5 days at 8°C and were then streaked on agar plates (M65 medium [11]). When colonies had developed on the plates, one colony from each agar plate was streaked on a new plate. This procedure was repeated three times. This gave us four bacterial isolates that had been isolated in different nutrient conditions (Table 1). The isolates were stored frozen in 15% glycerol at −80°C until the experiments were conducted.
TABLE 1.
Seawater medium in which bacterial strains were isolated
| Isolate no. | Nutrient addition (μM final concn)
|
|||
|---|---|---|---|---|
| KH2PO4 | KNO3 | Glucose | Trace metalsa | |
| 1 | 0 | 0 | 0 | − |
| 2 | 2 | 160 | 350 | + |
| 3 | 20 | 160 | 35 | + |
| 4 | 20 | 160 | 350 | + |
−, not added; +, added.
Growth experiments.
The media were based on aged seawater to which P, N, organic C, trace metals (f/2 medium [11]) and vitamins were added (f/2 medium [11]). Bacterial isolates were grown in media in which the bacterial growth in the end was limited by either organic C, N, or P. The nutrient content of the different media is presented in Table 2. The P content of the media was reduced compared with a typical bacterial C:N:P ratio (atomic) of 50:10:1 (7), since preliminary tests showed that the bacteria reached stationary phase later in the P-limited treatments than in the other treatments.
TABLE 2.
Initial nutrient concentrations of phosphorus, nitrogen, and glucose and C:N:P ratios of the media used in the growth experiments in which phosphorus, nitrogen, or organic carbon finally became limiting for further growtha
| Isolate and limiting nutrient | Concn (mM)
|
C:N:P ratio (atom atom−1) | ||
|---|---|---|---|---|
| Na2HPO4 | KNO3 | Glucose | ||
| Isolates 1, 2, and 4 | ||||
| Phosphorus | 0.007 | 1.7 | 5.8 | 5,000:240:1 |
| Nitrogen | 0.07 | 0.17 | 5.8 | 500:2.4:1 |
| Organic carbon | 0.07 | 1.7 | 0.58 | 50:24:1 |
| Isolate 3 | ||||
| Phosphorus | 0.009 | 1.6 | 7 | 4,700:180:1 |
| Nitrogen | 0.09 | 0.16 | 7 | 470:1.8:1 |
| Organic carbon | 0.09 | 1.6 | 0.7 | 47:18:1 |
Trace metals and vitamins were added to all media.
Before the experiments started, the isolates were grown in full medium. Then, 5 ml of the bacterial culture was transferred to the experimental bottles at the late exponential or early stationary phase, giving a bacterial abundance varying between 0.25 × 106 and 1 × 106 cells ml−1 at the start of the experiments. The carryover of nutrients in the inoculum was negligible.
The experiments were carried out with batch cultures at 8°C and in the dark; 300 ml of sterile medium was added to 500-ml Erlenmeyer flasks and stirred magnetically at low speed to mix the cultures. All four isolates were grown in a medium so that their growth finally became limited by organic C. Samples for X-ray microanalysis were taken during exponential growth (with the exception of isolate 3) and at stationary phase (all isolates). Isolates 1, 2, and 3 were grown in a medium in which N became limiting for further growth. Samples for X-ray microanalysis were taken at stationary phase for all three isolates and at exponential growth for isolate 3. Isolates 2 and 3 were also grown in a medium in which P became limiting for further growth. Samples for X-ray microanalysis were taken at stationary phase. Each treatment was carried out in duplicate, but usually the X-ray microanalysis were conducted on only one of the duplicates.
Bacterioplankton abundance, biovolume, and cell shape.
Growth of the bacteria was measured by monitoring the optical density at 600 nm. Bacterial abundance was analyzed by epifluorescence microscopy after staining with DAPI (22). The bacterial cell volume was determined in a transmission electron microscope by measuring the length and width of the bacteria (21). This was conducted on the same cells that were analyzed by X-ray. The width-length ratio was used as a measure of cell shape.
X-ray microanalysis.
All major elements of individual particles, except hydrogen, were analyzed by X-ray microanalysis as described in detail by Norland et al. (21). Unfixed bacterial cells were harvested by centrifugation (17,000 × g, 15 min) on 100-mesh Al grids (Agar Scientific, Ltd.) supported with carbon-coated Formvar film. The grids were air dried on filter paper at room temperature. The elemental contents of individual bacterial cells were analyzed with a Joel 100CX transmission electron microscope equipped with a Tracor Z-MAX 30 X-ray detector featuring silicon crystal and Norvar single window for light element detection. An analysis of the carbon-coated film was made for each cell analysis. This background value was subtracted from the cell measurement. For each isolate and treatment, 8 to 16 cells were analyzed. The dry weight was calculated as the sum of all elements, assuming the hydrogen content was one-sixth of the C content (21). The elemental contents are reported as weights, and the elemental ratios are atom atom−1 ratios.
Statistical analyses.
The effects of growth conditions (exponential growth and limitation of P, N, and organic C) and differences among isolates in C, N, and P content, C:N, C:P, and N:P ratios, cell volume, dry weight, C:V ratio, and cell shape were determined by analyses of variance. The data were log10 transformed to obtain equal variance. Differences in growth conditions were tested as both differences among cells in exponential and stationary phase (all types of growth limitation as one group) and differences between different types of growth limitation (P, N, and organic C). Data from all four isolates were used in the former analyses. In the latter, only the two isolates that had been tested on all types of limitation were used.
RESULTS
Bacterioplankton growth phases.
The stationary phase was reached after 42 to 120 h, depending on the treatment (Fig. 1). There was some variation between the experiments when different isolates reached stationary phase, but the shapes of the growth curves were similar. The bacterial numbers varied among different treatments at stationary phase and were approximately 200 × 106, 30 × 106, and 70 × 106 ml−1 in C-, N-, and P-limited cultures, respectively. The samples from cultures growing exponentially were taken within the first 24 h. Samples from the stationary phase were taken after 42 or 72 h for C- and N-limited cultures and after 120 h for P-limited cultures.
FIG. 1.
Example of typical growth curves for bacterioplankton cultures that become growth limited by organic carbon, phosphorus, or nitrogen.
Elemental content and dry weight.
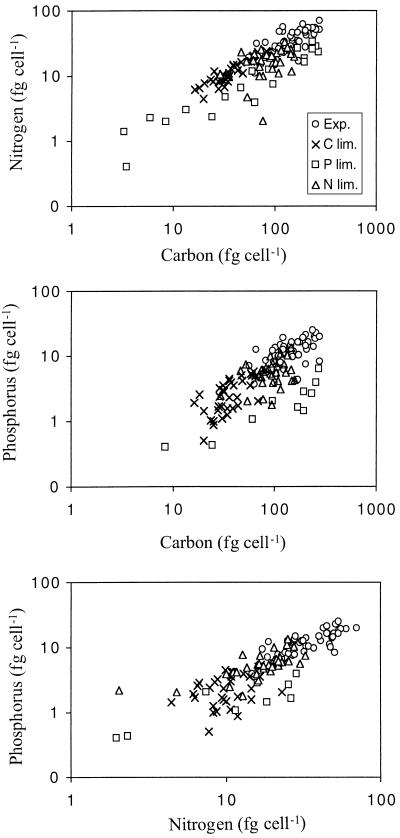
Different growth conditions resulted in large differences in C, N, and P content as well as dry weight (Fig. 2; Table 3). Overall, the C content per cell varied between 3 and 276 fg of C cell−1 (individual cells), the N content between 0.4 and 71 fg of N cell−1, the P content between 0.4 and 25 fg of P cell−1, and dry weight ranged from 39 to 884 fg cell−1. However, there was generally relatively low variation in elemental content and dry weight within the same isolate and treatment, with the exception of P limitation (Table 3).
FIG. 2.
Elemental content of individual bacterial cells that are growing exponentially (Exp.) or growth limited by organic carbon (C lim.), nitrogen (N lim.), or phosphorus (P lim.). Each point represents an individual bacterial cell. (A) Carbon versus nitrogen content; (B) carbon versus phosphorus content; (C) nitrogen versus phosphorus content.
TABLE 3.
Average dry weight, carbon content, nitrogen content, phosphorus content, and cell volume ± standard error of cells that are growing exponentially or growth limited by organic carbon, nitrogen, or phosphorus
| Growth phase | Isolate no. | No. of cells analyzed | Dry wt (fg cell−1) | Carbon (fg cell−1) | Nitrogen (fg cell−1) | Phosphorus (fg cell−1) | Vol (μm3) |
|---|---|---|---|---|---|---|---|
| Exponential | 1 | 11 | 391 ± 40 | 153 ± 17 | 33 ± 4 | 13 ± 1 | 2.17 ± 0.24 |
| 2 | 12 | 322 ± 36 | 128 ± 17 | 32 ± 4 | 13 ± 2 | 3.00 ± 0.33 | |
| 3 | 15 | 481 ± 36 | 156 ± 15 | 30 ± 3 | 8.5 ± 0.8 | 1.23 ± 0.22 | |
| 4 | 11 | 512 ± 49 | 160 ± 18 | 48 ± 4 | 16 ± 1 | 0.81 ± 0.10 | |
| Avg | 49 | 429 ± 22 | 149 ± 8 | 35 ± 2 | 12 ± 1 | 1.78 ± 0.17 | |
| Stationary | |||||||
| C limitation | 1 | 10 | 101 ± 11 | 32 ± 4 | 9.7 ± 1.1 | 1.8 ± 0.2 | 0.35 ± 0.03 |
| 2 | 10 | 76 ± 6 | 33 ± 3 | 9.8 ± 0.9 | 3.5 ± 0.3 | 0.33 ± 0.04 | |
| 3 | 8 | 147 ± 22 | 44 ± 8 | 14 ± 2 | 1.4 ± 0.2 | 0.91 ± 0.19 | |
| 4 | 9 | 114 ± 14 | 50 ± 7 | 16 ± 2 | 4.4 ± 0.4 | 0.28 ± 0.02 | |
| Avg | 37 | 107 ± 9 | 39 ± 3 | 12 ± 1 | 2.8 ± 0.2 | 0.45 ± 0.06 | |
| N limitation | 1 | 8 | 310 ± 25 | 90 ± 14 | 21 ± 2 | 8.3 ± 1.3 | 0.85 ± 0.16 |
| 2 | 10 | 207 ± 13 | 88 ± 9 | 14 ± 2 | 4.7 ± 0.6 | 1.99 ± 0.18 | |
| 3 | 16 | 312 ± 18 | 96 ± 8 | 20 ± 2 | 5.1 ± 1.6 | 1.31 ± 0.15 | |
| Avg | 34 | 281 ± 13 | 92 ± 5 | 18 ± 1 | 5.7 ± 0.4 | 1.40 ± 0.12 | |
| P limitationa | 2 | 11 | 268 ± 56 | 105 ± 33 | 13 ± 4 | 2.2 ± 0.6 | 1.70 ± 0.43 |
| 3 | 8 | 272 ± 65 | 106 ± 30 | 11 ± 3 | 2.3 ± 1.1 | 3.31 ± 0.56 | |
| Avg | 19 | 269 ± 41 | 106 ± 23 | 12 ± 2 | 2.3 ± 0.6 | 2.38 ± 0.38 |
Cells that had a P content below the detection limit were omitted from the calculation of P content (n = 8).
The C, N, and P contents as well as dry weight per cell were higher in growing cells than in growth-limited cells (Table 3). The different types of nutrient limitation also had a significant effect on the C, N, and P content. Among the growth-limited treatments, the C-limited cells also had on average the lowest C content and dry weight. C-limited cells had on average only 26% of the C content of exponentially growing cells. There were no significant differences in C content per cell among isolates, but the dry weight varied (Table 4). Both C- and P-limited cells had lower N and P contents than N-limited cells. In nutrient-limited cells, there were significant differences in N and P content between different isolates.
TABLE 4.
Analyses of variance of differences in elemental content of bacteria in exponential growth and bacteria that are growth limited and between bacteria that are growth limited by organic carbon, nitrogen, or phosphorus
| Parameter |
P
|
|||||
|---|---|---|---|---|---|---|
| Exponential growth vs growth limitation
|
P, N, or C limitation
|
|||||
| GCa | Isolateb | GC × isolate | GC | Isolatec | GC × isolate | |
| Vol | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.004 | 0.0002 |
| Dry wt | <0.0001 | 0.005 | 0.04 | <0.0001 | 0.008 | n.s.d |
| Carbon | <0.0001 | n.s. | n.s. | 0.006 | n.s. | n.s. |
| Nitrogen | <0.0001 | n.s. | n.s. | <0.0001 | 0.008 | n.s. |
| Phosphorus | <0.0001 | 0.03 | n.s. | 0.04 | <0.0001 | 0.006 |
| C:V | n.s. | <0.0001 | <0.0001 | <0.0001 | n.s. | <0.0001 |
| C:N | n.s. | 0.0003 | n.s. | <0.0001 | n.s. | 0.002 |
| C:P | 0.001 | <0.0001 | n.s. | <0.0001 | 0.0001 | 0.0001 |
| N:P | 0.0001 | 0.005 | n.s. | <0.0001 | <0.0001 | <0.0001 |
| Cell shape | 0.0003 | 0.002 | n.s. | <0.0001 | 0.04 | n.s. |
GC, growth condition.
Isolates 1, 2, 3, and 4.
Isolates 1 and 2.
n.s., P > 0.05.
Elemental ratios.
The average atomic C:N:P ratio of exponentially growing cells was 32:6.4:1. C-limited cells had a C:N:P ratio of 34:9.2:1, N-limited cells had a ratio of 42:7:1, and P-limited cells had a ratio of 172:16:1. The average C:N:P ratio (all treatments) was 45:7.4:1. The C:N ratio of individual cells varied between 2.3 and 44, the C:P ratio varied between 14 and 358, and the N:P ratio varied between 2.0 and 36 (atom atom−1). The C:N, C:P, and N:P ratios varied significantly among isolates (Table 4). Different types of growth limitation had significant effects on the elemental ratios. In stationary phase, C limitation resulted in the lowest C:N and C:P ratios, P limitation resulted in the highest C:P and N:P ratios, and N limitation resulted in the lowest N:P ratio (average values) (Table 5).
TABLE 5.
Average C:V, C:N, C:P, and N:P ratios and cell shape (± standard error) of cells that are growing exponentially or growth limited by organic carbon, nitrogen, or phosphorus
| Growth phase | Isolate no. | No. of cells analyzed | C:V (fg μm−3) | C:N | C:P | N:P | Cell shape (width:length) |
|---|---|---|---|---|---|---|---|
| Exponential | 1 | 11 | 77 ± 10 | 5.4 ± 0.3 | 29 ± 1 | 5.5 ± 0.3 | 0.64 ± 0.05 |
| 2 | 12 | 52 ± 11 | 4.7 ± 0.4 | 29 ± 4 | 6.2 ± 0.6 | 0.59 ± 0.05 | |
| 3 | 15 | 209 ± 49 | 6.3 ± 0.5 | 50 ± 5 | 7.9 ± 0.5 | 0.72 ± 0.05 | |
| 4 | 11 | 241 ± 38 | 3.8 ± 0.3 | 26 ± 2 | 6.9 ± 0.4 | 0.51 ± 0.05 | |
| Avg | 49 | 148 ± 21 | 5.2 ± 0.2 | 35 ± 2 | 6.7 ± 0.3 | 0.62 ± 0.03 | |
| Stationary | |||||||
| C limitation | 1 | 10 | 92 ± 7 | 3.9 ± 0.3 | 52 ± 6 | 14 ± 2 | 0.68 ± 0.03 |
| 2 | 10 | 111 ± 14 | 4.1 ± 0.2 | 25 ± 1 | 6.1 ± 0.2 | 0.74 ± 0.03 | |
| 3 | 8 | 51 ± 4 | 3.6 ± 0.3 | 70 ± 3 | 21 ± 2 | 0.81 ± 0.04 | |
| 4 | 9 | 169 ± 15 | 3.6 ± 0.1 | 28 ± 1 | 7.9 ± 0.2 | 0.72 ± 0.05 | |
| Avg | 37 | 107 ± 10 | 3.8 ± 0.1 | 42 ± 3 | 12 ± 1 | 0.73 ± 0.02 | |
| N limitation | 1 | 8 | 135 ± 38 | 4.9 ± 0.4 | 29 ± 2 | 6.2 ± 0.7 | 0.69 ± 0.06 |
| 2 | 10 | 45 ± 3 | 11 ± 4 | 56 ± 8 | 6.6 ± 0.9 | 0.56 ± 0.04 | |
| 3 | 16 | 82 ± 7 | 6.5 ± 0.8 | 55 ± 8 | 8.7 ± 0.6 | 0.68 ± 0.03 | |
| Avg | 34 | 83 ± 11 | 7.5 ± 1.2 | 49 ± 5 | 7.5 ± 0.5 | 0.64 ± 0.02 | |
| P limitationa | 2 | 11 | 44 ± 9 | 7.7 ± 1.1 | 180 ± 36 | 19 ± 5 | 0.75 ± 0.03 |
| 3 | 8 | 33 ± 8 | 12 ± 1 | 176 ± 46 | 16 ± 4 | 0.74 ± 0.05 | |
| Avg | 19 | 39 ± 6 | 9.5 ± 1.0 | 178 ± 28 | 18 ± 3 | 0.75 ± 0.02 |
Cells that had a P content below the detection limit were omitted from the calculation of C:P and N:P ratios (n = 8).
Cell volume, cell shape, and C:V ratio.
The growth conditions had significant effects on bacterial cell volume (Fig. 3; Table 4). The cell volume also varied significantly among isolates. The bacterial cell size decreased when the growth of the cells became limited, with the exception of isolate 3 (Table 3). Cells of isolate 3 did not change or increase in cell size during growth limitation compared with exponential growth. Average cell volume was smallest in C-limited cells. However, the decrease in cell volume when the bacterial cells became C-limited varied among isolates from a 25% reduction (not significant, t test; P > 0.05) to a 90% reduction. The shape of the cells, measured as the ratio of width to length, varied both among isolates and by growth condition (Tables 4 and 5). The width-length ratio increased when the cells became C limited and P limited compared with when they were growing exponentially. The effect of N limitation varied among the isolates.
FIG. 3.
Cell volume versus carbon content of individual bacterial cells that are growing exponentially (Exp.) or growth limited by organic carbon (C lim.), nitrogen (N lim.), or phosphorus (P lim.).
The relatively small variation in C content and large variation in cell volume resulted in large variations in the C:V ratios (Table 5). The C:V ratio of individual cells varied from 12 to 605 fg of C μm−3. Exponentially growing cells had the highest C:V ratio (average for all isolates). However, there was no significant difference when comparing the C:V ratio of growing cells with that of cells in stationary phase (as one group) (Table 4). Among nutrient-limited cells, C limitation led to the highest C:V ratio (on average), even though it was lower than for cells in exponential phase. There were differences in the C:V ratios among isolates (Table 4). However, there were no trends in how the C:V ratio of different isolates varied due to different growth conditions, i.e., some isolates increased while others decreased the ratio when their growth became nutrient limited. The largest variation in the C:V ratio among isolates within the same treatment was during exponential growth (Table 5).
DISCUSSION
The growth conditions had significant effects on the C, N, and P content of the bacterial cells. The elemental content was lower in cells in stationary phase than in cells in exponential growth. The C, N, and P content of the cells were in the upper range or higher than have been reported previously for native bacteria (7, 9, 14, 15) and the same as or lower than reported for cultured bacteria (7, 20). Since it is cells in stationary phase that have elemental contents that are in the same range as for native bacteria, this indicates that organic carbon and/or inorganic nutrients are often in short supply in natural systems. This is in agreement with previously published data that show that limitation of bacterioplankton growth by inorganic nutrients and organic carbon is common in both freshwater and marine systems (6, 17, 29).
It has previously been shown that the C content is substantially reduced when bacterial cells reach stationary phase (7). Furthermore, Troussellier et al. (27) found a reduction in C content during the first 7 days of C starvation. Our results indicate that other types of growth limitation also result in lower C content, since the C content per cell decreased in the N- and P-limited treatments. Furthermore, the N and P content of the cells decreased when the cells became growth limited, regardless of the type of limitation (C, N, or P). Bacterial cells that are N or P limited have been reported to increase their relative amount of carbohydrates (31). This may contradict our results, but in the study of Wanner and Egli (31), the total cell C content was not measured. The increased amount of C-rich macromolecules may be due to a redistribution of C within the cell and not an increased uptake.
The C:N ratios varied between 3.6 and 12 (average of individual isolates), which is within the range that has been reported for both marine and freshwater native bacteria (7, 9, 15, 19). Exponentially growing bacteria had a C:N ratio of 5.2 (average of all isolates), which is close to the C:N ratio (average, 5.5) that was reported previously for newly produced bacterial biomass (4).
The C:P ratios of the bacteria were lower in all treatments, except at P limitation, than what have been reported for natural bacteria (4, 7). However, this can be expected because P was in surplus in all these treatments and bacteria have the ability to store phosphorus (3, 32). Vadstein and Olsen (28) suggested from chemostat experiments that bacteria were P limited at C:P ratios of 21 to 152 atom atom−1. The P-limited bacteria in the present study had a higher C:P ratio (178, average of treatment), which could be expected because the P limitation was probably very severe in stationary phase. The C:P ratios of the bacteria in the treatments with a surplus of P were lower (average of individual isolates varied between 25 and 70) but above the lower range of P limitation as reported by Vadstein and Olsen. Even though our data are based on only two isolates, they indicate that the C:P range of P limitation may be more narrow than has been suggested earlier.
The N:P ratios of the bacteria varied between 5.5 and 19 (average of individual isolates), which is within the range of what has been reported for natural systems (4, 7). Both the C:P and N:P ratios were lowest at exponential growth. This is in accordance with Chrzanowski et al. (4), who found that a growing bacterial population had lower C:P and N:P ratios than when the bacterial abundance was declining. The C:P and N:P ratios of newly produced bacterial biomass were higher (44.4 and 8.5, respectively) in the study of Chrzanowski et al. (4) than what was found in the present study of exponentially growing cells (35 and 6.7, respectively). However, the P concentrations were probably higher in the growth media in the present study, leading to lower C:P and N:P ratios, compared with the enrichment experiments of Chrzanowski et al. (4), which were conducted with concentrated lake water. The C:N ratios of the bacteria varied less than the C:P and N:P ratios when comparing N and P limitation. Low variation in the C:N ratio with respect to the N:P ratio of the medium has also been found in experiments with Pseudomonas fluorescens (3).
C limitation resulted in the lowest C:N ratios of all treatments. The relative amount of carbohydrate has been shown to be lower in C-limited bacteria than in N-limited bacteria (26), which may explain the low C:N ratio in our experiments. The C:P ratio increased during C limitation compared to exponential growth, which may contradict the above explanation. However, RNA is a large P pool in the cell, and the RNA content is correlated with growth rate (25). The higher C:P ratio may therefore be due to a lower RNA content.
The N:P ratio increased when the cells became C limited, which indicates that the relative amounts of N are less affected by C limitation than the amounts of P. RNA contains approximately the same amount of N and P. However, while RNA is the largest P pool of the cell, there are other N-rich macromolecules, such as proteins, that constitute a larger fraction of cell dry weight than RNA (2, 26) and therefore constitute a larger proportion of the N content of the cell. A reduction in RNA content will therefore not affect the total content of N as much as the total content of P. Protein can also be degraded during starvation, but this seems to occur in a later starvation phase (13) and probably did not occur in our experiments, which studied the effect of short-term starvation.
Growth limitation by N resulted in higher C:N ratios than C limitation. This can be expected because the protein content in N-limited cells can be lower than that in C-limited cells (26). When the bacterial cultures became P limited, the C:P, C:N, and N:P ratios were highest, and the opposite pattern was found at exponential phase. This can be explained by high RNA content in growing cells (25) and by the fact that RNA can be used as a P reserve when the cells become P limited (18). Since bacteria can store P as polyphosphate (32), one can expect lower C:P and N:P ratios when elements other than P are limiting the growth and P is in excess, which was also the case in our experiments. However, this storage capacity of P was not so large that it could cover the P losses due to a reduction in RNA content that probably occurred during growth limitation.
The cell volume varied significantly both between growth conditions and among isolates. The large differences in cell size between C- and P-limited cells resulted in a much higher optical density for P-limited cells even though the cell numbers in the P-limited treatments were lower. Several studies have shown that C limitation causes a reduction in the cell volume and can result in ultramicrocells (12, 16, 27). Growth limitation by N and P has also been shown to decrease the cell volume of marine bacteria (16). In contrast, only minor changes in cell volume have been reported for a marine ultramicrobacterium grown over a range of C concentrations (5). In the present study, with the exception of one isolate, the cell volume decreased when the cells became C limited. However, depending on isolate and treatment, nutrient limitation led to increased, decreased, or unchanged cell volume. Thus, the results show a large variation in how different bacterial isolates respond to growth limitation.
The shape of the cell (measured as the width-length ratio) differed significantly among both growth conditions and isolates. The average width-length ratio increased when the cells became nutrient limited, but there were differences among the isolates. Holmquist and Kjelleberg (12) have shown that N-limited cells can form filaments and P-limited cells become swollen and rod-shaped. Among the growth-limited cells in the present study, N-limited cells usually had the lowest width-length ratio, which indicates an increase in length and/or a decrease in width. However, the overall lowest width-length ratio was found among exponentially growing cells. This indicates that there may be differences among bacterial strains in how their morphology changes when they become growth limited.
The C:V ratios in the present study are in the same range as reported previously for natural bacteria (7, 19, 23, 24). Depending on the isolate, growth limitation resulted in an increased or decreased C:V ratio. Studies of natural bacterial communities show large variations in C content and in the C:V ratio (7, 9, 14, 19, 24). If our results are representative for a majority of aquatic bacteria, this indicates that a large fraction of the variation in C content is due to different growth conditions in natural environments.
Our results indicate that conversion factors should be used with caution. The elemental content of the cells as well as the elemental and C:V ratios showed large variability among isolates and under different growth conditions. Standard conversion factors may therefore not always be good descriptors of either native or cultured bacteria. This is especially important in manipulated systems (for example, in enrichment experiments), in which the growth conditions and the bacterial community composition are affected.
Acknowledgments
The electron microscopy work was done at the Laboratory for Electron Microscopy, University of Bergen. Egil S. Erichsen is acknowledged for assistance with the electron microscope and Evy Foss Skjoldal for assistance in the laboratory. We thank Tobias Vrede for comments on the manuscript.
This research was supported by postdoctoral grant B-SK 11871-301 from the Swedish Natural Science Research Council to K. Vrede.
REFERENCES
- 1.Bratbak, G. 1985. Bacterial biovolume and biomass estimations. Appl. Environ. Microbiol. 49:1488-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brock, T. D., M. T. Madigan, J. Martinko, and J. Parker. 1994. Biology of microorganisms, 7th ed. Prentice Hall, Englewood Cliffs, N.J.
- 3.Chrzanowski, T. H., and M. Kyle. 1996. Ratios of carbon, nitrogen and phosphorus in Pseudomonas fluorescens as a model for bacterial element ratios and nutrient regeneration. Aquat. Microb. Ecol. 10:115-122. [Google Scholar]
- 4.Chrzanowski, T. H., M. Kyle, J. J. Elser, and R. W. Sterner. 1996. Element ratios and growth dynamics of bacteria in an oligotrophic Canadian shield lake. Aquat. Microb. Ecol. 11:119-125. [Google Scholar]
- 5.Eguchi, M., T. Nishikawa, K. MacDonald, R. Cavicchioli, J. C. Gottschal, and S. Kjelleberg. 1996. Responses to stress and nutrient availability by the marine ultramicrobacterium Sphingomonas sp. strain RB2256. Appl. Environ. Microbiol. 62:1287-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elser, J. J., B. L. Stabler, and P. R. Hassett. 1995. Nutrient limitation of bacterial growth and rates of bacterivory in lakes and oceans: a comparative study. Appl. Environ. Microbiol. 9:105-110. [Google Scholar]
- 7.Fagerbakke, K. M., M. Heldal, and S. Norland. 1996. Content of carbon, nitrogen, oxygen, sulphur and phosphorus in native aquatic and cultured bacteria. Aquat. Microb. Ecol. 10:15-27. [Google Scholar]
- 8.Fry, J. C. 1988. Determination of biomass, p. 27-72. In B. Austin (ed.), Methods for aquatic bacteriology. John Wiley & Sons Ltd., London, England.
- 9.Fukuda, R., H. Ogawa, T. Nagata, and I. Koike. 1998. Direct determination of carbon and nitrogen content of natural bacteria assemblages in marine environments. Appl. Environ. Microbiol. 64:3352-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman, J. C., D. A. Caron, and M. R. Denett. 1987. Regulation of gross growth efficiency and ammonium regeneration in bacteria by substrate C:N ratio. Limnol. Oceanogr. 32:1239-1252. [Google Scholar]
- 11.Guillard, R. L. L. 1975. Culture of phytoplankton for feeding marine evertebrates, p. 29-60. In W. L. Smith and M. H. Chanley (ed.), Culture of marine invertebrate animals. Plenum Press, New York, N.Y.
- 12.Holmquist, L., and S. Kjelleberg. 1993. Changes in viability, respiratory activity and morphology of the marine Vibrio sp. strain S14 during starvation of individual nutrients and subsequent recovery. FEMS Microbiol. Ecol. 12:215-224. [Google Scholar]
- 13.Hood, M. A., J. B. Guckert, D. C. White, and F. Deck. 1986. Effect of nutrient deprivation on lipid, carbohydrate, DNA, RNA and protein levels in Vibrio cholerae. Appl. Environ. Microbiol. 52:788-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroer, N. 1994. Relationships between biovolume and carbon and nitrogen content of bacterioplankton. FEMS Microbiol. Ecol. 13:217-224. [Google Scholar]
- 15.Lee, S., and J. A. Fuhrman. 1987. Relationships between biovolume and biomass of naturally derived marine bacterioplankton. Appl. Environ. Microbiol. 53:1298-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mårdén, P., A. Tunlid, K. Malmcrona-Friberg, G. Odham, and S. Kjelleberg. 1985. Physiological and morphological changes during short term starvation of marine bacterial isolates. Arch. Microbiol. 142:326-332. [Google Scholar]
- 17.Morris, D. P., and W. M. Lewis, Jr. 1992. Nutrient limitation of bacterioplankton growth in Lake Dillon, Colorado. Limnol. Oceanogr. 37:1179-1192. [Google Scholar]
- 18.Mundry, C., and K.-P. Kuhn. 1991. Modelling and parameter identification for batch fermentation with Streptomyces tendae under phosphate limitation. Appl. Microbiol. Biotechnol. 35:306-311. [DOI] [PubMed] [Google Scholar]
- 19.Nagata, T., and Y. Watanabe. 1990. Carbon- and nitrogen-to-volume ratios of bacterioplankton grown under different nutritional conditions. Appl. Environ. Microbiol. 56:1303-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakano, S.-I. 1994. Carbon:nitrogen:phosphorus ratios and nutrient regeneration of a heterotrophic flagellate fed on bacteria with different elemental ratios. Arch. Hydrobiol. 129:257-271. [Google Scholar]
- 21.Norland, S., K. M. Fagerbakke, and M. Heldal. 1995. Light element analysis of individual bacteria by X-ray microanalysis. Appl. Environ. Microbiol. 61:1357-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter, K. G., and Y. S. Feig. 1990. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 343:943-948. [Google Scholar]
- 23.Scavia, D., and G. A. Laird. 1987. Bacterioplankton in Lake Michigan: dynamics, controls, and significance to carbon flux. Limnol. Oceanogr. 32:1017-1033. [Google Scholar]
- 24.Simon, M., and F. Azam. 1989. Protein content and protein synthesis rates of planktonic marine bacteria. Mar. Ecol. Prog. Ser. 51:201-213. [Google Scholar]
- 25.Tempest, D. W., and J. W. Dicks. 1967. Interrelationships between potassium, magnesium, phosphorus and ribonucleic acid in the growth of Aerobacter aerogenes in a chemostat, p. 140-154. In E. O. Powell, G. T. Evans, R. E. Strange, and D. W. Tempest (ed.), Microbial physiology and continuous culture. Proceedings of the Third International Symposium. Her Majesty’s Stationery Office, London, United Kingdom.
- 26.Tempest, D. W., D. Herbert, and P. J. Phipps. 1967. Studies on the growth of Aerobacter aerogenes at low dilution rates in a chemostat, p. 240-254. In E. O. Powell, C. G. T. Evans, R. E. Strange, and D. W. Tempest (ed.), Microbial physiology and continuous culture. Proceedings of the Third International Symposium. Her Majesty’s Stationery Office, London, United Kingdom.
- 27.Troussellier, M., M. Bouvy, C. Courties, and C. Dupuy. 1997. Variation of carbon content among bacterial species under starvation condition. Aquat. Microb. Ecol. 13:113-119. [Google Scholar]
- 28.Vadstein, O., and Y. Olsen. 1989. Chemical composition and phosphate uptake of limnetic bacterial communities cultured in chemostats under phosphorus limitation. Limnol. Oceanogr. 34:939-946. [Google Scholar]
- 29.Vrede, K., T. Vrede, A. Isaksson, and A. Karlsson. 1999. Effects of nutrients (P, N, C) and zooplankton on bacterioplankton and phytoplankton—a seasonal study. Limnol. Oceanogr. 44:1616-1624. [Google Scholar]
- 30.Vrede, T. 1998. Elemental composition (C:N:P) and growth rates of bacteria and Rhodomonas grazed by Daphnia. J. Plankton Res. 20:455-470. [Google Scholar]
- 31.Wanner, U., and T. Egli. 1990. Dynamics of microbial growth and cell composition in batch culture. FEMS Microbiol. Rev. 75:19-44. [DOI] [PubMed] [Google Scholar]
- 32.Weltin, D., D. Hoffmeister, W. Dott, and P. Kämpfer. 1996. Studies on polyphosphate and poly-β-hydroxyalkanoate accumulation in Acinetobacter johnsonii 120 and some other bacteria from activated sludge in batch and continuous culture. Acta Biotechnol. 16:91-102. [Google Scholar]