Abstract
Besifovir dipivoxil maleate (BSV) has potent antiviral efficacy against chronic hepatitis B (CHB). This study investigated the efficacy of BSV in reducing hepatocellular carcinoma (HCC) development compared to other antiviral therapy (AVT) agents. We conducted a retrospective cohort study on treatment-naïve patients with CHB who initiated an AVT between 2017 and 2022 with BSV (n = 486), entecavir (ETV) (n = 852), tenofovir alafenamide (TAF) (n = 801), or tenofovir disoproxyl fumarate (TDF) (n = 750). The incidence and hazard ratio (HR) of HCC were calculated. The incidence of HCC in BSV users (n = 6, 4.3 per 1000 person-years [PYs]) was similar to that in TAF users (n = 21, 9.2 per 1000 PYs, log-rank P = 0.086, HR = 2.191, 95% confidence interval [CI] 0.884–5.434), but significantly lower than that in ETV users (n = 38, 12.5 per 1000PYs, log-rank P = 0.026, HR = 2.627, 95% CI 1.103–6.255) and TDF users (n = 32, 12.3 per 1000PYs, log-rank P = 0.028, HR = 2.623, 95% CI 1.090–6.311). Similarly, compared to BSV users, the adjusted HRs for ETV, TAF, and TDF users were higher after stabilized inverse probability of treatment weighting (2.836, 2.784, and 3.294, respectively) and pairwise propensity score matching (3.200, 3.250, and 3.750, respectively) (all P < 0.05). BSV demonstrated comparable efficacy in HCC reduction compared to other AVTs.
Keywords: Hepatitis B, chronic; Carcinoma, hepatocellular; Besifovir; Tenofovir; Entecavir
Subject terms: Hepatitis B, Hepatocellular carcinoma, Liver fibrosis
Introduction
Chronic hepatitis B (CHB) infection contributes to the development of more than half of hepatocellular carcinoma (HCC) cases in the endemic areas1. Direct mechanisms, such as hepatitis B virus (HBV) DNA integration into the host genome, expression of the viral regulatory or mutant proteins dysregulating cell proliferation control and hepatocyte transformation, and epigenetic changes affecting tumor suppressor genes, and indirect mechanism, such as chronic inflammation, fibrosis, and cirrhosis development, and other host and environmental factors, are all involved in HBV-related carcinogenesis1,2. The current mainstay of CHB treatment is antiviral therapy (AVT) using the oral nucleos(t)ide analogs (NAs) with high-genetic barriers, among current guidelines, such as entecavir (ETV), tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide (TAF)3–6. Although NAs fail to eradicate HBV due to the limited activities on the reverse transcriptase domain of the viral polymerase7, NA treatments improve patients’ prognosis by suppressing viral replication, thereby attenuating liver-related complications and HCC development8,9.
Besifovir dipivoxil maleate (BSV), an oral acyclic nucleotide phosphonate, is a novel potent AVT approved in May 2017 in South Korea10. BSV has provided a similar antiviral efficacy and a lower incidence of kidney and bone damage compared to those of TDF through the phase 3 trial11. Moreover, BSV provided histologic improvement and a decrease in intrahepatic cccDNA in the treatment-naïve patients with CHB who underwent paired biopsy12. Hence, BSV is recommended as the first-line AVT in treatment-naïve patients with CHB with or without cirrhosis in the Korean guideline6. However, data is limited regarding the influence of BSV on hepatocarcinogenesis. Yim et al. suggested that the decreased incidence of HCC after BSV therapy was through the decreased standardized incidence ratio calculated by the existing HCC prediction models13. Kim et al. presented that BSV offers comparable efficacy to TAF using national data (n = 41,949)14. Considering that the long-term viral suppression by ETV and TDF reduces the risk of hepatocarcinogenesis15,16, BSV may also have comparable effects. However, a direct comparison analysis of hepatocarcinogenesis among the potent NAs, including BSV, has not been conducted.
Therefore, this study aimed to reveal the long-term risk reduction of HCC development with BSV use in treatment-naïve patients with CHB through a retrospective comparative analysis among the potent NAs.
Results
Patient characteristics
According to the inclusion and exclusion criteria, 2,889 patients from the six tertiary hospitals were retrospectively recruited (Fig. 1). Of the study population, 486 (16.8%) patients started BSV, 852 (29.5%) started ETV, 801 (27.7%) started TAF, and 750 (26.0%) started TDF at the index date. The proportion of males (61.3% vs. 50.2%–55.3%), cirrhosis (38.5% vs. 31.1%–34.4%), and significant alcohol intake (11.9% vs. 5.5%–10.2%) were higher in the BSV group than the other groups (all P < 0.05). Patients in the ETV group were significantly older (median 54 years vs. 47–50 years), and had a higher proportion of hypertension (28.3% vs. 14.8%–17.1%), diabetes mellitus (19.5% vs. 9.9%–10.4%), chronic kidney disease (9.0% vs. 1.2%–1.9%), and negativity for HBeAg (64.2% vs. 44.1%–45.6%) (all P < 0.05) (Table 1).
Fig. 1.
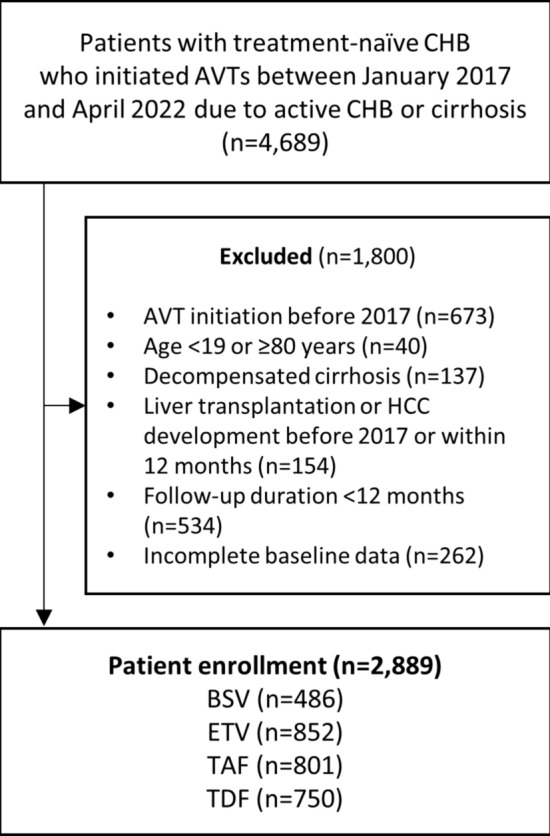
Flowchart of patient selection. Abbreviation: CHB, chronic hepatitis B; AVT, antiviral therapy; HCC; hepatocellular carcinoma; BSV, besifovir dipivoxil maleate; ETV, entecavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Table 1.
Baseline characteristics and development of HCC in patients with chronic hepatitis B.
| Variable (n = 2,889) | BSV (n = 486) | ETV (n = 852) | ASD* | TAF (n = 801) | ASD* | TDF (n = 750) | ASD* | P value | MASD** |
|---|---|---|---|---|---|---|---|---|---|
| Male sex | 298 (61.3) | 428 (50.2) | 0.225 | 443 (55.3) | 0.122 | 400 (53.3) | 0.162 | 0.001 | 0.225 |
| Age (year) | 50.0 (42.0, 57.0) | 54.0 (46.0, 61.0) | 0.379 | 49.0 (40.0, 57.0) | 0.063 | 47.0 (37.0, 54.0) | 0.258 | < 0.001 | 0.608 |
| Body mass index (kg/m2) | 24.0 (22.1, 26.0) | 23.2 (21.5, 25.6) | 0.182 | 23.5 (21.5, 25.6) | 0.156 | 23.4 (21.4, 25.7) | 0.152 | 0.013 | 0.182 |
| Hypertension | 72 (14.8) | 241 (28.3) | 0.332 | 125 (15.6) | 0.022 | 128 (17.1) | 0.062 | < 0.001 | 0.332 |
| Diabetes mellitus | 48 (9.9) | 166 (19.5) | 0.274 | 83 (10.4) | 0.016 | 74 (9.9) | 0.000 | < 0.001 | 0.274 |
| Chronic kidney disease | 6 (1.2) | 77 (9.0) | 0.359 | 31 (3.9) | 0.168 | 14 (1.9) | 0.051 | < 0.001 | 0.359 |
| Dyslipidemia | 32 (6.6) | 64 (7.5) | 0.036 | 64 (8.0) | 0.054 | 29 (3.9) | 0.122 | 0.005 | 0.175 |
| Cirrhosis | 187 (38.5) | 293 (34.4) | 0.085 | 253 (31.6) | 0.145 | 233 (31.1) | 0.156 | 0.029 | 0.156 |
| Significant alcohol intake | 58 (11.9) | 47 (5.5) | 0.229 | 82 (10.2) | 0.054 | 61 (8.1) | 0.127 | < 0.001 | 0.229 |
| Platelet count (× 109/L) | 181.0 (143.0, 218.0) | 182.0 (138.5, 227.0) | 0.028 | 179.0 (144.0, 219.0) | 0.002 | 181.0 (135.5, 229.0) | 0.052 | 0.998 | 0.052 |
| AST (IU/L) | 61.0 (41.0, 96.0) | 43.0 (27.0, 76.0) | 0.042 | 60.0 (39.0, 102.0) | 0.036 | 55.0 (33.0, 103.8) | 0.141 | < 0.001 | 0.156 |
| ALT (IU/L) | 84.0 (44.0, 139.0) | 43.5 (23.0, 89.0) | 0.178 | 88.0 (39.0, 151.0) | 0.015 | 67.5 (34.0, 133.8) | 0.036 | < 0.001 | 0.189 |
| Gamma-GT (IU/L) | 44.0 (26.0, 83.0) | 42.0 (22.0, 87.5) | 0.008 | 41.0 (26.0, 77.8) | 0.011 | 43.0 (23.0, 94.0) | 0.056 | 0.324 | 0.056 |
| Serum albumin (g/dL) | 4.3 (4.1, 4.5) | 4.2 (3.8, 4.5) | 0.364 | 4.3 (4.1, 4.5) | 0.016 | 4.2 (3.9, 4.5) | 0.303 | < 0.001 | 0.364 |
| Total bilirubin (mg/dL) | 0.8 (0.7, 1.0) | 0.8 (0.5, 1.1) | 0.087 | 0.8 (0.6, 1.1) | 0.064 | 0.8 (0.6, 1.1) | 0.146 | < 0.001 | 0.146 |
| BUN (mg/dL) | 13.4 (11.0, 15.5) | 13.8 (11.2, 17.4) | 0.306 | 13.0 (11.0, 15.6) | 0.068 | 12.3 (9.9, 15.0) | 0.172 | < 0.001 | 0.392 |
| Serum creatinine (mg/dL) | 0.80 (0.70, 0.91) | 0.79 (0.67, 0.94) | 0.226 | 0.81 (0.68, 0.94) | 0.090 | 0.79 (0.64, 0.91) | 0.004 | 0.013 | 0.226 |
| Prothrombin time (INR) | 1.03 (0.97, 1.09) | 1.03 (0.98, 1.11) | 0.104 | 1.03 (0.97, 1.09) | 0.004 | 1.03 (0.98, 1.11) | 0.098 | 0.262 | 0.104 |
| Positive for HBeAg | 261 (54.5) | 298 (35.8) | 0.382 | 439 (55.9) | 0.027 | 398 (54.4) | 0.002 | < 0.001 | 0.410 |
| Log10 HBV DNA (IU/mL) | 6.39 (5.23, 7.73) | 5.17 (3.17, 6.75) | 0.634 | 6.33 (4.95, 7.84) | 0.072 | 6.00 (3.91, 7.52) | 0.320 | < 0.001 | 0.634 |
| 4—7 Log10 HBV DNA (IU/mL) | 237 (48.8) | 349 (41.0) | 0.157 | 373 (46.6) | 0.044 | 314 (41.9) | 0.139 | 0.011 | 0.157 |
| ALBI grade 2 | 94 (19.4) | 234 (27.6) | 0.279 | 161 (20.2) | 0.038 | 208 (27.8) | 0.278 | < 0.001 | 0.279 |
| ALBI grade 3 | 1 (0.2) | 19 (2.2) | 3 (0.4) | 16 (2.1) | |||||
| FIB-4 | 1.9 (1.3, 3.1) | 2.0 (1.3, 3.6) | 0.150 | 1.9 (1.2, 3.2) | 0.012 | 1.8 (1.1, 3.5) | 0.101 | 0.103 | 0.150 |
| LS value (kPa) (n = 1,517) | 8.1 (5.6, 12.1) | 6.7 (4.9, 10.9) | 0.078 | 7.4 (5.5, 11.5) | 0.074 | 7.2 (5.1, 12.0) | 0.035 | 0.003 | 0.101 |
| CAP value ≥ 239 dB/m (n = 1,437) | 135 (48.4) | 182 (42.4) | 0.120 | 163 (40.8) | 0.154 | 135 (41.0) | 0.148 | 0.196 | 0.154 |
| CAP value ≥ 274 dB/m (n = 1,437) | 61 (21.9) | 81 (18.9) | 0.074 | 76 (19.0) | 0.071 | 63 (19.1) | 0.067 | 0.757 | 0.074 |
| Development of HCC | 6 (1.2) | 38 (4.5) | 0.195 | 21 (2.6) | 0.101 | 32 (4.3) | 0.186 | 0.004 | 0.195 |
| Follow-up duration (months) | 35.6 (25.4, 42.9) | 45.0 (28.0, 58.2) | 0.550 | 32.6 (20.7, 48.9) | 0.027 | 43.5 (25.1, 56.7) | 0.453 | < 0.001 | 0.550 |
Values are expressed as number (%) and median (interquartile range).
*ASD between the BSV group and each antiviral therapy group.
**MASD among all pairwise comparisons of the four groups.
Abbreviations: HCC, hepatocellular carcinoma; BSV, besifovir dipivoxil maleate; ETV, entecavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; ASD, absolute standardized difference; MASD, maximum ASD; AST, aspartate aminotransferase; ALT, alanine aminotransferase; Gamma-GT, gamma-glutamyl transferase; BUN, blood urea nitrogen; INR, international normalized ratio; HBeAg, hepatitis B e antigen; ALBI, Albumin-bilirubin,; FIB-4, fibrosis-4 index; LS, liver stiffness; CAP, controlled attenuation parameter.
Comparison of HCC development between groups before matching
Over a median follow-up period of 38.6 months (IQR, 23.9–53.4: 35.6, 45.0, 32.6, and 43.5 in the BSV, ETV, TAF, and TDF groups, respectively), HCC developed in 97 (3.4%) patients (Table 1). The cumulative 2-, 3-, and 5-year incidence rates were 1.5%, 3.1%, and 6.0%, respectively, with an incidence rate of 10.4 per 1000 person-years (PYs). In the subgroup with cirrhosis, HCC developed in 81 (8.4%) patients over a median follow-up period of 39.6 (IQR, 25.3–53.8) months, with the cumulative 2-, 3-, and 5-year incidence rate of 3.9%, 7.7%, and 14.6%, respectively.
Patients in the BSV group developed HCC with an incidence rate of 4.3 per 1000 PYs, which was statistically similar to that in the TAF group (9.2 per 1000 PYs, log-rank P = 0.086, crude HR = 2.191, P = 0.090), however, significantly lower than those in the ETV group (12.5 per 1000 PYs, log-rank P = 0.026, crude HR = 2.627, P = 0.029) and the TDF group (12.3 per 1000 PYs, log-rank P = 0.028, crude HR = 2.623, P = 0.031) (Table 2 and Fig. 2A). ETV, TAF, and TDF use (vs. BSV, HR = 2.938, 2.810, and 3.553, respectively) was significantly associated with an increased risk of HCC development, together with older age, hypertension, cirrhosis, higher level of gamma-glutamyl transferase, and baseline HBV DNA within 4–7 log10 IU/mL (all P < 0.05) (Table S2 and Table 3).
Table 2.
Comparisons of cumulative incidence of HCC and HR between treatment groups.
| Treatment | HCC (%) | Cumulative incidence (%) | Incidence rate* | log-rank P value |
Univariable Cox regression | E value (lower**) |
|||
|---|---|---|---|---|---|---|---|---|---|
| 2-year | 3-year | 5-year | Crude HR (95% CI) | P value | |||||
| All (n = 2,889) | 97 (3.4) | 1.5 | 3.1 | 6.2 | 10.4 | 0.135 | - | - | - |
| BSV (n = 486) | 6 (1.2) | 1.2 | 1.2 | 1.8 | 4.3 | - | Reference | - | - |
| ETV (n = 852) | 38 (4.5) | 1.2 | 3.6 | 6.8 | 12.5 | 0.026 | 2.627 (1.103, 6.255) | 0.029 | 4.694 (1.441) |
| TAF (n = 801) | 21 (2.6) | 1.7 | 3.4 | 7.4 | 9.2 | 0.086 | 2.191 (0.884, 5.434) | 0.090 | NA |
| TDF (n = 750) | 32 (4.3) | 1.9 | 3.4 | 6.9 | 12.3 | 0.028 | 2.623 (1.090, 6.311) | 0.031 | 4.686 (1.403) |
*Incidence rate, per 1000 person-years.
**lower confidence limit.
Abbreviation: HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; BSV, besifovir dipivoxil maleate; ETV, entecavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Fig. 2.
Kaplan–Meier curves for the cumulative incidence of hepatocellular carcinoma of unweighted groups (A), after stabilized IPTW (B), after pairwise propensity score matching between BSV and ETV (C), BSV and TAF (D), and BSV and TDF (E). Abbreviations: IPTW, inverse probability treatment weighting, BSV, besifovir dipivoxil maleate; ETV, entecavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Table 3.
Multivariable Cox regression analyses.
| Variables | Univariable P value |
Mutivariable Cox regression | |
|---|---|---|---|
| P value | HR (95 CI) | ||
| BSV | - | - | Reference |
| ETV vs. BSV | 0.029 | 0.026 | 2.938 (1.139, 7.575) |
| TAF vs. BSV | 0.090 | 0.040 | 2.810 (1.048, 7.536) |
| TDF vs. BSV | 0.031 | 0.009 | 3.553 (1.366, 9.242) |
| Age (year) | < 0.001 | 0.013 | 1.030 (1.006, 1.054) |
| Hypertension | < 0.001 | 0.018 | 1.722 (1.098, 2.699) |
| Cirrhosis | < 0.001 | < 0.001 | 5.876 (3.087, 11.18) |
| Significant alcohol intake | 0.002 | 0.084 | 1.699 (0.931, 3.101) |
| Platelet count (× 109/L) | < 0.001 | 0.053 | 0.996 (0.992, 1.000) |
| ALT (IU/L) | < 0.001 | 0.077 | 0.997 (0.994, 1.000) |
| Gamma-GT (IU/L) | 0.019 | 0.03 | 1.002 (1.000, 1.004) |
| Serum creatinine (mg/dL) | 0.388 | 0.236 | 0.667 (0.342, 1.303) |
| 4—7 Log10 HBV DNA (IU/mL)† | < 0.001 | 0.030 | 1.631 (1.048, 2.537) |
†The proportional hazard assumption is not met. The hazard ratio is provided for descriptive purposes.
Abbreviation: HR, hazard ratio; CI, confidence interval; BSV, besifovir dipivoxil maleate; ETV, entecavir; BSV, besifovir dipivoxil maleate; ETV, entecavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; ALT, alanine aminotransferase; Gamma-GT; gamma-glutamyl transferase.
Comparisons after four-group stabilized inverse probability of treatment weighting (IPTW)
After stabilized IPTW, regardless of chronic kidney disease, and the baseline HBV DNA, the differences between the four groups were balanced (maximum absolute standardized mean differences (ASD) < 0.2) (Table S3). The incidence rate of HCC development was significantly lower in the weighted BSV group (3.4 per 1000 PYs) than those in the ETV, TAF, and TDF groups (11.6, 9.7, and 13.6 per 1000 PYs, respectively, log-rank P = 0.005) (Table 4 and Fig. 2B). IPTW-adjusted Cox regression analyses including the unbalanced variables showed that the risk of HCC development was independently higher in the ETV (aHR = 2.836, P = 0.023, E-value = 5.118), TAF (aHR = 2.784, P = 0.031, E-value 5.013), and the TDF groups (aHR = 3.294, P = 0.010, E-value = 6.044) than that in the BSV group (Table 4).
Table 4.
Cumulative incidence of HCC after IPTW and each 1:1 PSM.
| IPTW | n | HCC (%) | Follow-up (PYs) | Incidence rate* | log-rank P value |
Cox regression | E value (lower**) |
|
|---|---|---|---|---|---|---|---|---|
| Adjusted HR† (95 CI) | P value† | |||||||
| BSV | 312 | 3 (1.0) | 891.6 | 3.4 | 0.005 | Reference | - | - |
| ETV | 726 | 31 (4.3) | 2670.0 | 11.6 | 2.836 (1.151,6.986) | 0.023 | 5.118 (1.569) | |
| TAF | 719 | 20 (2.8) | 2051.5 | 9.7 | 2.784 (1.097,7.069) | 0.031 | 5.013 (1.422) | |
| TDF | 610 | 29 (4.8) | 2135.0 | 13.6 | 3.294 (1.336,8.121) | 0.010 | 6.044 (2.007) | |
| 1:1 PSM | Pairs*** | HCC (%) | Follow-up (PYs) | Incidence rate* |
log-rank P value |
Cox regression |
E value (lower**) |
|
| Adjusted HR (95 CI) | P value | |||||||
| BSV | 486 | 6 (1.2) | 1405.0 | 4.3 | - | Reference | - | - |
| ETV | 486 | 28 (5.8) | 1835.1 | 15.3 | 0.011 | 3.200 (1.163,8.803)‡ | 0.024‡ | 5.853 (1.599) |
| TAF | 486 | 17 (3.5) | 1436.6 | 11.8 | 0.029 | 3.250 (1.060,9.967) | 0.039 | 5.954 (1.311) |
| TDF | 486 | 27 (5.6) | 1724.5 | 15.7 | 0.012 | 3.750 (1.245,11.299) | 0.019 | 6.961 (1.796) |
*Incidence rate, per 1000 person-years.
**lower confidence limit.
***For every 1:1 PSM, 486 pairs are yielded.
†IPTW-adjusted HR, adjusted with chronic kidney disease, and HBV DNA.
‡PSM-adjusted HR, adjusted with serum albumin.
Abbreviations: IPTW, inverse probability treatment weighting; PSM, propensity-score matching; n, number; HCC, hepatocellular carcinoma; PYs, person-years; HR, hazard ratio; CI, confidence interval; BSV, besifovir dipivoxil maleate; ETV, entecavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Comparisons between the propensity score (PS)-matched groups
Because the baseline characteristics were not significantly different among the unmatched groups, each 1:1 PS matching yielded well-balanced pairs, with all BSV users (n = 486) included in the matching (all ASD < 0.2), except for serum albumin (ASD = 0.255) between the BSV and TAF groups (Table S4). Compared to the matched BSV group, the matched ETV, TAF, and TDF groups showed significantly higher incidence rates (4.3 vs. 15.3, 11.8, and 15.7 per 1000PYs, respectively, all P < 0.05) and a higher risk of HCC development (aHR and E-value, 3.200 and 5.853, 3.250 and 5.954, and 3.750 and 6.961, respectively, all P < 0.05) (Table 4 and Figs. 2C–E).
Subgroup analyses with baseline vibration-controlled transient elastography (VCTE) and cirrhosis
To further clarify the potential bias due to liver fibrosis, we conducted IPTW and PSM analyses on a subgroup of patients who had reliable baseline VCTE results (324 BSV, 431 ETV, 431 TAF, and 331 TDF users) (Table S5). ETV use (vs. BSV, HR = 3.828, P = 0.032) was associated with an increased risk of HCC development in the multivariable analysis, together with hypertension, cirrhosis, lower platelet count, baseline HBV DNA within 4–7 log10 IU/mL, and higher liver stiffness (LS) values (all P < 0.05) (Table S6). After four-group IPTW, TAF and TDF users had a similar risk of HCC development: however, ETV users had a higher risk compared to BSV users (aHR = 4.404, P = 0.009, E-value = 8.277) (Tables S7, S8).
In patients with cirrhosis (n = 966), stepwise Cox regression analyses compared to the BSV group showed that the risk of HCC was significantly higher in the TDF group (aHR = 3.270, P = 0.018) (Table S9). There was also a trend toward a higher risk in the ETV and TAF groups (aHR = 2.746–2.748, P ≤ 0.051) (Table S9).
AVT switch
AVT switches were applied in 13 cases in the BSV group (3 to ETV, 6 to TAF, and 4 to TDF), 51 cases in the ETV group (3 to BSV, 26 to TAF, and 22 to TDF), 16 cases in the TAF group (6 to ETV and 10 to TDF), and 51 cases in the TDF group (1 to BSV, 12 to ETV, and 38 to TAF). Most switches from BSV occurred due to persistent low-level viremia (LLV) (1–2 log10 IU/mL of HBV DNA after BSV use), except for one patient who switched to TDF due to pregnancy. No significant adverse drug reactions were reported in the BSV group. Among patients who switched AVTs, only one TDF user who had received treatment for 30.9 months developed HCC 31.3 months after switching to TAF. AVT was discontinued in three patients (2 ETV users and 1 TDF user) after achieving a functional cure, and none of these patients developed HCC during the follow-up period.
Decompensation and liver transplantation (LT)
During the follow-up periods, 9 non-BSV users experienced decompensation events such as ascites or varieal bleeding, of whom 2 patients after the HCC development. None of them were died or underwent LT. Three patients underwent LT, of whom 2 patients after the HCC development. One patients underwent emergent LT due to acute liver failure followed by cholangitis, and immediately died.
Discussion
To date, it has been known that BSV has similar clinical efficacy to ETV and TDF, including achieving undetectable HBV DNA levels, ALT normalization, and HBeAg seroconversion, with the added advantage of causing fewer side effects such as osteoporosis or renal dysfunction6,11,17. Moreover, the similar efficacy and clinical outcome between BSV and TAF users was already reported by Kim et al.18 In the study, BSV showed similar biochemical and virological response rates compared to TAF, and the cumulative probabilities of HCC was not significantly different between the two AVT groups (P= 0.31)18. Yim et al. also reported that BSV users without cirrhosis have a decreased incidence of HCC than the predicted incidence calculated by existing HCC prediction models, indicating a further risk reduction13. Kim et al. showed that the incidence of HCC was statistically similar between the matched BSV and TDF groups (1.6 vs. 2.2 per 1000 PYs, P= 0.284) using the Health Insurance Review and Assessment Service in South Korea14. The present study incorporated a wide range of clinical variables, including LS measurements, allowing for a more precise comparison in real-world settings.
Considering that debates persist about whether TDF is significantly more advantageous than ETV in reducing the risk of hepatocarcinogenesis for patients with CHB, although both are beneficial19,20,{Choi, 2019 #40} we conducted a multicenter large-volume retrospective cohort-based direct comparison between BSV and other potent NAs for the risk of hepatocarcinogenesis. Therefore, BSV users showed similar or significantly lower cumulative incidence and HR of HCC development compared to ETV, TAF, and TDF users, both before and after four-group IPTW and pairwise PSM analyses.
Our study had several important clinical implications. First, to the best of our knowledge, our study is the first to directly compare the benefits of BSV in the development of HCC with other potent AVTs in a large cohort (n = 2,889) with treatment-naïve CHB patients. ETV, TAF, and TDF use had significantly higher HR of hepatocarcinogenesis in the IPTW-weighted and pairwise PS-matched cohorts. Moreover, the calculated high E-values (5.013–6.961) suggested that any unmeasured confounders not addressed would need to increase the risk of HCC by at least 5–6 times to affect the findings of our study. Considering that fibrosis is a clinically significant factor, we conducted a subgroup analysis of patients who underwent TE, which is more accurate than Fibrosis-4 index. This analysis also demonstrated that BSV users have a similar or lower risk of developing HCC, especially compared to ETV users (HR 3.556–4.404, E value 6.571–8.277). Besides the use of AVT, cirrhosis, high LS value, and moderate levels of baseline HBV DNA were associated with HCC development. This is similar to recent studies suggesting a binomial on-treatment HCC risk associated with baseline HBV DNA levels21,22.
Second, while the short follow-up period (median 38.6 months) makes it difficult to fully trust BSV’s superiority, the following mechanisms may account for our findings. BSV is an acyclic nucleotide phosphonate with a chemical structure similar to acyclic nucleoside phosphonates such as TDF and TAF. Current experimental evidence suggests that TDF and TAF attenuate liver fibrosis and hepatocarcinogenesis via various non-viral effects that ETV does not. Tenofovir prevents progression and promotes the regression of liver fibrosis by regulating inflammasome signaling pathways and the differentiation, activation, and proliferation of hepatic stellate cells23,24. Moreover, TDF and TAF upregulate expression of the tumor suppressor p7 trans-regulated protein 3, thereby attenuating carcinogenesis by the Wnt/β-catenin signaling pathway25. Although this experimental evidence is not directly available for BSV, it may share the additional advantages of TDF and TAF, such as interferon-λ3 induction in the gastrointestinal tract induced, immunomodulation of lipopolysaccharides-mediated cytokine production, and inhibition of Akt and mTOR pathways26, which may result in the reported histologic improvement and decrease in intrahepatic cccDNA12, as well as an attenuated risk of HCC development in our study.
Third, another consideration for the attenuated hepatocarcinogenesis in BSV users is the concurrent administration of L-carnitine supplementation due to L-carnitine depletion, a significant side effect observed in the phase IIb results27. While this administration strategy may cause inconvenience for patients, it could potentially improve the outcomes of patients with CHB. The potential utility of L-carnitine for preventing hepatocarcinogenesis has been proposed in the literature. In an animal model, L-carnitine administration to Long-Evans Cinnamon rats prevented mitochondrial injury induced by chronic inflammation, resulting in significantly lower degrees of hepatic damage and inhibition of hepatocarcinogenesis28. Moreover, a high-fat diet-induced steatohepatitis mouse model receiving L-carnitine showed substantially improved liver histology and a reduced incidence of liver tumors, with decreased activation of oncogenic signaling pathways29,30. Clinically, patients in the highest quartile of serum L-carnitine concentration had a reduced risk of overall cancer (odds ratio = 0.67) compared to those with low L-carnitine concentration31. Therefore, L-carnitine supplementation might benefit BSV users more than patients with CHB who initiate other NAs, in addition to the antiviral efficacy of BSV Whether the combination of L-carnitine with other potent NAs also provides additional benefits requires further prospective studies.
Fourth, although most regimen changes from BSV occurred due to detectable HBV DNA, these BSV users maintained LLV without exacerbation or hepatocarcinogenesis. In in vitro drug susceptibility assays, the lamivudine-resistance mutations rtL180M (M) and rtM204V (V) were potentially associated with BSV resistance32. However, HBV mutants with primary mutations conferring resistance to adefovir or tenofovir were found to be susceptible to BSV33. Therefore, BSV may serve as an alternative drug for patients with tenofovir-resistant HBV mutations that do not include the MV sequence33. Despite debates, LLV during potent AVT with good adherence is not a predictive factor for HCC8. Our study also demonstrates that only one case of regimen change from TDF to ETV developed HCC, indicating that first-line BSV use has non-inferiority compared to the other AVTs in the reduction of HCC risk in treatment-naïve patients with active CHB.
Several limitations remained. First, the retrospective nature of our study had inevitable selection bias. Second, although patients were recruited during the same study period, we could not exclude the possibility that differences in follow-up durations between BSV or TAF users and ETV or TDF users may have influenced the incidence of HCC. Moreover, the limited events in BSV group (n = 6) may reduce statistical reliability. Notably, another recent study using Korean health insurance data also reported a similarly short median follow-up period (2.3–2.4 years)14. Since BSV is a relatively newly introduced agent, these data represent the most up-to-date evidence currently available. Third, the limited availability of BSV in South Korea mitigated the direct application of the present results to countries with different genotype dominance where BSV was currently unavailable. Fourth, the beneficial influence of BSV on other clinical outcomes, such as cirrhosis development, kidney function decline, and osteopenia, was not evaluated in the present study.
In conclusion, BSV showed comparable efficacy in reducing HCC development in patients with CHB compared to TAF, ETV, and TDF, indicating promising efficacy. Given the small number of events and the relatively short follow-up duration observed in BSV users, further long-term analysis is warranted.
Methods
Study population
This multicenter, retrospective cohort study recruited treatment-naïve patients with CHB who initiated AVT with ETV, TDF, TAF, or BSV, from six tertiary academic medical institutes in South Korea (Severance Hospital, Seoul National University Hospital, Korea University Anam Hospital, Korean University Ansan Hospital, Soonchunhyang University Bucheon Hospital, and Catholic University of Korea Bucheon St. Mary’s Hospital) between January 2017 and April 2022. Exclusion criteria were as follows: (1) prior experience of AVT or interferon-based treatment before 2017; (2) age < 19 or ≥ 80 years; (3) decompensated cirrhosis; (4) HCC development or liver transplantation before or within 12 months from AVT initiation; (5) follow-up duration < 12 months; (6) incomplete baseline data; (7) other viral hepatitis such as hepatitis C virus infection; (8) uncontrolled malignancy; and (9) other significant illnesses. This study conformed to the ethical guidelines of the Declaration of Helsinki (1975) and was approved by the Institutional Review Board of the Yonsei University College of Medicine, Seoul, South Korea. The written informed consent waived by the Institutional Review Board of the Yonsei University College of Medicine due to the retrospective nature of the study.
AVT initiation and data collection
AVT was initiated in accordance with the practice guidelines of the Korean Association for the Study of the Liver6,34 (Table S1). An index date was defined as the date AVT was initiated. Cirrhosis was histologically or clinically diagnosed as follows: 1) platelet count of < 150 × 103/μL or imaging findings suggestive of cirrhosis, including a blunted, nodular surface accompanied by splenomegaly (> 12 cm); or 2) clinical signs of portal hypertension35. ETV and TDF were administered at 0.5 mg and 300 mg daily, respectively, and the doses were appropriately reduced according to the patient’s kidney function. TAF was administered at 25 mg daily as a fixed dose. BSV was administered at 150 mg daily as a fixed dose with supplementary L–carnitine at 600 mg daily27.
Patients with CHB routinely visited the outpatient clinic at 3–6 month intervals, and information on their laboratory data (such as blood chemistry test, viral marker, and serum levels of HBV DNA), abdominal image study (such as abdominal ultrasonography), change in their AVT from the index date until loss of follow–up or HCC development were collected6,8. LS value and controlled attenuation parameter (CAP) was measured using VCTE (FibroScan®, EchoSens, Paris, France) in a standard way36. Only LS and CAP values with at least 10 valid measurements, a success rate of at least 60%, and an interquartile range (IQR) to median ratio of < 30% were considered reliable. Patients with CAP values greater than 239 dB/m and 274 dB/m were considered to have mild and moderate steatosis, respectively37.
The primary outcome was HCC development between the index date and April 2023. HCC was diagnosed based on histological evidence or by dynamic computed tomography and/or magnetic resonance imaging findings (nodule > 1 cm with arterial hypervascularity and portal-/delayed-phase washout)38–40..
Statistical analysis
Baseline characteristics and the primary outcome were described as medians (IQRs) for continuous variables and numbers (percentages) for categorical variables. Kruskal–Wallis test was used for continuous variables, and Chi-squared test or Fisher’s exact test was used for categorical variables to assess statistical differences among the treatment groups. The cumulative incidence of HCC was evaluated using the Kaplan–Meier method, and were compared using the log-rank test. Cox proportional hazards models were used to assess the association among the treatment groups and outcome. The proportional hazards assumption was examined using the Schoenfeld residuals tests. To account for possible factors that could have influenced the attending physician’s choice of AVT, stabilized IPTW was adopted to create a “pseudo-sample” that is balanced in the distribution of baseline characteristics among the treatment groups. Furthermore, we conducted a sensitivity analysis using PS matching with a 1:1 optimal matching algorithm for pairwise comparisons. Cox proportional hazards models were performed, including imbalanced confounders (ASDs ≥ 0.2) after weighting for doubly robust estimation. An E-value with their corresponding 95% confidence interval lower limits was calculated to evaluate the robustness of the primary outcome to potential unmeasured confounders23. Subgroup analysis of patients with VCTE results were performed before and after the IPTW analysis. For further details, see the Supplementary information. All statistical analyses were performed using R (version 4.3.3; http://cran.r-project.org/). Two-sided P values of < 0.05 were considered to indicate statistical significance.
Supplementary Information
Acknowledgements
This research was supported by the National Institute of Health (NIH) research project in South Korea (2025-E1903-00).
Author contributions
JS Lee, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing; SW Lee, Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Writing – original draft, Writing – review & editing HL Lee, Data curation, Investigation, Resources, Writing – review & editing; JJ Yoo, Data curation, Investigation, Resources, Writing – review & editing; YS Seo, Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Writing – review & editing; SJ Yu, Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – review & editing; HJ Yim, Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – review & editing; YK Jung, Data curation, Investigation, Resources, Writing – review & editing; J Moon, Data curation, Formal analysis, Methodology, Validation, Visualization; HW Lee, Resources, Writing – review & editing; MN Kim, Resources, Writing – review & editing; BK Kim, Resources, Writing – review & editing; JY Park, Resources, Writing – review & editing; DY Kim, Resources, Writing – review & editing; SH Ahn, Resources, Writing – review & editing; SG Kim, Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing, Funding acquisition; SU Kim, Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing, Funding acquisition.
Funding
This study was supported by ILDONG Pharmaceutical, Inc. The funders played no role in the study design, data collection, analysis, interpretation, or the writing of the manuscript.
Data availability
The data that support the findings of this study are available from the co-corresponding authors upon reasonable request.
Declaration
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jae Seung Lee, Sung Won Lee equally contributed as co-1st authors.
Sang Gyune Kim, Seung Up Kim equally contributed as co-corresponding authors.
Contributor Information
Sang Gyune Kim, Email: mcnulty@schmc.ac.kr.
Seung Up Kim, Email: ksukorea@yuhs.ac.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-13456-8.
References
- 1.Lin, C. L. & Kao, J. H. Development of hepatocellular carcinoma in treated and untreated patients with chronic hepatitis B virus infection. Clin. Mol. Hepatol.29, 605–622. 10.3350/cmh.2022.0342 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levrero, M. & Zucman-Rossi, J. Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol.64, S84-s101. 10.1016/j.jhep.2016.02.021 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Terrault, N. A. et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology67, 1560–1599. 10.1002/hep.29800 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EASL. Clinical practice guidelines on the management of hepatitis B virus infection. J. Hepatol.67(370–398), 2017. 10.1016/j.jhep.2017.03.021 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Sarin, S. K. et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol. Int.10, 1–98. 10.1007/s12072-015-9675-4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol25 93–159 10.3350/cmh.2019.1002 (2019). [DOI] [PMC free article] [PubMed]
- 7.Hsu, Y. C., Tseng, C. H. & Kao, J. H. Safety considerations for withdrawal of nucleos(t)ide analogues in patients with chronic hepatitis B: First, do no harm. Clin. Mol. Hepatol.29, 869–890. 10.3350/cmh.2022.0420 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee, S. B. et al. Low-level viremia and cirrhotic complications in patients with chronic hepatitis B according to adherence to entecavir. Clin. Mol. Hepatol.26, 364–375. 10.3350/cmh.2020.0012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong, G. L. et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology58, 1537–1547. 10.1002/hep.26301 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Song, J. E. & Park, J. Y. Besifovir dipivoxil maleate: A novel antiviral agent with low toxicity and high genetic barriers for chronic hepatitis B. Expert Opin. Pharmacother.22, 2427–2433. 10.1080/14656566.2021.1967321 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Song, D. S. et al. Continuing besifovir dipivoxil maleate versus switching from tenofovir disoproxil fumarate for treatment of chronic hepatitis B: Results of 192-week phase 3 trial. Clin. Mol. Hepatol.27, 346–359. 10.3350/cmh.2020.0307 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yim, H. J. et al. Besifovir therapy improves hepatic histology and reduces covalently closed circular DNA in chronic hepatitis B patients. J. Gastroenterol. Hepatol.37, 378–386. 10.1111/jgh.15710 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Yim, H. J. et al. Reduced risk of hepatocellular carcinoma in patients with chronic hepatitis b receiving long-term besifovir therapy. Cancers (Basel)10.3390/cancers16050887 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, H. et al. Comparison of hepatocellular carcinoma incidence after long-term treatment with besifovir vs tenofovir AF. Sci. Rep.15, 5637. 10.1038/s41598-025-89325-1 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, W. R. et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer121, 3631–3638. 10.1002/cncr.29537 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Hosaka, T. et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology58, 98–107. 10.1002/hep.26180 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Yuen, M. F. et al. A randomized placebo-controlled, dose-finding study of oral LB80380 in HBeAg-positive patients with chronic hepatitis B. Antivir. Ther.11, 977–983 (2006). [PubMed] [Google Scholar]
- 18.Kim, T. H. et al. Noninferiority outcomes of besifovir compared to tenofovir alafenamide in treatment-naïve patients with chronic hepatitis B. Gut Liver18, 305–315. 10.5009/gnl220390 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, S. U. et al. A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naïve chronic hepatitis B in South Korea. J. Hepatol.71, 456–464. 10.1016/j.jhep.2019.03.028 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Choi, J. et al. Risk of hepatocellular carcinoma in patients treated with entecavir vs tenofovir for chronic hepatitis B: A Korean nationwide cohort study. JAMA Oncol.5, 30–36. 10.1001/jamaoncol.2018.4070 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi, W. M. et al. Non-linear association of baseline viral load with on-treatment hepatocellular carcinoma risk in chronic hepatitis B. Gut73, 649–658. 10.1136/gutjnl-2023-330225 (2024). [DOI] [PubMed] [Google Scholar]
- 22.Chun, H. S. et al. PAGE-B incorporating moderate HBV DNA levels predicts risk of HCC among patients entering into HBeAg-positive chronic hepatitis B. J. Hepatol.80, 20–30. 10.1016/j.jhep.2023.09.011 (2024). [DOI] [PubMed] [Google Scholar]
- 23.Zhao, J. et al. TAF and TDF attenuate liver fibrosis through NS5ATP9, TGFβ1/Smad3, and NF-κB/NLRP3 inflammasome signaling pathways. Hepatol. Int.14, 145–160. 10.1007/s12072-019-09997-6 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Lee, S. W. et al. Tenofovir disoproxil fumarate directly ameliorates liver fibrosis by inducing hepatic stellate cell apoptosis via downregulation of PI3K/Akt/mTOR signaling pathway. PLoS ONE16, e0261067. 10.1371/journal.pone.0261067 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao, J. et al. TDF and TAF inhibit liver cancer cell migration, invasion via p7TP3. Sci. Rep.14, 8161. 10.1038/s41598-024-58807-z (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murata, K. & Mizokami, M. Possible biological mechanisms of entecavir versus tenofovir disoproxil fumarate on reducing the risk of hepatocellular carcinoma. J. Gastroenterol. Hepatol.38, 683–691. 10.1111/jgh.16178 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Lai, C. L. et al. Phase IIb multicentred randomised trial of besifovir (LB80380) versus entecavir in Asian patients with chronic hepatitis B. Gut63, 996–1004. 10.1136/gutjnl-2013-305138 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Chang, B., Nishikawa, M., Nishiguchi, S. & Inoue, M. L-carnitine inhibits hepatocarcinogenesis via protection of mitochondria. Int. J. Cancer113, 719–729. 10.1002/ijc.20636 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Lyu, J. et al. Potential utility of l-carnitine for preventing liver tumors derived from metabolic dysfunction-associated steatohepatitis. Hepatol. Commun.10.1097/hc9.0000000000000425 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishikawa, H. et al. L-carnitine prevents progression of non-alcoholic steatohepatitis in a mouse model with upregulation of mitochondrial pathway. PLoS ONE9, e100627. 10.1371/journal.pone.0100627 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, T. et al. The association of serum L-carnitine concentrations with the risk of cancer in chinese adults with hypertension. Nutrients10.3390/nu14234999 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim, J. C. et al. Identification and characterization of besifovir-resistant hepatitis B virus isolated from a chronic hepatitis B patient. Biomedicines10.3390/biomedicines10020282 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Won, J. et al. Susceptibility of drug resistant hepatitis B virus mutants to besifovir. Biomedicines10.3390/biomedicines10071637 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yim, H. J. et al. Comparison of clinical practice guidelines for the management of chronic hepatitis B: When to start, when to change, and when to stop. Clin. Mol. Hepatol.26, 411–429. 10.3350/cmh.2020.0049 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.KASL clinical practice guidelines for liver cirrhosis. Varices, hepatic encephalopathy, and related complications. Clin. Mol. Hepatol.26, 83–127. 10.3350/cmh.2019.0010n (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim, G., Kim, M. Y. & Baik, S. K. Transient elastography versus hepatic venous pressure gradient for diagnosing portal hypertension: A systematic review and meta-analysis. Clin. Mol. Hepatol.23, 34–41. 10.3350/cmh.2016.0059 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen, J. et al. Controlled attenuation parameter for the detection of hepatic steatosis in patients with chronic hepatitis B. Infect Dis. (Lond)48, 670–675. 10.3109/23744235.2016.1165860 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Heimbach, J. K. et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology67, 358–380. 10.1002/hep.29086 (2018). [DOI] [PubMed] [Google Scholar]
- 39.EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J. Hepatol.69, 182–236. 10.1016/j.jhep.2018.03.019 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Lee, S., Kim, S. S., Chang, D. R., Kim, H. & Kim, M. J. Comparison of LI-RADS 2018 and KLCA-NCC 2018 for noninvasive diagnosis of hepatocellular carcinoma using magnetic resonance imaging. Clin. Mol. Hepatol.26, 340–351. 10.3350/cmh.2020.0004 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the co-corresponding authors upon reasonable request.



