Abstract
A number of hydrothermal vent sites exist on the summit of the Loihi Seamount, a shield volcano that is part of the Hawaiian archipelago. The vents are 1,100 to 1,325 m below the surface and range in temperature from slightly above ambient (10°C) to high temperature (167°C). The vent fluid is characterized by high concentrations of CO2 (up to 17 mM) and Fe(II) (up to 268 μM), but there is a general paucity of H2S. Most of the vents are surrounded by microbial mats that have a gelatinous texture and are heavily encrusted with rust-colored Fe oxides. Visually, the Fe oxides appeared homogeneous. However, light microscopy revealed that the oxides had different morphologies, which fell into three classes: (i) sheaths, (ii) twisted or irregular filaments, and (iii) amorphous oxides. A morphological analysis of eight different samples indicated that the amorphous oxides were overall the most abundant; however, five sites had >50% sheaths and filamentous oxides. These latter morphologies are most likely the direct result of microbial deposition. Direct cell counts revealed that all of the oxides had abundant microbial populations associated with them, from 6.9 × 107 to 5.3 × 108 cells per ml of mat material. At most sites, end point dilution series for lithotrophic Fe oxidizers were successful out to dilutions of 10−6 and 10−7. A pure culture was obtained from a 10−7 dilution tube; this strain, JV-1, was an obligate, microaerophilic Fe oxidizer that grew at 25 to 30°C. A non-cultivation-based molecular approach with terminal-restriction fragment length polymorphism also indicated the common presence of Fe-oxidizing bacteria at Loihi. Together, these results indicate that Fe-oxidizing bacteria are common at the Loihi Seamount and probably play a major role in Fe oxidation. A review of the literature suggests that microbially mediated Fe oxidation at hydrothermal vents may be important globally.
Ferrous iron is a common and often abundant constituent of hydrothermal vent fluids; however, we know little about the involvement of microbes in iron oxidation at deep-sea vents. Most of the research on Fe oxides and Fe oxidation at hydrothermal vents has focused on the iron mineral assemblages associated with low-temperature venting sites. These studies have demonstrated that the signature remains of putative Fe-oxidizing bacteria, in the form of Fe oxide-encrusted sheaths and filaments, are associated with both active and extinct vents (20, 22). Microbiological studies have shown that active microbial communities, including Mn-oxidizing bacteria, inhabit particulate iron and manganese oxides associated with vent plumes (4, 28). While these reports provide circumstantial evidence for the presence and activity of Fe oxidizers associated with hydrothermal vents, no systematic studies demonstrating the presence and activity of neutrophilic, lithotrophic Fe oxidizers have been carried out.
As a group, neutrophilic, aerobic Fe-oxidizing bacteria have been recognized for a very long time; however, since they have proven difficult to cultivate in the laboratory, evidence for their existence and importance in Fe transformations has largely been circumstantial (9). The signature characteristics of Fe-oxidizing bacteria are the unique morphological structures they produce, such as sheaths or stalks, that act as organic matrices upon which the deposition of hydrous ferric oxides (HFOs) can occur (13). Most characteristic among these are the Fe(III)-encrusted sheaths of Leptothrix ochracea and the helical stalklike filaments of Gallionella spp. L. ochracea has not been cultured axenically in the laboratory. However, Gallionella ferruginea has, and the prevailing wisdom is that this organism grows lithoautotrophically on Fe(II) (14, 16). Recently, novel lithotrophic Fe oxidizers have been isolated from freshwater environments; these organisms grow microaerobically at circumneutral pH but do not form morphologically distinct Fe oxide structures (10, 11, 34). In marine environments, the direct evidence for Fe-oxidizing bacteria is not well documented. The one notable exception was the finding of abundant Gallionella-like stalk material and microscopic identification of putative G. ferruginea cells from a shallow water volcanic system near Santorini Island in the Mediterranean Sea (15, 16).
Loihi Seamount is the newest shield volcano that is part of the Hawaiian archipelago. Low temperature hydrothermal venting was discovered at Loihi by deep-sea submersible in 1988 (24). Analysis of the vent waters at that time showed them to be highly enriched in Fe(II), Mn, and CO2 but to have quite low concentrations of H2S. The impact of the high Fe(II) concentrations was readily apparent in the large mats of HFOs that formed around the vent orifices; microscopic analysis showed that the empty Fe-encrusted sheath casts of L. ochracea-like iron-oxidizing bacteria were abundant (23). Subsequent molecular analysis of the microbial community at Loihi (29, 31) fortuitously found that some of the most abundant phylotypes based on small-subunit (SSU) ribosomal DNA (rDNA) analysis were very closely related to recently described freshwater lithotrophic Fe oxidizers (10).
Conditions at the Loihi Seamount changed dramatically in 1996 when a major eruption at the summit altered both the terrain and chemistry of the vent water (5). A 300-m-deep pit crater (Pele's Pit) formed at the summit; at the bottom of the pit, new vent sites formed that had rapid flow rates and temperatures up to 200°C. The chemistry of the vent water changed as well; in Pele's Pit the Fe/Mn ratios were much lower than at the lower flow rate, low-temperature vents (10 to 20°C) on the summit (39). The waters of the high-temperature vents also had low sulfide concentrations, were enriched in CO2, and had pHs of around 5.6. Visual observations revealed that abundant HFO-encrusted microbial mat communities formed in the immediate proximity of multiple vent orifices.
The goal of this research was to investigate both the low- and high-temperature vent sites at the summit of Loihi and to determine the extent to which Fe-oxidizing bacteria inhabit these sites. Using new culture techniques, we attempted to grow these organisms and isolate putatively lithotrophic representatives. Our findings indicate that microaerobic, Fe-oxidizing bacteria are present at all the vents we visited. They are often abundant and may contribute substantially to the formation of the extensive deposits of HFOs at the site.
MATERIALS AND METHODS
Site.
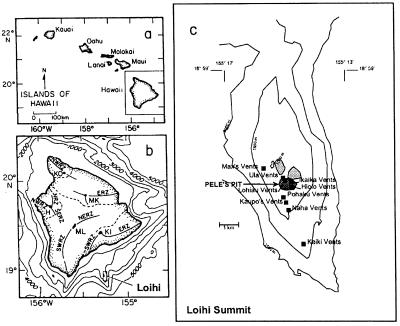
Loihi Seamount and the vent sites associated with both Pele's Pit and the flanking slopes are illustrated in Fig. 1. Since 1988, the summit of Loihi has been monitored closely by various oceanographic techniques, including remote sensing and submersible operations. As a result, there is a good record of the physical and geochemical conditions at the summit. At the time of this sampling trip in October 1998, the maximum temperature measured in Pele's Pit was 167°C; the temperatures in 1996 and 1997 were 200°C. The maximum Fe(II) concentrations measured directly in the vent water were 268, 296, and 1,934 μM in 1998, 1997, and 1996, respectively (G. Wheat, personal communication)
FIG. 1.
(a) Hawaiian Islands, showing location of the island of Hawaii. (b) Location of Loihi Seamount relative to the island of Hawaii and its five volcanic shields. (c) Summit bathymetry of Loihi Seamount, showing location of observed hydrothermal vents. Pele's Pit formed during the 1996 eruption. Depths are in meters. Shaded areas indicate the summit pit craters.
Sampling.
Sampling was done with the manned deep-sea submersible Pisces V. All microbiological samples were collected with a suction pump, or slurp gun, that was very effective at vacuuming up the Fe mats from the sea floor. The slurp gun was coupled to a rotating carousel of acrylic collection bottles. Upon return to the surface, the bottles were removed from the carousel and the samples settled for 1 to 2 h before the overlaying water was poured off, leaving a mixture of HFOs. A portion of this Fe oxide sample was treated as a live sample and stored at 4°C for enrichment studies. Other aliquots were fixed with 2% glutaraldehyde, stored at 4°C, and subsequently used for cell counts and morphological analysis; the remainder of each sample was frozen at −80°C for nucleic acid extraction and molecular analysis.
Iron analysis.
To determine the total iron concentration from fixed samples of microbial mat, aliquots were removed and diluted 500-fold in 0.5 M hydroxylamine-HCl and incubated on a rotary shaker (150 rpm) at 30°C for at least 12 h and then a subsample was diluted, either 50- or 100-fold, into a ferrozine solution to determine the Fe(II) concentration colorimetrically (36). Visually, it appeared that the Fe oxides from all of the Loihi vent sites were readily reduced under these conditions because the rust-colored solutions cleared completely. To determine the density of mat material at each site, a dry weight determination was done by placing duplicate aliquots of mat material into tared aluminum weigh boats. The wet weights were determined to the nearest 0.1 mg; the sample was oven dried at 80°C for 72 h and weighed again.
Total cell counts and morphological analysis.
Total cell numbers for each vent sample were determined by total direct cell counting as described previously (1). This consisted of diluting the samples, usually 50-fold, in sterile artificial seawater (see below); 10 μl of the diluent was smeared evenly within a 1-cm-diameter circle inscribed on a microscope slide (fluorescent antibody slides; Gold Seal Products, Highland Park, Ill.) that had been coated with 1% agarose (Sigma Chemical Co, St. Louis, Mo.). After air drying, the samples were stained with 25 μM Syto (Molecular Probes, Eugene, Oreg.) and 15 fields/circle were counted by using an Olympus BX60 microscope equipped for epifluorescence. At least 4 smears (ca. 300 to 600 cells) were counted for each vent sample.
To quantitate the morphotypes of iron oxides from each of the different sites, samples of the iron mat were mixed by vortexing for 1 min and then diluted to the extent that the different HFO morphotypes were distributed individually with minimal clumping when viewed on a microscope slide. Photomicrographs of 15 to 20 microscope fields per sample were taken at random with a charge-coupled device camera (Optronics DEI 750). Each image was viewed with NIH Image (http://www.rsb.info.nih.gov/nih-image) on an Apple Macintosh G3 computer. The trace tool of NIH Image was used to manually outline each oxide morphotype in each field, and then the area of that particle was calculated automatically. Area values were imported into Microsoft Excel, and each oxide area was binned into one of three types: amorphous, filament, or sheath (see below). Using the appropriate dilution factor, the total area of each oxide category was determined for each sample.
Growth media.
Two variations of an Fe oxidizer growth medium were used: gradient tube and liquid. The gradient tube method has been described previously (10). Briefly, this culture method exploits opposing gradients of O2 and Fe(II) that allow the Fe-oxidizing microbes to grow at the oxic-anoxic interface in a gel-stabilized medium that contains a ferrous sulfide plug at the bottom that acts as a source of Fe(II) and reducing power. For the present work, the growth conditions were modified to use a modified Wolfe’s minimal medium-artificial seawater (MWMM/ASW) mineral salts medium composed of the following (per liter): 27.5 g of NaCl, 5.38 g of MgCl2 · 6H2O, 6.78 g of MgSO4 · 7H2O, 0.72 g of KCl, 0.2 g of NaHCO3, 1.4 g of CaCl2 · 2H2O, 1 g of NH4Cl, and 0.05 g of K2HPO4 · 3H2O. Wolfe's vitamins and trace element solutions (40) were added to a final concentration of 1 ml/liter. Sodium bicarbonate was added at a concentration of 10 mM to serve as a buffering agent and C source. The gradient tubes used 0.15% (wt/vol) agarose (low melt agarose; Fisher Scientific, Pittsburgh, Pa.) as a gel-stabilizing agent.
A liquid enrichment technique was also developed, the principle of which was to maintain low concentrations of Fe(II) and O2 through the regular addition of small amounts of air and FeCl2 to a liquid medium in a closed vessel. The same basal salts medium described above was used, except agarose was omitted. For the dilution series, 10 ml of liquid in 25-ml Balch tubes was used; for larger scale growth studies, 60-ml serum bottles containing 40 ml of medium were used. The tubes or bottles were prepared by gassing the ASW medium, containing 10 mM NaHCO3, with N2 for 5 min, then gassing with CO2 for an appropriate time to reduce the pH to 6.4 to 6.5. This was determined empirically with a pH meter. The medium was then gassed for an additional 2 to 3 min with N2. Butyl rubber septa were placed in the tubes and sealed with aluminum crimps, and then the medium was autoclaved at 121°C for 20 min. Just prior to inoculation, a sterile syringe with a 22-gauge needle was used to deliver approximately 200 μM FeCl2 from a stock solution and enough sterile air to yield approximately 1% O2 in the headspace.
A sterile FeCl2 stock solution was prepared by bubbling deionized water with N2 for 15 min and then adding enough FeCl2 to make a 100 mM solution. This solution was filter sterilized through a 0.22-μm syringe filter into a presterilized serum bottle that had been flushed with N2 gas. The solution was bubbled with sterile N2 gas for an additional 5 min. This FeCl2 solution was a golden color, over time (weeks) some precipitation of Fe oxides occurred.
Enrichments.
Some enrichments were started onboard ship by doing dilution series with gradient tubes. In this case, a series of 10-fold dilutions of each original sample was done in tubes containing sterile ASW. From these dilutions, gradient tubes were inoculated (10 μl each) in 10× steps from 10−2 to 10−8. In liquid medium, serial dilutions were done starting with a vent sample diluted to 10−2, followed by a series of 10× dilutions to a final dilution of 10−7. The tubes were incubated in the dark at temperatures of 12, 20 to 24 (room temperature), 60, or 80°C. Every 24 or 48 h, additional air and FeCl2 were added to the tubes. The presence or absence of cell growth was determined by epifluorescence microscopy with either acridine orange or Syto to reveal the cells that were bound to the Fe oxides.
To obtain purified enrichments or pure cultures, subsequent dilution series were done with an inoculum from the highest dilution tube that yielded growth. To obtain pure cultures, at least two additional dilutions to extinction were carried out until a uniform cell morphology was confirmed. To check for purity, heterotrophic medium, consisting of R2A (Difco, Detroit, Mich.) or nutrient agar plates, both amended with 2.5% NaCl were streaked out and incubated both aerobically and microaerobically at room temperature.
Pure cultures.
Pure cultures were assessed for growth on a variety of substrates in liquid ASW under microaerobic conditions. The substrates included thiosulfate, tetrathionate, formate, pyruvate, acetate, succinate, glucose, and galactose, all at 5 mM, and 0.5% (wt/vol) yeast extract. A growth curve of the strain JV-1 was carried out in liquid media. For this study, the organism was grown in serum bottles amended each day with FeCl2 and air, as described above. Each day a sample was withdrawn and a direct cell count by epifluorescent microscopy was done to determine the total number of cells.
T-RFLP analysis.
Terminal-restriction fragment length polymorphism (T-RFLP) is a direct DNA fingerprinting technique that employs fluorescently labeled PCR primers to amplify selected regions of microbial SSU rDNA from specific groups of taxa. PCR products are then digested with tetrameric restriction enzymes, and the fluorescently labeled terminal restriction fragments are precisely measured and quantified (27). Samples of the Fe mat were subjected to extraction and isolation of total genomic DNA followed by multitemplate PCR (30) with an annealing temperature of 56°C and a total reaction volume of 50 μl. A pair of universal bacterial primers were used. The forward primer (5′-TNA NAC ATG CAA GTC GRR CG) corresponds to positions 49 to 68 of Escherichia coli rRNA, and the reverse primer (5′-RGY TAC CTT GTT ACG ACT T) corresponds to positions 1510 to 1492, where R is purine analog K, Y is pyrimidine analog P, and N is an equal mixture of both analogs at a single position (Glen Research, Sterling, Va.). The 68F primer was fluorescently labeled with 6-FAM (6-carboxyfluorescein) on the 5′ end, and both primers were polyacrylamide gel electrophoresis purified (ResGen, Huntsville, Ala.). PCR amplification products (5 μl) were visualized and assayed for size by 1% gel electrophoresis against a 1-kb ladder DNA standard. Only reaction mixtures yielding no amplification of the negative controls were used. Fluorescently labeled PCR products (15 μl of each PCR) were then digested for 6 to 8 h with 5 U of HhaI (New England Biolabs, Beverly, Mass.) in a total volume of 30 μl. The array of end-labeled SSU rDNA fragments was separated by polyacrylamide gel electrophoresis against the Genescan-500 ROX size standard with an ABI model 377 automated DNA sequencer, and the data were analyzed with the Genescan software (Applied Biosystems, Foster City, Calif.).
RESULTS
Microbial mats at Loihi.
All of the vent sites that were investigated at Loihi had deposits of HFOs associated with them (Table 1), these were primarily associated with flocculent mats, which often extended several meters from the vent orifices (Fig. 2). In a few cases, the oxides precipitated as loose crusts that formed <1 m from the vent orifice. The depths of these HFO mats were difficult to accurately determine due to the rough substrata and inherent lack of precision of the sampling methods; however, estimated depths of 1 to 5 cm were common. When normalized to a dry weight basis, all of the mats had very high concentrations of total reducible iron, ranging from 55 to 183 mg of iron·g (dry weight) of mat−1 (Table 1). Correlation analysis of the data showed no evident associations between the temperature and the amount of Fe oxides (results not shown). Extensive microscopic observations and total cell counts of all of the Fe mat samples collected from each of the sites revealed that in every case between 107 and 108 cells per ml of mat were present (Table 1). Most of these cells appeared tightly associated with the Fe oxides, as observed by epifluorescence microscopy. Furthermore, many of these oxides had distinctive morphotypes, indicating the involvement of Fe-oxidizing bacteria (Fig. 3 and see below).
TABLE 1.
Characteristics of Fe mats at Loihi, including total number of cells and oxide morphotypee
| Sample location | Sample no. | Depth (m below sea level) | Temp (°C)a | Mat density (g/ml) | Total Fe (mg/g [dry wt]) | No. of cells (SD)b | Dilution seriesc | Dominant morphotype(s)d |
|---|---|---|---|---|---|---|---|---|
| Pele's Pit, Ikaika vents, marker 20 | 393, 1-4 | 1,317 | 68 | 0.170 | 55 | 2.93 × 108 (4.2 × 107) | 10−4 | Filaments |
| Pele's Pit, Ikaika vents, lower jets | 393, 5-8 | 1,298 | 167 | 0.268 | 81 | 6.9 × 107 (1.6 × 107) | 10−7 | Amorphous |
| South rift, Naha vents, marker 1 | 396, 2-4 | 1,325 | 10 | 0.097 | 183 | 2.45 × 108 (7.94 × 107) | 10−6 (10−4 at 12°C) | Filaments |
| Upper South rift, Pohaku vents, marker 27 | 396, 6 | 1,196 | 17 | 0.049 | 77 | 9.33 × 107 (8.6 × 106) | 10−5 | Amorphous, sheaths |
| Upper South rift, Pohaku vents, marker 27 | 396, 7-8 | 1,196 | 17 | 0.157 | 153 | 1.26 × 108 (3.1 × 107) | 10−7 | Amorphous, filaments |
| Pele's Pit, Ikaika vents, lower jets | 397, 1-4 | 1,295 | 165 | 0.140 | 141 | 4.61 × 108 (6.5 × 107) | 10−6 | Amorphous |
| Pele's Pit, Ikaika vents, upper jets | 397, 5 | 1,289 | 167 | ND | ND | ND | ND | Amorphous |
| Pele's Pit, Ikaika vents, near marker 15 | 398, 5-6 | 1,290 | 113 | 0.115 | 152 | 1.54 × 108 (4 × 107) | 10−6 | Amorphous, filaments |
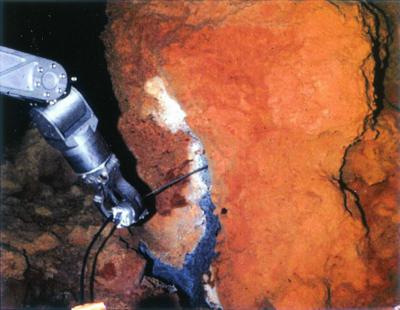
FIG. 2.
Loihi hydrothermal vent site. A temperature probe is being inserted into the vent orifice; note the extensive deposits of Fe oxides surrounding the vent opening.
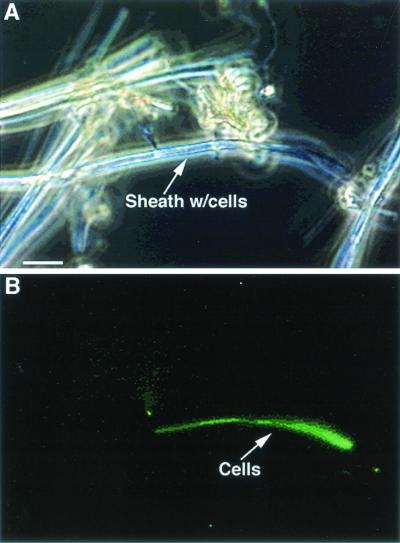
FIG. 3.
L. ochracea-like sheaths collected at the Pohaku vents near marker 27. The sample has been stained with Syto. Panel B is the same image as in panel A but viewed by epifluorescence to reveal a filament of cells inside the iron-encrusted sheath. The cells are only visible when stained; most of the sheaths are empty. Bar, 5 μm.
All dilution series that were initiated from samples collected at Loihi yielded positive results for putative lithotrophic Fe-oxidizing bacteria (Table 1). At the Ikaika vents lower jets site (393, 5-8) and the Pohaku vents site (396, 7-8), growth was detected in 10−7 dilution tubes, which was the end dilution for these series. The least abundant growth was represented by the 10−4 dilution at the Ikaika vents marker 20 site (393, 1-4). Gradient dilution tubes inoculated onboard ship within a few hours after sampling from another Pohaku vents sample (396, 7-8) yielded growth in 10−6 dilutions compared to growth in 10−7 dilutions in the liquid series. In general, we found that dilutions done in liquid medium tended to yield higher recoveries of Fe oxidizers than dilution series done in gradient tubes.
Several dilution series were also attempted at temperatures of 60 and 80°C. At 80°C there was no evidence for microbial growth, and at 60°C evidence for sustained growth by microbial iron oxidation was ambiguous. No pure cultures of thermophilic, microaerobic Fe oxidizers have been obtained. At the other temperature extreme, a dilution series incubated under psychrotrophic conditions at 12°C exhibited growth at the 10−4 dilution.
Morphological studies.
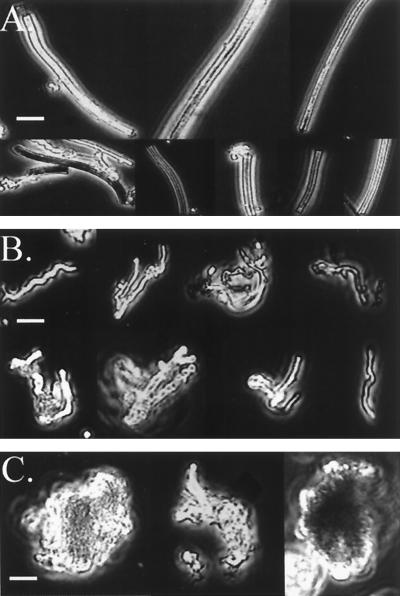
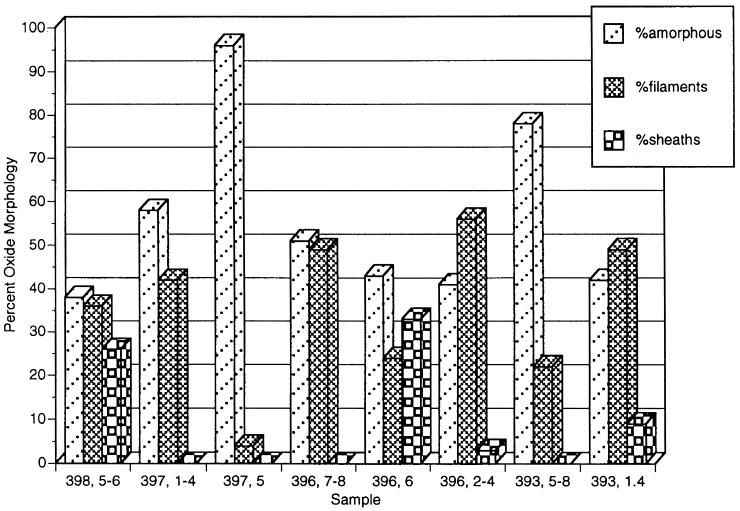
As mentioned above, light microscopy indicated that the distinctive morphological remains of Fe-oxidizing bacteria were present at all sites. Due to their abundance, it became apparent that it should be possible to type and quantify the oxides based on morphology. For this purpose, the oxides were grouped into three types: particulate oxides, sheathlike oxides, and filamentous oxides. Examples of each type are shown in Fig. 4. Particulate oxides were amorphous with no defining shape. The sheath-associated oxides were those formed by L. ochracea-like microbes and are hollow straight tubes of HFO that are 1 to 2 μm in diameter. The filamentous oxides were classified as thin (≤1 μm in diameter) solid filaments of iron oxides that were either twisted or curved. These appeared to be like the oxides formed by strain PV-1 that was isolated previously from Loihi (see below). The results of this morphological analysis are shown in Fig. 5. Of the three oxide types, amorphous oxides were the most common and were observed at all sites. At the Ikaika vents upper jets site (397, 5), they comprised over 90% of the oxides present. This sample was collected right in the vent orifice and had a high flow rate (>1 m · s−1). The filamentous oxides comprised >25% of the oxide morphotypes at five of the sites and were the dominant morphotype in the Naha vents (396, 2-4) and the Ikaika vents marker 20 (393, 1-4) site samples which were both characterized by slow flow rates. Sheaths comprised 25 and 33% of the oxide morphologies at sites at the Ikaika vents upper jets site (398, 5-6) at 113°C and at the Pohaku vents (396, 6), respectively, while at the other sites they were either not present or represented <10% of the total Fe particulates.
FIG. 4.
Montage of the three primary morphotypes of oxides found at the different vent sites at Loihi, see the text for details. (A) Sheaths; (B) filaments; (C) amorphous particulate oxides. Bars, 5 μm.
FIG. 5.
Percentages of different oxide morphotypes associated with different sites at Loihi, see the text for details.
Filament formation by PV-1.
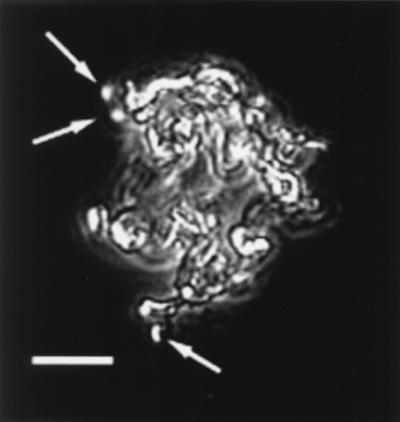
The strain PV-1 was isolated from a sample collected in 1996 at Loihi from a low-temperature vent field near the Naha vents site on the south rift of Loihi. Details of the isolation of this strain and its characterization will be described elsewhere. A unique characteristic of PV-1 is that it forms a filamentous oxide structure as it grows. Based on evidence from light microscopy, it appears that the cells grow at the termini of the filaments; the filaments are probably the primary locations for Fe oxidation (Fig. 6). When the HFOs associated with the filaments are reduced with hydroxylamine, a fine matrix remains; presumably this is organic material (results not shown). This mode of growth is analogous to that of G. ferruginea, whose cells grow at the apical ends of a twisted stalk composed principally of HFO (16, 17). Under the growth conditions that we have tried, strain PV-1 has never formed the regular, ribbonlike helical stalks formed by Gallionella. What is notable is that the filaments formed by strain PV-1 do bear a striking resemblance to the filaments commonly found at Loihi (compare Fig. 6 with Fig. 4B).
FIG. 6.
Strain PV-1 and filament formation. This pure culture was grown in liquid MWMM/ASW medium. This image is a composite of a phase-contrast image and an epifluorescence image. The preparation was stained with Syto to reveal the cells, denoted with arrows, that are attached to the Fe oxides. Note that the cells appear to grow at the termini of the filaments. Compare the morphology of these bacterially formed filaments to that of the filaments observed directly from the Loihi site (Fig. 4B).
Isolation of strain JV-1.
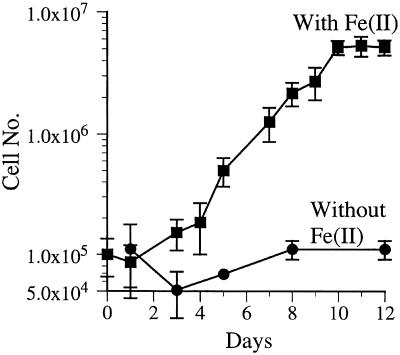
This strain was enriched from a 10−7 liquid dilution tube from the Ikaika vents lower jets site (393, 5-8). This enrichment was subjected to two sequential dilution series to extinction that resulted in a morphologically homogeneous culture that did not grow heterotrophically. A growth curve in liquid medium indicated that JV-1 required Fe(II) for growth and had a doubling time of approximately 24 h under the growth conditions used (Fig. 7). This strain did not form a well-defined filamentous oxide as was the case for strain PV-1 (data not shown). The only substrate that JV-1 grew on was Fe(II); it did not grow on thiosulfate, tetrathionate, formate, acetate, pyruvate, succinate, glucose, galactose, or yeast extract. A more extensive description of this organism will be published elsewhere.
FIG. 7.
Growth curve of strain JV-1. This strain, isolated from the lower jet vents, was grown on liquid MWMM/ASW medium amended with Fe(II) and air on a daily basis, as described in the text. Aliquots were removed daily for cell enumeration by direct counting.
Molecular analysis.
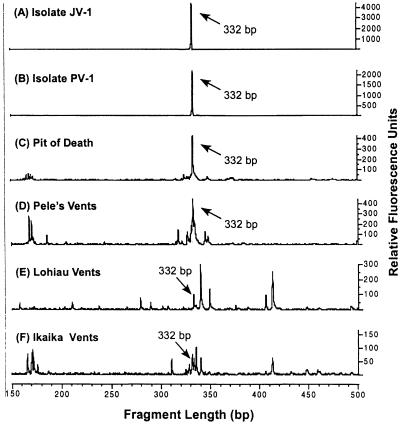
A community analysis by T-RFLP of samples collected from Loihi during the 1998 sampling trip (Fig. 8F), a posteruption sampling trip in 1996 (Fig. 8E), and preeruptive sampling trips (Fig. 8D and C) indicated that there were microbial communities of various complexities present at different vent sites. An analysis of the two pure cultures JV-1 and PV-1 (Fig. 8A and B) showed a single peak of 332 bp, as expected for the axenic cultures. This 332-bp peak was present in all of the community samples as well, actually being most dominant in the preeruption samples. Without knowing the exact sequence of the 332-bp fragments from the environmental samples, the linkage between these samples and the pure cultures remains circumstantial; however, combined with other evidence, it strongly suggests that JV-l-like Fe oxidizers have been, and remain, common inhabitants of the Loihi site.
FIG. 8.
T-RFLP profile of isolates JV-1 and PV-1 along with four hydrothermal vent bacterial communities sampled from Loihi Seamount with the Pisces V submersible. Terminal fragments were generated from SSU rDNA amplicons digested with HhaI. (A) Isolate JV-1; (B) isolate PV-1; (C) Pit of Death site, dive 244, September 1993; (D) Pele's vents “Boulder Patch” site, dive 247, September 1993; (E) Lohiau vents site, dive 311, October 1996; (F) Ikaika vents lower jets site, dive 393, October 1998. Terminal fragments of 332 bp are congruous with the JV-1-PV-1 phylotype and are prevalent in all of the environmental samples, suggesting that the presence of this Fe-oxidizing phylotype is common throughout this hydrothermal system.
DISCUSSION
Our results indicate that Fe-oxidizing bacteria are both ubiquitous and abundant at the Loihi Seamount. The results confirm that L. ochracea-like organisms remain a common inhabitant of Loihi and have colonized vents in the pit crater following the 1996 eruption. It is also apparent that other morphological types of Fe-oxidizing bacteria are important members of the microbial community at Loihi. Most significant in this regard was the isolation of strain PV-1 and the discovery of the unique filamentous form of the Fe oxides that it produced. The subsequent findings that this morphotype was present at most of the Loihi vents and that it is dominant at several of them suggest that this organism, or ones like it, plays an important role in iron deposition at Loihi.
Impact of Fe oxidizers at Loihi.
In general, bacterial morphology is not considered a reliable indicator of microbial community dynamics; however, correlating microbial activity with the formation of unique HFO morphotypes is compelling. The most plausible explanation is that these morphotypes are directly the result of microbial activity, since there is no evidence that oxide structures like sheaths or filaments can form abiologically under the chemical conditions at Loihi (12). It should also be stressed that the amorphous particulate oxides that are the most abundant morphotype found at Loihi (Fig. 4) may also be the result of microbial activity. The strain JV-1 described here formed an amorphous iron oxide indistinguishable by light microscopy from amorphous oxides formed by other iron oxidizers isolated from freshwater habitats (7, 10, 34). If it is assumed that the densities of the different oxide morphotypes are nearly the same, then we can conservatively estimate that up to 60% of the iron oxide deposition (the combination of sheaths and filaments) at a number of the Loihi vents is directly attributable to microbial activity. This omits any contribution from the particulate oxides. This value falls in the range of 50 to 80% of the iron-oxidizing activity accounted for by microbes in a freshwater microcosm experiment (8) where the laboratory microcosms had mixed populations of sheathed and nonfilamentous iron oxidizers. Sobolev and Roden reported recently that a pure culture of an Fe-oxidizing bacterium accounted for up to 90% of the Fe oxidation (34). Another recent report demonstrated that neutrophilic Fe oxidizers growing in a cave substantially accelerated the rate of Fe oxidation (25). However, there is one further caveat, as it is also important to distinguish between the amount of Fe oxidation that may be catalyzed directly by the microbes, putatively for energy-yielding lithotrophic growth, versus the proportion that may result from indirect auto-oxidation on the preformed HFOs. It has been shown that the microbially formed oxides remain good catalysts for continued Fe oxidation after the microbes that formed them are gone (17). Thus, it is reasonable to conclude that microbial activity is responsible for the bulk of the HFO precipitation at Loihi, although the percentage of the Fe oxidation that could potentially support lithotrophic growth remains to be determined.
Biology of pure cultures.
The isolation of the strains PV-1 and JV-1 add to our understanding of this novel group of neutrophilic Fe-oxidizing bacteria. While the PV-1 morphotype is reminiscent of the helical stalks formed by Gallionella spp., we have never witnessed it forming a true ribbonlike helix even in young cultures. Phylogenetically, based on SSU rDNA, neither JV-1 nor PV-1 has a high degree of similarity with G. ferruginea; instead, they cluster with other neutrophilic Fe oxidizers like ES-1 and ES-2 (10; D. Emerson and C. L. Moyer, unpublished results). The JV-1 strain was isolated from a 10−7 dilution culture tube and, as such, probably represents a dominant, nonfilamentous type of Fe oxidizer at Loihi. Our non-cultivation-based T-RFLP method also provides strong circumstantial evidence that this group of Fe oxidizers is present at Loihi and that by cultivating PV-1 and JV-1 we have isolated some of the dominant members of the Fe-oxidizing community.
Based on substrate tests, both JV-1 and PV-1 appear to be obligate Fe oxidizers, unable to obtain energy from organic C sources, which is consistent with the findings for other Fe-oxidizing bacteria (10, 34). They are adapted to growing in seawater, as neither strain will grow in freshwater medium. Both strains are mesophiles, growing optimally between 25 and 30°C. Thus far, we have been unsuccessful in isolating a thermophilic, microaerophilic Fe-oxidizing bacterium from Loihi or from other hydrothermal vent sites. At the other end of the temperature spectrum, we have been successful in enriching Fe oxidizers that grow at 12 but not at 20°C. At Loihi, as at other vent sites where extensive Fe precipitates have been found, low-temperature vents (10 to 15°C) are common. These psychrotrophic organisms would be well adapted for growing at these cold temperatures.
Fe oxidation at other hydrothermal systems.
Our results are consistent with a number of reports from geologists and geochemists who have reported Fe oxyhydroxides of biological origin associated with hydrothermal venting systems. Furthermore, the results presented here strengthen the biological context for the formation of these oxides. Specific examples include the finding of abundant tubelike microbial filaments with a very high Fe content (up to 42% by weight) in the Coriolis troughs in the southwestern Pacific (19). Abundant evidence of Fe mats dominated by putative Fe-oxidizing bacteria have been found in investigations of the Franklin Seamount in the Woodlark Basin and the MacDonald Seamount located off the coast of New Guinea (3, 33, 35).
The Red Seamount located near 21°N on the East Pacific Rise is reported to have extensive deposits of Fe oxides, which range from hardened crusts to fluffy, orange HFOs (2). These oxide deposits are associated with lower temperature venting sites that are only a few degrees above ambient seawater. Scanning electron micrographs revealed that this material had filamentous morphologies similar to the material at Loihi; Fe oxyhydroxide-encrusted sheaths also appeared to be present. Juniper and collaborators have reported finding filamentous and sheathlike structures at a number of hydrothermal vent sites, both in the Pacific Ocean and along the mid-Atlantic Ridge. At one site they investigated on the Explorer Ridge in the Northeastern Pacific, they found evidence for an abundance of sheaths and filamentous oxide-type structures (20-22).
We have also observed a thin gelatinous coating of Fe oxides on volcanic rocks associated with venting activity at the East Pacific Rise crustal spreading center at 21°N. This site was typical of a sulfur-dominated venting system with black smokers, macrofaunal communities of tube worms and clams, and massive pyritic deposits. At 21°N, the gelatinous HFOs appeared to be only a few millimeters thick and beneath them was a hard crust of Fe oxides. These amorphous overlying oxides were difficult to collect since they rapidly dispersed as soon as the mechanical arm of the submarine touched them. Nevertheless it was possible to collect small amounts of this material, and non-filament-forming Fe-oxidizing lithotrophs were enriched from these samples. Microscopically, these oxides consisted of amorphous particulate material and sheaths and filaments were not observed (D. Emerson, unpublished results)
Very recently, high-temperature hydrothermal venting sites were discovered at a crustal spreading center in the Indian Ocean. At the Edmond vent field, which had a variety of vents ranging from diffuse vents to black smokers, iron concentrations of 14 mmol·kg−1 were measured, and it was reported that centimeter-thick mats of HFO were present near the vents (38). In total, these results indicate that microbial Fe oxidation at hydrothermal vents may be under appreciated for the common process that it probably is.
Microfossil evidence.
In addition to reports of Fe-oxidizing-like morphologies associated with extant hydrothermal vent sites, a number of recent reports have shown that microfossil remains of putative Fe-oxidizing bacteria have been found at ancient hydrothermal vent sites as well as other ancient environments where Fe-rich minerals have accreted and form part of the fossil record (6, 20, 26, 32, 37). Hofmann and Farmer carried out a systematic search of museum mineral collections, supplemented with their own field work, and found evidence for potential biogenic fossilized filaments at over 140 sites (18). They referred to these as fabrics consisting of tubular filamentous structures with core diameters of 1 to 2 μm. Geochemical evidence indicated that these structures formed at temperatures of <100°C and that they were comprised of a variety of iron minerals, with goethite and hematite being the most common. These mineral assemblages were most often associated with either volcanism or oxidized ore bodies. Microscopically, some of these structures bear striking resemblance to extant Gallionella spp. stalks, although most appear more tubular and while not identical to extant L. ochracea-type sheaths or PV-1-like filaments, there is more than a rudimentary similarity. In all cases, the authors of these reports suggest that a biogenic origin is the most likely explanation for these fossilized minerals. The striking similarities with modern day Fe-oxidizing organisms, such as those found at Loihi, suggest a modern day analog for these ancient structures.
Acknowledgments
We thank the captain and crew of the R/V Kaimikai-o-Kanaloa operated by the University of Hawai'i and especially Terry Kerby, chief pilot of the DSRV Pisces V, for help in sample collection. The technical assistance of Chris Bradburne is gratefully acknowledged. We also thank Cris Little of the University of Leeds for bringing to our attention the literature on microfossils and Geoff Wheat, University Alaska, Fairbanks, for providing data on Fe chemistry.
This work was funded in part by grants from the National Science Foundation (MCB-9723459) and the NASA Astrobiology Institute to D.E., by NOAA's Hawaiian Undersea Research Laboratory, and by a summer research grant provided by Western Washington University to C.L.M.
REFERENCES
- 1.Adams, L. F., and W. C. Ghiorse. 1985. Influence of manganese on growth of a sheathless strain of Leptothrix discophora. Appl. Environ. Microbiol. 49:556-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alt, J. C. 1988. Hydrothermal oxide and nontronite deposits on seamounts in the Eastern Pacific. Mar. Geol. 81:227-239. [Google Scholar]
- 3.Bogdanov, Y. A., A. P. Lisitzin, R. A. Binns, A. I. Gorshkov, E. G. Gurvich, V. A. Dritz, G. A. Dubinina, O. Y. Bogdanova, A. V. Sivkov, and V. M. Kuptsov. 1997. Low-temperature hydrothermal deposits of Franklin Seamount, Woodlark Basin, Papua New Guinea. Mar. Geol. 142:99-117. [Google Scholar]
- 4.Cowen, J. P., and M. W. Silver. 1984. The association of iron and manganese with bacteria on marine macroparticulate material. Science 224:1340-1342. [DOI] [PubMed] [Google Scholar]
- 5.Duennebier, F. K., N. C. Becker, J. Caplan-Auerbach, D. A. Clague, J. Cowen, M. Cremer, M. Garcia, F. Goff, A. Malahoff, G. M. McMurtry, B. P. Midson, C. L. Moyer, M. Norman, P. Okubo, J. A. Resing, J. M. Rhodes, K. Rubin, F. J. Sansone, J. R. Smith, K. Spencer, X. Wen, and C. G. Wheat. 1997. Researchers rapidly respond to submarine activity at Loihi volcano, Hawaii. EOS Trans. Am. Geophys. Union 78:229-233. [Google Scholar]
- 6.Duhig, N. C., G. J. Davidson, and J. Stolz. 1992. Microbial involvement in the formation of Cambrian sea-floor silica-iron oxide deposits, Australia. Geology 20:511-514. [Google Scholar]
- 7.Emerson, D., and N. P. Revsbech. 1994. Investigation of an iron-oxidizing microbial mat community located near Aarhus, Denmark: field studies. Appl. Environ. Microbiol. 60:4022-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emerson, D., and N. P. Revsbech. 1994. Investigation of an iron-oxidizing microbial mat community located near Aarhus Denmark: laboratory studies. Appl. Environ. Microbiol. 60:4032-4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emerson, D. 2000. Microbial oxidation of Fe(II) at circumneutral pH, p. 31-52. In D. R. Lovley (ed.), Environmental microbe-metal interactions. ASM Press, Washington, D.C.
- 10.Emerson, D., and C. Moyer. 1997. Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl. Environ. Microbiol. 63:4784-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emerson, D., J. V. Weiss, and J. P. Megonigal. 1999. Iron-oxidizing bacteria are associated with ferric hydroxide precipitates (Fe-plaque) on the roots of wetland plants. Appl. Environ. Microbiol. 65:2758-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Ruiz, J. M. 1988. Carbonate precipitation into alkaline silica-rich environments. Geology 26:843-846. [Google Scholar]
- 13.Ghiorse, W. C. 1984. Biology of iron- and manganese-depositing bacteria. Annu. Rev. Microbiol. 38:515-550. [DOI] [PubMed] [Google Scholar]
- 14.Hallbeck, L., and K. Pederson. 1991. Autotrophic and mixotrophic growth of Gallionella ferruginea. J. Gen. Microbiol. 137:2657-2661. [Google Scholar]
- 15.Hanert, H. H. 1981. Bakterielle und chemische Eisen(II)-oxidation auf Palaea Kameni-stereoscan, elektronenestrahl-mikroanalyse (FeKa) und photometrie von in situ-experimenten. In E. S. H. Puchelt (ed.), Genesis of marine iron sediments from Santorini, Greece. Springer-Verlag, Berlin, Germany.
- 16.Hanert, H. H. 1992. The genus Gallionella, p. 4082-4088. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schliefer (ed.), The prokaryotes, 2nd ed., vol. 4. Springer-Verlag, New York, N.Y.
- 17.Heldal, M., and O. Tumyr. 1983. Gallionella from metaliminion in an eutrophic lake: morphology and X-ray energy-dispersive microanalysis of apical cells and stalks. Can. J. Microbiol. 29:303-308. [Google Scholar]
- 18.Hofmann, B. A., and J. D. Farmer. 2000. Filamentous fabrics in low-temperature mineral assemblages: are they fossil biomarkers? Implications for the search for a subsurface fossil record on the early Earth and Mars. Planet. Space Sci. 48:1077-1086. [Google Scholar]
- 19.Iizasa, K., K. Kawasaki, K. Maeda, T. Matsumoto, N. Saito, and K. Hirai. 1998. Hydrothermal sulfide-bearing Fe-Si oxyhydroxide deposits from the Coriolis Troughs, Vanuatu backarc, southwestern Pacific. Mar. Geol. 145:1-21. [Google Scholar]
- 20.Juniper, S. K., and Y. Fouquet. 1988. Filamentous iron-silica deposits from modern and ancient hydrothermal sites. Can. Mineral. 26:859-869. [Google Scholar]
- 21.Juniper, S. K., and J. Sarrazin. 1995. Interaction of vent biota and hydrothermal deposits: present evidence and future experimentation. Seafloor hydrothermal systems: physical, chemical, biological, and geological interactions. Geophys. Monogr. 91:178-193. [Google Scholar]
- 22.Juniper, S. K., and B. M. Tebo. 1995. Microbe-metal interactions and mineral deposition at hydrothermal vents, p. 219-253. In D. M. Karl (ed.), The microbiology of deep-sea hydrothermal vents. CRC Press, Boca Raton, Fla.
- 23.Karl, D. M., A. M. Brittain, and B. D. Tilbrook. 1989. Hydrothermal and microbial processes at Loihi Seamount, a mid-plate hot-spot volcano. Deep-Sea Res. 36:1655-1673. [Google Scholar]
- 24.Karl, D. M., G. M. McMurtry, G. M. Malahoff, and M. O. Garcia. 1988. Loihi seamount, Hawaii: a mid-plate volcano with a distinctive hydrothermal system. Nature 335:532-535. [Google Scholar]
- 25.Kasama, T., and T. Murakami. 2001. The effect of microorganisms on Fe precipitation rates at neutral pH. Chem. Geol. 180:117-128. [Google Scholar]
- 26.Little, C. T. S., R. J. Herrington, R. M. Haymon, and T. Danelian. 1999. Early Jurassic hydrothermal vent community from the Franciscan Complex, San Rafael Mountains, California. Geology 27:167-170. [Google Scholar]
- 27.Liu, W.-T., T. L. Marsh, H. Cheng, and L. J. Forney. 1997. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl. Environ. Microbiol. 63:4516-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandernack, K. W., and B. M. Tebo. 1993. Manganese scavenging and oxidation at hydrothermal vents and in vent plumes. Geochim. Cosmochim. Acta 57:3907-3923. [Google Scholar]
- 29.Moyer, C. L., F. C. Dobbs, and D. M. Karl. 1994. Estimation of diversity and community structure through restriction fragment polymorphism distribution analysis of bacterial 16S rRNA genes from a microbial mat at an active, hydrothermal vent system, Loihi Seamount, Hawaii. Appl. Environ. Microbiol. 60:871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moyer, C. L. 2001. Molecular phylogeny: applications and implications for marine microbiology. Methods Microbiol. 30:375-394. [Google Scholar]
- 31.Moyer, C. L., F. C. Dobbs, and D. M. Karl. 1995. Phylogenetic diversity of the bacterial community from a microbial mat at an active, hydrothermal vent system, Loihi Seamount, Hawaii. Appl. Environ. Microbiol. 61:1555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preat, A., B. Mamet, C. De Ridder, F. Boulvain, and D. Gillan. 2000. Iron bacterial and fungal mats, Bajocian stratotype (mid-Jurassic, northern Normandy, France). Sediment. Geol. 137:107-126. [Google Scholar]
- 33.Puteanus, D., G. P. Glasby, P. Soffers, and H. Kunzendorf. 1991. Hydrothermal iron-rich deposits from the teahitia-mehitia and MacDonald hot-spot areas, Southwest Pacific. Mar. Geol. 98:389-409. [Google Scholar]
- 34.Solobev, D., and E. E. Roden. 2001. Suboxic deposition of ferric iron by bacteria in opposing gradients of Fe(II) and oxygen at circumneutral pH. Appl. Environ. Microbiol. 67:1328-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoffers, P., G. P. Glasby, D. Stuben, R. M. Renner, T. G. Pierre, J. Webb, and C. M. Cardile. 1993. Comparative mineralogy and geochemistry of hydrothermal iron-rich crusts from the Pitcairn, Teahitia-Mehetia, and MacDonald hot-spot areas of the SW Pacific. Mar. Georesour. Geotechnol. 11:45-89. [Google Scholar]
- 36.Stookey, L. L. 1970. Ferrozine-a new spectrophotometric reagent for iron. Anal. Chem. 42:779-781. [Google Scholar]
- 37.Trewin, N. H., and A. H. Knoll. 1999. Preservation of Devonian chemotrophic filamentous bacteria in calcite veins. Palaios 14:288-294. [Google Scholar]
- 38.Van Dover, C. L., S. E. Humphris, D. Fornari, C. M. Cavanaugh, R. Collier, S. K. Goffredi, J. Hashimoto, M. D. Lilley, A. L. Reysenbach, T. M. Shank, K. L. Von Damm, A. Banta, R. M. Gallant, D. Gotz, D. Geen, J. Hall, T. L. Harmer, L. A. Hurtado, P. Johnson, Z. P. McKiness, and C. Meredi. 2001. Biogeography and ecological setting of Indian Ocean hydrothermal vents. Science 294:818-823. [DOI] [PubMed] [Google Scholar]
- 39.Wheat, G. C., H. W. Jannasch, J. N. Plant, C. L. Moyer, F. J. Sansone, and G. M. McMurtry. 2000. Continuous sampling of hydrothermal fluids from Loihi seamount after the 1996 event. J. Geophys. Res. 105:19353-19367. [Google Scholar]
- 40.Wolin, E. A., M. J. Wolin, and R. S. Wolfe. 1963. Formation of methane by bacterial extracts. J. Biol. Chem. 238:2882-2886. [PubMed] [Google Scholar]