Abstract
BACKGROUND
Toxoplasma gondii is a zoonotic protozoan capable of infecting warm-blooded animal species and humans. Although toxoplasmosis presents mostly as mild or asymptomatic infection in immunocompetent individuals, in unborn children and people with weakened immune systems, the disease can be severe with ocular, neurological or multi-systemic manifestations and even death.
AIM
We aimed to collate and analyse data on T. gondii seroprevalence in humans to model and compare age-dependent prevalence in geographic regions in Europe.
METHODS
A systematic review identified 1,822 scientific publications, from which seroprevalence data were extracted from 69 studies. Data were analysed using a Bayesian hierarchical model.
RESULTS
The modelling of the seroprevalence indicated the highest incidence rates in eastern (50%) and western (48%) Europe, with the lowest estimates in northern Europe (18%) and the United Kingdom (UK) (18%). Eastern and western Europe were regions where T. gondii infections occurred earliest in life, with half of the population expected to be seropositive by the age of 44 and 47 years, respectively. In contrast, in northern Europe and the UK the modelled median time to infection exceeded 170 years.
CONCLUSION
Results of the study provide a robust baseline for future epidemiological research on human T. gondii infections in Europe and may be useful to validate subsequent research, such as risk assessment studies.
Keywords: Europe, Human, Toxoplasma gondii, Seroprevalence, Systematic review
Introduction
Toxoplasma gondii, a protozoan parasite with a worldwide distribution, is capable of infecting humans and potentially all warm-blooded vertebrates [1]. Felids serve as the definitive hosts for T. gondii [2]. When ingested, the parasite replicates in the felid’s intestine, followed by shedding of the oocysts via faeces into the environment. The oocysts can sporulate and survive for long periods in the environment [3]. Ingestion of sporulated T. gondii oocysts present in contaminated water, soil or fresh produce can lead to formation of tissue cysts in all susceptible hosts, including humans [4]. The bradyzoites in these tissue cysts are infective, allowing transmission through the consumption of undercooked or raw meat from infected hosts [5,6]. Humans can become infected both via the environmental route and via consumption of undercooked or raw meat of infected animals. Another route is transplacental transmission to a fetus, causing congenital infection potentially resulting in abortion or stillbirth [7]. Moreover, T. gondii can be transmitted via blood transfusions or organ transplants [5,8].
Acquired T. gondii infections in humans are generally asymptomatic or cause non-specific and self-limiting symptoms, but they can also present as ocular toxoplasmosis. Severe acute toxoplasmosis, although rare, may manifest as myocarditis, polymyositis, pneumonitis, retinitis, hepatitis or encephalitis, and mainly occurs in people with severely weakened immune systems [5]. Prevalence of T. gondii infection is influenced by factors such as climate, cultural habits such as consumption of raw meat, hygiene practices and socioeconomic conditions [9].
Serological testing for T. gondii infection in humans is performed for several reasons. In some countries, screening is conducted during pregnancy to monitor seroconversion and guide treatment. Testing also helps to assess population seroprevalence and aids in diagnosing clinical toxoplasmosis. Both, infection with T. gondii and the presence of detectable antibodies, are assumed to persist lifelong, with the prevalence of anti-T. gondii IgG in humans increasing with age [9].
The aim of this work was to collate available published data on human T. gondii seroprevalence to model and compare the age-dependent prevalence of the infection in Europe.
Methods
Literature screening and study selection
A structured literature search was carried out according to the PRISMA guidelines [10], using Emtree terms within the Embase literature database. The search string can be found in Supplementary Table S1. The search terms were chosen to cover human seroprevalence and risk factors of infections with T. gondii in Europe. For the study area, 41 countries in Europe were considered, including the 27 European Union (EU) countries. The list of the countries included is presented in Supplementary Table S2. The publication period of interest was set from January 2000 to May 2021. The search strategy had no limitations on publication language. The search was conducted in May 2021.
A group of 17 scientists with expertise in T. gondii and toxoplasmosis from 12 countries across Europe assessed the eligibility of the publications identified. The screening of the publications was performed within Cadima [11], an open-access online software tool for conducting systematic reviews. The systematic review was done in two stages based on a set of predefined criteria: an article was eligible if (i) it reported a study based on original data; (ii) the study was on human seroprevalence and/or risk factors for T. gondii infection in the included European countries, with at least part of the data collected from the year 2000 onwards; and (iii) had been published in a peer-reviewed journal. Reviews, meta-analyses and other articles not reporting original data were excluded, as were studies investigating the prevalence of T. gondii in particular risk groups and studies investigating T. gondii infection as a risk factor for another condition. First, the title and abstract were screened by two randomly chosen scientists from the group, after which consensus on inclusion had to be reached in case of initial disagreement. All inconsistencies were solved between the two scientists without the need of a third person. Second, full text of the remaining publication was screened and again consensus on inclusion was reached. The same criteria were used in both screening steps.
The next step involved extracting relevant data from the selected articles. Per article, data were extracted by one scientist and then checked by another. Thirteen scientists from the previous group were involved in this process, and four additional scientists helped extracting data from articles in different languages. The data were gathered in a template file created in Microsoft Excel. For each study, data on study design, period, population, serological tests used, and results were registered. Extracted data on seroprevalence were harmonised and categorised for modelling. For this, countries were assigned to one of five European regions (western, northern, eastern, southeastern, southwestern), as described previously [12,13].
Data analysis
Some publications presented more than one set of results on seroprevalence for the same population (e.g. results for the total study population, as well as for various subcategories based on, for example, age, sex and/or region), resulting in several rows of data for those populations. In those cases, the subpopulations were weighted by their probability to ensure that they contributed proportionally to the actual number of participants in the study. The meta-analysis was done using the data with specification on age, as reported in the publications. Since the exact ages of individual participants at the time of sampling were not provided, we defined an uncertainty distribution based on the estimates of the minimum, maximum and most probable age at sampling. If a median or mean age was given per age range, this was used as most probable age; otherwise, the median age was calculated from the minimum and maximum age of the age range. Data from studies that did not specify age of the participants or only reported data for all ages combined were excluded from data analysis.
A Bayesian hierarchical model built for estimating the age-dependent seroprevalence of T. gondii in animal species [13] was adapted to the human data collected in the present review. This model consists of an age-dependent Susceptible-Infected-Susceptible (SIS) framework, where individuals move from susceptible (i.e. seronegative) to infected (i.e. seropositive), with the possibility of reversion to susceptible (i.e. loss of detectable antibody response). Individuals were considered born susceptible, and hence able to move into the infected compartment based on a constant force of infection ( , incidence rate, i.e. the rate at which individuals acquire infection measured in new infections per year) and with the reversion to seronegative at rate γ.
The Bayesian hierarchical model was built to be able to estimate the parameters through partial pooling, granting the possibility to overcome data gaps. Variables used in the model are shown in Table 1.
Table 1. Data used in the Bayesian hierarchical model of Toxoplasma gondii seroprevalence in humans, Europe, 2000–2021.
| Variablea | Values |
|---|---|
| region[i] | Eastern, Northern, Southeastern, Southwestern, Western Europe |
| pop[i] | A unique identifier for a population |
| ntot[i] | Total number of participants tested |
| npos[i] | Total number of participants test positive |
| agemin[i] | Lower bound of the age range |
| agemax[i] | Upper bound of the age range |
| agemean[i] | The most probable age at sampling |
a Variables as included in the model with corresponding values and the index [i] corresponding to the i-th data point.
In the process of model fitting by means of Bayesian inference, the age distribution for each population was updated, i.e. a posterior age distribution was obtained. Differences between regions were considered using a hierarchical model and modelled as linear contributions to the logarithmic baseline force of infection (λ). No regional differences were considered for the reversion rate (γ). Model fitting was performed using Stan (https://mc-stan.org) (interfaced with R version 4.1.3 (https://www.r-project.org/). Trace plots of the Markov chains were visually assessed to confirm the convergence of the model.
Results
Data collection
A total of 1,822 publications were identified, of which 12 were removed as duplicates. After screening titles and abstracts, 367 articles were selected for full-text screening, of which 142 articles met the inclusion criteria. During data extraction, a further 67 articles were excluded because of missing or incomplete information. Of the remaining 75 publications, 69 provided seroprevalence data [14-83] and 22 contained data on risk factors. A PRISMA flow diagram is presented in Supplementary Figure S1, and a list of the 22 references is also included in the Supplementary Material. The further analyses focused solely on seroprevalence. Relevant seroprevalence data could be recovered from 25 of the 41 countries considered in the search strategy.
Regional seroprevalence of Toxoplasma gondii in Europe
The average T. gondii seroprevalence per region and by age derived from the model is presented in Figure 1 and Table 2. The United Kingdom (UK) was initially a country included in the western region. However, the analysis per region and age showed a deviant seroprevalence for the UK compared with the other countries included in our categorisation of western Europe. Therefore, the UK was analysed separately. The highest prevalence estimates were seen in eastern (50%), western (48%), southeastern (45%) and southwestern (38%) Europe. The seroprevalence was markedly lower in northern Europe (18%) and the UK (18%). Thus, while the seroprevalence increased with age from 13% to 16% in those aged ≤ 25 years to above 50% in those aged > 50 years in most regions, it increased from 4% to 26–27% in northern Europe and the UK.
Figure 1.
Susceptible-Infected-Susceptible (SIS) model fit for age-dependent seroprevalence of Toxoplasma gondii in humans, Europe, 2000–2021
In total, 69 studies with data from 25 countries were included in the model. The line indicates the fitted seroprevalence by age, with the orange area being the 95% confidence interval. The grey dots represent seroprevalence data points at the best estimate of age in the data for the studied populations. The size of the dots reflects the number of persons tested. The dots are shifted horizontally along the grey lines extending from the minimum to the maximum possible age, to the best fitting age (coloured dots).
Table 2. Modelled average seroprevalence estimates of Toxoplasma gondii in humans, by age group and age at infection, Europe, 2000–2021.
| Regiona | Seroprevalence (%), by age group | Age at infection (years) | |||||
|---|---|---|---|---|---|---|---|
| 0–25 years | 26–50 years | > 50 years | Mean | 10% quantile | Median | 90% quantile | |
| Eastern | 16 | 43 | 68 | 63.96 | 6.74 | 44.34 | 147.28 |
| Northern | 4 | 14 | 27 | 249.95 | 26.33 | 173.25 | 575.53 |
| Southeastern | 13 | 38 | 62 | 77.19 | 8.13 | 53.51 | 177.75 |
| Southwestern | 10 | 30 | 52 | 101.66 | 10.71 | 70.46 | 234.08 |
| UK | 4 | 13 | 26 | 259.07 | 27.30 | 179.58 | 596.54 |
| Western | 15 | 41 | 66 | 68.46 | 7.21 | 47.45 | 157.63 |
| Overall | NA | 136.72 | 14.40 | 94.76 | 314.80 | ||
NA: not applicable; UK: United Kingdom.
a Eastern: Belarus, Czechia, Estonia, Hungary, Latvia, Lithuania, Poland, Romania, Slovakia, Ukraine; Northern: Denmark, Finland, Iceland, Norway, Sweden; Western: Austria, France, Germany, Ireland, Liechtenstein, Luxembourg, the Netherlands, Switzerland; Southeastern: Albania, Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, Greece, Kosovo, Moldova, North Macedonia, Serbia, Slovenia; Southwestern: Andorra, Italy, Malta, Portugal, San Marino, Spain.
A total of 69 studies were included in the analysis (two studies with results for more than one region): eastern (26 studies, 7 countries), northern (4 studies, 4 countries), southeastern (9 studies, 5 countries), southwestern (18 studies, 3 countries), western (11 studies, 5 countries), UK (3 studies).
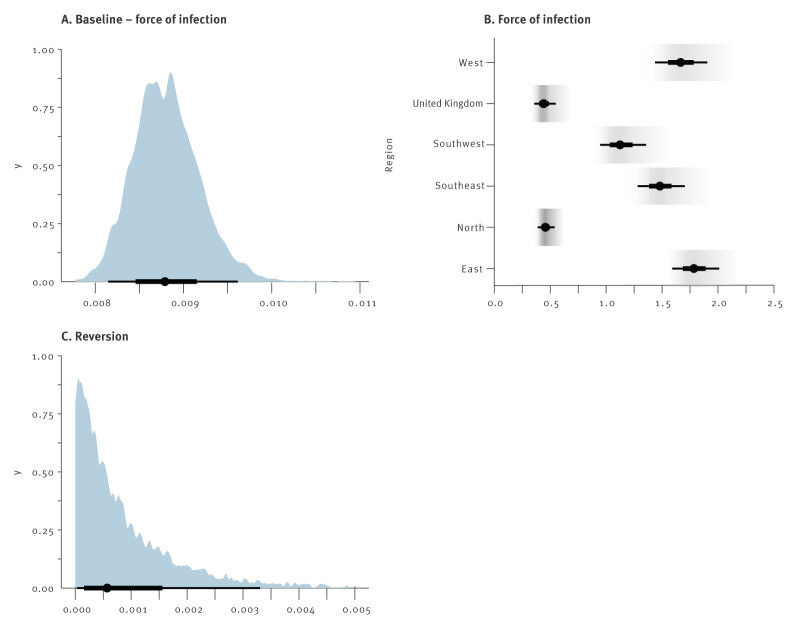
In addition to regional seroprevalence estimates, the model also provides estimates for the force of infection and the rate of reversion to seronegative status (Figure 2). For the force of infection, the inverse of the posterior coefficient represents the average waiting time in years until the event. For T. gondii infection, this results in an average waiting time of 1/0.009 ca 137 years (Figure 2), with a median waiting time of 95 years (Table 2). The lowest force of infection in Europe was observed in the UK (exp(λregion) = 0.445) and the northern region (exp(λregion) = 0.459), followed by the southwestern (exp(λregion) = 1.13) and the southeastern region (exp(λregion) = 1.48). Highest forces of infection were seen for the western (exp(λregion) = 1.67) and the eastern region (exp(λregion) = 1.79).
Figure 2.
Bayesian hierarchical model outcomes (i.e. posterior probabilities) for the force of infection (λ) and reversion rate (γ) to seronegative status of Toxoplasma gondii in humans, Europe, 2000–2021
Values for the force of infection in the regions are all exponentiated, which means that a value of 1 indicates the absence of an effect on the baseline probability. Grey area represents the uncertainty distribution, the thin and thick black lines indicate the 95% and 50% Bayesian credible intervals, respectively, with dots indicating the mean of exponentiated forces of infection per region. In total, 69 studies with data from 25 countries were included in the model.
To reconstruct the total force of infection per year for each region, the baseline force of infection was multiplied by the region-specific exponentiated contributions. For example, in the eastern region, the average time until infection was estimated to be ca 64 years (1/(0.009 × 1.79)), with 50% of the population becoming infected by age 44 years (the 50% quantile of the exponential distribution with parameter 0.009 × 1.79). In contrast, the average time until infection in the northern region and the UK exceeded 250 years, and the time 50% of the population becoming infected exceeding 170 years (Table 2). Despite this long average, 10% of the population would be infected at the age of 26 years, reflecting the skewed distribution of the infection age distribution.
The reversion rate was estimated at γ = 9.0 × 10−4 (3.0 × 10−4, 3.5 × 10−3), which sets an average waiting time of > 1,000 years. However, at the upper end of the credible interval this average waiting time was 275 years, with 10% of the population reverted at 29 years and 20% at 60 years. Hence, considerable reversion is realistic within the credible interval.
Discussion
The aim of this study was to model the age-dependent prevalence of T. gondii in the human population in Europe. To achieve this, a systematic review of seroprevalence studies in humans published in 2000–2021 was conducted. Data were extracted from 69 papers selected from 1,822 publications identified by the search strategy and, where possible, analysed using a Bayesian hierarchical model. Compared with conventional analytical techniques, Bayesian methods may perform better in meta-analyses, particularly by dealing better with uncertainty [84]. This is especially important when analysing population-based studies, where heterogeneity may arise from factors such as study design, geographic region, serological test used or missing data.
Human T. gondii seroprevalence data were obtained for 25 of the 41 countries included in the search. A similar geographic coverage of European T. gondii seroprevalence studies was achieved in a previous systematic review [85], indicating geographic gaps in available data. Meta-analysis was performed on a regional scale, by aggregating countries into five geographic areas. Albeit at the cost of loss of detail, grouping the data allowed for a broader geographic coverage, making it easier to identify regional differences and establish regional correlations. By using partial pooling of prevalence distributions from regions with a larger amount of data, the Bayesian hierarchical model allowed to provide seroprevalence estimates for regions lacking data, though with a larger uncertainty. Furthermore, age information given in the studies was notoriously incomplete, with differing age ranges used to categorise data, missing age data or use of categories that only gave an indication of age (e.g. children or pregnant women). The Bayesian hierarchical model helped to address these gaps by incorporating uncertainty on this variable, which made it possible to derive posterior predictive distributions for the age-dependent seroprevalence in each region in Europe. Modelling the prevalence of T. gondii by age provides a better understanding of infection dynamics, from birth to any given age, and holds the potential for developing age-specific prevention strategies. Thus far, most systematic review and meta-analysis studies have focused primarily on identifying sources of infection in outbreaks [86] and sporadic toxoplasmosis [3] or assessing prevalence in specific risk groups [87,88]. To our knowledge, one other study has reviewed seroprevalence data for the European general population [85], but age was not considered in the subgroup analysis in that study.
When estimating the age-dependent seroprevalence of T. gondii, we used the SIS model which includes the possibility that individuals can return to the susceptible state (some time) after infection. This approach was used before to model the age-dependent prevalence of T. gondii in animals [13]. In that study, the plateau in seroprevalence observed in animals at higher age was better explained by the SIS model, than the SI (Susceptible-Infected) model (where 100% of susceptible individuals would become infected if living long enough). Although hardly reported in literature, evidence of seroreversion in humans was, for example, reported in a cohort of blood donors followed over 4 years [23]. Nevertheless, the reversion rate estimated in the present study was negligible, supporting the hypothesis of lifelong persistence of anti-T. gondii antibodies in humans [89].
Results from this study revealed considerable differences in seroprevalence between geographic regions across Europe. The estimates for T. gondii seroprevalence were highest in eastern, western, and southeastern Europe, with a mean seroprevalence of 45–50%, followed by the southwestern region (38%), and were lowest in the UK and northern Europe where the model predicted a mean infection rate of 18%. Based on our results, seroprevalence in the age group 25–50 years varied between 13% and 43% in Europe. Worldwide, highest seroprevalence in pregnant women has been observed in South America (53–56%), mostly based on Brazil, and Africa (47–49%); the seroprevalence in Europe in this group was 25–31%, whereas the seroprevalence in North America was 20–28% [87,90,91]. Importantly, measured seroprevalences between countries within a continent can vary largely, as they can even within countries [92]. In line with the assumption of lifelong infection, the seroprevalence increased between the three age groups, with as few as 4% of individuals seropositive in the youngest age group in the UK and northern Europe and a several-fold increase up to 68% by the age of > 50 years in eastern Europe. Measuring the force of infection, i.e. the rate at which individuals acquire infection, is a key to understanding the epidemiology of infectious diseases and estimating disease burden. Estimates for this parameter clearly showed that individuals in eastern and western Europe became infected at a younger age, compared with the other regions. Translated into time until infection, this means that on average half of the population in eastern and western Europe was expected to be infected by the age of 47 years, while in northern Europe and the UK this was expected at an unreachable age of 173 and 179 years, respectively. Noteworthily, the strong force of infection estimated for western Europe is in agreement with the results from a recent large-scale T. gondii serosurvey in female children and adolescents in Germany, showing that with each year of life the chance of becoming seropositive increased by 1.2 [93].
Comparing T. gondii prevalence and force of infection in humans and animals in the same geographic area may provide One Health insights into potential sources. Interestingly, results obtained for humans in the present study parallel the force of infection trends among geographic regions disclosed in the previous review of the animal prevalence in Europe [13], except for western Europe, where the force of infection ranked second highest in humans but was lowest in animals. Although results from the two studies do not provide direct evidence of specific sources of human infection, a high prevalence in herbivorous animals that have outdoor access or are raised as free-range (e.g. sheep, wild ruminants) suggests that environmental contamination of T. gondii is a contributing factor [94,95]. While there is a possible association between high prevalence in animals with outdoor access and increased risk of human exposure from the environment, this does not necessarily translate into a higher seroprevalence in humans, as direct environmental exposure is not the only possible route of infection to humans.
To understand seroprevalence differences between countries or regions, more data are needed on the cultural differences in behaviours that can influence the risk of T. gondii infection. Seroprevalence in humans is likely influenced by consumption habits and consumed products, including imported products from other countries. For example, the frequency, amount, preparation and types of meat or meat products consumed, and especially local preferences for specific products made of raw or undercooked meat are important. Previously, two quantitative microbiological risk assessment (QMRA) models suggested that filet américain, a typical Dutch raw beef spread, was the most important source of T. gondii infection in the Netherlands [96,97]. Similarly, Hackepeter or Mett, a German dish made of raw minced pork meat, is more popular and consumed more frequently in eastern Germany compared with western Germany. This dietary preference was linked to a higher T. gondii seroprevalence in the human adult population in eastern Germany [81]. Moreover, exposure to oocysts can vary locally due to differences in soil exposure, frequency of consumption of raw vegetables, fruits and shellfish, or drinking water treatment [89].
Yet, studies assessing the relative contribution of the various sources of infection in Europe are scarce. Underlining the varying importance of animals raised for human consumption in the transmission of T. gondii, a previous multicentre case-control study attributed 30–63% of infections to the consumption of undercooked or cured meat products and 6–17% to soil contact [98]. Also, several studies identifying significant foodborne risk factors have found a link between the consumption of livestock-derived foods, such as raw or undercooked meat [17,21,34,37,40,50,77,99], types of processed meat [43,77], unpasteurised milk and raw milk cheese [17,34], and T. gondii infection. With regards to oocyst-driven infections, eating raw or unwashed vegetables or fruits [17,43], contact with cats [17,37,40,50,55,77,81] and contact with soil [21,43,66,74,77] were reported as risk factors. However, for a risk factor to be an important source of infection at a population level, exposure to the factor also needs to be common. Exposure behaviour can vary between and within populations, and these data are often lacking. The recent implementation of a harmonised European survey tailored to capture variation in the frequency and amount of meat and vegetable products of known risk consumed, as well as consumers’ behaviour associated with an increased risk of infection (e.g. preference for raw or undercooked meat, washing of vegetables) will bring new data for T. gondii food-borne risk assessment (https://onehealthejp.eu/projects/foodborne-zoonoses/jrp-toxosources).
In this study, only region and age were included in the model. Any differences related to sex will therefore be missed. Nevertheless, in most studies reporting results on sex, differences between sexes were not observed [15,17,70,100], except for one study where males were more often seropositive [81]. Other study limitations were mainly related to the absence of seroprevalence data for some European countries, the small scale of some studies and incomplete or inconsistent data reporting. Also, policies towards screening for T. gondii infections differ between countries. Although the Bayesian model could address uncertainty in both prevalence and age data, the lack of diagnostic performance characteristics of in-house serological tests and commercial kits used in the selected studies made it impossible to estimate ‘true prevalence’ [101]. To fill this gap, data on test sensitivity and specificity could potentially be sourced from validation studies by the manufacturer or diagnostic accuracy studies. However, previous attempts to retrieve this information from the literature have shown inconsistent results due to a variety of factors, including the characteristics of the sample population (e.g. immune status, time since infection, potentially cross-reacting pathogens or rheumatic factors), study design, quality of the reference standard used, or different cutoffs employed [102,103]. Thus, even when data on test characteristics are available, it is important to keep in mind that such data may not be constant over populations and that the reproducibility of serological test results is not warranted [102,104,105].
For future work to overcome the hurdles associated with data heterogeneity in serological studies, we emphasise the importance of the primary data source when publishing original research. A main challenge is assuring that raw data are reported in a way that allows their accessibility and their further reuse in reviews and meta-analyses [106]. A solution to this challenge is the development and adoption of standardised templates that enable harmonised reporting of data. Ideally, results from serological surveys should be provided on an individual basis, when possible, and include demographic information on age and sex, together with test performance characteristics, cutoffs, as well as titres or OD values. Additional details are desirable, e.g. the ELISA plate identification or analysis date, which allows for plate-to-plate correction when using binary mixture models [107]. A spreadsheet for epidemiological data reporting that could function as such a template was previously proposed [13]. These data templates could be used, for example, in the online supplementary files of research papers or made available in public repositories.
Conclusion
Knowing the prevalence of infection is key information in assessing disease burden and estimating costs of illness and prevention efforts in a population. The results of this review revealed a considerably higher seroprevalence in eastern and western Europe, compared with northern Europe and the UK, and that T. gondii infection is occurring at an earlier age in these regions. The commonly held belief that the infection is lifelong is corroborated by our results, as the current best estimate of the reversion rate equates to infection durations that far exceed a human lifespan. However, the uncertainty in the estimate is large, and reversion rates that correspond to infection durations of decades are within the 95% credible interval. Prevalence distributions derived by the model contribute to a better understanding of disease burden caused by T. gondii and provide a baseline for future epidemiological research in the different European regions.
Ethical statement
Ethical approval was not required as only pooled data from other studies were used in the review and modelling.
Use of artificial intelligence tools
None declared.
Acknowledgements
We would like to express our appreciation to Rob van Spronsen for his expertise and guidance with the literature search, Sara Monteiro Pires, Lea lovric and Vitomir Djokic for help with data extraction in specific languages, and members of the TOXOSOURCES consortium for their continuous support and ideas.
Supplementary Data
Authors’ contributions: Ingrid HM Friesema: Conceptualisation, Data curation, Formal analysis, Methodology, Validation, Supervision, Writing – original draft, Writing – review & editing. Helga Waap: Writing – original draft, Writing – review & editing. Arno Swart: Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. Adriana Györke: Data curation, Formal analysis, Methodology, Writing – review & editing. Delphine Le Roux: Data curation, Formal analysis, Methodology, Writing – review & editing. Francisco Evangelista: Data curation, Formal analysis, Methodology, Writing – review & editing. Furio Spano: Data curation, Formal analysis, Methodology, Writing – review & editing. Gereon Schares: Data curation, Formal analysis, Methodology, Writing – review & editing. Gunita Deksne: Data curation, Formal analysis, Methodology, Writing – review & editing. Maria João Gargaté: Data curation, Formal analysis, Methodology, Writing – review & editing. Rafael Calero-Bernal: Data curation, Formal analysis, Methodology, Writing – review & editing. Pikka Jokelainen: Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing. Frank Seeber: Data curation, Formal analysis, Methodology, Writing – review & editing. Jacek Sroka: Data curation, Formal analysis, Methodology, Writing – review & editing. Anna Lundén: Data curation, Formal analysis, Methodology, Writing – review & editing. Oda van den Berg: Data curation, Formal analysis, Methodology, Writing – review & editing. Solveig Jore: Data curation, Formal analysis, Methodology, Writing – review & editing. Henk J Wisselink: Data curation, Formal analysis, Methodology, Writing – review & editing. Filip Dámek: Data curation, Formal analysis, Methodology, Writing – review & editing. Lasse S Vestergaard: Data curation, Formal analysis, Methodology, Writing – review & editing. Marieke Opsteegh: Conceptualisation, Data curation, Formal analysis, Methodology, Validation, Supervision, Writing – original draft, Writing – review & editing.
Conflict of interest: None declared.
Funding statement: This work was performed within the projects TOXOSOURCES and ToxSauQMRA that received funding from the European Union’s Horizon 2020 Research and Innovation Programme, under grant agreement No. 773830: One Health European Joint Programme.
Data availability
Supplementary data for the reported results, including publicly archived datasets analysed or generated during the study, can be found at GitHub, an online data repository, reachable through the following URL: https://github.com/rivm-syso.
References
- 1. Dubey JP. The history of Toxoplasma gondii--the first 100 years. J Eukaryot Microbiol. 2008;55(6):467-75. 10.1111/j.1550-7408.2008.00345.x [DOI] [PubMed] [Google Scholar]
- 2. Dubey JP. History of the discovery of the life cycle of Toxoplasma gondii. Int J Parasitol. 2009;39(8):877-82. 10.1016/j.ijpara.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 3. Thebault A, Kooh P, Cadavez V, Gonzales-Barron U, Villena I. Risk factors for sporadic toxoplasmosis: a systematic review and meta-analysis. Microb Risk Anal. 2021;17:100133. 10.1016/j.mran.2020.100133 [DOI] [Google Scholar]
- 4. López Ureña NM, Chaudhry U, Calero Bernal R, Cano Alsua S, Messina D, Evangelista F, et al. Contamination of soil, water, fresh produce, and bivalve mollusks with Toxoplasma gondii oocysts: a systematic review. Microorganisms. 2022;10(3):517. 10.3390/microorganisms10030517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363(9425):1965-76. 10.1016/S0140-6736(04)16412-X [DOI] [PubMed] [Google Scholar]
- 6. Sullivan WJ, Jr, Jeffers V. Mechanisms of Toxoplasma gondii persistence and latency. FEMS Microbiol Rev. 2012;36(3):717-33. 10.1111/j.1574-6976.2011.00305.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McAuley JB. Congenital Toxoplasmosis. J Pediatric Infect Dis Soc. 2014;3(Suppl 1) Suppl 1;S30-5. 10.1093/jpids/piu077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8(10):634-40. 10.1046/j.1469-0691.2002.00485.x [DOI] [PubMed] [Google Scholar]
- 9. de Barros RAM, Torrecilhas AC, Marciano MAM, Mazuz ML, Pereira-Chioccola VL, Fux B. Toxoplasmosis in human and animals around the world. Diagnosis and perspectives in the One Health approach. Acta Trop. 2022;231:106432. 10.1016/j.actatropica.2022.106432 [DOI] [PubMed] [Google Scholar]
- 10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kohl C, McIntosh EJ, Unger S, Haddaway NR, Kecke S, Schiemann J, et al. Online tools supporting the conduct and reporting of systematic reviews and systematic maps: a case study on CADIMA and review of existing tools. Environ Evid. 2018;7(8). [Google Scholar]
- 12. Bouwknegt M, Devleesschauwer B, Graham H, Robertson LJ, van der Giessen JW, Euro-FBP workshop participants . Prioritisation of food-borne parasites in Europe, 2016. Euro Surveill. 2018;23(9):17-00161. 10.2807/1560-7917.ES.2018.23.9.17-00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dámek F, Swart A, Waap H, Jokelainen P, Le Roux D, Deksne G, et al. Systematic review and modelling of age-dependent prevalence of Toxoplasma gondii in livestock, wildlife and felids in Europe. Pathogens. 2023;12(1):97. 10.3390/pathogens12010097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bartolomé Alvarez J, Martínez Serrano M, Moreno Parrado L, Lorente Ortuño S, Crespo Sánchez MD. Prevalencia e incidencia de la infección por Toxoplasma gondii en mujeres en edad fértil en Albacete (2001-2007). [Prevalence and incidence in Albacete, Spain, of Toxoplasma gondii infection in women of childbearing age: differences between immigrant and non-immigrant (2001-2007)]. Rev Esp Salud Publica. 2008;82(3):333-42. Spanish. 10.1590/S1135-57272008000300009 [DOI] [PubMed] [Google Scholar]
- 15. Antolová D, Janičko M, Halánová M, Jarčuška P, Gecková AM, Babinská I, et al. Exposure to Toxoplasma gondii in the Roma and non-Roma inhabitants of Slovakia: A cross-sectional seroprevalence study. Int J Environ Res Public Health. 2018;15(3):408. 10.3390/ijerph15030408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antoniou M, Tzouvali H, Sifakis S, Galanakis E, Georgopoulou E, Tselentis Y. Toxoplasmosis in pregnant women in Crete. Parassitologia. 2007;49(4):231-3. [PubMed] [Google Scholar]
- 17. Asencio MA, Herraez O, Tenias JM, Garduño E, Huertas M, Carranza R, et al. Seroprevalence survey of zoonoses in Extremadura, southwestern Spain, 2002-2003. Jpn J Infect Dis. 2015;68(2):106-12. 10.7883/yoken.JJID.2014.181 [DOI] [PubMed] [Google Scholar]
- 18. Berger F, Goulet V, Le Strat Y, Desenclos JC. Toxoplasmosis among pregnant women in France: risk factors and change of prevalence between 1995 and 2003. Rev Epidemiol Sante Publique. 2009;57(4):241-8. 10.1016/j.respe.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 19. Birgisdóttir A, Asbjörnsdottir H, Cook E, Gislason D, Jansson C, Olafsson I, et al. Seroprevalence of Toxoplasma gondii in Sweden, Estonia and Iceland. Scand J Infect Dis. 2006;38(8):625-31. 10.1080/00365540600606556 [DOI] [PubMed] [Google Scholar]
- 20. Bobić B, Milosavić M, Guzijan G, Djurković-Djaković O. First report on Toxoplasma gondii infection in Bosnia and Herzegovina: study in blood donors. Vector Borne Zoonotic Dis. 2016;16(12):807-9. 10.1089/vbz.2016.2028 [DOI] [PubMed] [Google Scholar]
- 21. Bobić B, Nikolić A, Klun I, Vujanić M, Djurković-Djaković O. Undercooked meat consumption remains the major risk factor for Toxoplasma infection in Serbia. Parassitologia. 2007;49(4):227-30. [PubMed] [Google Scholar]
- 22. Brkić S, Gajski G, Bogavac M, Marić D, Turkulov V, Tomić S. Серопреваленција токсоплазмозе у Војводини. [Seroprevalence of toxoplasmosis in Vojvodina]. Srp Arh Celok Lek. 2010;138(5-6):333-6. Serbian. 10.2298/SARH1006333B [DOI] [PubMed] [Google Scholar]
- 23. Burrells A, Opsteegh M, Pollock KG, Alexander CL, Chatterton J, Evans R, et al. The prevalence and genotypic analysis of Toxoplasma gondii from individuals in Scotland, 2006-2012. Parasit Vectors. 2016;9(1):324. 10.1186/s13071-016-1610-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Capretti MG, De Angelis M, Tridapalli E, Orlandi A, Marangoni A, Moroni A, et al. Toxoplasmosis in pregnancy in an area with low seroprevalence: is prenatal screening still worthwhile? Pediatr Infect Dis J. 2014;33(1):5-10. 10.1097/INF.0000000000000006 [DOI] [PubMed] [Google Scholar]
- 25. Czyzewski K, Fraczkiewicz J, Salamonowicz M, Pieczonka A, Zajac-Spychala O, Zaucha-Prazmo A, et al. Low seroprevalence and low incidence of infection with Toxoplasma gondii (Nicolle et Manceaux, 1908) in pediatric hematopoietic cell transplantation donors and recipients: Polish nationwide study. Folia Parasitol (Praha). 2019;66:66. 10.14411/fp.2019.019 [DOI] [PubMed] [Google Scholar]
- 26. Daković Rode O, Židovec Lepej S, Vodnica Martucci M, Lasica Polanda V, Begovac J. Prevalencija protutijela na Toxoplasma gondii u bolesnika zaraženih virusom humane imunodeficijencije u Hrvatskoj. [Prevalence of antibodies against Toxoplasma gondii in patients infected with human immunodeficiency virus in Croatia]. Infektol Glas. 2010;30(1):5-10. Croatian. [Google Scholar]
- 27. De Paschale M, Agrappi C, Clerici P, Mirri P, Manco MT, Cavallari S, et al. Seroprevalence and incidence of Toxoplasma gondii infection in the Legnano area of Italy. Clin Microbiol Infect. 2008;14(2):186-9. 10.1111/j.1469-0691.2007.01883.x [DOI] [PubMed] [Google Scholar]
- 28. de Witte LD, Snijders G, Litjens M, Kamperman AM, Kushner SA, Kahn RS, et al. Are infectious agents involved in the pathogenesis of postpartum psychosis? J Affect Disord. 2018;229:141-4. 10.1016/j.jad.2017.12.069 [DOI] [PubMed] [Google Scholar]
- 29. de Witte LD, van Mierlo HC, Litjens M, Klein HC, Bahn S, Osterhaus AD. The association between antibodies to neurotropic pathogens and schizophrenia: a case-control study. NPJ Schizophr. 2015;1(1):15041. 10.1038/npjschz.2015.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diza E, Frantzidou F, Souliou E, Arvanitidou M, Gioula G, Antoniadis A. Seroprevalence of Toxoplasma gondii in northern Greece during the last 20 years. Clin Microbiol Infect. 2005;11(9):719-23. 10.1111/j.1469-0691.2005.01193.x [DOI] [PubMed] [Google Scholar]
- 31. Dzitko K, Malicki S, Komorowski J. Effect of hyperprolactinaemia on Toxoplasma gondii prevalence in humans. Parasitol Res. 2008;102(4):723-9. 10.1007/s00436-007-0824-0 [DOI] [PubMed] [Google Scholar]
- 32. Fanigliulo D, Marchi S, Montomoli E, Trombetta CM. Toxoplasma gondii in women of childbearing age and during pregnancy: seroprevalence study in Central and Southern Italy from 2013 to 2017. Parasite. 2020;27:2. 10.1051/parasite/2019080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Findal G, Barlinn R, Sandven I, Stray-Pedersen B, Nordbø SA, Samdal HH, et al. Toxoplasma prevalence among pregnant women in Norway: a cross-sectional study. APMIS. 2015;123(4):321-5. 10.1111/apm.12354 [DOI] [PubMed] [Google Scholar]
- 34. Flatt A, Shetty N. Seroprevalence and risk factors for toxoplasmosis among antenatal women in London: a re-examination of risk in an ethnically diverse population. Eur J Public Health. 2013;23(4):648-52. 10.1093/eurpub/cks075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Flegr J, Hrusková M, Hodný Z, Novotná M, Hanusová J. Body height, body mass index, waist-hip ratio, fluctuating asymmetry and second to fourth digit ratio in subjects with latent toxoplasmosis. Parasitology. 2005;130(Pt 6):621-8. 10.1017/S0031182005007316 [DOI] [PubMed] [Google Scholar]
- 36. Gutiérrez-Zufiaurre N, Sánchez-Hernández J, Muñoz S, Marín R, Delgado N, Sáenz MC, et al. Seroprevalencia de anticuerpos frente a Treponema pallidum, Toxoplasma gondii, virus de la rubéola, virus de la hepatitis B y C y VIH en mujeres gestantes. [Seroprevalence of antibodies against Treponema pallidum, Toxoplasma gondii, rubella virus, hepatitis B and C virus, and HIV in pregnant women]. Enferm Infecc Microbiol Clin. 2004;22(9):512-6. Spanish. 10.1016/S0213-005X(04)73152-3 [DOI] [PubMed] [Google Scholar]
- 37. Hofhuis A, van Pelt W, van Duynhoven YTHP, Nijhuis CDM, Mollema L, van der Klis FRM, et al. Decreased prevalence and age-specific risk factors for Toxoplasma gondii IgG antibodies in The Netherlands between 1995/1996 and 2006/2007. Epidemiol Infect. 2011;139(4):530-8. 10.1017/S0950268810001044 [DOI] [PubMed] [Google Scholar]
- 38. Holec-Gasior L, Kur J. Badania epidemiologiczne populacji kobiet gminy Przodkowo w kierunku toksoplazmozy. [Epidemiological studies of toxoplasmosis among women from Przodkowo commune]. Przegl Epidemiol. 2009;63(2):311-6. Polish. [PubMed] [Google Scholar]
- 39. Kaňková Š, Procházková L, Flegr J, Calda P, Springer D, Potluková E. Effects of latent toxoplasmosis on autoimmune thyroid diseases in pregnancy. PLoS One. 2014;9(10):e110878. 10.1371/journal.pone.0110878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kolbekova P, Kourbatova E, Novotna M, Kodym P, Flegr J. New and old risk-factors for Toxoplasma gondii infection: prospective cross-sectional study among military personnel in the Czech Republic. Clin Microbiol Infect. 2007;13(10):1012-7. 10.1111/j.1469-0691.2007.01771.x [DOI] [PubMed] [Google Scholar]
- 41. Lassen B, Janson M, Viltrop A, Neare K, Hütt P, Golovljova I, et al. Serological evidence of exposure to globally relevant zoonotic parasites in the Estonian population. PLoS One. 2016;11(10):e0164142. 10.1371/journal.pone.0164142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lobo ML, Patrocinio G, Sevivas T, DE Sousa B, Matos O. Portugal and Angola: similarities and differences in Toxoplasma gondii seroprevalence and risk factors in pregnant women. Epidemiol Infect. 2017;145(1):30-40. 10.1017/S0950268816001904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lopes AP, Dubey JP, Moutinho O, Gargaté MJ, Vilares A, Rodrigues M, et al. Seroepidemiology of Toxoplasma gondii infection in women from the North of Portugal in their childbearing years. Epidemiol Infect. 2012;140(5):872-7. 10.1017/S0950268811001658 [DOI] [PubMed] [Google Scholar]
- 44. López-Fabal F, Gómez-Garcés JL. Marcadores serológicos de gestantes españolas e inmigrantes en un área del sur de Madrid durante el periodo 2007-2010. [Serological markers of Spanish and immigrant pregnant women in the south of Madrid during the period 2007-2010]. Rev Esp Quimioter. 2013;26(2):108-11. Spanish. [PubMed] [Google Scholar]
- 45. Maggi P, Volpe A, Carito V, Schinaia N, Bino S, Basho M, et al. Surveillance of toxoplasmosis in pregnant women in Albania. New Microbiol. 2009;32(1):89-92. [PubMed] [Google Scholar]
- 46. Marcinek P, Nowakowska D, Szaflik K, Spiewak E, Małafiej E, Wilczyński J. Analiza wybranych powikłatń w przebiegu ciazy u kobiet z serologicznymi cechami ostrej toksoplazmozy lub ostrej parwowirozy. [Analysis of complications during pregnancy in women with serological features of acute toxoplasmosis or acute parvovirosis]. Ginekol Pol. 2008;79(3):186-91. Polish. [PubMed] [Google Scholar]
- 47. Mihu AG, Balta C, Marti DT, Paduraru AA, Lupu MA, Olariu TR. Seroprevalence of Toxoplasma gondii infection among women of childbearing age in an endemic region of Romania, 2016-2018. Parasite. 2020;27:59. 10.1051/parasite/2020057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mizgajska-Wiktor H, Jarosz W, Andrzejewska I, Krzykała M, Janowski J, Kozłowska M. Differences in some developmental features between Toxoplasma gondii-seropositive and seronegative school children. Folia Parasitol (Praha). 2013;60(5):416-24. 10.14411/fp.2013.044 [DOI] [PubMed] [Google Scholar]
- 49. Motoi S, Navolan DB, Malita D, Ciohat I, Nemescu D, Manciuc C, et al. A decreasing trend in Toxoplasma gondii seroprevalence among pregnant women in Romania - results of a large scale study. Exp Ther Med. 2020;20(4):3536-40. 10.3892/etm.2020.9012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nash JQ, Chissel S, Jones J, Warburton F, Verlander NQ. Risk factors for toxoplasmosis in pregnant women in Kent, United Kingdom. Epidemiol Infect. 2005;133(3):475-83. 10.1017/S0950268804003620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Niemiec KT, Raczyński P, Markiewicz K, Leibschang J, Ceran A. Czestość wystepowania zarazeń pierwotniakiem Toxoplasma gondii u 2016 kobiet ciezarnych oraz ich dzieci urodzonych w Instytucie Matki i Dziecka w Warszawie. [The prevalence of Toxoplasma gondii infection among 2016 pregnant women and their children in the Institute of Mother and Child in Warsaw]. Wiad Parazytol. 2002;48(3):293-9. Polish. [PubMed] [Google Scholar]
- 52. Nowakowska D, Stray-Pedersen B, Śpiewak E, Sobala W, Małafiej E, Wilczyński J. Prevalence and estimated incidence of Toxoplasma infection among pregnant women in Poland: a decreasing trend in the younger population. Clin Microbiol Infect. 2006;12(9):913-7. 10.1111/j.1469-0691.2006.01513.x [DOI] [PubMed] [Google Scholar]
- 53. Nowakowska D, Wujcicka W, Sobala W, Śpiewak E, Gaj Z, Wilczyński J. Age-associated prevalence of Toxoplasma gondii in 8281 pregnant women in Poland between 2004 and 2012. Epidemiol Infect. 2014;142(3):656-61. 10.1017/S0950268813001179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Olariu TR, Petrescu C, Darabus G, Lighezan R, Mazilu O. Seroprevalence of Toxoplasma gondii in Western Romania. Infect Dis (Lond). 2015;47(8):580-3. 10.3109/23744235.2015.1028098 [DOI] [PubMed] [Google Scholar]
- 55. Olariu TR, Ursoniu S, Hotea I, Dumitrascu V, Anastasiu D, Lupu MA. Seroprevalence and risk factors of Toxoplasma gondii infection in pregnant women from Western Romania. Vector Borne Zoonotic Dis. 2020;20(10):763-7. 10.1089/vbz.2019.2599 [DOI] [PubMed] [Google Scholar]
- 56. Piffer S, Lauriola AL, Pradal U, Collini L, Dell’Anna L, Pavanello L. Toxoplasma gondii infection during pregnancy: a ten-year observation in the province of Trento, Italy. Infez Med. 2020;28(4):603-10. [PubMed] [Google Scholar]
- 57. Pinto B, Castagna B, Mattei R, Bruzzi R, Chiumiento L, Cristofani R, et al. Seroprevalence for toxoplasmosis in individuals living in north west Tuscany: access to Toxo-test in central Italy. Eur J Clin Microbiol Infect Dis. 2012;31(6):1151-6. 10.1007/s10096-011-1422-8 [DOI] [PubMed] [Google Scholar]
- 58. Pribakovic JA, Katanic N, Radevic T, Tasic MS, Kostic M, Stolic B, et al. Serological status of childbearing-aged women for Toxoplasma gondii and cytomegalovirus in northern Kosovo and Metohija. Rev Soc Bras Med Trop. 2019;52:e20170313. 10.1590/0037-8682-0313-2017 [DOI] [PubMed] [Google Scholar]
- 59. Prusa AR, Kasper DC, Olischar M, Husslein P, Pollak A, Hayde M. Evaluation of serological prenatal screening to detect Toxoplasma gondii infections in Austria. Neonatology. 2013;103(1):27-34. 10.1159/000342625 [DOI] [PubMed] [Google Scholar]
- 60. Puccio G, Cajozzo C, Canduscio LA, Cino L, Romano A, Schimmenti MG, et al. Epidemiology of Toxoplasma and CMV serology and of GBS colonization in pregnancy and neonatal outcome in a Sicilian population. Ital J Pediatr. 2014;40(1):23. 10.1186/1824-7288-40-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ramos JM, Milla A, Rodríguez JC, Padilla S, Masiá M, Gutiérrez F. Seroprevalence of Toxoplasma gondii infection among immigrant and native pregnant women in Eastern Spain. Parasitol Res. 2011;109(5):1447-52. 10.1007/s00436-011-2393-5 [DOI] [PubMed] [Google Scholar]
- 62. Robinson E, de Valk H, Villena I, Le Strat Y, Tourdjman M. National perinatal survey demonstrates a decreasing seroprevalence of Toxoplasma gondii infection among pregnant women in France, 1995 to 2016: impact for screening policy. Euro Surveill. 2021;26(5):1900710. 10.2807/1560-7917.ES.2021.26.5.1900710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rodrigues FT, Sousa AP, Escoval MA, Condeço J, Cardoso L, Lopes AP. Seroepidemiology of Toxoplasma gondii in blood donors in Portugal. Transfus Apher Sci. 2020;59(4):102777. 10.1016/j.transci.2020.102777 [DOI] [PubMed] [Google Scholar]
- 64. Rudin C, Hirsch HH, Spaelti R, Schaedelin S, Klimkait T. Decline of seroprevalence and incidence of congenital toxoplasmosis despite changing prevention policy-three decades of cord-blood screening in north-western Switzerland. Pediatr Infect Dis J. 2018;37(11):1087-92. 10.1097/INF.0000000000001978 [DOI] [PubMed] [Google Scholar]
- 65. Sagel U, Krämer A, Mikolajczyk RT. Incidence of maternal Toxoplasma infections in pregnancy in Upper Austria, 2000-2007. BMC Infect Dis. 2011;11(1):348. 10.1186/1471-2334-11-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Salamon D, Bulanda M. Toxoplasma gondii and women of reproductive age: an analysis of data from the Chair of Microbiology, Jagiellonian University Medical College in Cracow. Ann Parasitol. 2014;60(4):291-6. [PubMed] [Google Scholar]
- 67. Sampedro A, Mazuelas P, Rodríguez-Granger J, Torres E, Puertas A, Navarro JM. Marcadores serológicos en gestantes inmigrantes y autóctonas en Granada. [Serological markers in immigrant and Spanish pregnant women in Granada]. Enferm Infecc Microbiol Clin. 2010;28(10):694-7. Spanish. 10.1016/j.eimc.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 68. Santiago B, Blázquez D, López G, Sainz T, Muñoz M, Alonso T, et al. Perfil serológico en gestantes extranjeras frente a VIH, VHB, VHC, virus de la rubéola, Toxoplasma gondii, Treponema pallidum, y Trypanosoma cruzi. [Serological profile of immigrant pregnant women against HIV, HBV, HCV, rubella, Toxoplasma gondii, Treponema pallidum, and Trypanosoma cruzi]. Enferm Infecc Microbiol Clin. 2012;30(2):64-9. Spanish. 10.1016/j.eimc.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 69. Scepanovic P, Alanio C, Hammer C, Hodel F, Bergstedt J, Patin E, et al. Human genetic variants and age are the strongest predictors of humoral immune responses to common pathogens and vaccines. Genome Med. 2018;10(1):59. 10.1186/s13073-018-0568-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Siponen AM, Kinnunen PM, Koort J, Kallio-Kokko H, Vapalahti O, Virtala AM, et al. Toxoplasma gondii seroprevalence in veterinarians in Finland: older age, living in the countryside, tasting beef during cooking and not doing small animal practice associated with seropositivity. Zoonoses Public Health. 2019;66(2):207-15. 10.1111/zph.12550 [DOI] [PubMed] [Google Scholar]
- 71. Sroka J, Zwoliński J, Dutkiewicz J. Czekstość wystepowania przeciwciał anty-Toxoplasma gondii wśród pracowników Zakładów Miesnych w Lublinie. [The prevalence of anti-Toxoplasma gondii antibodies among abattoir workers in Lublin]. Wiad Parazytol. 2003;49(1):47-55. Polish. [PubMed] [Google Scholar]
- 72. Strhársky J, Klement C, Hrubá F. Seroprevalence of Toxoplasma gondii antibodies in the Slovak Republic. Folia Microbiol (Praha). 2009;54(6):553-8. 10.1007/s12223-009-0081-y [DOI] [PubMed] [Google Scholar]
- 73. Studenicová C, Bencaiová G, Holková R. Seroprevalence of Toxoplasma gondii antibodies in a healthy population from Slovakia. Eur J Intern Med. 2006;17(7):470-3. 10.1016/j.ejim.2006.07.007 [DOI] [PubMed] [Google Scholar]
- 74. Studenicová C, Ondriska F, Holková R. Séroprevalencia Toxoplasma gondii u gravidných zien na Slovensku. [Seroprevalence of Toxoplasma gondii among pregnant women in Slovakia]. Epidemiol Mikrobiol Imunol. 2008;57(1):8-13. Slovakian. [PubMed] [Google Scholar]
- 75. Suvisaari J, Torniainen-Holm M, Lindgren M, Härkänen T, Yolken RH. Toxoplasma gondii infection and common mental disorders in the Finnish general population. J Affect Disord. 2017;223:20-5. 10.1016/j.jad.2017.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Szénási Z, Horváth K, Sárkány E, Melles M. Toxoplasmosis surveillance during pregnancy and quality assurance of methods in Hungary. Wien Klin Wochenschr. 2005;117(S4) Suppl 4;29-34. 10.1007/s00508-005-0444-6 [DOI] [PubMed] [Google Scholar]
- 77. Thaller R, Tammaro F, Pentimalli H. Fattori di rischio per la toxoplasmosi in gravidanzain una popolazione del centro Italia. [Risk factors for toxoplasmosis in pregnant women in central Italy]. Infez Med. 2011;19(4):241-7. Italian. [PubMed] [Google Scholar]
- 78. Tomasoni LR, Sosta E, Beltrame A, Rorato G, Bigoni S, Frusca T, et al. Antenatal screening for mother to child infections in immigrants and residents: the case of toxoplasmosis in northern Italy. J Immigr Minor Health. 2010;12(6):834-40. 10.1007/s10903-010-9321-0 [DOI] [PubMed] [Google Scholar]
- 79. Vilibic-Cavlek T, Ljubin-Sternak S, Ban M, Kolaric B, Sviben M, Mlinaric-Galinovic G. Seroprevalence of TORCH infections in women of childbearing age in Croatia. J Matern Fetal Neonatal Med. 2011;24(2):280-3. 10.3109/14767058.2010.485233 [DOI] [PubMed] [Google Scholar]
- 80. Wang H, Yolken RH, Hoekstra PJ, Burger H, Klein HC. Antibodies to infectious agents and the positive symptom dimension of subclinical psychosis: The TRAILS study. Schizophr Res. 2011;129(1):47-51. 10.1016/j.schres.2011.03.013 [DOI] [PubMed] [Google Scholar]
- 81. Wilking H, Thamm M, Stark K, Aebischer T, Seeber F. Prevalence, incidence estimations, and risk factors of Toxoplasma gondii infection in Germany: a representative, cross-sectional, serological study. Sci Rep. 2016;6(1):22551. 10.1038/srep22551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wójcik-Fatla A, Sroka J, Zając V, Zwoliński J, Sawczyn-Domańska A, Kloc A, et al. Study on Toxoplasma gondii, Leptospira spp., Coxiella burnetii, and Echinococcus granulosus infection in veterinarians from Poland. J Vet Res (Pulawy). 2018;62(4):477-83. 10.2478/jvetres-2018-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zajkowska A, Garkowski A, Czupryna P, Moniuszko A, Król ME, Szamatowicz J, et al. Seroprevalence of parvovirus B19 antibodies among young pregnant women or planning pregnancy, tested for toxoplasmosis. Przegl Epidemiol. 2015;69(3):479-82, 597-600. [PubMed] [Google Scholar]
- 84. Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res. 2001;10(4):277-303. 10.1177/096228020101000404 [DOI] [PubMed] [Google Scholar]
- 85. Calero-Bernal R, Gennari SM, Cano S, Salas-Fajardo MY, Ríos A, Álvarez-García G, et al. Anti-Toxoplasma gondii antibodies in European residents: a systematic review and meta-analysis of studies published between 2000 and 2020. Pathogens. 2023;12(12):1430. 10.3390/pathogens12121430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Meireles LR, Ekman CC, Andrade HF, Jr, Luna EJ. Human toxoplasmosis outbreaks and the agent infecting form. Findings from a systematic review. Rev Inst Med Trop São Paulo. 2015;57(5):369-76. 10.1590/S0036-46652015000500001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bigna JJ, Tochie JN, Tounouga DN, Bekolo AO, Ymele NS, Youda EL, et al. Global, regional, and country seroprevalence of Toxoplasma gondii in pregnant women: a systematic review, modelling and meta-analysis. Sci Rep. 2020;10(1):12102. 10.1038/s41598-020-69078-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rostami A, Riahi SM, Contopoulos-Ioannidis DG, Gamble HR, Fakhri Y, Shiadeh MN, et al. Acute Toxoplasma infection in pregnant women worldwide: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2019;13(10):e0007807. 10.1371/journal.pntd.0007807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264-96. 10.1128/CMR.05013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rostami A, Riahi SM, Gamble HR, Fakhri Y, Nourollahpour Shiadeh M, Danesh M, et al. Global prevalence of latent toxoplasmosis in pregnant women: a systematic review and meta-analysis. Clin Microbiol Infect. 2020;26(6):673-83. 10.1016/j.cmi.2020.01.008 [DOI] [PubMed] [Google Scholar]
- 91. Salari N, Rahimi A, Zarei H, Abdolmaleki A, Rasoulpoor S, Shohaimi S, et al. Global seroprevalence of Toxoplasma gondii in pregnant women: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2025;25(1):90. 10.1186/s12884-025-07182-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009;39(12):1385-94. 10.1016/j.ijpara.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 93. Giese L, Seeber F, Aebischer A, Kuhnert R, Schlaud M, Stark K, et al. Toxoplasma gondii infections and associated factors in female children and adolescents, Germany. Emerg Infect Dis. 2024;30(5):995-9. 10.3201/eid3005.231045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12-13):1217-58. 10.1016/S0020-7519(00)00124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Stelzer S, Basso W, Benavides Silván J, Ortega-Mora LM, Maksimov P, Gethmann J, et al. Toxoplasma gondii infection and toxoplasmosis in farm animals: Risk factors and economic impact. Food Waterborne Parasitol. 2019;15:e00037. 10.1016/j.fawpar.2019.e00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Opsteegh M, Prickaerts S, Frankena K, Evers EG. A quantitative microbial risk assessment for meatborne Toxoplasma gondii infection in The Netherlands. Int J Food Microbiol. 2011;150(2-3):103-14. 10.1016/j.ijfoodmicro.2011.07.022 [DOI] [PubMed] [Google Scholar]
- 97. Deng H, Swart A, Bonačić Marinović AA, van der Giessen JWB, Opsteegh M. The effect of salting on Toxoplasma gondii viability evaluated and implemented in a quantitative risk assessment of meat-borne human infection. Int J Food Microbiol. 2020;314:108380. 10.1016/j.ijfoodmicro.2019.108380 [DOI] [PubMed] [Google Scholar]
- 98. Cook AJC, Gilbert RE, Buffolano W, Zufferey J, Petersen E, Jenum PA, et al. Sources of toxoplasma infection in pregnant women: European multicentre case-control study. BMJ. 2000;321(7254):142-7. 10.1136/bmj.321.7254.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Said B, Halsby KD, O’Connor CM, Francis J, Hewitt K, Verlander NQ, et al. Risk factors for acute toxoplasmosis in England and Wales. Epidemiol Infect. 2017;145(1):23-9. 10.1017/S0950268816002235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. van den Berg OE, Stanoeva KR, Zonneveld R, Hoek-van Deursen D, van der Klis FR, van de Kassteele J, et al. Seroprevalence of Toxoplasma gondii and associated risk factors for infection in the Netherlands: third cross-sectional national study. Epidemiol Infect. 2023;151:e136. 10.1017/S095026882300122X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rogan WJ, Gladen B. Estimating prevalence from the results of a screening test. Am J Epidemiol. 1978;107(1):71-6. 10.1093/oxfordjournals.aje.a112510 [DOI] [PubMed] [Google Scholar]
- 102. Huertas-López A, Cantos-Barreda A, Sánchez-Sánchez R, Martínez-Carrasco C, Ibáñez-López FJ, Martínez-Subiela S, et al. A systematic review and meta-analysis of the validation of serological methods for detecting anti-Toxoplasma gondii antibodies in humans and animals. Vet Parasitol. 2024;328:110173. 10.1016/j.vetpar.2024.110173 [DOI] [PubMed] [Google Scholar]
- 103. Greiner M, Gardner IA. Epidemiologic issues in the validation of veterinary diagnostic tests. Prev Vet Med. 2000;45(1-2):3-22. 10.1016/S0167-5877(00)00114-8 [DOI] [PubMed] [Google Scholar]
- 104. Deng H, Devleesschauwer B, Liu M, Li J, Wu Y, van der Giessen JWB, et al. Seroprevalence of Toxoplasma gondii in pregnant women and livestock in the mainland of China: a systematic review and hierarchical meta-analysis. Sci Rep. 2018;8(1):6218. 10.1038/s41598-018-24361-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Berkvens D, Speybroeck N, Praet N, Adel A, Lesaffre E. Estimating disease prevalence in a Bayesian framework using probabilistic constraints. Epidemiology. 2006;17(2):145-53. 10.1097/01.ede.0000198422.64801.8d [DOI] [PubMed] [Google Scholar]
- 106. Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data. 2016;3(1):160018. 10.1038/sdata.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Swart A, Maas M, de Vries A, Cuperus T, Opsteegh M. Bayesian binary mixture models as a flexible alternative to cut-off analysis of ELISA results, a case study of Seoul Orthohantavirus. Viruses. 2021;13(6):1155. 10.3390/v13061155 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplementary data for the reported results, including publicly archived datasets analysed or generated during the study, can be found at GitHub, an online data repository, reachable through the following URL: https://github.com/rivm-syso.