Abstract
Research has indicated that PM2.5 exposure and low muscle strength may increase the risk of cardiovascular diseases (CVDs). However, inconsistent findings existed on PM2.5 constituents and CVDs, and little is known about the interplay between PM2.5 constituents and muscle strength. This study aimed to examine the associations of PM2.5 and its components with the incidence of CVDs and to further investigate the joint effects of pollutants and muscle strength on CVDs in a nationwide cohort from 2011–2018. PM2.5 and five constituents, including black carbon (BC), organic matter (OM), sulfate (SO42−), nitrate (NO3−), and ammonium (NH4+), were obtained from established spatiotemporal models and evaluated annually within the follow-up period. A time-varying Cox model was employed to investigate the impact of long-term exposure to PM2.5 and its components on CVDs. The joint effects on CVDs were examined under exposure to both pollutants (high vs. low) and muscle strength (normal vs. low). During the 7-year follow-up, 1971 cases of CVD occurred among the 10,413 participants. Significant associations of CVDs with a one standard deviation increase in each pollutant were observed, with HRs (95 % CIs) of 1.15 (1.09, 1.21) for PM2.5, 1.17 (1.11, 1.24) for OM, 1.16 (1.09, 1.22) for BC, 1.13 (1.07, 1.19) for NH4+, 1.14 (1.08, 1.19) for NO3− and 1.13 (1.07, 1.19) for SO42−. Higher risks of CVDs were obtained in participants exposed to high levels of PM2.5 constituents and low muscle strength. These findings suggest that reduced muscle strength may enhance the effects of PM2.5 constituents on cardiovascular damage.
Keywords: PM2.5 constituents, Cardiovascular disease, Muscle strength
Highlights
-
•
A nationwide cohort was used with spatiotemporal data of PM2.5 and constituents.
-
•
Long-term exposure to PM2.5 constituents elevated incidence of CVDs.
-
•
Nitrate was the most detrimental component to cardiovascular health.
-
•
Individuals with low muscle strength were susceptible to air pollution.
1. Introduction
The burden of cardiovascular diseases (CVDs) constitutes a significant proportion of the global burden of noncommunicable diseases (NCDs), encompassing over 44 % of all NCDs [1]. In China, the prevalence of CVDs has consistently increased since the 1990s, establishing CVD as the foremost cause of mortality and socioeconomic strain [2]. Identifying the risk factors for CVDs is critical for formulating targeted prevention strategies to mitigate their impact. Notably, fine particulate matter (i.e., PM2.5) is a widely recognized causal factor leading to increased incidence and mortality of CVD [3]. PM2.5 has undergone extensive scrutiny because of its adverse effects on cardiovascular health in epidemiological and toxicological research [3,4].
However, prior research on features of PM2.5 pollution and its source revealed that PM2.5 comprises a complex mixture of elements with varying concentrations, poisoning, and temporal fluctuations. For example, the primary chemical composition of PM2.5 varies seasonally in China, with nitrate as the principal pollutant in spring and organic carbon as the dominant contributor in winter [5]. Therefore, it is important to discern the specific toxic components within PM2.5 to enhance our comprehension of the biological mechanisms underlying cardiovascular damage attributable to particular PM2.5 components. Nevertheless, existing studies yield inconsistent results regarding the identification of major toxic components for cardiovascular health. Among all the PM2.5 components, only black carbon (BC) and nitrate (NO3−) were found to be significantly linked with increased mortality from ischemic heart disease, according to a cohort study conducted in California involving almost 100,000 women [6]. The US Medicare cohort study demonstrated a noteworthy association between nitrate (NO3−) exposure and an increased risk of CVD mortality [7], while findings from both the Women's Health Initiative cohort and the Multi-Ethnic Study of Atherosclerosis cohort only indicated that sulfate (SO42−) was associated with stronger CVD-related responses than other components [8]. These inconsistent findings suggest that predominant PM2.5 components linked to cardiovascular damage may vary across regions and countries, and it is imperative to conduct further research in China, given the country's complex pollution emissions and the existence of regional disparities in CVD burdens.
In addition, loss of muscle mass is a major sign of frailty and sarcopenia in elderly individuals, and it can have a variety of detrimental effects on health. For example, research has shown that a decrease in muscle strength is linked to an increased risk of CVDs [9,10]. Interestingly, recent research observed negative associations between air pollution and muscle strength, suggesting that muscle strength may modulate the deleterious effects of air pollution through potential pathways involving nervous system impairment and inflammation [11,12]. Moreover, experimental studies further suggest that air pollution and reduced muscle strength may influence the development of CVDs through shared biological mechanisms, including oxidative stress and inflammation [13]. However, population-based evidence estimating the combined effects of both low muscle strength and PM2.5 components on CVDs is lacking, especially in countries or regions with high PM2.5 pollution like China. It is also unclear whether enhanced muscle strength modifies the link between air pollution and CVDs, which might provide novel insights for mitigating the deleterious effects of PM2.5 components on cardiovascular health. Thus, using long-term survey results collected by the China Health and Retirement Longitudinal Study (CHARLS), this study seeks to quantify associations between PM2.5 and its chemical components and the incidence of CVDs and to further investigate the joint impact of muscle strength and PM2.5 components upon CVD onset.
2. Methods
2.1. Study population
The data of the subjects in our research were sourced from the CHARLS. The CHARLS constitutes a nationwide prospective cohort study focusing on Chinese middle-aged and older adults aged 45 years and above, with a baseline survey conducted in 2011, followed by subsequent surveys in 2013, 2015, and 2018. These surveys encompassed visits to 150 counties spread across 28 provinces throughout China. Further information on the design, methodology, and participant characteristics of the CHARLS study can be found elsewhere [14]. Each participant provided informed consent, and the study methodology was examined and approved by Peking University's Ethics Committee Ethical Review Committee.
For this retrospective cohort study, we analyzed CHARLS data from 2011 to 2018. Initially, the baseline assessment included 17,708 participants. We subsequently excluded individuals who were ineligible for age younger than 45 years (n = 602), those who had heart disease or stroke at 2011 (n = 2206), those whose follow-up data on CVDs were lacking in 2013, 2015, and 2018 (n = 1231), those with incomplete covariate information (n = 3218), and those lacking data on muscle strength (n = 38). Following these exclusions, the final analysis involved 10,413 individuals (Fig. S1).
2.2. Exposure assessment
The datasets of PM2.5 and its constituents were sourced from the Tracking Air Pollution in China (TAP) platform (http://tapdata.org.cn). A thorough description of the TAP dataset has been previously described in the literature [15]. In summary, TAP has developed a nearly real-time dataset on the estimated daily PM2.5 concentration and the chemical composition of PM2.5 since 2000 after integrating multisource fusion data [16]. The TAP dataset provides sulfate (SO42−), nitrate (NO3−), ammonium (NH4+), organic matter (OM), and black carbon (BC) data at a 10-km resolution. The annual average concentration of each pollutant was evaluated at the county level during the study period of 2011–2018 and further assigned to each participant to assess the individual exposure level during the follow-up.
2.3. Outcome
The outcome of this study focused on the incidence of CVD events, including heart disease and stroke, by self-reported questionnaire, which is consistent with previous research [17,18]. In cases where a participant was first reported to have physician-diagnosed CVDs in a subsequent survey, the midpoint between this survey and the nearest preceding survey was employed to calculate the follow-up time. For individuals without CVDs in all subsequent surveys, the date of the last survey was utilized to determine the end time of follow-up.
2.4. Muscle strength assessment
The muscle strength assessment included the evaluation of dominant hand grip strength and chair-rising time [19] using recommendations from the Asian Working Group for Sarcopenia 2019 guidelines. Within the CHARLS study, grip strength for each hand was measured twice, followed by recording the maximum value from the dominant hand. Chair-rising time was determined by timing the participant as they stood and sat down in a chair as quickly as possible without using their arms, with the total time taken for five repetitions recorded as the chair-rising time. The criteria for determining low muscle strength were a chair rise time of 12 s or more, or a dominant hand grip strength of less than 28 kg for males and less than 18 kg for females [20].
2.5. Covariates
Based on earlier research on the relationship between air pollution and CVD, potential confounders were taken into account throughout the statistical analysis [18,21]. The demographic factors included age, sex, marital status (married, unmarried, and other), and education level (primary school and below, middle school, and college and above). Body mass index (BMI), smoking status (yes or no), alcohol consumption (yes or no), and physician-diagnosed diabetes, dyslipidemia and hypertension were considered health variables. Additionally, the annual mean temperature was incorporated into the model because previous studies highlighted the impact of temperature on CVD [22].
2.6. Statistical analysis
For continuous variables, the mean and standard deviation (SD) were used to characterize the participants' baseline characteristics; and for categorical variables, the percentage was utilized. The Cox proportional hazards model was adopted with time-varying exposures on a 1-year (annual) scale [23], accounting for spatiotemporal variations in PM2.5 and its constituents [24]. Hazard ratios (HRs) and 95 % confidence intervals (95 % CIs) for the incidence of CVDs were calculated using each SD increase in the PM2.5, BC, OM, NH4+, NO3−, and SO42− concentrations. We constructed three models adjusting for different variables: the crude model included only pollutants; Model 1 was adjusted for age, sex, marital status, education, BMI, smoking, alcohol, history of diabetes, history of dyslipidemia, history of hypertension, and environmental temperature; and Model 2 was further adjusted for muscle strength. To explore the dose‒response relationships between pollutants and CVDs, we utilized restricted cubic spline (RCS) plots for analysis and selected 3 knots to fit RCS models based on the Akaike Information Criterion [25]. To validate associations between mixed exposure to PM2.5 compositions and the incidence of CVD, we employed the Quantile g-computation (QGC) model for mixture analysis. The QGC model is a parameter-based statistical method that combines Weighted Quantile Sum (WQS) and g computation. In contrast to other methods that estimate mixture effects, the QGC model not only effectively addresses the multicollinearity issue in mixed pollutant exposures but also assigns positive or negative weights to each pollutant in the mixture [26]. In the QGC model, the sum of positive and negative weights for each pollutant equals 1, enabling the identification of significant components within the PM2.5 mixture on the basis of the weights assigned by the model.
The associations between muscle strength and CVD incidence were also assessed. Moreover, we categorized participants into four subgroups based on their pollutant exposure and muscle strength to examine the joint associations with CVDs, and then a trend test across these four subgroups was conducted.
To evaluate the robustness of our findings, sensitivity analyses were conducted by excluding participants who developed CVDs in the two years preceding follow-up. Additionally, we employed the cumulative exposure concentration of the pollutants for each participant to assess the relationship between the pollutant and cardiovascular morbidity. Moreover, physical activity was additionally adjusted for in the multivariable models among participants with available physical activity data. Physical activity status was coded as a binary variable: ever vs. never.
All analyses were performed using R (version: 4.3.2; R Foundation for Statistical Computing), and all the statistical tests were two-sided, with P values < 0.05 considered statistically significant.
3. Results
3.1. Descriptive statistics
Table 1 describes the baseline characteristics of the study population. The study included 10,413 participants with an average age of 58.50 years (SD: 9.25 years). Among the participants, 48.48 % were male, 71.40 % had completed secondary school education or higher, 21.32 % had hypertension, 4.87 % had diabetes, and 7.45 % had hyperlipidemia. The mean BMI of all participants was 23.24 kg/m2 (SD: 3.54 kg/m2). Moreover, 6986 participants (67.09 %) had normal muscle strength, whereas 3427 participants (32.91 %) had low muscle strength.
Table 1.
Baseline characteristics of the study participants.
| Characteristic | Total (N = 10413) | Non-CVD (N = 8442) | Incident CVDs (N = 1971) |
|---|---|---|---|
| Age (years) | 58.50 ± 9.25 | 58.10 ± 9.28 | 60.26 ± 8.94 |
| BMI (kg/cm2) | 23.24 ± 3.54 | 23.08 ± 3.46 | 23.95 ± 3.74 |
| Gender | |||
| male | 5048 (48.48) | 4206 (49.82) | 842 (42.72) |
| female | 5365 (51.52) | 4236 (50.18) | 1129 (57.28) |
| Marriage status | |||
| married | 8682 (83.38) | 7055 (83.57) | 1627 (82.55) |
| unmarried | 81 (0.78) | 71 (0.84) | 10 (0.51) |
| other | 1650 (15.84) | 1316 (15.59) | 334 (19.94) |
| Education level | |||
| primary school and below | 2978 (28.60) | 2349 (27.83) | 629 (31.91) |
| middle school | 4208 (40.41) | 3446 (40.82) | 762 (38.66) |
| collage and above | 3227 (30.99) | 2647 (31.35) | 580 (29.43) |
| Hypertension | |||
| No | 8193 (78.68) | 6931 (82.10) | 1262 (64.03) |
| Yes | 2220 (21.32) | 1511 (17.90) | 709 (35.97) |
| Diabetes | |||
| No | 9906 (95.13) | 8090 (95.83) | 1816 (92.14) |
| Yes | 507 (4.87) | 352 (4.17) | 155 (7.86) |
| Hyperlipidemia | |||
| No | 9637 (92.55) | 7930 (93.94) | 1707 (86.61) |
| Yes | 776 (7.45) | 512 (6.06) | 264 (13.39) |
| Drinking status | |||
| No | 6263 (60.15) | 5029 (59.33) | 1234 (62.61) |
| Yes | 4150 (39.85) | 3413 (40.67) | 737 (37.39) |
| Smoking status | |||
| No | 6240 (59.93) | 5009 (59.33) | 1231 (62.46) |
| Yes | 4173 (40.07) | 3433 (40.67) | 740 (37.54) |
| Muscle strength | |||
| Normal | 6986 (67.09) | 5801 (68.72) | 1185 (60.12) |
| Low | 3427 (32.91) | 2641 (31.28) | 786 (39.88) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); CVD, cardiovascular disease; SD, standard deviation. The data are expressed as the means ± SDs or numbers (percentages).
The exposure levels of the study population to PM2.5 and its constituents are shown in Table S1 for the years 2011 through 2018. During the follow-up period, the average exposure concentrations for PM2.5, BC, OM, NH4+, NO3−, and SO42− were 50.69 μg/m3, 2.50 μg/m3, 12.35 μg/m3, 7.68 μg/m3, 10.96 μg/m3, and 9.69 μg/m3, respectively.
3.2. Relationships between PM2.5 and its components and the incidence of CVDs
During the follow-up period spanning from 2011 to 2018, 1971 participants developed CVDs in this study. The associations between PM2.5 and its five constituents and the incidence of CVDs are presented in Table 2. We identified a significant positive relationship between PM2.5 exposure and the incident risk of CVDs, with a HR for CVDs of 1.15 (95 % CI: 1.09, 1.21) for each SD increase in PM2.5. Each component of PM2.5 was also associated with incident CVDs, with an increase of 17 % (95 % CI: 11 %, 24 %) risk for OM, an increase of 16 % (95 % CI: 9 %, 22 %) for BC, an increase of 13 % (95 % CI: 7 %, 19 %) for NH4+, an increase of 14 % (95 % CI: 9 %, 22 %) for NO3−, and an increase of 13 % (95 % CI: 7 %, 19 %) for SO42−.
Table 2.
Associations of PM2.5 and its components (per SD increment) with the incidence of CVD.
| HR (95 % CI) |
|||
|---|---|---|---|
| Crude Mode | Model1 | Model2 | |
| PM2.5 | 1.19 (1.13–1.26) | 1.15 (1.10–1.22) | 1.15 (1.09–1.21) |
| BC | 1.16 (1.09–1.23) | 1.16 (1.10–1.23) | 1.16 (1.09–1.22) |
| OM | 1.18 (1.12–1.25) | 1.18 (1.12–1.24) | 1.17 (1.11–1.24) |
| NH4+ | 1.15 (1.09–1.21) | 1.13 (1.07–1.19) | 1.13 (1.07–1.19) |
| NO3− | 1.17 (1.12–1.23) | 1.14 (1.08–1.12) | 1.14 (1.08–1.19) |
| SO42− | 1.11 (1.05–1.18) | 1.13 (1.07–1.20) | 1.13 (1.07–1.19) |
Abbreviations: HR, hazard ratio; CI, confidence interval; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm; BC, black carbon; OM, organic matter; NH4+, ammonium; NO3−, nitrate; SO42−, sulfate.
Model 1 was adjusted for age, sex, marital status, educational level, smoking status, drinking status, body mass index and history of hypertension, dyslipidemia, diabetes and environmental temperature.
Model 2 was adjusted as Model 1 with further adjustment for muscle strength status.
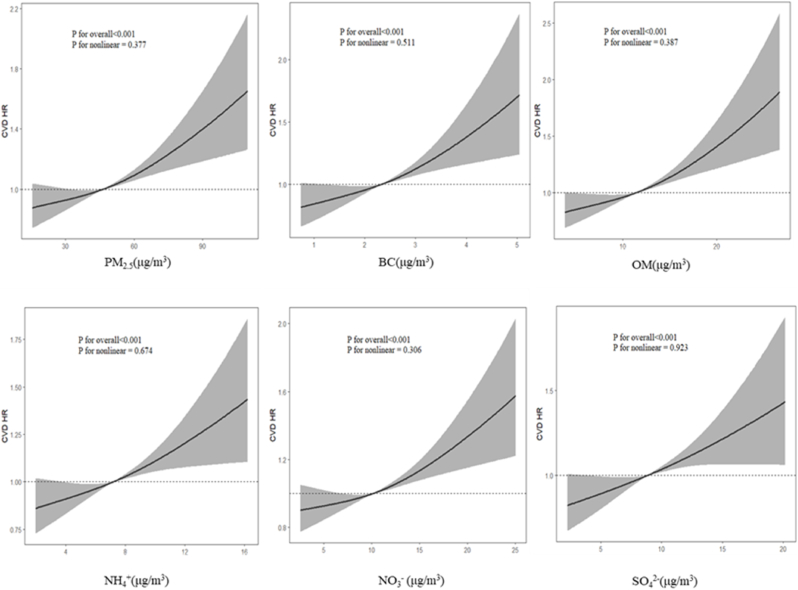
In the dose‒response relationships, Figure_1 illustrates a positive near-linear association for each air pollutant (P for nonlinearity >0.05), indicating that the risk of CVD development increases as the concentrations of pollutants increase.
Figure_1.
Dose‒response curves of PM2.5, BC, OM, NH4+, NO3− and SO42− with the incidence of CVD. All the models were adjusted for age, sex, marital status, educational level, smoking status, drinking status, body mass index, history of hypertension, dyslipidemia, diabetes, environmental temperature and muscle strength status.
The QGC modeling indicated that the five components of PM2.5 as a whole were associated with an increased incidence of CVDs. The HRs (95 % CI) for CVDs was 1.09 (95 % CI: 1.04, 1.14) for each simultaneous increase in the interquartile range for each of the five components of PM2.5 in Model 2 (Table S2). Furthermore, the QGC model revealed that NO3− had the largest positive weight, which may indicate that NO3− is the most detrimental component to cardiovascular health among the five components of PM2.5 (Fig. S2).
3.3. Joint associations of PM2.5 and its components and muscle strength with CVD incidence
In this study, a positive relationship was shown between decreasing muscle strength and an increased incidence of CVDs (Table S3). In the joint association analysis, individuals with high exposure to pollution and low muscle strength had the greatest risk of incident CVD (P for trend <0.05) (Figure_2).
Figure_2.
Joint effects of muscle strength and exposure to PM2.5 and its constituents on CVD. PM2.5 and its components are classified into high and low groups using cumulative exposure medians. All the models were adjusted for age, sex, marital status, educational level, smoking status, drinking status, body mass index, history of hypertension, dyslipidemia and diabetes and environmental temperature.
3.4. Sensitivity analyses
Sensitivity analyses showed that, even after individuals whose initial occurrence occurred in the first two years of follow-up were removed, the associations between PM2.5 and its chemical constituents and the risk of developing CVDs remained strong (Table S4–S5). Additionally, the associations did not substantially change when the 1-year scale exposure of each pollutant was replaced by the cumulative concentration of each pollutant during the whole period of follow-up (Table S6–S7). Furthermore, similar results were observed after further adjustment for physical activity (Table S8–S9).
4. Discussion
In a nationwide cohort study conducted among Chinese adults, prolonged exposure to PM2.5 and its components was linked to an increased risk of CVDs, with NO3− emerging as the primary contributor to this association. Additionally, diminished muscle strength seemed to augment the associations of pollutant exposure with CVDs. To the best of our understanding, this is the first prospective epidemiological investigation to document the negative effects of combined exposure to PM2.5 components and reduced muscle strength on CVD risk. These findings offer valuable insights into potential strategies for attenuating the detrimental effects of PM2.5 components on cardiovascular health through the enhancement of muscle strength.
This investigation revealed a significant relationship between the incidence of CVDs and prolonged exposure to PM2.5. However, the HR was lower in the main model (Model 2) than in previous studies [24,27]. For example, in a previous China-PAR study, which included 127,840 Chinese adults in 15 provinces across China, the HRs for CVD incidence was reported to be 1.25 (95 % CI: 1.22, 1.28) for a 10 μg/m3 increase in the PM2.5 concentration [24]. Similarly, in a separate investigation focusing on long-term PM2.5 exposure in the China Family Panel, which included more than 30,000 adults and 9000 children in 162 county-level units in 25 provinces and cities, the HR was 1.29 (95 % CI: 1.15, 1.45) for each IQR (27.90 μg/m3) increase in PM2.5 [28]. In contrast, our findings revealed an HR of 1.15 (95 % CI: 1.09, 1.21) for each SD (21.68 μg/m3) increase in the PM2.5 concentration. Potential explanations for this discrepancy may originate from various demographic features of the study populations and disparities in the delineation of outcome definitions.
For the five components of PM2.5 in this study, we found positive associations between them and the incidence of CVDs, with dose‒response relationships suggesting an approximately linear relationship. BC is derived mainly from inadequate combustion of fuels [29], and it has been well established in previous studies that long-term exposure to BC raises the risk of developing CVDs. According to experimental research, BC can cause oxidative stress and inflammation, which can have a negative impact on cardiovascular health [30]. A study encompassing nine cities in northern China reported a significant association between OM and CVD development, which aligns with our observations [28]. However, in another national cohort study in China, no discernible association between OM and CVD incidence was identified [27]. This incongruity could be attributed to variations in pollution sources and pathways, resulting in divergent effects of OM on CVD risk. NH4+, NO3−, and SO42− are categorized as secondary inorganic aerosol constituents, and their exposure could impact CVD events by activating the hypothalamus‒pituitary‒adrenal axis [31]. While the associations of these secondary aerosol constituents with acute cardiovascular mortality have been extensively documented, evidence regarding their long-term effects on cardiovascular health remains inadequate. Both our study and previous research support that long-term exposure to inorganic ions is significantly associated with elevated risks of CVD [28], and further investigations are warranted to comprehensively explore the chronic hazards associated with these ions.
People are usually exposed to various PM2.5 components in real-world situations, underscoring the importance of comprehending the collective effects of these exposures on CVD incidence. This understanding is pivotal in discerning which of the five components exert the most significant influence on cardiovascular health. Consequently, the Quantile g-Computation model was used to analyze the combined effects of PM2.5 component mixtures and to ascertain the contribution of each pollutant [26]. Our findings indicate a positive association between exposure to PM2.5 component mixtures and an elevated risk of CVDs, with NO3− emerging as the primary contributing component. This may be attributed to the overall downward trend in atmospheric pollutants following the implementation of air pollution control measures. However, there are significant differences in the rates of change between the chemical components closely related to primary emissions and secondary inorganic components. This has resulted in nitrate-dominated secondary pollutants becoming the primary harmful constituents in PM2.5. A recent review proposed that nitrate control strategies should prioritize the reduction of atmospheric oxidation and ammonia emissions [32], suggesting that these targeted interventions may yield more effective reductions in air pollution-induced CVD incidence.
Furthermore, this study investigated the combined effects of air pollution and muscle strength on CVD incidence. Individuals with elevated pollutant exposure and low muscle strength had an increased risk of CVDs. Research has demonstrated a protective association between muscle strength and the risk of cardiovascular disease, with potential underlying biological mechanisms encompassing inflammation, oxidative stress, muscle mitochondrial dysfunction, and metabolic disorders [[33], [34], [35]]. Concurrently, numerous studies have highlighted oxidative stress and inflammation as major mechanisms through which PM2.5 contributes to the onset of CVD [13]. These findings suggest that exposure to PM2.5 components, alongside decreased muscle strength, may influence CVD risk via several shared biological mechanisms. According to the findings of this study, maintaining normal muscle strength may be essential for reducing the risk of CVD caused by exposure to PM2.5 and its chemical constituents. Consequently, further randomized trials and superior cohort studies are needed to validate the effectiveness of enhancing muscle strength in diminishing adverse cardiovascular outcomes triggered by PM2.5 exposure.
This research has a number of advantages. First, the national samples from the CHARLS cohort study improved the generalization of our findings to other middle-aged and older groups in China. Furthermore, we employed the QGC model, adept at handling both linear and nonlinear relationships among various exposures, to examine the effects of co-exposure to the five components on the incidence of CVDs. This approach enabled us to discern which components exerted the most substantial impact on CVDs and to develop priority strategies for air quality improvement that target PM2.5 components. Finally, this study identified the combined effects of muscle strength and PM2.5 and its components on CVDs, offering novel insights into populations susceptible to air pollution.
Despite the aforementioned strengths, several limitations need to be addressed. First, the study did not account for greenness, proximity to main roads, or other potential covariates that could impact the characteristics of air pollution exposure. These factors should be taken into account in future studies. Second, we only investigated the impact of baseline muscle strength levels on the association between long-term PM2.5 exposure and CVDs. Future studies may explore the influence of changes in muscle strength during follow-up to provide a more comprehensive understanding of this relationship. Despite these limitations, the present study enhances our comprehension of the associations between PM2.5 and its components and CVDs and sheds light on the combined effects of muscle strength and air pollution on CVDs.
5. Conclusion
This study indicated a substantial connection between prolonged exposure to PM2.5 and its constituents and an elevated incidence of CVDs. Additionally, decreased muscle strength amplifies the detrimental effects of PM2.5 components on cardiovascular health. These findings shed light on the potential for maintaining normal muscle strength to lower the risk of CVDs related to PM2.5 and its components. To confirm these results and investigate the underlying processes underlying the combined impact of muscle strength and air pollution on the incidence of CVDs, more research is required.
CRediT authorship contribution statement
Guangbin Sun: Writing – original draft, Methodology, Formal analysis, Data curation. Zeyu Chen: Writing – original draft, Methodology, Formal analysis. Hongyue Sun: Writing – review & editing, Methodology, Data curation. Ze Yang: Methodology. Dongfang Zhang: Writing – review & editing. Liwen Zhang: Writing – review & editing. Miao Liu: Writing – review & editing, Supervision. Xueli Yang: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Patient consent for publication
Not applicable.
Availability of data and materials
The data that support the findings of this study are available from the China Health and Retirement Longitudinal Study (CHARLS) (https://charls.pku.edu.cn) and the Tracking Air Pollution in China (TAP) platform (http://tapdata.org.cn), but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are, however, available from the correspondence author Xueli Yang, upon reasonable request and with the permission of the CHARLS and TAP.
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations. The CHARLS protocol was approved by the Institutional Review Board of Peking University (No. IRB00001052-11015). Informed consent has been acquired from all participants before the participation.
Consent to publication
Not applicable.
Funding
This work was supported by the Fundamental Research Funds for Higher Education of Tianjin Municipal Education Commission (Grant No. 2021ZD038), and the National Key Research and Development Program of China (Grant No. 2022YFC2503605).
Declaration of competing interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgement
We thank all the participants, investigators, and assistants of the China Health and Retirement Longitudinal Study (CHARLS) for survey, design and data collection at Peking University.
Handling Editor: Dr D Levy
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcrp.2025.200495.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Roth G.A., Mensah G.A., Fuster V. The global burden of cardiovascular diseases and risks: a compass for global action. J. Am. Coll. Cardiol. 2020;76(25):2980–2981. doi: 10.1016/j.jacc.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Zhao D., Liu J., Wang M., Zhang X., Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat. Rev. Cardiol. 2019;16(4):203–212. doi: 10.1038/s41569-018-0119-4. [DOI] [PubMed] [Google Scholar]
- 3.Munzel T., Sorensen M., Hahad O., Nieuwenhuijsen M., Daiber A. The contribution of the exposome to the burden of cardiovascular disease. Nat. Rev. Cardiol. 2023;20(10):651–669. doi: 10.1038/s41569-023-00873-3. [DOI] [PubMed] [Google Scholar]
- 4.Lechner K., von Schacky C., McKenzie A.L., Worm N., Nixdorff U., Lechner B., et al. Lifestyle factors and high-risk atherosclerosis: pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 2020;27(4):394–406. doi: 10.1177/2047487319869400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H., Xiao Z., Chen K., Tang M., Zheng N., Li P., et al. Spatial and temporal distribution, chemical characteristics, and sources of ambient particulate matter in the Beijing-Tianjin-Hebei region. Sci. Total Environ. 2019;658:280–293. doi: 10.1016/j.scitotenv.2018.12.164. [DOI] [PubMed] [Google Scholar]
- 6.Ostro B., Hu J., Goldberg D., Reynolds P., Hertz A., Bernstein L., et al. Associations of mortality with long-term exposures to fine and ultrafine particles, species and sources: results from the California Teachers Study Cohort. Environ. Health Perspect. 2015;123(6):549–556. doi: 10.1289/ehp.1408565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kazemiparkouhi F., Honda T., Eum K.D., Wang B., Manjourides J., Suh H.H. The impact of Long-Term PM (2.5) constituents and their sources on specific causes of death in a US Medicare cohort. Environ. Int. 2022;159 doi: 10.1016/j.envint.2021.106988. [DOI] [PubMed] [Google Scholar]
- 8.Lippmann M. Toxicological and epidemiological studies of cardiovascular effects of ambient air fine particulate matter (PM2.5) and its chemical components: coherence and public health implications. Crit. Rev. Toxicol. 2014;44(4):299–347. doi: 10.3109/10408444.2013.861796. [DOI] [PubMed] [Google Scholar]
- 9.Gao K., Cao L.F., Ma W.Z., Gao Y.J., Luo M.S., Zhu J., et al. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: findings from the China health and retirement longitudinal study. eClinicalMedicine. 2022;44 doi: 10.1016/j.eclinm.2021.101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo X., Li X., Tang K., Zhao R., Wu M., Wang Y., et al. Sarcopenia and cardiovascular diseases: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14(3):1183–1198. doi: 10.1002/jcsm.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H., Zhang S., Yao X., Meng L., Lin Y., Guo F., et al. Does physical activity attenuate the association between ambient PM (2.5) and physical function? Sci. Total Environ. 2023;874 doi: 10.1016/j.scitotenv.2023.162501. [DOI] [PubMed] [Google Scholar]
- 12.Lin H., Guo Y., Ruan Z., Kowal P., Di Q., Zheng Y., et al. Association of indoor and outdoor air pollution with hand-grip strength among adults in six low- and middle-income countries. J Gerontol A Biol Sci Med Sci. 2020;75(2):340–347. doi: 10.1093/gerona/glz038. [DOI] [PubMed] [Google Scholar]
- 13.Benincasa G., Coscioni E., Napoli C. Cardiovascular risk factors and molecular routes underlying endothelial dysfunction: novel opportunities for primary prevention. Biochem. Pharmacol. 2022;202 doi: 10.1016/j.bcp.2022.115108. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y., Hu Y., Smith J.P., Strauss J., Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS) Int. J. Epidemiol. 2014;43(1):61–68. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng G., Xiao Q., Liu S., Liu X., Cheng J., Zheng Y., et al. Tracking air pollution in China: near real-time PM(2.5) retrievals from multisource data fusion. Environ. Sci. Technol. 2021;55(17):12106–12115. doi: 10.1021/acs.est.1c01863. [DOI] [PubMed] [Google Scholar]
- 16.Liu C., Cao G., Li J., Lian S., Zhao K., Zhong Y., et al. Effect of long-term exposure to PM (2.5) on the risk of type 2 diabetes and arthritis in type 2 diabetes patients: evidence from a national cohort in China. Environ. Int. 2023;171 doi: 10.1016/j.envint.2023.107741. [DOI] [PubMed] [Google Scholar]
- 17.Li H., Zheng D., Li Z., Wu Z., Feng W., Cao X., et al. Association of depressive symptoms with incident cardiovascular diseases in middle-aged and older Chinese adults. JAMA Netw. Open. 2019;2(12) doi: 10.1001/jamanetworkopen.2019.16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mai X., Zhou H., Li Y., Huang X., Yang T. Associations between ambient fine particulate (PM(2.5)) exposure and cardiovascular disease: findings from the China Health and Retirement Longitudinal Study (CHARLS) Environ. Sci. Pollut. Res. Int. 2022;29(9):13114–13121. doi: 10.1007/s11356-021-16541-3. [DOI] [PubMed] [Google Scholar]
- 19.Chen L.K., Woo J., Assantachai P., Auyeung T.W., Chou M.Y., Iijima K., et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020;21(3):300–307 e302. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Qiu S., Cai X., Yuan Y., Xie B., Sun Z., Wang D., et al. Muscle strength and prediabetes progression and regression in middle-aged and older adults: a prospective cohort study. J Cachexia Sarcopenia Muscle. 2022;13(2):909–918. doi: 10.1002/jcsm.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen N., Ma L.L., Zhang Y., Yan Y.X. Association of household solid fuel use and long-term exposure to ambient air pollution with estimated 10-year high cardiovascular disease risk among postmenopausal women. Environ. Pollut. 2023;342 doi: 10.1016/j.envpol.2023.123091. [DOI] [PubMed] [Google Scholar]
- 22.Liu M., Xue X., Zhou B., Zhang Y., Sun B., Chen J., et al. Population susceptibility differences and effects of air pollution on cardiovascular mortality: epidemiological evidence from a time-series study. Environ. Sci. Pollut. Res. Int. 2019;26(16):15943–15952. doi: 10.1007/s11356-019-04960-2. [DOI] [PubMed] [Google Scholar]
- 23.Andersen P.K., Gill R.D. Cox's regression model for counting processes: a large sample study. Ann. Stat. 1982;10(4):1100–1120. [Google Scholar]
- 24.Liang F., Liu F., Huang K., Yang X., Li J., Xiao Q., et al. Long-term exposure to fine particulate matter and cardiovascular disease in China. J. Am. Coll. Cardiol. 2020;75(7):707–717. doi: 10.1016/j.jacc.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz J., Coull B., Laden F., Ryan L. The effect of dose and timing of dose on the association between airborne particles and survival. Environ. Health Perspect. 2008;116(1):64–69. doi: 10.1289/ehp.9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keil A.P., Buckley J.P., O'Brien K.M., Ferguson K.K., Zhao S., White A.J. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ. Health Perspect. 2020;128(4) doi: 10.1289/EHP5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L., Zhang Y., Yang Z., Luo S., Zhang Y. Long-term exposure to fine particulate constituents and cardiovascular diseases in Chinese adults. J. Hazard Mater. 2021;416 doi: 10.1016/j.jhazmat.2021.126051. [DOI] [PubMed] [Google Scholar]
- 28.Wen F., Li B., Cao H., Li P., Xie Y., Zhang F., et al. Association of long-term exposure to air pollutant mixture and incident cardiovascular disease in a highly polluted region of China. Environ. Pollut. 2023;328 doi: 10.1016/j.envpol.2023.121647. [DOI] [PubMed] [Google Scholar]
- 29.Kirrane E.F., Luben T.J., Benson A., Owens E.O., Sacks J.D., Dutton S.J., et al. A systematic review of cardiovascular responses associated with ambient black carbon and fine particulate matter. Environ. Int. 2019;127:305–316. doi: 10.1016/j.envint.2019.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei X., Chen R., Wang C., Shi J., Zhao Z., Li W., et al. Personal fine particulate matter constituents, increased systemic inflammation, and the role of DNA hypomethylation. Environ. Sci. Technol. 2019;53(16):9837–9844. doi: 10.1021/acs.est.9b02305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niu Y., Chen R., Xia Y., Cai J., Ying Z., Lin Z., et al. Fine particulate matter constituents and stress hormones in the hypothalamus-pituitary-adrenal axis. Environ. Int. 2018;119:186–192. doi: 10.1016/j.envint.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 32.Wang H., Lu K., Tan Z., Chen X., Liu Y., Zhang Y. Formation mechanism and control strategy for particulate nitrate in China. J. Environ. Sci. (China) 2023;123:476–486. doi: 10.1016/j.jes.2022.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Cohen S., Nathan J.A., Goldberg A.L. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015;14(1):58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 34.de Lima T.R., Sui X., de Lima L.R.A., Silva D.A.S. Muscle strength and its association with cardiometabolic variables in adolescents: does the expression of muscle strength values matter? World J Pediatr. 2021;17(6):597–608. doi: 10.1007/s12519-021-00460-x. [DOI] [PubMed] [Google Scholar]
- 35.Schaap L.A., Pluijm S.M., Deeg D.J., Harris T.B., Kritchevsky S.B., Newman A.B., et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64(11):1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the China Health and Retirement Longitudinal Study (CHARLS) (https://charls.pku.edu.cn) and the Tracking Air Pollution in China (TAP) platform (http://tapdata.org.cn), but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are, however, available from the correspondence author Xueli Yang, upon reasonable request and with the permission of the CHARLS and TAP.