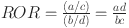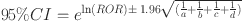Abstract
Aims
This study aims to investigate the safety profile of Esketamine, with a particular focus on comparing adverse events (AEs) between adults (< 65 years) and older adults (≥ 65 years) using data from the FDA Adverse Event Reporting System (FAERS).
Methods
We conducted a comprehensive analysis of FAERS data from 2019 to 2024, identifying 6,452 Esketamine-related AE reports. After removing data without age information, these reports were categorized into two age groups: 536 from older adults and 3,566 from younger adults. Reporting odds ratios (RORs) were calculated to determine the relative risk of specific AEs in each age group.
Results
At system Organ Class (SOC)level, the Esketamine-related AEs of both adults group and older adults group were seen in 17 organ systems. The analysis revealed significant differences in AE profiles of psychiatric disorders between the two age groups. Older adults had a higher incidence of dissociation, suicidal ideation, depression, and anxiety, compared to younger adults. Additionally, older adults reported more general and administration site conditions, suggesting a greater susceptibility to systemic and local reactions. Gastrointestinal and respiratory disorders were less frequent in older adults, but their potential impact remains critical.
Conclusions
The findings highlight a higher risk of severe psychiatric and general AEs in older adults treated with Esketamine, necessitating careful patient selection, monitoring, and tailored treatment protocols.
Keywords: Esketamine, FAERS, Real-world data analysis, Adverse events, Older adults
Introduction
Major Depressive Disorder (MDD), one of the most prevalent mental health conditions globally, has seen a 49.7% increase in incidence over the past three decades [1].Critically, 30–40% of MDD patients develop Treatment-Resistant Depression (TRD) [2], defined as failure to achieve remission after ≥ 2 adequate antidepressant trials. These individuals endure persistent symptoms, functional impairment in social/occupational domains, and a 2.8-fold elevated suicide mortality risk [3]. Esketamine is the S-enantiomer of ketamine, a US Food and Drug Administration (FDA)-approved anesthetic. Its nasal spray formulation was approved as an adjunctive treatment for TRD in adults by the FDA and the European Medicine Agency (EMA) in 2019 [4, 5]. As a non-selective, non-competitive N-methyl-D-aspartate receptor antagonist, Esketamine offers a distinct pharmacological profile compared to conventional antidepressants [6]. With its rapid onset of action and unique mechanism of action, several clinical trials have demonstrated the efficacy of Esketamine in rapidly reducing depressive symptoms, including suicidal ideation, compared to placebo [7]. Moreover, its effects appear to be sustained over time, offering a promising long-term treatment option for individuals struggling with TRD [8].
However, other researchers express significant concern regarding Esketamine’s abuse liability and potential for addiction. Such concerns are warranted given that its parent compound, ketamine (a common street drug also known as “special K”), is known to produce reinforcing psychoactive and behavioral effects that lead to abuse and dependence [9]. As a derivative of ketamine, Esketamine also carries a risk of addiction, particularly when administered intravenously [10]. Poor dosage control can easily lead to addiction in patients. Therefore, Esketamine treatment requires careful patient selection, monitoring, and management of potential adverse effects.
Furthermore, older patients with TRD typically have lower response and remission rates and poorer tolerability to antidepressant treatments compared to younger patients [11]. Significant concerns have arisen regarding the long-term safety and tolerability of Esketamine among the older adults following its approval. Presently, the bulk of evidence regarding Esketamine’s safety on older patients stems primarily from its development programs and the approval of randomized clinical trials (RCTs) [12]. These trials, while providing initial insights, were typically limited by small sample sizes and short monitoring durations. For instance, a Phase 3 study involving 137 older adults reported adverse events (AEs) in 70.8% of patients receiving Esketamine nasal spray plus antidepressant versus 60.0% in the antidepressant plus placebo group [13]. While the AE profiles were generally similar, some tolerability differences were noted. A post-hoc analysis comparing long-term safety in younger (n = 624) and older (n = 178) patients with treatment-resistant depression suggested comparable safety profiles between age groups within the context of these trials [11]. However, the inherent limitations of small patient numbers and short-term follow-up in these registration RCTs preclude definitive conclusions on the comprehensive safety and long-term tolerability of Esketamine in the older population, underscoring the critical need for post-marketing surveillance data.
The FDA Adverse Event Reporting System (FAERS), one of the largest global pharmacovigilance repositories, serves as the primary data source for this study. Established to support FDA post-marketing surveillance of pharmaceutical and therapeutic products, FAERS amasses spontaneous reports of adverse events from healthcare professionals, consumers, and manufacturers worldwide [14]. Over time, it has amassed a wealth of real-world data pertaining to adverse drug events. This database is particularly adept at identifying rare AEs, which may evade detection or reporting in RCTs due to their short follow-up durations, varied dosages, and dissimilar patient populations compared to real-world scenarios [15]. Given that Esketamine has been on the market for over five years, its safety profile among older adults has not been adequately examined. Thus, our study aims to analyze the AEs related to Esketamine among of adults group (< 65 years) and older adults group (≥ 65 years) based on the FAERS database and to investigate the differences in AEs between adult (< 65 years) and older adults.
Method
Data source and extraction
This retrospective pharmacovigilance investigation draws upon the FAERS database, specifically selecting ASCII data packages spanning from the second quarter of 2019 to first quarter of 2024. This timeframe aligns with the approval of Esketamine for marketing by the U.S. FDA in March 2019.
For the primarily suspected drug “Esketamine,” tailored to research requirements, we input specific fields to extract data concerning the occurrence of adverse events (AEs). The combination of PROD_AI terms (“Esketamine,” “Esketamine hydrochloride,” “Esketamine HCl,” “nasal spray Esketamine”) and DRUGNAME terms (“Spravato,” “Esketamine,” “Ketanests,” “S-Ketamine,” “Esketamine hydrochloride,” “Esketamine for pain,” “Esketamine injection/infusion liquid,” “Spravato nasal spray”) was employed to retrieve relevant information from the database.
In FAERS, duplicate reports from multiple sources are commonplace. To address this issue, duplications were identified and eliminated using the method recommended by the FDA. The most recent FDA_DT served as the temporal identifier when PRIMARYID matched. In instances where both FDA_DT and CASEID coincided, the higher PRIMARYID was prioritized to eliminate duplicate reports originating from distinct individuals or institutions. This study proceeded to encode, categorize, and localize signals for AEs based on the Medical Dictionary for Regulatory Activities (MedDRA), version 26.1, utilizing Preferred Terms (PT) and System Organ Class (SOC) classifications [16].
Data analysis
In this study, we performed a distinct disproportionality approach, reporting odds ratios (ROR), to detect adverse event (AE) signals [17]. ROR provides the advantage of identifying disproportionately high proportions of AE reporting, thereby illuminating the risks associated with Esketamine use. Additionally, we incorporated an interaction term into the model to estimate the interaction between Esketamine and age group, aiming to reveal disparities in adverse reactions between adult and older populations. Continuous variables were compared using Student t-test. P < 0.05 was considered statistically significant. The Benjamini-Hochberg false discovery rate (FDR) method was used to correct for multiple comparisons, with FDR < 0.05 considered statistically significant. Normally distributed continuous variables are presented as mean ± standard deviation (SD), non-normally distributed continuous variables are reported as median with interquartile range (IQR), categorical variables are summarized using frequencies and percentages (%). Detailed calculation formulas and specific operations are delineated in Tables 1 and 2.
Table 1.
A 2 × 2 contingency table for disproportionality analysis. Contingency table
| Target AEs | Non-target AEs | Total | |
|---|---|---|---|
| Esketamine | a | b | a + b |
| Non- Esketamine | c | d | c + d |
| Total | a + c | b + d | N = a + b + c + d |
Table 2.
ROR formulas and thresholds
| Method | Formula | Threshold |
|---|---|---|
| ROR |
|
a ≥ 3 and 95% CI (lower limit)>1 |
Results
General characteristics
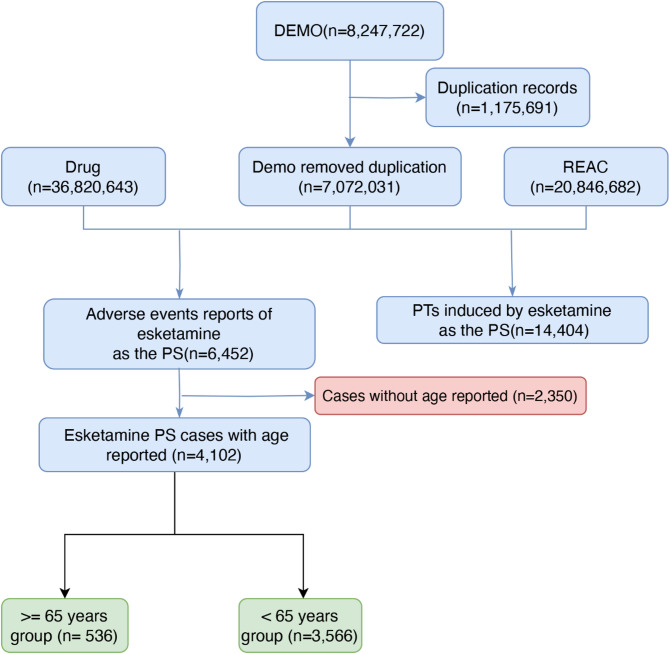
Between the second quarter of 2019 and the first quarter of 2024, we collected a total of 8,247,722 initial case reports from the FAERS database. In the initial data processing phase, duplicate records (n=1,175,691) were first excluded, resulting in 7,072,031 unique records after deduplication. As depicted in Fig. 1, through cross-referencing and screening against the Drug (n=36,820,643) and REAC (n=20,846,682) datasets, 6,452 case reports were identified as adverse event reports related to Esketamine adverse events (AEs) with Esketamine as the primary suspect (PS). Among these, cases without age reporting (n=2,350) were further excluded, leaving 4,102 Esketamine PS cases with age information. Finally, the remained cases were stratified into two age groups for further analysis, including 536 cases in the over 65 years group (≥ 65 years) and 3,566 cases in the less than 65 years group (< 65 years), as depicted in Fig. 1. Esketamine-related AE reports showed an annual increase in both adults and older adults group since 2019, likely attributable to the rising number of Esketamine users. The incidence of AEs was higher among females (63.10%) compared to males (35.51%), which was similarly reflected in both the adults group (< 65 years, female 63.12%, male 35.5%) and older adults group (≥ 65 years, female 62.69%, male 35.82%). The median age of adults group and older group were 45.0 years (33,55) and 70 years (67,74), respectively. The primary reporters were physicians (37.59%), followed by consumers (32.54%) and pharmacists (28.80%), which was also similarly reflected in both the adult group and older adults group. The majority of reports on adult and older adult group originated from the United States, totaling 2701 cases (75.74%) and 402 cases (75%), respectively. In terms of clinical outcomes, aside from the category of “other serious” events with ambiguous details, the highest proportion involved events leading to hospitalization (31.04% for adults group, 31.38% for older adults group), followed by death (7.59% for adults group, 6.88% for older adults group). The medium time to onset of adults group and older adults group was 45 days(6.50, 211)and 47.5 days (3.25,368.25), respectively. Further details are available in Table 3.
Fig. 1.
The flow diagram of selecting Esketamine-related AEs from FAERS database
Table 3.
Basic information in AEs related to Esketamine of adults group (< 65 years) and older adults group (≥ 65 years)
| All (4102) | Adults(3566) | Older adults(536) | |
|---|---|---|---|
| Year | |||
| 2019 | 288(7.00%) | 251(7.04%) | 37(6.90%) |
| 2020 | 611(14.90%) | 527(14.78%) | 84(15.67%) |
| 2021 | 676(16.46%) | 590(16.55%) | 86(16.04%) |
| 2022 | 828(20.20%) | 717(20.11%) | 111(20.71%) |
| 2023 | 1254(30.59%) | 1086(30.45%) | 168(31.34%) |
| 2024 | 445(10.85%) | 395(11.08%) | 50(9.33%) |
| Sex | |||
| Female | 2587(63.10%) | 2251(63.12%) | 336(62.69%) |
| Male | 1458(35.51%) | 1266(35.50%) | 192(35.82%) |
| Unknown | 57(1.39%) | 49(1.37%) | 8(1.49%) |
| Age, years (IQR) | 48(35.00,59.00) | 45(33.00,55.00) | 70(67.00,74.00) |
| Reporter | |||
| Physician | 1542(37.59%) | 1335(37.44%) | 207(38.62%) |
| Consumer | 1335(32.54%) | 1162(32.59%) | 173(32.28%) |
| Pharmacist | 1181(28.80%) | 1040(29.16%) | 141(26.31%) |
| Other health-professional | 38(0.93%) | 26(0.73%) | 12(2.24%) |
| Unknown | 6(0.15%) | 3(0.08%) | 3(0.56%) |
| Reported countries | |||
| United States | 3103(75.66%) | 2701(75.74%) | 402(75.00%) |
| Other | 554(13.51%) | 475(13.32%) | 79(14.74%) |
| France | 196(4.78%) | 172(4.82%) | 24(4.48%) |
| Brazil | 101(2.46%) | 94(2.64%) | 7(1.31%) |
| Spain | 79(1.93%) | 65(1.82%) | 14(2.61%) |
| Germany | 68(1.66%) | 59(1.65%) | 9(1.68%) |
| Outcomes | |||
| Other serious | 2073(56.33%) | 1793(56.22%) | 282(57.09%) |
| Hospitalization | 1144(31.09%) | 990(31.04%) | 155(31.38%) |
| Death | 276(7.50%) | 242(7.59%) | 34(6.88%) |
| Life threatening | 151(4.10%) | 137(4.30%) | 14(2.83%) |
| Disability | 28(0.76%) | 22(0.69%) | 6(1.21%) |
| Required intervention to Prevent Permanent Impairment/Damage | 8(0.22%) | 5(0.16%) | 3(0.61%) |
| Time to onset(TTO) | 45(6.00,217.00) | 45(6.50,211.00) | 47.50(3.25,368.25) |
Signal detection
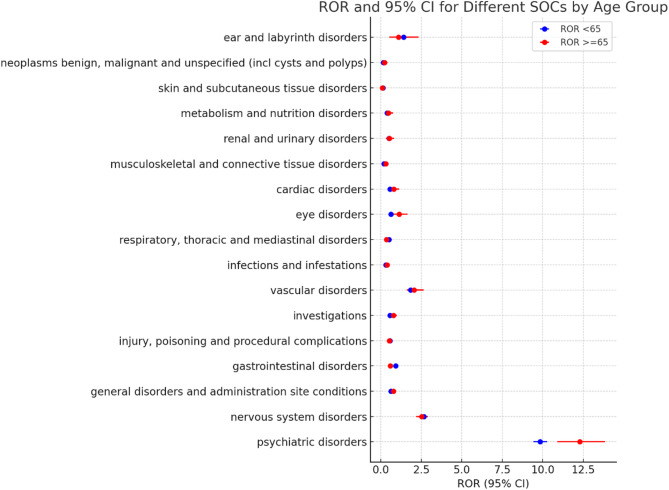
The signal reporting of Esketamine at the SOC level is shown in Fig. 2; Table 4. The Esketamine-related AEs of both adults group and older adults group were seen in 17 organ systems. According to P value, the adults group and older adults group showed significant difference on Psychiatric disorders(ROR 9.83vs12.29, P=0.0017), General disorders and administration site conditions(ROR 0.62vs0.78, P=0.0183), Gastrointestinal disorders(ROR 0.91vs0.59, P=0.0001),Respiratory, thoracic and mediastinal disorders(ROR 0.5vs0.34 P=0.031) and Eye disorders(ROR 0.62vs1.13, P=0.0252). This age-dependent disparity is visually reinforced in Fig. 2 (graphical representation of Table 4), which displays the Reporting Odds Ratios (RORs) with 95% confidence intervals across SOC.
Fig. 2.
The comparison of ROR for different SOC between adults and older adults group
Table 4.
The signal strength of AEs of Esketamine at the SOC level in adults and older adults group
| System Organ Class (SOC) | Case reports (Adults group) |
reporting odds ratios (ROR) (95%CI) |
Case reports (Older adults group) |
reporting odds ratios (ROR) (95%CI) |
P value |
|---|---|---|---|---|---|
| Psychiatric disorders | 3245 |
9.83 (9.42, 10.26) |
383 |
12.29 (10.9,13.85) |
0.0017 |
| Nervous system disorders | 1720 |
2.67 (2.53, 2.82) |
226 |
2.53 (2.19,2.92) |
0.4848 |
| General disorders and administration site conditions | 994 |
0.62 (0.58, 0.67) |
169 |
0.78 (0.66, 0.91) |
0.0183 |
| Gastrointestinal disorders | 696 |
0.91 (0.84, 0.98) |
70 |
0.59 (0.46, 0.75) |
0.0001 |
| Injury, poisoning and procedural complications | 637 |
0.56 (0.52, 0.61) |
82 |
0.53 (0.43, 0.67) |
0.6464 |
| Investigations | 327 |
0.57 (0.51, 0.64) |
68 |
0.77 (0.61, 0.99) |
0.0509 |
| Vascular disorders | 306 |
1.83 (1.63, 2.05) |
63 |
2.06 (1.6, 2.65) |
0.4253 |
| Infections and infestations | 179 |
0.30 (0.26, 0.35) |
32 |
0.39 (0.27, 0.55) |
0.2303 |
| Respiratory, thoracic and mediastinal disorders | 217 |
0.50 (0.44, 0.57) |
27 |
0.34 (0.24, 0.5) |
0.031 |
| Eye disorders | 102 |
0.62 (0.51, 0.75) |
28 |
1.13 (0.78, 1.64) |
0.0252 |
| Cardiac disorders | 100 |
0.57 (0.47, 0.69) |
30 |
0.79 (0.55, 1.14) |
0.1708 |
| Musculoskeletal and connective tissue disorders | 107 |
0.20 (0.16, 0.24) |
20 |
0.30 (0.19, 0.47) |
0.1783 |
| Renal and urinary disorders | 87 |
0.50 (0.41, 0.62) |
19 |
0.52 (0.33, 0.81) |
0.881 |
| Metabolism and nutrition disorders | 67 |
0.38 (0.3, 0.49) |
17 |
0.46 (0.29, 0.75) |
0.5286 |
| Skin and subcutaneous tissue disorders | 92 |
0.13 (0.11, 0.16) |
6 |
0.10 (0.04, 0.22) |
0.529 |
| Neoplasms benign, malignant and unspecified (incl cysts and p) | 55 |
0.15 (0.12, 0.2) |
12 |
0.23 (0.13, 0.41) |
0.2815 |
| Ear and labyrinth disorders | 48 |
1.40 (1.05, 1.86) |
7 |
1.10 (0.53, 2.32) |
0.5495 |
As Psychiatric disorders are the most common type of AEs to Esketamine, this study focused on analyzing the differences in Psychiatric disorder-related AEs between adults and older adults populations. As shown in Table 5, the adults group and older adults group showed significant difference on the following AEs(P<0.05): Dissociation(ROR 1336.65vs4207.59, P=3.11962E-06), Suicidal ideation(ROR 40.68vs86.05, P=0.001181655), Depression(ROR 8.63vs17.87, P=0.002482576), Anxiety(ROR 4.95vs9.74, P=0.010454363), Hallucination(ROR 11.5vs4.5, P=0.000327962), Visual hallucination(ROR 17.76vs8.16, P=0.040212903) and Agitation(ROR 2.21vs10.69, P=0.014622076).
Table 5.
The psychiatric disorder-related AEs among adults and older adults group
| Preferred Terms (PT) | Case reports (Adults group) |
reporting odds ratios (ROR) (95%CI) |
Case reports (Older adults group) |
reporting odds ratios (ROR) (95%CI) |
P value |
|---|---|---|---|---|---|
| Dissociation | 800 |
1336.65 (1201.26, 1487.29) |
102 |
4207.59 (3176.6,5573.19) |
3.11962E-06 |
| Suicidal ideation | 529 |
40.68 (37.2, 44.49) |
42 |
86.05 (63.06, 117.41) |
0.001181655 |
| Depression | 249 |
8.63 (7.61, 9.8) |
38 |
17.87 (12.93, 24.7) |
0.002482576 |
| Anxiety | 218 |
4.95 (4.32, 5.66) |
30 |
9.74 (6.78, 13.99) |
0.010454363 |
| Suicide attempt | 156 |
12.38 (10.56, 14.52) |
7 |
21.68 (10.29, 45.67) |
0.305826641 |
| Panic attack | 92 |
15.99 (13, 19.67) |
9 |
29.54 (15.3, 57.05) |
0.209004356 |
| Hallucination | 80 |
11.50 (9.22, 14.35) |
11 |
4.50 (2.48, 8.14) |
0.000327962 |
| Completed suicide | 102 |
6.84 (5.63, 8.32) |
6 |
5.38 (2.41, 12.01) |
0.565929916 |
| Insomnia | 51 |
1.50 (1.14, 1.98) |
9 |
2.06 (1.07, 3.98) |
0.468591486 |
| Major depression | 52 |
47.44 (35.89, 62.73) |
3 |
45.7 (14.62, 142.86) |
0.958481069 |
| Visual hallucination | 41 |
17.76 (13.03, 24.2) |
6 |
8.16 (3.66, 18.21) |
0.040212903 |
| Euphoric mood | 39 |
27.08 (19.69, 37.25) |
4 |
57.99 (21.57, 155.91) |
0.37114193 |
| Depressed mood | 28 |
3.08 (2.12, 4.46) |
7 |
7.80 (3.71, 16.4) |
0.151613219 |
| Agitation | 22 |
2.21 (1.45, 3.36) |
11 |
10.69 (5.90, 19.38) |
0.014622076 |
| Intentional self-injury | 27 |
4.08 (2.79, 5.95) |
3 |
28.02 (8.99, 87.36) |
0.231506972 |
| Confusional state | 19 |
1.07 (0.68, 1.69) |
7 |
1.47 (0.7, 3.1) |
0.547052775 |
| Emotional distress | 15 |
1.18 (0.71, 1.95) |
4 |
8.94 (3.35, 23.89) |
0.139332225 |
| Emotional disorder | 15 |
3.50 (2.11, 5.81) |
4 |
16.23 (6.07, 43.39) |
0.183319306 |
| Fear | 15 |
5.88 (3.54, 9.77) |
3 |
10.89 (3.5, 33.88) |
0.52655531 |
| Depressive symptom | 9 |
11.53 (5.97, 22.25) |
4 |
113.84 (42.05, 308.21) |
0.132579022 |
| Nervousness | 6 |
1.14 (0.51, 2.53) |
6 |
6.24 (2.8, 13.94) |
0.077426272 |
| Sleep disorder | 9 |
0.72 (0.37, 1.39) |
3 |
2.42 (0.78, 7.52) |
0.328275756 |
| Delirium | 4 |
0.98 (0.37, 2.61) |
6 |
4.78 (2.14, 10.68) |
0.091566943 |
| Catatonia | 5 |
4.62 (1.92, 11.13) |
4 |
45.88 (17.09, 123.14) |
0.128661073 |
Discussion
Esketamine, functioning as a non-competitive N-methyl D-aspartate (NMDA) receptor antagonist, exhibits promising clinical efficacy in treating TRD. Several studies were conducted to explore its long-term safety and tolerability. A previous systematic pharmacovigilance study based on the FAERS was published after 2 years of Esketamine was on the market, it identified a variety of new and unexpected signals, as well as several risk factors associated with AEs which indeed provided valuable medication guidance for medical professionals and patients [18]. However, due to the time constraints of market availability, this study only included reports recorded in FAERS from the second quarter (Q2) of 2019–2020 Q1. Another post-marketing safety study on Esketamine included more reports recorded in FAERS, but it only focused on mining and analysis of neurological AEs with Esketamine [19]. The latest post-marketing safety study on Esketamine was published on December 2023 and it collected data related to Esketamine adverse events from 2019 Q1 to 2023 Q1, which was an update for previous study [20].
Due to changes in physiological functions, older patients are at higher risk when using medications. Ensuring the safe use of medications in older patients has always been a clinical concern. Although the three studies mentioned above indicate that older patients have a higher risk of experiencing adverse events when using Esketamine, none of them conducted an in-depth analysis. Thus, we systematically analyzed AEs reports related to Esketamine for both adults group(<65 years) and older adults group(≥ 65years) in the FAERS database from 2019Q2 to 2024Q1 in this study, in order to obtain some new insights in the difference of AEs risk between adults group(<65 years) and older adults group(≥ 65years) and to provide a basis for the safe use of Esketamine in older patients.
As shown in Table 3, the AEs reports relevant to Esketamine increased annually from 2019 to 2023, except for 2024, as only the first quarter’s data was included for that year. This increasing trend was consistent between the adults group and the older adults group. The annual increase in the number of AEs reports may be related to the growing number of patients using Esketamine. As a self-reported system, the data form FAERS might be also affected by individual subjective factors [15], but the overall upward trend in AEs reports among older adults reminds us that it is essential to evaluate the types and distributions of adverse reactions in older patients using Esketamine.
Our results found that female patients significantly outnumbered male patients in reports related to Esketamine, which was observed in both adults and older adults group. This result might be related to gender differences in depression [21], specifically that the incidence of depression is higher in females than in males. Consequently, the proportion of females using Esketamine might be higher than that of males, leading to an increase in the number of adverse event reports due to the larger base of female patients. This is merely an epidemiological speculation, and it is still essential to explore whether there are gender differences in the risk of adverse reactions to Esketamine. Regardless, our study results indicate that the higher number of AEs reports for Esketamine in female patients compared to male patients does not vary with age difference. In addition, the primary reporters were physicians, followed by consumers and pharmacists, which was also similarly reflected in both the adult group and older adults group. The majority of reports on both adult and older adult group originated from the United States. However, the occurrence time of adverse reactions in the older population was a little bit longer than that in the adult population, the TTO of adults group and older adults group was 47.50 days (3.25,368.25) and 45.00 days (6.50,211.00), respectively. This might be related to changes in the sensitivity of older patients to Esketamine treatment.
In this study, ROR was applied to assess the disproportionality of AEs report of Esketamine from FAERS database between adults group and older adults group. For each subgroup, ROR was calculated and compared by P value for interaction. As we can see in Table 4; Fig. 2, Psychiatric disorders and Nervous system disorders were the top 2 System Organ Classes (SOC) in both adults group and older adults group, which was complying with the medication warning of Esketamine. Upon comparison using P-values, statistically significant disparities were observed in the occurrence of Psychiatric disorders(ROR 9.83vs12.29, P=0.0017), General disorders and administration site conditions(ROR 0.62vs0.78, P=0.0183), Gastrointestinal disorders(ROR 0.91vs0.59, P=0.0001),Respiratory, thoracic and mediastinal disorders(ROR 0.5vs0.34 P=0.031), as well as Eye disorders(ROR 0.62vs1.13, P=0.0252) between the adults and older adults group. This indicated that the risk of AEs related to Esketamine on above organ systems varied by age difference.
As Psychiatric disorders are the most common type of AEs to Esketamine, this study primarily analyzed the differences in Psychiatric Disorders-related AEs between the adult and older populations. As shown in Table 5, the adults group and older adults group showed significant difference on the following AEs(P<0.05): Dissociation(ROR 1336.65vs4207.59, P=3.11962E-06), Suicidal ideation(ROR 40.68vs86.05, P=0.001181655), Depression(ROR 8.63vs17.87, P=0.002482576), Anxiety(ROR 4.95vs9.74, P=0.010454363), Hallucination(ROR 11.5vs4.5, P=0.000327962), Visual hallucination(ROR 17.76vs8.16, P=0.040212903) and Agitation(ROR 2.21vs10.69, P=0.014622076). Specifically, the ROR of Dissociation, Suicidal ideation, Depression, Anxiety and Agitation on older adults group was significantly higher than those on adults group, while the ROR of Hallucination and Visual hallucination on older adults group was significantly lower than those on adults group. This indicated that the four AEs, Dissociation, Suicidal ideation, Depression, Anxiety and Agitation, were reported more frequently in older adults. The results of a RCT indicated that the efficacy and safety of Esketamine for treatment-resistant depression in older patients are comparable to those in younger patients [13], which appears to contradict our findings. However, another post-hoc analysis revealed that older patients experienced more adverse reactions, such as dizziness and dissociation, when using Esketamine [22]. As mentioned above, clinical trials may have limitations in monitoring adverse reactions due to factors such as sample size and duration of detection periods. Real-world studies, such as those utilizing the FAERS database, are a vital supplement to drug safety assessments based on clinical trials, and it aid in identifying new factors that impact the safe use of medications. Our results are consistent with previous post-hoc research, indicating that Esketamine exhibits age-related differences in certain adverse reactions.
Dissociation is one of the most common side effects of Esketamine treatment. In studies involving Esketamine treatment for patients with major depressive disorder and suicidal ideation, 29.2–38.6% of patients experienced dissociative symptoms [23]. Furthermore, in studies using intravenous racemic ketamine for treating TRD, approximately 72% reported dissociation, whereas this proportion was 36% in studies using non-intravenous racemic ketamine [24]. This discrepancy is likely related to differences in the route of administration. Interestingly, reports from TRD Esketamine trials indicated that the incidence of dissociation in adult patients ranges from 23 to 27.6%, while it was 12.5% in older patients [11]. However, our findings revealed that the frequency of reported dissociation was significantly higher in older patients compared to adult patients. This discrepancy may stem primarily from limitations in the Clinician-Administered Dissociative States Scale (CADSS). As the most common scale used in TRD trials to assess dissociation severity, the CADSS appears insufficient for capturing the full spectrum of psychotomimetic experiences induced by Esketamine. Its limited coverage likely underestimates the true frequency of dissociation [25]. Consequently, this measurement limitation in clinical trials may account for the observed difference in dissociation incidence between adult and older patient groups. Furthermore, the convergence of age-dependent decline in hepatic/renal function (slowing Esketamine clearance) and amplified neuropsychological susceptibility to anxiety likely constitutes the primary etiology for elevated dissociation risk in older Esketamine users [26]. This dual mechanism potentiates both drug exposure and perceptual sensitivity, culminating in increased reporting of dissociative phenomena among older adults group from FEARS. Although the risk posed by dissociation is relatively minor and it can be quickly resolved, it is important to note that based on our study data, older patients may have a higher risk of experiencing dissociation when using Esketamine.
Notably, the reporting of Major depression as an AE presents a paradoxical observation given its status as a primary therapeutic indication for Esketamine. This apparent contradiction may arise from either: (1) clinically significant worsening of the underlying depressive condition being treated - which falls within regulatory AE documentation requirements; or (2) potential infusion-related effects from off-label administration routes, as incomplete route documentation in the FAERS database prevents exclusion of this possibility. While these data limitations preclude definitive attribution of causality for these reported AEs, our analysis demonstrated no statistically significant difference in ‘Major depression’ AE incidence between adult and older adults group.
One of limitations of this study is its reliance on the FAERS database, which may be susceptible to reporting biases. The self-reported nature of FAERS data could result in the omission or inaccuracy of crucial patient information, potentially impacting the final statistical analysis results [15]. In this study, 2350 cases without age information were excluded. If age information were complete, our research data would be further expanded, potentially leading to more new discoveries. Moreover, the information on the method of administration of Esketamine was uncompleted in the FAERS database, thus it was not accounted in this study. But whether the administration routes are intranasal or intravenous indeed affects the occurrence, frequency, and types of AEs. Future studies should incorporate information on routes of administration to offer a comprehensive understanding of Esketamine’s safety profile. Additionally, another significant limitation arises from the inherent diagnostic heterogeneity within the FAERS data and our inability to precisely distinguish the specific indication for Esketamine use in reported cases. FAERS reports often lack detailed clinical information, making it difficult to reliably separate patients receiving Esketamine for TRD from those receiving it for major depressive episodes with acute suicidal ideation/behavior. These two patient populations likely differ substantially in baseline disease severity, comorbidity profiles, and prior treatment history. TRD patients typically have chronic illness and multiple medication failures, potentially with more complex comorbidities. Patients with acute suicidality represent an extremely severe, often agitated subset of MDD. This diagnostic heterogeneity introduces a substantial risk of confounding by indication. Observed differences in AE reporting patterns between age groups could be influenced by the underlying differences in the clinical characteristics and acuity of the populations being treated for different indications, rather than (or in addition to) a true age-related effect of Esketamine. For instance, certain AEs more common in older adults (e.g., falls, confusion) might reflect the frailty or comorbidities prevalent in older TRD patients [27], while AEs like agitation or dissociation reported in younger adults might be more associated with the acute, severe suicidal state itself. Our analysis, stratified only by age and lacking adjustment for specific indication or detailed baseline severity/comorbidity due to data constraints, cannot adequately disentangle these potential confounding effects. Future studies with prospectively collected, indication-specific data and rigorous adjustment for baseline characteristics are needed to clarify the age-associated safety profile of Esketamine within each approved indication. Despite these limitations, the large sample size and real-world nature of the FAERS data provide valuable insights into Esketamine’s safety profile across different age groups.
Conclusion
This study systematically analyzed AE reports associated to Esketamine of adults group (< 65 years) and older adults group (≥ 65 years) in the FAERS database from Q2 of 2019 to Q1 of 2024. The analysis of FAERS data reveals significant age-related differences in the safety profile of Esketamine. Older adults are at a higher risk for severe psychiatric and general AEs, necessitating careful patient selection, monitoring, and tailored treatment approaches. These findings highlight the critical need for ongoing pharmacovigilance and age-specific clinical guidelines to optimize the use of Esketamine in treating treatment-resistant depression. By addressing these safety concerns, we can better harness the therapeutic potential of Esketamine while safeguarding patient well-being.
Acknowledgements
This study was performed using the FAERS source that was provided by the FDA. The information, results, or interpretation of the current study do not represent any opinion of the FDA.
Author contributions
Bin Deng, Zhiwen Fu, Xuejia Zhai and Yongning Lv conceived the study; Zhiwen Fu, Linjie Li, Yusen Xu, Zihe Yang, Xuejia Zhai, Bin Deng and Yongning Lv collected the report; Zhiwen Fu, Bin Deng and Yongning Lv wrote the manuscript and edited the manuscript. All authors have approved the publication of the manuscript.
Funding
This work is supported by Key Research and Development Plan of Hubei Province, China(2023BCB030), Hubei Provincial Natural Science Foundation (2024AFB645 and 2022CFB092) and Project of Administration of Traditional Chinese Medicine of Hubei Province of China (ZY2025Q025).
Data availability
The data supporting the conclusion of this article will be made available from the corresponding authors upon on reasonable request.
Declarations
Ethical approval
This study utilized data from the FAERS, which is a publicly accessible and anonymized pharmacovigilance database. Because all records are fully de-identified and contain no personally identifiable information, this research did not involve direct interaction with human participants. Therefore, Institutional Review Board (IRB) approval and informed consent requirements were formally waived.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bin Deng and Zhiwen Fu both are equal first author.
Contributor Information
Xuejia Zhai, Email: zhaixuejia@163.com.
Yongning Lv, Email: luyn_union@163.com.
References
- 1.Monroe SM, Harkness KL. Major depression and its recurrences: life course matters. Annu Rev Clin Psychol. 2022;18:329–57. [DOI] [PubMed] [Google Scholar]
- 2.Kautzky A, et al. A new prediction model for evaluating Treatment-Resistant depression. J Clin Psychiatry. 2017;78(2):215–22. [DOI] [PubMed] [Google Scholar]
- 3.Darby I. Treatment-resistant depression. Aust Dent J. 2023;68(4):221. [DOI] [PubMed] [Google Scholar]
- 4.Swainson J, et al. Esketamine for treatment resistant depression. Expert Rev Neurother. 2019;19(10):899–911. [DOI] [PubMed] [Google Scholar]
- 5.Jalloh M. Esketamine (Spravato) for Treatment-Resistant depression. Am Fam Physician. 2020;101(6):339–40. [PubMed] [Google Scholar]
- 6.Bozymski KM, et al. Esketamine: a novel option for treatment-resistant depression. Ann Pharmacother. 2020;54(6):567–76. [DOI] [PubMed] [Google Scholar]
- 7.Canuso CM, et al. Esketamine nasal spray for the rapid reduction of depressive symptoms in major depressive disorder with acute suicidal ideation or behavior. J Clin Psychopharmacol. 2021;41(5):516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaur U, et al. Esketamine: a glimmer of hope in treatment-resistant depression. Eur Arch Psychiatry Clin Neurosci. 2021;271(3):417–29. [DOI] [PubMed] [Google Scholar]
- 9.Feeney A, Papakostas GI. Pharmacotherapy: ketamine and Esketamine. Psychiatr Clin North Am. 2023;46(2):277–90. [DOI] [PubMed] [Google Scholar]
- 10.Schatzberg AF. Mechanisms of action of ketamine and Esketamine. Am J Psychiatry. 2021;178(12):1130. [DOI] [PubMed] [Google Scholar]
- 11.Ochs-Ross R, et al. Comparison of long-term efficacy and safety of Esketamine nasal spray plus oral antidepressant in younger versus older patients with treatment-resistant depression: post-hoc analysis of SUSTAIN-2, a Long-Term Open-Label phase 3 safety and efficacy study. Am J Geriatr Psychiatry. 2022;30(5):541–56. [DOI] [PubMed] [Google Scholar]
- 12.Liu P, et al. Efficacy and safety of Esketamine combined with antidepressants for treatment-resistant depression: a meta-analysis. Neuropsychiatr Dis Treat. 2022;18:2855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochs-Ross R, et al. Efficacy and safety of Esketamine nasal spray plus an oral antidepressant in elderly patients with Treatment-Resistant Depression-TRANSFORM-3. Am J Geriatr Psychiatry. 2020;28(2):121–41. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez EM, Staffa JA, Graham DJ. The role of databases in drug postmarketing surveillance. Pharmacoepidemiol Drug Saf. 2001;10(5):407–10. [DOI] [PubMed] [Google Scholar]
- 15.Sakaeda T, et al. Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci. 2013;10(7):796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown EG. Using MedDRA. Drug Saf. 2004;27(8):591–602. [DOI] [PubMed] [Google Scholar]
- 17.Sakaeda T, et al. Commonality of drug-associated adverse events detected by 4 commonly used data mining algorithms. Int J Med Sci. 2014;11(5):461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gastaldon C, et al. Post-Marketing safety concerns with esketamine: A disproportionality analysis of spontaneous reports submitted to the FDA adverse event reporting system. Psychother Psychosom. 2020;90(1):41–8. [DOI] [PubMed] [Google Scholar]
- 19.Guo H, et al. Neurological adverse events associated with esketamine: a disproportionality analysis for signal detection leveraging the FDA adverse event reporting system. Front Pharmacol. 2022;13:849758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Y, et al. The correlation of Esketamine with specific adverse events: a deep dive into the FAERS database. European Archives of Psychiatry and Clinical Neuroscience; 2023. [DOI] [PubMed]
- 21.Parker G, Brotchie H. Gender differences in depression. Int Rev Psychiatry. 2010;22(5):429–36. [DOI] [PubMed] [Google Scholar]
- 22.d’Andrea G, et al. Investigating the effectiveness and tolerability of intranasal Esketamine among older adults with Treatment-Resistant depression (TRD): A Post-hoc analysis from the REAL-ESK study group. Am J Geriatr Psychiatry. 2023;31(12):1032–41. [DOI] [PubMed] [Google Scholar]
- 23.Daly EJ, et al. Efficacy of Esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Short B, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65–78. [DOI] [PubMed] [Google Scholar]
- 25.van Schalkwyk GI, et al. Acute psychoactive effects of intravenous ketamine during treatment of mood disorders: analysis of the clinician administered dissociative state scale. J Affect Disord. 2018;227:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turnheim K. Drug therapy in the elderly. Exp Gerontol. 2004;39(11–12):1731–8. [DOI] [PubMed] [Google Scholar]
- 27.Soares B, et al. Prevalence and impact of Treatment-Resistant depression in Latin america: a prospective, observational study. Psychiatr Q. 2021;92(4):1797–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusion of this article will be made available from the corresponding authors upon on reasonable request.