Abstract
Recent studies in our lab have demonstrated the ubiquity and diversity of microorganisms which couple growth to the reduction of chlorate or perchlorate [(per)chlorate] under anaerobic conditions. We identified two taxonomic groups, the Dechloromonas and the Dechlorosoma groups, which represent the dominant (per)chlorate-reducing bacteria (ClRB) in the environment. As part of these studies we demonstrated that chlorite dismutation is a central step in the reductive pathway of (per)chlorate that is common to all ClRB and which is mediated by the enzyme chlorite dismutase (CD). Initial studies on CD suggested that this enzyme is highly conserved among the ClRB, regardless of their phylogenetic affiliation. As such, this enzyme makes an ideal target for a probe specific for these organisms. Polyclonal antibodies were commercially raised against the purified CD from the ClRB Dechloromonas agitata strain CKB. The obtained antiserum was deproteinated by ammonium sulfate precipitation, and the antigen binding activity was assessed using dot blot analysis of a serial dilution of the antiserum. The titers obtained with purified CD indicated that the antiserum had a high affinity for the CD enzyme, and activity was observed in dilutions as low as 10−6 of the original antiserum. The antiserum was active against both cell lysates and whole cells of D. agitata, but only if the cells were grown anaerobically with (per)chlorate. No response was obtained with aerobically grown cultures. In addition to D. agitata, dot blot analysis employed with both whole-cell suspensions and cell lysates of several diverse ClRB representing the alpha, beta, and gamma subclasses of Proteobacteria tested positive regardless of phylogenetic affiliation. Interestingly, the dot blot response obtained for each of the ClRB cell lysates was different, suggesting that there may be some differences in the antigenic sites of the CD protein produced in these organisms. In general, no reactions were observed with cells or cell lysates of the organisms closely related to the ClRB which could not grow by (per)chlorate reduction. These studies have resulted in the development of a highly specific and sensitive immunoprobe based on the commonality of the CD enzyme in ClRB which can be used to assess dissimilatory (per)chlorate-reducing populations in environmental samples regardless of their phylogenetic affiliations.
Environmental contamination with oxyanions of chlorine, especially perchlorate (ClO4−), chlorate (ClO3−), chlorite (ClO2−), and chlorine dioxide (ClO2), has been a constantly growing problem over the last 100 years (33, 34). In general these compounds are not formed naturally, although one natural source of perchlorate has been identified associated with a nitrate deposit in the Atacama desert region in northern Chile (11, 17). These compounds have been introduced into the environment in large quantities in the form of disinfectants, bleaching agents, and herbicides (3, 12, 29) and as components of explosives and rocket propellants by the aerospace and defense industries (32). Legal discharge of perchlorate-containing wastestreams from munition manufacturing and handling facilities prior to 1997 has recently been identified as the predominant source of perchlorate found in major drinking water supplies in the United States (25, 34). Perchlorate contamination poses a significant health threat, as preliminary toxicological studies have demonstrated that it has a direct effect on iodine uptake by the thyroid gland and at higher concentrations (6 mg per kg of body weight per day) may result in fatal bone marrow disease. As a result, the California Environmental Protection Agency initiated a recommended maximum concentration limit of 18 μg/liter (25) which was later increased by the U.S. Environmental Protection Agency to 32 μg/liter (24). In 1998, perchlorate was added to the U.S. Environmental Protection Agency's drinking water candidate contaminant list.
The recent concerns over the environmental contamination of groundwaters and drinking waters with perchlorate have focused a significant amount of attention on microbial remediation (19, 24, 25, 33), as both chlorate and perchlorate can be reductively transformed into innocuous products by microorganisms in the absence of O2 (6, 9, 21, 26, 28, 30, 36). This reductive process was originally identified with chlorate (4) and was associated with nitrate-respiring organisms which simply used chlorate as a coincidental substrate for the nitrate reductase (10, 13, 14). Growth was not associated with this metabolism and chlorite was formed as a toxic end product (10, 13, 14, 27). Now it is known that specialized organisms have evolved which can grow by the anaerobic reductive dissimilation of (per)chlorate into innocuous chloride with simple organic or inorganic electron donors (6, 7, 9, 18, 21, 22, 26, 28, 30, 36). Recent studies have demonstrated that the ubiquity of dissimilatory microbial (per)chlorate respiration is much more extensive than was previously assumed (9). These studies resulted in the isolation and identification of more than 30 new dissimilatory (per)chlorate-reducing bacteria (ClRB) from a broad diversity of environments, including both pristine and contaminated soils and sediments (9, 22; J. Pollock, L. A. Achenbach, and J. D. Coates, unpublished results). The known ClRB isolates represent a broad phylogeny with members in the alpha, beta, gamma, and epsilon subclasses of Proteobacteria (1, 9, 21); however, the majority of the known ClRB are in the beta subclass of Proteobacteria (1, 9). 16S ribosomal DNA (rDNA) sequence analysis identified the Dechloromonas and Dechlorosoma species as the two dominant ClRB genera in the beta Proteobacteria (1), and several studies have demonstrated that members of these two groups are present in nearly all environments screened, including both field samples and ex situ bioreactors treating perchlorate-contaminated wastes (9, 20; Pollock et al., unpublished). Although pure culture studies have demonstrated that members of these genera can grow under a broad range of environmental conditions, in general they grow optimally at circum-neutral pH in freshwater environments (6, 9, 22). In support of this, field studies have shown that alternative ClRB are generally dominant in sites of adverse pH or salinity (Pollock et al., unpublished). As a result, it is difficult to rapidly determine which organisms are responsible for perchlorate removal in a given site. Although 16S rRNA primer sets have been designed which are specific for the Dechloromonas and Dechlorosoma species (8; Pollock et al., unpublished), they are of limited use if these organisms are not dominant in the contaminated environment. Due to the extreme phylogenetic diversity of ClRB, 16S rRNA primer sets can only be designed to detect a few specific genera in the environment; thus, there is a direct need for a more general probe that is specific to ClRB. This is especially true in light of the fact that several ClRB are essentially phylogenetically identical, based on 16S rDNA sequence analysis, to non-(per)chlorate-reducing relatives; thus, even specific 16S rRNA primer sets may prove unreliable, as they are not indicative of physiological functionality (2).
Previous studies (6, 9, 35) have demonstrated that chlorite dismutation is a central step in the reductive pathway of (per)chlorate that is common to all ClRB and is mediated by the enzyme chlorite dismutase (CD). If conserved, this enzyme would make an ideal target for a universal probe specific for these organisms. We previously purified to homogeneity the CD from Dechloromonas agitata strain CKB (9). The purified CD was a homotetramer with a molecular mass of 120 kDa and a specific activity of 1,928 μmol of chlorite dismutated per mg of protein per min (9). This is similar to the molecular mass and specific activity observed for the CD previously purified from the ClRB strain GR-1 (35). These data suggested that similar CD enzymes can be found in the phylogenetically diverse ClRB.
As part of these ongoing studies, polyclonal antibodies were commercially raised against the purified CD from D. agitata to develop an immunoprobe which would be universally selective for all ClRB regardless of their phylogenetic affiliation. Our results demonstrate the sensitivity, specificity, and applicability of this probe.
MATERIALS AND METHODS
Media and culturing techniques.
All freshwater ClRB cultures, including D. agitata strain CKB, Dechloromonas aromatica strain RCB, Dechlorosoma suillum strain PS, Pseudomonas sp. strain PK, and Dechlorospirillum anomolous strain WD were maintained on basal freshwater medium as previously described (6). The marine ClRB Dechloromarinus chlorophilus strain NSS was maintained on APW medium as previously described (9). All ClRB were grown with acetate (10 mM) as the electron donor and either chlorate or perchlorate (10 mM) as the electron acceptor unless otherwise stated. In addition, active cultures of Pseudomonas stutzeri, and Escherichia coli were grown aerobically on the same basal freshwater medium as the ClRB from which the (per)chlorate was omitted. Rhodocyclus tenuis was grown on RCVB medium as previously described (31).
In the laboratory, standard anaerobic culturing techniques were used (5, 16, 23). The anoxic basal medium was prepared by boiling under N2-CO2 (80:20 [vol/vol]) to remove dissolved O2 and dispensed under N2-CO2 (80:20 [vol/vol]) into anaerobic pressure tubes or serum bottles that were then capped with thick butyl rubber stoppers and sterilized by autoclaving (15 min at 121°C).
Cell lysates were prepared from 1-liter cultures of the various organisms as follows. The cells were harvested and resuspended in 5 ml of 10 mM phosphate buffer, pH 7.3, and lysed by passing through a French press at 20,000 lb/in2. The cell lysates were kept frozen at −80°C until required. The protein concentration of both cell lysates and whole cells was determined using a Total Protein Determination kit from Sigma (St. Louis, Mo.). The cell lysates and whole cells were probed with CD-specific immunoglobulin G (IgG) using the dot blot protocol outlined below. The lysates and whole cells were diluted such that each spot contained 5 μg of protein.
CD purification.
The CD was purified from the soluble fraction of a lysed cell preparation of D. agitata as previously described (9). Briefly, a 30-ml sample of cell-free extract was loaded onto a 2.5- by 10-cm column packed with Q-Sepharose Fast Flow medium (Amersham Pharmacia Biotech, Piscataway, N.J.) and the column was developed using a 0-to-300 mM KCl gradient in 50 mM Tris-HCl (pH 7.5). The CD active fractions were pooled and loaded onto a 2.5- by 20-cm column packed with hydroxyapatite (Bio-Rad Laboratories, Hercules, Calif.) and developed with a potassium phosphate buffer gradient (10 to 250 mM; pH 7.2). The resulting CD active fractions were pooled and augmented with ammonium chloride to a final concentration of 2 M NH4Cl prior to loading onto a 1- by 1-cm phenyl-Sepharose high-performance column. The phenyl-Sepharose column was developed with a descending gradient of ammonium chloride (2 to 0 M) in 50 mM Tris-HCl, pH 7.5. The eluted CD was concentrated by ultrafiltration (molecular mass cutoff of 30 kDa) and passed through a 1.6- by 60-cm column packed with Superdex 200 medium (Amersham Pharmacia Biotech). The pure CD was eluted with 150 mM NaCl in 50 mM potassium phosphate buffer (pH 7.2) and stored at −20°C until use.
CD-specific IgG.
Polyclonal antibodies to purified CD were commercially raised by Capralogics Inc. (Hardwick, Mass.), following a standard operating procedure for the production of rabbit polyclonal antibodies. The antigen was prepared by mixing CD with complete Freund's adjuvant and immunizing New Zealand-type rabbits with 400 μg of the antigen by subcutaneous injection. Further boosts of 200 μg of antigen were provided every 3 weeks. The initial test bleed 4 weeks after the first immunization indicated the presence of CD-active polyclonal antibodies. The rabbits were bled out after 12 weeks and the CD polyclonal antibodies were partially purified from the antisera by ammonium sulfate precipitation (15). One volume of a saturated solution of NH4SO4 was added to two volumes of antiserum to precipitate the polyclonal antibodies. The resulting pellet was resuspended in a 0.25-volume of phosphate-buffered saline (PBS) and reprecipitated with one volume of saturated NH4SO4. The resulting IgG pellet was resuspended in a 0.5 volume of PBS and was dialyzed for 24 h against PBS. The protein concentration of the dialysate was determined by the Bradford assay, and the purified CD polyclonal IgG was stored at −80°C in PBS at a final concentration of 15 mg/ml.
Dot blots.
Dot blots of the CD-specific IgG were prepared by spotting strips of Immobilon P transfer membranes (Millipore, Bedford, Mass.) with diluted pure CD (1 μl of a 5 μg/μl solution) and air dried. The membranes were then blocked for 1 h in 0.1% Tween 20-PBS (TPBS) and incubated with the CD-specific IgG for 1 h. The strips were washed four times in TPBS (5 min per wash) and were further incubated with goat anti-rabbit IgG (Sigma) labeled with horseradish peroxidase for 1 h in TPBS. The strips were washed as before and exposed to the SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, Ill.) for 3 min, after which the luminescence was recorded using Kodak BioMax film.
Western blotting.
Pure CD and cell lysates from both D. agitata and E. coli were run at 5 mA overnight on a sodium dodecyl sulfate (SDS)-12% polyacrylamide gel electrophoresis (PAGE) gel prepared as previously described (9), after which the gel was blotted onto Immobilon P transfer membrane, using Towbin buffer (192 mM Glycine-25 mM Tris base-20% methanol, pH 8.3) and a semidry transfer apparatus (Trans-Blot SD-Dry transfer cell; Bio-Rad) set at 20 V for 20 to 30 min. The resulting blot transfer was developed as outlined above for the dot blot protocol, while the posttransfer gel was stained with Coomassie blue.
Fluorescence micrographs.
Dry mounts of either D. agitata or E. coli cells were prepared for fluorescence microscopy by spreading a small volume (approximately 5 μl) of an active liquid culture onto an alcohol-cleaned glass slide. The slides were air dried at 40°C and rinsed briefly in PBS (0.05 M; pH 7.2). The primary antibody (rabbit polyclonal) was then applied at a dilution of 1:50 (diluted in PBS), and the slides were incubated for 30 min at room temperature after which the slide was again rinsed in PBS. The rinsed slides were then placed in a Coplin jar filled with PBS for an additional 10 min. After draining the slides of excess PBS, the fluorescein-labeled secondary IgG (goat anti-rabbit) was applied at a dilution of 1:100 and allowed to stand for 30 min at room temperature. The slide was rinsed with PBS as above and a coverslip was placed on the sample with a mountant composed of 9 parts glycerol and 1 part PBS (pH 8.5). The prepared slides were examined in a Leica Orthoplan 2 epifluorescence microscope system, and images were recorded using conventional photographic procedures or a Pixera 600CL Peltier-cooled charge-coupled device system.
RESULTS AND DISCUSSION
Sensitivity and specificity.
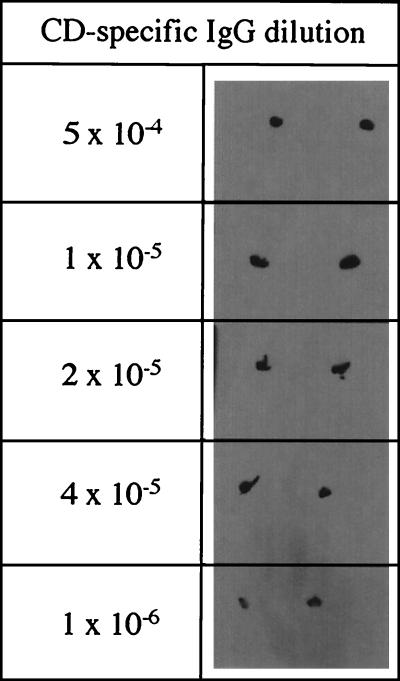
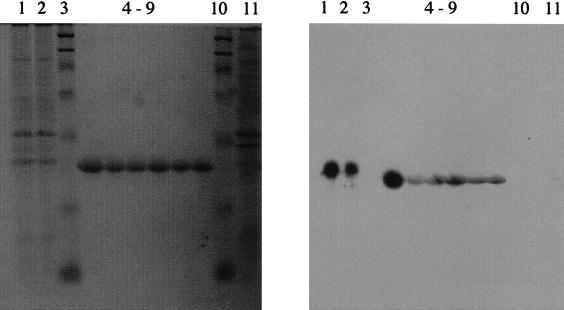
The commercially raised antiserum was partially purified by salt precipitation of serum proteins using ammonium sulfate cuts prior to determination of antigen binding activity. Dot blot analysis of a serial dilution of the partially purified IgG against the purified CD (5 μg μl−1) indicated that the IgG had a high affinity for CD, and activity was still observed at dilutions as low as 10−6 of the original IgG (Fig. 1). No response was observed in similar experiments performed with bovine serum albumin (5 μg μl−1) in place of the purified CD (data not shown). Western blot analysis of cell lysates of D. agitata indicated that the IgG only reacted with a single protein band in the cell lysate (Fig. 2). No cross-reactivity of the IgG was observed with cell lysates of E. coli (Fig. 2). The fact that the IgG reacted with the denatured protein is indicative that the antigenic site of the IgG is located in the subunits and does not need the native homotetramer structure. Comparison of the reactive protein band with the molecular mass marker indicated a molecular mass of 32 kDa (Fig. 2). This is similar to the migration of the CD previously observed in the cell lysates (9). Interestingly, the purified CD migrated slightly further than the 32-kDa band of the lysed cell preparations (Fig. 2). In contrast, previous results of SDS-PAGE studies performed with freshly purified D. agitata CD demonstrated that its mobility was identical to the 32-kDa band observed in the lysed cell preparation (9). The difference in the observed mobility is likely the result of the extended storage (18 months) of the purified CD at −20°C prior to use, which may have resulted in a slight alteration of the protein structure and subsequent SDS-PAGE mobility pattern. In support of this hypothesis, activity determination of the stored CD indicated that the specific activity had decreased to 569 μmol of chlorite dismutated per mg of protein per min, which is only 30% of the original activity (9).
FIG. 1.
Dot blot results of a range of dilutions of the CD-specific IgG. As shown, each sample analysis was performed in duplicate.
FIG. 2.
SDS-PAGE gel and Western blot analysis of cell lysates from D. agitata and E. coli as well as the purified CD enzyme. Lanes 1 and 2, cell lysate from D. agitata; lanes 3 and 10, molecular mass marker; lanes 4 through 9, dilutions of purified CD; lane 11, cell lysate from E. coli.
Reaction with other ClRB
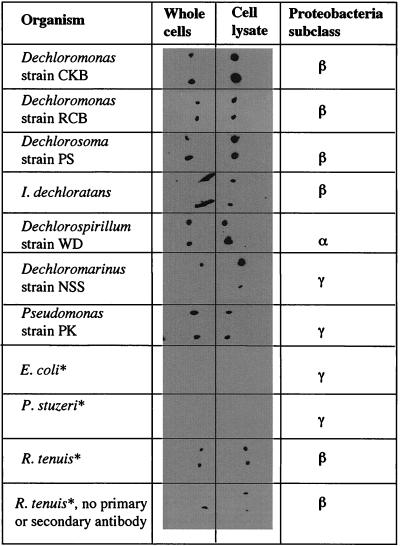
Microbial dissimilatory (per)chlorate reduction is a phylogenetically diverse metabolism, and microorganisms with this capability have been placed in four of the five subclasses of Proteobacteria (9). As such, the application of specific molecular probes based on signature nucleotides in the 16S rDNA sequence are of limited use, especially in light of the fact that 16S rDNA sequence analysis indicates that several ClRB are phylogenetically identical to non-(per)chlorate-reducing organisms (2). To test the universal applicability of the IgG, cell lysates of several phylogenetically distinct ClRB representing the alpha, beta, and gamma subclasses of the Proteobacteria, including species of Dechloromonas, Dechlorosoma, Dechlorospirillum, Dechloromarinus, Ideonella, and Pseudomonas genera, were screened. In addition, cell lysates of closely related organisms to the ClRB which do not grow by dissimilatory (per)chlorate reduction were also investigated. All the ClRB cell lysates tested positive regardless of phylogenetic affiliation (Fig. 3). Interestingly, the response obtained for each of the ClRB cell lysates was slightly different (based on dot blot intensity), suggesting that there may be some minor differences in the CD protein produced in these organisms (Fig. 3). Unsurprisingly, cell lysates of the Dechloromonas species, especially D. agitata, from which the CD was purified, showed the most reactivity. No reaction was observed with E. coli or with P. stutzeri, a phylogenetically identical species of the ClRB Pseudomonas sp. strain PK based on 16S rDNA sequence analysis, which cannot grow by (per)chlorate reduction (9) (Fig. 3). In contrast, a slight positive reaction was observed in the dot blot for the phototrophic beta proteobacterium R. tenuis, which is the closest non-(per)chlorate-reducing relative of the Dechloromonas genus (Fig. 3) (1, 6). However, if the dot blot procedure was repeated on this organism in the absence of the CD-specific IgG, the horseradish peroxidase-labeled goat anti-rabbit IgG, and the chemiluminescence substrate, a similar positive reaction was observed (Fig. 3). This suggests that the reaction was not a result of cross-reactivity of the IgG but was rather a reaction between the luminescent phototrophic centers of R. tenuis and the photographic film used.
FIG. 3.
Dot blot results of cell lysates and whole cells of a diverse range of ClRB and their non-(per)chlorate-reducing close relatives. As shown, each sample analysis was performed in duplicate. ∗, organism is incapable of reductive (per)chlorate respiration.
Reaction with whole cells
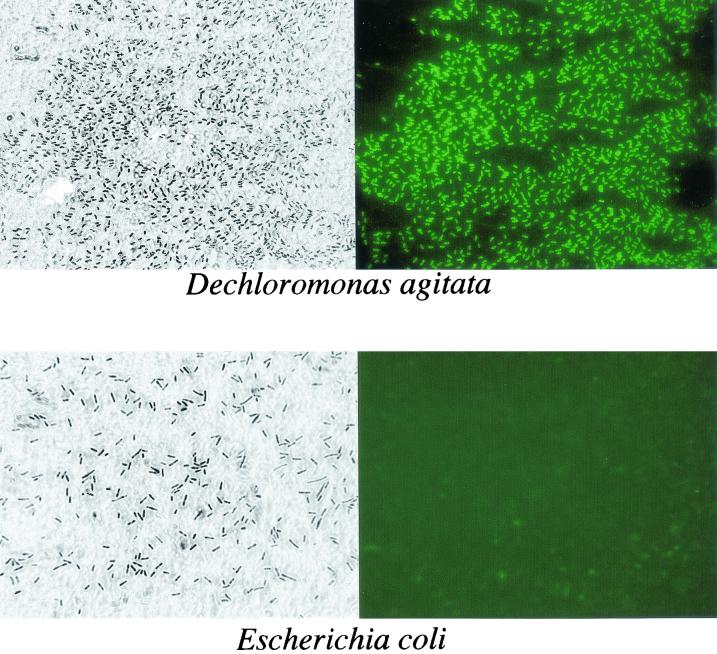
Interestingly, the IgG also reacted with whole-cell preparations of the individual ClRB and not with the negative controls (Fig. 3). In general, the reaction with the whole cells was less intensive than that observed with the cell lysates. Previous observations made in our laboratory have indicated that ClRB rapidly lyse in the absence of a suitable electron donor or acceptor (8, 18). In order to determine whether this reaction was the result of reaction of the IgG with CD released as a result of autolysis of the cells during the dot blot procedure, immunofluorescence micrographs were prepared from dry mounts of D. agitata by using fluorescein-labeled secondary goat anti-rabbit antibody. Phase-contrast microscopy of the dry mounts indicated that the cells of D. agitata were in good condition with little cell lysis (Fig. 4). Immunofluorescence microscopy revealed that the IgG readily reacted with the whole cells and clearly obviated the intact cell structure (Fig. 4). Similar studies performed with E. coli showed no fluorescence of the whole cells even after extended staining periods. The fact that the IgG reacted with the whole cells suggests that the CD may be present in the cell outer membrane, where it is accessible to the IgG. In support of this, previous studies demonstrated that the CD activity was associated with both the cell membrane and soluble fractions of a lysed-cell preparation of D. agitata when prepared using a French press (6, 9). This result suggested that the CD was loosely bound to the membrane and was sheared off by the French press procedure. In contrast, however, the CD activity of the ClRB strain GR-1 was located exclusively in the soluble fraction (35).
FIG. 4.
Phase contrast and immunofluorescence micrographs of whole cells of D. agitata and E. coli stained with CD-specific IgG primary antibody and fluorescein-labeled goat anti-rabbit IgG secondary antibody.
Functional response
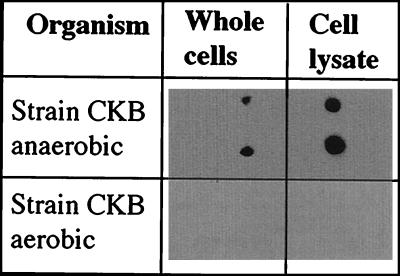
As the CD is a central enzyme in the pathway involved in the dissimilatory reduction of (per)chlorate, the CD-specific IgG may potentially be applied to determining the metabolic state of ClRB. Dot blot analysis indicated that the CD-specific IgG was active only against cells of D. agitata grown with either perchlorate or chlorate as alternative electron acceptor (Fig. 5). No reaction was observed when D. agitata was grown aerobically (Fig. 5). Previous kinetics studies by our lab on ClRB indicated that the enzymes involved in perchlorate reduction are induced only under anaerobic conditions in the presence of (per)chlorate (J. D. Coates, unpublished data). As part of these studies, we demonstrated that the CD activity was present only when the organisms were actively growing by (per)chlorate reduction. CD activity was not observed if the cells were growing aerobically or with nitrate as an alternative electron acceptor. This implies that the CD-specific IgG will only bind to ClRB which are actively metabolizing (per)chlorate, as it is only under these conditions that they produce an active CD. We could not test the reactivity of the CD-specific IgG with nitrate-grown cells of D. agitata strain CKB, as this organism is one of the few ClRB that cannot grow by nitrate reduction (6).
FIG. 5.
Dot blot results of whole cells of D. agitata grown aerobically or anaerobically with perchlorate as an electron acceptor. As shown, each sample analysis was performed in duplicate.
CONCLUSION
The present study resulted in the development of an immunoprobe which has a high affinity for CD, a central enzyme involved in the dissimilatory reduction of (per)chlorate by (per)chlorate-reducing bacteria. Previous studies indicated that this enzyme is unique to ClRB, and phylogenetically close relatives of the known ClRB which cannot grow by reduction of (per)chlorate are incapable of the dismutation of chlorite (2, 6, 9). In support of this, the developed immunoprobe was specific to ClRB and cross-reactivity with non-ClRB was not observed, regardless of their phylogenetic similarities. The probe was reactive with both cell lysates and whole cells of all ClRB tested but only when they were actively metabolizing (per)chlorate. Partiality based on phylogenetic affiliation was not observed, indicating that this probe has potential application for monitoring mixed (per)chlorate-reducing populations in environmental samples.
Acknowledgments
We thank John Bozzola of the IMAGE Center at Southern Illinois University for phase-contrast and immunofluorescence microscopy.
This work was supported by grant no. DACA72-00-C-0016 from the U.S. Department of Defense.
REFERENCES
- 1.Achenbach, L. A., R. A. Bruce, U. Michaelidou, and J. D. Coates. 2001. Dechloromonas agitata gen. nov., sp. nov. and Dechlorosoma suillum gen. nov., sp. nov., two novel environmentally dominant (per)chlorate-reducing bacteria and their phylogenetic position. Int. J. Syst. E vol. Microbiol. 51:527-533. [DOI] [PubMed] [Google Scholar]
- 2.Achenbach, L. A., and J. D. Coates. 2000. Disparity between bacterial phylogeny and physiology. ASM News 66:714-716. [Google Scholar]
- 3.Agaev, R., V. Danilov, V. Khachaturov, B. Kasymov, and B. Tishabaev. 1986. The toxicity to warm-blooded animals and fish of new defoliants based on sodium and magnesium chlorates. Uzb. Biol. Zh. 1:40-43. [Google Scholar]
- 4.Aslander, A. 1928. Experiments on the eradication of Canada thistle Cirsium arvense with chlorates and other herbicides. J. Agric. Res. 36:915-935. [Google Scholar]
- 5.Balch, W. E., G. E. Fox, L. J. Magrum, C. R. Woese, and R. S. Wolfe. 1979. Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 43:260-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce, R. A., L. A. Achenbach, and J. D. Coates. 1999. Reduction of (per)chlorate by a novel organism isolated from a paper mill waste. Environ. Microbiol. 1:319-331. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhuri, S. K., J. G. Lack, and J. D. Coates. 2001. Biogenic magnetite formation through anaerobic biooxidation of Fe(II). Appl. Environ. Microbiol. 67:2844-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coates, J. D., R. A. Bruce, J. A. Patrick, and L. A. Achenbach. 1999. Hydrocarbon bioremediative potential of (per)chlorate-reducing bacteria. Bioremed. J. 3:323-334. [Google Scholar]
- 9.Coates, J. D., U. Michaelidou, R. A. Bruce, S. M. O'Connor, J. N. Crespi, and L. A. Achenbach. 1999. The ubiquity and diversity of dissimilatory (per)chlorate-reducing bacteria. Appl. Environ. Microbiol. 65:5234-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Groot, G. N., and A. H. Stouthamer. 1969. Regulation of reductase formation in Proteus mirabilis. I. Formation of reductases and enzymes of the formic hydrogenlyase complex in the wild type and in chlorate resistant mutants. Arch. Microbiol. 66:220-233. [PubMed] [Google Scholar]
- 11.Erickson, G. E. 1961. The Chilean nitrate deposits. Am. Sci. 71:366-374. [Google Scholar]
- 12.Germgard, U., A. Teder, and D. Tormund. 1981. Chlorate formation during chlorine dioxide bleaching of softwood kraft pulp. Paperi Puu 3:127-133. [Google Scholar]
- 13.Hackenthal, E. 1965. Die Reduktion von Perchlorat durch Bacterien. II. Die Identitat der Nitratreduktase und des Perchlorat Reduzierenden Enzyms aus B. cereus. Biochem. Pharm. 14:1313-1324. [DOI] [PubMed] [Google Scholar]
- 14.Hackenthal, E., W. Mannheim, R. Hackenthal, and R. Becher. 1964. Die Reduktion von Perchlorat durch Bakterien. I. Untersucungen an Intaken Zellen. Biochem. Pharm. 13:195-206. [DOI] [PubMed] [Google Scholar]
- 15.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 16.Hungate, R. E. 1969. A roll tube method for cultivation of strict anaerobes. Methods Microbiol. 3:B117-B132. [Google Scholar]
- 17.Konnert, J. A., H. T. J. Evans, Jr., J. J. McGee, and G. E. Ericksen. 1994. Mineralogical studies of the nitrate deposits of Chile: two new saline minerals with the composition K6(Na, K)aNa6Mg10(XO4)12(IO)12.12(H2): fuenzalidaite (X = S) and carlosruizite (X = Se). Am. Mineral. 79:1003-1008. [Google Scholar]
- 18.Lack, J. G., S. K. Chaudhuri, R. Chakraborty, L. A. Achenbach, and J. D. Coates. Anaerobic biooxidation of Fe(II) by Dechlorosoma suillum. Microb. Ecol., in press. [DOI] [PubMed]
- 19.Logan, B. E. 2001. Assessing the outlook for perchlorate remediation. Environ. Sci. Technol. 35:483A-487A. [DOI] [PubMed] [Google Scholar]
- 20.Logan, B. E., H. Zhang, P. Mulvaney, M. G. Milner, I. M. Head, and R. F. Unz. 2001. Kinetics of perchlorate- and chlorate-respiring bacteria. Appl. Environ. Microbiol. 67:2499-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malmqvist, A., T. Welander, E. Moore, A. Ternstrom, G. Molin, and I.-M. Stenstrom. 1994. Ideonella dechloratans gen. nov., sp. nov., a new bacterium capable of growing anaerobically with chlorate as an electron acceptor. Syst. Appl. Microbiol. 17:58-64. [Google Scholar]
- 22.Michaelidou, U., L. A. Achenbach, and J. D. Coates. 2000. Isolation and characterization of two novel (per)chlorate-reducing bacteria from swine waste lagoons, p. 271-283. In E. D. Urbansky (ed.), Perchlorate in the environment. Kluwer Academic/Plenum, New York, N.Y.
- 23.Miller, T. L., and M. J. Wolin. 1974. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl. Microbiol. 27:985-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renner, R. 1999. EPA draft almost doubles safe dose of perchlorate in water. Environ. Sci. Technol. 33:110A-111A. [DOI] [PubMed] [Google Scholar]
- 25.Renner, R. 1998. Perchlorate-tainted wells spur government action. Environ. Sci. Technol. 9:210A. [DOI] [PubMed] [Google Scholar]
- 26.Rikken, G., A. Kroon, and C. van Ginkel. 1996. Transformation of (per)chlorate into chloride by a newly isolated bacterium: reduction and dismutation. Appl. Microbiol. Biotechnol. 45:420-426. [Google Scholar]
- 27.Roldan, M. D., F. Reyes, C. Moreno-Vivian, and F. Castillo. 1994. Chlorate and nitrate reduction in the phototrophic bacteria Rhodobacter capsulatus and Rhodobacter sphaeroides. Curr. Microbiol. 29:241-245. [Google Scholar]
- 28.Romanenko, V. I., V. N. Korenkov, and S. I. Kuznetsov. 1976. Bacterial decomposition of ammonium perchlorate. Mikrobiologiya 45:204-209. [PubMed] [Google Scholar]
- 29.Rosemarin, A., K. Lehtinen, and M. Notini. 1990. Effects of treated and untreated softwood pulp mill effluents on Baltic sea algae and invertebrates in model ecosystems. Nord. Pulp Paper Res. J. 2:83-87. [Google Scholar]
- 30.Stepanyuk, V., G. Smirnova, T. Klyushnikova, N. Kanyuk, L. Panchenko, T. Nogina, and V. Prima. 1992. New species of the Acinetobacter genus Acinetobacter thermotoleranticus sp. nov. Mikrobiologiya 61:347-356. [Google Scholar]
- 31.Tayeh, M. A., and M. T. Madigan. 1987. Malate dehydrogenase in phototrophic purple bacteria: purification, molecular weight, and quaternary structure. J. Bacteriol. 169:4196-4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urbanski, T. 1984. Chemistry and technology of explosives, vol. 4, p. 602-620. Pergamon Press, Oxford, United Kingdom.
- 33.Urbansky, E. T. 1998. Perchlorate chemistry: implications for analysis and remediation. Bioremed. J. 2:81-95. [Google Scholar]
- 34.U.S. Environmental Protection Agency. 2000. The effects of ammonium perchlorate on thyroids. Pathology Working Group Report. [Online.] http://www.epa.gov/ncea/perch.htm.
- 35.van Ginkel, C., G. Rikken, A. Kroon, and S. Kengen. 1996. Purification and characterization of chlorite dismutase: a novel oxygen-generating enzyme. Arch. Microbiol. 166:321-326. [DOI] [PubMed] [Google Scholar]
- 36.Wallace, W., T. Ward, A. Breen, and H. Attaway. 1996. Identification of an anaerobic bacterium which reduces perchlorate and chlorate as Wolinella succinogenes. J. Ind. Microbiol. 16:68-72. [Google Scholar]