Abstract
A new method for the rapid and accurate detection of pathogenic Naegleria fowleri amoebae in surface environmental water was developed. The method is based on an immunofluorescent assay combined with detection by solid-phase cytometry. In this study we developed and compared two protocols using different reporter systems conjugated to antibodies. The monoclonal antibody Ac5D12 was conjugated with biotin and horseradish peroxidase, and the presence of cells was revealed with streptavidin conjugated to both R-phycoerythrin and cyanine Cy5 (RPE-Cy5) and tyramide-fluorescein isothiocyanate, respectively. The RPE-Cy5 protocol was the most efficient protocol and allowed the detection of both trophozoite and cyst forms in water. The direct counts obtained by this new method were not significantly different from those obtained by the traditional culture approach, and results were provided within 3 h. The sensitivity of the quantitative method is 200 cells per liter. The limit is due only to the filtration capacity of the membrane used.
The free-living amoeba Naegleria fowleri (3, 16), found in diverse freshwater environments, produces a rapidly fatal primary amoebic meningoencephalitis after exposure to contaminated water (7, 11, 12). N. fowleri infects mostly young and healthy people swimming in contaminated water. Symptoms occur in a few days, followed by a dramatic clinical course and death. Therefore, risk prevention is essential and necessitates environmental monitoring using a rapid and accurate assay to distinguish pathogenic N. fowleri from other free-living amoeba in water samples.
Current methods for detection and enumeration of Naegleria species are based on culture techniques (8) followed by identification using monoclonal antibodies (19, 21), PCR (10, 20), or enzyme electrophoresis (15). Additionally, isolates are tested for pathogenicity in mice. These methods are time-consuming, and novel methods are being developed to increase the sensitivity and rapidity of detection and thus reduce the amount of time required to obtain results. The main challenges for the development of an assay are to provide tools for the real-time monitoring of the pathogen in the aquatic environment which are highly quantitative and sensitive.
Epifluorescence microscopy and flow cytometry are commonly used for the detection and enumeration of cells after fluorescent staining (1, 6). However, none of these techniques can be applied to the detection of low concentrations of pathogens in the aquatic environment because of their low quantitative sensitivity (10). The ChemScan system (Chemunex, Ivry, France) is a recently developed solid-phase cytometer that uses fluorescent labeling of microorganisms after concentration of organisms by filtration on a membrane in combination with an automated detection and counting system (13, 23). Solid-phase cytometry is the only technique that allows the accurate enumeration of rare events (down to one cell on a filtration membrane), providing the same sensitivity as traditional culture methods (10). This system can be applied to the detection of specific microorganisms when combined with the use of taxonomic probes such as fluorescent antibodies (17, 19).
The aim of this work was to develop an immunofluorescent assay for the detection of N. fowleri in water by solid-phase cytometry. We have developed and compared two staining procedures using the monoclonal antibody Ac5D12, which specifically reacts with the three forms of N. fowleri: cysts, trophozoites, and flagellates (19). The two staining procedures were based on the use of two conjugated antibodies. One antibody was conjugated with biotin and revealed by streptavidin labeled with a tandem conjugate: R-phycoerythrin (RPE) conjugated with the cyanine dye Cy5 (RPE-Cy5). The second antibody was conjugated with horseradish peroxidase (HRP) and revealed by fluorescein isothiocyanate (FITC)-conjugated tyramide. Both protocols were optimized and compared on pure cultures before being applied to the detection of free-living amoebae in naturally contaminated waters. Direct counts were compared to those obtained by culture.
MATERIALS AND METHODS
Amoeba strains and growth conditions.
Two environmental strains of N. fowleri were used in this study: Na420c and By 99.2.3.f15a isolated from the Bugey and Cattenom sites, two nuclear power stations of Electricité de France (Paris, France) located on the Rhône and Moselle rivers, respectively. Na420c was maintained axenized in Chang's medium (5) and incubated in 50-ml Erlenmeyer flasks at 37°C, whereas By 99.2.3.f15a was grown at 43°C on nonnutrient agar (NNA) plates spread with Escherichia coli (CAPSIS, Les Ulis, France).
Cultures and natural samples.
Amoebae were harvested from axenic cultures to collect vegetative forms. After decantation and elimination of Chang's medium, cells were resuspended in phosphate-buffered saline (PBS; pH 7.2), stored on ice for 15 min in order to reduce cell attachment to the flask walls, and then vortexed for 5 min. This procedure allows reduction of membrane damage and cellular fragmentation. Cysts could not be obtained from axenic cultures. For cyst production, By 99.2.3.f15a was grown on NNA plates previously spread with E. coli and incubated at 43°C for 5 days. Cysts were harvested in 2 to 3 ml of Ringer's solution (Merck, Darmstadt, Germany) by gentle scraping of encysted areas using a Pasteur pipette with a tapered tip bent at 90°. In all cases, cell concentrations were determined by counting four replicate samples on a Thoma hemacytometer. Then cell suspensions were fixed (2% formaldehyde, final concentration) and stored at 4°C in the dark until analysis.
For natural samples, contaminated water samples were collected during the summers of 2000 and 2001 at different power plants. Samples were collected in the cooling effluents of two nuclear power stations located on the Seine river (Nogent) and on the Rhône river (Bugey). Samples were collected in the cooling system of these stations with a 1-liter plastic bottle. An aliquot was used immediately to determine the viable counts of the amoebae and the remaining volume was fixed with 2% formaldehyde (final concentration) and stored at 4°C in the dark until further analyses.
Spread plate count.
The most-probable-number (MPN) method was used to determine the concentration of viable N. fowleri in natural waters (4). Immediately after collection, the sample was suspended by magnetic stirring, and 10 replicate subsamples of 1 and 0.1 ml were spread onto NNA plates previously overlaid with E. coli. Plates were incubated at 43°C, and the presence of amoebae was determined daily for 5 days by microscopic examination (14). To confirm the Naegleria genus, positive samples were further analyzed to determine enflagellation induced by suspending vegetative forms in demineralized water at 37°C for 2 h (2). Then the species was identified by an enzyme-linked immunosorbent assay (Indicia Biotechnology, Oullins, France) using monoclonal antibody 5D12.
Filtration procedure.
Natural water samples were treated with ultrasonic irradiation to eliminate cell adherence on bottle walls for 30 min with a Branson 2510 sonicator (Bransonic) operated at 42 kHz ± 6%. Fixed samples were filtered under a maximum vacuum of 70 mm of mercury through polycarbonate white membranes (pore size, 2 μm; diameter, 25 mm; Millipore). The membranes were chosen after a preliminary comparison study in which different types and porosities of membranes were compared (data not shown). The volume of sample was dependent on the concentration of suspended matter. For cultures, the filtered volume was determined from the concentration of the suspension in order to obtain 50 to 150 trophozoites or cysts on the membrane. For natural samples, the procedure consisted of filtering the highest possible volume which was determined by the concentration of suspended matter. This volume was generally in the range of 2 to 10 ml.
Labeling techniques.
Two immunofluorescence staining procedures were compared. Amoebae were revealed by using specific monoclonal antibody 5D12 (Indicia Biotechnology) described by Sparagano et al. (20) and Reveiller et al. (18). The antibody was diluted at different concentrations in PBS (8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4, and 0.24 g of KH2PO4 per liter, pH 7.2) containing 0.1% Tween 20 and 2% bovine serum albumin (PBS-T-BSA). All reagents were from Sigma Chemical Co. (St. Louis, Mo.). The antibody was conjugated with biotin or HRP and revealed by streptavidin conjugated with RPE-Cy5 or FITC-conjugated tyramide, respectively (see below for protocols). All incubations were performed in petri dishes to keep the membrane humid.
Streptavidin-RPE-Cy5 detection.
After filtration, the membrane was transferred onto a 100-μl drop of a labeling solution: a 1:250 dilution of biotin-antibody in PBS-T-BSA containing 4′,6′-diamidino-2-phenylindole (DAPI; 2.5 μg. ml−1, final concentration; Sigma). DAPI was included in the staining solution for DNA labeling. Incubation was performed in a petri dish (Gelman Sciences, Arbor, Mich.) for 30 min at room temperature (RT). Cells were washed by transferring the membrane onto an absorbent pad (Chemunex, Ivry-sur-Seine, France) saturated with 650 μl of PBS-T-BSA solution for 1 min. Then the membrane was incubated in a 100-μl drop of a 1:20 solution of RPE-Cy5-conjugated streptavidin (Dako, Glostrup, Denmark) in PBS-T-BSA. Incubation was carried out for 30 min at RT in the dark, and the membrane was rinsed by single transfer onto a pad soaked with 650 μl of PBS-T-BSA.
Tyramide-FITC detection.
The use of tyramide conjugates for fluorescence labeling is sometimes difficult or impossible due to the presence of natural organisms producing peroxidase and contributing to the precipitation of tyramide conjugates. In preliminary assays, we observed the presence of naturally occurring microorganisms producing peroxidase activity (data not shown). Therefore, to avoid the possible overlap between fluorescent signals provided by both sources of peroxidase, the cells were pretreated with hydrogen peroxide to eliminate natural peroxidase activity before the labeling step. After filtration, the membrane was incubated onto 100 μl of a H2O2 (Sigma) solution (3%, vol/vol) and incubated at RT for 15 min. Then the membrane was rinsed by transfer onto an absorbent pad soaked with 650 μl of PBS-T-BSA and incubated for 1 min at RT to eliminate the excess H2O2 before labeling. For cell labeling, the membrane was incubated with 100 μl of labeling solution containing a 1:1,000 dilution of HRP-conjugated antibody and 2.5 μg of DAPI ml−1 (final concentration). The membrane was incubated for 30 min at RT and rinsed as described previously. The detection step was performed by transfer on 100 μl of a solution containing a 1:50 dilution of FITC-conjugated tyramide (NEN, Boston, Mass.). Incubation was stopped after 15 min in the dark at RT, and the membrane was rinsed by single transfer onto a pad soaked with 650 μl of PBS-T-BSA.
Enumeration of labeled cells by solid-phase cytometry.
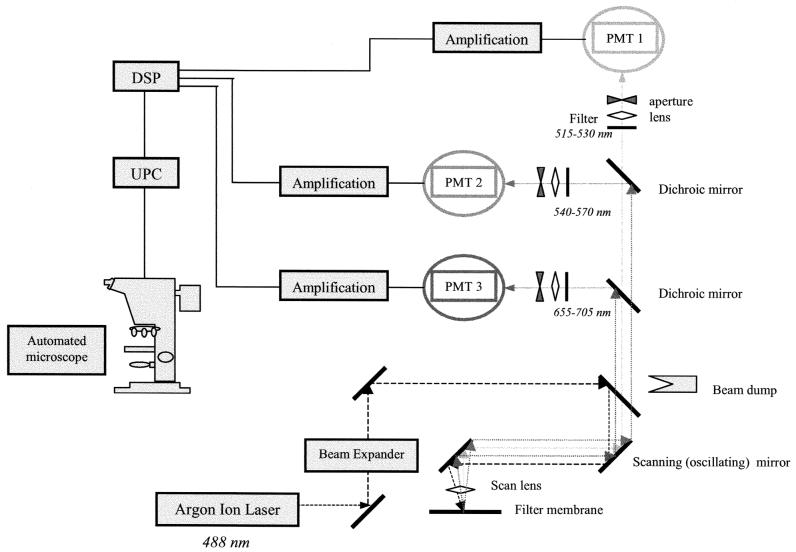
After labeling, the membrane was transferred onto the sample holder of the ChemScan solid-phase cytometer (Chemunex) (Fig. 1). The ChemScan laser scanning device is described elsewhere (9, 13). The system is able to differentiate between labeled microorganisms and autofluorescent particles present in the sample based on the optical and electronic characteristics of the generated signals (22). The scanning procedure ensures a full overlapping of each scan line, and therefore, a single labeled cell present on the membrane can be detected on different lines by the multiple detection channels within 3 to 4 min. The position of each detected event on the membrane is displayed. Then the ChemScan sample holder, with the membrane in place, can be transported to an epifluorescence microscope for validation (see below).
FIG. 1.
Optical configuration of the ChemScan system. PMT, photomultiplier tube; DSP, digital signal processor; UPC, user computer.
Light was provided by a water-cooled argon laser emitting at 488 nm. The fluorescence emission was collected in the green channel (500 to 530 nm) for FITC and in the red channel (655 to 705 nm) for RPE-Cy5. In this study, the holder was previously overlaid with a support pad (black membrane; Chemunex) soaked with 100 μl of mounting medium (Chemunex) and recovered with a CB04 membrane (Chemunex) for fluorescein labeling to optimize detection of fluorescent signals and the focusing procedure.
Two procedures of discrimination between amoebae and background could be used according to the manufacturer's instructions (13). The first used automatic validation. The signals were analyzed with MatLab-based software (Matworks, Natick, Mass.) for comparison with Gaussian curves and to remove non-Gaussian signals, which are sometimes generated by nonbacterial autofluorescent particles (13). A set of discriminants was applied to all fluorescent events detected after the entire surface of the membrane had been scanned, which then allows the differentiation of labeled microorganisms from autofluorescent particles present in the sample. At the end of the analysis procedure, the exact position of each detected cell on the membrane was displayed; this spatial information can be used to drive the motorized stage of the microscope, allowing immediate visual result confirmation by epifluorescence microscopy. All events positively selected after the discrimination process are validated by microscopic examination as true positive or false positive. In addition, when this automatic discrimination procedure was not used, all detected fluorescent events including background signals were counted by the cytometer and were then manually discriminated under the microscope using the motorized stage as described above. The two procedures were applied to all experiments, and data obtained from both cells and particles were used to optimize the setting of the instrument and the automatic discrimination procedure. When doubts as to the nature of the event observed during validation under the microscope with epifluorescence remain, it is possible to use the dichroic filter specific for DAPI and thus to confirm the nature of the event observed, cell or particle.
The calibration of the instrument was tested daily with fluorescent beads emitting green and red fluorescent signals. Standard C beads (Chemunex) were used to calibrate green fluorescence, whereas 1-μm-diameter transfluorosphere beads (T-8883; Molecular Probes, Leiden, The Netherlands) were used for red signals.
Expression of results and statistical analyses.
The mean numbers of cells were determined from three to five replicates, and mean values were compared by Student's t test. A regression model was used to compare viable counts (culture) from natural water with total counts obtained by cell labeling and direct enumeration by solid-phase cytometry. All analyses were performed with Statgraphics Plus software (Fontsoftware; Bitstream, Inc., Cambridge, Mass.) running on a PC.
RESULTS AND DISCUSSION
Comparison of two staining procedures.
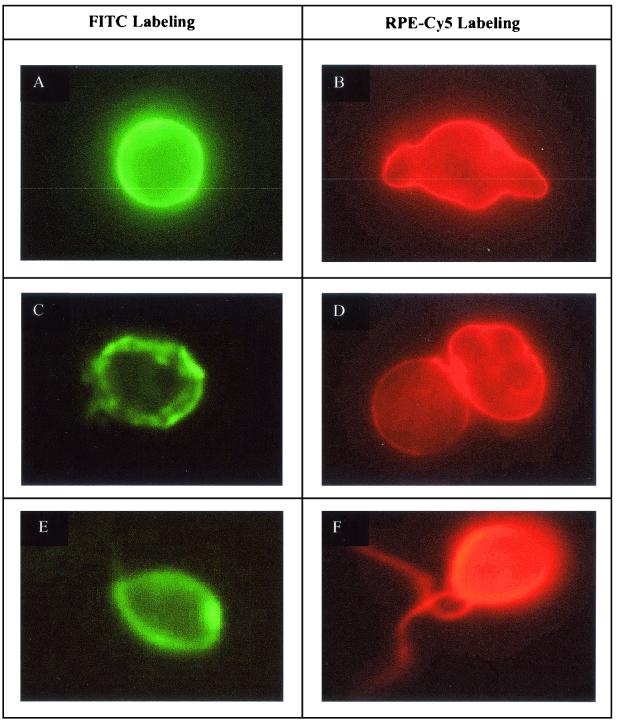
Two techniques were used to stain trophozoites (axenic cultures) and cysts (monoxenic cultures) forms of N. fowleri. Representative pictures of the two labeling procedures are shown in Fig. 2. The efficiency of each staining protocol was evaluated from two parameters determined by solid-phase cytometry: the peak of fluorescence intensity and the heterogeneity of fluorescence signals as determined by the coefficient of variation (CV) (mean value divided by the standard error). The best procedure was determined by optimizing the signal/noise ratio.
FIG. 2.
Microscopic photographs of N. fowleri stained by immunofluorescence and two fluorochromes, FITC and RPE-Cy5. (A and B) Labeled trophozoites from axenic culture samples; (C and D) labeled cyst from monoxenic samples; (E and F) labeled flagellates from natural water samples.
The results obtained from a wide diversity of samples were pooled and obtained from the analyses of approximately 1,000 cells (Table 1). The best labeling technique appears to be that generating the highest peak of fluorescence intensity, the lowest CV, and the lowest fluorescence background. Cells labeled with tyramide-FITC showed great differences between trophozoites and cysts and also an important heterogeneity of fluorescent signals for cysts. This heterogeneity may be due to the low reproducibility of the tyramide precipitation induced by enzymatic activity. However, the peak intensity of vegetative forms was the highest recorded in this assay. In contrast, the peak intensity of cysts was much lower and varied greatly. The lowest fluorescence intensity recorded for cysts was very similar to that of nonspecific fluorescent signals.
TABLE 1.
Comparison of peak intensity (PI) mean by the two staining procedures (FITC and RPE-Cy5) on vegetative and cystic forms by solid-phase cytometry
| Fluorescent dye | Morphological form | No. of N. fowleri amoebae validateda | PI | CV |
|---|---|---|---|---|
| RPE-Cy5 | Trophozoites | 1,063 | 5,647 | 3.11 |
| Cysts | 1,092 | 3,949 | 1.76 | |
| FITC | Trophozoites | 981 | 17,139 | 4.20 |
| Cysts | 1,049 | 3,085 | 0.95 |
Obtained after microscopic validation.
The difference between the two cellular forms was greatly reduced when staining was performed with streptavidin-RPE-Cy5. Nevertheless, although the mean fluorescence intensity of trophozoites was lower than that recorded for FITC-labeled cells, the mean fluorescence intensity of cysts was higher with RPE-Cy5. Furthermore, in all cases the CV was higher than or similar to those reported for the tyramide-FITC labeling procedure. This suggests that the cellular fluorescence is more homogeneously distributed within the amoeba population. Although the flagellated form was not systematically studied because of its transient nature, the cellular signals obtained from these cells were similar to those provided by trophozoites.
Comparison of cell counts obtained by the two labeling procedures.
Cell counts were also compared by applying the two protocols to cellular suspensions of trophozoites and cysts. At least three replicate analyses were performed on each sample. This approach was used also to assess the efficiency of solid-phase cytometry to detect all labeled cells. The numbers of cystic and vegetative forms obtained by staining with FITC were 118 ± 6.6 (n = 3) and 99.6 ± 15.6 (n = 5), respectively. When cells were stained with RPE-CY5, the numbers were 113.3 ± 7.15 (n = 3) and 101.25 ± 121.99 (n = 5), respectively. For both trophozoites and cysts, the counts determined by the two staining protocols were not significantly different (t test, P > 0.05). This suggests that all cells were labeled by both techniques and could be discriminated by the ChemScan. Furthermore, no significant differences between replicate values obtained for a given cellular form and protocol were reported, suggesting that the labeling procedure was very reproducible.
Detection of amoebae in natural waters.
Different water samples were collected in the cooling effluent from two nuclear power stations, and analyses were performed on five replicate samples using the two staining procedures (Tables 2 and 3).
TABLE 2.
Comparison of N. fowleri counts in natural water obtained with two labeling procedures (FITC and RPE-Cy5) and plate counts (MPN)a
| Sample date (date/mo./yr)b | Fluorescent dye | ChemScan resultc | No. of validated cellsd | No. of amoebae/liter
|
|
|---|---|---|---|---|---|
| ChemScan | MPN | ||||
| 06/07/00 | RPE-Cy5 | 244.20 ± 33.63 | 13.6 ± 4.72 | 3,047 ± 1,058 | 2,872 ± 1,054 |
| FITC | 121.00 ± 78.42 | 2.60 ± 1.34 | 583 ± 292 | 2,872 ± 1,054 | |
| 11/07/00 | RPE-Cy5 | 166.00 ± 69.45 | 2.80 ± 1.30 | 628 ± 292 | 428 ± 218 |
| FITC | 22.20 ± 6.94 | 0.40 ± 0.55 | 90 ± 110 | 428 ± 218 | |
Values are means ± standard deviations.
Water samples were collected from the Bugey nuclear power plant site.
Counts determined by the ChemScan system after discrimination.
Counts obtained after microscopic validation.
TABLE 3.
Detection of N. fowleri in natural waters by RPE-Cy5 labeling associated with solid-phase cytometry (ChemScan) and plate counts (MPN)a
| Samples date (day/mo./yr)b | ChemScan resultc | No. of validated cellsd | No. of N. fowleri amoebae/liter
|
|
|---|---|---|---|---|
| ChemScan | MPN | |||
| 07/05/01 | 141.20 ± 52.70 | 13.40 ± 3.51 | 7,504 ± 1,964 | 5,423 ± 2,336 |
| 12/05/01 | 68.40 ± 51.55 | 14.40 ± 4.39 | 16,128 ± 4,920 | 16,095 ± 6,324 |
| 15/05/01 | 33.80 ± 11.67 | 12.80 ± 4.02 | 6,048 ± 2,183 | 5,423 ± 2,336 |
| 17/05/01 | 426.60 ± 44.25 | 8.00 ± 1.58 | 4,480 ± 885 | 3,477 ± 1,337 |
| 18/05/01 | 321.60 ± 153.35 | 4.00 ± 2.35 | 2,240 ± 1,313 | 1,959 ± 689 |
Values are means ± standard deviations of five subsamples.
Water samples were collected from Nogent nuclear power plant site.
Counts determined by the ChemScan after discrimination.
Counts obtained after microscopic validation.
The counts determined by the ChemScan after the automatic discrimination by the instrument and those obtained after microscopic validation are reported in Table 2. For samples collected at the Bugey station, only 5 ml could be filtered on the membranes because of the presence of a large amount of organic and inorganic particles. The primary counts varied greatly between membranes for a given sample, and these variations were more important when the tyramide-FITC protocol was used (data not shown). Furthermore, results and validation counts were much higher when cells were stained with the streptavidin-RPE-Cy5 protocol and the amoeba counts were comparable to those obtained by the traditional plate count technique. A similar trend was found for samples collected on two different dates. The significant differences (t test, P > 0.05) between the two protocols can be explained by the fact that nonspecific particles occurring in the natural environment provide more important emissions in the green than in the red channel. The automatic discrimination used to generate the results was very efficient, since more than 95% of the nonspecific signals were eliminated (data not shown). However, this discrimination process alone was not enough to provide accurate and definitive cell counts. The microscopic examination was necessary to eliminate the remaining nonspecific events.
The microscopic examination of nonspecific signals detected in the green fluorescence channel (FITC labeling) and taken into account in the primary counts revealed that cells closely associated with particles are considered single particles by the ChemScan and are therefore not considered further in the results. Consequently, amoeba counts were underestimated when cells were stained with FITC. Inversely, when these particle-attached cells are detected in the red channel, they are considered cells and not particles, because the red fluorescence emission of the particle is very low. The most appropriate immunofluorescence protocol was the streptavidin-RPE-Cy5 staining procedure. This method was used to further compare viable counts obtained by the MPN method with those found by immunofluorescence and solid-phase cytometry (Table 3).
Precision of counts determined by solid-phase cytometry.
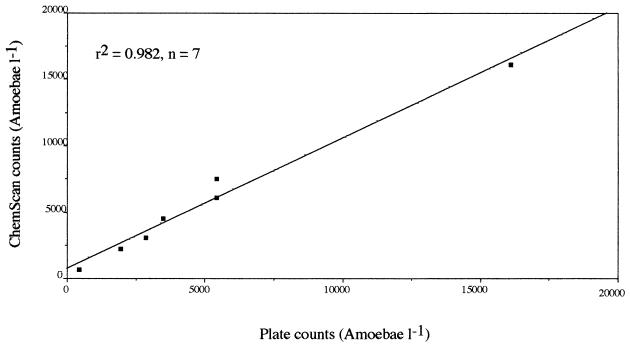
The accuracy of amoeba counts was estimated by comparing the two counting methods applied to natural samples (Tables 2 and 3). Although the ChemScan results were always higher than those determined by the MPN procedure, the results of the two methods were very close, and no significant difference was found (t test, P > 0.05). The correlation between counts obtained by cytometry and cell culture was very significant (Fig. 3). This suggests that the two methods provide similar results, due to the high standard deviations associated with the counts. In most cases, the standard deviation was higher for MPN results, suggesting a lower reproducibility of this counting procedure. However, the higher counts obtained by direct counting also may be explained by the detection of nonviable organisms.
FIG. 3.
Correlation between solid-phase cytometry and MPN counts of N. fowleri in natural water samples at different concentrations.
Conclusions.
The immunofluorescence method developed in this study proved very efficient for the direct and rapid detection of amoebae in natural waters. The solid-phase cytometer allows the detection of a single cell in a filtered volume in less than 3 h, whereas the traditional method requires at least 5 days to provide a diagnostic result. If we assume that the volume that can be filtered is in the range of 2 to 5 ml, the detection limit of amoebae is in the range of 200 to 500 cells per liter. One way to improve the detection limit is to increase the filtration capacity, which is limited by saturation of the membrane porosity by nonspecific particles; alternatively, an immunomagnetic sorting process could be used prior to filtration in order to physically separate the cells from nonspecific particles. The detection of N. fowleri in surface waters by solid-phase cytometry provides a very efficient method for the real-time monitoring of aquatic ecosystems. However, this immunofluorescent assay did not provide information on the viability of the detected cells. Further investigations will be performed to combine the antibody with a viability fluorescent probe. This multiparametric approach will be developed using the three fluorescent channels (PMT1 to PMT3) actually available on the solid-phase cytometer.
Acknowledgments
We are grateful to C. Oger and J. Deschamps for technical assistance, F. Marciano-Cabral for improving the English style and grammar of the article.
This work was supported by Electricité de France (Recherche et Développement). The ChemScan solid-phase cytometer at the Observatoire Océanologique of Banyuls-sur-Mer was funded by grants from Chemunex and the Région Languedoc-Roussillon.
REFERENCES
- 1.Boraziani, R. N., L. I. May, J. A. Noble, S. V. Avery, and D. G. Ahearn. 2000. Flow cytometry for determination of the efficacy of contact lens disinfection solutions against Acanthamoeba spp. Appl. Environ. Microbiol. 66:1057-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cable, B. L., and D. T. John. 1986. Conditions for maximum enflagellation in Naegleria fowleri. J. Protozool. 33:467-472. [DOI] [PubMed] [Google Scholar]
- 3.Carter, R. F. 1970. Description of a Naegleria sp. isolated from two cases of primary amoebic meningo-encephalitis and of the experimental pathological changes induced by it. J. Pathol. 100:217-244. [DOI] [PubMed] [Google Scholar]
- 4.Champsaur, H. 1996. Méthodes générales d'examen bactériologique des eaux, p. 755-756. In J. Rodier (ed.), L'analyse de l'eau. Dunod, Paris, France.
- 5.De Jonckeere, J. F. 1977. Use of an axenic medium for differentiation between pathogenic and nonpathogenic Naegleria fowleri isolates. Appl. Environ. Microbiol. 33:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faude, U. C., and M. G. Höfle. 1997. Development and application of monoclonal antibodies for in situ detection of indigenous bacterial strains in aquatic ecosystems. Appl. Environ. Microbiol. 63:4534-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.John, D. T. 1982. Primary amoebic meningoencephalitis and the biology of Naegleria fowleri. Annu. Rev. Microbiol. 36:101-123. [DOI] [PubMed] [Google Scholar]
- 8.John, D. T., and M. J. Howard. 1996. Techniques for isolating thermotolerant and pathogenic free-living amebae. Folia Parasitol. 43:267-271. [PubMed] [Google Scholar]
- 9.Joux, F., and P. Lebaron. 2000. Use of fluorescent probes to assess physiological functions of bacteria at single-cell level. Microb. Infect. 2:1523-1535. [DOI] [PubMed] [Google Scholar]
- 10.Kilvington, S., and J. Beeching. 1995. Identification and epidemiological typing of Naegleria fowleri with DNA probes. Appl. Environ. Microbiol. 61:2071-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemarchand, K., N. Parthuisot, P. Catala, and P. Lebaron. 2001. Comparative assessment of epifluorescence microscopy, flow cytometry and solid-phase cytometry used in the enumeration of specific bacteria in water. Aquat. Microb. Ecol. 25:301-309. [Google Scholar]
- 12.Marciano-Cabral, F. 1988. Biology of Naegleria spp. Microbiol. Rev. 52:114-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez, A. J. 1983. Free-living amoebae. Pathogenic aspects. A review. Protozool. Abstr. 7:293-306. [Google Scholar]
- 14.Mignon-Godefroy, K., J. G. Guillet, and C. Butor. 1997. Solid phase cytometry for detection of rare events. Cytometry 27:336-344. [PubMed] [Google Scholar]
- 15.Page, F. C. 1988. An illustrated key to freshwater and soil amoebae. Scientific publication no. 34. Freshwater Biological Association, Kendal, Cumberland, United Kingdom.
- 16.Pernin, P., M.-L. Cariou, and A. Jacquier. 1985. Biochemical identification and phylogenetic relationships in free-living amoebas of the genus Naegleria. J. Protozool. 32:592-603. [DOI] [PubMed] [Google Scholar]
- 17.Pyle, B. H., S. C. Broadaway, and G. A. McFeters. 1999. Sensitive detection of Escherichia coli O157:H7 in food and in water by immunomagnetic separation and solid-phase cytometry. Appl. Environ. Microbiol. 65:1966-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reveiller, F. L., F. Marciano-Cabral, P. Pernin, P. A. Cabanes, and S. Legastelois. 2000. Species specificity of a monoclonal antibody produced to Naegleria fowleri and partial characterization of its antigenic determinant. Parasitol. Res. 86:634-641. [DOI] [PubMed] [Google Scholar]
- 19.Rushton, P., B. M. Place, and N. F. Lightfoot. 2000. An evaluation of a laser scanning device for the detection of Cryptosporidium parvum in treated water samples. Lett. Appl. Microbiol. 30:303-307. [DOI] [PubMed] [Google Scholar]
- 20.Sparagano, O., E. Drouet, R. Brebant, E. Manet, G. Denoyel, and P. Pernin. 1993. Use of monoclonal antibodies to distinguish pathogenic Naegleria fowleri (cysts, trophozoites, or flagellate forms) from other Naegleria species. J. Clin. Microbiol. 31:2758-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sparagano, O., and A. Revol. 1995. Isolation of the freshwater Naegleria fowleri from a river using a monoclonal antibody and the polymerase chain reaction. Acta Hydrobiol. 37:29-32. [Google Scholar]
- 22.Visvesvara, G. S., M. J. Peralta, F. H. Brandt, M. Wilson, C. Aloisio, and E. Franko. 1987. Production of monoclonal antibodies to Naegleria fowleri, agent of primary amoebic meningoencephalitis. J. Clin. Microbiol. 25:1629-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walner, G., D. Tillman, K. Haberer, P. Cornet, and J. L. Drocourt. 1997. The ChemScanTM system: a new method for rapid microbiological testing of water. Eur. J. Parenter. Sci. 2:123-126. [Google Scholar]