Abstract
Abstract
Aggressive territoriality can have significant benefits for resource acquisition yet is a costly behaviour. Selection should therefore favour mechanisms that allow individuals to modify their behaviour to maintain and defend their territory whilst minimising costs. One such mechanism between intraspecific territorial competitors is neighbour-stranger discrimination. The familiarity hypothesis suggests that territory holders will respond less aggressively to neighbours they recognise than to strangers they do not recognise. Conversely, in systems where neighbours pose a greater threat to territory loss than strangers, the threat-level hypothesis predicts that neighbours will elicit a greater aggressive response. We tested these opposing hypotheses in territorial farming damselfishes Stegastes diencaeus using a common bottle presentation experiment design to initiate aggressive responses by territory holders to neighbouring and non-neighbour individuals. Neighbours that were smaller in body size than the territory holder elicited the greatest aggressive response, whereas larger neighbours elicited the weakest. The aggressive response elicited by non-neighbours did not vary greatly with body size difference between the stimulus fish and territory holder. We demonstrate that aggressive response in territorial farming damselfishes is influenced by both familiarity and potential threat determined by body size. These findings add to the growing pool of evidence that an understanding of multiple factors is needed to identify the drivers of neighbour-stranger discrimination.
Significance statement
Both familiarity and body size may mediate aggressive behaviour yet are not often included in the same study. Using manipulative field experiments, we investigated the interplay between familiarity and body size in shaping patterns of aggressive behaviour in farming damselfishes. We found that territory holders were less aggressive towards neighbours than non-neighbours, but only when they were larger than themselves. Our results showing an interaction between the effects of familiarity and body size on aggressive behaviour may hint at nuances in patterns of neighbour-stranger discrimination, such as dominance relationships.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00265-025-03636-x.
Keywords: Neighbour-stranger discrimination, Intraspecific aggression, Territoriality, Familiarity hypothesis, Dear enemy
Introduction
Territoriality is widespread across the animal kingdom yet carries significant cost in the form of aggression. Aggressive behaviour for the purpose of territory defence increases energy expenditure (Marler et al. 1995; Neat et al. 1998) and the risk of injury (Clutton-Brock and Huchard 2013). Thus, territory holders are expected to modify their behaviour in such a way that reduces these costs while still upholding their territory and minimising resource loss by intrusion. One such mechanism between intraspecific competitors is neighbour-stranger discrimination, where territory holders recognise and react differently towards neighbouring individuals than strangers (Ydenberg et al. 1988; Temeles 1994).
Territorial animals often demonstrate reduced aggression towards neighbours than strangers, termed the “dear enemy” effect. There are two alternative hypotheses that aim to explain this effect. The first, the familiarity hypothesis, predicts that frequent past encounters with the same individual will result in reduced conflict and aggression in subsequent encounters (Ydenberg et al. 1988; Temeles 1994). It is proposed that animals engage in fights to gain information about their opponent (Getty 1989). Neighbours have much less to learn about each other than strangers, resulting in fewer or reduced escalation of fights (Getty 1989). Similarly to familiarity, habituation to the same individual, such as neighbours who inevitably come into contact with each other more often, can decrease the intensity of subsequent encounters (Bee and Gerhardt 2001; Leiser 2003; Lehtonen and Wong 2017).
Alternatively, the threat-level hypothesis emphasises the importance of potential threat in determining aggressive response. When neighbours and strangers differ in their potential threat, the aggressive response of territory holders should be strongest towards the greatest threat (Temeles 1994). Established territory lines between individuals may lead to reduced conflict between neighbours, as observed in the root vole Microtus oeconomus (Rosell et al. 2008) and territorial cichlid fish Neolamprologus pulcher (Sogawa et al. 2016), conserving time and energy, whereas roving strangers seeking a territory of their own may be perceived as a greater threat to territory loss (Wilson 1975). Familiarity may also drive increased use of submissive behaviours (Hick et al. 2014).
Whilst the dear enemy effect has been widely documented (see Werba et al. 2022 for review), in some territorial species, neighbours elicit a stronger aggressive response than strangers, termed the “nasty neighbour” effect (e.g. Temeles 1990; Müller and Manser 2007; Newey et al. 2010; Munguía-Steyer et al. 2016). Typically, this effect is observed in systems where neighbours pose a greater threat than strangers. For example, intense competition may continue after territory boundaries are established if neighbours are continually trying to expand their territory (Müller and Manser 2007; Munguía-Steyer et al. 2016). The nasty neighbour effect may be more pronounced in species that hold multi-purpose, breeding territories, such as the Northern harrier Circus cyaneus (Temeles 1994), as territory holders stand to lose fitness as well as resources. Nasty neighbour effects may also be mediated by social status, as observed in crayfish Procambarus clarkii, where dominant females were preferred to fight with familiar subordinates (Tierney et al. 2013). It has also been suggested that social animals may exhibit nasty neighbour effects more often (Müller and Manser 2007; Newey et al. 2010), as strangers often represent smaller groups, and therefore a lower threat to territory takeover (Müller and Manser 2007).
A fundamental determinant of aggressive decisions, which is often overlooked in studies of neighbour-stranger discrimination, is differences in body size between opponents. Body size difference may mediate aggression by determining resource holding potential (RHP) (Green and Patek 2018) and social status (i.e. dominance). Larger individuals are expected to invest more in aggressive encounters than smaller individuals as they have a greater RHP and therefore a greater chance of winning a fight (Parker 1974). Social status is also often determined by differences in body size, as larger individuals with greater RHP gain dominance through past wins. As aggression is a costly behaviour, selection should favour aggression that is directed in such a way that maintains an individuals’ social status (Dehnen et al. 2022). Thus, it is expected that dominant individuals direct aggression to subordinates directly below them in the hierarchy (Dehnen et al. 2022). Both RHP and social status predict that individuals will display greater aggression towards smaller conspecifics (e.g. Green and Patek 2018; Tierney et al. 2013; Wright et al. 2019). Combining familiarity and body size differences in the same study will help to unearth potential nuances in neighbour-stranger discrimination.
Neighbour-stranger discrimination is observed in a variety of taxa, including birds (Temeles 1990; Godard 1993; Moser-Purdy et al. 2017), mammals (Müller and Manser 2007; Siracusa et al. 2017), invertebrates (Newey et al. 2010; Tierney et al. 2013; Munguía-Steyer et al. 2016) and fish (Leiser 2003; Lehtonen and Wong 2017; Sogawa and Kohda 2018). However, the mechanisms behind such discrimination have been inferred from studies limited in taxonomic breadth, with a disproportionate investment in birds, particularly breeding males (Werba et al. 2022). This imbalance limits our understanding of the generality of trends in neighbour-stranger discrimination across taxa (Werba et al. 2022). Marine species in particular have received very little attention, likely because of the challenges of underwater research. Moreover, the few studies testing the effect of familiarity on aggression in marine species have primarily worked in laboratory settings (e.g. Tricarico et al. 2011; Silveira et al. 2020). Though laboratory testing allows for greater manipulation and control and provides important insight into the drivers of such discrimination, investigating neighbour-stranger discrimination in the field, with the trade-off of smaller sample sizes, is critical to understanding how this phenomenon impacts processes in complex ecological systems.
Territorial farming damselfishes present an ideal model system to explore neighbour-stranger discrimination in the field. Individual farming damselfish of both sexes hold small contiguous territories which they aggressively defend from intra- and interspecific intruders. Encounters between neighbouring damselfishes along shared boundaries are frequent. Species of the genus Stegastes are some of the most aggressive (Ceccarelli et al. 2001) and hold multipurpose territories used for cultivating turf algae and, in the case of males, a space to care for and protect eggs. These species are highly site-attached (Itzkowitz et al. 1995; McDougall and Kramer 2007), taking occasional short forays (< 7 m; Stegastes planifrons; Itzkowitz 1978) outside of their territories. In Stegastes diencaeus, studies have found no evidence of nonterritorial roving individuals and infrequent relocation of territories by adults (McDougall and Kramer 2007) suggesting that individuals hold the same territory for long periods of time. This lack of relocation provides the opportunity for habituation and familiarity between neighbours.
We tested for the presence of dear enemy effects in the territorial farming damselfish species Stegastes diencaeus. In addition, we include differences in body size to investigate the interplay between familiarity and body size in mediating patterns of neighbour-stranger discrimination. We presented captured S. diencaeus neighbours and non-neighbours to territory holders and measured subsequent aggressive response of the free individual. This present study had two main objectives;
Determine whether S. diencaeus territory holders discriminate between neighbours and non-neighbours in their aggressive response (Do S. diencaeus exhibit dear enemy or nasty neighbour?).
Explore how body size differences between S. diencaeus territory holders and intruders mediates neighbour-stranger discrimination.
Materials and methods
Field methodology
We conducted our study between 01 June and 14 July 2023 at Coral View reef, Utila, Honduras (N 16.088233, W −86.910945). As our study involved focal animals in the field, it was not possible to record data blind. Eighteen focal S. diencaeus were chosen opportunistically (depth range 4.9–11.1 m), with the criteria of having at least one intraspecific neighbour with adjoining territory. The species were identified based on (1) dorsal and anal fins extending well beyond the base of the tail, and (2) an electric blue edge to the anal fin (differentiating them from other Stegastes species). Territories were marked with biodegradable flagging tape, which is a reliable method of identification (Snekser et al. 2009); Weimann et al. 2018) because this species is highly site-attached (McDougall and Kramer 2007). Previous work recorded a mean territory size of 0.55 m2 for S. diencaeus at this site (Sheppard et al. 2024) (maximum 1.19 m2; unpublished data). Therefore, focal S. diencaeus were located at least 5 m away from each other to maximise independence of the samples.
The sex of focal S. diencaeus could not be determined as there is no sexual dimorphism in this species. As both sexes hold territories for the purpose of farming algae, but only male territories are used for egg laying and guarding, it can be expected that sex affects aggressive behaviour relating to reproduction only, such as egg guarding or mating behaviour. No evidence of eggs or nests was observed, therefore differences between sexes in aggression should not affect our results substantially. Larval damselfish disperse on average around 50 km, with self-recruitment (ratio of larvae returning to their home reef compared with larvae from other reefs) averaging 15% (Hogan et al. 2012; Puebla et al. 2012). Given that genetic relatedness is unlikely to be significantly different between neighbours and non-neighbours, any effect of genetic relatedness on aggression was discounted.
Bottle presentations
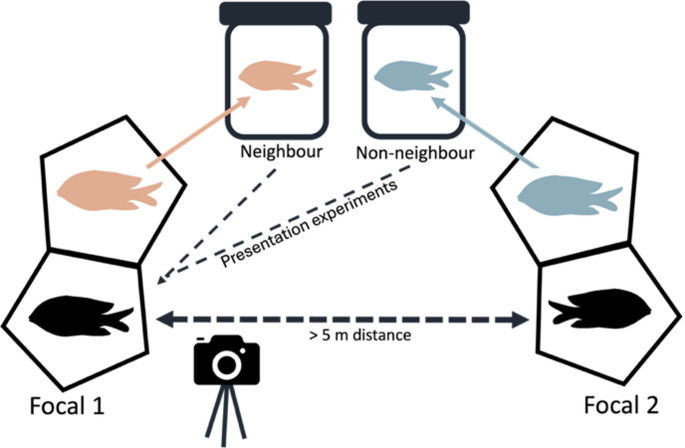
The aggressive response of S. diencaeus to intraspecific neighbours and non-neighbours was tested using a common presentation experiment (Harrington 1993; Haley and Müller 2002; Osório et al. 2006) which presents stimulus fish to focal individuals in clear bottles (Myrberg and Thresher 1974; Harrington 1993; Haley and Müller 2002; Osório et al. 2006; Fig. 1). We used 3 L clear plastic cylinders (14 × 24 cm) in which stimulus fish could swim freely, modified with mesh lids and perforated bottoms to allow waterflow. Teams of SCUBA divers captured two neighbouring S. diencaeus, one from each of two focal individuals, using barrier nets and spray bottles filled with a mix of ethanol and clove oil (3:1), a common fish anaesthetic (Whiteman and Côté 2002). Both captured S. diencaeus were used as stimulus fish for two focal individuals, offering a neighbour and non-neighbour stimulus, to reduce the number of animals required. Upon capture, stimulus fish within bottles were placed in their own territory, covered, and left to recover for at least 30 min. This also allowed any residual clove oil in the area to dissipate.
Fig. 1.
Bottle-presentation study design in respect to focal individual 1. Hexagons represent S. diencaeus territories. Black fish represent focal S. diencaeus, whilst orange and blue fish represent neighbouring S. diencaeus. Neighbouring S. diencaeus of two focal individuals (> 5 m apart), representing a neighbour and non-neighbour, were captured and contained in 3 L clear plastic bottles. After a period of acclimatisation, stimulus fish within their bottles were placed successively into the centre of the territory of the focal S. diencaeus and the aggressive response was video recorded for 3 min. The stimulus fish were presented to both focal individuals before being returned to their own territory
After the recovery period, stimulus fish within their bottles were placed successively into the centre of the territory of the focal S. diencaeus for 3 min, with the resultant behaviour recorded using a GoPro HERO camera (HERO Black 7|8|9) placed approximately 1 m away. Stimulus fish were presented from a different direction than their own territory. During presentations, SCUBA divers retreated to at least 2 m so as not to influence behaviour. This distance was deemed appropriate as S. diencaeus continue to display territorial behaviour in the presence of divers (per obvs), and teams had to be close enough on ethical grounds that they could observe the contained fish for signs of distress. The first ten focal S. diencaeus in our experiment were also presented with an empty bottle for 3 min to act as a control. As minimal response to the empty bottle was observed (see Results), control tests were discontinued, and subsequent focal individuals were presented with a neighbour and non-neighbour only. The order in which neighbours, non-neighbours and controls were presented was randomised using a random sequence generator and focal S. diencaeus were allowed to return to normal behaviour between presentations, confirmed by resumption of foraging or farming. Upon completion of the experiment, stimulus fish were returned and released back into their territories. During presentations to one focal S. diencaeus, a grouper, a known predator of damselfishes, intruded into the immediate area. This focal individual was removed from further analysis to exclude any effects of predator presence on aggressive behaviour. Our final dataset contained paired presentation experiments to 17 focal S. diencaeus. Due to the time restrictions of field experiments and SCUBA, each focal individual was tested once. The size of the presenting neighbour and non-neighbour was opportunistic.
Behavioural analysis and size measurement
Behavioural videos were analysed using the BORIS software V. 8.6.2 (Friard and Gamba 2016). We recorded the total number of bites and rams directed towards the stimulus over the 3 min presentations. As it was difficult to discern when contact with the bottle was made, the number of bites and rams were summed to give a total count of aggressive displays. The standard lengths of focal and stimulus S. diencaeus were measured using ImageJ software (Schneider et al. 2012). Screengrabs were taken from behavioural footage such that the fish were positioned parallel to the bottle, allowing the bottle to be used for scale. Due to the restricted accuracy of this method, body size differences between stimulus and focal fish were recorded binomially as smaller (mean: − 1.0 cm, range: − 2.5 to − 0.1 cm; Fig. S1) or larger (mean = 0.7 cm, range = 0 to 1.9 cm; Fig. S1), with respect to the focal. Given that containing individuals undoubtedly changes their behaviour, and our ability to correctly identify behaviours (e.g. attack or defence), the behaviour of the stimulus fish was not measured. Whilst the behaviour of the stimulus fish may influence the behaviour of the territory holder, this was not possible to control but should be minimised by their restricted movement and lack of physical contact. Due to practical constraints and the close familiarity between observer and test subjects, observations were not blinded.
Statistical analysis
All data manipulation and statistical analysis were conducted in R version 4.3.2 (R Core Team 2023). We ran Bayesian models using the brms package (Bürkner 2017) implemented in STAN (RStan 2023). We fitted total counts of aggressive displays against the type of stimulus (neighbour/non-neighbour) and body size difference (smaller/larger), with a Poisson distribution. The interaction effect between type of stimulus fish and body size difference was also included. We also fit total aggression against the type of stimulus fish and body size difference using subsets of data containing only larger or smaller conspecifics, and only neighbours and non-neighbours respectively (larger neighbours n = 6; smaller neighbours n = 11; larger non-neighbours n = 9; smaller non-neighbours n = 8). All models included focal damselfish ID as a grouping factor to account for individual variation in aggression. Weakly informative normal priors were used for all Bayesian models (Hadfield 2010). Models were run for 5000 iterations, with a warm-up of 1000 iterations over four chains. The adapt delta control parameter was increased to 0.95 or 0.99 to avoid any divergent transitions (Bürkner 2017). Model fit and convergence were visually validated using graphical posterior predictive checks, trace and density plots and Gelman-Ruban convergence diagnostic (R-hat) (Gelman and Rubin 1992). All models had an R-hat value of 1.00, signifying that the models had converged well. We tested our a priori hypotheses for our models using hypothesis testing. Hypothesis tests were two-way for comparing aggressive response to neighbours and non-neighbours (dear enemy or nasty neighbour) and one-way for comparing larger and smaller stimulus fish. For each test, we calculated the posterior probability (PP) and evidence ratios (ER). PP determines the probability to which our hypotheses were supported, and ER represents the extent to which the evidence supports our hypotheses compared with alternative hypotheses (Table 1). Note that two-way hypothesis testing tests whether two samples are the same, therefore posterior probability (PP) of below 95% indicates the samples are different.
Table 1.
Summary of bayesian hypothesis tests. Hypotheses relate to total aggression by territory holder towards stimulus conspecific
| Hypothesis | Median estimate (credible interval) | Posterior probability (PP) | Evidence ratio (ER) |
|---|---|---|---|
| Two-way hypothesis testing | |||
| Neighbour = non-neighbour | 0.36 (0.10–0.61) | 0.74 | 2.8 |
| Larger neighbour = larger non-neighbour | 0.13 (−0.54–0.79) | 0.98 | 40.25 |
| Smaller neighbour = smaller non-neighbour | 0.42 (0.15–0.69) | 0.58 | 1.37 |
| One-way hypothesis testing | |||
| Smaller conspecific > larger conspecific | 0.77 (0.45–1.1) | 1.0 | 7999 |
| Smaller neighbour > larger neighbour | 1.93 (0.09–4.03) | 0.96 | 22.77 |
| Smaller non-neighbour > larger non-neighbour | 1.04 (−0.95–3.02) | 0.82 | 4.63 |
Results
Hypothesis testing revealed that the total count of aggressive displays was mediated by both the type of stimulus fish and body size difference (Figs. 2 and 3; Table 1). Overall, there was strong evidence that S. diencaeus territory holders displayed greater aggression towards neighbours than non-neighbours (PP = 0.74, ER = 2.8; Fig. 2a). However, this trend was not consistent across body size difference. S. diencaeus territory holders were more aggressive towards smaller neighbours than smaller non-neighbours (PP = 0.58, ER = 1.37; Fig. 2c), but not towards larger neighbours than larger non-neighbours (PP = 0.98, ER = 40.25; Fig. 2b).
Fig. 2.
Bayesian posterior density plots of hypothesis testing, showing that (a) S. diencaeus territory holders are more aggressive towards neighbours than non-neighbours, (b) S. diencaeus territory holders are equally aggressive towards larger neighbours and larger non-neighbours, with body size being relative to themselves, and (c) S. diencaeus territory holders are more aggressive towards smaller neighbours than smaller non-neighbours. PP, ER and posterior density in orange represent the evidence to which S. diencaeus territory holders display greater total aggression
Fig. 3.
Bayesian posterior density plots of hypothesis testing, showing that S. diencaeus territory holders are (a) more aggressive towards smaller conspecifics than larger conspecifics, with body size being relative to themselves, (b) more aggressive towards smaller neighbours than larger neighbours, and (c) more aggressive towards smaller non-neighbours than larger non-neighbours. PP, ER and posterior density in orange represent the evidence to which S. diencaeus territory holders display greater total aggression
S. diencaeus territory holders displayed greater aggression towards smaller conspecifics relative to themselves than larger conspecifics (PP = 1.00, ER = 7999; Fig. 3a). This was consistent across neighbours (PP = 0.96, ER = 22.77; Fig. 3b) and non-neighbours (PP = 0.82, ER = 4.63; Fig. 3c). Extended hypothesis testing results are given in Table 1.
Smaller neighbours elicited the greatest aggressive response (Median estimated posterior prediction = 7.97, 90% highest posterior density interval (HPDI) = 2.32 to 15.09; Fig. 4, S2; Table 2), whereas larger neighbours elicited the weakest aggressive response (Median estimated posterior prediction = 3.65, HPDI = 1.03 to 7.26; Fig. 4, S2; Table 2).
Fig. 4.
Total aggressive displays directed towards intraspecific stimulus fish by S. diencaeus territory holders is influenced by both familiarity and body size difference between territory holder and stimulus fish. Expected posterior predictions presented for S. diencaeus neighbours and non-neighbours. Point intervals represent median estimates and lines represent 90 and 70% highest posterior density intervals (HPDIs). Note x-axis limited to 30 for ease of viewing
Table 2.
Median estimates and 90% highest posterior density intervals of total aggression by territory holder
| Stimulus type | Median estimate | 90% Highest posterior density interval (HPDI) |
|---|---|---|
| Smaller neighbour | 7.85 | 2.62–14.79 |
| Larger neighbour | 3.63 | 1.03–6.89 |
| Smaller non-neighbour | 5.48 | 1.61–10.26 |
| Larger non-neighbour | 4.84 | 2.54–9.44 |
Only one focal S. diencaeus was observed to aggressively ram the empty control bottle once, no other aggressive response was elicited by the empty control bottles. This demonstrates the lack of response to empty bottles, and that aggressive responses were elicited by stimulus fish.
Discussion
The aggressive response elicited by intraspecific neighbours and non-neighbours in S. diencaeus territory holders was determined by both familiarity and body size difference between the territory holder and stimulus fish. Overall, aggressive response was highest towards neighbours, suggestive of nasty neighbour effects, yet the exact response was mediated by difference in body size. When looking at larger conspecifics only, S. diencaeus displayed similar levels of aggression towards larger neighbours as larger non-neighbours. We propose that the difference between level of threat posed by neighbours and non-neighbours, rather than familiarity, may offer a plausible explanation for our results.
The familiarity hypothesis suggests that familiarity between neighbouring individuals based on past encounters reduces aggressiveness of responses in subsequent interactions (Ydenberg et al. 1988). However, one key aspect that the familiarity hypothesis overlooks is that familiarity does not always confer potential threat (Temeles 1994). The threat-level hypothesis reasons that aggressive response should be strongest when directed towards the greatest threat (Temeles 1994). Typically, established territory lines between neighbours is expected to reduce conflict as neighbours are seen as a lower threat than roving strangers seeking their own territory (Temeles 1994; Rosell et al. 2008; Sogawa et al. 2016). However, in some territorial species, the opposite may be true. Neighbours may pose a greater threat to territory loss than strangers if neighbours are continually trying to expand their territory (Müller and Manser 2007; Munguía-Steyer et al. 2016). It has also been suggested that neighbours pose a greater threat when territories are used for reproductive purposes (Temeles 1990), or during the breeding season when mates are fertile (Moser-Purdy et al. 2017). In such circumstance, neighbours are predicted to elicit a greater aggressive response (e.g. Müller and Manser 2007; Newey et al. 2010; Munguía-Steyer et al. 2016). However, this assumes that all neighbours pose the same level of threat.
We found that the aggressive response by S. diencaeus to both neighbours and non-neighbours depended on the body size difference between opponents. One explanation for this may be that the body size of an intruder influences their potential threat in terms of RHP. Assessment of relative RHP relies on an individual’s assessment of their own RHP and that of their opponent and is expected to mediate the escalation of fights and the strength of aggressive response (Arnott and Elwood 2009). Larger individuals with greater RHP are expected to win fights, and therefore invest more into fighting than smaller individuals (Parker 1974). In terms of territorial species, this would suggest that territory holders respond more aggressively to smaller conspecifics. Indeed, we found evidence that S. diencaeus territory holders respond more aggressively to smaller conspecifics. This finding may suggest that S. diencaeus can assess the RHP and fighting ability of both familiar and unfamiliar individuals and adjust their behaviour accordingly.
Nasty neighbour effects alongside assessment of body size and associated RHP offers one plausible explanation for our findings. Nasty neighbour effects predict that neighbours elicit a greater aggressive response than strangers, whereas assessment of RHP predicts greater aggression is directed towards smaller conspecifics. Here, we found evidence that S. diencaeus respond more aggressively to neighbours than non-neighbours overall, but no such evidence was observed when looking at larger conspecifics only. S. diencaeus responded similarly to larger neighbours as larger non-neighbours, suggesting that size-mediated nasty neighbour effects may be present in this species.
An alternative explanation for our findings may be the presence of size-determined hierarchies between neighbours (Hemelrijk 2000; Hobson 2020; Tibbetts et al. 2022). Dominance hierarchies are widespread in group-living and aggregating species and are commonly established based on previous wins and losses during competitive encounters between neighbouring individuals (Tibbetts et al. 2022). Larger individuals with greater RHP are expected to win more fights and become dominants (Parker 1974), therefore dominance rank is often strongly correlated with body size (Favre et al. 2008; Clutton-Brock 2017; Wright et al. 2019). Typically, larger dominant individuals are expected to be most aggressive towards smaller subordinates (Tierney et al. 2013; Wright et al. 2019; Dehnen et al. 2022).
The application of these ideas to territorial farming damselfishes, such as Stegastes, has not been widely explored. Although farming damselfishes are described as solitary species, individual territories are often contiguous, forming intraspecific aggregations (Itzkowitz 1978; Robertson and Lassig 1980; McDougall and Kramer 2007). Though dominance hierarchies are typically present in group-living species, it has been argued that in territorial species, individuals with neighbouring territories may form dominant-subordinate relationships (rather than hierarchies) based on previous encounters (Rubenstein 1981; Fernö 1987). In Stegastes partitus, a territorial damselfish, dominance relationships have been found to be size-based (Sadovy 1985). Past examination of the spatial organisation of Stegastes planifrons, a closely related sister species of S. diencaeus, revealed that larger individuals, considered to be more dominant, held more preferable central territories than smaller individuals (Itzkowitz 1978). Following the expectation that dominant individuals direct greater aggression towards subordinates (Dehnen et al. 2022), we would expect territory holders to direct stronger aggressive responses towards neighbours that are smaller than themselves compared with those that are larger. We found strong evidence that S. diencaeus territory holders were more aggressive towards smaller neighbours, and therefore likely subordinate, than larger neighbours. Although this finding may suggest dominance relationships between neighbouring S. diencaeus, the aggressive response directed towards non-neighbouring S. diencaeus by territory holders was also associated with body size difference. Therefore, assessment of RHP between unfamiliar opponents may also be present.
An alternative perspective on the determinants of aggressive behaviour is to focus on the costs of aggressive encounters rather than the potential threat posed by an opponent. Costs of aggressive encounters are higher when the asymmetry in RHP between opponents is lower (i.e. opponents are more closely matched) (Arnott and Elwood 2009) and, intuitively, for individuals with lower RHP, such as those of smaller body size. Previous encounters with neighbours offer territory holders more information regarding their opponents RHP (fight to learn; Getty 1989), allowing for more accurate mutual assessment of the costs of subsequent interactions (Taylor and Elwood 2003; Arnott and Elwood 2009). In contrast, when faced with unfamiliar individuals, territory holders have less information on their opponent (Taylor and Elwood 2003; Arnott and Elwood 2009) and consequently, the costs are less predictable. Applying cost assessment theory, we might expect a weaker aggressive response (1) between unfamiliar individuals and (2) towards opponents with a larger RHP than the territory holder, as we observed in S. diencaeus. Assessment of the cost of an aggressive encounter may therefore mediate neighbour-stranger discrimination and provide an explanation to our findings.
Our findings rely on measuring aggressive response as the number of aggressive displays, in terms of bites and rams, directed by the territory holder to the stimulus fish. These behaviours are highly detectable to a human observer. However, damselfishes are known to communicate through a wide array of signals and displays which are more difficult to observe. For example, the presence and absence of UV patterns affects aggression between intraspecific Pomacentrus amboinensis (Siebeck 2004), and colouration patterns are integral to individual recognition in Amphiprion hicinctus (Fricke 1973) and Stegastes planifrons (Thresher 1979). Acoustic differences have also been shown to aid intruder discrimination between species (Weimann et al. 2018). The function of these more cryptic communications is largely unknown (however see Fricke 1973; Siebeck 2004) but there is potential for these signals to aid the assessment of a competitor’s RHP. Experiments to investigate the role of more cryptic signals in the communication of fighting ability and subsequent impact on aggressive territorial behaviour could add significant insight into these behaviours.
Aggressive behaviour associated with territoriality carries costs (Marler et al. 1995; Neat et al. 1998; Clutton-Brock and Huchard 2013). Natural selection should therefore favour any mechanism or strategy that allows a territory holder to minimise these costs whilst upholding their territory and preventing resource loss from intrusion (Arnott and Elwood 2009). We demonstrate that familiarity, interpreted with the added information provided by body size, predicts the level of aggressive response by S. diencaeus territory holders. Studies on neighbour-stranger discrimination are common (see Werba et al. 2022). However, our findings add to the growing pool of evidence that argue the need to include additional factors (e.g. dominance or body size: Tierney et al. 2013; Wright et al. 2019; density: Morales et al. 2014) when investigating the drivers of variation in territorial aggression between neighbours and non-neighbours. In doing so, we will be able to better predict not only individual behavioural responses but also how these responses scale up to influence population and community dynamics.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr Rachel Gunn, as well as the staff and volunteers at Coral View Research Centre and Operation Wallacea Honduras during summer 2023 for their assistance and support in the field. We thank the reviewers for their constructive feedback on this manuscript.
Author contributions
Study conception, design, data collection and analysis were performed by CES. The first draft of the manuscript was written by CES. All authors commented on subsequent drafts of the manuscript, and read and approved the final manuscript.
Funding
This work was supported by the Natural Environment Research Council (SAK, grant number NE/S00050X/1 and CES, grant number NE/S007423/1), with CES’s studentship through the Envision Doctoral Training Partnership. Fieldwork was supported by project partner Operation Wallacea.
Data availability
All data and code associated with this study is available at https://github.com/cesheppard/damselfish_NSD. Raw videos available on request.
Declarations
Ethical approval
This study approved by the Animal Welfare and Ethical Review Body (AWERB), considering the three R’s principle: replacement, reduction, and refinement. Each captured fish was presented to two focal individuals, reducing the number of animals needed for the experiment. Using a mix of clove oil and ethanol prior to capture reduced the stress caused by this process. After capture, individuals in their containers were placed back into their own territories and covered in a small tarp to allow them to destress and for the effects of the clove oil to wear off. The fish used in this study were contained for no more than 180 min and were released in the same area from which they were caught. Contained fish were presented for 3 min to two focal individuals, equating to 6 min in total per animal. These time limits minimised the duration with which they were subject to aggressive displays and reduced distress to the animals. During presentations, the behaviour of both captured and focal individuals was continually monitored for signs of unnaturally high stress (e.g. swimming rapidly in circles, or withdrawing into a corner and remaining still). The health status of the captured fish was checked after capture and release. Data were collected under permit number DE-MP-108-2023 issued by the Honduran government’s Instituto de Conservación Forestal (ICF). The use of animals adheres to the guidelines set forth by the Association for the Study of Animal Behaviour.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arnott G, Elwood RW (2009) Assessment of fighting ability in animal contests. Anim Behav 77:991–1004. 10.1016/j.anbehav.2009.02.010 [Google Scholar]
- Bee MA, Gerhardt HC (2001) Habituation as a mechanism of reduced aggression between neighboring territorial male bullfrogs (Rana catesbeiana). J Comp Psychol 115(1):68–82. 10.1037/0735-7036.115.1.68 [DOI] [PubMed] [Google Scholar]
- Bürkner P-C (2017) Brms: an R package for bayesian multilevel models using Stan. J Stat Softw 80:1–28. 10.18637/jss.v080.i01 [Google Scholar]
- Ceccarelli DM, Jones GP, McCook LJ (2001) Territorial damselfishes as determinants of the structure of benthic communities on coral reefs. Oceanogr Mar Biol 39:355–389 [Google Scholar]
- Clutton-Brock TH (2017) Reproductive competition and sexual selection. Phil Trans R Soc Lond B Biol Sci 372:20160310. 10.1098/rstb.2016.0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH, Huchard E (2013) Social competition and selection in males and females. Phil Trans R Soc Lond B Biol Sci 368:20130074. 10.1098/rstb.2013.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2023) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
- Dehnen T, Papageorgiou D, Nyaguthii B, Cherono W, Penndorf J, Boogert NJ, Farine DR (2022) Costs dictate strategic investment in dominance interactions. Phil Trans R Soc Lond B Biol Sci 377:20200447. 10.1098/rstb.2020.0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre M, Martin JGA, Festa-Bianchet M (2008) Determinants and life-history consequences of social dominance in Bighorn ewes. Anim Behav 76:1373–1380. 10.1016/j.anbehav.2008.07.003 [Google Scholar]
- Fernö A (1987) Aggressive behaviour between territorial cichlids (Astatotilapia burtoni) in relation to rank and territorial stability. Behaviour 103:241–258. 10.1163/156853987X00189 [Google Scholar]
- Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7:1325–1330. 10.1111/2041-210X.12584 [Google Scholar]
- Fricke HW (1973) Individual partner recognition in fish: field studies on Amphiprion bicinctus. Naturwissenschaften 60:204–204. 10.1007/BF00599441 [DOI] [PubMed] [Google Scholar]
- Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7:457–472. 10.1214/ss/1177011136 [Google Scholar]
- Getty T (1989) Are dear enemies in a war of attrition? Anim Behav 37:337–339. 10.1016/0003-3472(89)90125-5 [Google Scholar]
- Godard R (1993) Tit for tat among neighboring hooded warblers. Behav Ecol Sociobiol 33:45–50. 10.1007/BF00164345 [Google Scholar]
- Green PA, Patek SN (2018) Mutual assessment during ritualized fighting in mantis shrimp (Stomatopoda). Proc R Soc B Biol Sci 285:20172542. 10.1098/rspb.2017.2542 [Google Scholar]
- Hadfield JD (2010) MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw 33:1–22. 10.18637/jss.v033.i02 [PMC free article] [PubMed] [Google Scholar]
- Haley MP, Müller CR (2002) Territorial behaviour of beaugregory damselfish (Stegastes leucostictus) in response to egg predators. J Exp Mar Biol Ecol 273:151–159. 10.1016/S0022-0981(02)00144-2 [Google Scholar]
- Harrington ME (1993) Aggression in damselfish: adult-juvenile interactions. Copeia 1993:67–74. 10.2307/1446296 [Google Scholar]
- Hemelrijk CK (2000) Towards the integration of social dominance and spatial structure. Anim Behav 59:1035–1048. 10.1006/anbe.2000.1400 [DOI] [PubMed] [Google Scholar]
- Hick K, Reddon A, O’Connor C, Balshine S (2014) Strategic and tactical fighting decisions in cichlid fishes with divergent social systems. Behaviour 151:47–71. 10.1163/1568539X-00003122 [Google Scholar]
- Hobson EA (2020) Differences in social information are critical to understanding aggressive behavior in animal dominance hierarchies. Curr Opin Psychol 33:209–215. 10.1016/j.copsyc.2019.09.010 [DOI] [PubMed] [Google Scholar]
- Hogan JD, Thiessen RJ, Sale PF, Heath DD (2012) Local retention, dispersal and fluctuating connectivity among populations of a coral reef fish. Oecologia 168:61–71. 10.1007/s00442-011-2058-1 [DOI] [PubMed] [Google Scholar]
- Itzkowitz M (1978) Group organization of a territorial damselfish, Eupomacentrus planifrons. Behaviour 65:25–137. 10.1163/156853978X00576 [Google Scholar]
- Itzkowitz M, Itzkowitz DE, Shelly D (1995) Territory use and disuse in the beaugregory damselfish. Bull Mar Sci 57:653–662 [Google Scholar]
- Lehtonen TK, Wong BBM (2017) Males are quicker to adjust aggression towards heterospecific intruders in a cichlid fish. Anim Behav 124:145–151. 10.1016/j.anbehav.2016.12.013 [Google Scholar]
- Leiser JK (2003) When are neighbours ‘dear enemies’ and when are they not? The responses of territorial male variegated pupfish, Cyprinodon variegatus, to neighbours, strangers and heterospecifics. Anim Behav 65:453–462. 10.1006/anbe.2003.2087 [Google Scholar]
- Marler CA, Walsberg G, White ML, Moore M, Marler CA (1995) Increased energy expenditure due to increased territorial defense in male lizards after phenotypic manipulation. Behav Ecol Sociobiol 37:225–231. 10.1007/BF00177401 [Google Scholar]
- McDougall PT, Kramer DL (2007) Short-term behavioral consequences of territory relocation in a Caribbean damselfish, Stegastes diencaeus. Behav Ecol 18:53–61. 10.1093/beheco/arl055 [Google Scholar]
- Morales MB, Casas F, García de la Morena E, Ponjoan A, Calabuig G, Martínez-Padilla J, García JT, Mañosa S, Viñuela J, Bota G (2014) Density dependence and habitat quality modulate the intensity of display territory defence in an exploded lekking species. Behav Ecol Sociobiol 68:1493–1504. 10.1007/s00265-014-1758-z [Google Scholar]
- Moser-Purdy C, MacDougall-Shackleton EA, Mennill DJ (2017) Enemies are not always dear: male song sparrows adjust dear enemy effect expression in response to female fertility. Anim Behav 126:17–22. 10.1016/j.anbehav.2017.01.009 [Google Scholar]
- Müller CA, Manser MB (2007) Nasty neighbours’ rather than ‘dear enemies’ in a social carnivore. Proc R Soc Lond B 274:959–965. 10.1098/rspb.2006.0222 [Google Scholar]
- Munguía-Steyer R, Córdoba-Aguilar A, Maya-García JS (2016) Rubyspot territorial damselflies behave as nasty neighbors. J Insect Behav 29:143–152. 10.1007/s10905-016-9548-2 [Google Scholar]
- Myrberg AA Jr, Thresher RE (1974) Interspecific aggression and its relevanceto the concept of territoriality in reef fishes. Am Zool 14:81–96 [Google Scholar]
- Neat FC, Taylor AC, Huntingford FA (1998) Proximate costs of fighting in male cichlid fish: the role of injuries and energy metabolism. Anim Behav 55:875–882. 10.1006/anbe.1997.0668 [DOI] [PubMed] [Google Scholar]
- Newey PS, Robson SKA, Crozier RH (2010) Weaver ants Oecophylla Smaragdina encounter nasty neighbors rather than dear enemies. Ecology 91:2366–2372. 10.1890/09-0561.1 [DOI] [PubMed] [Google Scholar]
- Osório R, Rosa IL, Cabral H (2006) Territorial defence by the Brazilian damsel Stegastes fuscus (Teleostei: Pomacentridae). J Fish Biol 69:233–242. 10.1111/j.1095-8649.2006.01095.x [Google Scholar]
- Parker GA (1974) Assessment strategy and the evolution of fighting behaviour. J Theor Biol 47:223–243. 10.1016/0022-5193(74)90111-8 [DOI] [PubMed] [Google Scholar]
- Puebla O, Bermingham E, McMillan WO (2012) On the spatial scale of dispersal in coral reef fishes. Mol Ecol 21:5675–5688. 10.1111/j.1365-294X.2012.05734.x [DOI] [PubMed] [Google Scholar]
- Robertson DR, Lassig B (1980) Spatial distribution patterns and coexistence of a group of territorial damselfishes from the Great Barrier Reef. Bull Mar Sci 30:187–203 [Google Scholar]
- Rosell F, Gundersen G, Le Galliard J-F (2008) Territory ownership and familiarity status affect how much male root voles (Microtus oeconomus) invest in territory defence. Behav Ecol Sociobiol 62:1559–1568. 10.1007/s00265-008-0585-5 [Google Scholar]
- RStan (2023) RStan: the R interface to Stan. R package version 2.26.1 (Stan Development Team). 10.32614/CRAN.package.rstan
- Rubenstein DI (1981) Population density, resource patterning, and territoriality in the Everglades pygmy sunfish. Anim Behav 29:155–172. 10.1016/S0003-3472(81)80162-5 [Google Scholar]
- Sadovy Y (1985) Field analysis of the dominance hierarchy of the bicolor damselfish Stegastes partitus. In: Reaka ML (ed) The ecology of coral reefs. NOAA, Philadelphia, PA, pp 129–137 [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to imageJ: 25 years of image analysis. Nat Methods 9:671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard CE, Boström-Einarsson L, Williams GJ, Exton DA, Keith SA (2024) Variation in farming damselfish behaviour creates a competitive landscape of risk on coral reefs. Biol Lett 20:20240035. 10.1098/rsbl.2024.0035
- Siebeck UE (2004) Communication in coral reef fish: the role of ultraviolet colour patterns in damselfish territorial behaviour. Anim Behav 68:273–282. 10.1016/j.anbehav.2003.11.010 [Google Scholar]
- Silveira MM, Silva PF, Ferreira RG, Luchiari AC (2020) Fighting off the intruder: context-dependent territory defence in the damselfish Stegastes fuscus. Environ Biol Fish 103:1091–1104. 10.1007/s10641-020-01011-5 [Google Scholar]
- Snekser JL, Leese J, Ganim A, Itzkowitz M (2009) Caribbean damselfish with varying territory quality: correlated behaviors but not a syndrome. Behav Ecol 20:124–130. 10.1093/beheco/arn123
- Siracusa E, Boutin S, Humphries MM, Gorrell JC, Coltman DW, Dantzer B, Lane JE, McAdam AG (2017) Familiarity with neighbours affects intrusion risk in territorial red squirrels. Anim Behav 133:11–20. 10.1016/j.anbehav.2017.08.024 [Google Scholar]
- Sogawa S, Kohda M (2018) Tit for tat in the dear enemy relationship between territorial females of a cichlid fish. Front Ecol Evol 6:44. 10.3389/fevo.2018.00044 [Google Scholar]
- Sogawa S, Ota K, Kohda M (2016) A dear enemy relationship in a territorial cichlid: evidence for the threat-level hypothesis. Behaviour 153:387–400. 10.1163/1568539X-00003351 [Google Scholar]
- Taylor PW, Elwood RW (2003) The mismeasure of animal contests. Anim Behav 65:1195–1202. 10.1006/anbe.2003.2169 [Google Scholar]
- Temeles EJ (1990) Northern harriers on feeding territories respond more aggressively to neighbors than to floaters. Behav Ecol Sociobiol 26:57–63. 10.1007/BF00174025 [Google Scholar]
- Temeles EJ (1994) The role of neighbours in territorial systems: when are they dear enemies? Anim Behav 47:339–350. 10.1006/anbe.1994.1047 [Google Scholar]
- Thresher RE (1979) The role of individual recognition in the territorial behaviour of the threespot damselfish, Eupomacentrus planifrons. Mar Behav Physiol 6:83–93. 10.1080/10236247909378556 [Google Scholar]
- Tibbetts EA, Pardo-Sanchez J, Weise C (2022) The establishment and maintenance of dominance hierarchies. Phil Trans R Soc Lond B Biol Sci 377:20200450. 10.1098/rstb.2020.0450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AJ, Andrews K, Happer KR, White MKM (2013) Dear enemies and nasty neighbors in crayfish: effects of social status and sex on responses to familiar and unfamiliar conspecifics. Behav Process 99:47–51. 10.1016/j.beproc.2013.06.001 [Google Scholar]
- Tricarico E, Borrelli L, Gherardi F, Fiorito G (2011) I know my neighbour: individual recognition in Octopus vulgaris. PLoS One 6:e18710. 10.1371/journal.pone.0018710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimann SR, Black AN, Leese J, Richter ML, Itzkowitz M, Burger RM (2018) Territorial vocalization in sympatric damselfish: acoustic characteristics and intruder discrimination. Bioacoustics 27:87–102. 10.1080/09524622.2017.1286263 [Google Scholar]
- Werba JA, Stuckert AM, Edwards M, McCoy MW (2022) Stranger danger: a meta-analysis of the dear enemy hypothesis. Behav Process 194:104542. 10.1016/j.beproc.2021.104542 [Google Scholar]
- Whiteman EA, Côté IM (2002) Cleaning activity of two Caribbean cleaning gobies: intra- and interspecific comparisons. J Fish Biol 60:1443–1458. 10.1111/j.1095-8649.2002.tb02439.x [Google Scholar]
- Wilson EO (1975) Sociobiology: the new synthesis. Harvard University Press, Cambridge, MA [Google Scholar]
- Wright E, Galbany J, McFarlin SC, Ndayishimiye E, Stoinski TS, Robbins MM (2019) Male body size, dominance rank and strategic use of aggression in a group-living mammal. Anim Behav 151:87–102. 10.1016/j.anbehav.2019.03.011 [Google Scholar]
- Ydenberg RC, Giraldeau LA, Falls JB (1988) Neighbours, strangers, and the asymmetric war of attrition. Anim Behav 36:343–347. 10.1016/S0003-3472(88)80004-6 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and code associated with this study is available at https://github.com/cesheppard/damselfish_NSD. Raw videos available on request.