Abstract
Calcium-phosphate cement (CPC), a paste-like artificial bone, is a material form that allows minimally invasive treatment. However, CPC is not infection resistant, which may lead to surgical site infections. We recently developed a paste-like organic/inorganic hybrid artificial bone that is compatible with the bone remodeling cycle. In this study, we added silver-loaded tricalcium phosphate, which has antibacterial properties, to the hybrid CPC and fabricated a prototype “antibacterial CPC”. Antibacterial and non-antibacterial CPCs were implanted into a rabbit jaw defect model in which infection could occur, and the in vivo responses were compared. In cement specimens retrieved from rabbit jaws, residual material was observed with the non-antibacterial CPC, whereas with the antibacterial CPC, almost all of the material was resorbed and replaced with host bone. These results suggest that placement of antibacterial CPC in a rabbit jaw bone defect model susceptible to bacterial infection promotes material resorption and bone formation. The antibacterial CPC developed in this study is thus a novel paste artificial bone exhibiting good bioresorption and osteogenic potential in biological hard tissues.

Introduction
As global societies continue to age, the demand for implant treatments in the fields of dental and orthopedic surgery is increasing. The oral cavity is highly exposed to bacteria; thus, the development of implant-related infections (IRIs) in treatments involving implant materials such as dental implants is a significant public health concern, whether the IRIs occur during the early postoperative period or as a delayed-onset complication [1]. Currently, the ideal treatment for IRIs in dental implants involves the physical removal of biofilm adhering to the implant surface and stimulating the regeneration of lost bone [2, 3]. Although these approaches could be promising in some cases, the challenging environment of the oral cavity, which is continually exposed to microorganisms, poses risks that can impede treatment success, such as reinfection. Therefore, developing novel approaches and technologies that integrate infection control with bone regeneration is essential for improving long-term treatment outcomes.
Two types of bone grafting have been described: autologous bone grafting, in which healthy bone is transplanted, and artificial bone grafting, in which artificial bone is transplanted. Autologous bone has high osteogenic potential and a low risk of rejection; however, there are limits to the amount of bone that can be harvested, and there is a risk of secondary invasion. Artificial bone, by contrast, is difficult to adapt to different shapes. Various types of artificial bone materials are available, including dense, porous, granular, and paste materials. From the viewpoint of minimally invasive surgery, we focused on paste-like artificial bone [4] that can be injected using a syringe.
We previously developed a paste-like artificial bone composed primarily of bioresorbable β-tricalcium phosphate (β-Ca3(PO4)2; β-TCP) by utilizing chelating inositol phosphate (IP6) [5]. Simultaneous addition of lactic acid–glycolic acid copolymer (PLGA) particles and calcium sulfate hemihydrate (CSH) to this paste-like artificial bone enabled the generation of fully resorbable and replaceable CPCs [6–8]. However, as clinical application of paste-like artificial bone can lead to surgical site infections, infection-resistant biomaterials are needed. We therefore synthesized silver-loaded tricalcium phosphate powder (Ag-TCP) using an ultrasonic spray-pyrolysis technique and characterized the antibacterial properties of the powder [9]. Antimicrobial CPCs can be prepared by adding Ag-TCP to the above-mentioned CPCs.
Depending on the research objectives, a variety of animal experimental models are available for studying bone regeneration in the jaw [10–12]. Notably, when evaluating materials useful in the treatment of IRIs, it is essential to consider clinical scenarios in which the test materials are exposed to bacteria from saliva and food debris. Therefore, the selected animal model must not only accommodate the implantation of appropriately sized biomaterials but also replicate the susceptibility to infection characteristic of the oral environment. In this context, Shah et al. demonstrated the usefulness of rabbit models for evaluating therapies aimed at promoting tissue regeneration in defects potentially contaminated by saliva- and food-derived bacteria [13].
The overall aim of the present study was to impart infection resistance to implant materials as a means of preventing IRIs. Thus, antibacterial Ag-TCP was added to the above-mentioned fully resorbable and replacement paste-like artificial bone to prepare an antibacterial paste-like artificial bone (hereinafter referred to as “antibacterial CPC”). The material properties of the antibacterial CPC were evaluated, including the crystalline phase, initial setting time (IST), compressive strength, microstructure, and calcium ion solubility. In addition, the resulting antibacterial CPC was implanted into a rabbit jawbone model [13] in which infection was likely to occur, and the in vivo response was compared with that of non-antimicrobial CPC implants using histological and X-ray micro–computed tomography (micro-CT) methods.
Materials and methods
Preparation of CPC paste
Preparation of PLGA particles
PLGA particles were prepared using the oil-in-water (w/o/w) double emulsion solvent evaporation method in water based on the method reported by Habraken et al. [14]. First, 3 g of polyvinyl alcohol (Fujifilm Wako Pure Chemical Industries, Ltd., Osaka, Japan) was placed in a beaker containing 1000 cm³ of pure water and stirred at 800 rpm and 50 °C for 1 week to generate a 0.3 mass% polyvinyl alcohol (PVA) solution. Next, 1 g of PLGA (Sigma-Aldrich, average molecular weight [Mw] 34,000-54,000, lactic acid:glycolic acid = 50:50 [w/w]) powder was dissolved in 4 cm3 of chloroform (Fujifilm Wako Pure Chemical Industries) in a 50-mL centrifuge tube. Pure water (0.5 cm3) was added to the centrifuge tube and mixed by vortexing for 90 s. Subsequently, 6 cm3 of 0.3 mass% PVA solution was added to the 50-mL tube containing PLGA and mixed by vortexing for 90 s. The contents of the 50-mL centrifuge tube were then added while stirring to a 1000-mL beaker containing 400 cm3 of 2 mass% isopropyl alcohol (Fujifilm Wako Pure Chemical Industries Co). In addition, 394 cm3 of 0.3 mass% PVA solution was slowly added to the 1000-mL beaker. A suspension consisting of PLGA, chloroform, and PVA was stirred at 700 rpm for 1 h at 25 °C, allowed to stand, and then decanted and centrifuged at 1000 rpm for 2 min. The resulting supernatant was aspirated for solid-liquid separation. Finally, the PLGA particles were frozen and lyophilized for 24 h.
Preparation of Ag-TCP powders
Ag-TCP powders were prepared according to a previous report [9]. The sample solution for synthesis of Ag-TCP powders was prepared using calcium nitrate (Ca(NO3)2; 0.9 mol ∙ dm−3), diammonium hydrogen phosphate ((NH4)2HPO4; 0.9 mol ∙ dm−3), nitric acid (HNO3; 0.9 mol ∙ dm−3), and silver nitrate (AgNO3; 0.9 mol ∙ dm−3). The molar concentrations of the solutes are shown in parentheses. The content of added silver was 20 mol% of TCP.
Fine droplets of the sample solution for spray pyrolysis were generated using an ultrasonic oscillator. The droplets were introduced into the heating zone at an air flow rate of 1.5 dm3·min−1. The introduced droplets were transformed into powder via removal of the solvent and subsequent pyrolysis of the precipitate. In order to remove residual nitric acid in the powder, approximately 1.0 g of the synthesized powder was aliquoted into a 50-mL centrifuge tube and stirred to wash with approximately 40 cm3 of ultrapure water for approximately 1 min using a vortex mixer. The suspension was then separated into supernatant and powder via centrifugation. After three washing and separation cycles, the suspension was suspended and separated in 40 cm3 of acetone. The precipitate was frozen at −80 °C for 1 day. The frozen powder was then freeze-dried for 1 day using a Labconco Free Zone® lyophilizer (Labconco, USA). Hereafter, the resulting product is designated “Ag-TCP powder”.
Preparation and characterization of starting CPC powders
Raw powder for the CPC was prepared according to the protocol described in our previous report [5]. Briefly, a solution of 3000 ppm aqueous IP6 (50 mass% phytic acid, Fujifilm Wako Pure Chemical Industries) was prepared and adjusted to pH 7.3 with NaOH (0.1 mol·dm−3). Subsequently, 10 g of commercial β-TCP powder (β-TCP-100, Taihei Chemical Industry Co., Japan) and 40 cm3 of 3000 ppm IP6 solution were placed in a zirconia (ZrO2) pot. The β-TCP powder was then finely ground by ball milling, and the IP6 was surface-modified on the finely ground TCP particles. Ball milling was performed using a planetary ball mill operated at 300 rpm for 3 h in a ZrO2 pot containing 180 g of 2-mm-diameter ZrO2 beads. After ball milling, the resulting slurry was suction filtered and freeze-dried for 24 h using a Labconco Free Zone lyophilizer to obtain β-TCP powder surface-modified with IP6 (hereafter, IP6/β-TCP powder). The resulting powder was sieved through 200 mesh (150 μm under).
The crystalline phase of the IP6/β-TCP powder was identified using an X-ray diffractometer equipped with a CuKα source (Ultima IV, Rigaku Co., Ltd., Japan). Measurements were carried out in the scanning range 2θ: 10°–50°, step size: 0.02°, and counting time: 1.2 s·step−1. The crystalline phase was identified as β-TCP (#09-0169) using the International Center for Diffraction Data (ICDD) database.
The particle morphology of the generated IP6/β-TCP powder was observed using scanning electron microscopy (SEM; JSM6390LA, JEOL Co., Ltd.) at an acceleration voltage of 10 kV. Samples for observation were coated with platinum using a JFC-1500 ion sputtering device (JEOL Co., Ltd.). The IP6/β-TCP powder was then mixed with CSH and PLGA using a V-type mixer (Tsutsui Scientific Instruments Co., Ltd., Japan) for 5 min to prepare a mixed powder. The amount of PLGA particles added was set to “5 mass%” based on a previous report [7]. The composition of the mixed powder is given in Table 1. The mixed powder was prepared so that the ratio of calcium phosphate component (IP6/β-TCP and Ag-TCP powders) to CSH was 8:2 [w/w] [8]. Hereafter, the prepared mixed powders are designated “Ag(0)” and “Ag(5)” based on the Ag-TCP content (mass%); Ag(0) was a non-antibacterial CPC, whereas Ag(5) was an antibacterial CPC.
Table 1.
Nominal composition of raw powders for cement specimens
| Sample | Nominal composition/mass% | P/L [w/v] | |||
|---|---|---|---|---|---|
| IP6/b-TCP | Ag-TCP | CSH | PLGA | ||
| Ag(0) | 76 | 0 | 19 | 5 | 1.0/0.7 |
| Ag(5) | 71 | 5 | 19 | 5 | 1.0/0.7 |
Preparation of mixing liquid for cement pastes
The mixing solution was prepared according to our previous report [15]. Initially, 95 g of pure water, 2.5 g of disodium hydrogen phosphate (Na2HPO4), 1.0 g of sodium alginate ((C6H7O6Na)n), and 1.5 g of citric acid (C6H8O7) were added in order. The mixed solution was then adjusted to pH 7.0 using aqueous NaOH (0.1 mol·dm−3).
Preparation and properties of cement pastes
Cement pastes for Ag(0) and Ag(5) were prepared by blending mixed powder with the kneaded liquid for 2 min using an agate mortar and rubber spatula at the appropriate powder-liquid ratio (1.0/0.7 [g/cm3]).
IST was measured using a Gilmore needle (113.4 g) in accordance with JIS T 0330-4. The prepared cement paste was placed in a plastic mold (8 mm diameter, 2 mm height), and the IST was measured for the desired period of time until the cement paste set. The Gilmore needle was gently lowered into the cement paste in the plastic mold, and the end of setting was defined as the time point at which the indentation disappeared completely.
Properties of the CPCs after setting
Compressive strength (CS)
Cement specimens for CS measurements were fabricated by filling 6-mm diameter and 12-mm high cylindrical Teflon molds with prepared cement paste, which was then cured in an incubator (MIR-H163, PHC Holdings, Inc.) at 37 °C and 100% humidity for 24 h. CS was measured using a universal testing instrument (AG-5KNXplus, Shimadzu Corp., Kyoto, Japan). The crosshead speed was set to 0.5 mm·min−1, and a 5-kN load cell was used. The mean value and standard deviation were determined from measurements of 3 to 4 specimens.
Crystalline phase and microstructure of the fracture surface
The crystalline phase of the CPC was identified using X-ray diffraction (XRD) analysis, as described in Section 2.1.3. The crystalline phases were identified as β-TCP (#09-0169), HAp (#09-0432), and CSH (#43-605) according to the ICDD database. Sample powders used for XRD measurements were prepared by grinding in a mortar after determination of the CS of cement cured for 7 days. After measurement of the CS, the fracture surface of the broken cement specimen was observed using SEM.
Solubility of the resulting CPCs
Calcium ion solubility tests of Ag(0) and Ag(5) were performed according to JIS T 0330-3 and our previous report [16]. A 0.08 mol·dm⁻³ acetic acid–sodium acetate buffer (200 cm³, pH 5.5) was used to mimic the acidic environment generated by osteoclasts. The stirring speed was set at 430 ± 15 rpm, and the measurement time was set to 180 min. In addition to Ag(0) and Ag(5), commercially available granular artificial bone, Osferion® (0.15 cm3; Olympus Terumo Biomaterials, Japan), was used as a control for the measured specimens.
Antibacterial properties of the CPCs
The antibacterial activity of hardened cement specimens was evaluated using the inhibition zone method in accordance with JIS L 1902. Cement paste was placed into a plastic mold (15 mmϕ × 2 mm) and cured in an incubator (37 °C, 100% relative humidity) for 24 h to generate cement specimens. The cement specimens were then sterilized using an ethylene oxide gas sterilizer (CT-190C, Toho Manufacturing Co., Ltd., Japan).
Staphylococcus aureus was used as the bacterial species for evaluation. Bacteria were cultured in Wako Pure Chemicals LB medium “Daigo” (Wako Pure Chemicals) dissolved in 100 cm3 of ultrapure water, and the medium was autoclaved before use. Colonies appearing on LB agar medium dried in a dish were pre-cultured in LB liquid medium at 37 °C overnight. After incubation, the turbidity (OD600) of the bacterial suspension was measured, and a bacterial suspension with a concentration of 1 × 107 colony-forming units (CFU)·cm−3 was prepared. A UV-visible spectrophotometer was used to measure the turbidity, and the following formula (1) was used to convert the concentration:
| 1 |
Bacteria were precultured to a concentration of 1 × 107 CFU·cm−3, and 0.1 cm3 of the suspension was added to 6 cm3 of top agar and poured into a sample placed on LB agar medium. After solidification, the plates were incubated at 37 °C for 24 h and observed for the formation of an inhibition zone.
Cytotoxicity of CPCs
In this study, the cytotoxicity of CPC specimens was examined using a Transwell® kit (Corning). First, cement paste was placed into plastic molds (4 mmϕ × 8 mm) and cured in an incubator (37 °C, 100% relative humidity) for 24 h to obtain cement specimens. The cement specimens were then sterilized using an ethylene oxide gas sterilizer (CT-190C, Toho Manufacturing Co., Ltd.). Cell proliferation was determined by counting the number of cells co-cultured with the cement using the Transwell® kit. Cells were seeded into 12-well plates at a seeding density of 6 × 104 cells/well and pre-incubated for 1 day, after which the medium was removed, and adherent cells were counted and designated seeded cells (day 0 culture). Next, 1 cm3 of medium was added to each well, a hardened cement specimen was placed in the Transwell® insert, and an additional 0.8 cm3 of medium was added to the insert. Cells cultured on polystyrene plates without Transwell® inserts were used as controls. After placement of the Transwell® insert, the cells were incubated for 1 and 3 days, after which the cells on the plate were peeled off and counted. The culture medium was changed daily during the 3-day incubation period.
Surgical procedures for potentially infectious conditions in the rabbit model
All procedures were conducted in accordance with protocols approved by the Institutional Guidelines on Animal Experimentation and the Keio University Institutional Animal Care and Use Committee. Each group consisted of 3 New Zealand white rabbits (adult males) weighing 3.0–3.3 kg (Oriental Yeast, Tokyo, Japan). A full-thickness defect model in the molar/premolar region of the rabbit mandible was utilized, simulating clean-contaminated bacterial inoculations from saliva and tooth plaque (e.g., bone augmentation for dental implant surgery) [13]. Anesthesia was induced via a subcutaneous injection of a combination anesthetic comprising medetomidine hydrochloride (0.5 mg/kg), midazolam (2 mg/kg), and butorphanol tartrate (0.5 mg/kg). After sterile preparation and local anesthesia with 2% lidocaine, a 3-cm skin incision was made along the left mandibular inferior border, exposing the mandibular periosteum. The periosteum was carefully dissected to expose the mandible. A bicortical 8-mm circular bone defect was introduced using a trephine bur (Implatex, Tokyo, Japan). A cross-cut bur was subsequently used to introduce a 2- to 3-mm notch in the superior aspect of the defect. The overlying crown was removed to generate a model in which the bone defect communicated with the oral cavity via the extraction socket. This approach generated a mandibular defect that allowed bacterial contamination from saliva and food debris. The defect was then implanted with the developed antibacterial CPC (Ag(5)) or a control material (Ag(0)). The CPCs were mixed on-site and placed manually into the defect. The CPC powders used in this experiment were sterilized by ethylene oxide gas, and the mixing solution was filter-sterilized using a 0.2-μm filter (As-One Co., Ltd., Japan). To prevent pathological fractures, the mandible was reinforced using a 6-hole, 0.75-mm titanium plate with 2.0-mm screws (Stryker, Portage, MI, USA) (Fig. 1). Rabbits implanted with CPCs were housed for 12 weeks. Postoperatively, buprenorphine (0.02–0.03 mg/kg) and meloxicam (0.5 mg/kg) were administered subcutaneously every 12 h for 48 h and once daily for 3 days, respectively.
Fig. 1.

Procedure for generating the jawbone defect model. After exposing the left mandible, a bicortical 8-mm circular bone defect was introduced using a trephine bur (A). Subsequently, a molar (B) was extracted from the defect side using a cross-cut bur, resulting in the introduction of a 2- to 3-mm notch on the superior aspect of the defect (C). The defect was then filled with the respective material, followed by placement of a reinforcing titanium plate secured with 2.0-mm screws to prevent mandibular fracture (D). The images in (A, B) and (C, D) were obtained from cases 4 and 6, respectively (not specified in the manuscript)
Histological evaluation using hematoxylin-eosin (HE) and Villanueva bone (VB) staining
For histological evaluation, specimens were dehydrated using an increasing ethanol gradient, starting at 70%. Specimens were stained with HE and VB to analyze bone regeneration in each region of interest (ROI). Hematoxylin nuclear staining was substituted with VB staining. Subsequently, sagittal pathological tissue sections without decalcification were prepared from the central buccolingual portion of the mandible.
In addition to standard HE staining, VB staining was also employed in this study, as VB is frequently used to stain non-decalcified specimens of hard tissues, such as bones and teeth. VB-stained specimens can be viewed under normal or fluorescent light and used to distinguish the calcification stage of bone (e.g., osteoid or calcified). Under fluorescent light, osteoid bone stains red, hypocalcified bone orange, and calcified bone green. The specimens were observed histologically and imaged under normal and fluorescent light using an Olympus BX51 microscope and DP70 digital microscope camera (Olympus).
Post-surgical evaluation using X-ray micro-CT
Rabbits were sacrificed 12 weeks after surgery. Following sacrifice, intraoral gross observations were performed to confirm that the extraction socket was covered by oral mucosa. Subsequently, segmental jawbone resection was conducted, focusing on the ROI, for radiological and histological evaluation of the specimens. The specimens were scanned using an RmCT2 scanner (Rigaku, Tokyo, Japan), with X-rays set to a voltage of 90 kV, current of 160 μA, and 20-mm field of view.
Analysis of newly formed bone volume
To calculate new bone formation within the ROI, solid jawbone, elongated teeth, residual materials, and newly formed bone were semi-automatically extracted from micro-CT data obtained 12 weeks after the procedure using Mimics software (version 25.0, Materialise, Leuven, Belgium) at a threshold range of 950–7309 Hounsfield unit. The extracted data were subsequently converted into 3D models. A model representing the ROI was generated using ProPlan CMF (version 3.0, Materialise). The data extracted using Mimics were then subtracted using this model, enabling calculation of the volumes of the jawbone, elongated teeth, residual materials, and newly formed bone within the ROI (Fig. 2). The regenerated bone volume was then determined. The remaining tooth roots at the lower border of the mandible extended back into the ROI, which was taken into account when calculating the bone regeneration rate within the ROI. Therefore, the percentage of newly formed bone within the ROI was calculated using the following formula:
Fig. 2.
Protocol for determining the volume of areas with bone grafting. At 12 weeks after the procedure, the jawbone was carefully extracted using a threshold and then solidified to fill hollow areas in the STL file (A). Subsequently, the data before solidification were semi-automatically divided into elongated teeth, residual materials, and newly formed bone areas (B). A model representing the region of interest (ROI) was then generated (C) to subtract these data within the ROI. Finally, the solid jawbone (white), elongated teeth (red), residual materials (yellow), and newly formed bone areas (blue) within the ROI were subtracted using this model (D)
Statistical analysis
The volume and percentage of newly formed bone were compared between the two groups using Welch’s t-test and the Mann-Whitney U test, performed using RStudio 2024.12.0, Build 467 (posit, Boston, MA, USA).
Results
Material properties of cement pastes and their hardened products for in vivo evaluations
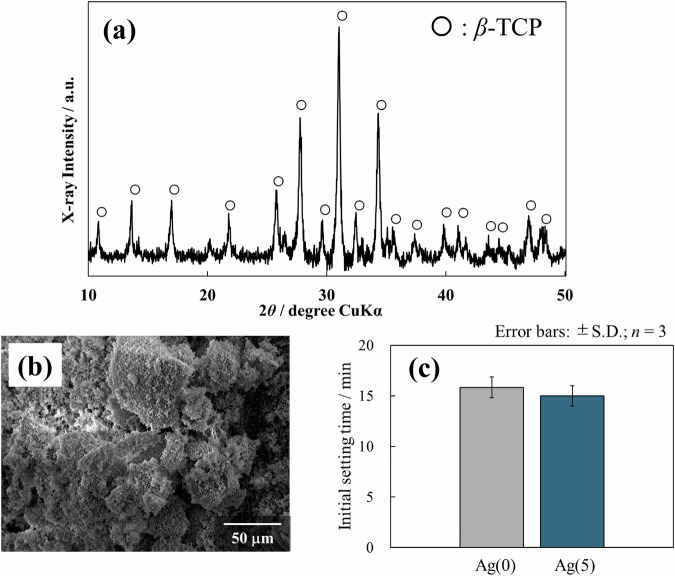
Figure 3a, b shows the XRD patterns and SEM images, respectively, of IP6/β-TCP powder. The XRD results showed that the crystalline phase of IP6/β-TCP powder is β-TCP single phase. The SEM images revealed that the IP6/β-TCP powder consists of an aggregate of fine particles.
Fig. 3.
Crystalline phase (a) and particle morphology (b) of IP6/β-TCP powder, together with initial setting time of the prepared paste (c)
Figure 3c shows the IST of cement pastes prepared based on the IP6/β-TCP powder. Both non-antibacterial Ag(0) and antibacterial Ag(5) pastes generally reached initial setting in approximately 15 min, indicating that the addition of Ag-TCP had little effect on the setting time.
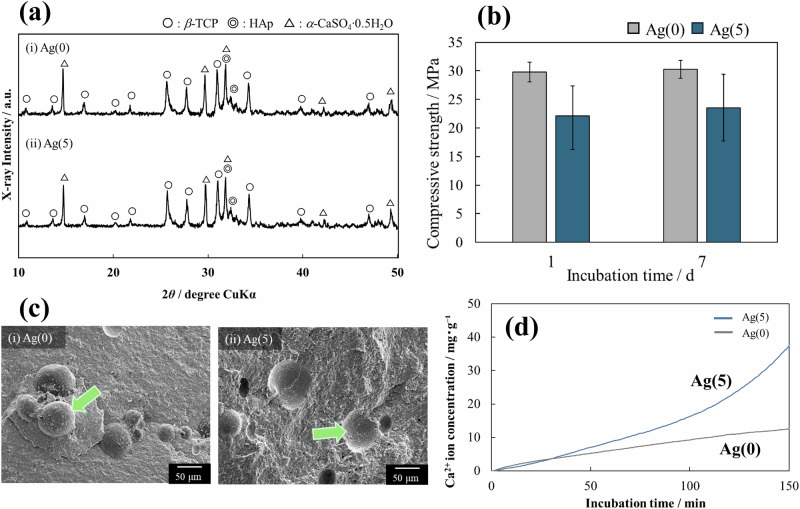
Figure 4 summarizes some material properties of the cured CPC. First, Fig. 4a shows the XRD diagram after 7 days of curing; for both Ag(0) and Ag(5), various crystalline phases attributable to the starting materials listed in Table 1 were identified. Hydroxyapatite (Ca10(PO4)6(OH)2; HAp) was detected in part due to the hydration conversion of some of the fine β-TCP particles to HAp.
Fig. 4.
Some properties of the resulting CPCs: a crystalline phase, b compressive strength (error bar, ± S.D., n = 3), c microstructure (arrows indicate PLGA particles), and d solubility of Ca2+ ions determined using a calcium ion electrode
The Ag content in Ag(5) was determined using inductively coupled plasma–atomic emission spectrometry, which revealed that Ag(5) contained 0.285 mass% of Ag. The theoretical value of Ag content determined from the composition ratios given in Table 1 was 0.354 mass%, which means that Ag(5) contained roughly 80% of the composition of the preparation.
Figure 4b shows the CS of the specimens at day 1 and day 7 of curing; Ag(5) with Ag-TCP tended to exhibit lower CS than Ag(0). However, the average compressive strength of Ag(5) exceeded 20 MPa, which is sufficient for clinical applications. In addition, minimal change in strength was observed over time from day 1 to day 7. This result indicates that the curing of the paste was sufficiently complete on the first day of curing.
Figure 4c shows the results of SEM observations of the fracture surface of specimens on day 7 of curing. No large pores or cracks were observed in either Ag(0) or Ag(5), and the specimens appeared sufficiently cured. The arrow in Fig. 4ci indicates PLGA particles. The arrow in Fig. 4cii shows the area where PLGA particles added as pore-forming material have dropped out.
Figure 4d shows the release behavior of calcium ions from Ag(0) and Ag(5). Ag(5) with Ag-TCP was more soluble than Ag(0) due to the low crystallinity of Ag-TCP particles [9]. Although detailed data are not shown, the leaching of Ag+ ions from Ag(5) specimens was examined in the neutral and weak acid regions. When specimens were immersed in neutral or weakly acidic solutions (10 cm3), 0.298 µmol·dm−3 and 21.66 µmol·dm−3 of Ag+ ions were released during the 28-day immersion period, respectively. HEPES buffer (pH 7.3) was used for the neutral solution, and sodium acetate buffer (pH 5.5) was used for the weakly acidic solution.
Antibacterial properties and cytotoxicity of the resulting CPCs
Figures 5a, b shows the results of antibacterial and cytotoxicity tests, respectively, of Ag(0) and Ag(5). In the antibacterial tests, Ag(0) produced no inhibition zone, whereas Ag(5) produced a clear inhibition zone (Fig. 5a). In the cytotoxicity tests, the effect of Ag(5) on cell proliferation was similar to that of Ag(0) without Ag. A polystyrene cell culture plate was used as a control. This result indicates that Ag(5) is not cytotoxic (Fig. 5b). Therefore, it can be concluded that Ag(5) functions as a paste-like artificial bone that has antibacterial properties but does not exhibit cytotoxicity.
Fig. 5.

Antibacterial properties and cytotoxicity of the resulting CPCs: a antibacterial tests of cement specimens determined using the inhibition zone method (scale bars: 5 mm); red dotted circle indicates the inhibition zone area; b cytotoxicity test using Transwell® kit
Intraoral gross observation
One of the three cases in the non-antibacterial group exhibited exposure of the implanted materials through the extraction site, whereas the other two cases in the non-antibacterial group and three cases in the antibacterial group (a total of five cases) exhibited coverage by soft tissue at the extraction sites.
Results of histological analyses using HE and VB staining
Residual graft material was observed in all specimens. In the HE-stained sections, foreign body reactions were observed in all three cases from the non-antibacterial group, whereas no foreign body reactions were observed in the antibacterial group. In two of the three cases in the non-antibacterial group, marked infiltration of inflammatory cells and encapsulation around the graft material were observed (Fig. 6). In one of the three cases, granulomas containing multinucleated giant cells were observed, which was considered indicative of a foreign body reaction. Mild inflammation was observed in all three cases in the antibacterial group, but no foreign body reactions such as encapsulation were noted. Instead, absorption of the graft material was accompanied by tooth progression into the ROI and bone regeneration (Fig. 7).
Fig. 6.

Pathological findings of the non-antibacterial group. In the HE-stained section, abscess formation (**) and extensive infiltration of inflammatory cells (arrowheads) were observed around the graft material (*). The material was encapsulated by fibrous tissue (arrows), indicating a foreign body reaction
Fig. 7.
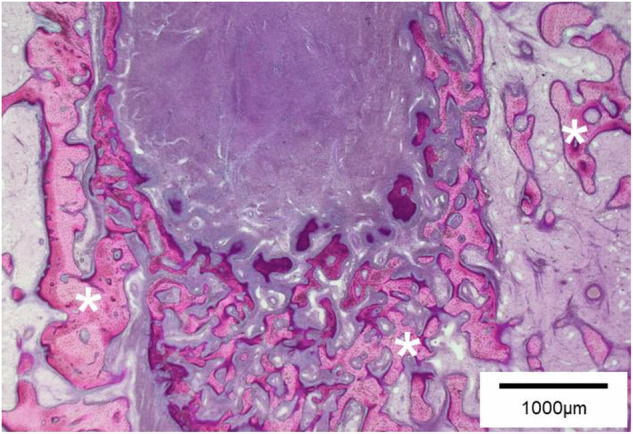
Pathological findings of the antibacterial group. In the HE-stained section, bone regeneration (*) was observed within the ROI. Infiltration of inflammatory cells was minimal
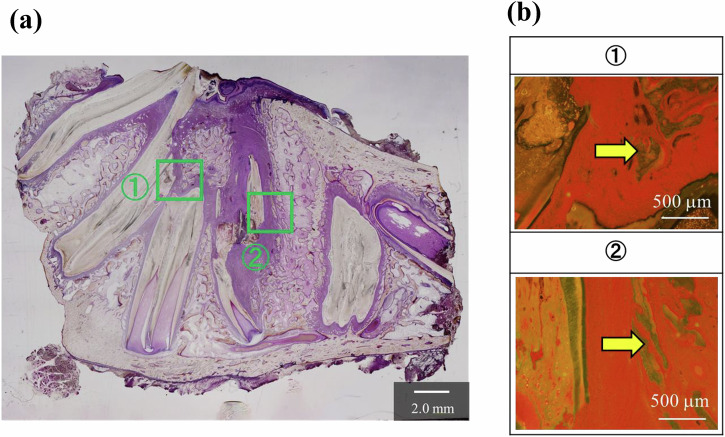
Figure 8a shows an image of the overall jaw tissue of a VB-stained section of Ag(0) under normal light. Brown-stained areas indicate remaining material, and Fig. 8b shows the results of fluorescent observation of the enlarged areas (1) and (2). Almost no calcified areas were observed in the vicinity of the material; however, calcified areas were confirmed at a distance from the material.
Fig. 8.
Pathological findings of the non-antibacterial group (Ag(0)) on the basis of VB staining: a normal light (×1.25), b fluorescent light (×4), regions of ① and ② in (a) were magnified; “M” indicates remaining CPCs
Figure 9a shows an image of the overall jaw bone tissue of VB-stained sections of Ag(5) under normal light. In the Ag(5)-implantation experimental group, the implanted material was almost completely absorbed, and no remaining intact defects were observed. Figure 9b shows the results of fluorescent observation of the enlarged images of the area around the host tooth. In (1) and (2), osteoconduction originating from the host tooth and jawbone was observed, and new bone formation was confirmed. The green coloration under fluorescent observation indicates a high level of calcification. Rabbit teeth are everlasting and continue to grow throughout life [17]. In the Ag(5)-implantation experimental group in which infection can occur, it was concluded that the bone defects healed when the antibacterial properties were expressed, and a normal in vivo environment was established, which allowed the teeth to continue to grow.
Fig. 9.
Pathological findings of the antibacterial group (Ag(5)) on the basis of VB staining: a normal light (×1.25), b fluorescent light (×4), regions of ① and ② in (a) were magnified; arrows indicate calcified areas
X-ray micro-CT analysis of the volume of newly formed bone
The median volume of newly formed bone in the antibacterial and non-antibacterial groups was 102.65 mm³ and 74.28 mm³, respectively (p = 0.4) (Fig. 10). Additionally, the mean percentage of newly formed bone in the antibacterial and non-antibacterial groups was 40.77 ± 10.14% and 21.50 ± 10.51%, respectively (p = 0.0843) (Fig. 11).
Fig. 10.

Volume comparison between the two groups. The median volume of regenerative bone within the ROI in the antibacterial group was greater than that in the non-antibacterial group
Fig. 11.

Comparison of the percentage of regenerative bone between the two groups. The mean percentage of regenerative bone in the antibacterial group tended to be higher than that in the non-antibacterial group
Discussion
We successfully developed a paste-like organic/inorganic hybrid artificial bone prosthesis composed of IP6/β-TCP powder with PLGA particles as a pore-forming agent and CSH as a curing accelerator [6–8]. This cement conforms to the bone remodeling cycle, and a previous study using a porcine tibial defect model showed that it was almost completely resorbed and replaced by host bone within 3 months of implantation. In the present study, Ag-TCP [9] was added to the hybrid type of CPC as an antimicrobial inorganic filler in order to characterize some of the material properties of the resulting CPCs and compare the in vivo responses of antimicrobial and non-antimicrobial CPCs using an infection-susceptible rabbit jaw bone defect model [10].
First, it was found that the IST and CS required for CPC were maintained regardless of the addition of Ag-TCP. Furthermore, the addition of Ag-TCP improved the solubility of the final cured material. This can be explained by considering that Ag-TCP prepared via the ultrasonic spray-pyrolysis route is less crystalline than the commercial β-TCP powder used, as described in a previous report [9]; thus, the solubility of calcium ions improves depending on the content of low crystalline Ag-TCP in CPCs.
The solubility of calcium ions from CPC affects the dissolution of the material itself. The solubility of the material has an important impact on the resorption replacement of living hard tissue. Matching the rate of resorption on the material side with the rate of bone formation on the living tissue side would facilitate the development of artificial bones that are consistent with the bone remodeling cycle [6–8]. In terms of solubility, Ag(5) is more effective than Ag(0). In addition, as the Ag-TCP in Ag(5) dissolves, leaching of Ca2+ ions as well as Ag+ ions incorporated within the crystal structure of the TCP occurs. These Ag+ ions contribute to the antibacterial properties of the material. The Ag(5) used in this study is a bioresorbable artificial bone replacement material with relatively high solubility, and it also exhibits antibacterial properties due to the release of Ag+ ions. The results of histological evaluations demonstrated a near-normal progression of bone formation in rabbit jaw defects implanted with Ag(5) (Figs. 6 and 7), which could be attributed to the solubility and antibacterial properties of Ag(5). However, antibacterial activity and cytotoxicity exhibit a trade-off relationship [18]. In the present study, Ag(5) was not cytotoxic but did exhibit antibacterial properties, which suggests that Ag(5) is a useful antibacterial bone replacement material exhibiting a good balance between antibacterial and cytotoxic properties.
The above findings of this study indicate that the prototype materials Ag(0) and Ag(5) are appropriate models for non-antibacterial and antibacterial CPCs, respectively. Therefore, these two types of CPC were implanted into a rabbit jaw bone defect model susceptible to infection, and the in vivo responses of the materials were examined histologically. Bone formation, as well as a variety of other parameters, were quantified using X-ray micro-CT analysis. As previously mentioned, the oral cavity environment is prone to bacterial exposure. When cement-like artificial bone materials, such as CPC, are implanted in oral regions affected by periodontal disease, fibrous tissue encapsulation of the graft material may occur, potentially resulting in long-term retention of the material and hindering of new bone formation [19, 20]. These well-documented reactions are the result of immunoreactive responses to inflammation [21, 22]. Such undesirable reactions associated with graft material implantation also tend to occur in the treatment of conditions such as IRIs. A previous study in dogs demonstrated that bone augmentation using artificial bone materials for peri-implantitis increased the inflammatory response [23]. Indeed, no bone regeneration therapies for dental implant–associated IRIs have been firmly established to date, even for cases in which the infected implant surface was appropriately decontaminated [24, 25]. We previously developed a “chelate-setting β-TCP cement” and reported its superior material strength and high osteoconductivity compared with other cement-like artificial bone materials in an in vivo study using pigs [6–8]. However, considering the use of non–infection-resistant artificial bone graft materials (including the non-antibacterial cement developed in this study) in reconstructive procedures for conditions such as IRIs, further improvements in material properties seem necessary. In our study, implants with Ag(5) exhibited no clear signs of infection, and the implanted material was properly resorbed and replaced by bone according to the bone remodeling cycle. By contrast, Ag(0) did not promote bone formation favorably, differing from the findings of previous studies examining pig tibia [6–8]. Among three cases examined, one showed exposure of the bone graft material within the oral cavity. In all three cases, histological analysis revealed findings characteristic of a foreign body reaction, such as encapsulation of residual materials and granulation tissue containing multinucleated giant cells. These findings indicate that chelate-setting β-TCP cements exhibiting antibacterial properties would hold significant clinical value for addressing potentially infectious conditions such as IRIs.
The above findings from a bone defect model in which infection could occur indicate that the use of antibacterial CPCs promotes normal bone regeneration more strongly than non-antibacterial CPCs. This can be explained by the release of Ag+ ions from Ag-TCP, which blocks bacterial infection, in addition to the good solubility of organic/inorganic hybrid cement composed of PLGA and β-TCP, which is compatible with the bone remodeling cycle.
Conclusions
An antibacterial Ag-TCP was added to a paste-like organic/inorganic hybrid artificial bone compatible with the bone remodeling cycle to create an antibacterial CPC. Antibacterial and non-antibacterial CPCs were implanted into a rabbit jaw defect model in which infection could occur, and the in vivo responses of the CPCs were compared. Non-antibacterial CPC specimens retrieved from rabbit jaws showed residual material, whereas almost all of the antibacterial CPC material was resorbed and replaced with host bone. These results suggest that placement of an antibacterial CPC in a jaw defect susceptible to bacterial infection promotes material resorption and bone formation in a more normal fashion. The findings of this study demonstrate the usefulness of artificial bone materials that exhibit antibacterial properties. In addition, paste-like bone grafts represent a novel type of artificial bone graft exhibiting good resorption and osteogenic potential in hard tissues while also demonstrating resistance to infection.
Acknowledgements
This work was supported by JSPS KAKENHI grant number JP24K20006.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
These authors contributed equally: H. Miyashita, Y. Kamaya.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
H. Miyashita, Email: miyashita@keio.jp
M. Aizawa, Email: mamorua@meiji.ac.jp
References
- 1.Quirynen M, De Soete M, Van Steenberghe D. Infectious risks for oral implants: a review of the literature. Clin Oral Implants Res. 2002;13:1–19. [DOI] [PubMed] [Google Scholar]
- 2.Schwarz F, Sahm N, Bieling K, Becker J. Surgical regenerative treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane: a four-year clinical follow-up report. J Clin Periodontol. 2009;36:807–14. [DOI] [PubMed] [Google Scholar]
- 3.Aghazadeh A, Persson GR, Renvert S. A single-centre randomized controlled clinical trial on the adjunct treatment of intra-bony defects with autogenous bone or a xenograft: results after 12 months. J Clin Periodontol. 2012;39:666–73. [DOI] [PubMed] [Google Scholar]
- 4.Tsuru K, Sugiura Y, Ishikawa K. Chapter 6—Bone cements utilised for the reconstruction of hard tissue: basic understanding and recent topics. In: Thian ES, Huang J, Aizawa M, editors. Nanobioceramics for healthcare applications. Singapore: World Scientific; 2017. p. 151–86.
- 5.Konishi T, Takahashi S, Zhuang Z, Nagata K, Mizumoto M, Honda M, et al. Biodegradable β-tricalcium phosphate cement with anti-washout property based on chelate-setting mechanism of inositol phosphate. J Mater Sci Mater Med. 2013;24:1384–94. [DOI] [PubMed] [Google Scholar]
- 6.Ando A, Kamikura M, Takeoka Y, Rikukawa M, Nakano K, Nagaya M, et al. Bioresorbable porous β-tricalcium phosphate chelate-setting cements with poly lactic-co-glycolic acid particles as pore-forming agent: fabrication, material properties, cytotoxicity, and in vivo evaluation. Sci Technol Adv Mater. 2021;22:511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamaya Y, Ando A, Suzuki K, Nakano K, Nagaya M, Nagashima H, et al. Development of paste-like organic/inorganic artificial bones compatible with bone remodeling cycles, consisting of β-tricalcium phosphate, calcium sulfate hemihydrate, and poly(lactic-co-glycolic acid) particles. New J Chem. 2024;4:8545–55. [Google Scholar]
- 8.Kamaya Y, Kato S, Ando A, Suzuki K, Nakano K, Nagaya M, et al. Development of fully-resorption replacement paste-like organic/inorganic artificial bones compatible with bone remodeling cycles. Biomater Biosyst. 2025;17:100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honda M, Kawanobe Y, Nagata K, Ishii K, Matsumoto M, Aizawa M. Potential application of protamine for antimicrobial biomaterials in bone tissue engineering. Int J Mol Sci. 2020;21:3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrel JP, Wiskott A, Scherrer S, Durual S. Large bone vertical augmentation using a three-dimensional printed TCP/HA bone graft: a pilot study in dog mandible. Clin Implant Dent Relat Res. 2016;18:1183–92. [DOI] [PubMed] [Google Scholar]
- 11.Lundgren AK, Sennerby L, Lundgren D. Guided jaw-bone regeneration using an experimental rabbit model. Int J Oral Maxillofac Surg. 1998;27:135–40. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki S, Venkataiah VS, Yahata Y, Kitagawa A, Inagaki M, Njuguna MM, et al. Correction of large jawbone defect in the mouse using immature osteoblast–like cells and a 3D polylactic acid scaffold. PNAS Nexus. 2022;1:pgac151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah SR, Young S, Goldman JL, Jansen JA, Wong ME, Mikos AG. A composite critical-size rabbit mandibular defect for evaluation of craniofacial tissue regeneration. Nat Protoc. 2016;11:1989–2009. [DOI] [PubMed] [Google Scholar]
- 14.Habraken WJEM, Wolke JGC, Mikos AG, Jansen JA. Injectable PLGA microsphere/calcium phosphate cements: physical properties and degradation characteristics. J Biomater Sci Polym Ed. 2016;17:1057–74. [DOI] [PubMed] [Google Scholar]
- 15.Nagata K, Fujioka K, Konishi T, Honda M, Nagaya M, Nagashima H, et al. Evaluation of resistance to fragmentation of injectable calcium-phosphate cement paste using X-ray microcomputed tomography. J Ceram Soc Jpn. 2017;125:1–6. [Google Scholar]
- 16.Ito A, Sogo Y, Yamazaki A, Aizawa M, Osaka A, Hayakawa S, et al. Interlaboratory studies on in vitro test methods for estimating in vivo resorption of calcium phosphate ceramics. Acta Biomater. 2015;25:347–55. [DOI] [PubMed] [Google Scholar]
- 17.Okuda A. Clinical dental examination and radiological evaluation of rabbit. J Anim Clin Med. 2002;11:39–43. [Google Scholar]
- 18.Kakinuma H, Ishii K, Ishihama H, Honda M, Toyama Y, Matsumoto M, et al. Antibacterial polyetheretherketone implants immobilized with silver ions based on chelate-bonding ability of inositol phosphate: Processing, material characterization, cytotoxicity, and antibacterial properties. J Biomed Mater Res A. 2014;103A:57–64. [DOI] [PubMed] [Google Scholar]
- 19.Baldock WT, Hutchens LH, McFall WT, Simpson DM. An evaluation of tricalcium phosphate implant in human periodontal osseous defects of two patients. J Periodontol. 1985;56:1–7. [DOI] [PubMed] [Google Scholar]
- 20.Froum S, Stahl SS. Human intraosseous healing responses to the placement of tricalcium phosphate ceramic implants. II. 13 to 18 months. J Periodontol. 1987;58:103–9. [DOI] [PubMed] [Google Scholar]
- 21.Mariani E, Lisignoli G, Borzì RM, Pulsatelli L. Biomaterials: foreign bodies or tuners for the immune response?. Int J Mol Sci. 2019;20:636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carnicer-Lombarte A, Chen ST, Malliaras GG, Barone DG. Foreign body reaction to implanted biomaterials and its impact in nerve neuroprosthetics. Front Bioeng Biotechnol. 2021;15:622524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solderer A, Pippenger BE, Donnet M, Wiedemeier D, Ramenzoni LL, Schmidlin PR. Evaluation of air polishing with a sterile powder and mechanical debridement during regenerative surgical periimplantitis treatment: a study in dogs. Clin Oral Investig. 2021;25:2609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoshkam V, Chan HL, Lin GH, MacEachern MP, Monje A, Suarez F, et al. Reconstructive procedures for treating peri-implantitis: a systematic review. J Dent Res. 2013;92:131S–138S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roccuzzo M, Layton DM, Roccuzzo A, Heitz-Mayfield LJ. Clinical outcomes of peri-implantitis treatment and supportive care: a systematic review. Clin Oral Implants Res. 2018;29:331–50. [DOI] [PubMed] [Google Scholar]