Abstract
For the detection of six groups of anaerobic bacteria in human feces, we designed seven new 16S rRNA-based oligonucleotide probes. This set of probes extends the current set of probes and gives more data on the composition of the human gut flora. Probes were designed for Phascolarctobacterium and relatives (Phasco741), Veillonella (Veil223), Eubacterium hallii and relatives (Ehal1469), Lachnospira and relatives (Lach571), and Eubacterium cylindroides and relatives (Ecyl387), and two probes were designed for Ruminococcus and relatives (Rbro730 and Rfla729). The hybridization conditions for the new probes were optimized for fluorescent in situ hybridization, and the probes were validated against a set of reference organisms. The probes were applied to fecal samples of 11 volunteers to enumerate their target bacterial groups. The Phasco741 and Veil223 probes both detected average numbers below 1% of the total number of bacteria as determined with the bacterial kingdom-specific Bact338 probe. The Ecyl387 probe detected about 1.4%, the Lach571 and Ehal1469 probes detected 3.8 and 3.6%, respectively, and a combination of the Rbro730 and Rfla729 probes detected 10.3%. A set of 15 probes consisting of probes previously described and those presented here were evaluated in hybridization with the fecal samples of the same volunteers. Together, the group-specific probes detected 90% of the total bacterial cells.
The human gut flora is a complex ecosystem involved in human nutrition and health (6). Encouraged by medicine and the food industry, research is currently being undertaken to stimulate that fraction of the microbiota that is beneficial for human health (9-11). For evaluation of such studies, accurate analysis of the intestinal microbiota is required. This microbiota consists mostly of anaerobic bacteria that are not easy to enumerate by conventional culturing techniques (12, 23). Therefore, for the last few years, interest in molecular analysis of human gut microbiota has been rapidly growing. Techniques such as sequence analysis of clone libraries from amplified fecal ribosomal DNA (rDNA) and denaturing or temperature-gradient gel electrophoresis (DGGE/TGGE) analysis of the amplified rDNA and rRNA have demonstrated the enormous diversity of species that thrive in the human gut (32, 36, 38). For quantitative analysis of human gut and fecal flora, 16S rRNA-based oligonucleotides were designed that were applied as either primers in PCR (27) or as probes in fluorescent in situ hybridization (FISH) (8, 13, 15). For this purpose, a large set of probes that covers around 80% of the total microbiota has already been described (8). However, detection of the remaining 20% is still a challenge. Analysis of clone libraries of 16S rDNA amplified from total fecal DNA showed the presence of sequences related to Phascolarctobacterium, Ruminococcus flavefaciens, and Eubacterium cylindroides (32). However, no group-specific probes for in situ hybridization existed to detect the bacteria corresponding to these sequences, and quantitative data about the numbers of these bacteria in feces are scarce. Veillonella can be cultured from feces of newborn children (14, 18, 19) and therefore might be present in feces from adults as well. Furthermore, the specific probe Erec482 for the Eubacterium rectale-Clostridium coccoides group detects about one-third of the total microbiota (8). This group might be too large to detect subtle variations in microbiota and therefore needs to be divided into smaller subgroups. Some probes that divide the Erec482 group have already been described, but this development needs to be continued (29). In this paper, we describe seven new group-specific probes to investigate the composition of the human gut microbiota. Together with the previously described probes with which other major groups of human gut bacteria can be detected, they form an extensive probe set for analysis of human gut microbiota (8, 13, 15). This probe set consisting of 15 probes was used to describe the composition of the fecal microbiota of 11 healthy human volunteers.
MATERIALS AND METHODS
Organisms and culture conditions.
All reference strains used in this study are listed in Table 1. The strains were obtained from different sources as indicated in the table: DSM is Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany), ATCC is the American Type Culture Collection (Rockville, Md.), NIZO is The Netherlands Institute for Dairy Research (Ede, The Netherlands), and MMB is the Laboratory for Medical Microbiology (Groningen, The Netherlands). DSM or ATCC strains were cultivated on the media described in the respective catalogues. All other strains were cultivated in anoxic peptone-yeast extract-glucose (PYG) medium (15) under anaerobic conditions at 37°C or, in the case of facultative anaerobes, on brain heart infusion agar (Oxoid, Basingstoke, United Kingdom). All MMB strains are clinical or human fecal isolates from local and regional public health laboratories and have been identified by routine procedures.
TABLE 1.
Organisms used in the FISH specificity studies and the results of the FISH specificity tests
| Strain | Code | Probe result
|
||||||
|---|---|---|---|---|---|---|---|---|
| Phasco741 | Veil223 | Ehal1469 | Lach571 | Ecyl387 | Rbro730 | Rfla729 | ||
| Succiniclasticum ruminis | DSM 9236 | + | − | − | − | − | − | − |
| Acidaminococcus fermentans | MMB | + | − | − | − | − | − | − |
| Veillonella atypica subsp. rodentium | DSM 20737 | − | + | − | − | − | − | − |
| Veillonella parvula | MMB | − | + | − | − | − | − | − |
| Clostridium polysaccharolyticum | DSM 1801 | − | − | + | − | − | − | − |
| Lachnospira multipara | DSM 3073 | − | − | − | + | − | − | − |
| Eubacterium eligens | MMB | − | − | − | + | − | − | − |
| Eubacterium cylindroides | DSM 20477 | − | − | − | − | + | − | − |
| Clostridium innocuum | MMB | − | − | − | − | + | − | − |
| Clostridium leptum | DSM 753 | − | − | − | − | − | + | − |
| Clostridium sporosphaeroides | DSM 1294 | − | − | − | − | − | + | − |
| Ruminococcus bromii | ATCC 27255 | − | − | − | − | − | + | − |
| Ruminococcus albus | ATCC 27210 | − | − | − | − | − | + | + |
| Ruminococcus flavefaciens | ATCC 19208 | − | − | − | − | − | − | + |
| Ruminococcus callidus | ATCC 27760 | − | − | − | − | − | − | + |
| Eubacterium siraeum | DSM 3996 | − | − | − | − | − | − | + |
| Atopobium parvulum | MMB | − | − | − | − | − | − | − |
| Bacteroides fragilis | DSM2151 | − | − | − | − | − | − | − |
| Bacteroides ovatus | MMB | − | − | − | − | − | − | − |
| Bacteroides splanchnicus | MMB | − | − | − | − | − | − | − |
| Bacteroides thetaiotaomicron | MMB | − | − | − | − | − | − | − |
| Bacteroides uniformis | MMB | − | − | − | − | − | − | − |
| Bifidobacterium adolescentis | MMB | − | − | − | − | − | − | − |
| Bifidobacterium dentium | NIZO B679 | − | − | − | − | − | − | − |
| Bifidobacterium infantis | MMB | − | − | − | − | − | − | − |
| Bifidobacterium longum | MMB | − | − | − | − | − | − | − |
| Bifidobacterium pseudolongum | MMB | − | − | − | − | − | − | − |
| Clostridium aminovalericum | MMB | − | − | − | − | − | − | − |
| Clostridium beijerinckii | MMB | − | − | − | − | − | − | − |
| Clostridium butyricum | MMB | − | − | − | − | − | − | − |
| Clostridium carnis | MMB | − | − | − | − | − | − | − |
| Clostridium difficile | DSM 1296 | − | − | − | − | − | − | − |
| Clostridium histolyticum | MMB | − | − | − | − | − | − | − |
| Clostridium perfringens | MMB | − | − | − | − | − | − | − |
| Clostridium putrificum | DSM 1734 | − | − | − | − | − | − | − |
| Clostridium sporogenes | MMB | − | − | − | − | − | − | − |
| Clostridium tyrobutyricum | MMB | − | − | − | − | − | − | − |
| Collinsella aerofaciens | DSM 13713 | − | − | − | − | − | − | − |
| Eubacterium moniliforme | MMB | − | − | − | − | − | − | − |
| Eubacterium plautii | DSM 4000 | − | − | − | − | − | − | − |
| Eubacterium tenue | DSM 20695 | − | − | − | − | − | − | − |
| Eubacterium ventriosum | DSM 3988 | − | − | − | − | − | − | − |
| Lactobacillus brevis | DSM 20468 | − | − | − | − | − | − | − |
| Peptostreptococcus prevotii | DSM 20548 | − | − | − | − | − | − | − |
| Prevotella intermedia | ATCC 49046 | − | − | − | − | − | − | − |
| Sporomusa paucivorans | DSM 3697 | − | − | − | − | − | − | − |
Design and testing of oligonucleotide probes.
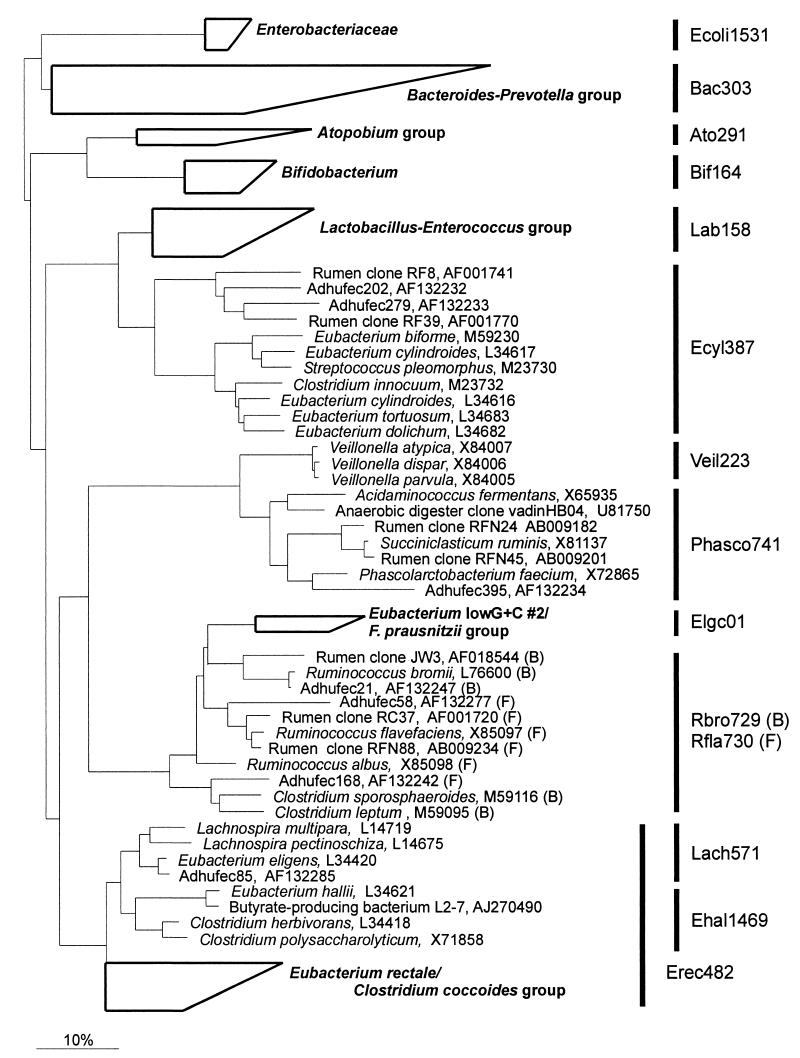
Oligonucleotide probes were designed with the ARB software package (24), and rRNA sequences were obtained in an aligned form from the Ribosomal Database Project (RDP) (25) supplemented with newly deposited rRNA sequences from GenBank. Fluorescein-labeled oligonucleotides against selected group-specific target sequences were synthesized commercially (Eurogentec, Seraing, Belgium) and tested for specificity against a set of reference organisms listed in Table 1. For this purpose, paraformaldehyde (PFA)-fixed cells of the reference strains were applied to slides and hybridized overnight at 50°C in hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl [pH 7.2], 0.1% sodium dodecyl sulfate [wt/vol]) containing 5 ng of labeled probe μl−1 as described previously (8). If more stringent conditions were needed, formamide was added to the hybridization buffer in concentrations ranging from 0 to 60% (vol/vol). For hybridization with the Rbro730 and Rfla729 probes, cells were incubated prior to hybridization with 10 μl of 1 mg of lysozyme ml−1 in 100 mM Tris-HCl (pH 8.5) for 10 min at room temperature. Phylogenetic trees illustrating the target groups of the extensive probe set (Fig. 1) were generated with the ARB software package by applying the neighbor-joining method to a relevant selection of sequences from the database of the RDP comprising around 20,000 sequences.
FIG. 1.
Phylogenetic tree based on 16S rRNA sequences illustrating the target groups of the newly described probes and the other probes of the extensive gut microbiota probe set. The tree shows the relationship between the major genera, groups, and organisms known to be present in the human gut. The species or clone sequences with their corresponding accession numbers are the target organisms that have a full match with the newly described oligonucleotide probes depicted behind the vertical bars on the right. Boxes and group names in boldface indicate that the target groups of the probes have been described previously. The sizes of the boxes reflect the number of sequences used in the tree construction, and their shape reflects the phylogenetic depth of the corresponding groups. The size bar at the bottom represents 10% sequence divergence.
Enumeration of bacteria in fecal samples by FISH.
Eleven healthy volunteers ranging from 20 to 55 years provided fresh fecal stools. Portions (0.5 g) of each stool were fixed with PFA as described previously (8). Dry weights were determined by lyophilizing a weighed portion of each homogenized fecal sample.
Fecal samples were applied to glass slides by the protocol described previously (16), except that the dilution of the PFA-fixed fecal samples was made in phosphate-buffered saline and not in 5% Tween solution. The slides were hybridized with the (newly designed) probes or stained with diamidino-2-phenylindole (DAPI) as described previously (8, 16). The fluorescent cells in the samples were counted automatically (16) with a Leica DMRXA epifluorescence microscope (Leica, Wetzlar, Germany), except when the number of cells was lower than 4 × 108 cells g−1 (wet weight): in that situation, the cells were counted visually with an Olympus BH2 epifluorescence microscope.
RESULTS
Design and specificity of oligonucleotide probes.
Seven specific probes were designed to extend the existing set of probes for fecal bacteria. These probes are listed together with their target organisms in Table 2. A schematic representation of the probes and their target groups is shown in Fig. 1 as a phylogenetic tree. The probe Phasco741 was for the Phascolarctobacterium group, which includes the species Phascolarctobacterium faecium, Acidaminococcus fermentans, and Succiniclasticum ruminis, members of the Clostridium cluster IX, as described before (3). The probe Veil223 was used for members of the genus Veillonella (e.g., V. dispar, V. parvula, and V. atypica). The remaining two members of the genus, V. ratti and V. criceti, have one G-T mismatch on position 9 from the 5′ end of the probe and will probably hybridize with the probe under the hybridization conditions in Table 2. The probe Ehal1469 is for the Eubacterium hallii group, including E. hallii, Clostridium herbivorans, and Clostridium polysaccharolyticum, which are members of the Clostridium cluster XIVa (3). The probe Lach571 is specific for other members of cluster XIVa: the Lachnospira group, which includes the species Lachnospira multipara, Eubacterium eligens, and Lachnospira pectinoschiza. The probe Ecyl387 is specific for members of Clostridium cluster XVI, referred to here as the Eubacterium cylindroides group. The Rfla729 and the Rbro730 probes are specific for members of Clostridium cluster IV, Clostridium leptum, Clostridium sporosphaeroides, and ruminococci related to these bacteria, including Ruminococcus flavefaciens, Ruminococcus albus, and Ruminococcus bromii. Ruminococcus callidus and Eubacterium siraeum, both members of cluster IV, have only one mismatch to the Rfla729 probe. Probe design was based on 16S rRNA sequences of known bacteria and on sequences from human fecal clone libraries (32). In order to test the specificity of the newly designed probes, a selection of target and nontarget bacterial strains was hybridized in situ with the probes. This included strains with one or two mismatches in the target sequence of the 16S rRNA. The strains were hybridized and washed at 50°C. The specificity was optimized with a range of formamide concentrations in the hybridization buffer. After hybridization, the strains were screened for fluorescent signals and compared with those obtained after hybridization with bacterial probe Bact338 as a positive control and its complement non-Bact338 as the negative control. Table 1 shows the results of these hybridizations under the optimized conditions described in Table 2. All probes were specific for their target organisms, except for the Rbro730 probe and the Rfla729 probe. The Rbro730 probe also hybridized to R. albus, which has one U-G mismatch on position 13 from the 5′ end of the probe. If Rbro730 is used in conjunction with the Rfla729 probe, this will not cause any problems, because the target groups overlap. The Rfla729 probe cross-reacted with R. callidus and E. siraeum, which both have one G-T mismatch on position 10 from the 5′ end of the probe. Because of their close relationship with the target organisms, this cross-reaction is favorable. It would be possible to include these two species in the target group by introducing a wobble base on the mismatching position. However, this creates new single mismatches with unwanted species, and therefore this idea was rejected. The Lach571 probe has only one mismatch with many Bacteroides species. However at 60% formamide, the probe did not show fluorescence with these Bacteroides species. In addition, it was found that lowering the salt concentration from 0.9 to 0.4 M NaCl in combination with 40% formamide effected brighter signals with the target species without giving rise to fluorescence with the Bacteroides species. This was chosen as the optimal hybridization condition.
TABLE 2.
Seven new probes, their target species, probe sequences, and optimal hybridization conditions for the detection of human fecal bacteria
| Probe | Target species | Probe sequence | Hybridization conditions
|
|
|---|---|---|---|---|
| % of formamide | Lysozyme treatment (min) | |||
| Phasco741 | Phascolarctobacterium faecium, Acidaminococcus fermentans, and Succiniclasticum ruminis | 5′TCAGCGTCAGACACAGTC | 0 | NTa |
| Veil223 | Veillonella dispar, Veillonella parvula, and Veillonella atypica | 5′AGACGCAATCCCCTCCTT | 0 | NT |
| Ehal1469 | Eubacterium hallii, Clostridium herbivorans, and Clostridium polysaccharolyticum | 5′CCAGTTACCGGCTCCACC | 20 | |
| Lach571 | Lachnospira multipara, Eubacterium eligens, and Lachnospira pectinoschiza | 5′GCCACCTACACTCCCTTT | 40 (400 mM NaCl) | NT |
| Ecyl387 | Eubacterium cylindroides, Clostridium innocuum, Eubacterium biforme, Eubacterium tortuosum, Eubacterium dolichum and Streptococcus pleomorphus | 5′CGCGGCATTGCTCGTTCA | 20 | NT |
| Rfla729 | Ruminococcus albus and Ruminococcus flavefaciens | 5′AAAGCCCAGTAAGCCGCC | 20 | 15 |
| Rbro730 | Clostridium sporosphaeroides, Ruminococcus bromii, and Clostridium leptum | 5′TAAAGCCCAGYAGGCCGC | 20 | 15 |
NT, no treatment.
Application of the probe in enumeration of the bacterial group in human feces.
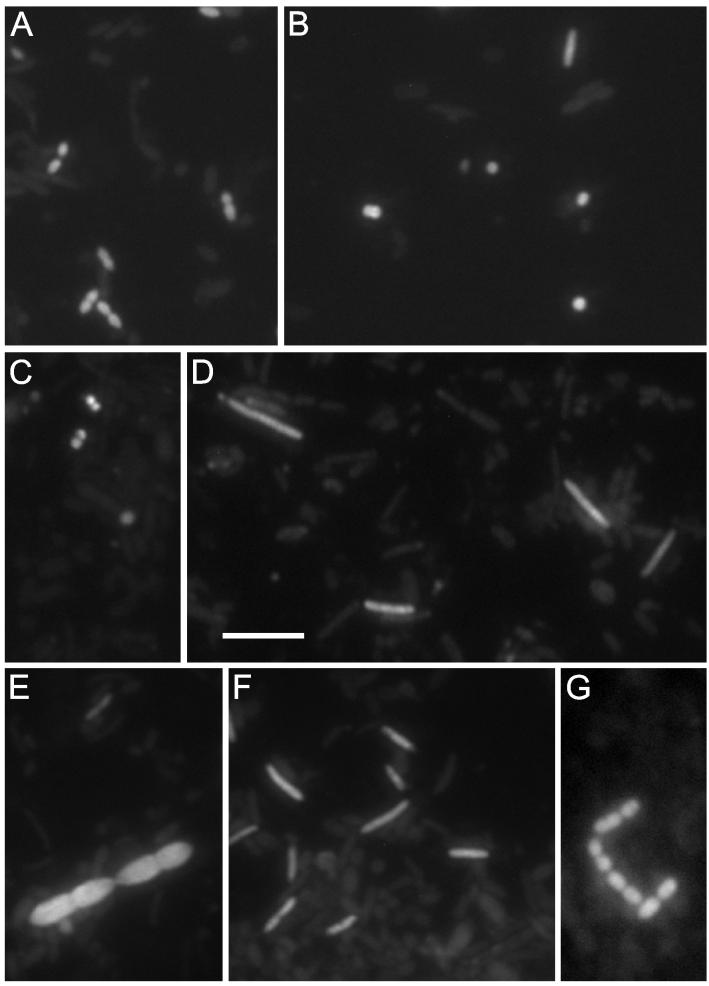
The newly designed probes were used in FISH experiments to enumerate the numbers of their target groups in fecal samples from 11 healthy volunteers. The results were listed in Table 3. The Ecyl387 probe detected 1.5 × 109 cells per g (dry weight), representing 1.8% of the total (Bact338) (range, 0.05 to 7.4%). The probes detected mostly cylindrical small rods, presumably E. cylindroides (Fig. 2A), and sometimes detected C. innocuum-like thin rods. A combination of the Rbro729 and the Rfla730 probes detected a mean number of 1.4 × 1010 cells per g (dry weight) of feces. This was 10% of the total hybridizable cell count (Bact338), which ranged from 0.6 to 28%. The probes detected brightly fluorescing cocci—presumably ruminococci—and some weakly fluorescing rods, presumably clostridia of group IV (Fig. 2B). The Veil223 probe detected 108 cells per g (dry weight). The only morphology detected was represented by small Veillonella-like cocci (Fig. 2C); on average, 0.08% of the total bacterial cells were within the range not detectable to 0.5% detectable. The Phasco741 probe detected 9.0 × 108 cells per g (dry weight), representing 0.6% of the total (Bact338) and ranging from not detectable to 2.6% detectable. The bacteria that were detected by the Phasco741 probe were all rods about 4 to 6 μm long—presumably Phascolarctobacterium or Succiniclasticum cells (Fig. 2D). The Lach571 probe also detected 5.1 × 109 cells per g (dry weight), representing 3.6% of the total (Bact338) and ranging from 1.6 to 13.6%. The morphology detected by the Lach571 probe was more diverse, ranging from large oval dividing rods (Fig. 2E) to thin rods (Fig. 2F). The Ehal1469 probe detected 5.1 × 109 cells per g (dry weight), representing 3.8% of the total (Bact338) and ranging from 0.9 to 8.2%. Their main morphology was represented by pairs of rods about 2 μm in length (Fig. 2G), although in some cases, longer rods were seen.
TABLE 3.
Numbers and percentages of fecal microorganisms from 11 volunteers as determined by DAPI staining (total cells) and by hybridization with bacterial probe Bact338 (total bacteria) or newly designed probes
| Population | Stain or probe | No. of cells/g (dry wt) in volunteer no.a:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Total cells | DAPI | 1.4 × 1011 | 2.9 × 1011 | 3.2 × 1011 | 1.5 × 1011 | 1.4 × 1011 | 2.5 × 1011 | 2.0 × 1011 | 3.7 × 1011 | 2.4 × 1011 | 1.6 × 1011 | 8.6 × 1010 |
| Total bacteria | Bact 338 | 7.9 × 1010 | 1.7 × 1011 | 2.2 × 1011 | 1.3 × 1011 | 6.2 × 1010 | 8.4 × 1010 | 1.7 × 1011 | 2.2 × 1011 | 1.2 × 1011 | 6.0 × 1010 | 8.3 × 1010 |
| Ruminococcus group | Rbro729/Rfla730 | 2.0 × 109 (2.5) | 3.1 × 1010 (18.4) | 1.7 × 1010 (8.1) | 8.0 × 108 (0.6) | 1.7 × 109 (2.8) | 1.4 × 1010 (16.9) | 2.7 × 109 (1.6) | 4.4 × 1010 (19.8) | 3.5 × 1010 (28.4) | 6.6 × 109 (10.9) | 3.1 × 109 (3.8) |
| E. cylindroides group | Ecyl387 | 2.6 × 108 (0.3) | 2.0 × 108 (0.1) | 1.9 × 108 (0.09) | 9.3 × 109 (7.4) | 3.3 × 108 (0.5) | 3.4 × 109 (4.1) | 7.8 × 107 (0.05) | 1.3 × 108 (0.06) | 8.0 × 108 (0.6) | 8.5 × 108 (1.4) | 5.2 × 108 (0.6) |
| Phascolarctobacterium group | Phasco 741 | 3.9 × 108 (0.5) | 1.5 × 108 (0.1) | 2.8 × 109 (1.3) | 2.1 × 108 (0.2) | NDb | ND | 4.3 × 109 (2.6) | 1.0 × 107 (0.005) | 4.7 × 106 (0.004) | 7.4 × 106 (0.01) | 2.1 × 109 (2.5) |
| Veillonella | Veil223 | 6.0 × 107 (0.07) | ND | 6.1 × 106 (0.003) | ND | 6.1 × 106 (0.01) | 3.4 × 107 (0.04) | 7.8 × 108 (0.5) | 3.7 × 107 (0.02) | 1.3 × 107 (0.01) | 6.0 × 107 (0.1) | 1.4 × 108 (0.2) |
| E. hallii group | Ehal1469 | 5.7 × 109 (7.1) | 1.4 × 1010 (8.2) | 7.2 × 109 (3.4) | 7.1 × 109 (5.7) | 2.4 × 109 (3.9) | 8.8 × 108 (1.0) | 1.1 × 1010 (6.7) | 4.6 × 109 (2.0) | 1.3 × 109 (1.1) | 5.6 × 108 (0.9) | 1.4 × 109 (1.7) |
| Lachnospira group | Lach571 | 3.1 × 109 (3.9) | 7.6 × 109 (4.6) | 6.2 × 109 (2.9) | 2.1 × 109 (1.7) | 1.3 × 109 (2.1) | 1.3 × 109 (1.6) | 2.3 × 1010 (13.6) | 5.8 × 109 (2.6) | 2.8 × 109 (2.3) | 1.1 × 109 (1.8) | 2.3 × 109 (2.8) |
| % Dry matter of the fecal sample | 30 | 36 | 26 | 25 | 37 | 33 | 17 | 24 | 35 | 33 | 22 | |
Values in parentheses represent the percentage relative to the total number of cells determined by hybridization with probe Bact338.
ND, not detected.
FIG. 2.
FISH experiments with the newly designed probes on fecal samples from different volunteers. (A) Epifluorescent images of a hybridization with the Ecyl387 probe specific for the E. cylindroides group showing small fluorescent rods. (B) Hybridization with a combination of the Rbro729 and the Rfla730 probes specific for bacteria of the Ruminococcus group; the image shows one rod and different cocci. (C) Hybridization with the Veil223 probe specific for Veillonella showing small cocci. (D) Hybridization with the Phasco741 probe detecting rods of the Phascolarctobacterium group. (E and F) Two images of different fecal samples hybridized with the Lach571 probe specific for the Lachnospira group showing big oval dividing rods and one thin rod (E) or only thin rods (F). (G) Hybridization with the Ehal1469 probe showing rods of the E. hallii group. Bar, 5 μm.
Evaluation of the coverage of the extensive probe set for detecting specific bacterial groups in human feces.
A set of 15 probes were used to evaluate how much of the total microbiota was detected with these probes specific for the major bacterial groups in human feces. Furthermore, differences in counts with the various probes between individuals were analyzed. The extensive set of probes consists of the probes presented in Fig. 1 and the Bact338 probe specific for virtually all bacteria (1). Apart from the newly described seven probes, probes for other fecal bacterial groups designed and validated elsewhere were used to enumerate bacteria in the same fecal samples as described above. These were (i) Bact338 to detect the total hybridizable bacterial cells; (ii) the Erec482 probe for most members of the Clostridium group XIVa (8); (iii) Bac303 for most Bacteroides and Prevotella bacteria (26); (iv) Bif164 for the genus Bifidobacterium (23); (v) Elgc01 for Fusobacterium prausnitzii-related eubacteria (8, 36); (vi) Ato291 for the Atopobium group, with Collinsella aerofaciens as the predominant fecal species (13); (vii) Ecoli1531 for Escherichia coli and related species (28); and (viii) Lab158 for enterococci and lactobacilli (15). For this enumeration, the same 11 fecal samples described above were used. DAPI staining was used to enumerate the total amount of cells in these samples. The mean results for the 11 individuals with the 15 probes are presented in Table 4. The mean counts are given in cells per gram in both dry weight and wet weight to make the results more comparable with data from the literature.
TABLE 4.
Mean numbers per gram of feces, CVs, and percentages of total microbiota from 11 volunteersa
| Population | Stain or probe | Mean no. of cells/g of feces (SD)b
|
CVinterc | % Microbiota by:
|
||
|---|---|---|---|---|---|---|
| Dry wt | Wet wt | DAPI | Bact338 | |||
| Total cells | DAPI | 2.1 (0.8) × 1011 | 6.2 × 1010 | 0.38 | 100 | |
| Total bacteria | Bact338 | 1.3 (0.6) × 1011 | 3.5 × 1010 | 0.46 | 60.9 | 100 |
| Bacteroides/Prevotella | Bac303 | 3.6 (2.3) × 1010 | 9.5 × 109 | 0.62 | 18.4 | 27.7 |
| E. rectale/C. coccoides group | Erec482 | 2.9 (1.9) × 1010 | 7.9 × 109 | 0.62 | 14.0 | 22.7 |
| Eubacterium low G+C2 | Elgc01 | 1.4 (1.2) × 1010 | 3.6 × 109 | 0.86 | 7.1 | 10.8 |
| Atopobium group | Ato291 | 1.4 (0.9) × 1010 | 4.1 × 109 | 0.67 | 6.9 | 11.9 |
| Ruminococcus group | Rbro729/Rfla730 | 1.4 (1.6) × 1010 | 4.4 × 109 | 1.07 | 5.5 | 10.3 |
| Bifidobacterium | Bif164 | 6.0 (4.0) × 109 | 1.7 × 109 | 0.61 | 2.7 | 4.8 |
| E. cylindroides group | Ecyl387 | 1.5 (2.8) × 109 | 4.0 × 108 | 1.89 | 0.9 | 1.4 |
| Phascolarctobacterium group | Phasco741 | 9.0 (15) × 108 | 2.0 × 108 | 1.62 | 0.5 | 0.6 |
| Enterobacteriaceae | Ecoli1531 | 3.2 (9.1) × 108 | 6.1 × 107 | 2.84 | 0.2 | 0.2 |
| Veillonella | Veil223 | 1.0 (2.3) × 108 | 2.1 × 107 | 2.20 | 0.06 | 0.08 |
| Lactobacillus/Enterococcus | Lab158 | 1.2 (2.6) × 107 | 4.1 × 106 | 2.11 | 0.01 | 0.01 |
| Sum of specific probes | 56.2 | 90.5 | ||||
| E. hallii group | Ehal1469 | 5.1 (4.4) × 109 | 1.4 × 109 | 0.85 | 2.5 | 3.8 |
| Lachnospira group | Lach571 | 5.1 (6.2) × 109 | 1.3 × 109 | 1.21 | 2.5 | 3.6 |
Values represent microbiota from the same 11 volunteers in Table 3, as determined by DAPI staining and FISH with the extensive probe set for the predominant fecal microflora.
Mean numbers from 11 fecal samples were calculated assuming that the numbers below the detection limit were zero.
CVinter, CV due to normal differences in microbiota composition between the 11 human volunteers corrected for assay error.
We determined the coefficient of variation due to the enumeration assay itself (CVassay) by repeating the assay, including sample preparation, 12 times on the same stool sample. The fluorescent cells were enumerated automatically on the Leica DMRXA epifluorescence microscope. The CVassays with a 1,600× dilution of the fecal sample were 0.15 for DAPI staining and 0.09 for hybridization with Bact338. The CVassays were 0.16 for Erec482 at a 400 times dilution, 0.28 for Bif164 at a 160 times dilution, and 0.12 for Lach571 at a 40 times dilution. For the other probes, the average CVassay (0.16) of the four aforementioned FISH probes was used. The CV between individuals (CVinter) was determined by correcting the total variation (CVtotal) between the individuals for the CVassay (16). For all probes combined, the CVinter was more than two times higher than the CVassay, showing that the variation between samples can be determined by this FISH method. A high CVinter indicates a large variation between the samples of the volunteers. In general, the CVinter is high when the percentage of bacteria detected with a specific probe is low, especially those of probes that do not detect bacteria in all individual samples. The CVinter (1.07) found with the Rbro729 and Rfla730 probes was remarkably high, which indicates that there are large differences between volunteers with respect to the numbers of bacteria of this group. Summation of the percentages of the cells detected and identified with group-specific probes resulted in 56.2% for DAPI-stained cells or 90.5% for the total bacterial cells enumerated with the Bact338 probe. The Ehal1469 and Lach571 probes were excluded from this summation, since they detect a group of bacteria already covered by the Erec482 probe.
DISCUSSION
A set of seven new oligonucleotide probes is presented to extend the set of probes for the predominant microbiota of the human gastrointestinal tract and in particular for fecal bacteria. Five of the seven probes each detected an average of more than 1% of the total bacterial microbiota. Especially the probes for the ruminococci and related Clostridium group IV bacteria are valuable, since these probes detected 10% of the fecal bacteria, and this group of bacteria is likely to have interesting metabolic features, such as degradation of complex carbohydrates. The numbers of the ruminococcus group are within the range earlier estimated in human feces by culture techniques (7), in which a mean of 1.6 × 1010 g−1 (dry weight) was found. Also the numbers of the Eubacterium cylindroides group are within the reported ranges. The numbers of C. innocuum, E. cylindroides, and Eubacterium dolichum enumerated previously showed a large variation between the subjects with mean numbers of 4 × 108, 4 × 109, and 4 × 108 · g−1 (dry weight), respectively (7). FISH with three species-specific probes for E. cylindroides, Eubacterium biforme, and E. dolichum detected 9 × 107, 2 × 108, and 0 cells g−1 (dry weight), respectively (28). Out of 12 volunteers, 2 possessed E. cylindroides cells and 6 possessed E. biforme cells (29). Although the Veil223 and the Phasco741 probes detected a minor percentage of the total bacteria, they are interesting as well. Veillonella may play a role in microbiota development at an early age (14), and the Phascolarctobacterium group is a fairly unknown group of bacteria with interesting metabolic properties, such as succinate decarboxylation (17). The number of Veillonella cells reported here is in the same order of magnitude as that reported earlier in feces of adults in which 8 × 107 cells per g were enumerated (7). The Ehal1469 and Lach571 probes specific for subgroups within the Erec482 group detect 3.8 and 3.6% of the total fecal bacteria, respectively. When comparing fecal samples, significant changes in the target groups of these probes would be difficult to detect with Erec482, which accounts on average for 22.7% of the bacterial microbiota.
The newly designed probes described in this study are additional to the existing set of probes, complementing it to an extensive set of 15 probes. Several other probes that can be useful for gut microbiota studies exist. For instance, a new group-specific probe was proposed for Fusobacterium prausnitzii and related species (33) that could be an alternative for the Elgc01 probe. Also the group-specific probe of the Clostridium leptum group (30) would be very useful, because it detects even more target species than the Ruminococcus group probes described here. However, this probe is used in dot blot hybridization and does not function well in FISH (results not shown). Species-specific probes have been designed for members of the genera Bacteroides (5), Bifidobacterium (37), Eubacterium (29, 31), and Ruminococcus (21, 22). Although all of these probes are useful to specifically detect these species in the gut microbiota, for total microbiota analysis, the use of probes with narrow specificity is too laborious.
With the extensive set of group-specific probes used in this study, we can detect 90.5% of the total bacterial microbiota detected with the Bact338 probe. However, of the total DAPI-stained cells, only 56.2% are detected by this extensive probe set and 60.9% are detected by the bacterial probe Bact338. This might indicate that still 38% of cells remains undetected. Several reasons why these cells are not detected come to mind. They may belong to the Archaea or the Eucarya. They may simply be dead cells. They may be not permeable or metabolically active. Finally, the target site for the single Bact338 probe used here (1) may be absent in some bacterial species (4). The specific probes detect bacteria also detected by the Bact338 probe. However, some groups of bacteria need lysozyme permeabilization for effective hybridization, such as ruminococci and lactobacilli. These bacteria are detected by the specific probes, since we use lysozyme in the protocol, but not by the Bact338 probe, in which no lysozyme treatment is used. Despite these considerations, the extensive probe set still does not cover all bacterial cells. Therefore, the need for further probe development remains.
The results obtained in this study with the probes described earlier are in agreement with data we presented previously (8), although the mean counts are now on average 1.5 times lower. The CVinter values presented here are in the same range as those determined previously (8), indicating the reliability of these values. High CVinter values indicate large differences between the volunteers. This makes these probes useful as sensitive parameters in the analysis of variations in composition of microbiota in relation to health and disease or as a result of modulation by pre- or probiotics. The probes described earlier have already been successfully applied in microbiota analysis to study the relationship between microbiota development and atopy in children (18), the relationship between microbiota and milk hypersensitivity (2), and to study the effects of prebiotics (20, 34, 35). Currently, the new probes are applied to study the gut microbiota composition in relation to age, health, and disease. This extensive probe set will make FISH a more valuable and sensitive tool to study the human gut microbiota.
Acknowledgments
We thank A. C. M. Wildeboer-Veloo and R. H. J. Tonk for technical assistance and all of the volunteers for providing a stool sample.
This work was supported by the European Research Project Fair-CT-97-3035.
REFERENCES
- 1.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apostolou, E., L. Pelto, P. V. Kirjavainen, E. Isolauri, S. J. Salminen, and G. R. Gibson. 2001. Differences in the gut bacterial flora of healthy and milk-hypersensitive adults, as measured by fluorescence in situ hybridization. FEMS Immunol. Med. Microbiol. 30:217-221. [DOI] [PubMed] [Google Scholar]
- 3.Collins, M. D., P. A. Lawson, A. Willems, J. J. Cordoba, J. Fernandez-Garayzabal, P. Garcia, J. Cai, H. Hippe, and J. A. E. Farrow. 1994. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int. J. Syst. Bacteriol. 44:812-826. [DOI] [PubMed] [Google Scholar]
- 4.Daims, H., A. Bruhl, R. Amann, K. H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 5.Doré, J., A. Sghir, G. Hannequart-Gramet, G. Corthier, and P. Pochart. 1998. Design and evaluation of a 16S rRNA-targeted oligonucleotide probe for specific detection and quantitation of human faecal Bacteroides populations. Syst. Appl. Microbiol. 21:65-71. [DOI] [PubMed] [Google Scholar]
- 6.Falk, P. G., L. V. Hooper, T. Midtvedt, and J. I. Gordon. 1998. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol. Mol. Biol. Rev. 62:1157-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finegold, S. M., V. L. Sutter, and G. E. Mathisen. 1983. Normal indigenous intestinal flora, p. 3-31. In D. J. Hentges (ed.), Human intestinal microflora in health and disease. Academic Press, NewYork, N.Y.
- 8.Franks, A. H., H. J. M. Harmsen, G. C. Raangs, G. J. Jansen, F. Schut, and G. W. Welling. 1998. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 64:3336-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 10.Goldin, B. R., S. L. Gorbach, M. Saxelin, S. Barakat, L. Gualtieri, and S. Salminen. 1992. Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig. Dis. Sci. 37:121-128. [DOI] [PubMed] [Google Scholar]
- 11.Gorbach, S. L., and B. R. Goldin. 1992. Nutrition and the gastrointestinal microflora. Nutr. Rev. 50:378-381. [DOI] [PubMed] [Google Scholar]
- 12.Harmsen, H. J., G. R. Gibson, P. Elfferich, G. C. Raangs, A. C. Wildeboer-Veloo, A. Argaiz, M. B. Roberfroid, and G. W. Welling. 2000. Comparison of viable cell counts and fluorescence in situ hybridization using specific rRNA-based probes for the quantification of human fecal bacteria. FEMS Microbiol. Lett. 183:125-129. [DOI] [PubMed] [Google Scholar]
- 13.Harmsen, H. J. M., A. C. M. Wildeboer-Veloo, J. Grijpstra, J. Knol, J. E. Degener, and G. W. Welling. 2000. Development of 16S rRNA-based probes for the Coriobacterium group and the Atopobium cluster and their application for enumeration of Coriobacteriaceae in human feces from volunteers of different age groups. Appl. Environ. Microbiol. 66:4523-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harmsen, H. J. M., A. C. Wildeboer-Veloo, G. C. Raangs, A. A. Wagendorp, N. Klijn, J. G. Bindels, and G. W. Welling. 2000. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 30:61-67. [DOI] [PubMed] [Google Scholar]
- 15.Harmsen, H. J. M., P. Elfferich, F. Schut, and G. W. Welling. 1999. A 16S rRNA-targeted probe for detection of lactobacilli and enterococci in fecal samples by fluorescent in situ hybridization. Microb. Ecol. Health Dis. 11:3-12. [Google Scholar]
- 16.Jansen, G. J., A. C. Wildeboer-Veloo, R. H. Tonk, A. H. Franks, and G. W. Welling. 1999. Development and validation of an automated, microscopy-based method for enumeration of groups of intestinal bacteria. J. Microbiol. Methods 37:215-221. [DOI] [PubMed] [Google Scholar]
- 17.Janssen, P. H., and K. A. O'Farrell. 1999. Succinispira mobilis gen. nov., sp. nov., a succinate-decarboxylating anaerobic bacterium. Int. J. Syst. Bacteriol. 49:1009-1013. [DOI] [PubMed] [Google Scholar]
- 18.Kalliomäki, M., P. Kirjavainen, E. Eerola, P. Kero, S. Salminen, and E. Isolauri. 2001. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 107:129-134. [DOI] [PubMed] [Google Scholar]
- 19.Kleessen, B., H. Bunke, K. Tovar, J. Noack, and G. Sawatzki. 1995. Influence of two infant formulas and human milk on the development of the faecal flora in newborn infants. Acta Paediatr. 84:1347-1356. [DOI] [PubMed] [Google Scholar]
- 20.Kleessen, B., L. Hartmann, and M. Blaut. 2001. Oligofructose and long-chain inulin: influence on the gut microbial ecology of rats associated with a human faecal flora. Br. J. Nutr. 86:291-300. [DOI] [PubMed] [Google Scholar]
- 21.Krause, D. O., R. J. Bunch, L. L. Conlan, P. M. Kennedy, W. J. Smith, R. I. Mackie, and C. S. McSweeney. 2001. Repeated ruminal dosing of Ruminococcus spp. does not result in persistence, but changes in other microbial populations occur that can be measured with quantitative 16S-rRNA-based probes. Microbiology 147:1719-1729. [DOI] [PubMed] [Google Scholar]
- 22.Krause, D. O., B. P. Dalrymple, W. J. Smith, R. I. Mackie, and C. S. McSweeney. 1999. 16S rDNA sequencing of Ruminococcus albus and Ruminococcus flavefaciens: design of a signature probe and its application in adult sheep. Microbiology 145:1797-1807. [DOI] [PubMed] [Google Scholar]
- 23.Langendijk, P. S., F. Schut, G. J. Jansen, G. C. Raangs, G. R. Kamphuis, M. H. F. Wilkinson, and G. W. Welling. 1995. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 61:3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig, W., O. Strunk, S. Klugbauer, N. Klugbauer, M. Weizenegger, J. Neumaier, M. Bachleitner, and K. H. Schleifer. 1998. Bacterial phylogeny based on comparative sequence analysis. Electrophoresis 19:554-568. [DOI] [PubMed] [Google Scholar]
- 25.Maidak, B. L., J. R. Cole, C. T. J. Parker, G. M. Garrity, N. Larsen, B. Li, T. G. Lilburn, M. J. McCaughey, G. J. Olsen, R. Overbeek, S. Pramanik, T. M. Schmidt, J. M. Tiedje, and C. R. Woese. 1999. A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res. 27:171-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manz, W., R. Amann, W. Ludwig, M. Vancanneyt, and K. H. Schleifer. 1996. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga-flavobacter-bacteroides in the natural environment. Microbiology 142:1097-1106. [DOI] [PubMed] [Google Scholar]
- 27.Matsuki, T., K. Watanabe, R. Tanaka, M. Fukuda, and H. Oyaizu. 1999. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl. Environ. Microbiol. 65:4506-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poulsen, L. K., T. R. Licht, C. Rang, K. A. Krogfelt, and S. Molin. 1995. Physiological state of Escherichia coli BJ4 growing in the large intestines of streptomycin-treated mice. J. Bacteriol. 177:5840-5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwiertz, A., G. Le Blay, and M. Blaut. 2000. Quantification of different Eubacterium spp. in human fecal samples with species-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 66:375-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sghir, A., G. Gramet, A. Suau, V. Rochet, P. Pochart, and J. Doré. 2000. Quantification of bacterial groups within human fecal flora by oligonucleotide probe hybridization. Appl. Environ. Microbiol. 66:2263-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simmering, R., B. Kleessen, and M. Blaut. 1999. Quantification of the flavonoid-degrading bacterium Eubacterium ramulus in human fecal samples with a species-specific oligonucleotide hybridization probe. Appl. Environ. Microbiol. 65:3705-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suau, A., R. Bonnet, M. Sutren, J.-J. Godon, G. R. Gibson, M. D. Collins, and J. Doré. 1999. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 65:4799-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suau, A., V. Rochet, A. Sghir, G. Gramet, S. Brewaeys, M. Sutren, L. Rigottier-Gois, and J. Doré. 2001. Fusobacterium prausnitzii and related species represent a dominant group within the human fecal flora. Syst. Appl. Microbiol. 24:139-145. [DOI] [PubMed] [Google Scholar]
- 34.Tuohy, K. M., R. K. Finlay, A. Wynne, and G. R. Gibson. 2001. A human volunteer study on the prebiotic effects of HP-inulin: faecal bacteria enumerated using fluorescent in situ hybridisation (FISH). Anaerobe 7:113-118. [Google Scholar]
- 35.Tuohy, K. M., S. Kolida, A. M. Lustenberger, and G. R. Gibson. 2001. The prebiotic effects of biscuits containing partially hydrolysed guar gum and fructo-oligosaccharides—a human volunteer study. Br. J. Nutr. 86:341-348. [DOI] [PubMed] [Google Scholar]
- 36.Wilson, K. H., and R. B. Blitchington. 1996. Human colonic biota studied by ribosomal DNA sequence analysis. Appl. Environ. Microbiol. 62:2273-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto, T., M. Morotomi, and R. Tanaka. 1992. Species-specific oligonucleotide probes for five Bifidobacterium species detected in human intestinal microflora. Appl. Environ. Microbiol. 58:4076-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zoetendal, E. G., A. D. L. Akkermans, and W. M. De Vos. 1998. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 64:3854-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]