Abstract
Liver steatosis can be measured with ultrasound techniques such as the controlled attenuation parameter (CAP) on an equipped FibroScan. For more widespread screening and quantitative evaluation of liver steatosis, a predictive model using body composition data obtained by body bioelectrical impedance analysis (BIA) was developed. In the training cohort including 365 patients suspected of having metabolic dysfunction-associated steatotic liver disease, a stepwise selection method was used to determine the BIA-related variables associated with CAP. Using the significant variables, a predictive formula was developed, and the estimated CAP (eCAP) was obtained. The diagnostic performance of eCAP was tested to predict liver steatosis with receiver operating characteristic (ROC) curve analysis in the training, validation (n = 408) and liver biopsy (n = 158) cohorts. The body fat mass of the trunk, skeletal muscle index and age were significant variables associated with CAP. eCAP was obtained as 219.1 − 0.4479 × age + 3.476 × BFM of trunk + 7.045 × SMI. The area under the ROC curve was 0.814 in the training cohort and 0.808 in the validation cohort. The sensitivity and specificity were 72.5% and 82.1% with a cut-off value of eCAP = 281 dB/m. For sensitivity ≥ 90%, the cut-off of eCAP was 266 dB/m. In the liver biopsy cohort, the presence of pathological steatosis was predicted with eCAP as an area under the ROC curve = 0.826, which was not statistically different from CAP (0.871). Completely non-invasive BIA-based eCAP could predict liver steatosis.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-17396-1.
Keywords: Bioelectrical impedance analysis, Body composition, Controlled attenuation parameter, Metabolic dysfunction-associated steatotic liver disease
Subject terms: Gastroenterology, Medical research
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as nonalcoholic fatty liver disease, is associated with overweight and lifestyle-related diseases; the cardiometabolic criteria for diagnosis of MASLD have been defined1. Metabolic dysfunction-associated steatohepatitis (MASH) is pathologically diagnosed on the basis of steatosis, inflammation and hepatocyte ballooning2. MASH with significant liver fibrosis is known as at-risk MASH and is considered to have a high risk of disease progression3. MASLD/MASH is globally prevalent and increasingly becoming an etiology of liver cancer4. Therefore, efficient surveillance of the general public is an urgent issue.
Steatosis is the accumulation of lipid droplets within hepatocytes. For the diagnosis of MASLD, steatosis should be observed in more than 5% of hepatocytes5. Obesity is one risk factor for MASLD. The hepatic steatosis of MASLD is one manifestation of overweight and is defined as ectopic fat accumulation that is associated with visceral fat accumulation and insulin resistance6,7. Skeletal muscle plays an important role in metabolic homeostasis, and skeletal muscle mass, function and myosteatosis are associated with the pathological severity of the liver and the prognosis of MASLD8–10. Therefore, in the management of MASLD, the patient’s body composition provides beneficial information regarding systemic adiposity and skeletal muscle mass. Indeed, a recent study indicates that impaired body composition, such as visceral fat accumulation and reduced skeletal muscle mass, is associated with severe steatosis, inflammation and fibrosis of the liver in MASLD11. Accumulating evidence also indicates that bioelectrical impedance analysis (BIA) is a useful and accessible method to measure body composition and shows a good correlation with the dual-energy X-ray absorptiometry method12. BIA is a noninvasive, simple and low-cost procedure and can be applied broadly and repeatedly in health check screenings and clinical settings.
In clinical practice, imaging modalities rather than liver biopsies are generally performed to evaluate hepatic steatosis because of their noninvasiveness, reliability and quantitativity3. B-mode ultrasound is the most common examination procedure to detect liver steatosis. However, it is impractical to perform ultrasound on all people with MASLD risk. Ultrasound technique-based examination measuring the attenuation of ultrasound is used for quantitative evaluation of hepatic steatosis3,13. FibroScan is one of the most widely used transient elastography techniques and can be used to perform controlled attenuation parameter (CAP) measurement and liver stiffness measurement (LSM)14. Using the CAP and LSM values, the FibroScan-Based Score (FAST Score) was also developed to predict at-risk MASH15. According to recent guidelines, FibroScan is included in the surveillance flow chart as the 2nd -step examination following the Fibrosis-4 index to identify advanced liver fibrosis3,5. CAP is simultaneously measured in this step. Therefore, in general, quantitative evaluation of liver steatosis such as CAP measurement is not provided to health checkup examinees and patients in the screening step, whereas information about the presence of and severity of liver steatosis would encourage health checkup examinees, patients and medical providers to perform further evaluation of MASLD.
Considering the connective pathogenesis between MASLD and body composition, we hypothesized that BIA-related variables are able to predict the severity of liver steatosis. In this study, we aim to develop a completely noninvasive formula using the variables obtained from BIA to predict CAP. In addition, the prediction of LSM and the FAST Score using BIA-related variables was attempted.
Materials and methods
Study design and patients
For the training cohort, 365 patients who visited Saga University Hospital from December 2018 to December 2021 were included (Fig. 1). All the patients were suspected of having MASLD and received BIA and FibroScan examinations. In this cohort, formulas to predict the CAP, LSM and FAST Score were developed, and the estimated CAP (eCAP), estimated LSM (eLSM) and estimated FAST Score (eFAST Score) were obtained. The diagnostic performances of the eCAP, eLSM and eFAST Score were tested using the actual CAP, LSM and FAST Score measured with FibroScan as the gold standard. Four hundred eight patients who visited Saga University Hospital from January 2022 to December 2024 were included in the validation cohort. The diagnostic accuracy of eCAP was similarly tested as in the training cohort. The liver biopsy cohort was independently organized and included 158 patients who received liver biopsy, FibroScan examination and BIA within a month from December 2018 to December 2024. The diagnostic performances of the eCAP and CAP were tested using the pathological steatosis score as the gold standard. All patients were adult and older than 20 years old. No patients in any of the cohorts had other liver disease etiologies, including habitual alcohol intake (daily ethanol consumption of < 30 g in men and < 20 g in women), positivity for hepatitis B surface antigen or hepatitis C virus antibody and abnormal serum thyroid hormone levels. Additionally, no patients had autoimmune liver disease, drug-induced hepatotoxicity, hemochromatosis or Wilson’s disease. The study protocol was approved by the Clinical Research Ethics Review Committee of Saga University Hospital and was performed in accordance with the principles of the 1975 Declaration of Helsinki (revised in 2013). The participants provided informed consent to participate in the study.
Fig. 1.
Study design. Using BIA parameters, a formula to predict CAP, LSM and FAST Score was developed in the training cohort, and eCAP, eLSM and eFAST Score were obtained. The diagnostic performance of these factors was tested in the training cohort. The diagnostic performance of eCAP was also tested in the validation cohort and liver biopsy cohort. Abbreviations: BIA, bioelectrical impedance analysis; CAP, controlled attenuation parameter; eCAP, estimated controlled attenuation parameter; LSM, liver stiffness measurement; eLSM, estimated liver stiffness measurement; FAST Score, FibroScan-Based Score; eFAST Score, estimated FibroScan-Based Score.
Physical examination and serum biochemical measurements
The body mass and height of the participants were measured, and body mass index (BMI) was calculated as body mass (kg) divided by height squared (m2). Venous blood samples were obtained after an overnight fast and were used to measure albumin, aspartate aminotransferase (AST), alanine transaminase (ALT), γ-glutamyl transpeptidase (GGT), total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglyceride and fasting plasma glucose concentrations, platelet count and hemoglobin A1c (HbA1c) using conventional laboratory techniques. Hepatic steatosis index (HSI) was calculated as follows: HSI = 8 × ALT/AST ratio + BMI (+ 2, if diabetes; +2, if female)16. Diabetes was diagnosed in the patients with both fasting plasma glucose ≥ 126 mg/dL and HbA1c ≥ 6.5%.
Body composition measurement
For body composition measurement, after an overnight fast, BIA was performed using Inbody 770® (Biospace Co, Seoul, Republic of Korea). Parameters included body fat mass (BFM), fat-free mass (FFM), skeletal muscle mass (SMM), total body water (TBW), intracellular water (ICW), extracellular water (ECW) and whole-body phase angle (50 kHz). Percent body fat (PBF) was obtained by dividing BFM by body weight (kg). The skeletal muscle index (SMI) was obtained by dividing appendicular muscle mass by height (m)2. Detail explanations for individual parameters are available in the product manual at https://uk.inbody.com/about-inbody/result-sheet-interpretation/.
Liver stiffness measurement and controlled Attenuation parameter measurement
Experienced operators who had performed at least 500 examinations assessed the LSM and CAP in the right liver lobe using a FibroScan® 502. Patients were examined after an overnight fast using the M or XL probes within three months before and after BIA. The probe was selected based on the skin–liver capsule distance (SCD): XL probe for patients with SCD ≥ 25 mm and M probe for patients with SCD < 25 mm17,18. After we measured the SCD using ultrasound B-mode, the LSM and CAP measurements were performed using FibroScan® until the first 10 valid measurements were obtained for each patient. The median values were used to quantify liver fibrosis and steatosis. Based on previous reports, we defined measurement failure as examinations in which 10 valid LSMs were not obtained after 10 or more attempts, or < 60% success rate. In patients with 10 valid LSMs, LSM ≥ 7.1 kPa and interquartile range-to-median ratio > 30% were defined as unreliable values14. Patients who had measurement failure or unreliable values were excluded from this study. Patients with CAP ≥ 248 dB/m were considered to have liver steatosis (pathological steatosis ≥ S1)19, and patients with LSM ≥ 8.9 kPa were considered to have advanced fibrosis (pathological fibrosis stage ≥ 3)20. The FAST Score was calculated in accordance with the following previously reported formula15: FAST = (e – 1.65 + 1.07 × In (LSM) + 2.66*10‾⁸ × CAP³ – 63.3 × AST‾¹)/(1 + e – 1.65 + 1.07 × In (LSM) + 2.66*10‾⁸ × CAP³ – 63.3 × AST‾¹). Patients with a FAST Score of > 0.67 were considered to have at-risk MASH (MASH with fibrosis stage ≥ 2)15.
Evaluation of liver biopsy
Ultrasonography-guided liver biopsy was performed using a 16-gauge biopsy needle. All liver biopsies were approximately ≥ 20 mm in length. Liver biopsy slides, stained with hematoxylin–eosin and Azan or Masson stain, were independently evaluated by experienced central pathologist (S.A.) specializing in liver pathology. The central pathologists were blinded to the clinical data. Hepatic steatosis, lobular inflammation and hepatocyte ballooning were evaluated based on the NAFLD activity score21.
Statistical analysis
For the prediction of CAP, LSM and FAST Score using BIA, statistically independent predictive variables were selected by the forward–backward stepwise selection method from the variables obtained from BIA, age, sex and height. The predictive formula was developed with the selected variables. The variables tested in the stepwise selection method were age, sex, height, body weight, BMI and BIA-related variables (Supplementary Table S1). Correlations were tested using the Spearman’s rank correlation coefficient. The Steel-Dwass test was used for multiple comparisons. The diagnostic performance of individual tests was determined using receiver operating characteristic (ROC) curves. Optimal cut-off values were chosen to maximize the sum of the sensitivity and specificity, known as the Youden index22. Cut-off values with at least 90% sensitivity and specificity were also individually chosen. Comparisons of the area under the ROC curve (AUROC) between the tests were performed using the DeLong test23. We considered a p-value < 0.05 to be statistically significant. All statistical analyses were performed using JMP ver. 14 (SAS Institute Japan; Tokyo, Japan).
Results
Patient characteristics
The patient characteristics of the training cohort are summarized in Supplementary Table S2, and the BIA-related variables are summarized in Supplementary Table S1. Regarding the BIA-related variables, the mean PBF and mean SMI were 36.7% and 7.2 kg/m2. Regarding FibroScan examinations, the mean LSM, CAP and FAST Score were 6.1 kPa, 299 dB/m and 0.322, respectively. The patient characteristics of the validation cohort and liver biopsy cohort are summarized in Supplementary Table S3 and S4.
Formula to predict CAP, LSM and FAST score
Among the 34 variables including age, sex, height, body weight, BMI and 29 BIA-related variables, significant variables were selected by stepwise analysis (Table 1). For the prediction of CAP, age (p = 0.008), BFM of trunk (p < 0.0001) and SMI (p = 0.0012) were selected. The predictive formula is as follows: eCAP = 219.1 − 0.4479 × age + 3.476 × BFM of trunk + 7.045 × SMI. For the prediction of LSM, ECW/TBW (p < 0.0001), BFM Control (body fat mass normalized to the appropriate body composition, p < 0.0001), TBW/FFM (p = 0.0034), whole-body phase angle (p < 0.0001) and SMI (p = 0.0001) were significant. The predictive formula is as follows: eLSM = 20.75 + 967.9 × ECW/TBW − 0.2932 × BFM Control − 5.478 × TBW/FFM + 9.942 × whole-body phase angle − 2.436 × SMI. For the prediction of FAST Score, BFM% of trunk (p < 0.0001) was solely significant, and the predictive formula is as follows: eFAST = 0.1754 + 0.0006315 × BFM% of trunk.
Table 1.
Stepwise analysis to predict CAP, LSM and at-risk MASH.
| Variable to be predicted | Factor | Regression coefficient | p value | 95% confidence interval |
|---|---|---|---|---|
| CAP | Age | −0.4479 | 0.0079 | −0.777 - −0.118 |
| BFM of Trunk | 3.476 | < 0.0001 | 2.386–4.564 | |
| SMI | 7.045 | 0.0012 | 2.184–11.275 | |
| LSM | ECW/TBW | 967.9 | < 0.0001 | 662.6–1273.2 |
| BFM Control | −0.2932 | < 0.0001 | −0.3979 - −0.1885 | |
| TBW/FFM | −5.748 | 0.0034 | −9.582 - −1.915 | |
| Whole-Body Phase Angle | 9.942 | < 0.0001 | 6.226–13.658 | |
| SMI | −2.436 | 0.0001 | −3.669 - −1.202 | |
| FAST Score | BFM% of Trunk | 0.0006315 | < 0.0001 | 0.0004157–0.0008474 |
Regression coefficient and p value were obtained by stepwise analysis. Regression coefficient was shown as four significant digits. Abbreviations: CAP, controlled attenuation parameter; LSM, liver stiffness measurement; FAST Score, FibroScan-Based Score; BFM, body fat mass; SMI, skeletal muscle mass index; TBW, total body water; ECW, extracellular water; FFM, fat-free mass.
Correlation between predicted and actual CAP, LSM and FAST score values
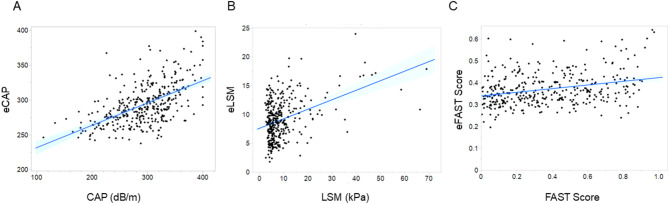
Using the formulas developed above, the predicted values, that is, eCAP, eLSM and eFAST Score, were obtained and correlated with the actual measured values of CAP, LSM and FAST Score (Fig. 2). A significant positive correlation was identified between CAP and eCAP (ρ = 0.5589, p < 0.0001). (Fig. 2A). The correlations between LSM and eLSM (ρ = 0.2677, p < 0.0001) and between FAST Score and eFAST Score (ρ = 0.2577, p < 0.0001) were weak (Fig. 2B and C).
Fig. 2.
Linear regression plot with 95% confidence intervals showing the correlation between estimated values based on BIA- and FibroScan-related data: eCAP and CAP (A), eLSM and LSM (B), and eFAST and FAST (C). Abbreviations: CAP, controlled attenuation parameter; eCAP, estimated controlled attenuation parameter; LSM, liver stiffness measurement; eLSM, estimated liver stiffness measurement; FAST Score, FibroScan-Based Score; eFAST Score, estimated FibroScan-Based Score.
ROC analysis of the training cohort
The diagnostic performances to detect the presence of liver steatosis (CAP ≥ 248 dB/m), advanced fibrosis (LSM ≥ 8.9 kPa) and at-risk MASH (FAST Score > 0.67) were tested by ROC analysis (Fig. 3; Table 2). The AUROCs of eCAP, eLSM and eFAST Score were 0.814, 0.700 and 0.657. On the basis of the Youden index, the optimal cut-off values for the diagnosis of steatosis, advanced fibrosis and at-risk MASH were eCAP = 281 dB/m, eLSM = 10.69 kPa and eFAST Score = 0.381. Based on this study’s aim to contribute to the surveillance of MASLD, cut-off values with a sensitivity ≥ 90% were also obtained: eCAP = 266 dB/m (sensitivity = 90.3%), eLSM = 6.67 kPa (sensitivity = 90%) and eFAST Score = 0.455 (sensitivity = 90.1%). Correlation between eCAP and HSI was tested, and diagnostic performance was also compared. There was significant positive correlation between eCAP and HSI (ρ = 0.8946, p < 0.0001) (Supplementary Figure S1). The AUROC of HSI was 0.827, which was not statistically different from eCAP (p = 0.4357) (Supplementary Figure S2). AUROC of eCAP was analyzed in the subgroups stratified by BMI and metabolic parameters (Supplementary Table S5). AUROC in the subgroup with BMI < 25 kg/m2 was 0.678, which was lower than other subgroups.
Fig. 3.
Receiver operating characteristic (ROC) curves demonstrating the diagnostic performance of eCAP (A), eLSM (B) and eFAST Score (C).
Table 2.
Diagnostic performance of eCAP, eLSM and eFAST Score.
| eCAP | eLSM | eFAST Score | |
|---|---|---|---|
| AUROC | 0.814 | 0.700 | 0.657 |
| 95% confidence interval | 0.767–0.861 | 0.639–0.761 | 0.622–0.778 |
| Optimal cut-off (Youden index) | 281 dB/m | 10.69 kPa | 0.381 |
| Sensitivity | 72.5% | 50.9% | 68.3% |
| Specificity | 82.1% | 79.2% | 57.4% |
| Optimal cut-off (Sensitivity ≥ 90%) | 266 dB/m | 6.67 kPa | 0.455 |
| Sensitivity | 90.3% | 90% | 90.1% |
| Specificity | 44.8% | 32.2% | 29.5% |
Abbreviations: AUROC, area under the receiver operating characteristics curve; eCAP, estimated controlled attenuation parameter; eLSM, estimated liver stiffness measurement; eFAST Score, estimated FibroScan-Based Score.
ROC analysis of the validation cohort
In accordance with the ROC analysis in the training cohort, the diagnostic performances of eLSM and eFAST Score were poor. Therefore, further analysis was performed to test eCAP. In the validation cohort, eCAP showed a significant positive correlation with CAP (ρ = 0.563, p < 0.0001) (Fig. 4A). The AUROC of eCAP was 0.808 (Fig. 4B). Using the cut-off value of eCAP obtained in the training cohort analysis, the sensitivity was 65.9% and the specificity was 79% with a cut-off value = 281 dB/m, and the sensitivity was 86.4% and the specificity was 58% with a cut-off value = 266 dB/m.
Fig. 4.
Analysis of the validation cohort. Linear regression plot with 95% confidence intervals showing the correlation between eCAP and CAP (A) and receiver operating characteristic (ROC) curves demonstrating the diagnostic performance of eCAP (B) in the validation cohort. Abbreviations: CAP, controlled attenuation parameter; eCAP, estimated controlled attenuation parameter.
Correlation with pathological steatosis and ROC analysis in the liver biopsy cohort
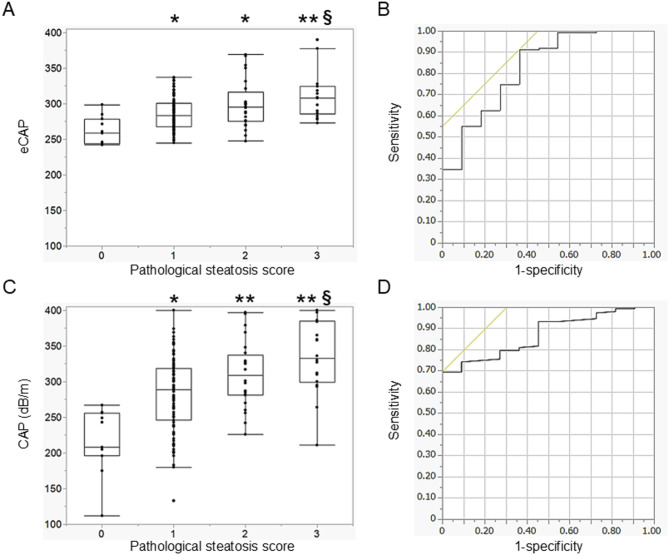
In the liver biopsy cohort, the correlation of eCAP or CAP with the pathological steatosis score was tested. The diagnostic performances of eCAP and CAP were also tested and compared. eCAP showed a significant positive correlation with the pathological steatosis score (ρ = 0.379, p < 0.0001), and a significant difference in eCAP was identified between the pathological scores (score 0 vs. score 1, p = 0.0061; score 0 vs. score 2, p = 0.0044; score 0 vs. score 3, p = 0.0007; score 1 vs. score 3, p = 0.0052) (Fig. 5A). The AUROC of eCAP for the diagnosis of pathological steatosis score ≥ 1 was 0.826 (Fig. 5B). The correlation between CAP and the pathological steatosis score was also significant (ρ = 0.429, p < 0.0001), and a significant difference in CAP was identified between the pathological scores (score 0 vs. score 1, p = 0.0015; score 0 vs. score 2, p = 0.0002; score 0 vs. score 3, p = 0.0002; score 1 vs. score 3, p = 0.0017) (Fig. 5C). The AUROC of CAP for the diagnosis of pathological steatosis score ≥ 1 was 0.871 (Fig. 5D). The AUROC of CAP was greater than that of eCAP, but it was not statistically significant (p = 0.4426).
Fig. 5.
Analysis of the liver biopsy cohort. Box plots showing the association between the pathological steatosis score and eCAP (A) or CAP (C). Receiver operating characteristic (ROC) curves demonstrating the diagnostic performance of eCAP (B) and CAP (D). Error bars in the box plots represent the quantiles. *p < 0.05, **p < 0.001 in comparison with steatosis score = 0. §p < 0.05, §§p < 0.001 in comparison with steatosis score = 1. Abbreviations: CAP, controlled attenuation parameter; eCAP, estimated controlled attenuation parameter.
Discussion
In this study, CAP was successfully predicted using BIA-related parameters and denoted as eCAP. The predictive performance of eCAP was validated in the validation cohort, and eCAP could predict the presence of pathological steatosis. eCAP is completely noninvasive and does not require liver biopsy, blood tests, radiation exposure or imaging examination. Emulating CAP, the most popular and well-validated parameter of FibroScan, eCAP enables the collection of intuitive and quantitative information regarding liver steatosis.
Early diagnosis of steatosis helps to prevent the progression of MASLD. Therefore, simple and easy-to-use serum tests are becoming crucial in disease diagnosis24. According to previous reports, there are several indexes for predicting steatotic liver, including the fatty liver index (FLI), HSI, Zhejiang University index, NAFLD liver fat score and Framingham steatosis index16,25–28. All these indexes require blood tests such as liver function tests, fasting glucose levels and HbA1c for calculation. Recent technological innovations have made it possible to evaluate liver steatosis by non-invasive imaging methods, such as ultrasonography, computed tomography, and magnetic resonance imaging29. Regarding the imaging modalities, B-mode ultrasound has been the most common procedure to identify liver steatosis. Ultrasonographical findings of liver–kidney contrast, vascular blurring and deep attenuation enable the identification of liver steatosis but are not quantitative30. Recent developments in ultrasound-based techniques measuring the attenuation of ultrasound in the liver with steatosis can provide quantitative results representing the severity of liver steatosis. Attenuation imaging (ATI) and the attenuation coefficient (ATT) represent the application of conventional ultrasound machines to quantitatively evaluate liver steatosis31,32. Clinically, CAP is the most widely studied algorithm for measuring the attenuation of A-mode ultrasound beams33. Magnetic resonance imaging-estimated proton density fat fraction (MRI-PDFF) is a reliable and representative method for measuring liver steatosis and evaluating treatment efficacy34–36. However, the low availability and high costs of these imaging modalities limit their wide use for screening3. eCAP, developed in this study, is a completely noninvasive and low-cost procedure to evaluate liver steatosis.
In general, skeletal muscle mass shows protective against liver steatosis. According to the meta-analysis including 19 studies with MASLD (n = 7934) and healthy participants (n = 29,533) shows that low SMI is a risk of MASLD (odds ratio = 1.77)37. On the other hand, in our study, BFM of trunk and SMI were independently and positively associated with liver steatosis and used in the predictive formula. The value of the regression coefficient was positive and highest for SMI, suggesting that liver steatosis was strongly affected by the SMI. This means that a higher SMI results in severe liver steatosis. This paradoxical association was observed in previous studies. Wan et al. performed computed tomography (CT) imaging-based body composition analysis and identified that the SMI, visceral fat index, visceral fat-to-muscle ratio and visceral fat-to-subcutaneous fat ratio were independently and positively associated with the degree of pathological liver steatosis in adult obesity11. Schmitz et al. reported that the SMI measured by BIA was significantly higher in patients with biopsy-proven MASH than in non-MASH patients38. These studies and our study indicate the undesirable effect of the SMI on liver steatosis. The association between skeletal muscle mass and liver steatosis may vary depending on the study cohort. An undesirable effect of skeletal muscle mass on liver steatosis was observed in patients with obesity and in cohorts characterized by a high prevalence of MASLD. The mean BMI in the study by Wan et al. was 36.8 kg/m2 with all patients diagnosed with MASLD11. In the study by Schmitz et al., the mean BMI was 51 kg/m2, and the prevalence of MASLD was 63.8%38. In our training cohort, the median BMI was 27.8 kg/m2 and the prevalence of MASLD was 81.6%. We additionally examined the correlation between SMI and CAP, revealing that the positive correlation between SMI and CAP was not significant in patients with BMI < 25 kg/m2, while it was significant in the overall cohort and in patients with BMI ≥ 25 kg/m2 (Supplementary Figure S3). Taken together, patients with obesity and high BMI tend to exhibit high SMI; thus, if the prevalence of MASLD is high in a cohort, such as hospital-based patient populations, it may be difficult to statistically demonstrate the protective effect of skeletal muscle mass and instead observe an undesirable effect on liver steatosis. Another possible explanation for the unpreferable effect of skeletal muscle mass on liver steatosis is myosteatosis. Myosteatosis is fat infiltration in skeletal muscle, and it negatively affects the pathogenesis of MASLD39,40. By detecting the difference in the signal intensities of triglycerides and adipose tissue, MRI and CT scans can be used to measure myosteatosis separately from the muscle area39–41. However, BIA and simple measurement of the muscle area in a CT or MRI image cannot exclude myosteatosis, resulting in a higher muscle mass and SMI in obese patients, and thus, the SMI can be a risk factor for liver steatosis.
According to our subgroup analysis (Supplementary Table S5), AUROC ranged from 0.75 to 0.90 in all the subgroups except BMI < 25 kg/m2 (AUROC = 0.678). This finding indicates a limited diagnostic performance of eCAP in non-obese patients. For the prediction of CAP, BFM of trunk and SMI are used. In predictive formula, BFM of trunk and SMI positively affect eCAP. Therefore, diagnostic performance of eCAP in predicting liver steatosis in the non-obese patients with low BFM of trunk and/or low SMI could be limited. To improve the prediction of liver steatosis in non-obese patients, other parameters such as liver function tests, glucose metabolism, and lipid profiles might be needed in addition to body component parameters.
The detection and prediction of liver steatosis can contribute to the management of lifestyle-related diseases as well as the diagnosis of MASLD. People with liver steatosis have a higher incidence of type 2 diabetes than that of people without liver steatosis42. Moreover, the risk of cardiovascular disease43, development of chronic kidney disease44 and risk of extrahepatic cancer45 are higher in the population with liver steatosis than in those without liver steatosis. Therefore, the earlier detection and prediction of liver steatosis using BIA in health checkup and primary care settings could contribute to improving the outcome of lifestyle-related diseases and promoting awareness of the associations between liver steatosis and the risk of extrahepatic disease.
As shown in this study, body composition is associated with MASH and liver fibrosis, but actual predictions of these conditions are challenging. In our study, BIA-related variables including the SMI were significantly associated with LSM, and BFM% of trunk was significantly associated with the FAST Score. According to recent reports, less skeletal muscle mass is independently associated with severe liver fibrosis and the presence of MASH11,46,47. Visceral fat mass is also associated with the presence of MASH11. Moreover, sarcopenia aggravates the prognosis of MASLD9. Taken together, body composition is consistently associated with the pathophysiological features and prognosis of MASLD. However, to our knowledge, no study has tried to develop a predictive model using body composition parameters. Recently, the development of biomarkers to predict liver fibrosis and the presence of MASH has become an emerging topic3,48. The combined use of BIA-related variables and blood tests or imaging examination might represent a future avenue for identifying reliable biomarkers to predict liver fibrosis and MASH.
There are several limitations in this study. The study cohort consisted of patients who were suspected of having MASLD. Therefore, the relatively high prevalence of MASLD may result in overestimation of positive predictive value and underestimation of negative predictive value of eCAP in general population. In terms of the study concept, investigation of the general population should be carried out. Differences in the version of the BIA modality might affect the values of the BIA-related parameters, and the representability should be tested using different modalities. In conclusion, BIA-related parameters are able to predict liver steatosis as well as the CAP value. BIA is a useful examination to evaluate liver steatosis as well as body composition.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely thank Maki Miyahara and Akiko Komine for their support with the BIA measurement and data collection. We thank Jenna MacArthur, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Abbreviations
- ALT
Alanine transaminase
- AST
Aspartate aminotransferase
- ATI
Attenuation imaging
- ATT
Attenuation coefficient
- AUROC
Area under the ROC curve
- BFM
Body fat mass
- BIA
Bioelectrical impedance analysis
- BMI
Body mass index
- CAP
Controlled attenuation parameter
- CT
Computed tomography
- eCAP
Estimated CAP
- ECW
Extracellular water
- eFAST Score
Estimated FAST Score
- eLSM
Estimated LSM
- FAST Score
FibroScan-Based Score
- FFM
Fat-free mass
- GGT
γ-glutamyl transpeptidase
- HbA1c
Hemoglobin A1c
- HDL cholesterol
High-density lipoprotein cholesterol
- ICW
Intracellular water
- LDL cholesterol
Low-density lipoprotein cholesterol
- LSM
Liver stiffness measurement
- MASH
Metabolic dysfunction-associated steatohepatitis
- MASLD
Metabolic dysfunction-associated steatotic liver disease
- MRI-PDFF
Magnetic resonance imaging-estimated proton density fat fraction
- PBF
Percent body fat
- ROC
Receiver operating characteristic
- SCD
Skin-liver capsule distance
- SMI
Skeletal muscle index
- SMM
Skeletal muscle mass
- TBW
Total body water
Author contributions
T.K., M.M and H.T. worked on the conceptualization, visualization and methodology of the study and wrote the original draft. Data curation and formal analysis were performed by T.K., S.O., Y.K., N.O. and H.T. H.T. and Y.E. contributed to funding acquisition. E.E., M.N., K.I., N.H., S.N., K.T. and S.Y. contributed to data acquisition. S.A. contributed to pathological data acquisition. A.K. and S.O supervised the statistical analysis. H.T. was the project administrator. This study was supervised by H.I. and T.K. The final draft of the manuscript was reviewed by K.A., S.Y. D.H and H.T.
Funding information
This research was supported by the Research Program on Hepatitis from Japan Agency for Medical Research and Development (AMED) [grant numbers 24015731 and 23808721] and JSPS KAKENHI, Grant-in-Aid for Scientific Research (C) [grant number 24K11171].
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol.79, 1542–1556 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Bedossa, P. et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology56, 1751–1759 (2012). [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver (EASL). European association for the study of diabetes (EASD); European association for the study of obesity (EASO). EASL-EASD-EASO clinical practice guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol.81, 492–542 (2024). [DOI] [PubMed] [Google Scholar]
- 4.Huang, D. Q., El-Serag, H. B. & Loomba, R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol.18, 223–238 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinella, M. E. et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology77, 1797–1835 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koda, M., Kawakami, M., Murawaki, Y. & Senda, M. The impact of visceral fat in nonalcoholic fatty liver disease: cross-sectional and longitudinal studies. J. Gastroenterol.42, 897–903 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Eguchi, Y. et al. The pathological role of visceral fat accumulation in steatosis, inflammation, and progression of nonalcoholic fatty liver disease. J. Gastroenterol.46 (Suppl 1), 70–78 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Wang, L. et al. Age and BMI have different effects on subcutaneous, visceral, liver, bone marrow, and muscle adiposity, as measured by CT and MRI. Obes. (Silver Spring). 32, 1339–1348 (2024). [DOI] [PubMed] [Google Scholar]
- 9.Moon, J. H., Koo, B. K. & Kim, W. Non-alcoholic fatty liver disease and sarcopenia additively increase mortality: a Korean nationwide survey. J. Cachexia Sarcopenia Muscle. 12, 964–972 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henin, G., Loumaye, A., Leclercq, I. A. & Lanthier, N. Myosteatosis: diagnosis, pathophysiology and consequences in metabolic dysfunction-associated steatotic liver disease. JHEP Rep.6, 100963 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan, Q. et al. Body composition and progression of Biopsy-Proven Non-Alcoholic fatty liver disease in patients with obesity. J. Cachexia Sarcopenia Muscle. 15, 2608–2617 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Völgyi, E. et al. Assessing body composition with DXA and bioimpedance: effects of obesity, physical activity, and age. Obes. (Silver Spring). 16, 700–705 (2008). [DOI] [PubMed] [Google Scholar]
- 13.En Li Cho, E. et al. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: an updated systematic review and meta-analysis. Gut72, 2138–2148 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Boursier, J. et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology57, 1182–1191 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Newsome, P. N. et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol. Hepatol.5, 362–373 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, J. H. et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis.42, 503–508 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Oeda, S. et al. Accuracy of liver stiffness measurement and controlled Attenuation parameter using FibroScan® M/XL probes to diagnose liver fibrosis and steatosis in patients with nonalcoholic fatty liver disease: a multicenter prospective study. J. Gastroenterol.55, 428–440 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Kumagai, E. et al. Appropriate use of virtual touch quantification and fibroscan M and XL probes according to the skin capsular distance. J. Gastroenterol.51, 496–505 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Karlas, T. et al. Individual patient data meta-analysis of controlled Attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol.66, 1022–1030 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Hsu, C. et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: A systematic review and pooled analysis of individual participants. Clin. Gastroenterol. Hepatol.17, 630–637e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology41, 1313–1321 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Youden, W. J. Index for rating diagnostic tests. Cancer3, 32–35 (1950). [DOI] [PubMed] [Google Scholar]
- 23.DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics44, 837–845 (1988). [PubMed] [Google Scholar]
- 24.Ahn, S. B. Noninvasive serum biomarkers for liver steatosis in nonalcoholic fatty liver disease: current and future developments. Clin. Mol. Hepatol.29, S150–S156 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bedogni, G. et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol.6, 33 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, J. et al. ZJU index: a novel model for predicting nonalcoholic fatty liver disease in a Chinese population. Sci. Rep.5, 16494 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotronen, A. et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology137, 865–872 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Long, M. T. et al. Development and validation of the Framingham steatosis index to identify persons with hepatic steatosis. Clin. Gastroenterol. Hepatol.14, 1172–1180e2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nogami, A. et al. Non-invasive imaging biomarkers for liver steatosis in non-alcoholic fatty liver disease: present and future. Clin. Mol. Hepatol.29, S123–S135 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yajima, Y. et al. Ultrasonographical diagnosis of fatty liver: significance of the liver-kidney contrast. Tohoku J. Exp. Med.139, 43–50 (1983). [DOI] [PubMed] [Google Scholar]
- 31.Tada, T. et al. Usefulness of Attenuation imaging with an ultrasound scanner for the evaluation of hepatic steatosis. Ultrasound Med. Biol.45, 2679–2687 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Koizumi, Y. et al. New diagnostic technique to evaluate hepatic steatosis using the Attenuation coefficient on ultrasound B mode. PLoS One. 14, e0221548 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eddowes, P. J. et al. Accuracy of fibroscan controlled Attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology156, 1717–1730 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Imajo, K. et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology150, 626–637e7 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Andersson, A. et al. Clinical utility of magnetic resonance imaging biomarkers for identifying nonalcoholic steatohepatitis patients at high risk of progression: A multicenter pooled data and Meta-Analysis. Clin. Gastroenterol. Hepatol.20, 2451–2461e3 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Jayakumar, S. et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: analysis of data from a phase II trial of Selonsertib. J. Hepatol.70, 133–141 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Cai, C., Song, X., Chen, Y., Chen, X. & Yu, C. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Hepatol. Int.14, 115–126 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitz, S. M. et al. Association of body composition and sarcopenia with NASH in obese patients. J. Clin. Med.10, 3445 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim, M. J. et al. Association between metabolic dysfunction-associated steatotic liver disease and myosteatosis measured by computed tomography. J. Cachexia Sarcopenia Muscle. 15, 1942–1952 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitajima, Y. et al. Severity of non-alcoholic steatohepatitis is associated with substitution of adipose tissue in skeletal muscle. J. Gastroenterol. Hepatol.28, 1507–1514 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Burakiewicz, J. et al. Quantifying fat replacement of muscle by quantitative MRI in muscular dystrophy. J. Neurol.264, 2053–2067 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lonardo, A., Nascimbeni, F., Mantovani, A. & Targher, G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J. Hepatol.68, 335–352 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Simon, T. G., Roelstraete, B., Hagström, H., Sundström, J. & Ludvigsson, J. F. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: results from a nationwide histology cohort. Gut71, 1867–1875 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Park, H., Dawwas, G. K., Liu, X. & Nguyen, M. H. Nonalcoholic fatty liver disease increases risk of incident advanced chronic kidney disease: a propensity-matched cohort study. J. Intern. Med.286, 711–722 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams, L. A., Anstee, Q. M., Tilg, H. & Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut66, 1138–1153 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Koo, B. K. et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol.66, 123–131 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Pan, X. Y. et al. Low skeletal muscle mass is associated with more severe histological features of non-alcoholic fatty liver disease in male. Hepatol. Int.16, 1085–1093 (2022). [DOI] [PubMed] [Google Scholar]
- 48.Kim, M. N. et al. KASL clinical practice guidelines for noninvasive tests to assess liver fibrosis in chronic liver disease. Clin. Mol. Hepatol.30, S5–S105 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.