Abstract
Intact core tetraether membrane lipids of marine planktonic Crenarchaeota were quantified in water column-suspended particulate matter obtained from four depth intervals (∼70, 500, 1,000 and 1,500 m) at seven stations in the northwestern Arabian Sea to investigate the distribution of the organisms at various depths. Maximum concentrations generally occurred at 500 m, near the top of the oxygen minimum zone, and the concentrations at this depth were, in most cases, slightly higher than those in surface waters. In contrast, lipids derived from eukaryotes (cholesterol) and from eukaryotes and bacteria (fatty acids) were at their highest concentrations in surface waters. This indicates that these crenarchaeotes are not restricted to the photic zone of the ocean, which is consistent with the results of recent molecular biological studies. Since the Arabian Sea has a strong oxygen minimum zone between 100 and 1,000 m, with minimum oxygen levels of <1 μM, the abundance of crenarchaeotal membrane lipids at 500 m suggests that planktonic Crenarchaeota are probably facultative anaerobes. The cell numbers we calculated from the concentrations of membrane lipids are similar to those reported for the Central Pacific Ocean, supporting the recent estimation of M. B. Karner, E. F. DeLong, and D. M. Karl (Nature 409:507-510, 2001) that the world's oceans contain ca. 1028 cells of planktonic Crenarchaeota.
The Archaea form one of the three domains of living organisms on Earth (43). Until recently, archaea were thought to inhabit only ecological niches characterized by extreme conditions such as high salinity, high temperatures, or anoxia. However, rRNA analyses of environmental samples have indicated that nonextremophilic archaea are widespread (3, 4, 7, 9, 10, 23, 24; K. L. Hershberger, S. M. Barns, A. L. Reysenbach, S. C. Dawson, and N. R. Pace, Letter, Nature 384:420, 1996) and abundant; they comprise up to 34% of the prokaryotic biomass (4) and account for 20% of marine picoplankton (17). Of the three major groups of nonthermophilic archaea identified so far but as yet uncultured, the so-called group 1 Crenarchaeota appear to be the most widely distributed, abundant, and ecologically diverse, and they form a phylogenetically distinct subgroup (5). Estimations indicate that there are ca. 1028 cells of planktonic Crenarchaeota in the ocean (17). Furthermore, their phylogenetic position suggests that these nonthermophilic and pelagic Crenarchaeota have thermophilic ancestries (5). These findings indicate the presence of a newly described and quantitatively important group of microbes in the marine water column, yet their ecology, physiology, and role in the marine carbon cycle are far from understood.
An independent line of evidence, the distribution of characteristic archaeal membrane lipids, supports the hypothesis that marine crenarchaeotes are ubiquitous in the marine water column. Cleavage of ether bonds in water column particulate and sedimentary organic matter has indicated that ether-bound cyclic biphytanes are abundant (15, 18, 35). Until a few years ago, the only known biological occurrence of such biphytanes was in the glycerol dibiphytanyl glycerol tetraethers (GDGTs) of hyperthermophilic archaea (8). Since ether-bound cyclic biphytanes were found in particulate matter throughout the marine water column, it was proposed that they derive from nonthermophilic, planktonic archaea (15). A direct link between the phylogenetic and membrane lipid data was established by the presence of these ether-bound cyclic biphytanes in the only available “culture” of a group 1 crenarchaeote, Cenarchaeum symbiosum (6). This archaeon lives in symbiosis with the sponge Axinella mexicana and can be harvested in sufficient quantity and purity to allow biochemical and genetic analysis.
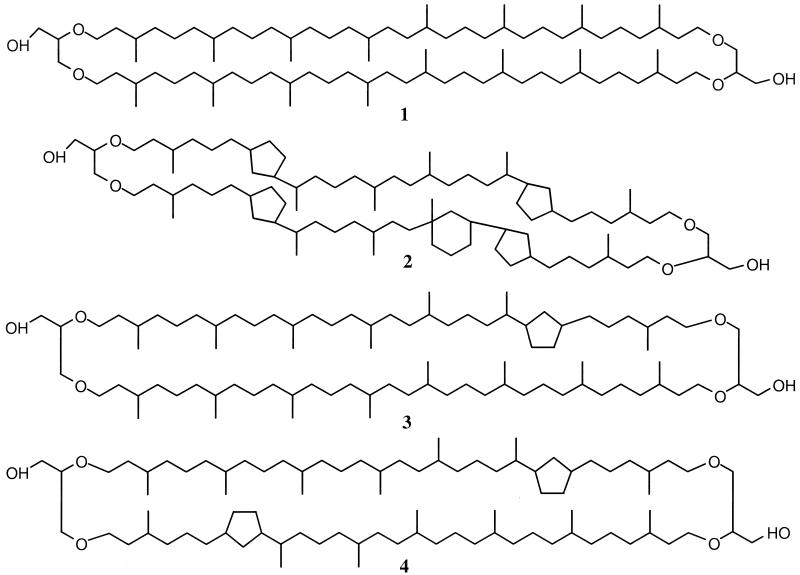
The development of a combined high-performance liquid chromatography-mass spectrometry (HPLC-MS) technique (16) has enabled us to characterize and determine concentrations of intact GDGTs. In addition to containing GDGTs with no rings (GDGT-0) (Fig. 1) and those with one to three cyclopentyl rings, marine surface sediments consistently contain another GDGT (36), which is also the most abundant GDGT in C. symbiosum (J. S. Sinninghe Damsté, S. Schouten, E. C. Hopmans, A. C. T. van Duin, and J. A. J. Geenevasen, unpublished data). Its structure (Fig. 1) has been identified by isolation and two-dimensional high-resolution nuclear magnetic resonance studies. It has been proposed that this compound be named “crenarchaeol,” because it appears to be derived largely from planktonic crenarchaeotes (Sinninghe Damsté et al., unpublished data). Crenarchaeol is composed of a dicyclic biphytane and a tricyclic biphytane, of which only the dicyclic biphytane is known from GDGTs of cultured hyperthermophilic archaea (8). The proposed tricyclic biphytane is structurally different from tricyclic biphytanes from cultured thermophilic archaea, since it possesses a cyclohexyl ring rather than a third cyclopentyl moiety. Note that the structure of this tricyclic biphytanyl of crenarchaeol is different from that of the tricyclic biphytanyl containing three cyclopentyl rings proposed previously for the GDGT of C. symbiosum (6) and for GDGTs found in a number of suspended-particulate-matter (SPM) and sediment samples (6, 15, 18, 35). This distinction is based on a recent structural elucidation by nuclear magnetic resonance, a technique that is more rigorous than the MS used in the past.
FIG. 1.
Structures of GDGTs present in water filtrates from the Arabian Sea. Shown are the structures of GDGT-0 (structure 1), crenarchaeol (structure 2), GDGT with one cyclopentane ring (structure 3), and GDGT with two cyclopentane rings (structure 4).
The ability to use crenarchaeol as a specific biomarker for nonthermophilic, planktonic Crenarchaeota in the marine water column allows a study of the distribution and ecological significance of crenarchaeotes as an important component of marine plankton. Here we report the results of a quantitative analysis of crenarchaeol and related GDGTs in water samples from the Arabian Sea. We compare the distribution of crenarchaeol in the water column with distributions of eukaryotic and bacterial biomarkers, and we use these data to shed further light on the ecology of cosmopolitan marine Crenarchaeota.
MATERIALS AND METHODS
Samples.
Samples of SPM were obtained at seven sites in the northwestern Arabian Sea during the U.S. Joint Global Ocean Flux Study (JGOFS) Arabian Sea Process Study cruise of the research vessel Thomas G. Thompson (cruise number TTN047) in May 1995. The sampling sites were as follows: station 2 (15°58′N, 61°29′E), station 4 (17°12′N, 59°35′E), station 5 (17°24′N, 58°49′E), station 6 (17°41′N, 57°50′E), station 7 (17°40′N, 57°41′E), station 10 (17°44′N, 57°29′E), and station 13 (19°13′N, 58°31′E). All stations (except station 13) form a transect perpendicular to the coast of Oman, with the most remote station being ca. 580 km from the coast, and this transect corresponds to the southern section of the U.S. JGOFS Arabian Sea Process Study, for which a large set of oceanographic data are available (http://usjgofs.whoi.edu). Strong coastal upwelling of cold, nutrient-rich bottom waters induced by the southeast and northwest monsoons during winter and summer, respectively, result in large numbers of phytoplankton. Our samples were taken during the spring intermonsoon period in mid-May 1995 (21).
At specific depths in the water column (Table 1), SPM samples were collected by filtration through 292-mm-diameter, precombusted (at 450°C for 4 h) glass fiber filters (nominal pore size, 0.7 μm) by use of a Challenger Oceanic Mark II in situ pump. For each sample, a large volume of water (2,400 to 3,000 liters) was filtered in situ over a period of ∼2 h. Upon collection of the sample and retrieval of the pump, the filter was immediately cut in half and each half was frozen (−20°C) and stored in a polycarbonate petri dish wrapped in aluminum foil until needed for chemical analysis. Each biomarker analysis in this study was done with one filter half. We recognized that filtration through 0.7-μm-pore-size filters may lead to an undersampling of archaeal cells unless the cells are associated with larger aggregates (22), so the calculated concentrations of archaeal lipids may represent lower-than-normal limits.
TABLE 1.
Description of field stations, hydrographic properties, and concentrations of GDGTs, cholesterol, and hexadecanoic acid
| Station | Location | Date(s) (mo/day) in 1995 | Depth (m) | Hydrographic propertya
|
Biomarker concentration (ng/liter)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp (°C) | [O2] (mg/liter) | [NO3−] (μM) | Crenarchaeol | GDGT-0 | Other GDGTs | Cholesterol | Hexadecanoic acid | ||||
| 2 | 15°58′N, | 5/5-5/6 | 60 | 25.1 | 4.2 | 3.0 | 0.4 | 0.1 | 0.0 | 45 | 161 |
| 61°29′E | 500 | 12.0 | 0.05 | 30 | 6.8 | 0.8 | 2.2 | 7.5 | 3.3 | ||
| 1,000 | 8.8 | 0.16 | 38 | 1.8 | 0.4 | 0.7 | 6.9 | 1.1 | |||
| 1,500 | 5.3 | 1.0 | 40 | 2.5 | 0.9 | 1.4 | 4.3 | 6.2 | |||
| 4 | 17°12′N, | 5/8-5/9 | 70 | 23.6 | 2.8 | 12 | 6.9 | 2.3 | 3.0 | 28 | 104 |
| 59°35′E | 500 | 12.5 | 0.08 | 29 | 7.9 | 2.2 | 3.2 | 6.8 | 3.9 | ||
| 1,000 | 9.0 | 0.15 | 38 | 5.2 | 1.6 | 2.3 | 3.4 | 5.7 | |||
| 1,500 | 5.1 | 1.0 | 40 | 2.7 | 1.1 | 1.3 | 4.0 | 1.7 | |||
| 5 | 17°24′N, | 5/10-5/11 | 90 | ∼20 | ∼0.5 | ∼20 | 34 | 9.6 | 13 | 55 | 182 |
| 58°49′E | 500 | 12.3 | 0.18 | 31 | 14 | 2.4 | 4.0 | 9.2 | 1.6 | ||
| 1,500 | 5.7 | 0.89 | 40 | 3.2 | 1.0 | 1.3 | 3.3 | 1.0 | |||
| 6 | 17°41′N, | 5/13 | 500 | 12.1 | 0.20 | 31 | 13 | 1.9 | 4.1 | 6.3 | 1.4 |
| 57°50′E | 1,000 | 9.1 | 0.23 | 37 | 5.2 | 1.6 | 2.2 | 5.7 | 1.6 | ||
| 7 | 17°40′N, 57°40′E | 5/14 | 85 | 24.2 | 3.2 | 11 | 1.4 | 0.3 | 0.5 | 34 | 46 |
| 10 | 17°44′N, | 5/16 | 80 | 24.2 | 3.2 | 11 | 8.2 | 2.7 | 3.5 | 29 | 103 |
| 57°29′E | 450 | 12.5 | 0.12 | 29 | 1.8 | 0.6 | 0.8 | 12 | 7.5 | ||
| 13 | 19°13′N, | 5/16-5/17 | 35 | NAb | NA | NA | 12 | 6.2 | 3.6 | 53 | 82 |
| 58°31′E | 500 | NA | NA | NA | 12 | 2.9 | 4.7 | 7.1 | 2.4 | ||
| 1,000 | NA | NA | NA | 8.3 | 2.4 | 2.8 | 7.0 | 2.1 | |||
Data are from nearby stations S7 (for station 2), S4 (for station 4), S3 (for station 5), and S2 (for stations 6, 7, and 10) of the U.S. JGOFS Arabian Sea Process Study cruise 2 (see http://usjgofs.whoi.edu) in early April 1995.
NA, not available.
Lipid analyses.
Filters containing SPM were ultrasonically extracted with methanol, dichloromethane-methanol (1:1, vol/vol), and dichloromethane (three times). The combined extracts were concentrated with a rotary evaporator and dried over a small pipette filled with anhydrous Na2SO4. For GDGT analysis, a subsample of the total extract was dissolved in a known volume of hexane-propanol (99:1, vol/vol) and subsequently filtered. An aliquot was analyzed by HPLC-atmospheric-pressure-positive ion chemical ionization-MS under the conditions described previously (16). GDGTs were quantified by the integration of peaks in summed mass chromatograms of [M + H]+ and [M + H]+ + 1 ions, and the values were compared with a standard curve obtained with a GDGT-0 standard. Repeated measurements were within 10% of each other. The GDGTs analyzed did not contain polar head groups. Treatment of the extracts or residual filters with acid did not result in the formation of significant additional quantities of GDGTs.
Sterols and fatty acids were analyzed by methylation (diazomethane in diethyl ether) of an aliquot of the total extract followed by chromatography over SiO2 and subsequent silylation of alcohols with BSTFA [N,O-bis(trimethylsilyl)trifluoroacetamide] in pyridine. Twenty micrograms of an internal standard was added to allow quantitation of the compounds. The obtained fractions were analyzed by gas chromatography (GC) (model HP 6890 chromatograph equipped with a CP-Sil 5 capillary column [inside diameter, 0.25 mm; length, 25 m; film thickness, 0.25 μm]) with He as the carrier gas and the temperature programmed to increase from 70 to 130°C at 20°C·min−1 and then from 130 to 320°C at 4°C·min−1; at 320°C, the chromatograph was maintained at the same temperature for 20 min. Repeated measurements of the concentrations were within 10% of each other. For identification purposes, the total lipid fractions were also analyzed by GC and MS with an HP 5890 series II gas chromatograph (with a CP-Sil 5 column identical to that of the HP 6890 model) coupled to a VG Autospec Q Ultima mass spectrometer (cycle time, 1.7 s; resolution, 1,000).
RESULTS AND DISCUSSION
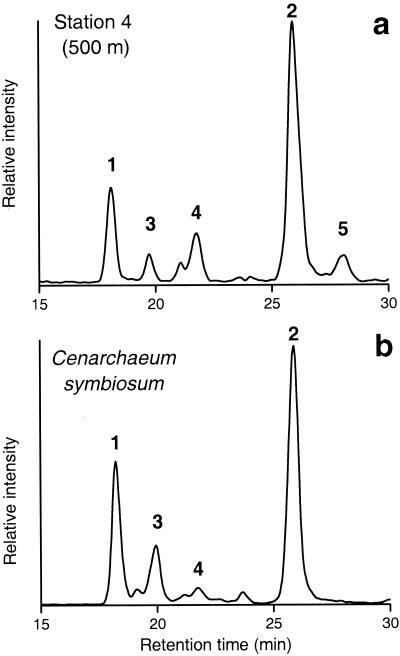
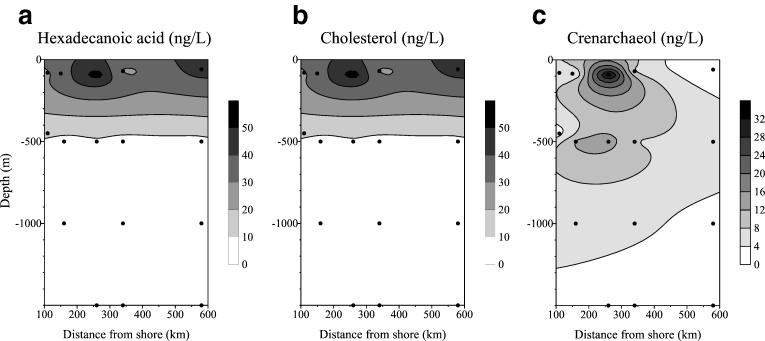
All SPM samples contained a series of GDGTs, which were dominated by crenarchaeol (Fig. 2a). In addition, smaller amounts of GDGT-0 and GDGTs with one to three cyclopentane rings were identified. Crenarchaeol concentrations ranged from <1 to 35 ng/liter, whereas concentrations of GDGT-0 and of other GDGTs with cyclopentane rings were typically an order of magnitude lower (Table 1). For comparison, the concentrations of cholesterol and hexadecanoic acid were also determined by GC and GC-MS analyses. Hexadecanoic acid concentrations varied from 1 to 180 ng/liter, with the highest values being obtained from the surface water samples (Table 1). Cholesterol concentrations were also at their highest in the upper water column but showed a slightly smaller range, from 3 to 55 ng/liter (Table 1).
FIG. 2.
Shown are a partial base peak chromatogram obtained by HPLC-atmospheric-pressure-positive ion chemical ionization-MS of the extract of the SPM obtained from station 4 (water depth, 500 m) indicating the distribution of crenarchaeol GDGTs (a) and, for comparison, a partial base peak chromatogram of the acid extract of C. symbiosum (Sinninghe Damsté et al., unpublished data) (b). The occurrence of GDGT-0 (peak 1), crenarchaeol (peak 2), GDGT with one cyclopentane ring (peak 3), GDGT with two cyclopentane rings (peak 4), and an isomer of crenarchaeol (peak 5) is indicated.
Distribution of archaeal membrane lipids in the marine water column.
The concentrations of crenarchaeol and other GDGTs were highest at most sites at a water depth of 500 m. This is in strong contrast to the concentration profiles of hexadecanoic acid, a lipid that occurs ubiquitously in bacteria and eukaryotes (34), and cholesterol, a lipid restricted to eukaryotes (40). These lipids show a 5-fold to 10-fold drop in concentration between surface waters and water at a depth of 500 m (Table 1; Fig. 3). The trends for the hexadecanoic acid and cholesterol concentrations are consistent with the fact that most biological productivity, zooplankton grazing, and bacterial decomposition takes place in surface waters, as indicated, for example, by the distributions of carotenoids (20), phytoplankton (1), zooplankton grazing activity (38), and bacterial activity (12) along the southern section covered by the Arabian Sea Process Study. The drop in the cholesterol concentration between surface waters and water at 500 m is less than that of the hexadecanoic acid concentration. This is because sterols are generally more stable than fatty acids (41) and most cholesterol is derived from zooplankton from ingested algal steroids and is rapidly transported into deep waters (41) by fecal pellets (2, 13). GDGTs are, however, only slightly more stable than sterols (37), so the abundance of crenarchaeol at a greater depth is unlikely to result from differences in turnover.
FIG. 3.
Contour plots of concentrations (in nanograms per liter of seawater) of hexadecanoic acid (a), cholesterol (b), and crenarchaeol (c) at various depths in the water column and distances from shore on a northwest-to-southeast transect off Oman in the Arabian Sea (stations 2, 4, 5, 6, 7, and 10). The black dots indicate positions where samples were collected. These data clearly reveal that marine Crenarchaeota are more abundant at greater water depths than eukaryotes and bacteria.
The concentrations of organic carbon and lipids associated with suspended particles at a given depth depend on the amounts of suspended living biomass and suspended detrital particles produced by disaggregation of larger particles sinking from above. Most organic carbon and lipids produced in surface waters are mineralized in the upper ocean, as indicated by the decreases in flux of 1 to 2 orders of magnitude measured by sediment traps in the Arabian Sea (21, 42) and by the decreases in the suspended particulate cholesterol and hexadecanoic acid concentrations described above. Deepwater concentration maxima for suspended lipids probably do not result, therefore, from the sinking and subsequent disaggregation of detrital aggregates. Rather, we suspect that the high deepwater concentrations of GDGTs derived from pelagic archaea result from in situ production. If so, then this indicates that the source crenarchaeotes are distributed over the entire water column and are not restricted to the upper water column as are most eukaryotic and bacterial cells.
Our biomarker-derived observation that pelagic crenarchaeotes are widely distributed throughout the water column is in good agreement with recent data of DeLong and coworkers (7) and Karner and coworkers (17). Those authors used fluorescent in situ hybridization to determine the cell counts of pelagic marine Crenarchaeota at station ALOHA in the Central Pacific Ocean over the annual cycle. They found that waters shallower than 500 m contained about 4 × 104 crenarchaeotal cells·ml−1, while waters below 1,000 m contained 0.3 × 104 to 0.7 × 104 cells · ml−1. The number of bacterial cells, in contrast, decreased from about 3 × 105 cells · ml−1 to 0.3 × 104 to 0.7 × 104 cells·ml−1 over the same depth intervals. Consequently, the global oceans contain approximately 1.3 × 1028 archaeal cells and 3.1 × 1028 bacterial cells. However, the proportion of crenarchaeotal to bacterial microorganisms changes with depth, with the 1:10 ratio of archaea to bacteria in surface waters shifting to 1:1 in deep waters.
If all GDGTs are derived from living cells, then it is possible to estimate the number of crenarchaeotal cells in the waters of the Arabian Sea. This calculation requires knowledge of the amount of GDGTs per cell. If we assume that the cell dimensions of marine Crenarchaeota are identical to those of C. symbiosum (0.8- by 0.5-μm rods [33]), which is supported by field data (7), and that 1 μm2 of archaeal cell membrane contains ca. 1.7 × 105 GDGT molecules (11), then marine Crenarchaeota would contain 1.0 × 10−3 pg of GDGT per cell. This estimate of cellular GDGT is remarkably similar to the 0.5 × 10−3 pg of hexadecanoic acid per cell reported for bacterial membranes (14). Using the summed concentrations of GDGTs, we find that there are, on average, 1.5 × 104 and 0.7 × 104 cells · ml−1 in waters that are <500 m and >500 m deep, respectively. These values are similar to those reported by Karner et al. (17) for the Central Pacific (4 × 104 cells · ml−1 at depths of <500 m and 0.3 × 104 to 0.7 × 104 cells · ml−1 at depths of >1,000 m) and support their estimation that there are ca. 1028 cells of this specific clade of planktonic Crenarchaeota in the ocean.
Ecology of marine Crenarchaeota.
Despite the fact that marine Crenarchaeota are abundant in the ocean, very little is known about their ecology and role in the carbon cycle. Attempts to culture these organisms have been unsuccessful to date, and thus we have to rely on available field-based ecological studies. The depth distributions of marine Crenarchaeota in the coastal waters of Antarctica (27), the Santa Barbara Channel (7, 28), the Central Pacific (17), and the Arabian Sea (this study) show that these organisms are not restricted to the photic zone and are likely not photoautotrophs. There is evidence that marine Crenarchaeota are chemoautotrophs. Compound-specific radiocarbon analyses of biphytanyl moieties of crenarchaeol suggest that marine Crenarchaeota use “old” 14C-depleted dissolved inorganic carbon from well below the photic zone in the water column (32). The 13C contents of biphytanyl moieties of crenarchaeol from both present-day (15) and ancient (15, 19) marine environments indicate that marine Crenarchaeota probably fix bicarbonate.
However, chemoautotrophy by marine Crenarchaeota seems to be at odds with the results of the study of Ouverney and Fuhrman (31), who showed that marine planktonic archaea take up amino acids, suggesting that they are heterotrophs. In a detailed study of the spatial and temporal variability of marine Crenarchaeota in the Santa Barbara Channel, however, Murray et al. (28) showed that their abundance is inversely correlated with the level of chlorophyll a. Such a correlation was not expected for a heterotrophic organism that lives on photoautotrophically produced organic matter.
Our data now add at least one important piece to the puzzle: marine Crenarchaeota can cope with extremely low oxygen levels. The Arabian Sea is characterized by a strong oxygen minimum zone in water at depths between 100 and 1,000 m, with minimum oxygen levels of <1 μM (25, 26) (Table 1). We found that concentrations of crenarchaeol (and probably cell counts) at water depths of 500 m are generally higher than those at the surface. This suggests that some marine Crenarchaeota are facultative anaerobes. In fact, some marine Crenarchaeota do not need oxygen at all for their cell metabolism. King et al. (18) reported biphytanyl moieties of crenarchaeol in SPM from anoxic waters of the Black Sea in concentrations similar to those in SPM from oxic surface waters. In contrast to the waters of the oxygen minimum zone in the Arabian Sea, Black Sea waters below the oxycline do not contain traces of oxygen but have sulfide levels of around 20 μM. These data indicate that marine Crenarchaeota, like most other cultivated Crenarchaeota, do not need oxygen for their cell metabolism.
The question regarding the energy source for marine Crenarchaeota remains unanswered. Murray et al. (28) noted a positive correlation between the hybridization signal of marine Crenarchaeota and combined nitrate and nitrite concentrations in the Santa Barbara Channel and suggested a potential connection. The high concentrations of crenarchaeol that we observed in the Arabian Sea also correspond to the nitrate minimum and nitrite maximum concentrations at water depths of 200 to 300 m observed during the spring intermonsoon period (25, 26). In the studied transect, the subsurface nitrite maximum concentration is most well developed far from the coast, where it may occur throughout the year (25, 26, 29). The cooccurrence of suboxic conditions and the maximum nitrite concentration indicates that denitrification is the dominant respiratory pathway under these conditions. Naqvi et al. (30) noted that the denitrification zone in the Arabian Sea is geographically separate from the zone of highest primary production and pointed out that lateral transport of organic matter is required to sustain denitrification.
Are the Crenarchaeota that generate the subsurface crenarchaeol involved in denitrification in the Arabian Sea? Denitrification is known in the kingdom Crenarchaeota: the hyperthermophilic autotrophic archaeon Pyrobaculum aerophilum reduces nitrate to nitrite by use of hydrogen (39). This archaeon is actually one of the few archaea of the Crenarchaeota that are aerophilic, which we infer to be the case for some marine Crenarchaeota. Perhaps marine Crenarchaeota and P. aerophilum evolved from common ancestors and marine Crenarchaeota adjusted to a cold-water habitat (19) but still use a cell metabolism similar to that of P. aerophilum, as they have preserved their lipid biosynthetic pathways. Clearly, this is speculation that suggests that more work is needed. However, if true, these chemoautotrophic archaea may play an important role in the global marine carbon cycle.
Conclusions.
(i) Intact core tetraether lipids derived from membranes of nonthermophilic marine Crenarchaeota have been identified in the marine water column of the Arabian Sea. (ii) The depth distribution of these lipids indicates that marine Crenarchaeota are not restricted to the upper water column as are most marine organisms but that they also thrive in the deepwater column below the photic zone. (iii) Since maximum concentrations of crenarchaeotal lipids occur where the oxygen concentration is at a minimum and the nitrite concentration is at a maximum, we suggest that these marine Crenarchaeota can cope with very low oxygen levels and thus are facultative anaerobes, and we further speculate that they may be chemoautotrophs involved in denitrification.
Acknowledgments
We are grateful to Chi Meredith (OSU) for assistance in collecting all high-volume in situ pump samples, and we thank E. F. DeLong for his generous gift of C. symbiosum cell material. We also thank the three anonymous referees for their helpful comments.
We are grateful to the U.S. National Science Foundation for support (grant OCE-9310686 to F.G.P. and grant OCE-9310364 to S.G.W.) of the sampling. This study was partially supported by the Research Council for Earth and Life Sciences (ALW) and by financial aid from The Netherlands Organization for Scientific Research (grant ALW 809.33.001).
Footnotes
Contribution 3660 from the Royal Netherlands Institute for Sea Research.
REFERENCES
- 1.Caron, D. A., and M. R. Dennett. 1999. Phytoplankton growth and mortality during the 1995 northeast monsoon and spring intermonsoon in the Arabian Sea. Deep-Sea Res. Part II 46:1665-1690. [Google Scholar]
- 2.Corner, E. D. S., S. C. M. O'Hara, A. C. Neal, and G. Eglinton. 1986. Copepod faecal pellets and the vertical flux of biolipids, p. 260-321. In E. D. S. Corner and S. C. M. O'Hara (ed.), The biological chemistry of marine copepods. Oxford Scientific, Oxford, England.
- 3.DeLong, E. F. 1992. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 89:5685-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeLong, E. F., K. Ying Wu, B. B. Prezelin, and R. V. M. Jovine. 1994. High abundance of Archaea in Antarctic marine picoplankton. Nature 371:695-697. [DOI] [PubMed] [Google Scholar]
- 5.DeLong, E. F. 1998. Everything in moderation: Archaea as “non-extremophiles.” Curr. Opin. Genet. Dev. 8:649-654. [DOI] [PubMed] [Google Scholar]
- 6.DeLong, E. F., L. L. King, R. Massana, H. Cittone, A. Murray, C. Schleper, and S. G. Wakeham. 1998. Dibiphytanyl ether lipids in nonthermophilic crenarchaeotes. Appl. Environ. Microbiol. 64:1133-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLong, E. F., L. T. Taylor, T. L. Marsh, and C. M. Preston. 1999. Visualization and enumeration of marine planktonic archaea and bacteria by using polyribonucleotide probes and fluorescent in situ hybridization. Appl. Environ. Microbiol. 65:5554-5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Rosa, M., and A. Gambacorta. 1988. The lipids of archaebacteria. Prog. Lipid Res. 27:153-175. [DOI] [PubMed] [Google Scholar]
- 9.Fuhrman, J. A., K. McCallum, and A. A. Davis. 1992. Novel major archaebacterial group from marine plankton. Nature 356:148-149. [DOI] [PubMed] [Google Scholar]
- 10.Fuhrman, J. A., K. McCallum, and A. A. Davis. 1993. Phylogenetic diversity of subsurface marine microbial communities from the Atlantic and Pacific Oceans. Appl. Environ. Microbiol. 59:1294-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabriel, J. L., and P. L. Chong. 2000. Molecular modeling of archaebacterial bipolar tetraether lipid membranes. Chem. Phys. Lipids 105:193-200. [DOI] [PubMed] [Google Scholar]
- 12.Garrison, D. L., M. M. Gowing, M. P. Hughes, L. Campbell, D. A. Caron, M. R. Dennett, A. Shalapyonok, R. J. Olson, M. R. Landrey, S. L. Brown, H.-B. Liu, F. Azam, G. F. Steward, H. W. Ducklow, and D. C. Smith. 2000. Microbial food web structure in the Arabian Sea: a U.S. JGOFS study. Deep-Sea Res. Part II 47:1387-1422. [Google Scholar]
- 13.Grice, K., W. C. M. Klein Breteler, S. Schouten, V. Grossi, J. W. de Leeuw, and J. S. Sinninghe Damsté. 1998. The effects of zooplankton herbivory on biomarker proxy records. Paleoceanography 13:686-693. [Google Scholar]
- 14.Guckert, J. B., C. P. Antworth, P. D. Nichols, and D. C. White. 1985. Phospholipid, ester-linked fatty acid profiles as reproducible assays for changes in prokaryotic community structure of estuarine sediments. FEMS Microbiol. Ecol. 31:147-158. [Google Scholar]
- 15.Hoefs, M. J. L., S. Schouten, L. King, S. G. Wakeham, J. W. de Leeuw, and J. S. Sinninghe Damsté. 1997. Ether lipids of planktonic archaea in the marine water column. Appl. Environ. Microbiol. 63:3090-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopmans, E. C., S. Schouten, R. D. Pancost, M. J. T. van der Meer, and J. S. Sinninghe Damsté. 2000. Analysis of intact tetraether lipids in archaeal cell material and sediments by high performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 14:585-589. [DOI] [PubMed] [Google Scholar]
- 17.Karner, M. B., E. F. DeLong, and D. M. Karl. 2001. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409:507-510. [DOI] [PubMed] [Google Scholar]
- 18.King, L. L., T. K. Pease, and S. G. Wakeham. 1998. Archaea in Black Sea water column particulate matter and sediments—evidence from ether lipid derivatives. Org. Geochem. 28:677-688. [Google Scholar]
- 19.Kuypers, M. M. M., P. Blokker, J. Erbacher, H. Kinkel, R. D. Pancost, S. Schouten, and J. S. Sinninghe Damsté. 2001. Massive expansion of marine archaea during a mid-Cretaceous oceanic anoxic event. Science 293:92-95. [DOI] [PubMed] [Google Scholar]
- 20.Latasa, M., and R. R. Bidigare. 1998. A comparison of phytoplankton populations of the Arabian Sea during the spring intermonsoon and southwest monsoon of 1995 as described by HPLC-analyzed pigments. Deep-Sea Res. Part II 45:2133-2170. [Google Scholar]
- 21.Lee, C., D. W. Murray, R. T. Barber, K. O. Buesseler, J. Dymond, J. I. Hedges, S. Honjo, S. J. Manganini, J. Marra, C. Moser, M. L. Peterson, W. L. Prell, and S. G. Wakeham. 1998. Particulate organic carbon fluxes: compilation of results from the 1995 US JGOFS Arabian Sea Process Study. Deep-Sea Res. Part II 45:2489-2501. [Google Scholar]
- 22.Lee, S., Y.-C. Kang, and J. A. Fuhrman. 1995. Imperfect retention of natural bacterioplankton cells by glass fiber filters. Mar. Ecol. Prog. Ser. 119:285-290. [Google Scholar]
- 23.MacGregor, B. J., D. P. Moser, E. W. Alm, K. H. Nealson, and D. A. Stahl. 1997. Crenarchaeota in Lake Michigan sediment. Appl. Environ. Microbiol. 63:1178-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massana, R., E. F. DeLong, and C. Pedros-Alio. 2000. A few cosmopolitan phylotypes dominate planktonic archaeal assemblages in widely different oceanic provinces. Appl. Environ. Microbiol. 66:1777-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison, J. M., L. A. Codispoti, S. Gaurin, B. Jones, V. Manghnani, and Z. Zheng. 1998. Seasonal variation of hydrographic and nutrient fields during the US JGOFS Arabian Sea Process Study. Deep-Sea Res. Part II 45:2053-2101. [Google Scholar]
- 26.Morrison, J. M., L. A. Codispoti, S. L. Smith, K. Wishner, C. Flagg, W. D. Gardener, S. Gaurin, S. W. A. Naqvi, V. Manghnani, L. Prosperie, and J. S. Gundersen. 1999. The oxygen minimum zone in the Arabian Sea during 1995. Deep-Sea Res. Part II 46:1903-1931. [Google Scholar]
- 27.Murray, A. E., C. M. Preston, R. Massana, L. T. Taylor, A. Blakis, K. Wu, and E. F. DeLong. 1998. Seasonal and spatial variability of bacterial and archaeal assemblages in the coastal waters near Anvers Island, Antarctica. Appl. Environ. Microbiol. 64:2585-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray, A. E., A. Blakis, R. Massana, S. Strawzewski, U. Passow, A. Alldredge, and E. F. DeLong. 1999. A time series assessment of planktonic archaeal variability in the Santa Barbara Channel. Aquat. Microb. Ecol. 20: 129-145. [Google Scholar]
- 29.Naqvi, S. W. A. 1991. Geographical extent of denitrification in the Arabian Sea in relation to some physical processes. Oceanol. Acta 14:281-290. [Google Scholar]
- 30.Naqvi, S. W. A., R. J. Noronha, M. S. Shailaja, K. Somasundar, and R. Sen Gupta. 1992. Some aspects of the nitrogen cycling in the Arabian Sea, p. 285-311. In B. N. Desai (ed.), Oceanography of the Indian Ocean. I. B. H. Publishing, Inc., New Delhi, India.
- 31.Ouverney, C. C., and J. A. Fuhrman. 2000. Marine planktonic archaea take up amino acids. Appl. Environ. Microbiol. 66:4829-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearson, A. 2000. Ph.D. thesis. Woods Hole Oceanographic Institution, Woods Hole, Mass.
- 33.Preston, C. M., K. Y. Wu, T. F. Molinski, and E. F. DeLong. 1996. A psychrophilic crenarchaeon inhabits a marine sponge: Cenarchaeum symbiosum gen. nov., sp. nov. Proc. Natl. Acad. Sci. USA 93:6241-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratledge, C., and S. G. Wilkinson. 1988. Microbial lipids, vol. 1. Academic Press, London, England.
- 35.Schouten, S., M. J. L. Hoefs, M. P. Koopmans, H.-J. Bosch, and J. S. Sinninghe Damsté. 1998. Structural characterization, occurrence and fate of archaeal ether-bound acyclic and cyclic biphytanes and corresponding diols in sediments. Org. Geochem. 29:1305-1319. [Google Scholar]
- 36.Schouten, S., E. C. Hopmans, R. D. Pancost, and J. S. Sinninghe Damsté. 2000. Widespread occurrence of structurally diverse tetraether membrane lipids: evidence for the ubiquitous presence of low-temperature relatives of hyperthermophiles. Proc. Natl. Acad. Sci. USA 97:14421-14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinninghe Damsté, J. S., W. I. C. Rijpstra, and G. J. Reichart. The influence of oxic degradation on the sedimentary biomarker record II. Evidence from Arabian Sea sediments. Geochim. Cosmochim. Acta, in press.
- 38.Smith, S., M. Roman, I. Prusova, K. Wishner, M. Gowing, L. A. Codispoti, R. Barber, J. Marra, and C. Flagg. 1998. Seasonal response of zooplankton to monsoonal reversals in the Arabian Sea. Deep-Sea Res. Part II 45:2369-2403. [Google Scholar]
- 39.Völkl, P., R. Huber, E. Drobner, R. Rachel, S. Burggraf, A. Trincone, and K. O. Stetter. 1993. Pyrobaculum aerophilum sp. nov., a novel nitrate-reducing hyperthermophilic archaeum. Appl. Environ. Microbiol. 59:2918-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volkman, J. K. 1986. A review of sterol markers for marine and terrigenous organic matter. Org. Geochem. 9:83-99. [Google Scholar]
- 41.Wakeham, S. G., C. Lee, J. I. Hedges, P. J. Hernes, and M. L. Peterson. 1997. Molecular indicators of diagenetic status in marine organic matter. Geochim. Cosmochim. Acta 61:5363-5369. [Google Scholar]
- 42.Wakeham, S. G., M. L. Peterson, J. I. Hedges, and C. Lee. Lipid biomarker fluxes in the Arabian Sea: with a comparison to the equatorial Pacific Ocean. Deep-Sea Res. Part II, in press.
- 43.Woese, C. R. 1987. Bacterial evolution. Microbiol. Rev. 51:221-271. [DOI] [PMC free article] [PubMed] [Google Scholar]